Abstract
Sex-dependent thermogenesis during reproductive organ development in the inflorescence is a characteristic feature of some of the protogynous arum species. One such plant, skunk cabbage (Symplocarpus renifolius), can produce massive heat during the female stage but not during the subsequent male stage in which the stamen completes development, the anthers dehisce, and pollen is released. Unlike other thermogenic species, skunk cabbage belongs to the bisexual flower group. Although recent studies have identified the spadix as the thermogenic organ, it remains unclear how individual tissues or intracellular structures are involved in thermogenesis. In this study, reproductive organ development and organelle biogenesis were examined during the transition from the female to the male stage. During the female stage, the stamens exhibit extensive structural changes including changes in organelle structure and density. They accumulate high levels of mitochondrial proteins, including possible thermogenic factors, alternative oxidase, and uncoupling protein. By contrast, the petals and pistils do not undergo extensive changes during the female stage. However, they contain a larger number of mitochondria than during the male stage in which they develop large cytoplasmic vacuoles. Comparison between female and male spadices suggests that mitochondrial number rather than their level of activity correlates with thermogenesis. Their spadices, even in the male, contain a larger amount of mitochondria that had greater oxygen consumption, compared with non-thermogenic plants. Taken together, our data suggest that the extensive maturation process in stamens produces massive heat through increased metabolic activities. The possible mechanisms by which petal and pistil metabolism may affect thermogenesis are also discussed.
Keywords: Alternative oxidase, bisexual flower, mitochondrial density, respiration, stamen, thermogenesis, thermoregulation, ultrastructure, uncoupling protein, vacuole
Introduction
Heat production is found in several members of the protogynous arum lily family (Araceae) (Gibernau et al., 2005; Thien et al., 2009). These thermogenic arum species are divided into two main groups, the ones with unisexual flowers and the ones with bisexual flowers (Bown, 2000; Cabrera et al., 2008). In the unisexual inflorescence, male and female florets reside in separate parts of a floral chamber. Usually, female florets are located at the base of the spadix of the inflorescence, and male florets are located in its upper part (Meeuse and Raskin, 1988). The Eastern Asian skunk cabbage (Symplocarpus renifolius) is the only bisexual flower belonging to thermogenic arum species studied to data, and has almost the same thermogenic and thermoregulatory characteristics as the eastern North American skunk cabbage (Symplocarpus foetidus) (Uemura et al., 1993; Wen et al., 1996; Seymour and Blaylock, 1999; Ito et al., 2004; Seymour, 2004). The two species, S. renifoilius and S. foetidus, are usually found in swampy habitats in the north temperate region (Mayo et al., 1997; Nie et al., 2006). Bisexual flowers of skunk cabbage comprise a large number of florets arranged on the surface of the spadix. Each flower is composed of one pistil surrounded by four stamens and four petals. The most striking feature of skunk cabbage is that it can keep the spadix temperature between 22–26 °C for 6.8±5.8 d even when the ambient temperature falls as low as –10 °C (Knutson, 1974; Uemura et al., 1993; Seymour, 2004). Thermoregulation in skunk cabbage is more precise and durable than other thermoregulatory plants studied to date, such as Philodendron sellom (Nagy et al., 1972; Seymour et al., 1983) and Nelumbo nucifera (Seymour and Schultze-Motel, 1998; Seymour et al., 1998). Many species, which are not thermoregulatory, are generally able to produce heat at most for 24 h. The robust thermoregulation observed in skunk cabbage makes it a great model organism to unravel the mechanism underlying plant thermogenesis (Ito et al., 2004; Ito and Ito, 2005; Ito-Inaba et al., 2008a, b).
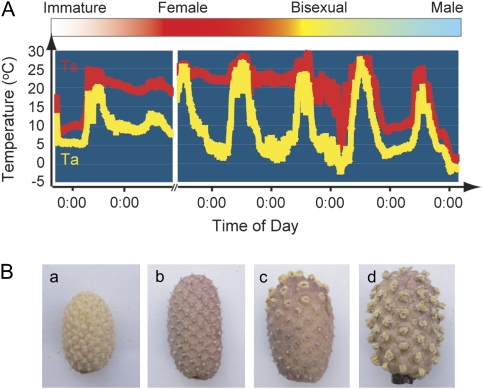
Development of skunk cabbage inflorescence can be divided into four stages: immature, female, bisexual, and male (Fig. 1A, B) (Uemura et al., 1993). At the immature stage, the inflorescence is just emerging, and heat production has not occurred yet. At the female stage, the petals open slightly, and the stigmas become exserted. At the same time, the spadix starts generating massive heat and can maintain its internal temperature around 20 °C even if the air temperature falls below freezing temperatures. This female phase usually lasts 6.8±5.8 d (Uemura et al., 1993). At the bisexual stage, stamens at the base of each stigma start emerging from the surface of the spadix. At this stage, the spadix can produce heat, but not enough to maintain the internal temperature constant. Subsequently, ripening of the whole spadix and release of pollen is completed at the male stage and thermogenesis ends. Inflorescence development in skunk cabbage appears to be closely associated with the thermogenic stages, suggesting that thermogenesis might be required for the efficient development of reproductive organs.
Fig. 1.
Floral development closely associated with the transition of thermogenic stage in skunk cabbage. (A) Variations in ambient air (Ta) and spadix (Ts) temperatures. At the immature stage (white bar), heat production has not occurred. During the female stage (red bar), spadices produce extensive heat, and maintain their internal temperature around 20 °C. At the bisexual stage (yellow bar), the spadix can produce heat, but not enough to keep a constant body temperature. At the male stage (light blue bar), spadices are no longer able to produce heat. (B) Photographs of spadices from each stage (immature (a), female (b), bisexual (c), male (d)).
Recent progress in thermographical techniques has made it possible to visualize specific organs directly involved in heat production. It was shown that heat is generated from the appendix and male florets in some species, such as Arum maculatum (Bermadinger-Stabentheiner and Stabentheiner, 1995), Arum italicum (Skubatz et al., 1990; Albre et al., 2003), dead horse (Helicodiceros muscivorus) (Seymour et al., 2003a), and voodoo lily (Sauromatum guttatum) (Skubatz et al., 1991), and the male florets in dragon lily (Dracunculus vulgaris) (Ito and Seymour, 2005), the male and sterile male florets in Philodendron melinonii (Barabe et al., 2002; Seymour and Gibernau, 2008), and the spadix in skunk cabbage (Ito et al., 2003). It should be noted that in such well-known arum species, heat is generated by the male florets consisting of dense stamens, and not by the female florets consisting of dense pistils. In addition, their male florets contain no developed petals (Bown, 2000). Hence, the stamens in thermogenic arum species might be important for generating the massive heat. In skunk cabbage, however, it remains unclear which tissues, among the petal, stamen, and pistil, are responsible for thermogenesis.
Ecological and physiological studies of many arum species have revealed that thermogenesis is positively correlated to their oxygen consumption rate (Seymour and Blaylock, 1999; Seymour and Schultze-Motel, 1999; Seymour et al., 2003a, b; Seymour and Gibernau, 2008). The possible involvement in thermogenesis of mitochondrial factors, such as the alternative oxidase (AOX), the uncoupling protein (UCP), and the reduction levels of ubiquinone (UQ), has also been studied (Wagner et al., 1998, 2008; Crichton et al., 2005; Sluse et al., 2006; Onda et al., 2007; Ito-Inaba et al., 2008a, b). These studies suggest that mitochondrial respiratory activity mediated by thermogenesis-related factors might play a major role in thermogenesis. However, very little is known about whether their mitochondrial number or density is correlated with heat production in higher plants as they are in mammals. Mammalian brown adipose tissues (BAT), where non-shivering thermogenesis takes place, have abundant mitochondria (Ghadially, 1988). Ultrastructural analyses of voodoo lily have revealed that mitochondria only accumulate osmophilic material between the inner and outer membranes during the thermogenic stage (Skubatz and Kunkel, 2000).
In this work, differentiation in the individual tissues and their intracellular structure during inflorescence development of skunk cabbage is examined in detail. A mechanism is proposed as to how the individual tissues or intracellular structures are correlated to the thermogenesis. In addition, the possible implication of mitochondrial density and function in thermogenesis of skunk cabbage is also shown by comparative analysis.
Materials and methods
Plant materials
The skunk cabbages (Symplocarpus renifolius) sampled for these experiments were grown in the marshlands in Shizukuishi and Nishiwaga town in Iwate, and Kawabe town in Akita. Potato tubers (Solanum tuberosum L. cv. Danshaku) and cauliflowers (Brassica oleracea L. botrytis) were purchased from a local market.
Microscopic observation
Light microscopy analyses were performed as previously described (Endo et al., 2004; Masuko et al., 2006; Suwabe et al., 2008). Briefly, material was fixed with 4% paraformaldehyde and 0.25% glutaraldehyde in 0.05 M sodium phosphate buffer (pH 7.2) overnight at 4 °C. After dehydration of the tissue pieces in a graded BuOH/EtOH/H2O-DEPC series, sections were stained with toluidine-blue O. Microscopy pictures were taken with a Nikon E800 microscope (Nikon, Tokyo, Japan) and Discovery V20 (Carl Zeiss) microscope. The developmental stages of microspores and pollen grains were determined by staining nuclei with DAPI (Watanabe et al., 1991) and microscopy pictures were taken with a Nikon E800 microscope. Procedures for ultrastructural analysis were conducted as previously described (Toyooka et al., 2000). Briefly, petals, pistils, and stamens were picked up by using clamping forceps, and the pith was cut into ∼1 mm cubes (1 mm3). Tissues were fixed with 4% paraformaldehyde and 2% glutaraldehyde in 100 mM sodium cacodylate buffer (pH 7.4) for 2 h at RT. They were post-fixed with 1% osmium tetroxide in 100 mM cacodylate buffer (pH 7.4) for 4 h at RT. After dehydration of the tissue pieces in a graded methanol series, the pieces were further dehydrated in methanol/propylene oxide (1:1 v/v), 100% propylene oxide, and propylene oxide/Epon812 resin (Taab) (1:1 v/v), and finally embedded in 100% Epon812 resin. Ultrathin sections (70 nm) were obtained by cutting with a diamond knife on an Ultracut UCT ultramicrotome (Leica), and were transferred to Formvar-coated grids. The sections were stained with 4% uranyl acetate for 12 min and then with lead citrate solution for 3 min and examined with a transmission electron microscope (JEM-1011; JEOL). Images were acquired using a Gatan DualView CCD camera and Gatan Digital Micrograph software or films. Mitochondrial density was determined by the ratio of the mitochondrial numbers to the total cytoplasmic area per cell. Total cytoplasmic areas were quantified by using Photoshop CS3 software.
Isolation of mitochondria from skunk cabbage, potato tuber, and cauliflower
Mitochondria were isolated from skunk cabbage, potato tubers, and cauliflower using differential centrifugation and Percoll density gradient centrifugation as previously described (Ito-Inaba et al., 2008a).
Oxygen consumption assay
Oxygen consumption was monitored using a Oxytherm(OXT)-1 oxygen electrode (Hansatech). For the standard assay, 200 μg of mitochondria was added in a 1.2 ml of 0.3 M mannitol, 1 mM MgCl2, 100 mM KCl, 10 mM KH2PO4, 10 mM HEPES-KOH, pH 7.0., containing 1 mM NADH. For the alternative respiration assay, 150 μg of mitochondria was added in 1.2 ml of 0.3 M sucrose, 1 mM MgCl2, 10 mM KCl, 5 mM KH2PO4, 20 mM MOPS-KOH, pH 7.2, and 0.1% BSA. 1 mM NADH or 10 mM succinate, 0.5 mM ADP, 1 mM potassium cyanide (KCN), an inhibitor of the cytochrome pathway, and finally 0.1 M n-propyl gallate (nPG), an inhibitor of the alternative pathway, were added successively. The addition of the substrate (NADH or succinate) and ADP lead to state2 and state 3 respiration, respectively. AOX activity was estimated from the ratio of oxygen consumption in the presence of KCN. Assays containing succinate also included 0.1 mM ATP to activate succinate dehydrogenase (Norman et al., 2004).
Temperature measurements
All temperatures were measured with a thermal recorder TR-52 (T & D).
RT-PCR of SrUCPA and SrAOX transcripts
RT-PCR analyses were carried out as described previously (Ito-Inaba et al., 2008a).
Extraction of total protein from various tissues
Total protein was extracted as described previously (Ito-Inaba et al., 2008a). Briefly, petals, pistils, stamens, and pith tissues were ground in liquid nitrogen using a mortar and pestle. 50 mg of powder were suspended in 0.45 ml extraction buffer (330 mM TRIS-HCl, 7.5% glycerol, 5% SDS, pH 6.8) containing 0.05% protein inhibitor (Sigma, P-9599). After 10 min incubation on ice, the supernatant was recovered by centrifugation at 10 000 g for 5 min at 4 °C. The protein extract was clarified by TCA precipitation, and the TCA precipitate was suspended in 150 μl of sample buffer (33 mM TRIS-HCl, 10% glycerol, 1.5% SDS, 1% mercaptoethanol, pH 6.8) containing 0.05% protease inhibitor (Sigma, P-9599) and boiled for 5 min.
Antibodies
Polyclonal antibodies against SrUCPA were described previously (Ito-Inaba et al., 2008b). Monoclonal antibody against AOX is a gift from Elthon T (Elthon et al., 1989). Anti-Cytochrome C (BD Biosciences) and anti-HSP60 (Stressgen) antibody were purchased.
Results
Alterations of individual tissues in the spadix during the inflorescence development
Thermogenesis in skunk cabbage is closely associated with the stages of inflorescence development (Fig. 1). To examine the correlation between inflorescence development and heat production further, the changes in the spadix temperature, the mass of each tissue, and the number of flowers during inflorescence development were investigated (Table 1). The temperature data clearly show that massive heat production in spadices occurs only during the female stage. Thermogenesis is weak at the bisexual stage, and heat production is virtually undetectable at the immature or the male stages. During the transition from the female to the male stage, petals or pistils increase their mass progressively. By contrast, the weights of stamens decrease, because anther dehiscence and pollen release occurs at the male stage. The pith, which is the central stalk of the spadix, increases its mass progressively, and so does the total mass of the spadix. The number of flowers (approximately 100) remains unchanged during the stage transition. These data show that the stamens undergo a reduction in mass in contrast with all the other tissues of the spadix.
Table 1.
The spadix temperature, tissue weights, and the number of flowers according to developmental stages in skunk cabbage inflorescence
| Inflorescence stage |
||||
| Immature | Female | Bisexual | Male | |
| Ts (°C)a | 5.7±0.90 | 20.4±2.89 | 8.0±1.31 | 5.7±0.78 |
| Ta (°C)b | 5.0±1.60 | 4.7±0.20 | 5.0±0.00 | 5.1±0.90 |
| Petal (mg) | – | 635.8±149.1 | 809.1±329.2 | 1208.1±246.2 |
| (34.3±4.3% w/w)c | (32.2±2.5% w/w) | (42.9±5.4% w/w) | ||
| Pistil (mg) | – | 129.4±37.0 | 149.0±108.9 | 263.3±100.0 |
| (7.0±1.5% w/w) | (5.4±1.6% w/w) | (9.7±3.8% w/w) | ||
| Stamen (mg) | – | 425.6±89.8 | 522.4±235.2 | 208.0±78.2 |
| (23.2±3.0% w/w) | (20.9±3.8% w/w) | 7.7±2.8 % w/w) | ||
| Pith (mg) | – | 276.7±105.4 | 455.3 ±288.21 | 507.3±163.5 |
| (14.7±2.2% w/w) | (17.1±3.7% w/w) | (17.1±2.8% w/w) | ||
| Spadix (g) | 0.77±0.057 | 1.9±0.43 | 2.5±1.05 | 2.9±0.64 |
| (100% w/w) | (100% w/w) | (100% w/w) | ||
| The number of flowers | – | 111.0±22.7 | 98.0±14.8 | 112.5±24.3 |
Data are the means ±SD of five spadices at least per each stage.
Spadix temperature.
Ambient temperature.
The percentage given in parenthesis represents the ratio of each tissue to the total spadix weight.
Extensive anther development at different developmental stages of spadices
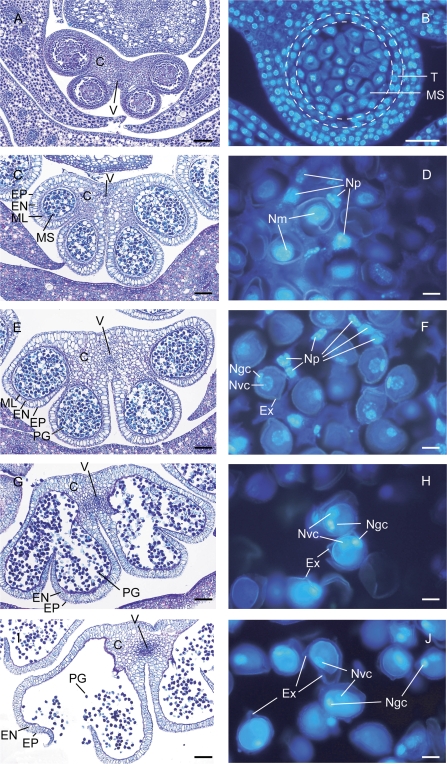
To examine the structural changes in the stamens, equatorial cross-sections of anthers stained with toluidine blue O and DAPI were observed in detail (Fig. 2). Toluidine blue O staining clearly showed the developmental changes of anthers during the inflorescence development (Fig. 2, left panels). The connective cell wall is well developed at the immature (Fig. 2A) and the early phase of the female stage (Fig. 2C). It becomes thinner at the later phases of the female stage (Fig. 2E), and begins to break down at the bisexual stage (Fig. 2G). The breakdown of the connective cell wall is completed and pollen grains are released at the male stage (Fig. 2I). DAPI staining gives a clear image of the nucleus (Fig. 2, right panels). At the immature stage (Fig. 2B), the tapetum, the nutritive layer of cells that lines the inner wall of the pollen sac, is visible. Since the behaviour of the tapetum in arum species is known to be amoeboid (Anger and Weber, 2006), it is conceivable that the tapetum of skunk cabbage develops to form the multinucleated periplasmodium enclosing the microspores (Fig. 2D, F) which degenerates and finally disappears (Fig. 2H, J). DAPI staining also clearly shows that microspores containing one nucleus (Fig. 2B, D) developed into pollen grains containing vegetative and generative cells (Fig, 2F, H, J). These observations indicate that extensive anther development occurs during inflorescence development. It is noteworthy that many developmental changes in the periplasmodium and microspores/pollen grains occur in the female-stage spadix when heat is produced.
Fig. 2.
Anther development during the transition between thermogenic stages. Toluidine blue O staining (A, C, E, G, I) and DAPI staining (B, D, F, H, J) of cross-sections from anthers in immature (A, B), female (C, D, E, F); bisexual (G, H), and male stages (I, J). C, connective cell wall; V, vascular bundle; EP, epidermis; EN, endothecium; ML, middle layer; MS, microspore; PG, pollen grain; T, tapetum; Ex, exine; N, nucleus; m, microspore; p, periplasmodium; gc, generative cell; vc, vegetative cell. Bars (A, B, C, E, G, I) 100 μm; (D, F, H, J) 10 μm.
Ultrastructural analyses of anther cells during female stage
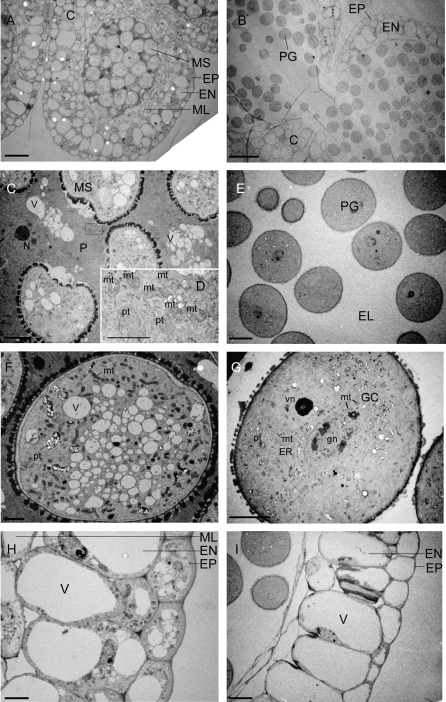
Since anther structure changes significantly during the female stage, ultrastructural analyses of the anthers were carried out at two stages during the development of the female stage, designated as the mature phase (Fig. 3A, C, D, F, H) and the later phase (Fig. 3B, E, G, I). In the transition from the mature to the later phase, well-developed connective cell walls gradually degenerate (from A to B), and the amoeboid structures of the tapetum, the periplasmodium surrounding the microspores, also degenerates and finally disappears resulting in the appearance of an ‘electron-lucent’ area (from C to E). The multinucleated periplasmodium cells contain dense cytoplasm with abundant mitochondria and plastids (Fig. 3C, D). During the development from microspore to pollen grain (from F to G), a large number of vacuoles disappear. During the development, the middle layers have degenerated (from H to I). In the epidermis and endothecium, vacuoles developed into larger ones, and other organelles, such as mitochondria and plastids, became invisible (from H to I). Since some of the characteristics (i.e. degenerated periplasmodium tissues and the pollen grains containing two nuclei) of the later phase at the female stage are also found at the bisexual stage as shown in Fig. 2G and H, this phase might be very near the bisexual stage. These data indicate that the tissues such as the connective cell wall, the tapetum/periplasmodium, the microspores/pollen, and the epidermis/endothecium/middle layer changes significantly during the thermogenic female stage. These extensive changes in the intracellular structure of stamens with abundant mitochondria might be correlated with the massive heat production in skunk cabbage.
Fig. 3.
Electron photographs showing anther development during the female stage. Cross-sections of anthers in the mature (A, C, D, F, H) or late (B, E, G, I) female stage. (A) Connective cells divides anther into two pollen sacs. (B) Degenerated connective cell wall. (C) Microspores surrounded by well-developed periplasmodium. (D) Detail in the square of (C). (E) Pollen grains surrounded by electron-lucent space. (F) Microspores containing many vacuoles and mitochondria. (G) Disappearance of vacuoles in functional pollen grains. (H) Well-developed anther epidermis and endothecium; (I) Degenerated anther endothecium. C, connective cell wall; MS, microspore; PG, pollen grain; EP, epidermis; EN, endothecium; ML, middle layer; P, periplasmodium; EL, electron lucent space; mt, mitochondria; V, vacuole; pt, plastid; ER, endoplasmic reticulum; GC, generative cell; gn, generative nucleus; vn, vegetative nucleus. Bars (A, B) 50 μm; (C, G, H) 5 μm; (E, I) 10 μm; (F) 2 μm; (D) 1 μm.
Distribution of SrAOX and SrUCPA in the female spadix
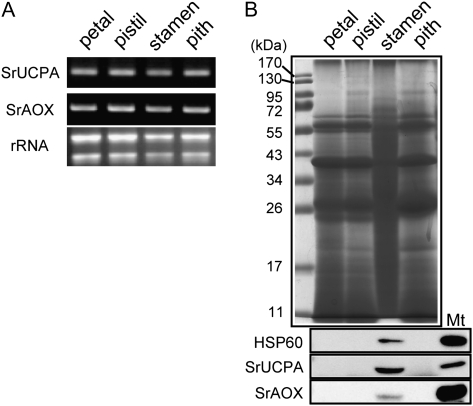
It is of great interest to know whether stamen cells of the thermogenic skunk cabbage highly accumulate alternative oxidase (AOX) and uncoupling protein (UCP), which have both been proposed to be putative thermogenic factors (McIntosh, 1994; Ito-Inaba et al., 2008a). Therefore, it was decided to examine the expression of SrUCPA and SrAOX in the various spadix tissues. First, their mRNA expression in petal, pistil, stamen, and in the pith at the female stages were examined by semi-quantitative RT-PCR using a SrAOX or SrUCPA specific primer. As shown in Fig. 4A, both SrUCPA and SrAOX mRNA were constitutively expressed in the different tissues. Next, the accumulation of various proteins in each tissue was examined (Fig. 4B). 40 μg of total protein extract from each tissue were separated by SDS-PAGE, and analysed by Coomassie Brilliant Blue (CBB) staining and immunoblotting with antibodies specific to HSP60, SrUCPA, and SrAOX. Interestingly, the CBB staining pattern observed in stamens was significantly different from those in other tissues (Fig. 4B, upper). Immunoblotting showed that HSP60, SrUCPA, and SrAOX are highly expressed in stamens, and undetectable in other tissues (Fig. 4B, lower). These results demonstrate that HSP60, SrUCPA, and SrAOX, are highly accumulated in the stamens of the female spadix and suggest that accumulation of the mitochondrial proteins AOX and UCP in the stamens might be associated with thermogenesis in skunk cabbage.
Fig. 4.
Expression study of SrUCPA and SrAOX in the female spadix. (A) Transcriptional expression of SrUCPA and SrAOX in petals, pistils, stamens, and pith was examined by RT-PCR. To prevent saturation of amplification, SrUCPA and SrAOX were amplified for 30 and 26 cycles, respectively. (B) 40 μg of total protein fractions extracted from each tissue were analysed by CBB staining, and by immunoblotting with either SrUCPA, SrAOX, or HSP60 antibodies.
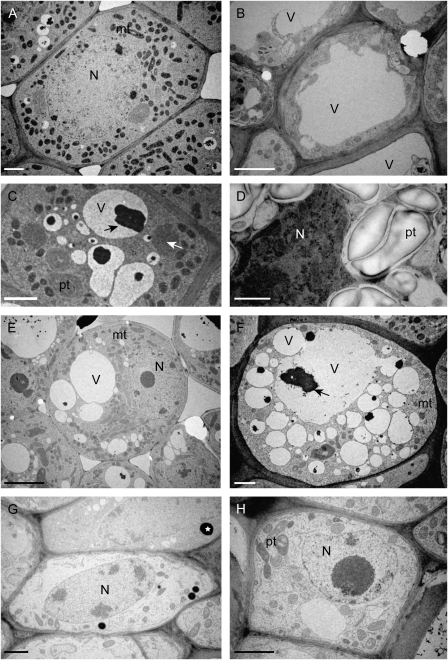
Organelle structure in the petal, pistil and pith at the female and male stage
Our microscopic analysis did not reveal significant changes in petals, pistils, and the pith during the transition from the thermogenic female stage to the non-thermogenic male stage. To investigate further the connection between the morphological change of these tissues and thermogenesis, the ultrastructure of the petals, pistils, and the pith were analysed by electron microscopy. As shown in Fig. 5A and C, petal cells at the female stage contain a large number of mitochondria which possess electro-dense cristae structures, and a small number of vacuoles containing an electro-dense black material which has not been characterized yet. By contrast, petal cells at the male stage contain large vacuoles but only a small number of mitochondria (Fig. 5B) and a lot of plastids containing large grains of starch were observed in the epidermal cells (Fig. 5D). In pistil cells (Fig. 5E, F), a large number of mitochondria were also observed at the female stage (Fig. 5E), but few at the male stage (Fig. 5F), and the size and the number of vacuoles at the male stage were larger than those at the female stage. By contrast, pith cells in both the female and the male stages contained only a small number of mitochondria, but vacuoles developed significantly into larger ones (Fig. 5G, H). Electron dense structures were observed only in the pith in both stages, but they have not been identified yet (Fig. 5G, H). These results suggest that there are significant differences in intracellular structure of petal and pistil cells between female and male stages. Therefore, these changes in petals and pistils might be also associated with thermogenesis in skunk cabbage.
Fig. 5.
Ultrastructural comparison between female (A, C, E, G) and male tissues (B, D, F, H). Petal (A, B), epidermis in petal (C, D), pistil (E, F), and pith (G, H). White and black arrows and asterisks in (C, F, G) indicate unidentified electron dense material. N, nucleus; V, vacuole; mt, mitochondria; pt, plastid. Bars (A, B, C, F, G, H) 2 μm; (D) 1 μm; (E) 5 μm.
Mitochondrial density and vacuolar content at the female and male stages
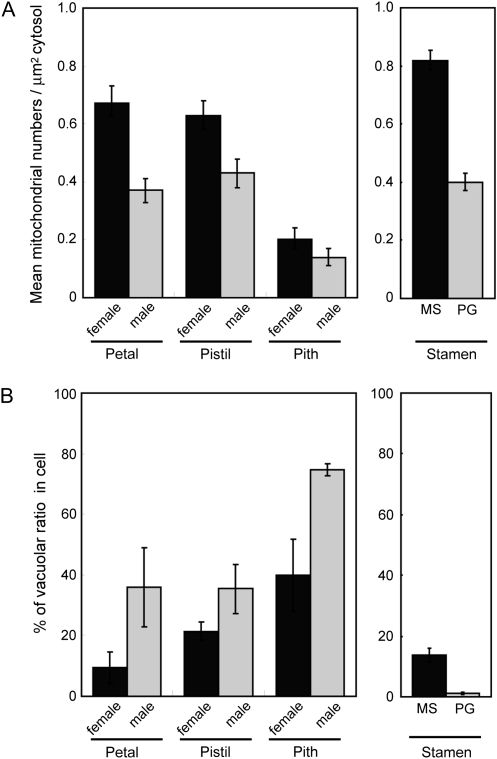
It is of interest to determine the number of mitochondria in the cells of each tissue during the female and male stages in skunk cabbage. To this end, high-resolution images of cells in each tissue during the female and male stages were captured, and mitochondrial number and surface area of the cytosol were determined. Cytosol was defined as all areas within the cytoplasm, excluding the nucleus and vacuoles. Mitochondrial density was expressed as the number of mitochondria μm−2 cytosol (Fig. 6A). In petals and pistils, mitochondrial density in females is higher than in males, and therefore, their density decreases during the development from the female to the male stages. In pith, however, the density in females is almost the same as in males and even lower than in the petals and pistils of the male flower. These data suggest that petals and pistils rather than the pith might be more involved in the massive energy metabolism in skunk cabbage. Besides mitochondria, vacuolar content in each cell was estimated using the same thin sections (Fig. 6B). The vacuolar ratio in cells of female-stage flowers is lower than that in all the male tissues examined. In the case of the pith, the vacuolar ratio in females reaches almost the same value as that in male-stage petals and pistils. Taken together, these results suggest that the decrease in mitochondrial number and the increase in vacuole volume in the petal and pistil may be associated with the transition from the thermogenic female stage to the non-thermogenic male stage. In stamens, due to the considerable variation of cellular types, it was only possible to estimate the mitochondrial density and vacuolar ratio in microspore and pollen cells (Fig. 6A, B, right panels). Both the mitochondrial density and vacuolar ratio are higher in the microspore cell (mature female stage) than in the pollen grain cell (end of the female or bisexual stage). It should be noted that mitochondrial density in the microspore cell is the highest among all the other tissues. Again, these results suggest that the decrease in mitochondrial number in stamens might be associated with the transition to the thermogenic stage.
Fig. 6.
Quantification of mitochondria and vacuolar ratio in each tissue at different developmental stages. (A) Average density (mitochondrial numbers μm−2 cytosol) in thin sections of cells during the female and male stages for the petal, pistil, pith, and for the microspore (MS) and pollen grain (PG) in the stamen. Cytosol was defined as all areas within the cytoplasm, excluding the nucleus and vacuole. (B) Average vacuolar surface ratio (% of cell surface) in cells in thin sections during the female and male stages for the petal, pistil, pith, and for the microspore (MS) and pollen grain (PG) in the stamen. For this analysis, 10 cells in petals, 5 cells in pistils, piths, and stamens were examined. Error bars represent standard error obtained in different replicates.
Comparisons of mitochondrial density in various plant tissues
The positive correlation between the energy metabolism and total mitochondrial number is a well known feature in brown adipose tissues (Rodriguez-Cuenca et al., 2002). Hence, it is be possible that thermogenic female-stage spadices contain a larger number of mitochondria than non-thermogenic males. To confirm this hypothesis further, mitochondrial content was determined biochemically. As shown in Table 2, 6.68 mg and 3.57 mg of mitochondrial proteins were recovered from about the same amount (12 g) of female- and male-stage spadices, respectively. Thus, the mitochondrial amount contained in female-stage spadices is estimated to be almost twice the amount found in male-stage spadices. In addition, compared to a non-thermogenic plant, such as potato and cauliflower, the mitochondrial amount in the female spadix is about 25 times greater (Table 2). This suggests that higher mitochondrial density in thermogenic spadices might be important for the thermogenic ability of skunk cabbage.
Table 2.
The recovery of mitochondria from female- and male-stage spadix of skunk cabbage, potato, and cauliflower
| Skunk cabbage |
Potato | Cauliflower | ||
| Female-stage spadix | Male-stage spadix | |||
| Plant materials (g) | 12.43±0.24 (6.3)a | 12.23±0.46 (5) | 1265.26±751.07 | 547.16±250.20 |
| Recovery of mitochondrial protein (mg) | 6.68±0.50 | 3.57±0.46 | 27.14±16.40 | 0.74±0.14 |
| Mitochondrial protein/plant materials | 0.54±0.033 | 0.29±0.010 | 0.022±0.0011 | 0.0015±0.00042 |
Data are the means±SD of three independent processes of mitochondrial isolation in each group.
The number given in parenthesis represents the averaged total number of spadix used for mitochondrial isolation in three independent experiments.
Comparisons of mitochondrial activity in various plant tissues
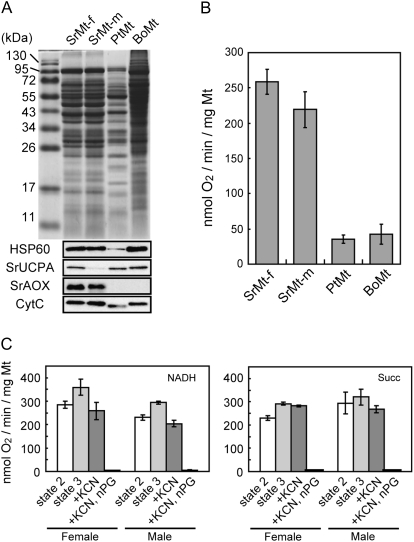
Although the large amount of mitochondria in female-stage spadices seems to be important for thermogenesis in skunk cabbage, it remains unclear whether mitochondrial activity is also correlated with thermogenesis. To address this question, the functional differences in mitochondria between female and male spadices were examined first. Mitochondrial proteins prepared from female- and male-stage spadices were separated by SDS-PAGE, followed by CBB staining and immunoblotting (Fig. 7A). There are little differences in the migration patterns between mitochondrial proteins from female- and male-stage spadices. Content of HSP60 and CytC, two mitochondrial protein controls, and SrAOX were not different between the female and the male while SrUCPA levels were higher in the female than in the male. Their oxygen consumption capacity was compared next (Fig. 7B). The respiration levels in female spadices (258.31±43.63 nmol O2 min−1 mg−1 Mt) were similar to that in male spadices (219.23±61.97 nmol O2 min−1 mg−1 Mt). Alternative oxidase activity (oxygen consumption in the presence of KCN) was also determined using two substrates, NADH and succinate (Fig. 7C). Under NADH oxidation, KCN partly inhibited state3 respiration (the rapid oxygen uptake in the presence of ADP) in female- and male-stage spadices, and the alternative respiration levels in female-stage spadices (258.82±37 nmol O2 min−1 mg−1 Mt) were similar to that in male-stage spadices (203.82±13.6 nmol O2 min−1 mg−1 Mt). Under succinate oxidation, KCN did not inhibit state3 respiration, and the levels in female-stage spadices (282.2±2.9 nmol O2 min−1 mg−1 Mt) were also similar to those in male-stage spadices (268.2±15.1 nmol O2 min−1 mg−1 Mt). Despite the higher expression of SrUCPA in mitochondria from the female-stage spadices, no other differences in respiratory activity between mitochondria from female- and male-stage spadices could be found. Therefore, our results suggest that higher mitochondrial amount, rather than activity, might be more important for regulating thermogenesis in skunk cabbage.
Fig. 7.
Comparative analyses of mitochondrial activity in the female-stage and male-stage skunk cabbage spadix, potato, and cauliflower. (A) Skunk cabbage mitochondria in the female-stage spadix (SrMt-f) and in the male-stage spadix (SrMt-m), potato mitochondria (PtMt), and cauliflower mitochondria (BoMt). Mitochondrial proteins were separated by SDS-PAGE, and analysed by CBB staining and immunoblotting with antibodies specific for HSP60, SrUCPA, SrAOX, and CytC (B) Oxygen consumption assay for SrMt-f, SrMt-m, PtMt, and BoMt. Error bars represent standard error obtained in different replicates. (C) Alternative respiration in female- and male-stage spadix. Oxygen uptake was measured in the presence of substrates (1 mM NADH or 10 mM succinate). 0.5 mM ADP, 1 mM KCN, and 0.1 M nPG were successively added. Error bars represent standard error obtained in different replicates.
Next, the mitochondrial function of skunk cabbage spadices was compared with that of non-thermogenic plants, such as potato and cauliflower. In the non-thermogenic plants, the CBB staining pattern is strikingly different from the spadices of skunk cabbage (Fig. 7A), and proteins were detected by anti-HSP60, SrUCPA, CytC antibodies, but not by anti-AOX antibodies (Fig. 7A). The levels of mitochondrial respiration in potato (35.17±7.33 nmol O2 min−1 mg−1 Mt) and cauliflower (42.2±24.4 nmol O2 min−1 mg−1 Mt) are much lower than in female- and male-stage spadices (Fig. 7B). These results also suggest that the significant differences in mitochondrial activity between thermogenic and non-thermogenic plants might explain their differences in thermogenic potential.
Discussion
In this study, the morphological and biochemical changes in the skunk cabbage spadix during the development of inflorescences have been examined in detail. By contrast with petals, pistils, and the pith, the stamens undergo significant structural changes during the transition of thermogenic stage (Fig. 1; Table 1). It is shown that stamen structure, especially anthers, was significantly changed during the thermogenic female stages (Fig. 2). Ultrastructural analyses of anther tissues indicated that the intracellular structure in microspore/pollen, tapetum/periplasmodium, and anther wall were extensively altered during the female stage (Fig. 3; Table 3). In addition, it is demonstrated that expression profiles of stamen proteins differed significantly from other tissues, and mitochondrial proteins, such as AOX and UCP, were enriched in the stamen tissues compared to other tissues (Fig. 4). AOX and UCP abundantly expressed in stamens may be involved in such thermogenesis. The mitochondrial density in microspore cells of the stamen is higher than in all other tissues, such as petals, pistils, and the pith (Figs 5, 6). Taken together, stamen tissues appear to increase their mitochondrial mass or metabolic activity in order to enable them to develop progressively during the female stage. These increased metabolic activities in stamen tissues might lead to the massive heat production in skunk cabbage, because heat is generated by many reactions in energy metabolism, which are exothermic in the forward direction (Lowell and Spiegelman, 2000).
Table 3.
The morphological features of spadix tissues during their developmental changes
| Female (thermogenic phase) | Male (non-thermogenic phase) | ||
| Stamen | From earlier to mature stage | Later stage | Anther dehiscence and pollen release has occurred. No electron micrographs were taken due to their impaired structure |
| Connective cell walls are well-developed; anther loculus contain microspores surrounded by periplasmodium; periplasmodium contains dense cytoplasm with nucleus, mitochondria and plastids; microspores contain abundant mitochondria, plastids and vacuoles; anther wall has thick surface layers consisting of epidermis, endothecium and middle layer | Connective cell walls and periplasmodium have degenerated; pollen grains contain mitochondria, plastid and few vacuoles; generative and vegetative nucleus have appeared in pollen; anther wall has thinned due to the degeneracy of middle layers | ||
| Petal | Dense cytoplasm with abundant mitochondria containing electron-dense cristae structure; relatively small vacuoles containing an electron-dense black material which has not been characterized yet; larger cytoplasmic vacuoles, and a few plastids in the epidermis cells | Overall, a few mitochondria, but instead a large cytoplasmic vacuole; a lot of plastids containing large grains of starch in the epidermis cells | |
| Pistil | Dense cytoplasm with abundant mitochondria and relatively small vacuoles | A few mitochondria, but instead a large cytoplasmic vacuole containing electron-dense black materials of as yet unknown function | |
| Pith | A few mitochondria and a large amount of cytoplasmic vacuoles during both the female and male stages | ||
Interestingly, in the dead horse, dragon lily, voodoo lily, and philodendron, which belong to the unisexual flower group, heat is generated by male florets on the spadix (Skubatz et al., 1991; Seymour et al., 2003a; Ito and Seymour, 2005; Seymour and Gibernau, 2008). Since those male florets contain developed stamens, but not any pistils and developed petals, stamens in those arum species might also play a central role in the heat production, as expected in skunk cabbage. The detailed analyses of stamens in thermogenic arum species may potentially solve the mechanism underlying heat generation. In skunk cabbage, each floret is composed of one pistil, four petals and four stamens. Since petals and pistils contain higher levels of mitochondria at the thermogenic female stage than at the non-thermogenic male stage (Figs 5, 6), they might be responsible, with stamen, of heat production. However, petals and pistils might not play central roles in thermogenesis (assuming that the mitochondrial density in these tissues is much less than that in stamens; Fig. 4). Alternatively, petals could play a structural role in keeping the spadix warm. As shown in Fig. 1 and Supplementary Fig. S1 at JXB online, the petals could act as a shield for internal structures, especially stamens, to avoid dissipation of the heat. Such a tightly sealed structure may be instrumental in keeping the spadix temperature constant (about 20 °C) for long periods of time (6.8±5.8 d). In other words, the short transient thermogenesis of 1–2 d in male florets of other thermogenic arum species may be attributable to the lack of support from petals and pistils, because the male florets on the inflorescences are separated from the female florets and have no petals.
In mammalian BAT, which is involved in thermogenesis, it is known that there is a positive correlation between the energy metabolism and the morphological features of the mitochondria, such as their number, size, surface area, and cristae density (Ghadially, 1988). After acclimation to 22 °C, female rats, which exhibit greater oxygen consumption than male rats, show a higher number and larger size of mitochondria, a greater amount of cristae, and a higher UCP1 content than males (Quevedo et al., 1998; Rodriguez-Cuenca et al., 2002). These gender-related differences in mitochondrial morphology and function may be associated with a higher thermogenic capacity in females than males in mammals. In thermogenic plants, it remained to be elucidated whether thermogenesis is related to the mitochondrial number or morphology. But our data showed that mitochondrial content of female-stage spadices was 2-fold higher than that of males (Table 2). Furthermore, our electron microscopic studies indicate that stamens at the thermogenic female stage, especially microspore and plasmodium, have high densities of mitochondria (Fig. 3). It is also shown that pistils and petals from spadices of the female stage contain a larger number of mitochondria than those of the male one (Fig. 6; Table 3). Our results suggest that plants might produce the massive heat from their tissues by increasing the numbers of mitochondria like mammalian BAT.
The skunk cabbage spadix, irrespective of the female or male stage, contains larger amounts of mitochondria than non-thermogenic plants (Table 2). Furthermore, the respiratory activity of mitochondria isolated from skunk cabbage was higher than in mitochondria from non-thermogenic plants (Fig. 7). Thus, it is conceivable that higher mitochondrial content and respiratory activity plays an important role in the higher thermogenic capacity in skunk cabbage. Although there are several reports studying respiratory activity of plant mitochondria using thermogenic and non-thermogenic plants (Weaire and Kekwick, 1975; Wagner et al., 1998, 2008; Hourton-Cabassa et al., 2002), direct comparison between thermogenic and non-thermogenic plants has been lacking. Our data provide direct evidence that there are substantial differences in mitochondrial amounts and mitochondrial respiratory activity between thermogenic and non-thermogenic plants. According to another report, mitochondrial yield from Arabidopsis leaves was 0.024 mg g−1 of leaf tissue (Keech et al., 2005), and this value is similar to the mitochondrial content in potato. It is worth examining whether these features observed in skunk cabbage are common among thermogenic plants or not. Evidence was also obtained that the female-stage spadix contains a larger number of mitochondria than the male spadix (Table 2), but few functional differences were observed between mitochondria isolated from female- and male-stage spadices (Fig. 7). Therefore, it is currently speculated that mitochondrial amount in female-stage spadices, rather than the function, might be more important for the intensity of thermogenesis in skunk cabbage.
Based on these observations, a hypothetical model for heat production in the spadix is proposed. (i) During the female stage, various maturation processes in the anthers may produce heat by their increased metabolic activities and mitochondrial respiration. Petals and pistils seem to play a minor role in a heat production. Alternatively, petals might play a role to keep the spadix warm by forming the tightly sealed surface structure. (ii) During the bisexual stage, anther dehiscence and pollen release starts occurring from the upper part of the spadix, and therefore, thermogenesis in stamens gradually ceases from the top to the bottom of spadix. Together with these changes in the stamens, the increase of vacuoles, and the decrease of mitochondrial number in petals and pistils may start progressively. It is hypothesized that the morphological changes in stamens might be one of the triggers to induce the intracellular changes in petals and pistils, resulting in the termination of their synergistic effects on thermogenesis. (iii) At the male stage, anther dehiscence and pollen release has been completed throughout the spadix, and the cytoplasm in the petal and pistil cells is occupied by large vacuoles, leading to the loss of thermogenesis. However, the validity of this hypothetical model awaits more experimental evidence. The detailed analysis of thermogenic stamens in arum species and in-depth observation at regular intervals during the bisexual stage may potentially address whether this model is relevant or not. Additional comparative study with non-thermogenic plants will also be useful for unravelling molecular mechanisms in thermogenic plants, because morphological changes and gene expression/regulation during reproductive organ development in non-thermogenic higher plants has been extensively studied to date (Watanabe, 2008).
Although SrAOX and SrUCPA mRNAs were expressed in all tissues during the female stage, SrAOX and SrUCPA proteins were only detected in stamens (Fig. 4). In addition, while SrUCPA mRNAs were constitutively expressed in various tissues irrespective of thermogenic stage, SrUCPA protein was detected only in the thermogenic tissue or stage (Ito-Inaba et al., 2008a). Such discrepancy between mRNA and protein levels is also known for rat UCP1 mRNA/protein. At 22 °C, accumulation of the UCP1 protein in female rats was 2-fold higher than in male rats, while the accumulation of UCP1 mRNA in both female and male was similar (Quevedo et al., 1998). These results suggest that increased protein accumulation may result not only from the increased gene expression but also from mRNA and/or protein turnover (Pico et al., 1994). Recently, an mRNA export system in yeast has been proposed as one of the mechanisms underlying these discrepancies. Izawa et al. have reported that HSP transcripts induced by ethanol stress remained in the nucleus in a hyperadenylated state and were not translated into HSP proteins (Izawa et al., 2008). Thus, it is of great interest to unravel the mechanisms which regulates the accumulation of UCP in plant and animal cells.
Supplementary data
Supplementary data are available at JXB online.
Supplementary Fig. S1. Cross-sectional structure of the female-stage spadix.
Acknowledgments
We thank Ms Takako Kawai and Ms Mayumi Wakazaki (RIKEN Plant Science Center) for their assistance in experiments with electron microscopy. We also thank Mrs Hitomi Ito (Iwate University) for her technical support. We are also grateful to Dr TE Elthon for his generous gift of monoclonal antibody against alternative oxidase. This work was supported by the 21st Century Center of Excellence program, Grant-in-Aid for Special Research on Priority Areas (Nos 18075003 and 20678001), Grant-in-Aid for Young Investigator (Nos 20780070 and 20780236), and the New Energy and Industrial Technology Development Organization (NEDO).
References
- Albre J, Quikichini A, Gibernau M. Pollination ecology of Arum Italicum(Araceae) Botanical Journal of the Linnean Society. 2003;141:205–214. [Google Scholar]
- Anger EM, Weber M. Pollen-wall formation in Arum alpinum. Annals of Botany. 2006;97:239–244. doi: 10.1093/aob/mcj022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabe D, Gibernau M, Forest F. Zonal thermogenetic dynamics of two species of Philodendron from two different subgenera (Araceae) Botanical Journal of the Linnean Society. 2002;139:79–86. [Google Scholar]
- Bermadinger-Stabentheiner E, Stabentheiner A. Dynamics of thermogenesis and structure of epidermal tissues in inflorescences of Arum maculatum. New Phytologist. 1995;131:41–50. doi: 10.1111/j.1469-8137.1995.tb03053.x. [DOI] [PubMed] [Google Scholar]
- Bown D. AROIDS: plants of the Arum family. 2nd edn. North America: Timber Press; 2000. pp. 53–73. [Google Scholar]
- Cabrera LI, Salazar GA, Chase MW, Mayo SJ, Bogner J, Davila P. Phylogenetic relationships of aroids and duckweeds (Araceae) inferred from coding and noncoding plastid DNA. American Journal of Botany. 2008;95:1153–1165. doi: 10.3732/ajb.0800073. [DOI] [PubMed] [Google Scholar]
- Crichton PG, Affourtit C, Albury MS, Carre JE, Moore AL. Constitutive activity of Sauromatum guttatum alternative oxidase in Schizosaccharomyces pombe implicates residues in addition to conserved cysteines in α-keto acid activation. FEBS Letters. 2005;579:331–336. doi: 10.1016/j.febslet.2004.10.107. [DOI] [PubMed] [Google Scholar]
- Elthon TE, Nickels RL, McIntosh L. Monoclonal antibodies to the alternative oxidase of higher plant mitochondria. Plant Physiology. 1989;89:1311–1317. doi: 10.1104/pp.89.4.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M, Tsuchiya T, Saito H, et al. Identification and molecular characterization of novel anther-specific genes in Oryza sativa L. by using cDNA microarray. Genes and Genetic Systems. 2004;79:213–226. doi: 10.1266/ggs.79.213. [DOI] [PubMed] [Google Scholar]
- Ghadially FN. Ultrastructural pathology of the cell and matrix. 3rd edn. London: Butterworth-Heinemann; 1988. [Google Scholar]
- Gibernau M, Barabe D, Moisson M, Trombe A. Physical constraints on temperature difference in some thermogenic aroid inflorescences. Annals of Botany. 2005;96:117–125. doi: 10.1093/aob/mci157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hourton-Cabassa C, Mesneau A, Miroux B, Roussaux J, Ricquier D, Zachowski A, Moreau F. Alteration of plant mitochondrial proton conductance by free fatty acids. Uncoupling protein involvement. Journal of Biological Chemistry. 2002;277:41533–41538. doi: 10.1074/jbc.M202805200. [DOI] [PubMed] [Google Scholar]
- Ito-Inaba Y, Hida Y, Ichikawa M, Kato Y, Yamashita T. Characterization of the plant uncoupling protein, SrUCPA, expressed in spadix mitochondria of the thermogenic skunk cabbage. Journal of Experimental Botany. 2008a;59:995–1005. doi: 10.1093/jxb/ern024. [DOI] [PubMed] [Google Scholar]
- Ito-Inaba Y, Hida Y, Mori H, Inaba T. Molecular identity of uncoupling proteins in thermogenic skunk cabbage. Plant and Cell Physiology. 2008b;49:1911–1916. doi: 10.1093/pcp/pcn161. [DOI] [PubMed] [Google Scholar]
- Ito K, Ito T, Onda Y, Uemura M. Temperature-triggered periodical thermogenic oscillations in skunk cabbage (Symplocarpus foetidus) Plant and Cell Physiology. 2004;45:257–264. doi: 10.1093/pcp/pch038. [DOI] [PubMed] [Google Scholar]
- Ito K, Onda Y, Sato T, Abe Y, Uemura M. Structural requirements for the perception of ambient temperature signals in homeothermic heat production of skunk cabbage (Symlocarpus foetidus) Plant, Cell and Environment. 2003;26:783–788. doi: 10.1046/j.1365-3040.2003.00989.x. [DOI] [PubMed] [Google Scholar]
- Ito K, Seymour RS. Expression of uncoupling protein and alternative oxidase depends on lipid or carbohydrate substrates in thermogenic plants. Biology Letters. 2005;1:427–430. doi: 10.1098/rsbl.2005.0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Ito K. Nonlinear dynamics of homeothermic temperature control in skunk cabbage, Symplocarpus foetidus. Physical Review E. 2005;72 doi: 10.1103/PhysRevE.72.051909. 051909. [DOI] [PubMed] [Google Scholar]
- Izawa S, Kita T, Ikeda K, Inoue Y. Heat shock and ethanol stress provoke distinctly different responses in 3′-processing and nuclear export of HSP mRNA in Saccharomyces cerevisiae. Biochemical Journal. 2008;414:111–119. doi: 10.1042/BJ20071567. [DOI] [PubMed] [Google Scholar]
- Keech O, Dizengremel P, Gardestrom P. Preparation of leaf mitochondria from Arabidopsis thaliana. Physiologia Plantarum. 2005;124:403–409. [Google Scholar]
- Knutson RM. Heat production and temperature regulation in eastern skunk cabbage. Science. 1974;186:746–747. doi: 10.1126/science.186.4165.746. [DOI] [PubMed] [Google Scholar]
- Lowell BB, Spiegelman BM. Towards a molecular understanding of adaptive thermogenesis. Nature. 2000;404:652–660. doi: 10.1038/35007527. [DOI] [PubMed] [Google Scholar]
- Masuko H, Endo M, Saito H, et al. Anther-specific genes, which expressed through microsporogenesis, are temporally and spatially regulated in model legume, Lotus japonicus. Genes and Genetic Systems. 2006;81:57–62. doi: 10.1266/ggs.81.57. [DOI] [PubMed] [Google Scholar]
- Mayo SJ, Bogner J, Boyce PC. The genera of Araceae. Kew, London, UK: The Trustees, Royal Botanic Gardens; 1997. [Google Scholar]
- McIntosh L. Molecular biology of the alternative oxidase. Plant Physiology. 1994;105:781–786. doi: 10.1104/pp.105.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeuse BJD, Raskin I. Sexual reproduction in the arum lily family, with emphasis on thermogenicity. Sexual Plant Reproduction. 1988;1:3–15. [Google Scholar]
- Nagy KA, Odell DK, Seymour RS. Temperature regulation by the inflorescence of Philodendron. Science. 1972;178:1195–1197. doi: 10.1126/science.178.4066.1195. [DOI] [PubMed] [Google Scholar]
- Nie ZL, Sun H, Li H, Wen J. Intercontinental biogeography of subfamily Orontioideae (Symplocarpus, Lysichiton, and Orontium) of Araceae in Eastern Asia and North America. Molecular Phylogenetics and Evolution. 2006;40:155–165. doi: 10.1016/j.ympev.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Norman C, Howell KA, Millar AH, Whelan JM, Day DA. Salicylic acid is an uncoupler and inhibitor of mitochondrial electron transport. Plant Physiology. 2004;134:492–501. doi: 10.1104/pp.103.031039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onda Y, Kato Y, Abe Y, et al. Pyruvate-sensitive AOX exists as a non-covalently associated dimer in the homeothermic spadix of the skunk cabbage, Symplocarpus renifolius. FEBS Letters. 2007;581:5852–5858. doi: 10.1016/j.febslet.2007.11.061. [DOI] [PubMed] [Google Scholar]
- Pico C, Herron D, Palou A, Jacobsson A, Cannon B, Nedergaard J. Stabilization of the mRNA for the uncoupling protein thermogenin by transcriptional/translational blockade and by noradrenaline in brown adipocytes differentiated in culture: a degradation factor induced by cessation of stimulation? Biochemical Journal. 1994;302:81–86. doi: 10.1042/bj3020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevedo S, Roca P, Pico C, Palou A. Sex-associated differences in cold-induced UCP1 synthesis in rodent brown adipose tissue. Pflügers Archiv European Journal of Physiology. 1998;436:689–695. doi: 10.1007/s004240050690. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Cuenca S, Pujol E, Justo R, Frontera M, Oliver J, Gianotti M, Roca P. Sex-dependent thermogenesis, differences in mitochondrial morphology and function, and adrenergic response in brown adipose tissue. Journal of Biological Chemistry. 2002;277:42958–42963. doi: 10.1074/jbc.M207229200. [DOI] [PubMed] [Google Scholar]
- Seymour RS. Dynamics and precision of thermoregulatory responses of eastern skunk cabbage Symplocarpus foetidus. Plant, Cell and Environment. 2004;27:1014–1022. [Google Scholar]
- Seymour RS, Bartholomew GA, Barnhart CM. Respiration and heat production by the inflorescence of Philodendron selloum Koch. Planta. 1983;157:336–343. doi: 10.1007/BF00397405. [DOI] [PubMed] [Google Scholar]
- Seymour RS, Blaylock AJ. Switching off the heater: influence of ambient temperature on thermoregulation by eastern skunk cabbage Symplocarpus foetidus. Journal of Experimental Botany. 1999;50:1525–1532. [Google Scholar]
- Seymour RS, Gibernau M. Respiration of thermogenic inflorescences of Philodendron melinonii: natural pattern and responses to experimental temperatures. Journal of Experimental Botany. 2008;59:1353–1362. doi: 10.1093/jxb/ern042. [DOI] [PubMed] [Google Scholar]
- Seymour RS, Gibernau M, Ito K. Thermogenesis and respiration of inflorescences of the dead horse arum Helicodiceros muscivorus, a pseudothermoregulatory aroid associated with fly pollination. Functional Ecology. 2003a;17:886–894. [Google Scholar]
- Seymour RS, Schultze-Motel P. Physiological temperature regulation by flowers of the sacred lotus. Philosophical Transactions of the Royal Society B. 1998;353:935–943. [Google Scholar]
- Seymour RS, Schultze-Motel P. Respiration, temperature regulation and energetics of thermogenic inflorescences of the dragon lily Dracunculus vulgaris (Arceae) The Royal Society. 1999;266:1975–1983. [Google Scholar]
- Seymour RS, Schultze-Motel P, Lamprecht I. Heat production by sacred lotus flowers depends on ambient temperature, not light cycle. Journal of Experimental Botany. 1998;49:1213–1217. [Google Scholar]
- Seymour RS, White CR, Gibernau M. Environmental biology: heat reward for insect pollinators. Nature. 2003b;426:243–244. doi: 10.1038/426243a. [DOI] [PubMed] [Google Scholar]
- Skubatz H, Kunkel DD. Developmental changes in the ultrastructure of the Sauromatum guttatum (Araceae) mitochondria. Journal of Electron Microscopy. 2000;49:775–782. doi: 10.1093/oxfordjournals.jmicro.a023871. [DOI] [PubMed] [Google Scholar]
- Skubatz H, Nelson TA, Dong AM, Meeuse BJD, Bendich AJ. Infrared thermography of Arum lily inflorescences. Planta. 1990;182:432–436. doi: 10.1007/BF02411396. [DOI] [PubMed] [Google Scholar]
- Skubatz H, Nelson TA, Meeuse BJ, Bendich AJ. Heat production in the voodoo lily (Sauromatum guttatum) as monitored by infrared thermography. Plant Physiology. 1991;95:1084–1088. doi: 10.1104/pp.95.4.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluse FE, Jarmuszkiewicz W, Navet R, Douette P, Mathy G, Sluse-Goffart CM. Mitochondrial UCPs: new insights into regulation and impact. Biochimica et Biophysica Acta. 2006;1757:480–485. doi: 10.1016/j.bbabio.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Suwabe K, Suzuki G, Takahashi H, et al. Separated transcriptomes of male gametophyte and tapetum in rice: validity of a laser microdissection (LM) microarray. Plant and Cell Physiology. 2008;49:1407–1416. doi: 10.1093/pcp/pcn124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thien LB, Bernhardt P, Devall MS, Chen ZD, Luo YB, Fan JH, Yuan LC, Williams JH. Pollination biology of basal angiosperms (ANITA grade) American Journal of Botany. 2009;96:166–182. doi: 10.3732/ajb.0800016. [DOI] [PubMed] [Google Scholar]
- Toyooka K, Okamoto T, Minamikawa T. Mass transport of proform of a KDEL-tailed cysteine proteinase (SH-EP) to protein storage vacuoles by endoplasmic reticulum-derived vesicles is involved in protein mobilization in germinating seeds. Journal of Cell Biology. 2000;148:453–464. doi: 10.1083/jcb.148.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura S, Ohkawara K, Kudo G, Wada N, Higashi S. Heat-production and cross-polination of the asian skunk cabbage Symplocarpus renifolius. American Journal of Botany. 1993;80:635–640. [Google Scholar]
- Wagner AM, Krab K, Wagner MJ, Moore AL. Regulation of thermogenesis in flowering Araceae: the role of the alternative oxidase. Biochimica et Biophysica Acta. 2008;1777:993–1000. doi: 10.1016/j.bbabio.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Wagner AM, Wagner MJ, Moore AL. In vivo ubiquinone reduction levels during thermogenesis in Araceae. Plant Physiology. 1998;117:1501–1506. doi: 10.1104/pp.117.4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M. Towards a comprehensive understanding of molecular mechanisms of sexual reproduction in higher plants. Plant and Cell Physiology. 2008;49:1404–1406. doi: 10.1093/pcp/pcn138. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Shiozawa H, Isogai A, Suzuki A, Takeuchi T, Hinata K. Existence of S-glycoprotein-like proteins in anthers of self-incompatible species of Brassica. Plant and Cell Physiology. 1991;32:1039–1047. [Google Scholar]
- Weaire PJ, Kekwick RG. The synthesis of fatty acids in avocado mesocarp and cauliflower bud tissue. Biochemical Journal. 1975;146:425–437. doi: 10.1042/bj1460425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J, Jansen RK, Kilgore K. Evolution of the eastern Asian and eastern North American disjunct genus Symplocarpus (Araceae): insights from chloroplast DNA restriction site data. Biochemical Systematics and Ecology. 1996;24:735–747. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.