Abstract
Models seldom consider the effect of leaf-level biochemical acclimation to temperature when scaling forest water use. Therefore, the dependence of transpiration on temperature acclimation was investigated at the within-crown scale in climatically contrasting genotypes of Acer rubrum L., cv. October Glory (OG) and Summer Red (SR). The effects of temperature acclimation on intracanopy gradients in transpiration over a range of realistic forest growth temperatures were also assessed by simulation. Physiological parameters were applied, with or without adjustment for temperature acclimation, to account for transpiration responses to growth temperature. Both types of parameterization were scaled up to stand transpiration (expressed per unit leaf area) with an individual tree model (MAESTRA) to assess how transpiration might be affected by spatial and temporal distributions of foliage properties. The MAESTRA model performed well, but its reproducibility was dependent on physiological parameters acclimated to daytime temperature. Concordance correlation coefficients between measured and predicted transpiration were higher (0.95 and 0.98 versus 0.87 and 0.96) when model parameters reflected acclimated growth temperature. In response to temperature increases, the southern genotype (SR) transpiration responded more than the northern (OG). Conditions of elevated long-term temperature acclimation further separate their transpiration differences. Results demonstrate the importance of accounting for leaf-level physiological adjustments that are sensitive to microclimate changes and the use of provenance-, ecotype-, and/or genotype-specific parameter sets, two components likely to improve the accuracy of site-level and ecosystem-level estimates of transpiration flux.
Keywords: Intraspecific acclimation, MAESTRA, microclimate, modelling, red maple, temperature acclimation, transpiration
Introduction
Forest trees modify their canopy microclimate along a vertical gradient. Atmospheric temperature changes ranging from 0.25–1.8 °C every 1 m have been observed in both coniferous (Zweifel et al., 2002) and mixed hardwood–conifer forests (Harley et al., 1996). While air temperature generally declines with canopy depth due to within-crown shading, Martin et al. (1999) described how leaf temperatures can rise well above the ambient air temperature at the lower canopy depths. Leuzinger and Körner (2007) confirmed the findings of Martin et al. (1999) and showed that leaf temperature regimes in canopies varied enormously over short vertical distances in several conifer and deciduous broad-leaved species. In both cases, spatial variations in temperature are likely to cause concomitant changes in water exchange at the intracanopy scale.
Within-crown microclimate gradients can cause spatially explicit physiological acclimation (Harley et al., 1996; Bauerle et al., 1999; Zweifel et al., 2002; Cermak et al., 2007), however, transpiration models typically neglect temporally dynamic within-crown physiological changes. In fact, the parameters are often derived from temporally static measurements. Furthermore, they often use no more than short-term (diurnal and/or daily) data to validate estimates (Bauerle et al., 2002; Medlyn et al., 2007), potentially overlooking physiological changes brought about by leaf-level biochemical acclimation. Thus, the present study used spatially explicit measurements and modelling to integrate leaf-level biochemical acclimation to temperature when scaling water use.
Bauerle et al. (2007) previously reported that, for climatically contrasting genotypes of Acer rubrum L. (red maple), leaf photosynthetic characteristics acclimate to local microclimate temperature gradients. The present study draws on the red maple genotype differences to test the significance of within-crown growth temperature acclimation on canopy water flux. The focus of this study is not on the process of acclimation per se, but rather on the effects of leaf-level biochemical acclimation on scaling forest water flux. Our primary objective was to manipulate within-crown growth temperature to assess how within-crown temperature acclimation may affect crown and canopy transpiration over vertical distances. Consequently, the present study attempts to isolate and control the interplay among environmental variables to investigate the transpiration response to within-crown temperature gradients. We hypothesized that transpiration is controlled by a stomatal conductance (gs) feedback that results from the acclimation of photosynthesis to vertical gradients in growth temperature. This hypothesis was tested with a crown section warming experiment designed to mimic the relative natural crown daytime temperature gradient of tall trees (∼13 °C) to (i) separate temperature effects among crown layers, (ii) quantify the spatial effect of gs acclimation to growth temperature, and (iii) examine the importance of within-crown temperature acclimation on whole crown and canopy transpiration predictions. Because experimental systems for regulating tree-canopy temperature present logistical constraints, we are not aware of any studies that have decoupled light from the influence of temperature on transpiration within the foliage of individual trees under outdoor conditions. Thus, the present study looked at the within-crown growth temperature feedback together with water flux. The results were expanded with a three-dimensional individual tree process-based model (MAESTRO(A); Wang and Jarvis, 1990a) to test the net effect of scaling leaf-level and crown-section temperature-acclimated transpiration to tree and canopy levels.
Materials and methods
Plant material and study site layout
Measurements were carried out during the 2003 growing season in a 0.58 ha outdoor gravel pad of open terrain at the Clemson University Calhoun Field Laboratory in Clemson, SC, USA (latitude 34° 40′ 8″; longitude 82° 50′ 40″). A full description of the site is given in Bauerle et al. (2002). Two genotypes from thermally contrasting parentage were used for intensive sampling in this study: red maple cv. October Glory (OG), obtained from a tree growing within a Massachusetts natural population (latitude 40° 27′ 18″; longitude 74° 29′ 3″), USA, and red maple cv. Summer Red (SR), obtained from a tree growing within a southern Georgia natural population (latitude 31° 27′ 27″; longitude 83° 33′ 41″), USA. A row–column design was used, which resulted in two genotypes, two treatments (temperature-controlled and ambient outdoor conditions), and four replicate trees per treatment and genotype with randomly assigned temperature profiles. Each genotype was processed in canopy bags over two separate 50 d periods. To ensure that the ∼4 m tall 2.5-year old trees never experienced substrate water-limiting conditions, each tree was grown in oversized containers (114 l) and watered three times daily to near container capacity with 360° pressure-compensating micro emitters (ML Irrigation Inc., Laurens, SC, USA). Substrate volumetric water content was monitored daily in each container at 10 cm and 20 cm below the substrate surface in four pre-drilled locations on opposite sides of the container (Theta Probe type ML2, Delta- T Devices, Cambridge, UK) to verify that root zone volumetric water content was maintained within a previously determined well-watered range (0.4–0.5 m3 m−3).
Chamber construction and temperature control
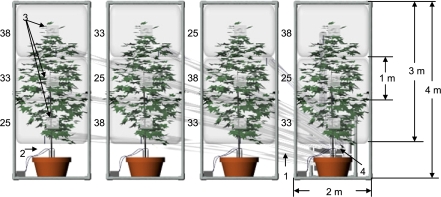
Whole crown chambers (n=4) were placed on four replicate trees per treatment. Crown chambers were subdivided by dividing each crown into three volumetrically equal area layers (Fig. 1). The daytime growth temperature of each subdivided crown section was controlled from 08.00–20.00 h. Temperature profiles were randomly assigned within each of four replicate tree crowns per treatment, thus, each crown in the temperature-controlled treatment had three different temperatures controlled at 25, 33, or 38 °C (Fig. 1). Each tree subcrown chamber (dimensions 1×1×2 m3) was constructed from 5 cm diameter polyvinyl chloride (PVC) tubing and covered with clear 0.025 mm Mylar® (DuPont, Wilmington, DE, USA). To create the self-contained crown sections, horizontal sheets of Mylar® divided each crown layer and were secured to the trunk with foam rubber gaskets. The Mylar® photon flux density (PFD) characteristics were checked with a spectroradiometer (model 1800, Li-Cor Inc., Lincoln, NE, USA). Results similar to Corelli-Grappadelli and Magnanini (1993) were found where PFD was >90% of the outside incident PFD, with midday levels exceeding 1800 μmol m−2 s−1, and the spectral composition was unchanged over the 400–900 nm range. Each subcrown chamber was plumbed independently to maintain growth temperature on an individual crown layer basis (Fig. 1). Three Twintemp 16300/10700 BTU/h cooling and heating air conditioners (model ES16, Friedrich Inc., San Antonio, TX, USA) were plumbed to the crown sections (three sections per crown) and together they continuously controlled temperature in each crown section. Each chamber's temperature was sampled at 10 s intervals with fine wire thermocouples (CR21X, Campbell Sci. Inc., Logan, UT, USA) and a control switch triggered the air conditioners to either heat, cool, or run at ambient temperature (fan only) to maintain the temperature within 1 °C of the set point. The airflow produced >3 volume exchanges min−1 per layer and created a slight positive pressure on the Mylar®, which kept it in a wrinkle-free state for maximum light penetration. A preliminary experiment found the amount of air exchange more than adequate to ensure that CO2 levels did not deviate from the outside ambient conditions. In addition, tree crowns were spaced to minimize lower crown shading and crown section treatment temperatures were randomized throughout all the canopy positions. The experimental setup, in combination with the independent temperature control per crown section, allowed us to consider each crown section a replicate (J Toler and L Grimes, personal communication). Daytime temperature was controlled for 50 continuous days per genotype (day of year 147–197, SR; 202–252, OG), however, vapour pressure deficit (VPD) was not controlled. At night, temperature was returned to ambient for two reasons: (i) to prevent variation in temperature acclimation of dark respiration (Turnbull et al., 2002), and (ii) to mimic natural diurnal conditions where temperature gradients primarily occur during the daytime due to absorbed irradiance. Leaf temperature was measured in all crown sections with Type T 0.255 mm diameter (Thermo Electric Wire and Cable, L.L.C., Newark, NJ, USA) thermocouples affixed to the abaxial leaf surface with breathable athletic tape (Johnson and Johnson Inc., New Brunswick, NJ, USA). Four leaves per crown section, one in each cardinal direction, were continuously monitored every minute and 15 min averages were computed and stored (CR7X, Campbell Scientific Inc., Logan, UT, USA).
Fig. 1.
A side view diagram of the Mylar® crown section temperature treatment chambers. Simultaneously, each of three prescribed temperatures were controlled on four separate replicate tree crowns per genotype. The controlled temperature of each crown section is denoted to the immediate left of the section in °C (25, 33, and 38). Arrows with reference numbers denote the following: (i) separately plumbed air ducts per crown section, (ii) micro irrigation emitters, (iii) ventilation and crown access ports, and (iv) location of air conditioners. A full description of individual crown section temperature control, measurement, and model application is provided in the Materials and methods section. Please note, the transparency of the chambers is darkened compared to the actual experimental conditions for visual clarity of the crown sections.
Gradients in leaf-intercepted radiation and age
Photosynthetically active radiation (PAR) absorption was estimated for each crown layer with MAESTRA, previously validated on red maple (Bauerle et al., 2004). Variation between the upper and lower crown position was <10%. One year prior to the experiment, a Western-blot analysis was conducted to determine within season relative Rubisco activase abundance among leaf age classes. No differences were found that would indicate the need to sample along a leaf age gradient (DJ Weston et al., unpublished data). In addition, leaf net photosynthesis values were monitored daily under saturated light conditions for 60 continuous days, resulting in no temporal difference in net photosynthesis values (WL Bauerle et al., unpublished data).
Leaf physiological properties and modelling
Leaf gas exchange was measured in an environmentally controlled cuvette (CIRAS-I, PP-Systems, Amesbury, MA, USA). A detailed description of protocols and parameter values derived from the leaf-level gas exchange measurements are described elsewhere (Bauerle et al., 2007). In this study, genotype-specific leaf temperature acclimation parameters and temperature dependencies reported in Bauerle et al. (2007) were used to parameterize gs in the Ball–Berry equation (Ball et al., 1987) and photosynthesis in the Farquhar and von Caemmerer (1982) model. The leaf model parameters in this study are reported in Table 1. In the gs scaling process, the Ball–Berry equation relies on the Farquhar and von Caemmerer (1982) model in that the Ball-Berry equation uses the Farquhar and von Caemmerer (1982) net photosynthesis (Anet) estimate, as well as relative humidity (h), and CO2 concentration at the leaf surface (Cs) (Ball et al., 1987):
| (1) |
where g1 is the empirical slope coefficient of the Ball–Berry equation and go is the y-intercept parameter that represents minimum stomatal conductance. These two parameters were calculated from the linear relationship between Anet and gs. Please note that the Leuning model was also tested (Leuning, 1995) in order to investigate the replacement of h with the more mechanistic VPD parameter and it was found that it performed in a very similar way (data not shown). We proceeded with the Ball–Berry equation for two primary reasons: (i) Van Wijk et al. (2000) found that the slope coefficient of the Ball–Berry model is related to both soil moisture and temperature (an important aspect in this study), while the Leuning model only showed a relationship with soil moisture and (ii) the Ball-Berry equation is a more commonly used approach that has received wide attention, analysis, acceptance, and incorporation into forest ecosystem models, thus broadening the applicability of the study (Running and Coughlan, 1988; Wang and Jarvis, 1990a; Baldocchi and Harley, 1995; Sellers et al., 1996; Medlyn et al., 2007).
Table 1.
Leaf photosynthetic temperature dependent parameters in leaves of Summer Red (SR) and October Glory (OG) red maple genotypes. Values and their thermodynamic properties were determined for each genotype and growth temperature and used in the temperature acclimated simulations (25 °C, 27 °C, 29.2 °C, 33 °C, and 38 °C). Details of parameter calculation and acclimation to temperature are reported elsewhere (Bauerle et al., 2007). Abbreviations: Vcmax, the maximal rate of Rubisco carboxylation; Jmax, the maximum rate of electron transport; Rd, leaf dark respiration rate, and Γ, the CO2 compensation point.
| SR | OG | ||||||||
| Parameter | 25 °C | 27 °Ca | 33 °C | 38 °C | 25 °C | 29.2 °Ca | 33 °C | 38 °C | Units |
| Vcmax | 69.7 | 83.7 | 125.6 | 158.2 | 60.2 | 71.8 | 104.9 | 103.3 | μmol CO2 m−2 s−1 |
| Jmax | 132.4 | 156.3 | 226.5 | 262.9 | 103.7 | 131.5 | 210.7 | 201.3 | μmol CO2 m−2 s−1 |
| Rd | 2.8 | 3.2 | 4.3 | 5.4 | 2.9 | 3.3 | 4.6 | 5.6 | μmol CO2 m−2 s−1 |
| Γ | 99.3 | 100.1 | 103.4 | 117.6 | 85.8 | 90.7 | 91.9 | 107.7 | μmol mol−1 |
Mean daytime growth temperature per 50 d genotype acclimation period.
Sap flow measurements
Three sap flow gauges (Dynamax Inc., Houston, TX, USA) were installed on the main stem of each tree, one immediately upstream of each crown section (encompassing transpiration measurement along the entire length of the live crown). In total, 12 gauges were placed on the temperature controlled trees (n=4) and a second set of 12 were placed on the ambient atmosphere controls (n=4). Along the vertical stem height gradient, the gauges encircled the stem with a flexible heating element equipped with a thermocouple above and below the element to measure vertical heat loss as water carries heat up the stem in the sap flow process (models SGB13-WS, SGB16-WS, and SGB19-WS). Saran Wrap® and a thin layer of silicon-based grease were placed between the stem and the heating element to ensure adequate contact with the stem as well as to exclude moisture. Weather-resistant insulation covered the gauges to approximately 15 cm above and below the heating element and aluminium foil covered the foam to exclude solar radiation. Data were collected by a CR10X data logger (Campbell Scientific, Logan, UT, USA) coupled to three multiplexers (AM416, Campbell Scientific, Logan, UT, USA) every 30 s, and 15 min means were logged.
Leaf area measurements
At the end of the 50 d temperature control periods, all trees fitted with sap flow sensors were felled and the crown vertical sections were separated. Leaves were removed from each section and bagged separately. Individual crown section leaf area was measured with a LI-3100 leaf area meter (Li-Cor, Lincoln, NE, USA).
Crown transpiration model
MAESTRA, an updated version of MAESTRO (Wang and Jarvis, 1990a), is a three-dimensional process-based model that computes transpiration, photosynthesis, and absorbed radiation within individual tree crowns. A full description of the model is beyond the scope of this article; however, detailed descriptions of model components can be found in Wang and Jarvis (1990a, b), Wang and Polglase (1995), Kruijt et al. (1999), Emhart et al. (2007), and Medlyn et al. (2007). In addition, a full bibliography for the model can be accessed at the website www.bio.mq.edu.au/maestra. Specific to this study, a modified version previously validated to estimate deciduous tree transpiration (Bauerle et al., 2002; Bowden and Bauerle, 2008) and within-crown light interception (Bauerle et al., 2004) was used.
Several MAESTRA characteristics were critical to scaling leaf level photosynthetic acclimation to the crown and quantifying vertical gradients in canopy transpiration. The spatial explicitness of the model allowed us to divide each crown into three layers, each layer comprising 36 equal area subvolumes made up of 12 sectors of 30o. Each genotypes’ genetic differences were parameterized with clonal-specific physiological parameters and control equations using coupled mechanistic submodels (Farquhar and von Caemmerer, 1982; Ball et al., 1987). During the scaling process, MAESTRA scaled up leaf level biochemical properties that were linked with stomatal gas-regulation both spatially and temporally (Medlyn et al., 2007; Bowden and Bauerle, 2008). This characteristic was also used to scale up genotype-specific temperature acclimation and to analyse the physiological regulation of transpiration between genotypes.
MAESTRA meteorological input data—photosynthetic photon flux (PPF), air temperature (Tair), h, and wind speed were measured once per minute at 0.3 m above the canopy and averaged every 15 min with a CR10X data logger (Campbell Scientific, Logan UT, USA). In addition, Tair and h were measured every 15 min within each temperature-controlled crown section (Onset Computer Corporation, Pocasset, MA, USA) and chamber specific Tair and h were used as model input.
Validation tests
For validation and scaling purposes, the vertical spatial distribution of all temperature acclimation responses were explicitly considered to predict transpiration rates on a m2 leaf area basis where MAESTRA calculates transpiration by applying the Penman–Monteith combination equation spatially within a crown and then summing over the within-crown estimates. The Penman–Monteith equation for a leaf with stomata only on the lower surface (e.g. red maple) can be defined as
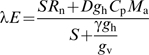 |
(2) |
where λ is the latent heat of evaporation of water (J kg−1), E is transpiration (mol m−2 s−1), S is the slope of the saturation VPD versus temperature at air temperature (kPa K−1), Rn is net radiation (W m−2), D is VPD (kPa), gh is leaf conductance to heat (mol m−2 s−1), Cp is the specific heat capacity of air (J kg−1 K−1), Ma is the molecular mass of air (kg mol−1), γ is the psychrometer constant (kPa K−1), and gv is leaf conductance to water vapour (mol m−2 s−1). The spatially explicit transpiration estimates from MAESTRA were compared with the measured values on a daily basis because the transpiration rate and leaf area of individual tree crown sections were known, thus permitting a one-to-one direct comparison of within-crown estimates and measurements.
Statistical analysis
Concordance correlation analysis was used to assess the agreement between measured and predicted transpiration and between genotypes under different temperature acclimation conditions. The concordance correlation coefficient (rc) provides a measure of reproducibility by evaluating the degree to which pairs of values (Yi1,Yi2), i=1,2,…,n, depart from a 45° line through the origin (Lin, 1989) and can be represented by
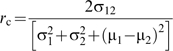 |
(3) |
where σ12 is the covariance of Y1 and Y2, σ12 is the variance of Y1, σ22 is the variance of Y2, μ1 is the mean of Y1, and μ2 is the mean of Y2. The rc contains measures of accuracy and precision and examines the strength of a 1:1 linear relationship between the measured and estimated values. A value of rc=1 corresponds to perfect agreement and a value of rc=0 corresponds to no agreement. Comparisons of concordance correlation coefficients were performed using the Fisher transformation (Zar, 1996). The 5% error probability level was used for hypothesis testing throughout. Between-genotype transpiration estimates were analysed by analysis of variance on the slopes of the transpiration versus temperature, and genotypes were compared with regression covariance analysis (SAS Institute, 2005).
Results
Leaf temperature conditions
Leaf temperatures in the controlled daytime temperature treatments of 25, 33, or 38 °C were within ±1.6 °C of the chamber set point. Leaf night temperatures (20.00 h–08.00 h) tracked ambient climatic conditions and average monthly night-time temperatures were similar over the course of the study. Ambient average daytime leaf temperatures were 27 °C and 29.2 °C for SR and OG, respectively.
Crown position versus microclimate temperature gradient within the crown
Independent effects of the canopy layer were not significant and regardless of the crown position (top, middle, or bottom), long-term exposure to different daytime growth temperatures resulted in leaf acclimation to a specific growth temperature (25, 27, 29.2, 33 or 38 °C). In addition, an analysis of time-dependent crown-section leaf characteristics under controlled daytime temperature did not show a significant effect at identical controlled temperatures and, therefore, the data were pooled. Mean temperature-dependent leaf photosynthetic parameters used in MAESTRA are reported in Table 1. The parameter values illustrate the difference between genotype acclimation and the change in response to temperature acclimation. Interested readers are referred to Bauerle et al. (2007) for a full description of the significant differences among these parameters and the variation in leaf-level temperature acclimation between the two genotypes.
Comparison of within-canopy spatial temperature acclimation on transpiration predictions
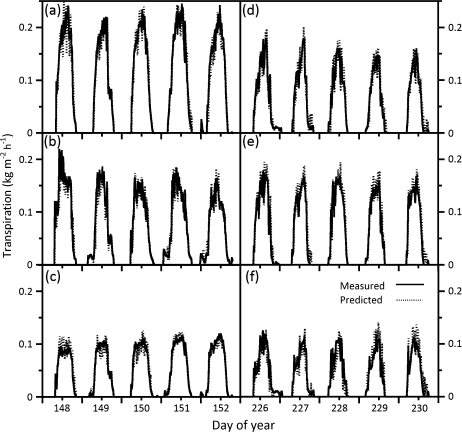
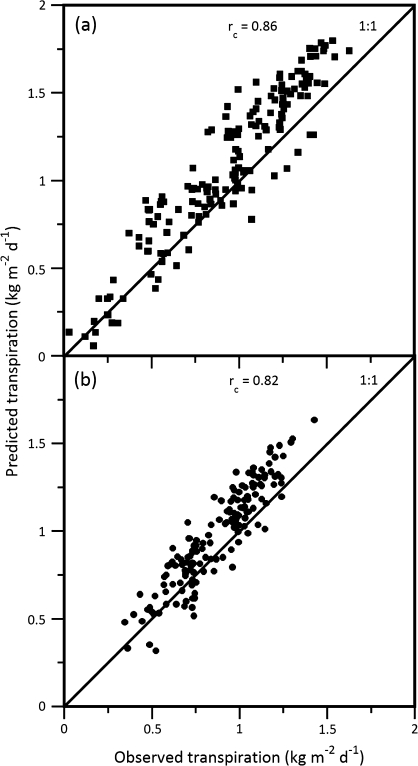
Figure 2 illustrates the diurnal relationship between measured and modelled water loss among three growth temperatures and between two genotypes. Measured water loss and that predicted by MAESTRA on a 15 min basis from Julian day 148–152 and 226–230, SR and OG, respectively, agree under various daily environmental conditions. The response to rapidly changing environmental variables, however, was generally underestimated by the model, which resulted in a water loss estimate higher than that measured by sap flow. A clear difference was observed in the transpiration due to elevated temperature acclimation (Fig. 2c versus d or Fig. 2a versus d). Transpiration calculated by MAESTRA with genotype-specific temperature-acclimated physiological parameters accurately described measured transpiration in the two genotypes. In addition, the model describes much of the variation in sap flow over time, where the rc value was 0.86 in SR and 0.82 in OG (SR 95% CI=0.83–0.90 and OG CI=0.77–0.87). The slightly higher rc value for SR reflects the higher reproducibility for SR as compared to OG (Fig. 3a versus b). Overall, daily observed transpiration rates versus model estimates, for both genotypes, followed a 45° line through the origin. However, regardless of genotype transpiration differences, the model performed better at lower to medium transpiration levels as opposed to higher levels, where the model tended slightly to overestimate genotype transpiration at rates > ∼1 kg m−2 d−1.
Fig. 2.
Diurnal time series comparison of measured (solid line) versus predicted (dashed line) transpiration over five representative consecutive days in 2003 for each Acer rubrum genotype from thermally contrasting parentage, cv. Summer Red (A–C) and cv. October Glory (D–F) at a growth temperature of (A) 38 oC, (B) 33 oC, (C) 25 oC, (D) 38 oC, (E) 33 oC, and (F) 25 oC.
Fig. 3.
Reproducibility of analyses of daily mean transpiration comparing Acer rubrum L. genotypes (a) Summer Red, closed squares and (b) October Glory, closed circles over three controlled daytime growth temperature conditions (25, 33, or 38 oC). Measured versus predicted transpiration concordance correlation coefficients (rc) are reported in each panel along with a 45° 1:1 line through the origin that represents perfect reproducibility. Each symbol represents the mean daily transpiration of four replicate trees in which tree crown sections were controlled at 25, 33, or 38 °C over a 50 d time period.
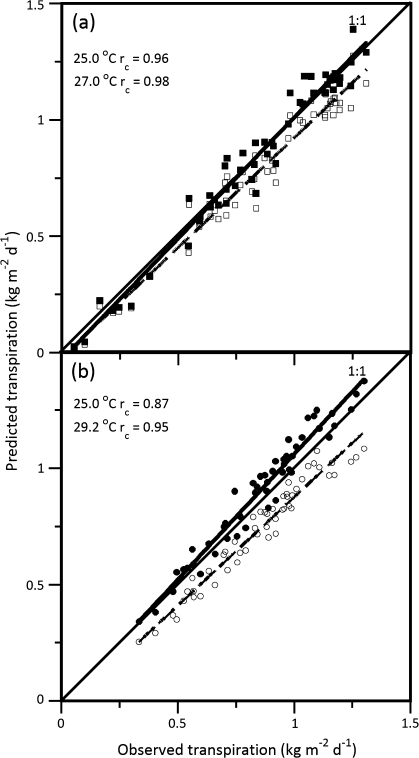
The comparison between sap-flow measurements (kg m−2 d−1) and MAESTRA estimates identified a potential systematic bias in model estimates of transpiration. Physiological parameters based on leaf acclimation to ambient daytime temperature (27 °C for SR and 29.2 °C for OG) performed better than those acclimated to 25 °C. Although the parameters and temperatures at which the leaves acclimated are not extremely different from 25 °C (2 oC for SR and 4.2 °C for OG), it had a significant impact on the reproducibility of the results both between temperatures and between genotypes (Fig. 4). MAESTRA parameterized for the SR genotype resulted in estimates that produced an rc value slightly closer to 1.0 (rc=0.98 versus 0.95), reflecting higher reproducibility for the SR as opposed to OG genotype (Fig. 2 versus 3 and Fig. 3a versus b). In addition, the model estimates described much of the variation in sap flow between the genotypes and over time (SR 95% CI=0.96–0.98 and OG CI–0.74–0.87). In Fig. 4, however, it was not as apparent as to whether a potential difference in model performance occurred at low, medium, or high transpiration estimation.
Fig. 4.
Reproducibility of analyses of daily transpiration comparing Acer rubrum L. genotypes (a) Summer Red (squares) and (b) October Glory (circles) under two temperature acclimation conditions: (i) average daytime ambient over the first 50 d (Summer Red, 27 oC and October Glory, 29.2 oC), closed symbols and (ii) acclimation to 25 oC, open symbols. Measured versus predicted transpiration concordance correlation coefficients (rc) are reported in each panel along with a 45° 1:1 line through the origin that represents perfect reproducibility. Each symbol represents the mean daily transpiration of 12 tree crown sections within four replicate trees over a 50 d time period.
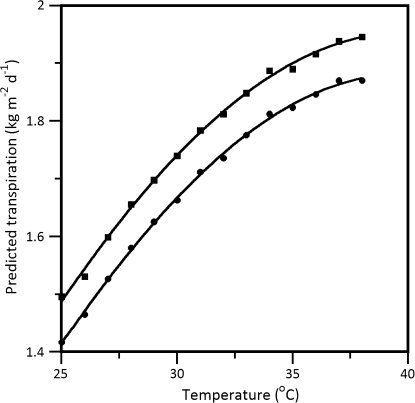
Figure 5 shows model estimates parameterized with contrasting temperature acclimation for the northern and southern genotypes. Model simulation behaviours were similar to the experimental data. The predicted genotype-specific difference in transpiration in response to temperature that is brought about by physiological variation between genotypes indicated that transpiration always remains at a higher level in SR as compared to OG (Fig. 5). Although transpiration is lower in the northern genotype relative to the southern genotype under well-watered, high light, and moderate temperature conditions, model simulations predict that all else being equal, acclimation to elevated temperature causes further separation in their transpiration rates.
Fig. 5.
Impact of physiological variation on predicted daily transpiration between Summer Red (closed squares) and October Glory (closed circles) genotypes response to temperature. Model simulations were run with each genotypes respective physiology on Julian day 182 (1 July) at an absorbed PAR of 25 mol m−2 d−1, a model controlled vapour pressure deficit of 1.25 kPa, and a wind speed of 0 m s−1. All other parameter values are listed in Table 1.
Comparison of intraspecific temperature-dependent transpiration
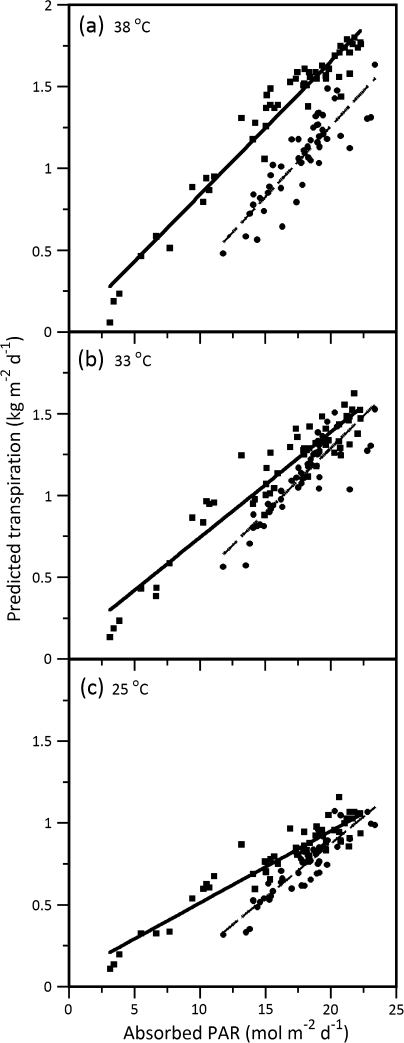
In response to increasing growth temperature and absorbed PAR, both genotypes showed a linear transpiration response that increased with temperature in response to absorbed PAR (Fig. 4), with significant differences between them at the lowest (25 °C) and the highest (38 °C) temperatures (Table 2). Compared with SR, OG had less transpiration per mol−1 absorbed PAR across all temperatures and the magnitude of transpiration increase was not as great in OG across the transition from lower to higher temperatures (Fig. 6). Moreover, at lower temperatures (25 °C and 33 °C versus 38 °C) OG transpired less under medium light conditions as opposed to SR (Fig. 6). This difference was obscured when light was high and temperature was lower than 38 °C. Based on a visual verification of best residual distribution, the relationships between transpiration and absorbed PAR were virtually linear regardless of temperature (Fig. 6). Upon comparison of genotype-specific transpiration across temperatures, a significant difference in slope means between genotypes occurred only at 25 °C (Table 2).
Table 2.
Analysis of co-variance with mean squares and temperature treatment significance on the predicted transpiration (kg m−2 d−1) of two thermally contrasting Acer rubrum L. genotypes (Summer Red and October Glory) as related to absorbed PAR and VPD and affected by three different temperature treatments (25, 33, and 38 oC) during the growing season of 2003 in Clemson, South Carolina, USA.
| Independent variable | Parameter | DF | Mean squares | P-value | ||||
| 25 oC | 33 °C | 38 °C | 25 °C | 33 °C | 38 °C | |||
| PAR | Genotype | 1 | 1.98 | 1.34 | 2.82 | <0.0001 | 0.057 | 0.028 |
| PAR×Genotype | 1 | 33.46 | 62.49 | 90.24 | <0.0001 | <0.0001 | <0.0001 | |
| Slopes | 1 | 0.95 | 0.11 | 0.06 | 0.005 | 0.58 | 0.75 | |
| Intercept | – | – | – | – | 0.086 | 0.32 | 0.91 | |
| Genotype | 1 | 3.72 | 3.99 | 26.75 | 0.0021 | 0.11 | 0.0005 | |
| VPD | VPD×Genotype | 2 | 21.05 | 2.06 | 7.74 | <0.0001 | 0.26 | 0.026 |
| Slopes | 1 | 3.55 | 4.11 | 4.29 | 0.0026 | 0.10 | 0.15 | |
| Intercept | – | – | – | – | 0.054 | <0.0001 | <0.0001 | |
Fig. 6.
Predicted daily transpiration versus absorbed photosynthetically active radiation (Absorbed PAR) in relation to growth temperature acclimation at (a) 38 °C, (b) 33 °C, and (c) 25 °C. The Summer Red estimates (closed squares; solid line) versus the October Glory estimates (closed circles; dashed line) are illustrated in each panel.
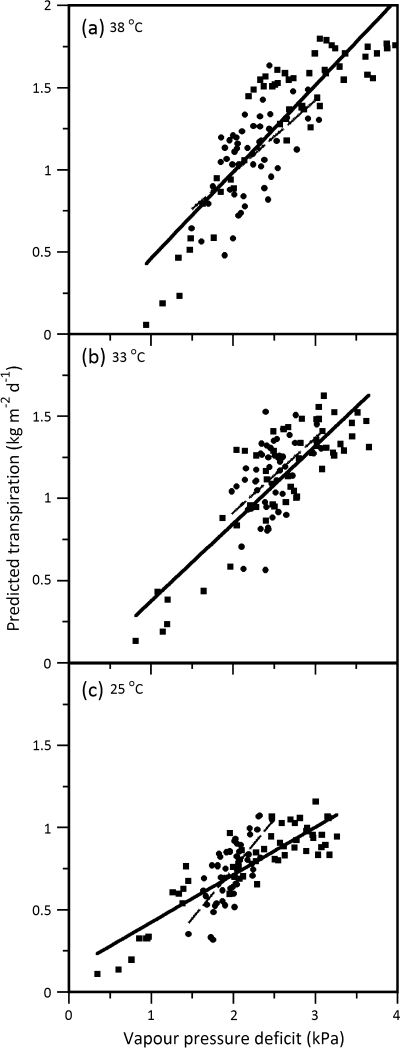
Both genotypes showed a linear transpiration response that increased with temperature in response to VPD (Fig. 7) with a significant difference between them at the lowest (25 °C) and the highest (38 °C) temperatures (Table 2). Transpiration when compared between genotypes, however, followed a slightly different pattern in response to VPD as opposed to absorbed PAR (Fig. 7). In response to VPD, both 38 °C and 33 °C resulted in a similar response between genotypes. At lower temperature (25 °C), however, OG transpired more than SR at higher VPD levels (above 2 kPa).
Fig. 7.
Predicted daily transpiration versus chamber vapour pressure deficit in relation to growth temperature acclimation at (a) 38 °C, (b) 33 °C, and (c) 25 °C. The Summer Red estimates (closed squares; solid line) versus the October Glory estimates (closed circles; dashed line) are illustrated in each panel.
Discussion
Comparison with other studies
Measuring micrometeorological variables temporally within canopies is relatively easy compared with measurements of climate-induced variation in biochemical parameters. Nonetheless, the seasonality of parameters such as the maximum rate of Rubisco carboxylation (Vcmax) is well established (Wilson et al., 2001; Kosugi et al., 2003; Bowden and Bauerle, 2008). The focus of this study, however, was not on the process of acclimation but rather on the effect that leaf-level biochemical acclimation has on scaling forest water flux. Transpiration estimates improved when parameters were used that characterized acclimation to daytime growth temperature. To regulate gs, the direct influence of photosynthetic capacity on transpiration was a consequence of the close relationship between gs and photosynthesis (Wong et al., 1979), where the coupled equations for carbon assimilation (Farquhar and von Caemmerer, 1982) and gs (Ball et al., 1987) describe the dependence of gs on net carbon assimilation. MAESTRA scaled the leaf-level physiological acclimation within a crown to show that temperature-acclimated parameters at the leaf-scale described transpiration. This finding has direct implications for efforts to improve global vegetation models that attempt to scale-up leaf-level transpiration because global models do not usually account for adjustments of temperature-dependent photosynthesis parameters (Thum et al., 2008). As a result, the variation in transpiration along a temperature gradient, possibly exacerbated by height within the canopy of tall trees, needs to be accounted for in canopy and ecosystem-level flux models.
It is widely known that temperature affects photosynthesis. However, the direct relationship between photosynthetic responses to temperature and global forest water flux requires an understanding of transpiration adjustments that helps to explain the variation in forest water exchange across climates and within canopies—two spatial temperature entities that are likely to diverge. Models that typically neglect within-canopy representation of fundamental physiological seasonal changes have adopted very simple parameterizations that are based on empirical functions of time (Wilson et al., 2001; Osborne and Beerling, 2002; Ito et al., 2006). However, empirical parameterizations may not be an effective tool for understanding the physiological significance of temperature acclimation. Mechanistic models, on the other hand, are well suited for interpreting the interactions between physiological phenomena and environmental stimuli among perennial plant genotypes (Martin et al., 2001) and have been successful at simulating transpiration (Medlyn et al., 2007). To date, however, they often use no more than short-term observational data (diurnal and/or daily) to parameterize and validate estimates (Bauerle et al., 2002; Medlyn et al., 2007). The significant differences in transpiration estimates, as a result of both growth-temperature acclimation and genotype-specific physiological responses, indicated that simulations of transpiration over the course of a season could benefit from the parameterization of temperature acclimation spatially, temporally, and genetically (α=0.05). Moreover, the MAESTRA model performed better when parameterized with physiological parameters that represented temperature acclimation; furthermore, the predictability of the stomatal response between genotypes was improved when genotype-specific physiology was characterized. Considering the fact that ecosystem behaviour is the integrated leaf, plant, species/genotype response, temperature-acclimatized parameterization may enable us to improve our ability to scale-up water exchange in order to estimate transpiration in simulation models that address larger-scale ecosystem functions.
Recently, biochemical parameters have been shown to result in a larger influence on transpiration flux as compared to meteorological variables (van der Tol et al., 2007). The study by van der Tol et al. (2007), which was conducted over a topographic temperature gradient (not a within-crown temperature gradient like this study), indicated that models with uniform biochemical parameters would not accurately predict transpiration along a topographically induced climate gradient. Similarly, this study identified the potential spatial transpiration fluxes within tree crowns and further supports the importance of capturing the variation and changes in transpiration as a result of physiological responses to gradients in growth temperature.
Toward better leaf and canopy parameterization
The seasonal variation of physiological parameters such as apparent quantum yield, maximum photosynthetic capacity, and respiration have been shown to be especially critical in characterizing the seasonality of temperate broad-leaved deciduous forests as opposed to evergreen forests (Zhang et al., 2006). The merit of incorporating seasonal model parameter changes, especially with the aim of scaling up to the stand or ecosystem level, has been expressed relative to estimating carbon exchange (Baldocchi et al., 2002; Janssens et al., 2005). Specifically, model predictions of temperate deciduous broad-leaved tree carbon exchange have been shown to be more accurate when seasonal physiological changes in Vcmax are accounted for (Wilson et al., 2001; Kosugi et al., 2003; Kosugi and Matsuo, 2006). MAESTRA was designed to simulate spatially explicit physiological processes by using species’ inherent physiology as a surrogate for genetic attributes. It was able to reproduce transpiration estimates that were more accurate with the use of leaf-level temperature-acclimated and genotype-specific parameters. Although the use of such methodology has a variety of applications, two that are important to the climate change community are the ability to capture the physiological acclimation response across the season and among species and/or genotypes. Thus, physiological adjustments can be applied to overcome the simplifying assumption that leaves are acclimated to a uniform ambient Tair. Lastly, our findings are in agreement with Kosugi et al. (2003) and Kosugi and Matsuo (2006), in that temporally dynamic seasonal response models should become integrated with larger ecosystem models to estimate long-term gas exchange.
Conclusions
The effect of photosynthetic temperature acclimation on the control of crown transpiration in a 3-D spatially explicit model (MAESTRA) has been demonstrated. MAESTRA was capable of predicting significant differences in transpiration both among spatial gradients in growth temperature and between genotypes. Model parameterization of temperature-acclimated physiology provided transpiration estimates that were precise and of acceptable accuracy. Although the study was carried out with individual trees, the results show that detailed leaf level parameterization of photosynthetic temperature acclimation can account for the differences in water exchange between genotypes and among growth temperatures. It is suggested that the results proffer a correction for scaling-up tree ecophysiological responses with more widely used terrestrial ecosystem models, because the Farquhar and von Caemmerer (1982) photosynthesis and Ball et al. (1987) gs models are commonly embedded in land surface models to describe the dependence of gs on net carbon assimilation (Gao et al., 2002). Moreover, the predicted versus measured water exchange comparison indicates that genotype-specific detail in leaf-level model parameterization can aid in accounting for the genetic diversity in mixed species, temperate, deciduous broad-leaved forests.
Acknowledgments
The authors are grateful to MF McLeod and A Hauseman for their dedicated help in instrumentation construction and maintenance. We thank Professors J Toler and L Grimes for their experimental statistics advice and EL Bauerle for helpful discussions. This work was partially funded by the South Carolina Agriculture Experiment Station project number SC-1700208, Colorado State Agriculture Experiment Station, and Tree Fund.
References
- Ball JT, Woodrow IE, Berry JA. A model predicting stomatal conductance and its contribution to the control of photosynthesis under different environmental conditions. In: Biggins J, editor. Progress in photosynthesis research. Dordrecht, The Netherlands: Martinus Nijhoff Publishers; 1987. pp. 221–224. [Google Scholar]
- Baldocchi DD, Harley PC. Scaling carbon dioxide and water vapour exchange from leaf to canopy in a deciduous forest. II. Model testing and applications. Plant, Cell and Environment. 1995;18:1331–1340. [Google Scholar]
- Baldocchi DD, Wilson KB, Gu L. How the environment, canopy structure and canopy physiological functioning influence carbon, water and energy fluxes of a temperate broad-leaved deciduous forest: an assessment with the biophysical model CANOAK. Tree Physiology. 2002;22:1065–1077. doi: 10.1093/treephys/22.15-16.1065. [DOI] [PubMed] [Google Scholar]
- Bauerle WL, Bowden JD, Wang GG. The influence of temperature on within-canopy acclimation and variation in leaf photosynthesis and respiration: spatial acclimation to microclimate gradients among thermally divergent Acer rubrum L. genotypes. Journal of Experimental Botany. 2007;58:3285–3298. doi: 10.1093/jxb/erm177. [DOI] [PubMed] [Google Scholar]
- Bauerle WL, Bowden JD, McLeod MF, Toler JE. Modeling intra-crown and intra-canopy interactions in red maple: assessment of light transfer on carbon dioxide and water vapor exchange. Tree Physiology. 2004;24:589–597. doi: 10.1093/treephys/24.5.589. [DOI] [PubMed] [Google Scholar]
- Bauerle WL, Post CJ, McLeod MF, Dudley JB, Toler JE. Measurement and modeling of the transpiration of a temperate red maple container nursery. Agricultural and Forest Meteorology. 2002;114:45–57. [Google Scholar]
- Bauerle WL, Hinckley TM, Cermak J, Kucera J, Bible K. The canopy water relations of old growth Douglas-fir trees. Trees: Structure and Function. 1999;13:211–217. [Google Scholar]
- Bowden JD, Bauerle WL. Measuring and modeling the variation in species-specific transpiration in temperate deciduous hardwoods. Tree Physiology. 2008;28:1675–1683. doi: 10.1093/treephys/28.11.1675. [DOI] [PubMed] [Google Scholar]
- Cermak J, Kucera J, Bauerle WL, Phillips N, Hinckley TM. Tree water storage and its diurnal dynamics related to sap flow and changes of stem volume in old-growth Douglas-fir trees. Tree Physiology. 2007;27:181–198. doi: 10.1093/treephys/27.2.181. [DOI] [PubMed] [Google Scholar]
- Corelli-Grappadelli L, Magnanini E. A whole tree system for gas exchange studies. HortScience. 1993;28:41–45. [Google Scholar]
- Emhart VI, Martin TA, White TL, Huber DA. Clonal variation in crown structure, absorbed photosynthetically active radiation and growth of loblolly pine and slash pine. Tree Physiology. 2007;27:421–430. doi: 10.1093/treephys/27.3.421. [DOI] [PubMed] [Google Scholar]
- Farquhar GD, von Caemmerer S. Modeling of photosynthetic response to environmental conditions. In: Lange O, Nobel PS, Osmond C, Ziegler H, editors. Encyclopedia of plant physiology. Berlin, Germany: Springer-Verlag; 1982. pp. 549–588. [Google Scholar]
- Gao Q, Zhao P, Zeng X, Cai X, Shen W. A model of stomatal conductance to quantify the relationship between leaf transpiration, microclimate and soil water stress. Plant, Cell and Environment. 2002;25:1373–1381. [Google Scholar]
- Harley P, Guenther A, Zimmerman P. Effects of light, temperature and canopy position on net photosynthesis and isoprene emission from sweetgum (Liquidambar styraciflua) leaves. Tree Physiology. 1996;16:25–32. doi: 10.1093/treephys/16.1-2.25. [DOI] [PubMed] [Google Scholar]
- Ito A, Muraoka H, Koizumi H, Saigusa N, Murayama S, Yamamoto S. Seasonal variation in leaf properties and ecosystem carbon budget in a cool-temperate deciduous broad-leaved forest: simulation analysis at Takayama site, Japan. Ecological Research. 2006;21:137–149. [Google Scholar]
- Janssens IA, Medlyn B, Gielen B, Laureysens I, Jack ME, Van Hove D, Ceulemans R. Carbon budget of Pinus sylvestris saplings after four years of exposure to elevated atmospheric carbon dioxide concentration. Tree Physiology. 2005;25:325–337. doi: 10.1093/treephys/25.3.325. [DOI] [PubMed] [Google Scholar]
- Kosugi Y, Matsuo N. Seasonal fluctuations and temperature dependence of leaf gas exchange parameters of co-occurring evergreen and deciduous trees in a temperate broad-leaved forest. Tree Physiology. 2006;26:1173–1184. doi: 10.1093/treephys/26.9.1173. [DOI] [PubMed] [Google Scholar]
- Kosugi Y, Shibata S, Kobashi S. Parameterization of the CO2 and H2O gas exchange of several temperate deciduous broad-leaved trees at the leaf scale considering seasonal changes. Plant, Cell and Environment. 2003;26:285–301. [Google Scholar]
- Kruijt B, Barton C, Rey A, Jarvis PG. The sensitivity of stand-scale photosynthesis and transpiration to changes in atmospheric CO2 concentration and climate. Hydrology and Earth Systems Science. 1999;3:55–69. [Google Scholar]
- Leuning R. A critical appraisal of a combined stomatal-photosynthesis model for C3 plants. Plant, Cell and Environment. 1995;18:339–355. [Google Scholar]
- Leuzinger S, Körner C. Tree species diversity affects canopy leaf temperatures in a mature temperate forest. Agricultural and Forest Meteorology. 2007;146:29–37. [Google Scholar]
- Lin LI-K. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45:255–268. [PubMed] [Google Scholar]
- Martin TA, Hinckley TM, Meinzer FC, Sprugel DG. Boundary layer conductance, leaf temperature and transpiration of Abies amabilis branches. Tree Physiology. 1999;19:435–443. doi: 10.1093/treephys/19.7.435. [DOI] [PubMed] [Google Scholar]
- Martin TA, Kurt H, White TL. Ideotype development in southern pines: rationale and strategies for overcoming scale-related obstacles. Forest Science. 2001;47:21–28. [Google Scholar]
- Medlyn BE, Pepper DA, O'Grady AP, Keith H. Linking leaf and tree water use with an individual-tree model. Tree Physiology. 2007;27:1687–1699. doi: 10.1093/treephys/27.12.1687. [DOI] [PubMed] [Google Scholar]
- Osborne CP, Beerling DJ. A process-based model of conifer forest structure and function with special emphasis on leaf lifespan. Global Biogeochemical Cycles. 2002;16 DOI 10.1029/2001GB001467. [Google Scholar]
- Running SW, Coughlan JC. A general model of forest ecosystem processes for regional applications. I. Hydrological balance, canopy gas exchange and primary production processes. Ecological Modelling. 1988;42:125–154. [Google Scholar]
- SAS Institute . SAS/STAT user's guide. Cary, NC: SAS Inst; 2005. [Google Scholar]
- Sellers PJ, Los SO, Tucker CJ, Justice CO, Dazlich DA, Collatz GJ, Randall DA. A revised land surface parameterization (SiB2) for atmospheric GCMs. II. The generation of global fields of terrestrial biophysical parameters from satellite data. Journal of Climate. 1996;9:706–737. [Google Scholar]
- Thum T, Aalto T, Laurila T, Aurela M, Lindroth A, Vesela T. Assessing seasonality of boreal coniferous forest CO2 exchange by estimating biochemical model parameters from micrometeorological flux observations. Biogeosciences Discussions. 2008;5:2707–2747. [Google Scholar]
- Turnbull MH, Murthy R, Griffin KL. The relative impacts of daytime and night-time warming on photosynthetic capacity in Populus deltoides. Plant, Cell and Environment. 2002;25:1729–1737. [Google Scholar]
- van der Tol C, Doman AJ, Waterloo MJ, Raspor K. Topography induced spatial variations in diurnal cycles of assimilation and latent heat of Mediterranean forest. Biogeosciences. 2007;4:137–154. [Google Scholar]
- Van Wijk MT, Dekker SC, Bouten W, Bosveld FC, Kohsiek W, Kramer K, Mohren GMJ. Modeling daily gas exchange of a Douglas-fir forest: comparison of three stomatal conductance models with and without a soil water stress function. Tree Physiology. 2000;20:115–122. doi: 10.1093/treephys/20.2.115. [DOI] [PubMed] [Google Scholar]
- Wang YP, Jarvis PG. Description and validation of an array model-MAESTRO. Agricultural and Forest Meteorology. 1990a;51:257–280. [Google Scholar]
- Wang YP, Jarvis PG. Influence of crown structural properties on PAR absorption, photosynthesis, and transpiration in Sitka spruce: application of a model (MAESTRO) Tree Physiology. 1990b;7:297–316. doi: 10.1093/treephys/7.1-2-3-4.297. [DOI] [PubMed] [Google Scholar]
- Wang YP, Polglase PJ. The carbon balance in the tundra, boreal and humid tropical forests during climate change: scaling up from leaf physiology and soil carbon dynamics. Plant, Cell and Environment. 1995;18:1226–1244. [Google Scholar]
- Wilson KB, Baldocchi DD, Hanson PJ. Leaf age affects the seasonal pattern of photosynthetic capacity and net ecosystem exchange of carbon in a deciduous forest. Plant, Cell and Environment. 2001;24:571–583. [Google Scholar]
- Wong S, Cowan I, Farquhar G. Stomatal conductance correlates with photosynthetic capacity. Nature. 1979;282:424–426. [Google Scholar]
- Zar JH. Biostatistical analysis. 3rd edn. Upper Saddle River, NJ, USA: Prentice Hall; 1996. [Google Scholar]
- Zhang GR, Yu GR, Sun XF, et al. Seasonal variations of ecosystem apparent quantum yield (α) and maximum photosynthesis rate (Pmax) of different forest ecosystems in China. Agricultural and Forest Meteorology. 2006;137:178–187. [Google Scholar]
- Zweifel R, Bohm JP, Hasler R. Midday stomatal closure in Norway spruce-reactions in the upper and lower crown. Tree Physiology. 2002;22:1125–1136. doi: 10.1093/treephys/22.15-16.1125. [DOI] [PubMed] [Google Scholar]