Abstract
Severe mucopolysaccharidosis type I (MPS I) is a fatal neuropathic lysosomal storage disorder with significant skeletal involvement. Treatment involves bone marrow transplantation (BMT), and although effective, is suboptimal, due to treatment sequelae and residual disease. Improved approaches will need to be tested in animal models and compared to BMT. Herein we report on bone marrow transplantation to treat feline mucopolysaccharidosis I (MPS I). Five MPS I stably engrafted kittens, transplanted with unfractionated bone marrow (6.3 × 107 to 1.1 × 109 nucleated bone marrow cells per kilogram) were monitored for 13–37 months post-engraftment. The tissue total glycosaminoglycan (GAG) content was reduced to normal levels in liver, spleen, kidney, heart muscle, lung, and thyroid. Aorta GAG content was between normal and affected levels. Treated cats had a significant decrease in the brain GAG levels relative to untreated MPS I cats and a paradoxical decrease relative to normal cats. The α-L-iduronidase (IDUA) activity in the livers and spleens of transplanted MPS I cats approached heterozygote levels. In kidney cortex, aorta, heart muscle, and cerebrum, there were decreases in GAG without significant increases in detectable IDUA activity. Treated animals had improved mobility and decreased radiographic signs of disease. However, significant pathology remained, especially in the cervical spine. Corneal clouding appeared improved in some animals. Immunohistochemical and biochemical analysis documented decreased central nervous system ganglioside storage. This large animal MPS I study will serve as a benchmark of future therapies designed to improve on BMT.
Keywords: Bone Marrow Transplantation, Disease Models, Animal, Lysosomal Storage Diseases, Iduronidase, Glycosaminoglycans, Mucopolysaccharidosis I
Introduction
The lysosomal acid hydrolase, α-L-iduronidase (IDUA, L-iduronidase, EC 3.2.1.76), is one of the obligate enzymes required for degradation of heparan and dermatan sulfates (HS, DS). Loss of activity leads to intracellular accumulation of HS and DS, a hallmark of the lysosomal storage disease (LSD) mucopolysaccharidosis type I (MPS I, OMIM 607014-16). The single most prevalent form of MPS [1], Hurler syndrome, has the most severe phenotype, leading to multisystem disease of CNS degeneration, dysostosis multiplex, corneal clouding, facial dysmorphia, organomegaly, cardiovascular disease, respiratory compromise, and premature death, usually within the first decade. Additional forms of MPS I include the attenuated Scheie syndrome, and Hurler/Scheie syndrome, intermediate in severity between Hurler and Scheie syndromes.
Similarly to most acid hydrolases, IDUA is targeted to lysosomes by both inter-and intracellular trafficking by the enzyme’s mannose-6-phosphate (MP6) moiety and its cognate receptors. The intercellular trafficking is the basis of “cross correction,” whereby diseased cells are corrected when exposed to enzyme from normal cells. Cross correction is the physiological basis of therapeutic response to bone marrow transplantation (BMT) or cord blood transplantation [3, 4], the standard of care for severe MPS I. Enzyme replacement therapy (ERT), the intravenous infusion of exogenous recombinant enzyme [2] is an additionally therapy from MPS I, albeit with limited application. Although ERT and/or transplantation are beneficial, significant hurdles remain in providing effective and uniform therapy for the CNS, which will require developments in other modes of therapy, including improved CNS-directed ERT, stem cell transplantation, and/or gene therapy. Animal models will prove critical in evaluating these future approaches.
Of the three species with in which MPS I models exist, the first characterized was a spontaneous feline model [5]. The feline mutation is a three base pair deletion causing the deletion of a conserved asparagine residue and leading to <5% normal IDUA activity [6]. The pathology has been characterized [7–15] and show this model to be consistent with human MPS I. A designation of Hurler, Hurler/Scheie, or Scheie for animal models is problematic, as this designation belies what is likely a continuous spectrum of signs seen in human patients which runs from most to least severe. Additionally animal models, even though they may be biochemically severe forms of a given LSD are often less clinically severe than the human counterpart. For example, none of the animal models for MPS I show routine pediatric mortality as seen with Hurler syndrome in patients. With this in mind, the feline model could be characterized as Hurler/Scheie, in that there is severe orthopedic disesase, stunted growth, histological brain disease, and premature mortality in the adult period. This model has been used to evaluate short-term ERT [16]. As future improvements of treatment are designed they will require validation of large animal models of MPS I, which would warrant comparison to BMT, the standard of care in severe human MPS I. Herein we report the results of (BMT) in cats with MPS I which will serve as such a benchmark..
Methods
Animals
Cats were produced and housed (University of Pennsylvania), under NIH and USDA guidelines for the care and use of animals in research.
BMT
Eleven MPS I kittens received 600–700cGy whole body irradiation (Siemens 6MV linear accelerator), and were transplanted from related MPS I heterozygotes with 6.3 × 107 to 1.1 × 109 unfractionated nucleated bone marrow cells/kg body weight (Table 1). Heterozygote donors were chosen since in matched human donors there is a significant chance that the matched donor will be a first degree relative and hence a heterozygote. Animals received two transplants at 1–2 and 5–7 days post irradiation. Kittens 3680 and 4289 received third transplants 16 and 20 days post-irradiation, respectively. Gastrointestinal preconditioning and support included neomycin (p.o., 5–7 days, 0–4 days pre- to 3–5 days post-irradiation), and 25 mg L-glutamine (p.o., twice daily, 11–25 days, 0–4 days pre- to 9–24 days post-irradiation). Beginning on 3-0 days pre-irradiation, cyclosporin was given at 25 mg/kg once a day, p.o. for three weeks, and then reduced by half every three weeks over two months. Graft versus host disease was treated using prednisone at dose of 1 mg/kg. From before irradiation until engraftment, kittens received antibiotics, and were housed in a laminar flow, hepa-filtered, class 100 room. Engraftment was considered successful if peripheral white blood cell counts exceeded 1,000/μl. Chimerism in transplants with non-sex matched donors was evaluated by karyotype of peripheral blood lymphocytes as described [17].
Table 1.
Bone Marrow Transplantion Data for Feline MPS I
| Recipient | Donor | Transplant Dose Bone Marrow Cells/kg |
Days to engraft ment |
Karyotype (% donor cells) |
Time post engraft -ment at euth. or death |
Transplant Results and Comments |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Animal Number | Sex | Age (m)* |
Coefficient of relatedness |
Sex | Age (m)* |
Dose 1 | Dose 2 | Dose 3 | Months post- engraftment % |
Months post- engraftment % |
|||||
| 3680 | M | 2.7 | 0.57 | F | 2.7 | 7.3 × 108 | 6.7 × 107 | 1.1 × 109 | 41–47 | 0.1 | ≥ 91 | 12.3 | ≤ 7 | 13.4 m | Graft lost by-12 m post engraftment |
| 4289 | M | 1.3 | 0.80 | M | 1.3 | 6.3 × 107 | 4.1 × 108 | 6.3 × 108 | 22–28 | 1 d** | Died 1 d post- engraftment/sub- capsular renal hemorhage | ||||
| 4296 | M | 5.4 | 0.77 | M | 5.4 | 4.0 × 108 | 7.4 × 107 | 10–14 | 35.8 m | Stable engraftment | |||||
| 4297 | F | 0.8 | 0.77 | M | 0.8 | 1.2 × 108 | 5.1× 108 | 11 | 2.2 m | Stable engraftment. Died 2.2 m post- engraftment/pylo- nephritis | |||||
| 4306 | F | 0.7 | 0.81 | M | 84.7 | Buffy Coat | 2 ml Bone Marrow | 4–10 | 2 d | Died 1 d post- engraftment/hydronephrosis | |||||
| 4347 | F | 0.8 | 0.77 | M | 87.2 | 3.0 × 108 | 1.0× 108 | 10–13 | 3.5 | ≥ 95 | 32.7 | ≥ 95 | 36.8 m | Stable engraftment. | |
| 4349 | M | 0.8 | 0.80 | F | 0.8 | 1.3 × 108 | NA† | Died post 2nd transplant/poss FIP‡ | |||||||
| 4366 | F | 1.1 | 0.77 | M | 4.7 | NA | 2.9× 108 | 6–10 | 3.2 | ≥ 95 | 32.4 | ≥ 95 | 36.3 m | Stable engraftment. History of seizure | |
| 4372 | M | 4.3 | 0.80 | F | 4.3 | 3.6 × 108 | NA | 9 | 17.7 m | Stable engraftment. IDDM 11 m post engraftment | |||||
| 4429 | F | 0.6 | 0.70 | M | 11.3 | 1.1 × 109 | 4.7× 108 | NA | 9 d | Died 9 days post engraftment/possibl e cardiomegaly | |||||
| 4583 | F | 3.3 | 0.89 | M | 3.3 | 1.1 × 109 | 4.5× 108 | 10–17 | 13.1 | ≥ 95 | 17.2 m | Stable engraftment | |||
m = months,
d = days,
NA = not available,
FIP = feline infectious peritonitis
Ophthalmological assessments by both direct and indirect ophthalmoscopy were made by a board-certified veterinary ophthalmologist with extensive experience with feline MPS I (GDA).
Radiographic Analysis
Treated animals were radiographed at 15 (4347 and 4366), 19 (4296) and 27 (4372) months. Radiographs of five male and five female untreated MPS I cats of similar ages were used for comparative analysis. Board-certified veterinary radiologists (VWK and SME) scored changes in the coxofemoral joint and cervical spine in a blinded fashion. Assessed abnormalities included femoral head morphology, coxofemoral joint laxity, acetabular morphology, vertebral body width, tipping (a change in the rostro-caudal axis of a vertebra relative to spine), beaking, (a vertebral remodeling response of the caudoventral aspect to instability caused by tipping), and smooth lateral vertebral proliferative changes, all recognized as elements of human and feline MPS I [5, 8, 18]. Findings for the spine and the femoral head were scored normal (0), mild changes (1), moderate changes (2) and severe change (3) based on criteria listed above; 0.5 increments were used to capture changes that were borderline between two categories. Findings for the coxofemoral joint laxity were graded normal (0), subluxated (1), luxated (2), severe luxation (3). Scores were subjected to an analysis using a general linear mixed model, and the SAS program PROC-GLIMMIX. This model included assessment by treatment status (affected versus BMT), with radiographer as a random variable.
Pathology
Euthanasia (barbiturate overdose) followed the AVMA guidelines, except for cats 4289, 4297, 4306, and 4429 that died (Table 1). Tissues were collected without perfusion, fixed in buffered 10% formalin (light microscopy), paraffin-embedded, sectioned, and stained with hematoxylin and eosin. Neuropathology was assessed on the right full-hemisphere coronal slabs and on the cervical spinal cord in a blinded fashion by a board-certified veterinary pathologist (MAC). Seven hematoxylin and eosin-stained sections including cerebral cortex at various locations, caudate nucleus, hippocampus, thalamus, brainstem, cerebellum and spinal cord were completely scanned for the presence of lesions. Findings in BMT cats were scored from normal (0) to marked (5) for frequency of vacuolated cells (absence = 0; presence in up to 10% of the cells = 1; up to 30% = 2; up to 50% = 3; up to 80% = 4; more than 80% = 5), the frequence of affected vessels by perivascular accumulation of mononuclear cells with foamy cytoplasm (absence = 0; presence in up to 10% of the vessels = 1; up to 30% = 2; up to 50% = 3; up to 80% = 4; more than 80% = 5) and the severity of perivascular vacuolated mononuclear cells (absence = 0; 1 per perivascular space = 1; 2–3 per space = 2; 4–5 per space = 3; 6–7 per space = 4; more than 7 per space = 5). Immunocytochemistry analysis of GM2 and GM3 ganglioside storage have been published [19]. The GM2 ganglioside immunoreactivity (GM2-IR) and GM3-IR were evaluated in cerebral cortex of four BMT-treated cats (4347, 4366, 4583, 4296), three MPS I cats, and one normal cat of equivalent age.
IDUA and Total β-Hexosaminidase (HEX) Assays
Activities of IDUA and HEX were assayed as described [16, 20]. Results, reported as percent normal of nmol 4-methylumbelliferone cleaved/h/mg protein, were analyzed by ANOVA and Tukey post-hoc analysis. Brain samples were cerebrum and consisted of samples of approximately equal amounts of gray and white matter.
GAG Assay
Total tissue sulfated GAGs were measured using a variation on the assay of Björnsson [21, 22]. The tissue GAG levels were evaluated by ANOVA and Tukey post-hoc analysis.
Ganglioside Quantitation
Ganglioside extraction and quantitation followed published protocols [23, 24] using prefrontal cortical gray matter from two BMT-treated (4296 and 4372), three normal, and three MPS I cats. Values are reported as mole %, i.e. a relative measure of moles of a given ganglioside per moles of total ganglioside, as calculated using densitometry values of HPTLC plates stained for N-acetylneuraminic acid (NeuAc) and the known number of NeuAc moieties associated with the various ganglioside species. Normal and untreated MPS I values were subjected to ANOVA.
Results
Bone Marrow Transplantation
The results of the BMT of the eleven affected MPS I kittens are presented in Table 1. Of 11 kittens transplanted, ten survived to engraftment, and of these, three died shortly after. Of the remaining seven kittens, 3680 required a third transplant and lost its graft by one year. The six remaining stably engrafted kittens were analyzed, with time from engraftment to death or euthanasia ranging from 2.2–36.8 months. Of this group, 4297 died two and a half months post engraftment from septicemia and bacterial pylonephritis. Of the long-term BMT survivors, cat 4366 had a history of seizures, while cat 4372 had a history insulin dependent diabetes mellitus (IDDM) beginning 11 months post engraftment. Three of the long-term stable engrafted cats all showed complete chimerism (≥95% donor lymphocytes).
Clinical and Radiographic Evaluation
Although not assessed in a quantitative manner, the clinical impression of the BMT cats was of greatly reduced disease. All long term BMT animals (4296, 4347, 4366, 4372, and 4583) were more mobile than untreated cats. Gait abnormalities were milder than untreated cats. The BMT animals lacked gross rib cage and sternum deformities present in MPS I cats (confirmed on radiographs, data not shown). Facial dysmorphia, while present, was less pronounced in the BMT cats than in untreated affected cats (confirmed on radiographs, data not shown). Skin of BMT cats was supple and pliable in contrast to the thickened and turgid skin of MPS I cats. Results of transplantation on growth were not assessed due to effects of total body irradiation. The mobility of the cervical spine in the BMT cats was clearly restricted, but less severely so than in MPS I cats.
All long-term BMT animals were examined ophthalmologically at varying time points post transplant. Two cats (4347 and 4366) showed no improvement in corneal cloudiness at 36 months post engraftment. One cat (4296) had mild corneal cloudiness for its age and disease 39 months post engraftment. Cat 4372, at 15 months post engraftment, had equatorial diabetic cataracts and mild corneal cloudiness for its age. Cat 4583 had very mild corneal cloudiness for its age and disease at 19 months post engraftment.
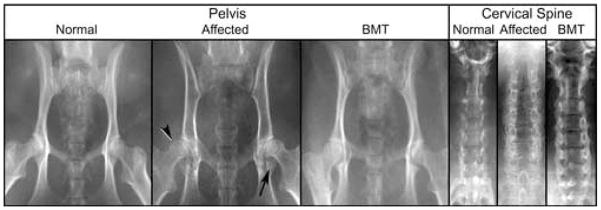
Although the radiographic evaluations were scored in a blinded fashion (Table 2) the correlation between the two radiologists were high (r2 of total hip score (0.87), total neck score (0.77), and overall total ((0.87). The total hip, total neck, and overall total scores of untreated and BMT-treated affected MPS I cats were statistically different (p < 1.0 × 10−4, p < 1.4 × 10−3, and p < 1.0 × 10−4, respectively). Visually, the radiographic improvement of the BMT-treated cats is clearly evident (Figure 1). The radiographic signs of MPS I, while not eliminated, were substantially reduced, seen especially in the scores of the coxofemoral joint, which were reduced from an average of 9.2 to 1.4. The cervical spine changes were less, the score being reduced only from 5.3 to 2.0.
Table 2.
Radiographic Analysis of BMT MPS I Cats
| Coxofemoral Joint |
Cervical Spine |
Totals |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Femora | Laxity | Acetabula | Sub-totals | Vertebral: | Sub-totals | By Radiologist | By Status | |||||||||||||
| Status | Animal num. | Radiologist | Rt | Lt | Rt | Lt | Rt | Lt | Ave | V-D width |
Tipping | Beaking | Prolif | Ave | Individ. | Ave | Ave | St Dev |
||
| Aff | 3894 | SME | 2.0 | 1.5 | 2.0 | 0.0 | 1.5 | 0.0 | 7.0 | 9.4 | 1.0 | 1.0 | 0.5 | 1.0 | 3.5 | 5.3 | 10.5 | 14.6 | 4.4 | |
| VK | 1.0 | 1.0 | 0.0 | 1.0 | 0.0 | 0.0 | 3.0 | 1.0 | 1.0 | 1.0 | 2.0 | 5.0 | 8.0 | 9.3 | ||||||
| 4693 | SME | 2.0 | 2.0 | 2.0 | 2.0 | 3.0 | 2.00 | 13.0 | 1.0 | 1.0 | 2.0 | 1.0 | 5.0 | 18.0 | ||||||
| VK | 1.0 | 1.0 | 1.0 | 1.0 | 3.0 | 3.0 | 10.0 | 2.0 | 1.0 | 1.0 | 1.0 | 5.0 | 15.0 | 15.0 | ||||||
| 4882 | SME | 2.5 | 2.5 | 1.0 | 1.0 | 2.0 | 2.0 | 11.0 | 1.5 | 0.0 | 1.0 | 0.0 | 2.5 | 13.5 | ||||||
| VK | 3.0 | 3.0 | 0.0 | 1.0 | 2.0 | 3.0 | 12.0 | 3.0 | 0.0 | 2.0 | 1.0 | 6.0 | 18.0 | 15.8 | ||||||
| 4993 | SME | 1.5 | 1.5 | 2.0 | 1.5 | 1.5 | 1.5 | 9.5 | 1.5 | 1.0 | 2.0 | 2.0 | 6.5 | 16.0 | ||||||
| VK | 1.0 | 1.0 | 1.0 | 1.0 | 2.0 | 1.0 | 7.0 | 2.0 | 2.0 | 1.0 | 2.0 | 7.0 | 14.0 | 15.0 | ||||||
| 5050 | SME | 2.5 | 2.5 | 3.0 | 2.0 | 3.0 | 3.0 | 16.0 | 2.0 | 2.0 | 2.0 | 1.0 | 7.0 | 23.0 | ||||||
| VK | 3.0 | 2.0 | 2.0 | 1.0 | 3.0 | 3.0 | 14.0 | 2.0 | 1.0 | 2.0 | 2.0 | 7.0 | 21.0 | 22.0 | ||||||
| 653 | SME | 0.5 | 1.0 | 2.0 | 2.0 | 1.0 | 0.0 | 6.5 | 2.0 | 0.5 | 1.0 | 1.0 | 4.5 | 11.0 | ||||||
| VK | 1.0 | 1.0 | 1.0 | 1.0 | 3.0 | 3.0 | 10.0 | 3.0 | 0.0 | 2.0 | 3.0 | 8.0 | 18.0 | 14.5 | ||||||
| 3896 | SME | 1.0 | 1.0 | 0.5 | 0.5 | 0.5 | 0.5 | 4.0 | 0.0 | 0.0 | 1.0 | 0.0 | 1.0 | 5.0 | ||||||
| VK | 1.0 | 1.0 | 0.0 | 0.0 | 1.0 | 1.0 | 4.0 | 1.0 | 0.0 | 1.0 | 2.0 | 4.0 | 8.0 | 6.5 | ||||||
| 472 | SME | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 12.0 | 2.0 | 0.0 | 1.0 | 1.5 | 4.5 | 16.5 | ||||||
| VK | 2.0 | 2.0 | 0.0 | 0.0 | 2.0 | 2.0 | 8.0 | 2.0 | 1.0 | 2.0 | 2.0 | 7.0 | 15.0 | 15.8 | ||||||
| 388 | SME | 0.0 | 1.5 | 0.0 | 2.0 | 0.0 | 1.0 | 4.5 | 2.0 | 0.0 | 1.0 | 0.5 | 3.5 | 8.0 | ||||||
| VK | 2.0 | 2.0 | 1.0 | 0.0 | 2.0 | 2.0 | 9.0 | 3.0 | 1.0 | 2.0 | 2.0 | 8.0 | 17.0 | 12.5 | ||||||
| 1240 | SME | 1.5 | 1.5 | 3.0 | 3.0 | 2.0 | 2.0 | 13.0 | 2.0 | 1.0 | 1.0 | 0.0 | 4.0 | 17.0 | ||||||
| VK | 3.0 | 3.0 | 1.0 | 1.0 | 3.0 | 3.0 | 14.0 | 2.0 | 1.0 | 1.0 | 2.0 | 6.0 | 20.0 | 18.5 | ||||||
| BMT | 4296 | SME | 0.0 | 0.0 | 1.0 | 0.0 | 0.0 | 0.0 | 1.0 | 1.41 | 0.0 | 0.0 | 0.0 | 0.5 | 0.5 | 2.02 | 1.5 | 3.41 | 1.4 | |
| VK | 0.0 | 0.0 | 0.0 | 1.0 | 0.0 | 0.0 | 1.0 | 1.0 | 1.0 | 0.0 | 1.0 | 3.0 | 4.0 | 2.8 | ||||||
| 4347 | SME | 0.5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.5 | 0.0 | 0.0 | 0.5 | 0.0 | 0.5 | 1.0 | ||||||
| VK | 0.0 | 0.0 | 0.0 | 0.0 | 1.0 | 1.0 | 2.0 | 0.0 | 0.0 | 1.0 | 1.0 | 2.0 | 4.0 | 2.5 | ||||||
| 4366 | SME | 0.0 | 0.0 | 3.0 | 0.0 | 0.5 | 0.5 | 4.0 | 0.0 | 1.0 | 0.0 | 0.0 | 1.0 | 5.0 | ||||||
| VK | 0.0 | 0.0 | 2.0 | 0.0 | 0.0 | 1.0 | 3.0 | 0.0 | 1.0 | 1.0 | 1.0 | 3.0 | 6.0 | 5.5 | ||||||
| 4372 | SME | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.5 | 0.0 | 0.5 | 0.0 | 1.0 | 1.0 | ||||||
| VK | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.0 | 1.0 | 1.0 | 2.0 | 5.0 | 5.0 | 3.0 | ||||||
0 = normal, 1 = mild, 2 = moderate, 3 = severe
1: General linear mixed model, p < 1.0 × 10−4
3: General linear mixed model, p <1.4 × 10−3
Figure 1. Composite Radiograph of MPS I Normal, Affected, and BMT-treated cats.
Images of the pelvis show characteristic changes in MPS I affected cats: coxofemoral joint subluxation (arrowhead), and acetabular flattening with bony proliferation (arrow). These changes are reduced in the BMT treated animal. The untreated MPS I cervical spine shows increased vertebral width and bony proliferation, both of which are reduced in the BMT treated animal.
Pathology
Liver, spleen, kidney, lung, heart, aorta, brain, and spinal cord were examined from six untreated and six BMT-treated MPS I affected cats. The most striking finding in the BMT-treated cats was a reduction of perivascular infiltration by vacuolated mononuclear cells in brain, liver, kidney, and myocardium (Figures 2 and 3). A substantial decrease of clear cytoplasmic vacuoles in smooth muscle cells with was seen in spleen (Figure 2, panels k and l) and lung of BMT-treated cats. The vacuolation in hepatocytes, Kupffer cells, and epithelial cells of proximal convoluted tubules was not reduced. However, the hydropic and fatty changes commonly observed in these organs, which is a common and normal finding in unaffected cats, reduced the sensitivity of detection. The vacuolation of distal convoluted tubules was improved (Figure 2, panels h and i). The number of affected neurons throughout the CNS was identical between MPS I BMT-treated and untreated cats. However, fewer neurons had a severe accumulation in MPS I BMT-treated cats (Figure 2, panels b and e, and Table 3). The choroid plexes, meninges, and parenchymal perivascular spaces of MPS I BMT-treated cats contained strikingly fewer mononuclear vacuolated cells.
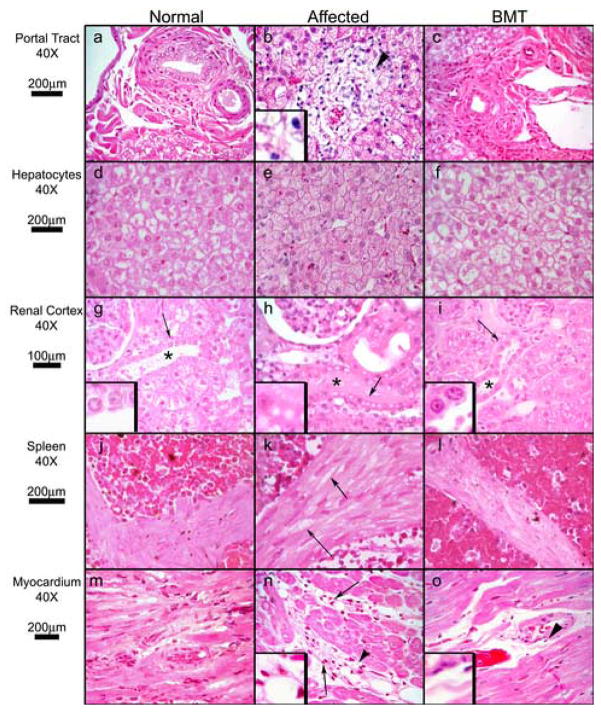
Figure 2. Histopathology of Somatic Tissues.
Hematoxylin and eosin-stained sections from normal, MPS I, and MPS I BMT-treated cats. Tissue, magnification, and micron bars located to the left of designated rows. Panels a, b, and c (liver portal tract): arrowhead (b) indicates vacuolated mononuclear cell infiltration of portal tract (3X inset) in an untreated MPS I cat, a finding absent in normal and BMT-treated cats. Panels d, e, and f (hepatocytes): the affected cat has enlarged hepatocytes with many small uniform cytoplasmic vacuoles. Normal and BMT samples have vacuolation typical of normal feline liver, are similar to each other, and differ strikingly from affected. Panels g, h, and i (renal cortex): asterisks indicate distal convoluted tubules. Cytoplasmic vacuoles in affected distal convoluted tubules are absent in normal and BMT cats (arrows indicate 3X insets). Panels j, k, and l (splenic trabeculae). Vacuolation of smooth muscle cells in MPS I (arrows) is decreased in BMT-treated cats. Panels m, n, and o (myocardium): arrows indicate perivascular infiltration by mononuclear cells in an affected cat, which is absent in the BMT animal. Arrowheads indicate 3X insets in panels n and o.
Figure 3. Histopathology of CNS Tissues.

Hematoxylin and eosin-stained sections from normal, MPS I, and MPS I BMT-treated cats. Tissue, magnification, and micron bars located to the left of designated rows. Panels a, b, and c (cortical neurons): affected and BMT cortical neurons are swollen with cytoplasmic vacuoles, however BMT neurons were slightly less so (insets are 2X images of neurons indicated by arrows). Panels d, e, and f (hippocampal neurons): severity of neuronal cytoplasmic vacuolation is decreased in BMT (insets are 4X images of neurons indicated by arrows). Panels g, h, and I (cerebral perivascular space): number of vacuolated mononuclear cells (arrow, panel h) is nearly absent in BMT (arrow, panel i). Panels j, k, and l (choroid plexus): infiltration of choroid plexus (asterisks) by vacuolated mononuclear cells and cytoplasmic vacuolation of epithelial cuboidal cells (arrows indicate 4X insets) present in affected cat are nearly absent in BMT-treated cat. Panels m, n, and o (leptomeninges): vacuolation of fibroblasts and mononuclear cell infiltrates in affected cat are nearly absent in BMT-treated cat (arrows).
Table 3.
Analysis of CNS Histopathology of BMT-treated MPS I Cats
| Animal # | 82766 | 4874 | 4296 | 4297 | 4347 | 4366 | 4372 | 4583 | BMT | BMT | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment Status | Untreated | Untreated | BMT | BMT | BMT | BMT | BMT | BMT | Average | St Dev | |
| Age at euthanasia (months) | 33.6 | 52.8 | 35.8 | 2.2 | 36.8 | 36.3 | 17.7 | 17.2 | |||
| Neuronal granulation1 |
|||||||||||
| Cerebral Cortex | Frequency | 5 | 5 | 4.0 | 3.0 | 3.0 | 2.0 | 3.0 | 3.0 | 3.0 | 0.6 |
| Intensity | 5 | 5 | 3.0 | 3.0 | 4.0 | 3.5 | 4.5 | 3.5 | 3.6 | 0.6 | |
| Caudate Nucleus | Frequency | 5 | 5 | 3.0 | NA | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 0.0 |
| Intensity | 5 | 5 | 4.0 | NA | 4.0 | 4.0 | 3.0 | 4.0 | 3.8 | 0.4 | |
| Hippocampus | Frequency | 5 | 5 | 4.0 | 4.0 | 4.0 | 4.0 | 3.0 | 3.0 | 3.7 | 0.5 |
| Intensity | 5 | 5 | 3.0 | 3.0 | 3.0 | 4.0 | 3.0 | 3.0 | 3.2 | 0.4 | |
| Thalamus | Frequency | 5 | 5 | 4.0 | 4.0 | 4.0 | 4.0 | 3.0 | 3.0 | 3.7 | 0.5 |
| Intensity | 5 | 5 | 3.0 | 4.0 | 3.0 | 4.0 | 3.0 | 3.0 | 3.3 | 0.5 | |
| Brain Stem | Frequency | 5 | 5 | 4.0 | NA | 4.0 | 4.0 | 3.0 | 3.0 | 3.6 | 0.5 |
| Intensity | 5 | 5 | 4.0 | NA | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 0.0 | |
| Cerebellum | Frequency | 5 | 5 | 4.0 | 4.0 | 4.0 | 4.0 | 3.0 | 4.0 | 3.8 | 0.4 |
| Intensity | 5 | 5 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 0.0 | |
| Spinal Cord | Frequency | 5 | 5 | 4.0 | NA | 3.0 | 4.0 | 3.0 | NA | 3.5 | 0.6 |
| Intensity | 5 | 5 | 4.0 | NA | 4.0 | 5.0 | 3.0 | NA | 4.0 | 0.8 | |
| Fibroblast Vacuolation1 and Mononuclear Cells Infiltration2 |
|||||||||||
| Perivascular Space | |||||||||||
| Vacuolation | Frequency | 5 | 5 | 4.0 | 4.0 | 4.0 | 4.0 | 2.0 | 4.0 | 3.7 | 0.8 |
| Intensity |
5 | 5 | 3.0 | 2.0 | 2.0 | 2.0 | 2.0 | 3.0 | 2.3 | 0.5 | |
| Infiltration | Frequency | 5 | 5 | 3.0 | 1.0 | 1.0 | 1.5 | 0.5 | 2.0 | 1.5 | 0.9 |
| Intensity | 5 | 5 | 2.5 | 1.0 | 1.0 | 1.5 | 0.5 | 2.0 | 1.4 | 0.7 | |
| Meninges | |||||||||||
| Vacuolation | Frequency | 5 | 5 | 2.5 | 2.0 | 2.0 | 1.0 | 2.0 | 2.0 | 1.9 | 0.5 |
| Intensity |
5 | 5 | 2.5 | 2.0 | 2.0 | 1.0 | 2.0 | 2.0 | 1.9 | 0.5 | |
| Infiltration | Frequency | 5 | 5 | 2.5 | 2.0 | 1.0 | 1.0 | 2.0 | 2.0 | 1.8 | 0.6 |
| Intensity | 5 | 5 | 2.5 | 2.0 | 2.0 | 1.0 | 2.0 | 2.0 | 1.9 | 0.5 | |
| Choroid Plexus | |||||||||||
| Vacuolation | Frequency | 5 | 5 | 3.0 | 2.0 | 2.0 | 2.0 | 3.0 | 1.0 | 2.2 | 0.8 |
| Intensity |
5 | 5 | 3.0 | 3.0 | 2.0 | 2.0 | 3.0 | 1.0 | 2.3 | 0.8 | |
| Infiltration | Frequency | 5 | 5 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 0.5 | 0.9 | 0.2 |
| Intensity | 5 | 5 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 0.5 | 0.9 | 0.2 | |
absence = 0; presence in up to 10% of the cells = 1; up to 30% = 2; up to 50% = 3; up to 80% = 4; more than 80% = 5
absence = 0; presence in up to 10% of the vessels = 1; up to 30% = 2; up to 50% = 3; up to 80% = 4; more than 80% = 5; NA = not assessed
Consistent with previous studies [25, 26], immunocytochemistry of normal adult cat cerebral cortex revealed no evidence of GM2 or GM3 gangliosides (Figure 4, panels a and d). In contrast, MPS I cats exhibited significant intracellular storage of these gangliosides (Figure 4, panels b and e; Figure 5, panels a and c), with GM3-IR being more abundant than GM2-IR. Some glial cells, particularly in white matter, appeared located near blood vessels (Figure 5, panel a), and showed significant GM3, but little or no GM2 ganglioside accumulation. In BMT-treated animals neurons and glial cells remained IR but the degree of staining was less intense (Figures 4, panels c and f). This difference was particularly evident for GM3 ganglioside, with fewer deep cortical neurons staining (Figure 5, panel d). In subcortical white matter, blood vessels associated GM3-IR glial cells, so conspicuous in untreated cats, were fewer in BMT-treated animals (Figure 5, panel b). The overall conclusion was that of a slight reduction in GM2 and GM3 IR in cerebral cortex and obvious reductions in glial GM3 IR in subcortical white matter.
Figure 4. GM2and GM3Ganglioside Storage in Supragranular Cerebral Cortex.

ICC stained sections from normal, MPS I, and MPS I BMT-treated cats. Top row panels (a, b, and c) were stained by ICC for GM2. Bottom row panels (d, e, and f) were stained for GM3. Images of the normal cat (a and d) show virtually no GM2 or GM3 staining. MPS I-affected sections (b and e) show significant intracellular storage of gangliosides, in neurons (arrows) and glia (arrows heads). Neurons and glia in BMT-treated animals remained immunoreactive but the degree of staining appears less intense, a difference particularly evident for GM3 ganglioside, with fewer deep cortical neurons appearing stained (panel f).
Figure 5. GM3Ganglioside Storage Reduced by BMT in Subcortical White Matter and Infragranular Cerebral Cortex.
GM3 ICC stained sections from MPS I (panels a and c), and BMT-treated cats (panels b and d). Tissues, magnification, and micron bars located to the left of designated rows. GM3-IR glial cells adjacent to blood vessels are conspicuous in untreated affected cats (arrowheads), but are much less frequently seen in BMT-treated cats. In the infragranular cerebral cortex the number and staining intensity of glial cells is reduced in BMT-treated cats (panel d).
Biochemistry
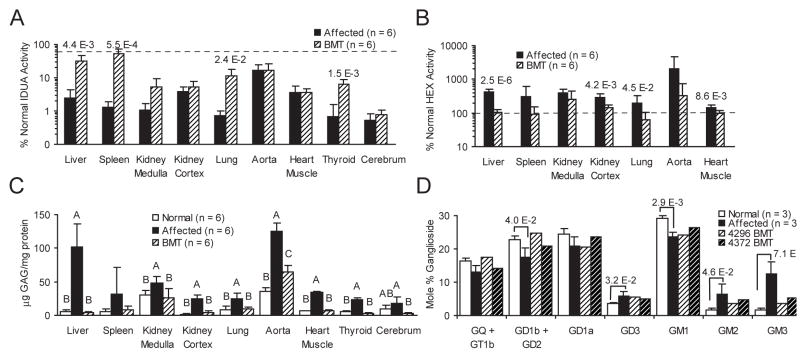
A semilog plot of percent normal enzyme activities (Figure 6, panels a and b) shows significant increases in mean IDUA levels, approaching those of heterozygous (donor) animals in liver spleen, lung, and thyroid of BMT-treated cats. Non-significant increases in mean IDUA levels were seen in kidney cortex and medulla, and the brain. No IDUA increase was seen in aorta or myocardium. What appears to be a relatively high level of residual activity in the aorta of affected animals is an artifact of means of reporting result (percent normal nmole 4MU/h/mg protein), and the low cellularity and high protein content of the tissue. Significant decreases in HEX activity levels, indicating improvement, were seen in liver, kidney cortex, lung, and myocardium (Figure 6, panel b). Of these all but kidney cortex levels were at or below normal. HEX activity in spleen, aorta, and kidney medulla in BMT-treated cats were not significantly decreased.
Figure 6. Enzyme, GAG, and Ganglioside Findings in Somatic and CNS Tissues in Normal, MPS I, and BMT-Treated Cats.
Panel A is mean percent normal IDUA activity (nmol 4MU/h/mg protein) of listed tissues from MPS I affected, BMT-treated cats (n = 6 for all groups, including normals), plotted as a semi-log bar graph (error bars represent +1 SD, dashed line represents 50% of normal levels). Brain tissue was cerebrum, consisting of approximately equal parts gray and white matter. Number above the bars are the P values of significant ANOVA comparisons. Panel B is mean percent normal HEX activity (nmol 4MU/h/mg protein) of listed tissues from MPS I affected, BMT-treated cats (n = 6 for all groups, including normals), plotted as a semi-log bar graph (error bars represent +1 SD, dashed line represents 100% of normal levels). Number above the bars are the P values of significant ANOVA comparisons. Panel C is mean tissue concentration (μg GAG/mg protein) of total sulfated GAGs in listed tissues from normal, MPS I, and BMT-treated cats (n = 6 for all groups). Error bars are +1 SD. Letters over the values indicate statistical group differences at P ≥ 0.05. Panel D is a bar graph of the mole percent of total gangliosides for from prefrontal cerebral gray matter of normal, MPS I (n of each = 3), and two BMT-treated cats (4296 and 4372). Brackets indicate statistical difference of normal and affected controls, and numbers above brackets are resultant P values.
Total sulfated GAG content (Figure 6, panel c) in BMT cats’ mean liver, kidney medulla and cortex, lung, myocardium, and thyroid, was statistically indistinguishable from normal. In aorta, a significant reduction was seen, but not to normal levels. Splenic GAG content was reduced to near normal levels, but was a non–significant reduction, likely due to the variability of untreated affected levels. In the brain, there was a paradoxical result. The BMT treated animals had total GAG concentration significantly lower than the MPS I animals. However, the GAG content of normal cats was intermittent between the levels of the untreated and BMT-treated MPS I affected cats.
The ganglioside content of prefrontal cortical gray matter (Figure 6, panel d) in normal and MPS I cats showed a number of differences. The untreated MPS I cats had significant relative elevations in GD3, GM3, and GM2 gangliosides. Concomitant decreases, often significant, were seen in all other gangliosides. Among the BMT-treated cats, there was a striking reduction of GM2, and especially GM3 gangliosides.
Discussion
Herein we have described a cohort of BMT-treated MPS I animals. Overall the response to therapy was good. However, there were some systems which saw a limited or negligible response to therapy. Although not designed to examine pediatric feline BMT, this study is among the largest involving BMT in kittens. When examining the data in Table 1, we concluded that transplanting kittens younger than 24 days of age, where only 20% of kittens survived long-term, or use of a third transplant, where both kittens failed to survive or maintain the graft, were of limited value. The finding of IDDM in one treated cat is unusual as this is rare in feline medicine, and may have been associated with the BMT, as there are reports of BMT-associated IDDM humans [27], and cats [28]. This study was not primarily a means to advance BMT transplant techniques, and the pre-transplant conditioning and treatment of donor cells used for transplantation are not state of the art in human medicine, although many early human MPS I patients have undergone similar therapy. The use of donor cells purified or enriched for hematopoetic precursors may limit therapeutic response, and hence also the conclusions to be drawn from this study, but the possibility also exists that the use of unfractionated whole bone marrow may have been relatively richer in mesenchymal stem cells, which could have exercised a positive therapeutic effect. While it may have been ideal to use methods for enrichment or purification, these remain undescribed in the cat. Regardless of the methods used, these results are a strong foundation from which to compare other improved methods to treat the bone and brain disease using the feline model.
The BMT-treated cats were assessed by a number of methods, including clinical, histological, and biochemical techniques. Most non-CNS soft tissues and organs except cornea and aorta saw near complete resolution of lesions. Corneal improvement was variable, and if present, was only mild. There was a clear improvement in the orthopedic aspects of the disease (Figure 1 and Table 2), especially in the major joints and limbs. Although the cervical spine in BMT-treated cats improved relative to untreated MPS I cats, there were still notable changes relative to normal cats. In the CNS, there was a nearly complete response to therapy in the meninges (which is not beyond the blood brain barrier) as well as the perivascular lesions, while the neuronal response to therapy was mild. Patients with MPS I often have a severe CNS component (mental retardation), to their disease, and assessment of the cats for behavioral improvement would have been desirable. However, the inherent difficulty in quantitatively assessing cat behavior in general, and the specific difficulty in evaluating such in animals for which control groups (MPS I affected cats) may have impaired sight, smell, and mobility, make any conclusions about functional CNS outcome impossible. Until biomarkers causally linked to CNS function in humans are developed and validated in the cat, assessment of functional CNS outcome in the feline model will be extremely problematic. Lacking such biomarkers, assessment of histology and primary and secondary biochemical changes must be used.
The response to BMT therapy was most striking in the soft tissue organs, especially in the liver and spleen. Enzyme levels approached donor levels, GAG and secondary enzyme elevations were reduced, and histological lesions were improved. The response was consistent with ERT in feline MPS I [16]. Some tissues, notably kidney, aorta, and myocardium showed a mixed response to therapy, with little to no increase in IDUA, but a partial or full response in substrate and secondary enzyme levels, and on histology. The myocardium showed nearly complete response to therapy in GAG and HEX levels and on histological sections, without an increase in IDUA levels. Clearly the therapeutic level of enzyme activity appears below the level of sensitivity of this assay. The response of the aorta was similar to the myocardium with no increased IDUA activity relative to untreated affected animals, but differed in that substrate and secondary enzyme levels did not decrease to normal, which may be related to the dense extracellular matrix. Although not assessed biochemically, the clinical response to therapy seen in the cornea is in contrast to the partial clinical response that was reported in BMT studies in the canine MPS I model [29]. Another tissue which saw a mixed response to therapy was the kidney.
The response of the CNS to BMT was of interest. No significant increase in brain IDUA was found in treated cats compared to untreated MPS I cats. However, there was a clear therapeutic response seen in the meninges, choroid plexus, and perivascular lesions. There was also a limited but consistent improvement of the neuronal lesions on histopathological analysis, supported by GAG and ganglioside levels. These findings are consistent with what had been noted in BMT using the canine MPS I model [30, 31]. The source of any correction in the CNS is unlikely due to serum enzyme, which is supported by the ERT studies in the feline and canine models of MPS I [32, 33]. The logical source of the correction are donor-derived macrophages or microglia.. However, these were clearly not enough to resolve the neuronal lesions, but resulted in a partial response, seen in GAG levels, ganglioside levels, ganglioside IC, and histopathology. If the response was from donor-derived microglia, we conclude that future approaches for CNS-directed therapy will need to rely on microglia which produce more enzyme. An approach which involves transplantation of genetically engineered cells could address this.
While enormous strides have been made in recent years in the therapy for MPS I using ERT [34], the complete resolution of the CNS and orthopedic disease remains an unachieved goal. The use of BMT in children can lead to some stabilization or improvement of cognitive function [35], but it remains unclear how lasting or complete the CNS response will be into mature adulthood. Clearly new approaches to therapy, involving combinations of techniques, such as ERT and gene therapy, as well as tissue directed therapies, especially to the bones and the CNS will be required before a full spectrum of resolution of disease can be achieved. The evaluation of such techniques and approaches will continue to require evaluation in large pre-clinical models, for which the MPS I cat is well suited, and for which the findings presented here will provide a baseline for comparison.
Acknowledgments
Appreciation is extended to Pat Miller-Wilson for irradiation, and to veterinary students for compassionate animal care. Supported by NIH: DK025759 and RR02512 (MEH), NME supported by RR007063.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Meikle PJ, Hopwood JJ, Clague AE, Carey WF. Prevalence of lysosomal storage disorders. Jama. 1999;281:249–254. doi: 10.1001/jama.281.3.249. [DOI] [PubMed] [Google Scholar]
- 2.Grabowski GA. Gaucher disease: lessons from a decade of therapy. J Pediatr. 2004;144:S15–19. doi: 10.1016/j.jpeds.2004.01.050. [DOI] [PubMed] [Google Scholar]
- 3.Peters C, Shapiro EG, Anderson J, Henslee-Downey PJ, Klemperer MR, Cowan MJ, Saunders EF, deAlarcon PA, Twist C, Nachman JB, Hale GA, Harris RE, Rozans MK, Kurtzberg J, Grayson GH, Williams TE, Lenarsky C, Wagner JE, Krivit W. Hurler syndrome: II. Outcome of HLA-genotypically identical sibling and HLA-haploidentical related donor bone marrow transplantation in fifty-four children. The Storage Disease Collaborative Study Group. Blood. 1998;91:2601–2608. [PubMed] [Google Scholar]
- 4.Staba SL, Escolar ML, Poe M, Kim Y, Martin PL, Szabolcs P, Allison-Thacker J, Wood S, Wenger DA, Rubinstein P, Hopwood JJ, Krivit W, Kurtzberg J. Cord-blood transplants from unrelated donors in patients with Hurler’s syndrome. N Engl J Med. 2004;350:1960–1969. doi: 10.1056/NEJMoa032613. [DOI] [PubMed] [Google Scholar]
- 5.Haskins ME, Jezyk PF, Desnick RJ, McDonough SK, Patterson DF. Alpha-L-iduronidase deficiency in a cat: a model of mucopolysaccharidosis I. Pediatr Res. 1979;13:1294–1297. doi: 10.1203/00006450-197911000-00018. [DOI] [PubMed] [Google Scholar]
- 6.He X, Li CM, Simonaro CM, Wan Q, Haskins ME, Desnick RJ, Schuchman EH. Identification and characterization of the molecular lesion causing mucopolysaccharidosis type I in cats. Mol Genet Metab. 1999;67:106–112. doi: 10.1006/mgme.1999.2860. [DOI] [PubMed] [Google Scholar]
- 7.Alroy J, Haskins M, Birk DE. Altered corneal stromal matrix organization is associated with mucopolysaccharidosis I, III and VI. Exp Eye Res. 1999;68:523–530. doi: 10.1006/exer.1998.0622. [DOI] [PubMed] [Google Scholar]
- 8.Haskins ME, Aguirre GD, Jezyk PF, Desnick RJ, Patterson DF. The pathology of the feline model of mucopolysaccharidosis I. Am J Pathol. 1983;112:27–36. [PMC free article] [PubMed] [Google Scholar]
- 9.Haskins ME, Otis EJ, Hayden JE, Jezyk PF, Stramm L. Hepatic storage of glycosaminoglycans in feline and canine models of mucopolysaccharidoses I, VI, and VII. Vet Pathol. 1992;29:112–119. doi: 10.1177/030098589202900203. [DOI] [PubMed] [Google Scholar]
- 10.Mollard RJ, Telegan P, Haskins M, Aguirre G. Corneal endothelium in mucopolysaccharide storage disorders. Morphologic studies in animal models. Cornea. 1996;15:25–34. [PubMed] [Google Scholar]
- 11.Sheridan O, Wortman J, Harvey C, Hayden J, Haskins M. Craniofacial abnormalities in animal models of mucopolysaccharidoses I, VI, and VII. J Craniofac Genet Dev Biol. 1994;14:7–15. [PubMed] [Google Scholar]
- 12.Stramm LE, Haskins ME, Aguirre GD. Retinal pigment epithelial glycosaminoglycan metabolism: intracellular versus extracellular pathways. In vitro studies in normal and diseased cells. Invest Ophthalmol Vis Sci. 1989;30:2118–2131. [PubMed] [Google Scholar]
- 13.Walkley SU, Baker HJ, Rattazzi MC, Haskins ME, Wu JY. Neuroaxonal dystrophy in neuronal storage disorders: evidence for major GABAergic neuron involvement. J Neurol Sci. 1991;104:1–8. doi: 10.1016/0022-510x(91)90208-o. [DOI] [PubMed] [Google Scholar]
- 14.Walkley SU, Haskins ME, Shull RM. Alterations in neuron morphology in mucopolysaccharidosis type I. A Golgi study. Acta Neuropathol (Berl) 1988;75:611–620. doi: 10.1007/BF00686207. [DOI] [PubMed] [Google Scholar]
- 15.Castagnaro M, Alroy J, Ucci AA, Glew RH. Lectin histochemistry and ultrastructure of feline kidneys from six different storage diseases. Virchows Arch B Cell Pathol Incl Mol Pathol. 1987;54:16–26. doi: 10.1007/BF02899193. [DOI] [PubMed] [Google Scholar]
- 16.Kakkis ED, Schuchman E, He X, Wan Q, Kania S, Wiemelt S, Hasson CW, O’Malley T, Weil MA, Aguirre GA, Brown DE, Haskins ME. Enzyme replacement therapy in feline mucopolysaccharidosis I. Mol Genet Metab. 2001;72:199–208. doi: 10.1006/mgme.2000.3140. [DOI] [PubMed] [Google Scholar]
- 17.O’Brien SJ, Nash WG. Genetic mapping in mammals: chromosome map of domestic cat. Science. 1982;216:257–265. doi: 10.1126/science.7063884. [DOI] [PubMed] [Google Scholar]
- 18.Tandon V, Williamson JB, Cowie RA, Wraith JE. Spinal problems in mucopolysaccharidosis I (Hurler syndrome) J Bone Joint Surg Br. 1996;78:938–944. doi: 10.1302/0301-620x78b6.1279. [DOI] [PubMed] [Google Scholar]
- 19.Zervas M, Walkley SU. Ferret pyramidal cell dendritogenesis: changes in morphology and ganglioside expression during cortical development. J Comp Neurol. 1999;413:429–448. doi: 10.1002/(sici)1096-9861(19991025)413:3<429::aid-cne6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 20.Sammarco C, Weil M, Just C, Weimelt S, Hasson C, O’Malley T, Evans SM, Wang P, Casal ML, Wolfe J, Haskins M. Effects of bone marrow transplantation on the cardiovascular abnormalities in canine mucopolysaccharidosis VII. Bone Marrow Transplant. 2000;25:1289–1297. doi: 10.1038/sj.bmt.1702448. [DOI] [PubMed] [Google Scholar]
- 21.Bjornsson S. Simultaneous preparation and quantitation of proteoglycans by precipitation with alcian blue. Anal Biochem. 1993;210:282–291. doi: 10.1006/abio.1993.1197. [DOI] [PubMed] [Google Scholar]
- 22.Ellinwood NM, Wang P, Skeen T, Sharp NJ, Cesta M, Decker S, Edwards NJ, Bublot I, Thompson JN, Bush W, Hardam E, Haskins ME, Giger U. A model of mucopolysaccharidosis IIIB (Sanfilippo syndrome type IIIB): N-acetyl-alpha-D-glucosaminidase deficiency in Schipperke dogs. J Inherit Metab Dis. 2003;26:489–504. doi: 10.1023/a:1025177411938. [DOI] [PubMed] [Google Scholar]
- 23.Fujita N, Suzuki K, Vanier MT, Popko B, Maeda N, Klein A, Henseler M, Sandhoff K, Nakayasu H. Targeted disruption of the mouse sphingolipid activator protein gene: a complex phenotype, including severe leukodystrophy and wide-spread storage of multiple sphingolipids. Hum Mol Genet. 1996;5:711–725. doi: 10.1093/hmg/5.6.711. [DOI] [PubMed] [Google Scholar]
- 24.Kyrklund T. Two procedures to remove polar contaminants from a crude brain lipid extract by using prepacked reversed-phase columns. Lipids. 1987;22:274–277. doi: 10.1007/BF02533991. [DOI] [PubMed] [Google Scholar]
- 25.Walkley SU. Cellular pathology of lysosomal storage disorders. Brain Pathol. 1998;8:175–193. doi: 10.1111/j.1750-3639.1998.tb00144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walkley SU. Pyramidal neurons with ectopic dendrites in storage diseases exhibit increased GM2 ganglioside immunoreactivity. Neuroscience. 1995;68:1027–1035. doi: 10.1016/0306-4522(95)00208-z. [DOI] [PubMed] [Google Scholar]
- 27.Lampeter EF, McCann SR, Kolb H. Transfer of diabetes type 1 by bone-marrow transplantation. Lancet. 1998;351:568–569. doi: 10.1016/S0140-6736(05)78555-X. [DOI] [PubMed] [Google Scholar]
- 28.Lothrop CD, Jr, al-Lebban ZS, Niemeyer GP, Jones JB, Peterson MG, Smith JR, Baker HJ, Morgan RA, Eglitis MA, Anderson WF. Expression of a foreign gene in cats reconstituted with retroviral vector infected autologous bone marrow. Blood. 1991;78:237–245. [PubMed] [Google Scholar]
- 29.Constantopoulos G, Scott JA, Shull RM. Corneal opacity in canine MPS I. Changes after bone marrow transplantation. Invest Ophthalmol Vis Sci. 1989;30:1802–1807. [PubMed] [Google Scholar]
- 30.Shull RM, Hastings NE, Selcer RR, Jones JB, Smith JR, Cullen WC, Constantopoulos G. Bone marrow transplantation in canine mucopolysaccharidosis I. Effects within the central nervous system. J Clin Invest. 1987;79:435–443. doi: 10.1172/JCI112830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shull RM, Breider MA, Constantopoulos GC. Long-term neurological effects of bone marrow transplantation in a canine lysosomal storage disease. Pediatr Res. 1988;24:347–352. doi: 10.1203/00006450-198809000-00015. [DOI] [PubMed] [Google Scholar]
- 32.Shull RM, Kakkis ED, McEntee MF, Kania SA, Jonas AJ, Neufeld EF. Enzyme replacement in a canine model of Hurler syndrome. Proc Natl Acad Sci U S A. 1994;91:12937–12941. doi: 10.1073/pnas.91.26.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kakkis ED, McEntee MF, Schmidtchen A, Neufeld EF, Ward DA, Gompf RE, Kania S, Bedolla C, Chien SL, Shull RM. Long-term and high-dose trials of enzyme replacement therapy in the canine model of mucopolysaccharidosis I. Biochem Mol Med. 1996;58:156–167. doi: 10.1006/bmme.1996.0044. [DOI] [PubMed] [Google Scholar]
- 34.Wraith JE, Clarke LA, Beck M, Kolodny EH, Pastores GM, Muenzer J, Rapoport DM, Berger KI, Swiedler SJ, Kakkis ED, Braakman T, Chadbourne E, Walton-Bowen K, Cox GF. Enzyme replacement therapy for mucopolysaccharidosis I: a randomized, double-blinded, placebo-controlled, multinational study of recombinant human alpha-L-iduronidase (laronidase) J Pediatr. 2004;144:581–588. doi: 10.1016/j.jpeds.2004.01.046. [DOI] [PubMed] [Google Scholar]
- 35.Grewal S, Shapiro E, Braunlin E, Charnas L, Krivit W, Orchard P, Peters C. Continued neurocognitive development and prevention of cardiopulmonary complications after successful BMT for I-cell disease: a long-term follow-up report. Bone Marrow Transplant. 2003;32:957–960. doi: 10.1038/sj.bmt.1704249. [DOI] [PubMed] [Google Scholar]