Abstract
Although the relationship between grassland productivity and soil water status has been extensively researched, the responses of plant growth and photosynthetic physiological processes to long-term drought and rewatering are not fully understood. Here, the perennial grass (Leymus chinensis), predominantly distributed in the Euro-Asia steppe, was used as an experimental plant for an irrigation manipulation experiment involving five soil moisture levels [75–80, 60–75, 50–60, 35–50, and 25–35% of soil relative water content (SRWC), i.e. the ratio between present soil moisture and field capacity] to examine the effects of soil drought and rewatering on plant biomass, relative growth rate (RGR), and photosynthetic potential. The recovery of plant biomass following rewatering was lower for the plants that had experienced previous drought compared with the controls; the extent of recovery was proportional to the intensity of soil drought. However, the plant RGR, leaf photosynthesis, and light use potential were markedly stimulated by the previous drought, depending on drought intensity, whereas stomatal conductance (gs) achieved only partial recovery. The results indicated that gs may be responsible for regulating actual photosynthetic efficiency. It is assumed that the new plant growth and photosynthetic potential enhanced by pre-drought following rewatering may try to overcompensate the great loss of the plant's net primary production due to the pre-drought effect. The present results highlight the episodic effects of drought on grass growth and photosynthesis. This study will assist in understanding how degraded ecosystems can potentially cope with climate change.
Keywords: Chlorophyll fluorescence, gas exchange, grassland ecosystem, relative plant growth rate (RGR), rewatering, water stress
Introduction
Water shortage has a major limiting effect on plant productivity in many terrestrial ecosystems, especially in arid and semi-arid areas, and the occurrence of drought usually fluctuates at different temporal and spatial levels (Knapp et al., 2001; Bai et al., 2004; Potts et al., 2006a, b; Sponseller, 2007; Swemmer et al., 2007). Global climatic change may result in not only a change in total precipitation but also the occurrence of episodic drought (IPCC, 2007; Wang et al., 2007). Clearly, a more robust assessment of the degree to which changes in water availability affect the ecological processes under present climatic conditions is urgently needed before predictions can be made for future responses to climatic scenarios (Sponseller, 2007). Moreover, how both continuous drought and rewatering induced by adverse climatic change events affect plant production, community composition, and plant function remains relatively unknown, especially for grassland plants that are more sensitive to drought.
Fernández (2007) indicated that information about the response of plants to rainfall events in some arid areas is often lacking. However, even a small rainfall pulse can trigger an increase in plant productivity in a desert ecosystem (Reynolds et al., 2004). For example, Siopongco et al. (2006) recently reported that rice plant growth may be remarkably stimulated by rewatering following drought. An investigation into the stimulated effect of pre-drought treatment following rewatering in wheat by Liu et al. (2001) indicated that severe water stress increased the leaf area more than moderate water stress after rewatering. Recently, however, Yahdjian and Sala (2006) reported that pre-drought can constrain the response of aboveground net primary production (NPP) to current precipitation in the Patagonian steppe. Thus, the effect of pre-drought and rewatering on plant growth and productivity must be further clarified.
The leaf net photosynthetic rate (A) of plants subjected to moderate drought (MD) may be largely attributed to stomatal limitation, rather than biochemical factors such as Rubisco activity, which is under debate (Lawlor and Cornic, 2002; Bota et al., 2004; Flexas et al., 2006; Galmés et al., 2007a). An obvious decrease in mesophyll conductance (gi) under severe drought (SD), however, can be partly responsible for an increase in non-stomatal limitations (Flexas et al., 2002; Grassi and Magnani, 2005; Galmés et al., 2007a). More SD may result in cell membrane damage (Benhassaine-Kesri et al., 2002) and decreased photosynthetic capacity, including photosystem II (PSII) activity (Ghannoum et al., 2003; Xu and Zhou, 2006b; Gallé and Feller, 2007). Recent reports indicated that discrete precipitation events can trigger brief but important episodes of biological activity, including both photosynthesis and respiration in water-limited ecosystems (Potts et al., 2006a; Loik, 2007). Leaf A of beech saplings can be completely restored after rewatering (Gallé and Feller, 2007), while stomatal conductance (gs) remains permanently lower in stressed plants than in control plants, resulting in increased intrinsic water use efficiency (WUE; Gallé and Feller, 2007; Pou et al., 2008) and thus demonstrating a high tolerance to episodic drought. However, Gomes et al. (2008) indicated that rewatering also leads to the incomplete recovery of leaf A rate, mainly due to photochemical impairment under SD. Thus, the issue remains debatable and needs to be elucidated.
Precipitation pulse obviously affects ecophysiological function processes, particularly in an arid ecosystem, from biochemical to ecosystem levels, and is of particular interest to many researchers (Reynolds et al., 2004; Yahdjian and Sala, 2006; Fernández, 2007; Yang et al., 2008). Nevertheless, the relationship between plant growth and ecophysiological performances in response to drought and rewatering is not yet fully understood (Wiegand et al., 2004; Yahdjian and Sala, 2006; Fernández, 2007; Xu and Zhou, 2008). Moreover, drought limitation and recovery following rewatering may have different adaptive mechanisms, as plants were subjected to erratic drought or rainfall in semi-arid regions (Gazanchian et al., 2007; Izanloo et al., 2008). Therefore, the main objective of the present experiment was to examine the responses of plant growth and photosynthesis to soil drought, particularly their recovery after rewatering, as plants were subjected to different intensities of long-term previous drought. It was hypothesized that although rewatering after long-term drought can overcompensate plant biomass limitation due to previous drought by increasing relative growth rate (RGR) and photosynthetic activity, the final biomass of plants that experienced previous SD may be less than the biomass of plants that were always under well-watered conditions.
Materials and methods
Plant culture
Grassland dominated by Leymus chinensis (Trin.) Tzvel., a native perennial grass with good palatability and high forage value, is widespread in the Euro-Asia steppe. However, an increase in grazing pressure and adverse climatic change have led to a substantial reduction in productivity throughout most of the grassland. When this condition is combined with drought and summer high temperature, soil moisture becomes more critical as daily temperatures increase during the summer. Thus, drought has become a major limiting factor for L. chinensis, especially in recent decades (Wang and Gao, 2003; Bai et al., 2004, 2008; Wang et al., 2007). During the last year of the experiment, seeds were obtained from a grassland in Xilinhot, Inner Mongolia, China (44°08′N, 117°05′E), at 1100 m above sea level. The region is continental temperate semi-dry grassland with mild temperatures during spring and autumn, cool and dry winters, and wet but hot summers. The annual mean temperature was 2 °C, and the annual precipitation was 350 mm over the previous 50 years.
Seeds of L. chinensis were sterilized by a 0.7% potassium permanganate solution for 8 min, rinsed, and then immersed in water for 7 d before being placed into a refrigerator below 0 °C. They were sown in plastic pots (5.1 l, 18 cm in diameter, 20 cm in height) wrapped with plastic film. Each plastic pot was filled with 4.08 kg of dry soil obtained from a natural field in the Xilinguole grassland (Inner Mongolia of China) and planted with a density of eight plants per pot. In the chestnut-coloured soil, the organic carbon concentration was 19.60±0.18 g kg−1 and the total nitrogen was 4.18±0.11 g kg−1. All experimental pots were placed in a naturally illuminated greenhouse at the daily maximum photosynthetic photon flux density (PPFD) of 1000 μmol m−2 s−1 above the plant canopy on a clear day, provided by a combination of cool-white fluorescent and incandescent lamps in the greenhouse, with a day/night temperature of 28–32/20–24 °C.
Soil water-withholding treatments were performed until a third leaf appeared (44 d after sowing). The soil relative water contents (SRWCs) (ratio between the present soil moisture and field capacity) were sorted into five levels: control (75–80%), light drought (LD) (60–75%), MD (50–60%), SD (35–50%), and extreme drought (ED) (25–35%). Water was added to return the soil moisture to the upper limit by irrigation at 17:00 h every 2–3 d. All additions of water were recorded for all treatments to obtain the amount of evapotranspiration. The SRWC is expressed as:
where Wsoil is the current soil weight, Wpot is the weight of the empty pot, DWsoil is the dry soil weight, and WFC is the soil weight at field capacity.
At 51 d after the start of water withholding, the treated pots were divided into two groups: one was rewatered, while the other continued water-withholding treatments. The experimental procedure, including the time-sampled biomass, is presented in Table 1.
Table 1.
Experimental procedure
| Date | 10 June | 24 July | 13 August | 13 September | 13 October | 2 November | 22 November |
| Period (d) | 0 | 44 | 20 | 31 | 30 | 20 | 20 |
| Activities | Sowing | Start of water withholding | Well treated | First harvestinga | Second harvesting | Third harvesting | Fourth harvesting |
On 13 September 2005, the treated pots were divided into two groups. One was rewatered, while the other continued water deficit treatments.
Biomass and leaf area measurements
Plant biomass was sampled in three or four pots per treatment at each harvesting time, and dried at 80 °C to a constant weight, then weighed. RGR (mg g−1 day−1) was expressed as [ln (harvest dry mass of t2)–ln(initial dry mass of t1)]/(t2–t1)×1000 (Lindroth et al., 2001).
In order to assess the limitation of pre-drought after rewatering, the percentage of pre-drought limitation (PDL) was estimated as:
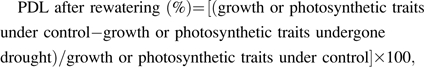 |
where growth or photosynthetic traits under control denoted the plants that had not experienced drought during the entire experimental period, and growth or photosynthetic traits undergone drought denoted those plants that had experienced pre-drought and were measured following recovery.
The plant leaf area was measured using a Li-3000 leaf area meter (Li-Cor, Inc., Lincoln, NE, USA); the specific leaf area (SLA) was calculated from the measurements of leaf area and dry matter.
Leaf gas exchange and chlorophyll fluorescence measurements
Leaf gas exchange measurements were made using an open gas exchange system (LI-6400, LI-COR Bioscience, Lincoln, NE, USA) concurrently with a leaf chamber fluorometer (LI-6400-40). The data were initially analysed with data acquisition software (OPEN Software version 5.1, LI-COR Bioscience). Illumination was supplied to the leaf from a red–blue light-emitting diode (LED) light source. Prior to measurement, leaves were acclimated in the chamber for >10 min at a relative consistent temperature (25–27 °C), an ambient CO2 concentration (380 μmol mol−1), and a PPFD of 900 μmol m−2 s−1 (a value at which photosynthesis is nearly saturated in the present experimental conditions). The gas exchange parameters were determined in the 3–4 youngest and fully expanded leaves of different individuals (one plant per pot, total of three pots) for all replicates, generally between 08:30 h and 14:30 h daily. For the actual measurement, the vapour pressure deficit (VPD) in the cuvette was maintained below 2.0 kPa to minimize its effect, showing that no significant changes in the slope of the initial part of the A/Ci curve occurred below the VPD threshold.
The leaves used for measurement of fluorescence parameters were the same leaves as used for determination of gas exchange; after 30 min of dark adaptation at the relative consistent temperature, the minimal fluorescence yield (F0) was measured by using modulated light that was sufficiently low (<0.1 μmol m−2 s−1), and the maximal fluorescence yield (Fm) was determined by a 0.8 s saturating pulse at 8000 μmol m−2 s−1 in dark-adapted leaves. The leaves were then continuously illuminated with white actinic light at an intensity of 900 μmol m−2 s−1 for 30 min. The steady-state value of fluorescence (Fs) was thereafter recorded, and the second saturating pulse at 8000 μmol m−2 s−1 was imposed to determine the maximal light-adapted fluorescence level (F′m). The fluorescence parameters were obtained from formulae (Genty et al., 1989; van Kooten and Snel, 1990): the maximal efficiency of PSII photochemistry [Fv/Fm=(Fm–F0)/Fm], actual PSII efficiency [ΦPSII=(F′m–Fs)/F′m], and non-photochemical quenching [NPQ=(Fm–′m)/F′m].
Estimation of light response parameters
After acclimation, the PPFD was sequentially lowered to 1200, 900, 800, 600, 400, 200, 100, 50, and 20 μmol m−2 s−1. The responses of photosynthesis parameters to light were estimated according to a quadratic equation (Prioul and Chartier, 1977; Long et al., 1993):
where A is the net photosynthetic rate (μmol m−2 s−1), Asat is the maximum CO2 accumulation rate (μmol m−2 s−1), PPFD is the photosynthetic photon flux density (μmol m−2 s−1), α is the leaf maximum apparent quantum yield of CO2 uptake, and θ is the convexity of the transit from light-limited to light-saturated photosynthesis. Instantaneous determinations of gs were obtained after illumination at saturating light (900 μmol photon m−2 s−1) for at least 10 min at 25–27 °C.
Estimation of a response to Ci
As detailed above, light acclimation was conducted before measurements of the A/Ci response. The CO2 concentration gradients to produce A/Ci curves were 380, 300, 200, 100, 50, 20, 380, 380, 600, 800, and 1000 μmol m−2 s−1 step by step. The two 380s in a row after the low value was not an error, but a trick to give the leaf some recovery time after the low CO2 measurement. Later, the first of those readings could be eliminated if it could not be fitted in. Curve-fitting software was used to analyse the A/Ci responses using the function of the form from the photosynthesis model of Farquhar et al. (1980) and the modification with gi by Ethier and Livingston (2004). The CO2 gi was measured by the methods of Loreto et al. (1992).
where Ac is the RuBP-saturated CO2 assimilation rate, Ci is the intercellular CO2 concentration, Vc,max is the maximum carboxylation velocity, and Rd is mitochondrial respiration in the light. The other parameters are indicated in Table 2.
Table 2.
Kinetic constants of Rubisco in vivo used to parameterize in the photosynthesis model (from Bernacchi et al., 2001, 2002)
| Γ* | 33.86 μmol mol−1 | Chloroplastic CO2 photocompensation point |
| Γ | 42.89 μmol mol−1 | CO2 photocompensation point |
| O | 210 mmol mol−1 | O2 concentration |
| Kc | 406.07 μmol mol−1 | Michaelis–Menten constant for RuBP carboxylation |
| Ko | 276.9 mmol mol−1 | Michaelis–Menten constant for oxygenation |
The electron transport rate (J) was expressed as J=ΦPSII×fIαleaf, where f is the fraction of absorbed quanta that is used by PSII and is typically assumed to be 0.5 for C3 plants (Ögren and Evan, 1993), I is actinic PPFD, and αleaf is the effective leaf absorptance, ranging from 0.88 to 0.95 (here assumed to be 0.95) (Flexas et al., 2007). This gi can be calculated by the variable J method (Harley et al., 1992):
Statistical analysis
All data analyses were conducted with SPSS 10.0 statistical software (SPSS, Chicago, IL, USA). The experiments were repeated at least three times and means ±SE of values are given. The parameters were analysed by one-/two-way analysis of variance (ANOVA) followed by Duncan's multiple range test (Duncan, 1955). In order to test the effect of gs on Asat, a three-component exponential function of the form by a non-linear regression estimate was used:
Using this function, Amax, sat in response to gs was expressed as a+c, and the slope ϕ was calculated as b(a+c). Thus, the value of the threshold of gs (the gs onset as Asat levels off at Amax, sat) estimate can be calculated by the linear function described by ϕ and c to its intersection with Amax, sat (i.e. a/ϕ). Otherwise, linear regression analysis was also used in this study.
Results
Responses of leaf water status to drought and rewatering
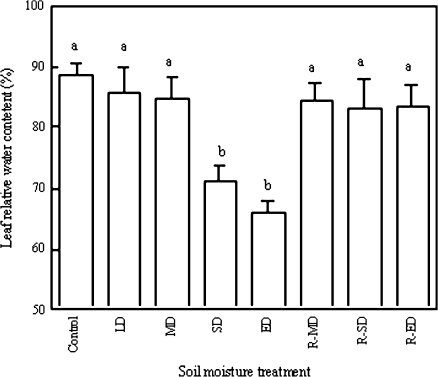
The effect of drought was determined by the leaf's RWC (F=28.065, P <0.001), and SD and ED led to significant RWC decreases, according to Duncan's multiple range test (P <0.05) (Fig. 1). As the plants under MD, SD, and ED were rewatered, leaf RWC exhibited no significant differences from well-watered plants (control treatment) (P >0.05) and within the three rewatered treatments (F=0.101, P=0.905), indicating that leaf RWC completely recovered following rewatering.
Fig. 1.
Leaf relative water content (RWC) under soil drought and rewatering (measured on 19 October 2005). Five SRWCs are indicated by control (75–80%), LD (6–75%), MD (50–60%), SD (35–50%), and ED (25–35%); R-MD, R-SD, and R-ED represent rewatering after the corresponding drought. All values are means ±SE for n=3–4. Bars with different lowercase letters are significantly different (P <0.05).
Recovery of new leaf following rewatering
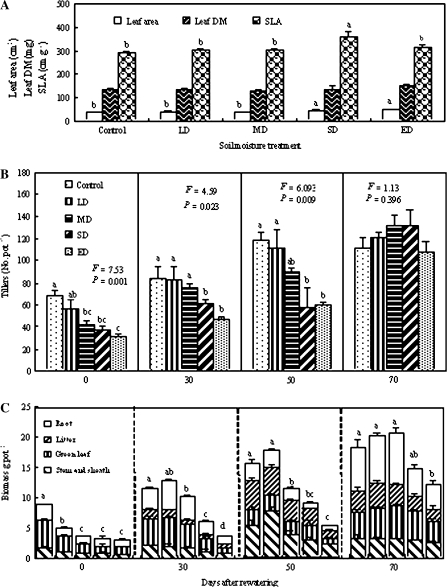
As shown in Fig. 2A, new leaf area per plant following rewatering in pots that had experienced previous SD and ED treatments was significantly affected by previous drought treatment, according to the F test (F=3.896, P=0.037), and was significantly higher (P <0.05) than that of the control; however, the pre-drought treatment did not result in a significant change in new leaf dry matter (F=1.012, P=0.446). For the SLA of the new leaves, the effect of drought was significant, and the effect of SD was significantly higher (P <0.05) than that of the control.
Fig. 2.
(A) Leaf area, leaf dry matter, and specific leaf area (SLA) of the new leaves following rewatering after plants experienced previous different drought severities; (B) plant tillers, and (C) plant biomass at soil drought, continual drought, and following rewatering (right panel) after plants experienced different previous drought severities. Five SRWCs are indicated by control (75–80%), LD (60–75%), MD (50–60%), SD (35–50%), and ED (25–35%). All values are means ±SE for n=3–6. Bars with different lowercase letters are significantly different (P <0.05).
Responses of plant growth to drought and rewatering
Figure 2B illustrates the changes in tiller number per pot from initial drought to continuous drought, after rewatering. A two-way ANOVA indicated significant effects on main factors (F=66.67, P <0.001 for time; F=10.03, P <0.001 for soil moisture) and their interaction (F=2.45, P=0.013). Under the three continuous drought conditions, withholding soil water led to significant variations (P <0.05); however, following rewatering, the obvious changes were negated due to previous soil drought (F=1.13, P=0.396). Compared with the effects of control treatment, the effects of SD and ED significantly declined at all water-withholding stages (P <0.05), but tillers of the plants subjected to previous SD and ED had already exceeded or approached the level of the control treatment following rewatering.
Figure 2C demonstrates the responses of biomass in various organs and whole plants to drought and rewatering. Before rewatering, during the initial water-withholding treatment, drought of more than LD (SRWC <75%) significantly decreased the biomass of various organs and the whole plant (P <0.05). However, with continual drought, LD no longer reduced the biomass, and even slightly increased root and whole plant biomass (P >0.05), although more than MD still decreased their biomass (P <0.05). On the 70th day following rewatering, the treatments still produced significant differences for the organs (F=7.304, P=0.005 for green leaf; F=3.823, P=0.039 for litter; F=4.667, P=0.022 for root; and F=3.649, P=0.044 for whole plant), except for the stem and sheath (F=1.029, P=0.438). Following rewatering, comparison between the treatments indicated that only ED significantly decreased the biomass of the green leaf, litter, root, and whole plant (P <0.05), but not that of the stem and sheath. However, previous drought of less than ED intensity did not reduce their biomass, indicating that MD did not limit growth following rewatering.
Responses of RGR to pre-drought and rewatering
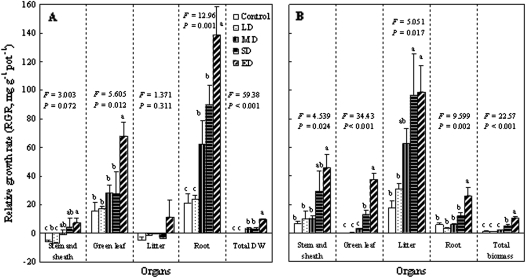
The RGR changes in different organs of the plants were measured after rewatering after the continuous soil drought treatment (101 d, Fig. 3A) and after rewatering from the starting soil drought treatment (81 d, Fig. 3B). It was found that the RGR was significantly stimulated by more than SD treatments when the two durations of drought treatments were completed, except for stem and litter organs with the previous long-term drought treatment. For the long-term drought treatment (Fig. 3A), the greatest RGR occurred in root, followed by green leaf; in contrast, for short-term drought (Fig. 3B), the maximum values were in litter, followed by stem and green leaf, possibly due to the lower amount of litter with the short-term drought treatment. Nevertheless, following rewatering, previous SD still caused a significant RGR increase of the whole plant for both durations of soil drought, indicating that plant RGR was remarkably stimulated by the previous SD.
Fig. 3.
(A) Relative growth rate (RGR) after rewatering from the continuous soil drought treatment (101 d), and (B) after rewatering from the start of soil drought treatment (81 d). Five SRWCs are indicated by control (75–80%), LD (60–75%), MD (5–60%), SD (35–50%), and ED (25–35%). All values are means ±SE for n=5. Bars with different lowercase letters are significantly different in the same organs (P <0.05).
PDL of both biomass and RGR due to previous soil drought
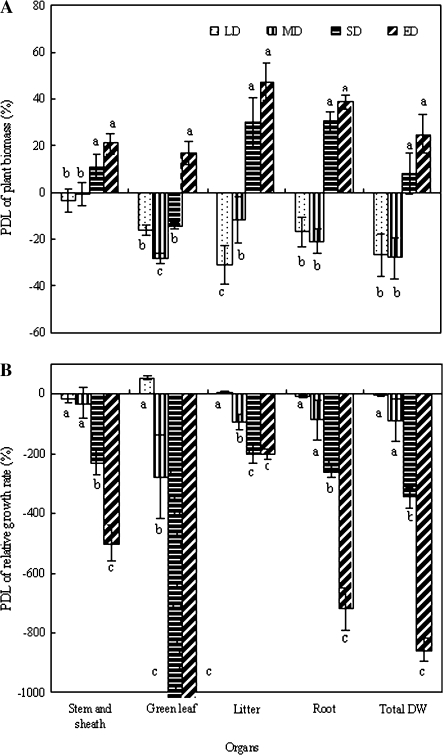
Figure 4A demonstrates the relative PDL biomass from the previous soil drought treatment. SD, especially ED, significantly increased the PDL in stem, green leaf, litter, root, and total biomass (P <0.05). However, LD and MD did not produce positive PDL values; in contrast, negative values resulted, indicating that LD and MD significantly stimulated plant productivity rather than limitation.
Fig. 4.
(A) Pre-drought limitation (PDL) of plant biomass and (B) relative growth rate (RGR) (40 d period after rewatering). Five SRWCs are indicated by control (75–80%), LD (60–75%), MD (50–60%), SD (35–50%), and ED (25–35%). All values are means ±SE for n=3. Bars with different lowercase letters are significantly different in the same organs (P <0.05)
However, when the RGR's PDL was determined, an obvious difference from the PDL of biomass was found (Fig. 4B). Previous drought led to significant changes in the RGR's PDL in stem (F=5.01, P=0.018), green leaf (F=33.00, P <0.001), litter (F=3.88, P=0.037), root (F=10.02, P=0.002), and whole plant (F=28.35, P <0.001). Values for all organs and whole plants were negative, except for green leaf at LD. SD and ED produced more negative values than LD and MD. For example, values >1000-fold for stimulating green leaf RGR and >300-fold for stimulating whole-plant RGR were observed under SD and ED conditions (P <0.01), clearly indicating that previous soil drought of <50% SRWC dramatically accelerated plant growth after rewatering.
Responses of photosynthesis and PSII function to drought and rewatering
Photosynthetic and PSII function performances were further determined under both soil drought and rewatering conditions. As shown in Table 3, significant changes in gs and gi resulted from soil moisture treatments (P <0.01), and significant reductions were induced by a drought of more than MD compared with the control treatment. Soil drought also significantly affected leaf photosynthetic potential indicated by Asat (F=83.63, P <0.01), and light use efficiency indicated by α (F=45.42, P <0.01). Compared with the control, SD decreased Asat by 22.3% and ED significantly decreased Asat by 74.9%; in contrast, LD and MD did not significantly affect Asat, although α significantly decreased due to the drought treatments of more than MD. Soil water-withholding treatments also resulted in significant changes in Vc,max (F=4.90, P=0.019), which significantly decreased at SD and ED, indicating that Rubisco activity in vivo was severely affected by severe water deficit stress. However, following watering, substantial increases were observed in gs, gi, Asat, Vc,max, and α compared with pre-irrigation values, leading to a lower variation from previous drought treatments (P >0.05), although gs and gi did not reach the control levels. These results indicated that the photosynthetic potential, the potential of light use efficiency, and Rubisco activity in vivo could be recovered following rewatering.
Table 3.
Photosynthetic and PSII function performances under soil drought and rewatering (measured on 15–18 October 2005), with soil relative water contents (SRWCs) of control (75–80%), LD (60–75%), MD (50–60%), SD (35–50%), and ED (25–35%)
| gs | gi | Asat | α | Vc,max | Fv/Fm | ΦPSII | NPQ | |
| Control | 0.415±0.076 a | 0.225±0.027 a | 17.01±0.99 a | 0.078±0.004 a | 103.34±5.68 a | 0.814±0.010 a | 0.393±0.002 a | 0.523±0.080 c |
| LD | 0.412±0.048 a | 0.217±0.029 a,b | 18.00±1.36 a | 0.080±0.003 a | 100.12±1.82 a,b | 0.816±0.003 a | 0.369±0.024 a | 1.064±0.081 b |
| MD | 0.269±0.017 b | 0.157±0.007 b,c | 17.82±0.57 a | 0.053±0.002 b | 88.63±3.25 a,b | 0.810±0.004 a | 0.309±0.011 b | 1.358±0.126 b |
| SD | 0.218±0.012 b,c | 0.120±0.017 c | 13.21±0.58 b | 0.030±0.001 c | 90.62±1.37b c | 0.791±0.002 b | 0.246±0.037 b | 2.090±0.070 a |
| ED | 0.146±0.019 c | 0.057±0.009 d | 4.27±0.46 c | 0.028±0.002 c | 84.89±3.43 c | 0.749±0.005 c | 0.203±0.007 b | 2.483±0.248 a |
| F | 7.91 | 12.58 | 45.42 | 83.63 | 4.79 | 24.12 | 16.42 | 32.90 |
| P | 0.004 | <0.01 | <0.01 | <0.01 | 0.020 | <0.01 | <0.01 | <0.01 |
| Performances after rewatering | ||||||||
| R-MD | 0.272±0.030 a* | 0.180±0.013 a | 18.18±0.95 a | 0.077±0.001 a | 107.71±20.68 a | 0.820±0.003 a | 0.312±0.012 a* | 1.625±0.147 a* |
| R-SD | 0.254±0.019 a* | 0.148±0.012 a,b* | 17.89±0.56 a | 0.070±0.004 a,b | 113.09±3.47 a | 0.819±0.003 a | 0.295±0.002 a* | 1.824±0.042 a* |
| R-ED | 0.249±0.009 a* | 0.126±0.005 b* | 18.95±1.49 a | 0.067±0.003 b* | 122.18±6.77 a* | 0.767±0.054 a | 0.268±0.021 a* | 1.935±0.153 a* |
| F | 0.94 | 5.88 | 0.26 | 2.64 | 0.32 | 0.96 | 2.52 | 1.58 |
| P | 0.441 | 0.069 | 0.778 | 0.150 | 0.736 | 0.44 | 0.160 | 0.28 |
| PDL (%) | ||||||||
| R-MD | 34.41±8.02 | 19.82±5.87 a | –6.88±5.64 | 1.28±4.95 b | –4.23±20.01 | –0.74±0.82 | 20.61±2.60 | –210.71±22.92 |
| R-SD | 38.75±8.20 | 34.26±5.87 a,b | –5.17±7.46 | 10.26±1.04 a,b | –9.44±3.36 | –0.61±0.98 | 24.94±0.22 | –248.76±41.52 |
| R-ED | 39.95±9.5 | 43.82±2.37 b | –11.41±10.43 | 14.10±1.5 2a | –18.24±7.28 | 5.77±5.94 | 31.81±4.97 | –269.98±34.91 |
R-MD, R-SD, and R-ED represent rewatering after the plants have undergone the corresponding pre-drought treatments.
Asat is the light-saturated CO2 accumulation rate (μmol mol−1); Fv/Fm is the maximal efficiency of PSII photochemistry (dimensionless); gs is stomatal conductance (mmol mol−1); gi is mesophyll conductance (mmol mol−1); NPQ is non-photochemical quenching (dimensionless); PDL is pre-drought limitation; Vc,max is maximum carboxylation velocity (μmol mol−1); α is the maximum photosynthetic quantum yield of CO2 uptake (dimensionless); and ΦPSII is the actual PSII efficiency (dimensionless). Asterisks indicate significance at the 0.05 level, compared with those of the control value.
The PSII function and its photochemistry status were also determined. As many reports have pointed out (e.g. Baker and Rosenqvist, 2004), the maximal efficiency of PSII photochemistry (Fv/Fm) can represent the greatest photochemical efficiency or the primary efficiency of light energy transitions; the actual quantum yield (ΦPSII) indicates the efficiency of transfer of absorbed photons to the reaction centre of PSII; and NPQ is the portion of light energy absorbed by antenna pigment but not used in electron transport and dissipated as thermal energy. As shown in Table 3, significant changes in Fv/Fm, ΦPSII, and NPQ were observed when the plants were subjected to soil moisture treatments (P <0.01). A significant decline in Fv/Fm was observed under more than SD, and a decline of ΦPSII was observed under more than MD (P <0.05); however, a significant increase in NPQ (P <0.05) occurred at SD and ED. Following rewatering, complete recovery of Fv/Fm and ΦPSII occurred in the leaves of plants subjected to pre-drought (P >0.05), but NPQ did not significantly change after rewatering.
The PDL of the photosynthetic and photochemical parameters was also calculated (bottom section in Table 3). The previous different drought levels did not lead to significant changes of the parameters except for α (F=5.4, P <0.05), but greater positive values of RDL of gs, gi, α, and ΦPSII indicated that inhibition of four parameters following rewatering existed from pre-drought. However, the PDLs of the NPQ were significant negative values, indicating that the dissipating heat mechanism of PSII induced by pre-drought was maintained following rewatering.
Relationships between photosynthetic parameters
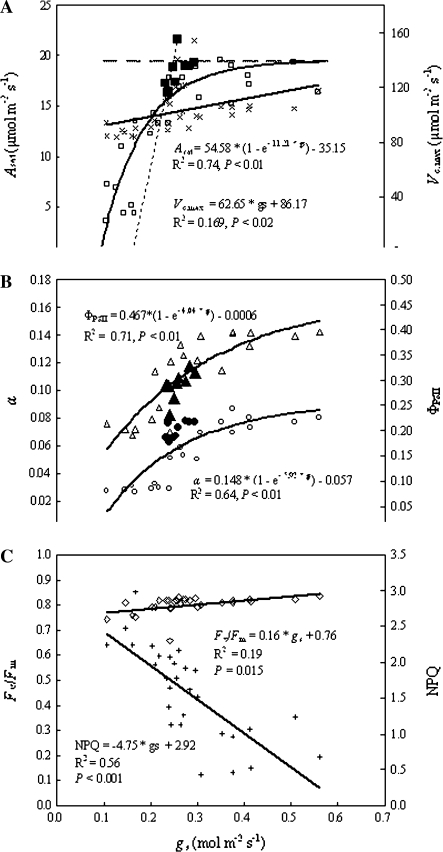
As shown in Fig. 5, the response of Asat to gs was better fitted with a three-component exponential function of the form estimated by non-linear regression [Asat=54.58×(1–e– 11.21×gs)–35.15; R2=0.74, P <0.01; Fig. 5A], rather than a linear function. Initially, Asat rapidly increased with increasing gs, then levelled off at a maximum of 19.4 μmol m−2 s−1 at a gs of 0.25 mol m−2 s−1. However, the correlation of Vc,max with gs was obviously scattered (R2=0.17), indicating that no close association existed.
Fig. 5.
Responses of photosynthetic potential and photochemical activities to stomatal conductance (gs). (A) Open squares, Asat light-saturated CO2 accumulation rate; filled squares, Asat after rewatering; crosses, Vc,max maximum carboxylation velocity. The dotted line denotes the initial Asat response to gs; the dashed line denotes Asat=constant value of Asat,maX estimated; and the intersection of the two lines represents a point at which Asat initially levelled off. (B) Open circles, α maximum photosynthetic quantum yield of CO2 uptake; filled circles, α after rewatering; open triangles, ΦPSII the actual PSII efficiency; filled traingles, ΦPSII after rewatering. (C) Open squares, the maximal efficiency of PSII photochemistry (Fv/Fm); open diamonds, non-photochemical quenching (NPQ).
The α and ΦPSII also had close relationships with gs, which are well expressed by the three-component exponential functions [α=0.148×(1–e–5.92×gs)–0.057; R2=0.64, P <0.01 for α; and ΦPSII=0.467×(1–e–4.04×gs)–0.0006; R2=0.71, P <0.01 for ΦPSII; Fig. 5B], with inflection points at MD soil moisture (gs was 0.275 with the maximum α of 0.091 and 0.248 with the maximum ΦPSII of 0.47), again indicating that the limitation to light use efficiency only occurred below the threshold. NPQ was significantly and negatively linearly related to gs along the soil moisture gradient (R2=0.56, P <0.001), but no strong relationship was observed between Fv/Fm and gs (R2=0.19, P=0.015) (Fig. 5C).
Discussion
The sensitivity of ecosystems to precipitation change plays a considerably important role in assessing and predicting ecological responses to climate change (Knapp et al., 2001; O'Connor et al., 2001; Yahdjian and Sala, 2006; Swemmer et al., 2007). The limitation to plant growth and productivity under current well-watered environmental conditions may be due to the drought effects of previous years (Wiegand et al., 2004; Yahdjian and Sala, 2006). However, in central North American grasslands, plant richness and growth increased most in wet years that followed dry years (Adler and Levine, 2007). The present results indicated that long-term SD remarkably leads to a dramatic decline in plant production, and that biomass resumption from the great loss caused by previous SD can be escalated by accelerating the growth of new parts of the plant and enhancing photosynthetic activity following rewatering.
The current experiment indicated that plant biomass was remarkably limited after rewatering (Fig. 2C), whereas RGR was stimulated drastically by previous SD (Figs 3, 4B). The limitation of the former has been emphasized by Yahdjian and Sala (2006). However, the stimulation of plant growth by rewatering has also been highlighted by other investigators (Liu et al., 2001; Reynolds et al., 2004; Siopongco et al., 2006). The rapid growth of new organs (e.g. new leaf) may contribute to the high RGR, which is favoured by easily available useful nutritional factors (e.g. nitrogen) in rewetted soil (Heckathorn et al., 1994; Xu and Zhou, 2006b). Moreover, plant growth is relatively sensitive to environmental water conditions (Hsiao, 1973). For example, upon rewatering, Lupinus albus plants could rapidly produce new leaves with quickly re-restored plant water status, although the levels of other metabolites (e.g. the sugar level) recovered more slowly (Pinheriro et al., 2004). The leaf length of maize plants that have experienced one or more days of drought stress could reach the levels of the control leaves after rewatering, but their growth rate could not exceed that of the latter, indicating that the growth may be only a resumption of the postponed event, not overcompensation (Acevedo et al., 1971). The extent and speed of resumption may depend on drought stress intensity and duration (Hsiao, 1973). Thus, the extent of compensation for the limitation of pre-drought by stimulating new growth following rewatering would determine the final plant biomass and RGR, which may be closely associated with the severity and duration of soil drought.
Benson et al. (2004) indicated that in a tallgrass prairie plant population, drought may limit the growth of the plant organs (e.g. roots and branches), thereby reducing meristems and finally decreasing the capacity of vegetation to respond to high resource availability. As a result of meristem limitation, it is suggested that grassland production may be lower in wet years preceded by dry years than in wet years preceded by wet or normal years (Benson et al., 2004; Yahdjian and Sala, 2006). Recently, a central North American grassland study by Adler and Levine (2007) suggested that ‘how quickly this long-term response develops may depend on the colonization rates of species better adapted to the altered rainfall regime’. In the present study, although SD led to declines in tiller and plant production (Fig. 2B, C), full recovery occurred as the plants were subjected to previous MD. Thus, the present results suggested that the limitation to meristems due to pre-drought following rewatering may also depend on pre-drought severity and duration.
SLA is a marker for the regulation of plant leaf following abiotic stress factors including drought (Monclus et al., 2006), implying that its high negative relationship with the leaf elastic modulus (ϵ) may be associated with leaf elasticity to water stress, depending on the species (Galmés et al., 2007a). Moreover, plants with a higher SLA can allocate a larger proportion of their leaf nitrogen to Rubisco instead of chlorophyll, enhancing their photosynthetic capacity and photosynthetic nitrogen use efficiency (PNUE), rather than their light capture (Poorter and Bongers, 2006). In previous reports, an SLA decrease under drought would be consistent with this suggestion (Xu and Zhou, 2006a); in the current experiment, an SLA increase in the new leaves of severely stressed plants after rewatering suggests that rewatering might cancel the elastic regulation mechanism of environmental stress to save energy for new growth (Fig. 2A).
The quantified changes in chlorophyll fluorescence parameters can indicate the PSII function in reponse to different environmental variables (Baker and Rosenqvist, 2004). For example, SD leads to a decline in PSII function, which may depend on species, growth stage, and stress intensity (Xu and Zhou, 2006a; Gallé et al., 2007). Consistent resilience of the maximal efficiency of Fv/Fm that declined only at SD below an SRWC of 50% (Marques da Silva and Arrabaca, 2004; Table 3) has been observed, but ΦPSII decreased concomitantly with declines in photosynthetic capacity from MD to ED intensification, depending on the species (Lu and Zhang, 1999; Tezara et al., 1999; Ghannoum et al., 2003; Liorens et al., 2003). The results of Gallé et al. (2007) indicated partial restoration of Fv/Fm and ΦPSII after only 1 d of rewatering, and complete restoration of all fluorescence parameters after 4 weeks. SD induced an increase in NPQ, and a high level remained after rewatering, implying that heat dissipation may involve photosynthetic acclimation to a change in water status (Table 3; Gomes et al., 2008). In the present experiment, a new insight is provided into the effects of different degrees of drought on recovery from rewatering, showing that only SD affected the PSII function, and overcompensation recovery was observed after rewatering (Table 3). It is suggested that the activity of the photosynthetic electron chain may cope with CO2 fixing under water stress and rewatering.
The gs response to water stress and rewatering largely depends on the species’ growth forms and leaf habits (Potts et al., 2006a; Galmés et al., 2007a; Brodribb and Cochard, 2009). Gallé and Feller (2007) reported a completely restored photosynthetic rate (A) 4 weeks after rewatering, but gs remained at a lower level, consequently resulting in an increase in WUE in Fagus sylvatica saplings. The asynchrony between A and gs appeared in Quercus pubescens in the first 2 weeks after rewatering, although both gs and photosynthesis could completely recover from drought-induced suppression 4 weeks after rewatering (Gallé et al., 2007). In the drought-adapted Vitis hybrid Richter-110, Pou et al. (2008) reported that gs maintained a lower level than in control plants, despite an increase in WUE. The lasting decrease in gs after rewatering was related to drastically decreased hydraulic conductivity, which differed from that in plants that were still drought stressed (Pou et al., 2008). A severely impaired vascular capacity for water transport due to previous drought may be responsible for the incomplete recovery of photosynthesis and gs in SD-stressed plants after rewatering (Resco, 2008; Brodribb and Cochard, 2009). Gallé and Feller (2007) indicated that non-stomatal limitations are mainly responsible for drought-induced photosynthetic inhibition seen after recent rewatering, but Marques da Silva and Arrabaca (2004) addressed the major role of stomatal limitation. In the present experiment, the initial rapid reduction in Asat, α, and ΦPSII occurred in parallel with a decrease in gs, but they levelled off as gs reached a threshold of 0.25–0.28 mol m−2 s−1 (Table 3, Fig. 5), consistent with reports of Ghannoum et al. (2003) and Flexas et al. (2006). This result suggests that stomatal limitation may lead to an inhibition of photosynthesis only below the gs threshold with a change in water status. Galmés et al. (2007b) reported that although stomatal limitation to photosynthesis under water stress is still a major factor, photosynthetic recovery of severely stressed plants after rewatering generally showed a major biochemical limitation rather than stomatal closure, and SD can lead to a decline in photosynthetic–biochemical capacity such as Rubisco activity (Xu and Baldocchi, 2003; Flexas et al., 2006). Furthermore, a decrease in gi may be responsible for decreased photosynthesis under drought (Flexas et al., 2002) and rewatering (Table 3). In an experiment by Huxman et al. (2004), following a pulse of precipitation, two grass species showed substantial increases in gs up to values that were three times higher than pre-pulse values, followed by a substantial increase in A. For the Great Basin Desert shrub species of the USA, Loik (2007) showed that both gs and A largely increased, particularly the latter, and their findings were confirmed by the current experiment (Table 3). However, Galmés et al. (2007b) found that the recovery of photosynthesis 24 h after rewatering ranged from only <10% to 70%, not up to the control level. Ignace et al. (2007) further reported that the photosynthetic recovery status of previous drought after a water pulse may depend on the temporal variation of antecedent soil moisture.
Plants in drying conditions may use different water use strategies to cope with fluctuation in water status; there may be a trade-off between physiological activity and biomass accumulation, such as the maintenance of photosynthesis as a cost of plant growth decline (Xu et al., 2007). A current major concern is that plant leaf photosynthetic activity may not always be associated with plant productivity (Long et al., 2006), implying that the trade-off may occur. Different sensitivities to water stress were found between plant growth and photosynthesis: the former may be more sensitive (Acevedo et al., 1971; Hsiao, 1973). Lizana et al. (2006) reported that crop varieties lacking plasticity to gs, photosynthetic rate, and resistance to photoinhibition can be compensated by an enhanced tendency for a morphological response, such as a rapid decrease in plant RGR. The present results showed that plant RGR and leaf photosynthetic potential were markedly stimulated by pre-drought following rewatering, depending on drought intensity. However, the light capture seemed to encounter a limitation from previous drought, and more heat dissipates (e.g. higher NPQ) (Table 3). This result might highlight the central role of the trade-off between physiological activity and biomass accumulation in plant growth, survival, and resource use processing. Furthermore, plant acclimation and tolerance to environmental stresses may also be associated with gene expression and molecular mechanisms in relation to signal transduction (Foyer et al., 1997), which should be investigated in the future.
In arid and semi-arid areas, persistent water limitation during periods of otherwise favourable metabolic conditions can maintain a reference state of minimal biological activity, such as maintaining a low gas exchange level (Potts et al., 2006a). Following wetting, however, plants immediately show a high rate of biological activity, including photosynthetic capacity and new organ growth, which can be considered as alternative functional states that may overcompensate for the limitation to plant growth and metabolic activity due to previous drought, in which processing may negate the constraints of dry conditions to NPP. Thus, plants exposed to well-watered conditions after a long-term drought may be able to increase both positive metabolic activity and growth rate. This current result may be significant because the synchrony of decreasing precipitation and rising temperature may lead to high potential evapotranspiration and low water availability, not only in the Northern Chinese steppe ecosystem (Wang and Gao, 2003; Cheng et al., 2006) but also in many of the semi-arid regions of the world, such as the Patagonian region of Argentina (Paruelo and Sala, 1995), the western European grassland (De Boeck et al., 2007), and the Chihuahuan Desert of the USA (Patrick et al., 2007). The world is facing drought that is likely to intensify; thus, in order to improve ecosystem management, the focus should be on recharging the soil profile with casual rainfall pulse events that may overcompensate the limitations resulting from previous drought.
Acknowledgments
This study was jointly funded by the National Key Basic Research Specific Foundation (2006CB400502), the National Natural Science Foundation of China (40625015, 90711001), and the Japan Society for the Promotion of Science (P07622). We thank Bing-Rui Jia, Yan-Ling Jiang, Jian Song, Yu-Hui Wang, Wen-Ping Yuan, Yu-Jin Zhang, and Wei Zeng for their immense help during the experiments.
References
- Acevedo E, Hsiao TC, Henderson DW. Immediate and subsequent growth responses of maize leaves to changes in water statues. Plant Physiology. 1971;48:631–636. doi: 10.1104/pp.48.5.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler PB, Levine JM. Contrasting relationships between precipitation and species richness in space and time. Oikos. 2007;116:221–232. [Google Scholar]
- Bai W-M, Wang Z-W, Chen Q-S, Zhang W-H, Li L-H. Spatial and temporal effects of nitrogen addition on root life span of Leymus chinensis in a typical steppe of Inner Mongolia. Functional Ecology. 2008;22:583–591. [Google Scholar]
- Bai Y, Han X, Wu J, Chen Z, Li L. Ecosystem stability and compensatory effects in the Inner Mongolia grassland. Nature. 2004;431:181–184. doi: 10.1038/nature02850. [DOI] [PubMed] [Google Scholar]
- Baker NR, Rosenqvist E. Applications of chlorophyll fluorescence can improve crop production strategies: an examination of future possibilities. Journal of Experimental Botany. 2004;55:1607–1621. doi: 10.1093/jxb/erh196. [DOI] [PubMed] [Google Scholar]
- Benhassaine-Kesri GB, Aid F, Demandre C, Kader JC, Mazliak P. Drought stress affects chloroplast lipid metabolism in rape (Brassica napus) leaves. Physiologia Plantarum. 2002;115:221–227. doi: 10.1034/j.1399-3054.2002.1150207.x. [DOI] [PubMed] [Google Scholar]
- Benson EJ, Hartnett DC, Mann KH. Belowground bud banks and meristem limitation in tallgrass prairie plant populations. American Journal of Botany. 2004;91:416–421. doi: 10.3732/ajb.91.3.416. [DOI] [PubMed] [Google Scholar]
- Bernacchi CJ, Portis AR, Nakano H, von Caemmerer S, Long SP. Temperature response of mesophyll conductance. Implications for the determination of Rubisco enzyme kinetics and for limitations to photosynthesis in vivo. Plant Physiology. 2002;130:1–7. doi: 10.1104/pp.008250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernacchi CJ, Singsaas EL, Pimentel C, Portis AR, Long SP. Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant, Cell and Environment. 2001;24:253–259. [Google Scholar]
- Bota J, Medrano H, Flexas J. Is photosynthesis limited by decreased Rubisco activity and RuBP content under progressive water stress? New Phytologist. 2004;162:671–681. doi: 10.1111/j.1469-8137.2004.01056.x. [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, Cochard H. Hydraulic failure defines the recovery and point of death in water-stressed conifers. Plant Physiology. 2009;149:575–584. doi: 10.1104/pp.108.129783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, An S, Li B, Chen J, Lin G, Liu Y, Luo Y, Liu S. Summer rain pulse size and rainwater uptake by three dominant desert plants in a desertified grassland ecosystem in northwestern China. Plant Ecology. 2006;184:1–12. [Google Scholar]
- De Boeck HJ, Lemmens CMHM, Gielen B, Bossuyt H, Malchair S, Carnol M, Merckx R, Ceulemans R, Nijs I. Combined effects of climate warming and plant diversity loss on above- and below-ground grassland productivity. Environmental and Experimental Botany. 2007;60:95–104. [Google Scholar]
- Duncan DB. Multiple range and multiple F test. Biometrics. 1955;11:1–42. [Google Scholar]
- Ethier GJ, Livingston NJ. On the need to incorporate sensitivity to CO2 transfer conductance into the Farquhar–von Caemmerer–Berry leaf photosynthesis model. Plant, Cell and Environment. 2004;27:137–153. [Google Scholar]
- Farquhar GD, von Caemmerer S, Berry JA. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta. 1980;149:78–90. doi: 10.1007/BF00386231. [DOI] [PubMed] [Google Scholar]
- Fernández RJ. On the frequent lack of response of plants to rainfall events in arid areas. Journal of Arid Environments. 2007;68:688–691. [Google Scholar]
- Flexas J, Bota J, Escalona JM, Sampol B, Medrano H. Effects of drought on photosynthesis in grapevines under field conditions: an evaluation of stomatal and mesophyll limitations. Functional Plant Biology. 2002;29:461–471. doi: 10.1071/PP01119. [DOI] [PubMed] [Google Scholar]
- Flexas J, Diaz-Espejo A, Galmés J, Kaldenhoff R, Medrano H, Ribas-Carbo M. Rapid variations of mesophyll conductance in response to changes in CO2 concentration around leaves. Plant, Cell and Environment. 2007;30:1284–1298. doi: 10.1111/j.1365-3040.2007.01700.x. [DOI] [PubMed] [Google Scholar]
- Flexas J, Ribas-Carbóm M, Bota J, Galmés J, Henkle M, Martínez-Cañellas S, Medrano H. Decreased Rubisco activity during water stress is not induced by decreased relative water content but related to conditions of low stomatal conductance and chloroplast CO2 concentration. New Phytologist. 2006;172:73–82. doi: 10.1111/j.1469-8137.2006.01794.x. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Lopez-Delgado H, Dat JF, Scott IM. Hydrogen peroxide- and glutathione-associated mechanism of acclimatory stress tolerance and signaling. Plant Physiology. 1997;100:241–254. [Google Scholar]
- Gallé A, Feller U. Changes of photosynthetic traits in beech saplings (Fagus sylvatica) under severe drought stress and during recovery. Physiologia Plantarum. 2007;131:412–421. doi: 10.1111/j.1399-3054.2007.00972.x. [DOI] [PubMed] [Google Scholar]
- Gallé A, Haldimann P, Feller U. Photosynthetic performance and water relations in young pubescent oak (Quercus pubescens) trees during drought stress and recovery. New Phytologist. 2007;174:799–810. doi: 10.1111/j.1469-8137.2007.02047.x. [DOI] [PubMed] [Google Scholar]
- Galmés J, Flexas J, Savér, Medrano H. Water relations and stomatal characteristics of Mediterranean plants with different growth forms and leaf habits: responses to water stress and recovery. Plant and Soil. 2007a;290:139–155. [Google Scholar]
- Galmés J, Medrano H, Flexas J. Photosynthetic limitations in response to water stress and recovery in Mediterranean plants with different growth forms. New Phytologist. 2007b;175:81–93. doi: 10.1111/j.1469-8137.2007.02087.x. [DOI] [PubMed] [Google Scholar]
- Gazanchian A, Hajheidari M, Sima NK, Salekdeh GH. Proteome response of Elymus elongatum to severe water stress and recovery. Journal of Experimental Botany. 2007;58:291–300. doi: 10.1093/jxb/erl226. [DOI] [PubMed] [Google Scholar]
- Genty B, Briantais JM, Baker NR. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta. 1989;990:87–92. [Google Scholar]
- Ghannoum O, Conroy JP, Driscoll SP, Paul MJ, Foyer CH, Lawlor D. Nonstomatal limitations are responsible for drought-induced photosynthetic inhibition in four C4 grasses. New Phytologist. 2003;159:599–608. doi: 10.1046/j.1469-8137.2003.00835.x. [DOI] [PubMed] [Google Scholar]
- Gomes FP, Oliva MA, Mielke MS, Almeida A-A Fde, Leite HG, Aquino LA. Photosynthetic limitations in leaves of young Brazilian Green Dwarf coconut (Cocos nucifera L. ‘nana’) palm under well-watered conditions or recovering from drought stress. Environmental and Experimental Botany. 2008;62:195–204. [Google Scholar]
- Grassi G, Magnani F. Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant, Cell and Environment. 2005;28:834–849. [Google Scholar]
- Harley PC, Loreto F, Marco GD, Sharkey TD. Theoretical considerations when estimating the mesophyll conductance to CO2 flux by analysis of the response of photosynthesis to CO2. Plant Physiology. 1992;98:1429–1436. doi: 10.1104/pp.98.4.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckathorn SA, Delucia EH. Drought-induced nitrogen retranslocation in perennial C4 grass of tallgrass prairie. Ecology. 1994;75:1877–1886. [Google Scholar]
- Hsiao TC. Plant responses to water stress. Annual Review of Plant Physiology. 1973;24:519–570. [Google Scholar]
- Huxman TE, Cable JM, Ignace DD, Eilts AJ, English N, Weltzin J, Williams DG. Response of net ecosystem gas exchange to a simulated precipitation pulse in a semiarid grassland: the role of native versus non-native grasses and soil texture. Oecologia. 2004;141:295–305. doi: 10.1007/s00442-003-1389-y. [DOI] [PubMed] [Google Scholar]
- Ignace DD, Huxman TE, Weltzin JF, Williams DG. Leaf gas exchange and water status responses of a native and non-native grass to precipitation across contrasting soil surfaces in the Sonoran Desert. Oecologia. 2007;152:401–413. doi: 10.1007/s00442-007-0670-x. [DOI] [PubMed] [Google Scholar]
- IPCC. Summary for policymakers of climate change 2007: the physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press; 2007. [Google Scholar]
- Izanloo A, Condon AG, Langridge P, Tester M, Schnurbusch T. Different mechanisms of adaptation to cyclic water stress in two South Australian bread wheat cultivars. Journal of Experimental Botany. 2008;59:3327–3346. doi: 10.1093/jxb/ern199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp AK, Briggs JM, Koelliker JK. Frequency and extent of water limitation to primary production in a mesic temperate grassland. Ecosystems. 2001;4:19–28. [Google Scholar]
- Lawlor DW, Cornic G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant, Cell and Environment. 2002;25:275–294. doi: 10.1046/j.0016-8025.2001.00814.x. [DOI] [PubMed] [Google Scholar]
- Lindroth RL, Roth S, Nordheim EV. Genotypic variation in response of quaking aspen (Populus tremuloides) to atmospheric CO2 enrichment. Oecologia. 2001;126:371–379. doi: 10.1007/s004420000521. [DOI] [PubMed] [Google Scholar]
- Liorens L, Peñuelas J, Estiarte M. Ecophysiological responses of two Mediterranean shrubs, Erica multiflora and Globularia alypum, to experimentally drier and warmer conditions. Physiologia Plantarum. 2003;119:231–243. [Google Scholar]
- Liu XY, Luo YP, Shi YC. The stimulating effects of rewatering in subjecting to water stress on leaf area of winter wheat. Scientia Agricultura Sinica. 2001;34:422–428. [Google Scholar]
- Lizana C, Wentworth M, Martinez JP, et al. Differential adaptation of two varieties of common bean to abiotic stress. I. Effects of drought on yield and photosynthesis. Journal of Experimental Botany. 2006;57:685–697. doi: 10.1093/jxb/erj062. [DOI] [PubMed] [Google Scholar]
- Loik ME. Sensitivity of water relations and photosynthesis to summer precipitation pulses for Artemisia tridentata and Purshia tridentata. Plant Ecology. 2007;191:95–108. [Google Scholar]
- Long SP, Bake NR, Raines CA. Analysing the responses of photosynthetic CO2 assimilation to long-term elevation of atmospheric CO2 concentration. Vegetatio. 1993;104/105:33–45. [Google Scholar]
- Long SP, Zhu XG, Naidu SL, Ort DR. Can improvement in photosynthesis increase crop yield? Plant, Cell and Environment. 2006;29:315–330. doi: 10.1111/j.1365-3040.2005.01493.x. [DOI] [PubMed] [Google Scholar]
- Loreto F, Harley PC, Di Marco G, Sharkey TD. Estimation of mesophyll conductance to CO2 flux by three different methods. Plant Physiology. 1992;98:1437–1443. doi: 10.1104/pp.98.4.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Zhang J. Effects of water stress on photosystem II photochemistry and its thermostability in wheat plants. Journal of Experimental Botany. 1999;50:1199–1206. [Google Scholar]
- Marques da Silva J, Arrabaca MC. Photosynthesis in the water-stressed C4 grass Setaria sphacelata is mainly limited by stomata with both rapidly and slowly imposed water deficits. Physiologia Plantarum. 2004;121:409–420. [Google Scholar]
- Monclus R, Dreyer E, Villar M, Delmotte FM, Delay D, Petit J-M, Barbaroux C, Thiec DLe, Bréchet C, Brignolas F. Impact of drought on productivity and water use efficiency in 29 genotypes of Populus deltoids×Populus nigra. New Phytologist. 2006;169:765–777. doi: 10.1111/j.1469-8137.2005.01630.x. [DOI] [PubMed] [Google Scholar]
- O'Connor TG, Haines LM, Snyman HA. Influence of precipitation and species composition on phytomass of a semi-arid African grassland. Journal of Ecology. 2001;89:850–860. [Google Scholar]
- Ögren E, Evan JR. Photosynthetic light-response curve: I. The influence of CO2 partial pressure and leaf inversion. Planta. 1993;189:180–190. [Google Scholar]
- Paruelo JM, Sala OE. Water losses in the Patagonian steppe: a modeling approach. Ecology. 1995;76:510–520. [Google Scholar]
- Patrick L, Cable J, Potts D, et al. Effects of an increase in summer precipitation on leaf, soil, and ecosystem fluxes of CO2 and H2O in a sotol grassland in Big Bend National Park, Texas. Oecologia. 2007;151:704–718. doi: 10.1007/s00442-006-0621-y. [DOI] [PubMed] [Google Scholar]
- Pinheriro C, Passarinho JA, Ricardo CP. Effect of drought and rewatering on the metabolism of Lupinus albus organs. Journal of Plant Physiology. 2004;161:1203–1210. doi: 10.1016/j.jplph.2004.01.016. [DOI] [PubMed] [Google Scholar]
- Poorter L, Bongers F. Leaf traits are good predictors of plant performance across 53 rain forest species. Ecology. 2006;87:1733–1743. doi: 10.1890/0012-9658(2006)87[1733:ltagpo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Potts DL, Huxman TE, Cable JM, English NB, Ignace DD, Eilts JA, Mason MJ, Weltzin JF, Williams DG. Antecedent moisture and seasonal precipitation influence the response of canopy-scale carbon and water exchange to rainfall pulses in a semi-arid grassland. New Phytologist. 2006a;170:849–860. doi: 10.1111/j.1469-8137.2006.01732.x. [DOI] [PubMed] [Google Scholar]
- Potts DL, Huxman TE, Enquist BJ, Weltzin JF, Williams DG. Resilience and resistance of ecosystem functional response to a precipitation pulse in a semi-arid grassland. Journal of Ecology. 2006b;94:23–30. [Google Scholar]
- Pou A, Flexas J, Alsina M, et al. Adjustments of water use efficiency by stomatal regulation during drought and recovery in the drought-adapted Vitis hybrid Richter-110 (V. berlandieri×V. rupestris) Physiologia Plantarum. 2008;134:313–323. doi: 10.1111/j.1399-3054.2008.01138.x. [DOI] [PubMed] [Google Scholar]
- Prioul JL, Chartier P. Partitioning of transfer and carboxylation components of intracellular resistance to photosynthetic CO2 fixation: a critical analysis of the methods used. Annals of Botany. 1977;41:789–800. [Google Scholar]
- Resco V, Ewers BE, Sun Wei, Huxman TE, Weltzin JF, Williams DG. Drought-induced hydraulic limitations constrain leaf gas exchange recovery after precipitation pulses in the C3 woody legume, Prosopis velutina. New Phytologist. 2008;181:672–682. doi: 10.1111/j.1469-8137.2008.02687.x. [DOI] [PubMed] [Google Scholar]
- Reynolds JF, Kemp PR, Ogle K, Fernández RJ. Modifying the ‘pulse-reserve’ paradigm for deserts of North America: precipitation pulses, soil water, and plant responses. Oecologia. 2004;141:194–210. doi: 10.1007/s00442-004-1524-4. [DOI] [PubMed] [Google Scholar]
- Siopongco JDLC, Yamauchi A, Salekdeh H, Bennett J, Wade LJ. Growth and water use response of doubled-haploid rice lines to drought and rewatering during the vegetative stage. Plant Production Science. 2006;9:141–151. [Google Scholar]
- Sponseller RA. Precipitation pulses and soil CO2 flux in a Sonoran Desert ecosystem. Global Change Biology. 2007;13:426–436. [Google Scholar]
- Swemmer AM, Knapp AK, Snyman HA. Intra-seasonal precipitation patterns and above-ground productivity in three perennial grasslands. Journal of Ecology. 2007;95:780–788. [Google Scholar]
- Tezara W, Mitchell VJ, Driscoll SD, Lawlor DW. Water stress inhibits plant photosynthesis by decreasing coupling factor and ATP. Nature. 1999;401:914–917. [Google Scholar]
- van Kooten O, Snel JFH. The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynthesis Research. 1990;25:147–150. doi: 10.1007/BF00033156. [DOI] [PubMed] [Google Scholar]
- Wang RZ, Gao Q. Climate-driven changes in shoot density and shoot biomass in Leymus chinensis (Poaceae) on the North-east China Transect (NECT) Global Ecology and Biogeography. 2003;12:249–259. [Google Scholar]
- Wang Y, Zhou G, Wang Y. Modeling responses of the meadow steppe dominated by Leymus chinensis to climate change. Climatic Change. 2007;82:437–452. [Google Scholar]
- Wiegand T, Snyman HA, Kellner K, Paruelo JM. Do grasslands have a memory: modeling phytomass production of a semiarid South African grassland. Ecosystems. 2004;7:243–258. [Google Scholar]
- Xu H, Li Y, Xu GQ, Zou T. Ecophysiological response and morphological adjustment of two Central Asian desert shrubs towards variation in summer precipitation. Plant, Cell and Environment. 2007;30:399–409. doi: 10.1111/j.1365-3040.2006.001626.x. [DOI] [PubMed] [Google Scholar]
- Xu LK, Baldocchi DD. Seasonal trends in photosynthetic parameters and stomatal conductance of blue oak (Quercus douglasii) under prolonged summer drought and high temperature. Tree Physiology. 2003;23:865–877. doi: 10.1093/treephys/23.13.865. [DOI] [PubMed] [Google Scholar]
- Xu ZZ, Zhou GS. Combined effects of water stress and high temperature on photosynthesis, nitrogen metabolism and lipid peroxidation of a perennial grass Leymus chinensis. Planta. 2006a;224:1080–1090. doi: 10.1007/s00425-006-0281-5. [DOI] [PubMed] [Google Scholar]
- Xu ZZ, Zhou GS. Nitrogen metabolism and photosynthesis in Leymus chinensis in response to long-term soil drought. Journal of Plant Growth Regulation. 2006b;25:252–266. [Google Scholar]
- Xu ZZ, Zhou GS. Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. Journal of Experimental Botany. 2008;59:3317–3325. doi: 10.1093/jxb/ern185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahdjian L, Sala OE. Vegetation structure constrains primary production response to water availability in the Patagonian steppe. Ecology. 2006;87:952–962. doi: 10.1890/0012-9658(2006)87[952:vscppr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Yang LH, Bastow JL, Spence KO, Wright AN. What can we learn from resource pulse? Ecology. 2008;89:621–634. doi: 10.1890/07-0175.1. [DOI] [PubMed] [Google Scholar]