Abstract
Apoptosis is a critical process in tissue homeostasis and results in immediate removal of the dying cell by professional phagocytes such as macrophages and dendritic cells. Phagocytosis of apoptotic cells actively suppresses production of pro-inflammatory growth factors and cytokines. Impaired phagocytosis of apoptotic cells has been implicated in the pathogenesis of chronic inflammatory and autoimmune diseases. In this study we found that, in addition to suppressing LPS-induced production of TNF-α and IL-6, phagocytosis of apoptotic cells by macrophages suppressed production of the chemokine CXCL10 that is activated by LPS-induced autocrine-acting type I IFNs. Inhibition of cytokine and chemokine production was not universally affected since LPS-induced production of IL-10 and IL-8 was not significantly affected. Apoptotic cells had minimal effects on LPS-induced activation of NF-κB and MAPKs, but induced expression of SOCS proteins and substantially suppressed induction of CXCL10 expression by IFN-α. In addition to suppressing LPS responses, apoptotic cells inhibited macrophage responses to another major macrophage activator IFN-γ by attenuating IFN-γ-induced STAT1 activation and downstream gene expression. These results identify suppressive effects of apoptotic cells on signal transduction, and extend our understanding of the anti-inflammatory effects of apoptotic cells to include suppression of Jak-STAT signaling.
Keywords: autoimmunity, inflammation, signaling, interferon, STAT
Introduction
Apoptosis is an evolutionarily conserved mechanism for removal of unwanted, old or damaged cells. The recognition and removal of apoptotic cells by macrophages represent a unique form of phagocytosis that actively induces an anti-inflammatory and immunosuppressive state (1). Production of anti-inflammatory mediators such as TGF-β, interleukin-10 (IL-10) and prostaglandin E2 (PGE2) during phagocytosis of apoptotic cells has been shown to mediate these effects (2–4). Many receptors and soluble factors have been implicated in the recognition and clearance of apoptotic cells by macrophages (5). These include the class A and class B of the scavenger receptors, receptors for oxidized LDL, certain integrins like the vitronectin receptor αvβ3, complement receptors CR3 and CR4, a phosphatidylserine receptor, the thrombospondin receptor CD36, the Mer tyrosine kinase receptor and CD14 (6–12). Exposure of the negatively charged phospholipid phosphatidylserine (PS) on the cellular membranes of apoptotic cells has been recognized as a specific feature of cells undergoing apoptosis. A number of soluble mediators such as thrombospondin, β2-glycoprotein I, protein S, histidine-rich glycoprotein (HRG), serum amyloid protein (SAP) and C-reactive protein (CRP) have been shown to play a role in the recognition and removal of apoptotic cells by macrophages (13–15).
The clearance of apoptotic cells by macrophages prevents their lysis and the consequent release of toxic and/or immunogenic intracellular components. Intra-articular injection of apoptotic cells prevented the development of an experimental immune complex inflammatory arthritis in mice (16) and in an animal model of acute pulmonary inflammation, direct apoptotic cell instillation enhanced the resolution of acute inflammation and decreased the production of pro-inflammatory chemokine levels in the bronchoalveolar lavage fluid (17). In a restrictive engraftment model of murine bone marrow transplantation, apoptotic leukocytes co-infused with allogeneic bone marrow cells had a graft-facilitating effect without causing graft-versus-host disease (18).
Deregulation of cytokine production or cytokine networks has been implicated in the pathogenesis of a number of human autoimmune/inflammatory diseases including rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) (19, 20). Bacterial lipopolysaccharide (LPS) and IFNγ are strong activators of inflammatory reactions and cellular immunity and major activators of macrophages. LPS signals by stimulating Toll-like receptor 4 (TLR4). Stimulation of TLR4 leads to the activation of two signaling pathways: the MyD88 (myeloid differentiation primary-response protein 88)-dependent and MyD88-independent pathways. The MyD88-dependent pathway involves the early phase of nuclear factor- κB (NF-κB) and MAPK activation, which leads to the production of inflammatory cytokines, such as TNF-α and IL-6. The MyD88-independent pathway activates interferon regulatory factor 3 (IRF3) and involves the late phase of NF-κB and MAPK activation, which leads to the production of IFN-β and the expression of IFN-inducible genes, such as the gene encoding CXC-chemokine ligand 10 (CXCL10; the product of which is also known as IFNγ-inducible 10 kDa protein (IP-10)) (21, 22). TLR4 stimulation activates all three members of the MAPKs, namely p38, JNK and ERK1/2, which play a role in inflammatory cytokine production (23, 24).
To extend our knowledge of the role of apoptotic cell phagocytosis on macrophage activation, we studied the effects of apoptotic cell phagocytosis on LPS-induced signal transduction and cytokine and chemokine production, including activation of the Jak-STAT pathway by autocrine action of type I IFNs. We found that, in parallel with inhibition of TNF-α and IL-6 production, apoptotic cell phagocytosis inhibited LPS-induced activation of STAT1 and CXCL10 production. Surprisingly, apoptotic cell phagocytosis directly inhibited IFN-γ-induced STAT1 activation both in vivo and in vitro. Thus, apoptotic cell phagocytosis achieves its anti-inflammatory effects in part by suppressing activation of the Jak-STAT pathway.
Materials and Methods
Reagents and cell culture
Experiments with animals were approved by the animal care and use committee of the Hospital for Special Surgery (New York, NY). C57BL/6 mice were purchased from the Jackson Laboratories. Peripheral blood mononuclear cells were isolated by density gradient centrifugation with Ficoll (Invitrogen) of whole blood from healthy volunteers with a protocol approved by the Institutional Review Board of the Hospital for Special Surgery (New York, NY). Human monocytes were purified from peripheral blood mononuclear cells immediately after isolation with anti-CD14 magnetic beads, as recommended by the manufacturer (Miltenyi Biotec) and were cultured in RPMI 1640 medium (Invitrogen) supplemented with 10% human serum and 10 ng/ml of macrophage colony-stimulating factor for 2–8 days and similar results were obtained regardless of the culture period. Recombinant human IFN-γ was from Roche Molecular Biochemicals; recombinant human IFN-αA was from Biosource International; recombinant human IL-10 and human macrophage colony-stimulating factor was from PeproTech; LPS was from Sigma. Jurkat T cells were cultured in RPMI 1640 medium supplemented with 10% FBS (Hyclone). Anti-Fas antibody (clone CH11) was from Upstate Biotechnology.
Apoptotic cell phagocytosis assays
Jurkat T cells were labeled with the red lipophilic dye PKH as recommended by the manufacturer (Sigma), and incubated with an agonistic anti-Fas IgM monoclonal antibody for 3 hours to induce apoptosis. Greater than 70% of cells were apoptotic as determined by flow cytometry with Annexin V. Murine thymocytes were treated with dexamethasone (100 μM) overnight to induce apoptosis. Control live Jurkat cells or apoptotic Jurkat cells were added at a ratio of 10:1 to human macrophages for 1 hour and non-phagocytosed cells were removed by gentle washes with HBSS. For microscopic analysis, macrophages were stained with anti-CD14-FITC antibody (BD Pharmingen, San Diego, CA) and then incubated with PKH-labeled Jurkat cells and imaged using a Leica DC 200 digital camera (Leica, Switzerland) attached to a Zeiss Axioplan microscope (Zeiss, Germany). For the in vivo experiments, mice were injected intraperitoneally with 2 ml of 3% thioglycollate medium to induce sterile peritonitis. Two days later mice were injected with apoptotic or viable syngeneic thymocytes. One hour later mice were euthanized and peritoneal cells were retrieved by peritoneal lavage with ice cold HBSS. Peritoneal cells were plated on plastic plates, macrophages were selected by adherence and stimulated with cytokines as noted.
EMSA and immunoblots
Total cell extracts were obtained and proteins were quantitated with the Bradford assay (BioRad), as described. (25). Cell extracts (5 μg) were incubated for 15 min at 25 °C with 0.5 ng of 32P-labeled double-stranded high-affinity sis-inducible element oligonucleotide (5′-GTCGACATTTCCCGTAAATC-3′) in a 15-μl binding reaction volume with 40 mM NaCl and 2 μg of poly(dI:dC) (Pharmacia); complexes were resolved by nondenaturing 4.5% PAGE. For immunoblots, cell lysates (10 μg) were fractionated by 7.5% SDS-PAGE. Monoclonal antibodies to STAT1 (clone 1) and STAT3 (clone 84) were from BD Transduction laboratories; polyclonal antibodies to p-38, ERK1/2 and JNK were from Santa Cruz Biotechnology and to IκB-α was from Cell Signaling Technology. Phosphorylation-specific antibodies to STAT1 (Tyr 701), STAT3 (Tyr 705), p-38, JNK and ERK1/2 were from Cell Signaling Technology.
Real Time Quantitative RT-PCR (qPCR)
For real time PCR, total RNA was extracted using an RNeasy Mini kit and 1 μg of RNA was reverse transcribed using a First Strand cDNA Synthesis kit (Fermentas, Hanover, MD). Real time, quantitative PCR was performed using iQ™ SYBR-Green® Supermix and an iCycler iQ™ thermal cycler (Biorad, Hercules, CA) following the manufacturer′s protocols. Triplicate reactions were run for each sample and expression of a tested gene was normalized relative to levels of CD14 such as to normalize relative to levels of macrophage RNA. The generation of only the correct size amplification products was confirmed by agarose gel electrophoresis. Sequences of primers used for amplification are: CXCL10 (IP-10) 5′-ATTTGCTGCCTTATCTTTCTG-3′ and 5′-TCTCACCCTTCTTTTTCATTGTAG-3′, CXCL9 (MIG) 5′-GCTTTTTCTTTTGGCTGACCTGTT-3′ and 5′-ATCAGCACCAACCAAGGGACTATC-3′, SOCS1 5′-CCCTTAGCGTGAAGATGGC-3′ and 5′-GCAGCTCGAAGAGGCAGTC-3′, SOCS3 5′-CACTCTTCAGCATCTCTGTCGGAAG-3′ and 5′-CATAGGAGTCCAGGTGGCCGTTGAC-3′.
Enzyme-linked immunosorbent assay (ELISA)
TNF-α, IL-6, IL-8, IL-10 and IP-10 concentrations were measured by sandwich ELISA, as recommended by the manufacturer (BD Pharmingen).
Results
Interaction of Apoptotic Jurkat Cells with Primary Macrophages in vitro
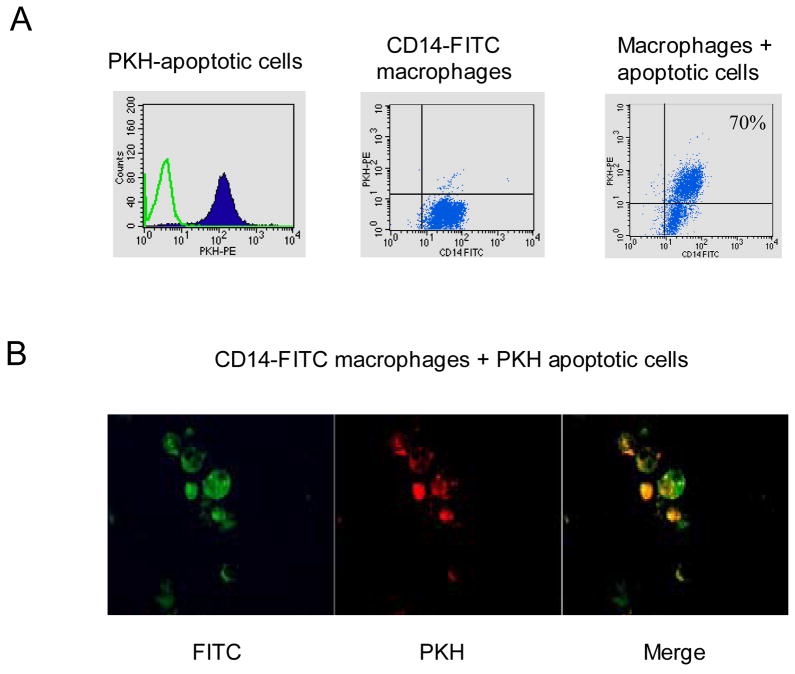
Apoptotic Jurkat cells were added in a ratio of 10:1 to macrophages and interaction of apoptotic cell phagocytosis with macrophages was evaluated using flow cytometry. Jurkat cells were labeled with PKH (a lipophilic red fluorescent dye that stains cell membranes) and macrophages were stained with an anti-CD14 antibody labeled with FITC (Fig. 1A). Typically 60 to 80% of the macrophages were double positive for CD14-FITC and PKH after one hour of interaction with apoptotic Jurkat cells (Fig. 1A) and were used for further experiments. Interaction of CD14-FITC-labeled macrophages (green) and PKH-labeled apoptotic cells (red) was also analyzed using fluorescence microscopy. Adherent macrophages were cultured with apoptotic Jurkat cells, Jurkat cells were washed away, and cells were imaged (Fig. 1B; a representative field is shown). In the merged view (right panel), five out of six macrophages contained internalized apoptotic cells (stained orange) whereas one out of six macrophages did not (stained green, bottom left corner of same panel). This result is consistent with the flow cytometry results shown in Fig. 1A. Primary human macrophages digest apoptotic cells rapidly, and thus we did not see discrete internalized cells but a punctate staining pattern where PKH-labeled apoptotic cell fragments localized within FITC-labeled macrophages (Fig. 1B). These results suggest that macrophages digest apoptotic cells.
Figure 1.
Interaction of Apoptotic Jurkat Cells with Human Macrophages. A. Apoptotic Jurkat T cells labeled with PKH and macrophages stained with CD14-FITC were cocultured for one hour at a ratio of 10:1 and then analyzed by FACS. B. Adherent macrophages stained with anti-CD14-FITC were incubated with PKH-labeled Jurkat cells, Jurkat cells were washed away, and macrophages were analyzed using fluorescence microscopy.
Apoptotic Cells Inhibit LPS-induced Cytokine and Chemokine Production by Macrophages
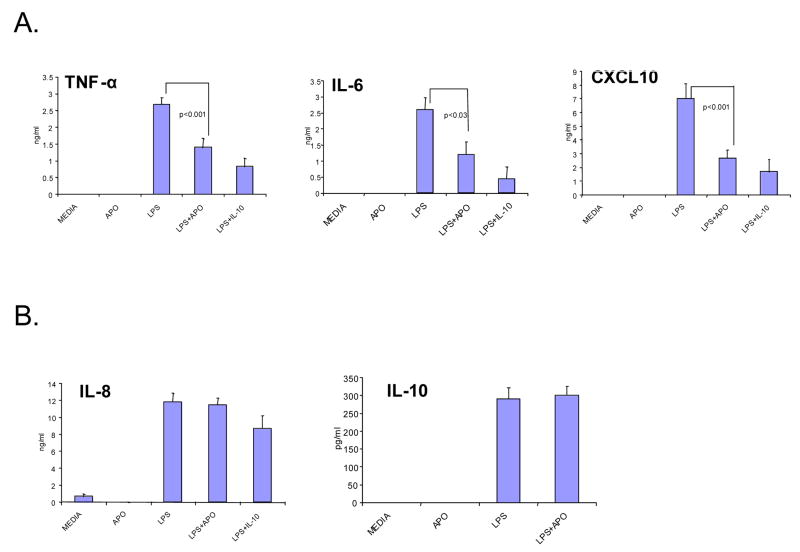
We investigated the effect of apoptotic cells on LPS-induced cytokine and chemokine production by macrophages in vitro. Consistent with previous reports, apoptotic cells significantly decreased LPS-induced production of TNF-α and IL-6 (Fig. 2A). In addition, apoptotic cells suppressed LPS induction of CXCL10, which is dependent on autocrine type I IFN production (Fig. 2A). This inhibition was specific since LPS-induced production of IL-8 was not significantly affected after apoptotic cell phagocytosis (Fig. 2B). Depending on the experimental system, production of the anti-inflammatory cytokines IL-10 and TGF-β by macrophages or by apoptotic cells has been shown to mediate inhibition of cytokine production (3, 4, 26). IL-10 production was not increased by apoptotic cells in our system (Fig. 2B), suggesting that apoptotic cells did not suppress cytokine production by inducing IL-10. Inhibition of TGF-β by blocking antibodies and latency associated peptide was not consistent in our system and thus the role of TGF-β in suppressing production of TNF-α and CXCL10 in our system is not clear at this time. Our results are in accordance with previously reported inhibition of LPS-induced TNF-α production by macrophages after apoptotic cell phagocytosis. In addition, we now show that apoptotic cells negatively regulate LPS-induced CXCL10 production by macrophages, a response that is dependent on autocrine type I IFN action (27).
Figure 2.
Selective Inhibition of LPS-induced Cytokine Production by Macrophages after Phagocytosis of Apoptotic Cells in vitro. Peripheral blood human monocyte derived macrophages (HMDMs) were stimulated with LPS (100 ng/ml) after culture with viable (control) or apoptotic Jurkat cells for 1 hr. Culture supernatants were collected 18 hours later and analyzed for cytokine and chemokine production using specific ELISAs. Pretreatment with IL-10 (25 ng/ml) before LPS stimulation was used as positive control. Bars represent means ± SD of 9 independent experiments. Student′s t-test was used for statistical analysis.
Apoptotic Cells Inhibit the LPS-induced Type I IFN-mediated Autocrine Loop in Macrophages
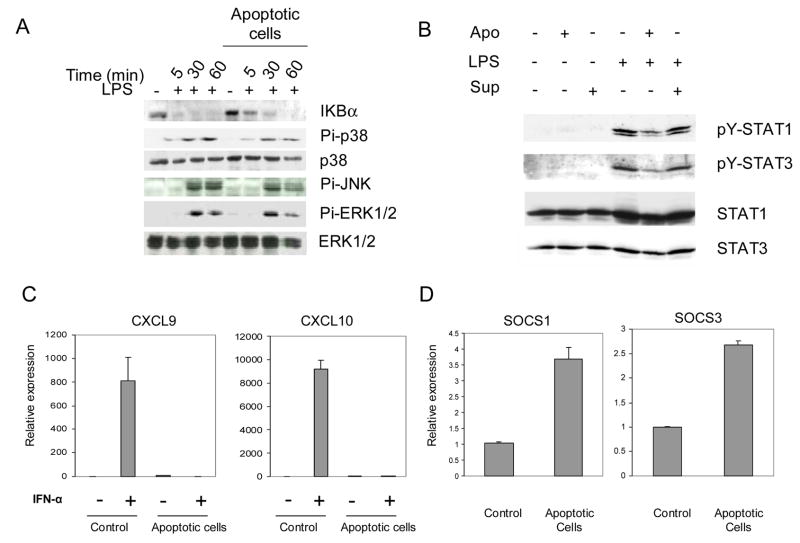
Production of the pro-inflammatory cytokines TNF-α and IL-6 after LPS stimulation is regulated by the TLR4-MyD88-dependent signaling pathway that results in the activation of NF-κB and p38, JNK and p42/44 ERK MAPKs. MyD88-deficient mice do not produce TNF-α or IL-6 when exposed to LPS, even though NF-κB and MAPK activation still occurs with delayed kinetics (28). To determine where apoptotic cell phagocytosis intersects the LPS-TLR4 signaling pathway, we examined the kinetics of activation of the three MAPKs and the NF-κB pathway, which are all downstream of TLR4 stimulation. We measured activation of the NF-κB pathway by examining the degradation of the inhibitor IκBα, which serves to retain NF-κB dimers in the cytoplasm, thereby preventing transcriptional activation by NF-κB. Apoptoic cells had a minor effect on the kinetics of IκBα degradation (Fig. 3A). The magnitude of p38 MAPK, JNK and p42/44 ERK phosphorylation was minimally affected by apoptotic cells (Fig. 3A, note that the last lane is underloaded). These results suggest that inhibition of TNF-α and IL-6 production could not be explained by apoptotic cell-induced changes in NF-κB and MAPK activation.
Figure 3.
Apoptotic Cells Suppress the LPS-induced IFN-mediated Autocrine Loop. A. Western blot analysis of IκB and MAPKs in LPS (10 ng/ml) activated macrophages after culture with viable (control) or apoptotic Jurkat cells for 1 hr. B. Phagocytosis of apoptotic cells inhibits LPS-induced STAT1 phosphorylation. Macrophages were stimulated with LPS (10 ng/ml) in the presence of viable (control) or apoptotic Jurkat cells for 18 hours. Cell extracts were analyzed using immunoblotting. C. Macrophages were cultured with viable (control) or apoptotic Jurkat cells for 90 min, stimulated with 8 ng/ml of IFN-α for 3 hours, and mRNA levels were measured using real time PCR. mRNA levels were normalized relative to the expression of CD14, a monocyte/macrophage marker, and results are shown as mean ± SD of triplicate determinants. D. Macrophages were cultured with viable (control) or apoptotic Jurkat cells for 90 min, and SOCS1 and SOCS3 mRNA was measured using real time PCR. mRNA levels were normalized relative to the expression of CD14 and results are shown as mean ± SD of triplicate determinants.
Transcription of the CXCL10 gene is regulated by the TLR4-MyD88-independent signaling pathway after LPS stimulation (27). Activation of the TLR4 MyD88-independent pathway induces type I IFN production, with downstream activation of STAT1 and its target genes such as CXCL10 (29). We assessed the effects of apoptotic cells on the MyD88-independent pathway by measuring the activation of STAT1 after LPS stimulation. Apoptotic cells induced no STAT1 phosphorylation in macrophages (Fig. 3B, second lane), whereas LPS stimulation induced tyrosine phosphorylation of STAT1 in control macrophages as expected (Fig. 3B, lane 4). Apoptotic cells attenuated LPS-induced tyrosine phosphorylation of STAT1 (Fig. 3B, lane 5). Phosphorylation of STAT3, another STAT activated by type I IFNs, was also decreased by apoptotic cells (Fig. 3B). It was possible that apoptotic cells induced expression of soluble or intracellular factors that suppressed LPS-induced STAT1 activation. To investigate the effect of soluble factors, we performed culture supernatant transfer experiments, where macrophages were cultured in supernatants obtained from cultures after apoptotic cell phagocytosis and then were stimulated with LPS. As shown in Fig. 3B, LPS-induced STAT1 phosphorylation was not affected after supernatant transfer, thereby excluding a role for a secreted factor(s) in the decreased phosphorylation of STAT1. Culture supernatants alone had no effect in the phosphorylation of STAT1 (Fig. 3B, lane 3).
We investigated the effects of apoptotic cells on gene activation by type I IFNs that mediate LPS induction of CXCL10 expression. Apoptotic cells effectively suppressed IFN-α-induced expression of CXCL10, and also of the STAT1 target gene CXCL9 (Fig. 3C). These results indicate that apoptotic cells inhibit macrophage gene induction by IFN-α and that suppression of IFN responses contributes to the inhibition of LPS-induced CXCL10 expression by apoptotic cells. Members of the SOCS protein family are potent suppressors of cytokine signaling and STAT activation, including signaling by type I IFNs, and therefore we tested whether apoptotic cells could induce SOCS expression. Apoptotic cells induced expression of SOCS1 and SOCS3 (Fig. 3D), suggesting that SOCS proteins contribute to apoptotic cell- mediated suppression of LPS- and IFN-α-induced gene expression. Overall, the results show that apoptotic cells suppressed LPS activation of the IFN-mediated autocrine loop that leads to activation of STATs and downstream target genes.
Inhibition of IFN-γ Responses in vitro and in vivo by Apoptotic Cell Phagocytosis
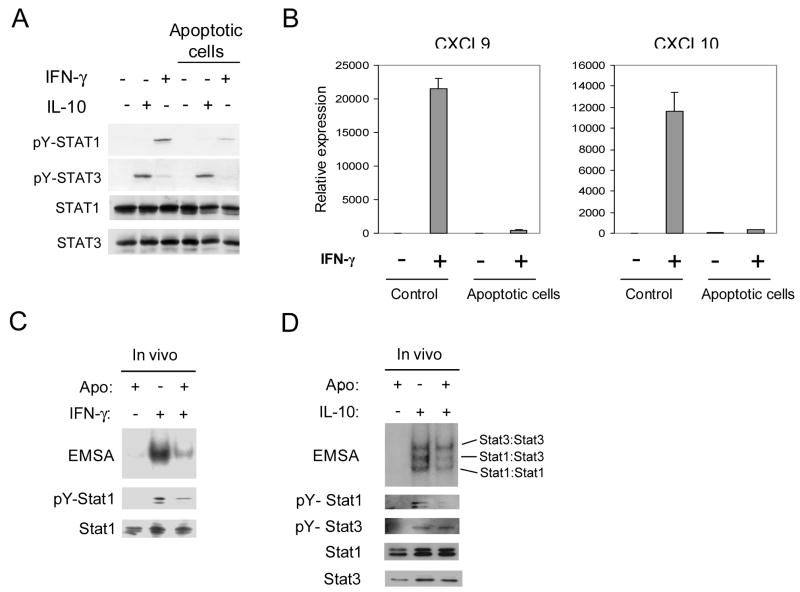
We wished to determine if apopotic cells suppressed macrophage activation more broadly than inhibition of LPS and type I IFN responses. Therefore we analyzed the effects of apoptotic cells on macrophage responses to the major activating factor IFN-γ. Apoptotic cells decreased IFN-γ-induced STAT1 tyrosine phosphorylation (Fig. 4A). This inhibition was specific, as activation of STAT3 by the anti-inflammatory cytokine IL-10 was not suppressed (Fig. 4A). However, inhibition of IFN-γ-induced STAT1 tyrosine phosphorylation was variable depending on the blood donor and strong inhibition was observed only in approximately one half of experiments (data not shown). We were unable to identify reasons for this variability, despite extensive troubleshooting of the in vitro experimental system. However, apoptotic cells effectively suppressed IFN-γ-induced expression of CXCL10 and CXCL9 (Fig. 4B), thereby supporting the biological significance of the signaling inhibition we had observed. In addition, we investigated the effects of apoptotic cell phagocytosis on IFN-γ and IL-10 signaling in vivo using a model of chemically-induced peritonitis in C57BL/6 mice. Apoptotic cells were injected intraperitoneally and peritoneal macrophages were removed and stimulated ex-vivo with IFN-γ and IL-10. Interaction of macrophages with apoptotic cells in vivo significantly inhibited IFN-γ-induced STAT1 DNA binding and STAT1 tyrosine phosphorylation (Fig. 4C). Apoptotic cell phagocytosis in vivo significantly inhibited IL- 10-induced STAT1 tyrosine phosphorylation and DNA binding of STAT1:STAT1 and STAT1:STAT3 dimers (Fig. 4D). Similar to the in vitro results (Fig. 4B), apoptotic cells only minimally affected IL-10-induced activation of STAT3 that mediates the anti-inflammatory effects of IL-10 (Fig. 4D). Thus, apoptotic cells preferentially suppress signaling mediated by the strongly activating factors IFN-γ and STAT1.
Figure 4.
Phagocytosis of Apoptotic Cell Inhibits IFN-γ Responses in Macrophages in vitro and in vivo. A. Macrophages were cultured in the presence of apoptotic or viable Jurkat cells at a ratio of 10:1 for 1 hour and then stimulated with IFN-γ (100 U/ml) or IL-10 (25 ng/ml) for 10 minutes. Cell extracts were analyzed using immunoblotting. B. Human macrophages were cultured with viable (control) or apoptotic Jurkat cells for 90 min, stimulated with 50 U/ml of IFN-γ for 3 hours and mRNA levels were measured using real time PCR. mRNA levels were normalized relative to the expression of CD14 and results are shown as mean ± SD of triplicate determinants. C and D. Thioglycolate elicited peritoneal macrophages from C57/BL6 mice were stimulated with murine IFN-γ (10 ng/ml) or IL-10 (25 ng/ml) for 10 min after exposure to apoptotic cells in vivo. Cell extracts were analyzed for STAT DNA binding activity using electrophoretic mobility shift assay (EMSA) with the hSIE radiolabeled oligonucleotide. Levels of tyrosine phosphorylation of STAT1 and STAT3 were analyzed using immunoblotting.
Discussion
There is considerable experimental evidence that phagocytic clearance of cells dying by apoptosis is much more than mere waste disposal. Depending on the context, the removal of apoptotic cells by phagocytes might suppress inflammation, modulate the macrophage directed deletion of invading pathogens and critically regulate immune responses (30). Inflammatory responses are vital for host defense against infection but when persistent they underlie important diseases such as rheumatoid arthritis. It has been shown in different experimental systems in vitro that phagocytosis of apoptotic cells actively suppresses the secretion from activated macrophages of pro-inflammatory mediators such as TNF-α, IL-1 and IL-12. In our present study we present evidence that uptake of apoptotic cells by macrophages inhibits LPS-induced production of CXCL10, a potent chemoattractant for the accumulation of Th1 lymphocytes at sites of inflammation (31). The inhibition LPS-induced cytokine production after apoptotic cell phagocytosis was not a universal phenomenon since macrophages produced equal amounts of IL-8 and IL-10 compared with control cells. IL-8 is a potent chemoattractant for neutrophils, a cell type that plays a vital role in the acute phase of inflammatory response against infections and IL-10 is a potent anti-inflammatory mediator that regulates the chronic phase of inflammatory responses. This pattern of cytokine and chemokine production by activated macrophages after the clearance of apoptotic cells would contribute to effective clearance of microorganisms while inhibiting the establishment of chronic unwanted inflammatory responses.
LPS-induced cytokine production is mainly mediated by activation of NF-κB, MAPKs, IRF-3 and by induction of a type I IFN-mediated, STAT1-dependent autocrine loop (22). We have shown that interaction of macrophages with apoptotic cells had minimal effects on LPS-induced NF-κB activation and activation of the MAPKs p38, JNK, and ERK1/2. In contrast, apoptotic cells suppressed the LPS-induced IFN-mediated autocrine loop by attenuating STAT1 activation and suppressing type I IFN activation of STAT1-dependent genes such as CXCL10 (27, 29). It is likely that apoptotic cell induction of SOCS1 and SOCS3 expression contributes to suppression of IFN-induced gene expression (32). LPS induces expression of inflammatory cytokines such as TNF-α and IL-6 via activation of NF-κB and MAPKs, and the mechanisms by which apoptotic cells suppress TNF-α and IL-6 expression have not been clarified. Our results suggest that inhibition of TNF-α and IL-6 expression by apoptotic cells does not occur at the level of NF-κB- and MAPK-mediated signal transduction. It is possible that apoptotic cells suppress alternative, as yet unknown, signaling pathways that lead to TNF-α and IL-6 expression, or work at the level of chromatin modification. In contrast, apoptotic cells suppressed Jak-STAT signaling and IFN-mediated responses downstream of TLR4.
IFN-γ is a key activator of macrophages and is mainly produced by NK cells and Th1 cells at later stages of the immune response and STAT1 mediates most of the activating effects of IFN-γ on macrophages. We analyzed the effect of apoptotic cells on the IFN-γ signaling pathway in macrophages both in vitro and in vivo. We found that IFN-γ-induced STAT1 activation, both at the tyrosine phosphorylation and DNA-binding level, was significantly inhibited in macrophages which had interacted with apoptotic cells both in vitro and in vivo in a chemically-induced peritonitis murine model of inflammation. Inhibition of STAT activation was somewhat selective for STAT1 relative to STAT3, which is activated by IL-10 and is strongly anti-inflammatory. This selective pattern of inhibition of cytokines and STATs would have the net effect of suppressing inflammatory macrophage activation while leaving deactivation pathways intact.
Acknowledgments
This work was supported by the National Institutes of Health (L.B.I.) and the Abbott Scholar Program (I.T.). This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program Grant Number C06-RR12538-01 from the National Center for Research Resources, National Institutes of Health.
Abbreviations
- Jak
janus kinase
- STAT
signal transducer and activator of transcription
- IFN
interferons
- TLR
Toll-like receptor
- LPS
lipopolysaccharide
- MAPK
mitogen activated kinase
- IL-10
interleukin-10
- TNF-α
tumor necrosis factor-α
- MyD88
myeloid differentiation factor 88
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fadok VA, Bratton DL, Henson PM. Phagocyte receptors for apoptotic cells: recognition, uptake, and consequences. J Clin Invest. 2001;108:957–962. doi: 10.1172/JCI14122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fadok VA, McDonald PP, Bratton DL, Henson PM. Regulation of macrophage cytokine production by phagocytosis of apoptotic and post-apoptotic cells. Biochem Soc Trans. 1998;26:653–656. doi: 10.1042/bst0260653. [DOI] [PubMed] [Google Scholar]
- 3.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 5.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 6.Lu Q, Lemke G. Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro 3 family. Science. 2001:306–311. doi: 10.1126/science.1061663. [DOI] [PubMed] [Google Scholar]
- 7.Mevorach D, Mascarenhas JO, Gershov D, Elkon KB. Complement-dependent clearance of apoptotic cells by human macrophages. J Exp Med. 1998;188:2313–2320. doi: 10.1084/jem.188.12.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor PR, Carugati A, Fadok VA, Cook HT, Andrews M, Carroll MC, Savill JS, Henson PM, Botto M, Walport MJ. A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo. J Exp Med. 2000;192:359–366. doi: 10.1084/jem.192.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verbovetski I, Bychkov H, Trahtemberg U, Shapira I, Hareuveni M, Ben-Tal O, Kutikov I, Gill O, Mevorach D. Opsonization of apoptotic cells by autologous iC3b facilitates clearance by immature dendritic cells, down-regulates DR and CD86, and up-regulates CC chemokine receptor 7. J Exp Med. 2002;196:1553–1561. doi: 10.1084/jem.20020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fadok VA, Bratton DL, Rose DM, Pearson A, Ezekewitz RA, Henson PM. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000;405:85–90. doi: 10.1038/35011084. [DOI] [PubMed] [Google Scholar]
- 11.Fadok VA, Warner ML, Bratton DL, Henson PM. CD36 is required for phagocytosis of apoptotic cells by human macrophages that use either a phosphatidylserine receptor or the vitronectin receptor (alpha v beta 3) J Immunol. 1998;161:6250–6257. [PubMed] [Google Scholar]
- 12.Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, Earp HS, Matsushima GK. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001:207–211. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- 13.Gorgani NN, Smith BA, Kono DH, Theofilopoulos AN. Histidine-rich glycoprotein binds to DNA and Fc gamma RI and potentiates the ingestion of apoptotic cells by macrophages. J Immunol. 2002;169:4745–4751. doi: 10.4049/jimmunol.169.9.4745. [DOI] [PubMed] [Google Scholar]
- 14.Anderson HA, Maylock CA, Williams JA, Paweletz CP, Shu H, Shacter E. Serum-derived protein S binds to phosphatidylserine and stimulates the phagocytosis of apoptotic cells. Nat Immunol. 2003:87–91. doi: 10.1038/ni871. [DOI] [PubMed] [Google Scholar]
- 15.Gershov D, Kim S, Brot N, Elkon KB. C-Reactive protein binds to apoptotic cells, protects the cells from assembly of the terminal complement components, and sustains an antiinflammatory innate immune response: implications for systemic autoimmunity. J Exp Med. 2000:1353–1364. doi: 10.1084/jem.192.9.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Lent PL, R Licht, Dijkman H, Holthuysen AE, Berden JH, van den Berg WB. Uptake of apoptotic leukocytes by synovial lining macrophages inhibits immune complex-mediated arthritis. J Leukoc Biol. 2001:708–714. [PubMed] [Google Scholar]
- 17.Huynh ML, V, Fadok A, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest. 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bittencourt MC, Perruche S, Contassot E, Fresnay S, Baron MH, Angonin R, Aubin F, Herve P, Tiberghien P, Saas P. Intravenous injection of apoptotic leukocytes enhances bone marrow engraftment across major histocompatibility barriers. Blood. 2001:224–230. doi: 10.1182/blood.v98.1.224. [DOI] [PubMed] [Google Scholar]
- 19.Ivashkiv LB, Hu X. The JAK/STAT pathway in rheumatoid arthritis: pathogenic or protective? Arthritis Rheum. 2003:2092–2096. doi: 10.1002/art.11095. [DOI] [PubMed] [Google Scholar]
- 20.Theofilopoulos AN, Koundouris S, Kono DH, Lawson BR. The role of IFN-gamma in systemic lupus erythematosus: a challenge to the Th1/Th2 paradigm in autoimmunity. Arthritis Res. 2001:136–141. doi: 10.1186/ar290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sabroe I, Read RC, Whyte MK, Dockrell DH, Vogel SN, Dower SK. Toll-like receptors in health and disease: complex questions remain. J Immunol. 2003:1630–1635. doi: 10.4049/jimmunol.171.4.1630. [DOI] [PubMed] [Google Scholar]
- 22.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 23.Huang Q, Yang J, Lin Y, Walker C, Cheng J, GLiu Z, Su B. Differential regulation of interleukin 1 receptor and Toll-like receptor signaling by MEKK3. Nat Immunol. 2004:98–103. doi: 10.1038/ni1014. [DOI] [PubMed] [Google Scholar]
- 24.Dumitru CD, Ceci JD, Tsatsanis C, Kontoyiannis D, Stamatakis K, HLin J, Patriotis C, Jenkins NA, Copeland NG, Kollias G, Tsichlis PN. TNF-alpha induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell. 2000:1071–1083. doi: 10.1016/s0092-8674(00)00210-5. [DOI] [PubMed] [Google Scholar]
- 25.Lee IH, Li WP, Hisert KB, Ivashkiv LB. Inhibition of interleukin 2 signaling and signal transducer and activator of transcription (STAT)5 activation during T cell receptor-mediated feedback inhibition of T cell expansion. J Exp Med. 1999:1263–1274. doi: 10.1084/jem.190.9.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen W, Frank ME, Jin W, Wahl SM. TGF-beta released by apoptotic T cells contributes to an immunosuppressive milieu. Immunity. 2001:715–725. doi: 10.1016/s1074-7613(01)00147-9. [DOI] [PubMed] [Google Scholar]
- 27.Kawai T, Takeuchi O, Fujita T, Inoue J, Muhlradt PF, Sato S, Hoshino K, Akira S. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J Immunol. 2001;167:5887–5894. doi: 10.4049/jimmunol.167.10.5887. [DOI] [PubMed] [Google Scholar]
- 28.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 29.Hoshino K, Kaisho T, Iwabe T, Takeuchi O, Akira S. Differential involvement of IFN-beta in Toll-like receptor-stimulated dendritic cell activation. Int Immunol. 2002;14:1225–1231. doi: 10.1093/intimm/dxf089. [DOI] [PubMed] [Google Scholar]
- 30.Savill J, Fadok V. Corpse clearance defines the meaning of cell death. Nature. 2000:784–788. doi: 10.1038/35037722. [DOI] [PubMed] [Google Scholar]
- 31.Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol. 2002:3195–3204. doi: 10.4049/jimmunol.168.7.3195. [DOI] [PubMed] [Google Scholar]
- 32.Yasukawa H, Sasaki A, Yoshimura A. Negative regulation of cytokine signaling pathways. Annu Rev Immunol. 2000:143–164. doi: 10.1146/annurev.immunol.18.1.143. [DOI] [PubMed] [Google Scholar]