Abstract
The Herpesviridae contain a group of highly conserved proteins designated the Herpes UL33 Superfamily (pfam03581). The Varicella-zoster virus (VZV) homolog, encoded by the ORF25 gene, was used to generate a GST-ORF25 fusion protein. Purified GST-ORF25 was used to generate a polyclonal rabbit antiserum that detected the 17.5 kDa ORF25 protein (pORF25) in VZV infected cells. In pull-down assays, GST-ORF25 interacted with a number of encapsidation proteins including ORF30, ORF42 (the second exon of ORF45/42) and itself. The self-interaction was confirmed via a yeast two-hybrid assay. Additionally, pORF25 and pORF30 were shown to co-immunoprecipitate from VZV infected cells. Our results suggest that pORF25 is part of the trimeric terminase complex for VZV. However, combined with data from previous studies on HSV-1 and Kaposi’s sarcoma associated herpesvirus (KSVH), we hypothesize that VZV pORF25 and the Herpes UL33 Superfamily homologs are not encapsidation proteins per se but instead work to bring viral proteins together to form functional complexes.
Keywords: Varicella-zoster virus, ORF25, encapsidation, UL33 Superfamily
Introduction
The VZV ORF25 gene product, pORF25, is one of a group of highly conserved proteins designated the Herpes UL33 Superfamily [Fig. 1, pfam03581] (Marchler-Bauer et al., 2007). Alignment of the C-terminal half of the 156 amino acid VZV pORF25 with the 8 human herpesviruses shows sequence similarities ranging from a high of 80% for pUL33 of Herpes simplex virus type 2 (HHV-2) to a low of 60% for pORF67.5 of KSVH. Little is known about the function of these proteins in infected cells.
Figure 1.
The Herpes UL33 Superfamily. HHV-1 (Human herpesvirus 1 or Herpes simplex virus type 1), HHV-2 (Human herpesvirus 2 or Herpes simplex virus type 2), HHV-3 (Human herpesvirus 3 or Varicella-zoster virus), HHV-4 (Human herpesvirus 4 or Epstein-Barr virus), HHV-5 (Human herpesvirus 5 or Human cytomegalovirus), HHV-6 (Human herpesvirus 6), HHV-7 (Human herpesvirus 7), and HHV-8 (Human herpesvirus 8 or Kaposi’s sarcoma associated virus). ClustalW2 alignment showing identical (*), conserved (:), or semi-conserved (.) amino acids within pfam 03581 for the eight human herpesviruses.
A recent systematic study by Uetz et al. (2005) suggested that VZV pORF25 interacted with at least 33 potential viral protein partners in a yeast two-hybrid assay. In the same study, the authors showed that KSHV pORF67.5, the pORF25 homolog, had twelve potential interacting viral protein partners. A subset of the interacting partners for pORF25 and pORF67.5 contained several orthologs. Hence, VZV pORF25 and KSHV pORF67.5 were designated as “central nodes” in their respective viral protein interaction networks and suggests that pORF25 and pORF67.5 perform similar functions in viral replication.
The HSV-1 pUL33 is the most well studied of the UL33 Superfamily. It encodes a 19 kDa protein in virus infected cells (Reynolds et al., 2000) and forms a complex with the putative HSV terminase subunits, pUL28 and pUL15 (Beard et al., 2002; Jacobson et al., 2006; Yang et al., 2007). Deletion of the HSV-1 UL33 gene results in the accumulation of empty capsids and a lack of infectious virus particles (al-Kobaisi et al., 1991). HSV-1 pUL28, pUL15, and pUL33 are thought to form a heterotrimeric terminase complex that directs DNA encapsidation in infected cells. The HSV-1 pUL28 and pUL15 proteins are homologs of Human cytomegalovirus (HCMV) pUL56 and pUL89 which have been shown to have characteristics consistent with that of viral terminases (Bogner et al., 1998; Hwang and Bogner, 2002; Scheffczik et al., 2002; Scholz et al., 2003; Visalli and van Zeijl., 2003). While HSV-1 pUL28 and pUL15 are considered the primary terminase subunits, it has been suggested that HSV-1 pUL33 can optimize the formation of the terminase complex in infected cells (Yang and Baines, 2006). The combination of (i) the association of HSV-1 pUL33 with the putative HSV terminase subunits and (ii) the absence of DNA containing capsids in UL33 mutant infected cells, resulted in the classification of HSV-1 pUL33 as an encapsidation protein. However, we hypothesize that the Herpes UL33 Superfamily homologs, including HSV-1 pUL33 and VZV pORF25, are not encapsidation proteins per se but instead play a role in bringing viral proteins together and/or optimizing viral protein complexes. This hypothesis is supported by numerous studies that include: (i) the role of HSV-1 pUL33 in optimizing the terminase complex in HSV infected cells (Yang and Baines, 2006), (ii) the interaction of VZV pORF25 and KSHV pORF67.5 with a large number of viral structural proteins (Uetz et al., 2005), (iii) the translocation of HSV-2 pUL33 and pUL14 (Yamauchi et al., 2001) or KSHV pORF67.5 and K10 (Sander et al., 2008) to the nucleus in co-transfected cells (pUL14 and K10 are not classified as DNA encapsidation proteins), and (iv) the interaction of VZV pORF25 with the pORF30 and pORF45/42 terminase subunits as well as at least one additional protein, pORF43 (this study). Combined, the data support a model where the Herpes UL33 Superfamily homologs act to bring viral proteins together to form functional complexes.
This study reports the identification of a 17.5 kDa polypeptide, pORF25, encoded by the VZV ORF25 gene. GST pull-down assays performed with in vitro translated protein products were used to identify interactions of pORF25 with itself and three additional putative VZV DNA encapsidation proteins, pORFs 30, 45/42, and 43. pORF42, exon II of pORF45/42 (Visalli et al., 2007), was shown to be sufficient for interaction with pORF25. Co-immunoprecipitation of pORF25 with pORF30 was observed from virus infected cells, and a yeast two-hybrid assay was used to confirm the pORF25-pORF25 self-interaction. The data suggest that the putative DNA encapsidation complex for VZV consists of at least three different proteins – analogous to that reported for HSV. The trimeric complex for HSV is reported to consist of pUL33, pUL15 and pUL28. Our studies suggest that the VZV complex consists of pORF25, pORF45/42 and pORF30 (Visalli et al., 2007).
Materials and Methods
Cells and virus
Monolayer cultures of human lung fibroblast (IMR-90) or human melanoma (MeWo) cells were used for propagation of VZV strain Ellen (ATCC VR-1367). IMR-90 cells were grown in Dulbecco’s Modified Eagles Medium (DMEM) supplemented with 10% fetal calf serum, 2 mM L-glutamine, 1.5 g/L sodium bicarbonate, 100 units/ml of penicillin, 100 mg/ml streptomycin sulfate, and 50 μg/ml ciprofloxacin. MeWo cells were grown in Minimal Essential Medium (MEM) supplemented with 8% fetal calf serum, 2 mM L-glutamine, 0.1 mM non-essential amino acids, 100 units/ml of penicillin, and 100 mg/ml streptomycin sulfate. Cells infected with VZV were incubated in either DMEM or MEM containing 3% serum. Infections were performed with VZV-infected cell stocks applied to monolayers of uninfected cells.
HSV-2 strain 186 was propagated and titered on African green monkey kidney (Vero) cells with complete DMEM containing 2% serum.
In vitro transcription/translation
In vitro transcription/translation of the pcDNA3.1D/V5-His-TOPO VZV ORF (Invitrogen) constructs was performed using a T7 coupled reticulocyte lysate system (Promega). Approximately 2 ug of plasmid was used for each 50 ul reaction as specified by the manufacturer. The in vitro products, containing the V5 epitope, were detected using an anti-V5 monoclonal antibody.
Antibodies and immunoblotting
Proteins were detected by SDS-PAGE and immunoblot analysis. In vitro translated products containing the V5 epitope were detected with an anti-V5 monoclonal antibody (1:5000) (Serotec). Infected cell proteins were detected using anti-ORF30 guinea pig (1:1000) or an anti-ORF25 rabbit (1:1000) sera. For proteins expressed in yeast, either anti-HA monoclonal (1:1000) (Santa Cruz) or anti-GAL4 DNA BD rabbit polyclonal (1:1000) (Santa Cruz) antibodies were used to detect proteins containing the GAL4 transcriptional activation domain (AD) or GAL4 DNA binding domain (BD) respectively. Secondary antibodies were either an anti-mouse (1:3333), anti-guinea pig (1:5000) or anti-rabbit (1:5000) HRP conjugate (Pierce). Chemiluminescent detection was performed using the SuperSignal West Pico Chemiluminescent Substrate System (Pierce).
Indirect immunofluorescence microscopy
MeWo cells grown on sterile glass cover slips were transfected with pcDNA3.1D/V5-ORF25 using Lipofectamine 2000 (Invitrogen). Forty-eight hours post-transfection, cells were fixed in a 50% methanol/50% acetone solution, blocked in the presence of 3% bovine serum albumin (BSA), washed with phosphate buffered saline (PBS), and incubated with anti-V5 monoclonal antibody (1:1000) for 1 hr. Subsequently, cover slips were washed with PBS containing 1% Triton X-100, incubated with fluorescein isothiocyanate-conjugated (FITC) goat anti-mouse secondary antibody (1;400) (Pierce) for 30 minutes, treated with 4′,6-diamidino-2-phenylindole (DAPI), and mounted for examination by fluorescence microscopy.
For transfection/infection experiments, MeWo cells were infected with ~0.5 MOI HSV-2 strain 186 24 hr after transfection and harvested10 hr post-infection. Cells were fixed as described above and were incubated simultaneously with anti-V5 rabbit serum (1:1000) (Sigma) and HSV-2 ICP8 specific monoclonal antibody (1:1000) (Virusys). Cover slips were washed with PBS containing 1% Triton X-100, incubated with FITC donkey anti-rabbit (1:400) and rhodamine red-X-conjugated donkey anti-mouse (1:400) secondary antibodies (Pierce) for 30 minutes, treated with 4′,6-diamidino-2-phenylindole (DAPI), and mounted for examination by fluorescence microscopy.
GST fusion protein purification
VZV ORF25 sequences were amplified from VZV strain Ellen genomic DNA using gene specific oligonucleotide primers (IDT Inc.): forward 5′-GGATCCCCATGTACGAATCGGAA-3′, reverse 5′-GGATCCCCCTTGGTTAAGCATCCT-3′. KOD HiFi DNA polymerase (TOYOBO/Novagen, EMD Biosciences Inc.) was used to generate a blunt-end PCR product that was subsequently digested with the restriction enzyme BamHI. A DNA fragment containing the entire coding region of VZV strain Ellen ORF25 (468 bp) was cloned into the pGEX-3X vector (GE Healthcare). DNA sequencing (SeqWright) confirmed that the GST and ORF25 sequences were fused in frame.
The resulting plasmid encoded a recombinant GST fusion protein, GST-ORF25, containing all 156 amino acids of pORF25. E. coli (JM109) transformed with pGEX-3X (GST) or pGEX-ORF25 were inoculated into 200 ml of 2x-YT media containing 50ug/ml ampicillin. Cultures were incubated at 30°C for 8 hr and induced with 0.1mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 16 hr. Cells were harvested by centrifugation at 7000×g for 10 minutes, washed twice with PBS, resuspended in lysis buffer (1.5% sarkosyl, 50 mg/ml lysozyme in PBS containing protease inhibitor cocktail) and sonicated briefly. Lysates were cleared by centrifugation at 10,000×g for 5 minutes at 4°C. Supernatants were adjusted to 2% Triton X-100, incubated at 4°C for 1 hr and purified on Sepharose 4B glutathione beads (GE Healthcare). Beads were washed five times for 10 minutes with cold PBS and resuspended in storage buffer containing 50mM Hepes, pH 7.4, 150mM NaCl, 5mM dithiothreitol (DTT), 10% (v/v) glycerol, and protease inhibitors (Proux-Gillardeaux et al., 2003). Proteins were fractionated by SDS-PAGE and analyzed for purity and integrity by Coomassie blue staining.
Purified fusion protein was used in GST pull-down assays and to prepare a rabbit polyclonal antiserum specific for VZV pORF25. Immunization protocols were overseen and reviewed by the Purdue Animal Care and Use Committee (PACUC). The Laboratory Animal Program assures that animal housing, environments, and husbandry practices are consistent with federal policies.
GST pull-down assays
GST or GST-ORF25 fusion protein immobilized on beads was removed from storage buffer and washed twice with binding buffer containing 50mM Hepes, pH 7.4, 150mM NaCl, 5mM DTT, 10% (v/v) glycerol, and 1% Triton X-100 (Proux-Gillardeaux et al., 2003). Equal concentrations of bead immobilized GST or GST-ORF25 were resuspended in 480 ml of binding buffer to which 20ul of various V5-tagged in vitro transcribed/translated protein was added. After mixing at room temperature overnight, beads were washed five times for 10 minutes with binding buffer, solubilized in 2X sample buffer, fractionated by SDS-PAGE and transferred to PVDF membrane. Western blot analysis was performed using an anti-V5 monoclonal antibody to detect potential interaction of the in vitro synthesized gene products with the GST-ORF25 fusion protein or the GST only control.
Co-immunoprecipitation of viral proteins
Mock or VZV infected MeWo cells were washed with cold phosphate buffered saline (PBS) and resuspended in radioimmunoprecipitation assay buffer (50mM Tris, pH 7.4, 150mM NaCl, 1% NP-40, 0.25% sodium deoxycholate, 1mM EDTA, and protease inhibitor cocktail). After incubation on ice for 30 minutes, the lysates were clarified at 14,000 rpm for 15 minutes at 4°C in a microcentrifuge. The supernatants were precleared by reaction with 25 ul of preimmune rabbit serum and 60 ul of a 50% slurry of Protein A/G-Sepharose beads (Amersham Pharmacia Biotech) for 2 hr at 4°C with constant rocking. The beads were pelleted by centrifugation and the supernatants were incubated with 25 ul of rabbit antiserum directed against pORF25 overnight at 4°C. An second aliquot of protein A/G beads was added and the mixture was incubated 3 hr at 4°C with constant rocking. The beads were washed five times with excess radioimmunoprecipitation assay buffer and immune complexes were boiled in loading buffer (62.5 mM Tris, pH 6.8, 2% sodium dodecyl sulfate [SDS], 5% β-mercaptoethanol, 12.5% glycerol). The immunoprecipitated material was separated on 10% SDS-PAGE and proteins were transferred to PVDF for immunoblot analysis.
Two-hybrid Analysis
The Matchmaker GAL4 two-hybrid system 3 (Clontech Laboratories) was used to verify protein-protein interactions. Upon binding and activation of GAL4 control sequences located upstream of the mel1 gene, yeast strain Y187 expresses the Mel1 gene product, alpha-galactosidase (α-gal). Interacting protein partners were identified with a sensitive colorimetric assay that measured the amount of α-gal secreted into the culture supernatant. A colorless compound, p-nitrophenyl-α-D-galactoside (PNP-α-gal) was added to the culture supernatants to detect α-gal activity. Hydrolysis of PNP-α-gal yielded a yellow product (p-nitrophenol) in the presence of α-gal.
VZV ORF25 sequences were amplified from VZV strain Ellen genomic DNA using gene specific oligonucleotide primers (IDT Inc.): forward 5′-GGATCCCCATGTACGAATCGGAA-3′, reverse 5′-GGATCCCCCTTGGTTAAGCATCCT-3′. KOD HiFi DNA polymerase (TOYOBO/Novagen, EMD Biosciences Inc.) was used to generate a blunt-end PCR product that was subsequently digested with the restriction enzyme BamHI. A DNA fragment containing the entire coding region of VZV strain Ellen ORF25 (468 bp) was cloned into the target and bait vectors: pGBKT7 expressed a fusion protein consisting of amino acids 1–147 of the GAL4 DNA binding domain (DNA-BD) plus the entire ORF25 coding region; pGADT7 expressed a fusion protein consisting of amino acids 768–881 of the GAL4 activation domain (AD) plus the entire ORF25 coding region. DNA sequencing (SeqWright) confirmed that the ORF25 sequences were fused in frame with the DNA binding or activation domains. Control plasmids included pGBKT7-53, pGBKT7-lam, and pGADT7-T.
Briefly, overnight cultures of co-transformed Y187 yeast were grown at 30°C in selective synthetic dropout medium lacking Leu and Trp to maintain the plasmids. Prior to starting the assay, the OD600 of each sample was recorded. Cells were pelleted and supernatants were incubated with assay buffer (2 volumes of 0.5M Sodium Acetate, pH 4.5, 1 volume 100mM PNP-α-gal) overnight at 30°C. Stop solution (1M Na2CO3) was added to each sample and the OD410 was recorded. To adjust for differences in cell density of the original culture, the OD410 was divided by the OD600 of each individual culture. Data is represented as the mean of 3 or 6 separate cultures for the control co-transformations or the double ORF25 co-transformation respectively. The standard error for each group is represented.
Results
Identification of VZV pORF25
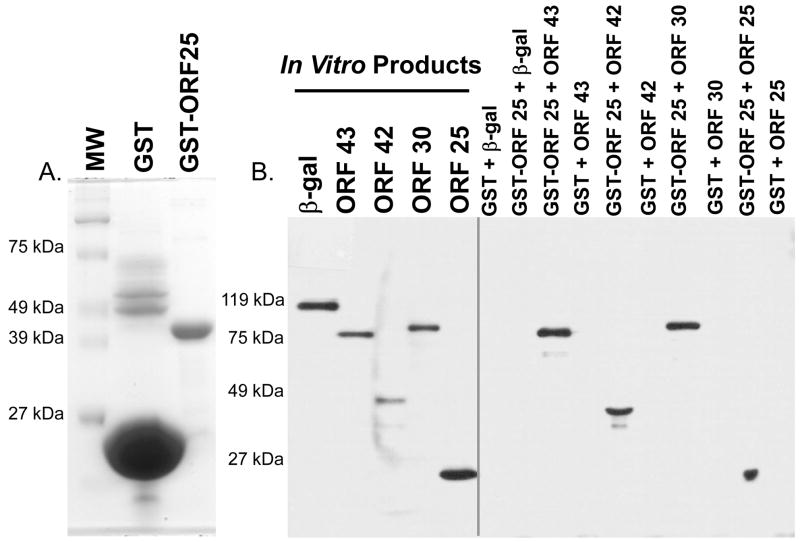
In order to determine if an ORF25 polypeptide (pORF25) was synthesized in VZV infected cells, the coding region of pORF25 (468 bp) was cloned into a GST fusion protein vector (pGEX-3X-ORF25). An approximately 43.5 kDa GST-ORF25 fusion protein was identified in E. coli transformed with pGEX-3X-ORF25. Total bacterial cell extracts were fractionated by SDS-PAGE and GST-ORF25 was eluted from gel slices (see Fig. 3A). Rabbits were immunized with the recombinant fusion protein (50–100 ug) to prepare an ORF25 specific polyclonal antiserum.
Figure 3.
GST pull-down assays were performed to detect potential protein-protein interactions. (A) GST or GST-ORF25 were purified on glutathione beads. Proteins were analyzed on Coomassie blue stained SDS-polyacrylamide gels to assess purity prior to performing pull-down assays. (B) pcDNA3.1D/V5–lacZ (β-gal), -ORF43, -ORF42, -ORF30, or -ORF25 were used in in vitro transcription/translation reactions to synthesize the respective protein products containing a C-terminal V5 epitope. Equal concentrations of GST or GST-ORF25 immobilized on beads were resuspended in binding buffer containing various pORF-V5s or β-gal-V5. Beads were washed, fractionated by SDS-PAGE and subjected to immunoblot analysis using an anti-V5 monoclonal antibody.
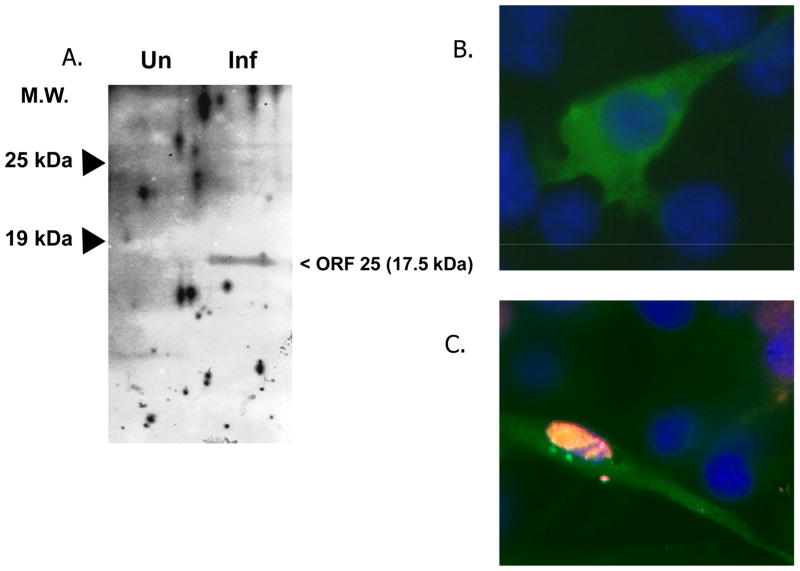
Immunoblot analysis was performed on detergent solubilized extracts from uninfected or VZV Ellen infected cells. Figure 2A shows a 17.5 kDa protein detected in virus infected IMR-90 cells. No polypeptide was detected migrating in this range for the uninfected control. The observed molecular mass of the polypeptide was consistent with that predicted for ORF25.
Figure 2.
Expression of VZV ORF25. (A) Immunoblot analysis was performed with the ORF25 rabbit polyclonal antiserum on mock infected or VZV infected IMR90 cell extracts. (B) Indirect immunofluorescence analysis of pORF25 expressed in transiently transfected cells. Cells were transfected with pcDNA3.1D/V5-ORF25 and 48 hr post-transfection fixed, stained with DAPI and incubated with an anti-V5 primary antibody followed by a FITC conjugated secondary antibody. (C) Indirect immunofluorescence analysis of pORF25 expressed in HSV-2 infected cells. Cells were transfected with pcDNA3.1D/V5-ORF25 and infected with HSV-2 24 hr post-transfection. Transfected, infected cells were fixed at 10 hr post-infection, stained with DAPI, and incubated with an anti-V5 rabbit and anti-HSV-2 ICP8 mouse antibodies followed by FITC anti-rabbit and rhodamine red-X conjugated anti-mouse secondary antibodies. pORF25 expressing cells appear green, HSV-2 infected cell nuclei appear red and DAPI stained nuclei appear blue.
Expression and localization of pORF25 in transfected MeWo cells
The ORF25 antiserum proved ineffective at consistently detecting pORF25 in VZV infected cells. This is not unlike the results recently reported by Fuchs et al. (2009) for the pORF25 homolog in pseudorabies virus, PrV pUL33. The authors speculated that PrV pUL33 was not detectable in infected cells using a PrV UL33 monospecific antiserum because PrV UL33 was in low abundance and/or suffered from poor immunogenicity. Hence, the sub-cellular localization of VZV pORF25 was examined via indirect immunofluorescence in transfected MeWo cells. Using an anti-V5 antibody, pORF25 tagged with a V5 epitope (ORF25-V5) was shown to localize to the cytoplasm of transfected MeWo cells (Fig. 2B). Little to no nuclear staining was observed in transfected cells. The results were consistent with those previously reported for the homologs of HSV-1 and -2 (pUL33), PrV (pUL33) and KSHV (pORF67.5).
Both HSV pUL33 and KSHV pORF67.5 localize primarily to the cytoplasm in the absence of other viral proteins (Yamauchi et al., 2001; Sander et al., 2008; Yamauchi et al., 2008). However, HSV-2 pUL33 was shown to translocate to the nucleus after co-expression with HSV-2 pUL14 (Yamauchi et al., 2001) and KSHV pORF67.5 was shown to translocate to the nucleus after co-expression with KSHV K10 (Sander et al., 2008). Similarly, we investigated whether VZV pORF25 would translocate to the nucleus in the presence of another viral gene product. It is inherently difficult to establish a synchronous infection at high MOI with VZV. Therefore, another alphaherpesvirus, HSV-2, was used to infect VZV ORF25-V5 transfected MeWo cells. We hypothesized that one or more HSV-2 gene products might substitute for homologous VZV protein(s) involved in translocating pORF25 to the nucleus. Figure 2C shows that HSV-2 infected MeWo cells stained positive for HSV-2 ICP8 expression in the nucleus (as expected). However, HSV-2 infected, VZV ORF25-V5 transfected cells that stained positive for both ICP8 and pORF25 did not show a clear pattern of pORF25 translocation to the nucleus (Fig. 2C). This result was not completely unexpected since Fuchs et al. (2009) recently showed that heterologous complementation could not be observed when infecting a PrV pUL33 expressing cell line with a UL33-negative HSV-1 mutant. Our results suggest that VZV pORF25 localization was not altered in the presence of HSV-2 infection. A partner involved in the translocation of VZV pORF25 has not yet been identified. We speculate that it could be the VZV homolog of HSV-2 UL14 (VZV ORF46) since pUL14 was previously shown to translocate HSV pUL33 from the cytoplasm to the nucleus (Yamauchi et al., 2001).
GST pull-down assays detect encapsidation protein interactions with VZV pORF25
GST pull-down assays were performed to determine if interactions could be observed for VZV pORF25 and the putative encapsidation proteins encoded by ORF25, ORF30, exon II of ORF45/42, and ORF43.
GST or GST-ORF25 were purified from E. coli extracts and immobilized on glutathione beads. Beta-galactosidase (a non-viral control protein) or viral proteins, each containing a C-terminal V5 epitope tag, were incubated with purified GST or GST-ORF25. Western blot analysis was performed on each pull-down assay to detect potential interactions between the V5 epitope tagged in vitro synthesized products and GST-ORF25 (Fig. 3B). All of the encapsidation proteins tested were observed to interact with GST-ORF25 but not with GST alone. β-gal-V5, did not interact with GST or GST-ORF25. The results show a strong and specific interaction for pORF25 and the four proteins tested. Additionally, ORF42 (exon II of ORF45/42) was sufficient to mediate interaction with pORF25 and pORF25 has the potential to form complexes with itself.
Yeast two-hybrid analysis confirms the self-interaction of VZV pORF25
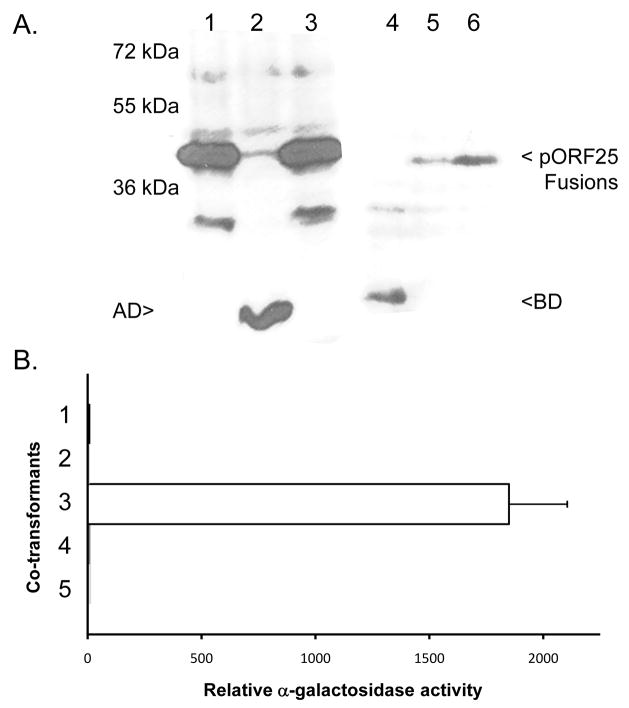
The GST pull-down assay results suggested that pORF25 forms complexes with itself. A yeast two-hybrid assay was used to validate this observation.
The full length 468 bp ORF25 gene was fused to either a Gal 4 DNA binding (BD) or transcriptional activation (AD) domain in the yeast expression vectors pGBKT7 or pGADT7 respectively. Y187 cells were co-transformed with pGBKT7+pGADT7pORF25 (Fig. 4, lanes 1 and 4), pGADT7+pGBKT7pORF25 (Fig. 4, lanes 2 and 5) or pGADT7pORF25+pGBKT7pORF25 (Fig. 4, lanes 3 and 6) and grown at 30°C for 24 hours. Total protein extracts were prepared for SDS-PAGE and immunoblot analysis with either activation domain specific (lanes 1–3) or binding domain specific (lanes 4–6) antibodies. Expression of the ~20 kDa binding or activation domains alone was observed in lanes 2 and 4 respectively. Proteins of ~38 kDa representing pORF25 fusions were observed in lanes 1, 3, 5, and 6 as expected. Lanes 3 and 6 represent co-expression of both ORF25 fusion proteins in the same cells.
Figure 4.
Two-hybrid analysis of pORF25 self-interaction. Target and bait vectors containing a DNA binding domain (BD) or transcriptional activation domain (AD) fused to the full length ORF25 gene were co-transformed into yeast. (A) Immunoblot analysis of yeast transformed with: pGBKT7 +pGADT7pORF25 (lanes 1 and 4), pGADT7+pGBKT7pORF25 (lanes 2 and 5) or pGADT7pORF25+pGBKT7pORF25 (lanes 3 and 6). Lanes 1–3 and lanes 4–6 were incubated with antibodies to the GAL4 activation (anti-HA) or binding (anti-GAL4 DNA BD) domains respectively. (B) Relative α-gal activity detected in the supernatants of yeast co-transformed with: 1-pGADT7 + pGBKT7pORF25, 2-pGBKT7 + pGADT7pORF25, 3-pGADT7pORF25 + pGBKT7pORF25, 4-pGBKT7-lam + pGADT7-T, or 5-pGADT7 + pGBKT7. A positive control using two known interacting partners, pGBKT7-53 and pGADT7-T, consistently yielded activity greater than 35,000 fold over baseline (data not shown).
Control vectors and pORF25 containing plasmids were paired and used to co-transform Y187 cells. After 24 hours of growth at 30°C, culture supernatants were tested for the secretion of α-gal. Figure 4B shows that co-transformants containing pGBKT7pORF25 and pGADT7pORF25 had measurable α-gal activity. Minimal activity was detected in cells containing any other combination of plasmids. Activation of the gene encoding α-gal in the presence of BD-pORF25 and AD-pORF25 suggest that pORF25 can form intermolecular complexes with itself.
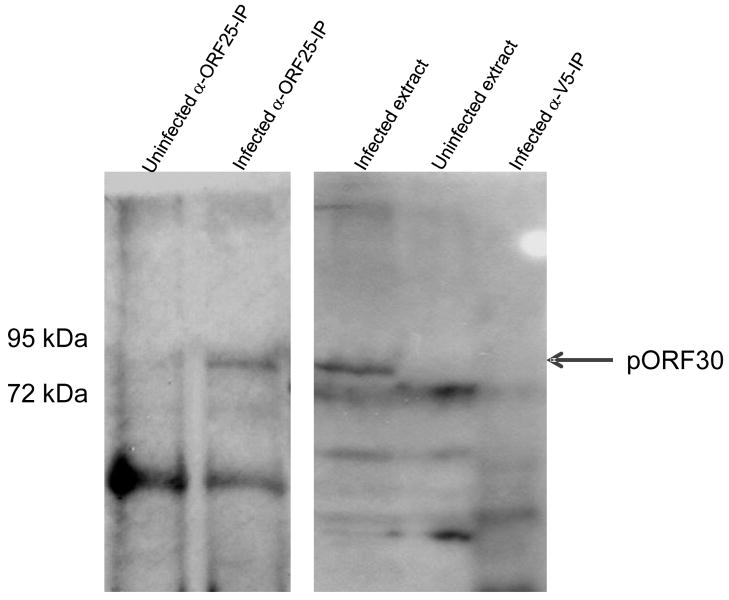
Co-immunoprecipitation of pORF25 and pORF30 from VZV infected cells
Virus infected or mock infected MeWo cell extracts were immunoprecipitated using either a pORF25 specific antiserum or a V5 epitope specific antibody. Potential co-precipitates were washed extensively, fractionated by SDS PAGE, and analyzed by immunoblotting with a pORF30 specific antiserum (Visalli et al., 2007). An 87 kDa protein co-immunoprecipitated with pORF25 from infected but not mock infected cell extracts (Fig. 5). The V5 epitope specific antibody did not precipitate the 87 kDa protein. The 87 kDa protein is consistent with the size previously reported for the putative terminase subunit pORF30, encoded by the VZV ORF30 gene (Visalli et al., 2007). This is the first evidence indicating that at least two VZV DNA encapsidation proteins form a complex in VZV infected cells and is consistent with the observation that the analogous HSV homologues co-precipitate from infected cell extracts (Yang and Baines, 2006).
Figure 5.
Co-immunoprecipitation of pORF25 and pORF30. Uninfected or VZV infected MeWo cell extracts were immunoprecipitated using an ORF25 specific antiserum or a V5 epitope specific antibody. Precipitated proteins were fractionated by SDS-PAGE and analyzed by western blot with an ORF30 specific antiserum. The arrow indicates the position of the 87 kDa pORF30.
Discussion
The Herpesviridae encode a family of protein homologs whose function is to cleave replicated viral DNA into genome-length units and insert the DNA into preformed capsids. This process of DNA encapsidation is best understood in HSV and HCMV where the genes encoding the encapsidation proteins were first characterized. The identification of encapsidation-specific antiviral inhibitors for HSV, HCMV, and VZV suggests that encapsidation is a valid antiviral target for herpesviruses (Underwood et al., 1998; van Zeijl et al., 2000; Buerger et al., 2001; Reefschlaeger et al., 2001; Bogner, 2002; Visalli et al., 2003; Di Grandi et al., 2004; Visalli, 2004, Biron, 2006). Hence, understanding the function and interplay between various encapsidation proteins may lead to strategies to prevent viral replication.
Previous reports have identified the HCMV terminase subunits pUL56 and pUL89 (Underwood et al., 1998; Buerger et al., 2001; Reefschlaeger et al., 2001; Bogner, 2002), as well as the capsid portal proteins VZV pORF54 (Visalli et al., 2003) and HSV pUL6 (van Zeijl et al., 2000), as viable antiviral targets. We hypothesize that other less studied encapsidation proteins are also putative targets. One such protein is the 156 amino acid polypeptide pORF25, encoded by the VZV ORF25 gene. The pORF25 homolog in HSV-1, pUL33, was shown to be essential for HSV replication (al-Kobaisi et al., 1991). Studies with HSV-1 have defined several interacting proteins that form a trimeric terminase complex. The HSV-1 subunits, pUL28, pUL15, and pUL33 (the VZV pORF30, pORF45/42, and pORF25 homologs respectively) were shown to interact via co-immunoprecipitation from infected cell extracts (Yang and Baines, 2006). The results showed a direct interaction between pUL28 and pUL15, pUL28 and pUL33, but an indirect interaction between pUL33 and pUL15. Yang and Baines (2006) suggested that in infected cells, the role of pUL33 is to optimize the pUL15/pUL28 interaction.
In this study, the 17.5 kDa pORF25 protein was identified in VZV infected cells. Using GST pull-down assays, pORF25 was shown to interact in vitro with the VZV DNA encapsidation proteins encoded by ORFs 25, 30, 45/42, and 43. Previously, pORF30 and pORF45/42 were shown to interact in vitro (Visalli et al., 2007). In the current study, two of the putative VZV terminase subunits, pORF30 and pORF25, were co-immunoprecipitated from infected cell extracts. Combined, the data suggests that VZV has a trimeric terminase complex consisting of pORF25, pORF30, and pORF45/42. Future studies will focus more precisely on defining the function of proteins involved in the VZV DNA encapsidation process.
The homologs of VZV pORF30 and pORF45/42 have functions and/or sequence characteristics associated with putative terminase proteins. However, there is no strong evidence that pORF25 or its homologs have a specific role in the actual enzymatic or mechanical processes of DNA encapsidation. In fact, Uetz et al. (2005) defined 29 additional potential interacting partners for VZV pORF25 and at least 9 interacting partners for the KSHV homolog, pORF66.5. Hence, VZV pORF25 may not be an encapsidation protein per se. Instead, pORF25 may play a more generic (yet still essential) role at multiple steps of the viral life cycle, including but not limited to optimizing the interaction of the terminase subunits. Sequence analysis shows that VZV pORF25 belongs to a family of proteins designated the Herpes UL33 Superfamily [Fig. 1, pfam03581] (Marchler-Bauer et al., 2007). We propose that the UL33 homologs act as protein “magnets” that aid in the formation of protein complexes during viral replication. Further investigation of the Herpes UL33 Superfamily, including VZV pORF25, may yield new targets for antiviral drug development.
Acknowledgments
These studies were supported by a National Institutes of Health grant (1 R15 AI062713-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- al-Kobaisi MF, Rixon FJ, McDougall I, Preston VG. The herpes simplex virus UL33 gene product is required for the assembly of full capsids. Virol. 1991;180(1):380–8. doi: 10.1016/0042-6822(91)90043-b. [DOI] [PubMed] [Google Scholar]
- Beard PM, Taus NS, Baines JD. DNA cleavage and packaging proteins encoded by genes U(L)28, U(L)15, and U(L)33 of herpes simplex virus type 1 form a complex in infected cells. J Virol. 2002;76(10):4785–91. doi: 10.1128/JVI.76.10.4785-4791.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron KK. Antiviral drugs for cytomegalovirus diseases. Antiviral Res. 2006;71(2–3):154–63. doi: 10.1016/j.antiviral.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Bogner E. Human cytomegalovirus terminase as a target for antiviral chemotherapy. Rev Med Virol. 2002;12(2):115–27. doi: 10.1002/rmv.344. [DOI] [PubMed] [Google Scholar]
- Bogner E, Radsak K, Stinski MF. The gene product of human cytomegalovirus open reading frame UL56 binds the pac motif and has specific nuclease activity. J Virol. 1998;72(3):2259–64. doi: 10.1128/jvi.72.3.2259-2264.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buerger I, Reefschlaeger J, Bender W, Eckenberg P, Popp A, Weber O, Graeper S, Klenk HD, Ruebsamen-Waigmann H, Hallenberger S. A novel nonnucleoside inhibitor specifically targets cytomegalovirus DNA maturation via the UL89 and UL56 gene products. J Virol. 2001;75(19):9077–86. doi: 10.1128/JVI.75.19.9077-9086.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Grandi MJ, Bloom JD, Curran KJ, Feigelson G, Prashad A, Ross AA, Visalli RJ, Feld B. Thiourea inhibitors of Herpesviruses Part 3: Inhibitors of VZV. Bioorg Med Chem Lett. 2004;16(14):4157–4160. doi: 10.1016/j.bmcl.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Fuchs W, Klupp BG, Granzow H, Leege T, Mettenleiter TC. Characterization of the pseudorabies virus (PrV) cleavage-encapsidation proteins and functional complementation of PrV pUL32 by the homologous protein of herpes simplex virus type 1. J Virol. 2009;83(8):3930–43. doi: 10.1128/JVI.02636-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JS, Bogner E. ATPase activity of the terminase subunit pUL56 of human cytomegalovirus. J Biol Chem. 2002;277(9):6943–8. doi: 10.1074/jbc.M108984200. [DOI] [PubMed] [Google Scholar]
- Jacobson JG, Yang K, Baines JD, Homa FL. Linker insertion mutations in the herpes simplex virus type 1 UL28 gene: effects on UL28 interaction with UL15 and UL33 and identification of a second-site mutation in the UL15 gene that suppresses a lethal UL28 mutation. J Virol. 2006;80(24):12312–23. doi: 10.1128/JVI.01766-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchler-Bauer A, Anderson JB, Derbyshire MK, DeWeese-Scott C, Gonzales NR, Gwadz M, Hao L, He S, Hurwitz DI, Jackson JD, Ke Z, Krylov D, Lanczycki CJ, Liebert CA, Liu C, Lu F, Lu S, Marchler GH, Mullokandov M, Song JS, Thanki N, Yamashita RA, Yin JJ, Zhang D, Bryant SH. CDD: a conserved domain database for interactive domain family analysis. Nucleic Acids Res. 2007;35:D237–40. doi: 10.1093/nar/gkl951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proux-Gillardeaux V, Galli T, Callebaut I, Mikhailik A, Calothy G, Marx M. D53 is a novel endosomal SNARE-binding protein that enhances interaction of syntaxin 1 with the synaptobrevin 2 complex in vitro. Biochem J. 2003;370(Pt 1):213–21. doi: 10.1042/BJ20021309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reefschlaeger J, Bender W, Hallenberger S, Weber O, Eckenberg P, Goldmann S, Haerter M, Buerger I, Trappe J, Herrington JA, Haebich D, Ruebsamen-Waigmann H. Novel non-nucleoside inhibitors of cytomegaloviruses (BAY 38-4766): in vitro and in vivo antiviral activity and mechanism of action. J Antimicrob Chemother. 2001;48(6):757–67. doi: 10.1093/jac/48.6.757. [DOI] [PubMed] [Google Scholar]
- Reynolds AE, Fan Y, Baines JD. Characterization of the U(L)33 gene product of herpes simplex virus 1. Virology. 2000;266(2):310–8. doi: 10.1006/viro.1999.0090. [DOI] [PubMed] [Google Scholar]
- Sander G, Konrad A, Thurau M, Wies E, Leubert R, Kremmer E, Dinkel H, Schulz T, Neipel F, Sturzl M. Intracellular localization map of human herpesvirus 8 proteins. J Virol. 2008;82(4):1908–1922. doi: 10.1128/JVI.01716-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffczik H, Savva CG, Holzenburg A, Kolesnikova L, Bogner E. The terminase subunits pUL56 and pUL89 of human cytomegalovirus are DNA-metabolizing proteins with toroidal structure. Nucleic Acids Res. 2002;30(7):1695–703. doi: 10.1093/nar/30.7.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz B, Rechter S, Drach JC, Townsend LB, Bogner E. Identification of the ATP-binding site in the terminase subunit pUL56 of human cytomegalovirus. Nucleic Acids Res. 2003;31(5):1426–33. doi: 10.1093/nar/gkg229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uetz P, Dong YA, Zeretzke C, Atzler C, Baiker A, Berger B, Rajagopala SV, Roupelieva M, Rose D, Fossum E, Haas J. Herpesviral protein networks and their interaction with the human proteome. Science. 2006;311(5758):239–42. doi: 10.1126/science.1116804. [DOI] [PubMed] [Google Scholar]
- Underwood MR, Harvey RJ, Stanat SC, Hemphill ML, Miller T, Drach JC, Townsend LB, Biron KK. Inhibition of human cytomegalovirus DNA maturation by a benzimidazole ribonucleoside is mediated through the UL89 gene product. J Virol. 1998;72(1):717–25. doi: 10.1128/jvi.72.1.717-725.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zeijl M, Fairhurst J, Jones TR, Vernon SK, Morin J, LaRocque J, Feld B, O’Hara B, Bloom JD, Johann SV. Novel class of thiourea compounds that inhibit herpes simplex virus type 1 DNA cleavage and encapsidation: resistance maps to the UL6 gene. J Virol. 2000;74(19):9054–61. doi: 10.1128/jvi.74.19.9054-9061.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visalli RJ. Novel compounds for the treatment of varicella-zoster virus infections. Expert Opin Ther Patents. 2004;14(3):355–365. [Google Scholar]
- Visalli RJ, Fairhurst J, Srinivas S, Hu W, Feld B, DiGrandi M, Curran K, Ross A, Bloom JD, van Zeijl M, Jones TR, O’Connell J, Cohen JI. Identification of small molecule compounds that selectively inhibit varicella-zoster virus replication. J Virol. 2003;77(4):2349–58. doi: 10.1128/JVI.77.4.2349-2358.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visalli RJ, van Zeijl M. DNA encapsidation as a target for anti-herpesvirus drug therapy. Antiviral Res. 2003;59(2):73–87. doi: 10.1016/s0166-3542(03)00108-6. [DOI] [PubMed] [Google Scholar]
- Visalli RJ, Nicolosi DM, Irven KL, Goshorn B, Khan T, Visalli MA. The Varicella-zoster virus DNA encapsidation genes: Identification and characterization of the putative terminase subunits. Virus Res. 2007;129:200–211. doi: 10.1016/j.virusres.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Baines JD. The putative terminase subunit of herpes simplex virus 1 encoded by UL28 is necessary and sufficient to mediate interaction between pUL15 and pUL33. J Virol. 2006;80(12):5733–9. doi: 10.1128/JVI.00125-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Homa F, Baines JD. Putative terminase subunits of herpes simplex virus 1 form a complex in the cytoplasm and interact with portal protein in the nucleus. J Virol. 2007;81(12):6419–33. doi: 10.1128/JVI.00047-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi Y, Wada K, Goshima F, Takakuwa H, Daikoku T, Yamada M, Nishiyama Y. The UL14 protein of herpes simplex virus type 2 translocates the minor capsid protein VP26 and the DNA cleavage and packaging UL33 protein into the nucleus of coexpressing cells. J Gen Virol. 2001;82(2):321–30. doi: 10.1099/0022-1317-82-2-321. [DOI] [PubMed] [Google Scholar]
- Yamauchi Y, Kiriyama K, Kubota N, Kimura H, Usukura J, Nishiyama Y. The UL14 tegument protein of herpes simplex virus type 1 is required for efficient nuclear transport of the alpha transinducing factor VP16 and viral capsids. J Virol. 2008;82(3):1094–106. doi: 10.1128/JVI.01226-07. [DOI] [PMC free article] [PubMed] [Google Scholar]