Summary
Purpose
Academic underachievement is common in pediatric epilepsy. Attempts to identify seizure and psychosocial risk factors for underachievement have yielded inconsistent findings, raising the possibility that seizure and psychosocial variables play a complex role in combination with other variables such as neuropsychological functioning. This study cross-validated a neuropsychological measurement model for childhood epilepsy, examined the relation between neuropsychological functioning and academic achievement, and tested the degree to which demographic, seizure, and psychosocial variables moderate that relation.
Methods
Children with chronic epilepsy (N = 173; ages 8 to 15 years; 49% girls; 91% white/non-Hispanic; 79% one seizure type; 79% taking one medication; 69% with active seizures) completed a comprehensive neuropsychological battery. Children diagnosed with mental retardation were excluded.
Results
Structural equation modeling identified a three-factor measurement model of neuropsychological function: Verbal/Memory/Executive (VME), Rapid Naming/Working Memory (RN/WM), and Psychomotor (PM). VME and RN/WM were strongly related to reading, math, and writing; PM predicted writing only. Family environment moderated the impact of neuropsychological deficits on writing (p ≤ 0.01) and possibly for reading (p = 0.05); neuropsychological deficits had a smaller impact on achievement for children in supportive/organized homes compared with children in unsupportive/disorganized homes.
Conclusions
These findings lend partial support for our theoretical model showing direct effects of neuropsychological function on achievement and the moderating role of family factors. This study suggests that a subgroup of children with epilepsy (those who have not only neuropsychological deficits but also disorganized/unsupportive home environments) are particularly at risk for adverse academic outcomes. Implications for intervention are discussed.
Keywords: Epilepsy, Children, Family, Cognition, Academic achievement
Children with epilepsy are at great risk for academic difficulties (1–7) and for underemployment as adults (6,8). Fastenau et al. (9) described a model to explain how various risk factors might contribute to academic problems. In that model, neuropsychological functioning appears to play an integral role, possibly mediating the effects of structural and electrophysiologic abnormalities (10–14). Other risk factors in the model include seizure, demographic, and psychosocial variables (9).
Studies to date have not been consistent in identifying seizure variables (e.g., seizure type, age at onset, seizure severity) that might influence the relation between neuropsychological functioning and academic achievement in children with epilepsy. For example, some studies have shown academic underachievement to be associated with absence epilepsy more so than with juvenile rheumatoid arthritis (15), and children with generalized seizures demonstrated worse outcomes than did those with partial seizures (5,16,17). Still, others found no association between seizure type and academic achievement (4,18,19), and no studies of academic achievement examined the interaction between seizure type and neuropsychological functioning.
An early age at onset for seizures was associated with worse cognitive function in many studies (e.g., 5,20–22); yet others failed to find a relation (18,23). Persistent seizures and greater seizure severity also have been associated with greater academic underachievement in children (1,15), but others have failed to find a relation between seizure severity and achievement (e.g., 4). The lack of consistency in this literature suggests a need for further evaluation of seizure variables in academic functioning, particularly as they might interact with other variables (i.e., moderate other relations).
In addition to seizure variables, demographic and psychosocial variables might serve as moderators in the relation between neuropsychological functioning and academic achievement. Past studies suggest that gender, family environment, and children’s perceptions influence academic achievement in children with epilepsy. With regard to gender, a study of children with epilepsy found males to be more at risk for academic underachievement than females (1). In contrast, Howe et al. (24) found male adolescents, including those with neurologic conditions, had higher achievement scores than did adolescent females. In addition, low stimulation and support in the family environment were related to poorer academic achievement in children with seizures (4). Negative child attitudes and perceptions have been associated with poorer academic performance in children with epilepsy (1). Negative attributions have been observed in epilepsy for both adults (25) and teens (26), and such attributions have been associated with poor academic achievement in school-age children (27). Despite the support for the roles of these psychosocial factors on classroom success, no studies have examined the extent to which psychosocial variables might interact with neuropsychological functioning to influence academic achievement in this population.
Past studies focusing on seizure and psychosocial predictors of achievement in childhood epilepsy have been limited in several ways. They tended to rely on group tests of achievement or parent report or both, rather than using well-validated individual achievement tests (e.g., 1). In addition, neuropsychological functioning in many of those studies was assessed with limited measures, or a large battery of tests was used with no attempt to reduce the data into fewer and more relevant constructs (e.g., 28). We identified only one study of neuropsychology and academic achievement with this population that included a factor analysis to reduce the test battery into fewer, more salient neuropsychological constructs (29). Most important, no study to date has modeled the complex moderating roles of demographic, seizure, and psychosocial variables on the relation between neuropsychological functioning and academic achievement.
The present study builds on past studies by (1) recruiting a large sample of school-age children with diverse seizure types, (2) using a more comprehensive battery of neuropsychological and achievement tests, (3) exploring the factor structure of that battery, (4) modeling the relative contributions of each of these cognitive deficits to specific outcomes in each major academic domain, and (5) testing whether demographic, seizure, and psychosocial variables serve as moderators of the relation between neuropsychological functioning and academic achievement.
METHODS
Sample
Participants were recruited from outpatient pediatric neurology clinics, private pediatric neurology practices, and school nurses in Indiana and neighboring areas. Letters, brochures, and flyers were sent to nurses at all schools on a mailing list that was provided by the State of Indiana. In addition, all child neurology clinics in the Greater Indianapolis area were contacted and were provided letters, brochures, and flyers describing the study. The sample size was determined based on power analyses conducted before initiation of the study. Of the 173 children who participated, 165 completed neuropsychological and achievement testing. Seven of the eight who did not complete neuropsychological testing did complete extensive interviews for the study by phone (or mail, in one case), which suggests that travel limited their involvement in the neuropsychological testing (which required coming to the medical center); the eighth family withdrew from the study shortly after enrollment and did not give a reason. Participants who did not complete neuropsychological testing were not used in any analyses in the article. Age ranged from 8 to 15 years. Each child entered the study with a diagnosis of epilepsy; all children were taking antiepileptic medications at the time of enrollment. Children were excluded if they had another chronic physical condition, or had been diagnosed with mental retardation, or had been classified by the schools as mentally handicapped (based on parent report of the school’s evaluation) before enrollment into the study. Demographic and clinical characteristics of the sample are presented Table 1. EEGs showed no spike–wave activity for 40% of our participants, consistent with literature documenting a false-negative rate as high as 70% after a single routine EEG (30).
TABLE 1.
Demographic and clinical characteristics of the sample
| Mean | SD | Median | Range | |
|---|---|---|---|---|
| Age (yr)a | 11.8 | 1.8 | 11.4 | 8.8–15.0a |
| Age at onset (yr) | 6.5 | 3.8 | 6.9 | 0.0–13.9 |
| Duration of disorder (yr) | 5.2 | 3.9 | 4.6 | 0.3–14.4 |
| IQ (estimated from K-BIT)b | 93.3 | 14.9 | 96.0 | 56–130 |
| Caregiver’s education (yr completed) | 13.5 | 2.3 | 13.0 | 8–20 |
| Percentage of sample | ||||
| Gender (% female) | 49.1 | |||
| Handedness (% left-handed) | 15.8 | |||
| Race | ||||
| White/Non-Hispanic | 91.3 | |||
| African-American | 5.8 | |||
| Other or multiracial | 2.9 | |||
| Number of seizure types per child | ||||
| 1 | 78.7 | |||
| 2 | 19.5 | |||
| Missing | 1.8 | |||
| Primary seizure typec | ||||
| Generalized tonic–clonic (GTC) | 20.2 | |||
| Atonic, akinetic, myoclonic (AAM) | 1.2 | |||
| Complex partial seizures (CPSs) | 33.9 | |||
| CPSs with secondary generalization | 16.7 | |||
| Simple partial | 7.1 | |||
| Simple partial with secondary generalization | 3.0 | |||
| Absence | 17.9 | |||
| Unknown/Unclassified | 0.6 | |||
| Etiology of seizures | ||||
| Idiopathic/Cryptogenic | 69.9 | |||
| Familial | 15.4 | |||
| Symptomatic | 14.7 | |||
| Seizure status (% of group) | ||||
| Active | 69.0 | |||
| Controlled | 31.0 | |||
| Number of current antiepileptic drugs (% of group) | ||||
| 0 | 4.6 | |||
| 1 | 78.6 | |||
| 2 | 14.5 | |||
| 3 | 2.3 | |||
N = 173.
Two children enrolled 2 months before their ninth birthday, and one child turned 15 years old between enrollment and neuropsychological testing.
Some children scored in the range of mild mental retardation (MR) on a brief IQ screening; however, these children were not diagnosed with MR or classified as having MR by the schools.
For analysis as a moderator variable, seizure type was reclassified into four groups: (a) absence; (b) GTC/AAM; (c) simple partial and complex partial seizures; and (d) simple partial with generalization and complex partial with generalization.
Procedure
An institutional review board approved the study; legal guardians of all participants signed informed consent statements before participation, and the children gave informed assent. Parents completed an extensive structured interview by phone with a carefully trained nurse or clinical research assistant; demographic data and seizure history were obtained as part of that interview. The children completed a structured interview by phone and completed a comprehensive neuropsychological evaluation at the medical center. In addition, a board-certified child neurologist (D.W.D.) reviewed EEGs, neuroimaging reports [magnetic resonance imaging (MRI) or computed tomography (CT) or both], and clinic notes to classify each child’s seizure types. Neuropsychological testing was conducted individually by trained psychometrists.
Instruments
Independent and dependent variables
The independent variables (neuropsychological test scores) and dependent variables (academic achievement test scores) are listed in Table 2 in the order of administration. Table 3 identifies the specific variables analyzed, organized by the neuropsychological domain. All instruments were administered according to the standardized procedures for each test; scores were converted to age-corrected standardized scores by using the best available national norms for all tests except Wide Range Assessment of Memory and Learning (WRAML) Design Copy, for which no norms were available.
TABLE 2.
Neuropsychological and academic achievement tests battery (in order of administration)
| Dependent variables |
| WJR Broad Reading Index (Letter-Word Identification and Passage Comprehension subtests) |
| WJR Broad Math Index (Calculation and Applied Problems subtests) |
| WJR Broad Written Language Index |
| (Dictation and Writing Samples subtests) |
| Independent variables |
| WJR Picture Vocabulary |
| Token Test for Children (TTC;61) |
| Stroop Color-Word Test (62) |
| Children’s Category Test (CCT;63) |
| Kaufman Brief Intelligence Test (K-BIT;64) |
| Wide Range Assessment of Memory and Learning (WRAML;65) |
| Conners’ Continuous Performance Test (CPT;66) |
| Attentional Capacity Test (ACT;67) |
| Trail Making Test (TMT;68) |
| Grooved Pegboard (69) |
WJR, Woodcock Johnson Psychoeducational Battery–Revised (70).
TABLE 3.
Test scores, by primary functional domain
| Domain Test variable | Observed |
Normative samplea |
||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Dependent variables: Academic achievement tests | ||||
| Reading | ||||
| WJR Letter-Word Identification | 92.6 | 17.1 | 100 | 15 |
| WJR Passage Comprehension | 94.8 | 17.3 | 100 | 15 |
| Math | ||||
| WJR Calculations | 89.6 | 20.3 | 100 | 15 |
| WJR Applied Problems | 98.7 | 16.3 | 100 | 15 |
| Writing | ||||
| WJR Dictation | 84.5 | 13.6 | 100 | 15 |
| WJR Writing Samples | 89.2 | 19.3 | 100 | 15 |
| Independent variables: Neuropsychological measures | ||||
| Processing speed | ||||
| CPT Hit Response Time (T score) | 37.9 | 14.2 | 50 | 10 |
| Attention | ||||
| CPT% Omissions (T score)b | 63.9b | 9.1 | 50 | 10 |
| CPT Hit RT SE (T score)b | 72.9b | 17.1 | 50 | 10 |
| Psychomotor and visual-spatial skills | ||||
| WRAML Design Copy (% correct)a | 94.1 | 10.2 | N/Aa | N/Aa |
| Trail Making Test, Part A (Z score)b | 0.3b | 1.5 | 0 | 1 |
| Grooved Pegboard, Dominant Hand (Z)b | 1.6b | 2.7 | 0 | 1 |
| Grooved Pegboard, Nondominant Hand (Z)b | 3.3b | 5.2 | 0 | 1 |
| Language | ||||
| Stroop Word Naming (T score) | 39.6 | 9.0 | 50 | 10 |
| Stroop Color Naming (T score) | 39.5 | 9.0 | 50 | 10 |
| Token Test for Children (“standard score”) | 495.9 | 8.6 | 500 | 5 |
| WJR Picture Vocabulary (standard score) | 91.3 | 15.1 | 100 | 15 |
| Attentional Capacity Test (Z score) | −1.3 | 1.4 | 0 | 1 |
| Memory and learning | ||||
| WRAML Design Memory (scaled score) | 7.4 | 3.2 | 10 | 3 |
| WRAML Story Memory (scaled score) | 8.0 | 3.7 | 10 | 3 |
| WRAML Verbal Learning (scaled score) | 9.6 | 3.6 | 10 | 3 |
| Executive functioning | ||||
| Children’s Category Test (T score) | 45.7 | 9.8 | 50 | 10 |
| Trail Making Test, Part B (Z score)b | 0.4b | 1.9 | 0 | 1 |
| Moderating variablesc | ||||
| Demographic variables (age, gender, caregiver’s education)c | ||||
| Seizure variables (seizure type, seizure status, duration of disorder, age at onset)c | ||||
| Psychosocial variables | ||||
| CATIS (mean item score)a | 3.3 | 0.7 | (range, 1–5) | |
| PH CSCS Happiness-Satisfactiona | 8.6 | 1.6 | (range, 0–10) | |
| CASQ Negative Compositea | 7.3 | 3.0 | (range, 0–24) | |
| FIRM Family Masterya | 2.0 | 0.5 | (range, 0–3) | |
WJR, Woodcock Johnson Psychoeducational Battery–Revised; CPT, Conners’ Continuous Performance Test; RT SE, Response Time Standard Error; CATIS, Child Attitude Toward Illness Scale; PH CSCS, Piers-Harris Children’s Self-Concept Scale; CASQ, Children’s Attributional Style Questionnaire; FIRM, Family Inventory of Resources for Management.
Normative data were not available for WRAML Copy, CATIS, PH CSCS, CASQ, or FIRM.
For CPT% Omissions, CPT Hit RT SE, Trail Making Test Parts A & B, and Grooved Pegboard, scores above the mean indicate worse performance.
Other moderator variables (Demographic and Seizure Variables) are reported in Table 1.
Moderating variables
Three groups of moderating variables were tested in this study: demographic, seizure, and psychosocial (child/family) variables. Demographic characteristics were age, gender, and the primary caregiver’s years of formal education.
Seizure variables consisted of seizure status, seizure type, duration of disorder, and age at onset. Seizure status was defined dichotomously depending on whether the child had had at least one seizure in the previous 12 months (active) or no seizures in the past 12 months (controlled). Seizure types (see Table 1) were reclassified into four groups: (a) absence; (b) generalized tonic–clonic (GTC) and atonic, akinetic, myoclonic (AAM); (c) simple partial and complex partial seizures (CPSs); and (d) simple partial with generalization and CPSs with generalization. Psychometric scales were used to measure psychosocial variables; these are described in the next section.
Psychosocial variables consisted of child attitude toward epilepsy, child self-concept, child attributional style, and family mastery. The Child Attitude Toward Illness Scale (CATIS, 31) measured the child’s attitude toward having epilepsy. This 13-item scale has shown good reliability and validity (31,32), and Cronbach’s alpha was good (α =0.78) in the present study; possible mean scores range from 1 to 5. For self-concept, the Happiness-Satisfaction subscale of the Piers-Harris Children’s Self-Concept Scale (33) was used. This 10-item subscale (possible scores range from 0 to 10) has shown good reliability and validity, including in children with epilepsy (33,34), and reliability was adequate in the present study (α = 0.63).
For attributional style, the Negative Composite scale of the Children’s Attributional Style Questionnaire (CASQ, 35) was used. The Negative Composite scale consists of 24 items (possible scores range from 0 to 24); higher scores indicate a stronger tendency to explain bad events in terms of internal, stable, and global causes. The CASQ has been found to have satisfactory validity with children who had a chronic condition and were similar in age (36), but the reliability of the Negative Composite score was weak in the present study (α = 0.44).
Family environment was measured by the primary care-givers’ ratings on the Family Inventory of Resources for Management (FIRM, 37). The present study used an abbreviated Family Mastery subscale consisting of the 18 items with the highest factor loadings in earlier studies. Possible mean scores range from 0 to 3; a higher score reflects a more supportive, organized family environment with few disruptions in daily routines and interactions. The 18 items measure family planning and problem solving, decision making, cooperation among family members, distribution of responsibilities for household tasks, organization, ability to complete important tasks, emotional support, and stress in the family. The subscale has shown good reliability and validity (37); reliability was very good in the present study (α = 0.88).
Statistical analysis
Data analyses followed a two-step procedure. In step 1, exploratory factor analysis (EFA) was performed to identify a factor structure model (measurement model, Fig. 1) for the neuropsychological variables. This was followed by confirmatory factor analysis (CFA) using LISREL 8 (38) to examine further the fit of the model and to identify potential modifications to the model.
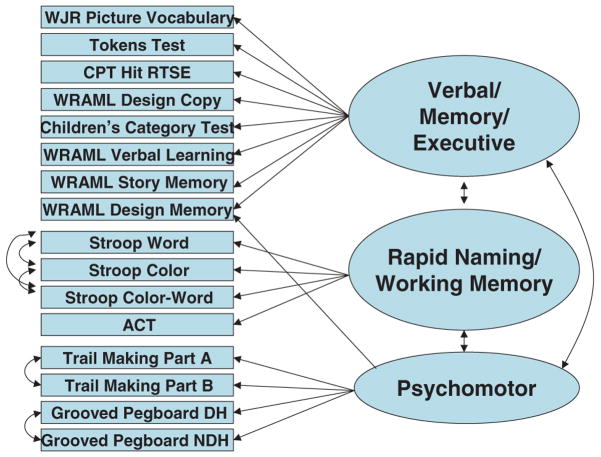
FIG. 1.
Measurement model. WJR, Woodcock-Johnson Psychoeducational Test Battery–Revised; WRAML, Wide Range Assessment of Memory and Learning; CPT, Conners’ Continuous Performance Test; DH, dominant hand; NDH, nondominant hand; ACT, Attentional Capacity Test.
In step 2, structural equation modeling (SEM) with LISREL 8 was used to test the direct links between neuropsychological function and academic achievement in the structural model (Fig. 2). Statistically, it would be ideal to test the moderator effects by incorporating moderators into the SEM models; unfortunately, our sample size was too small to fit the SEM models with stable estimates. Therefore conventional regression models were used to test the interactions between the neuropsychological factors and moderators. We first created a composite score for each neuropsychological factor based on the item factor loadings resulting from the CFA model. Because of the sample-size concerns, we tested the significance of each moderator variable in separate models instead of putting all moderators into a single model (after controlling for main effects of the three neuropsychological factors). SAS Version 8.2 software (39) was used for the conventional regression analyses. All models were fit by using the method of maximum likelihood.
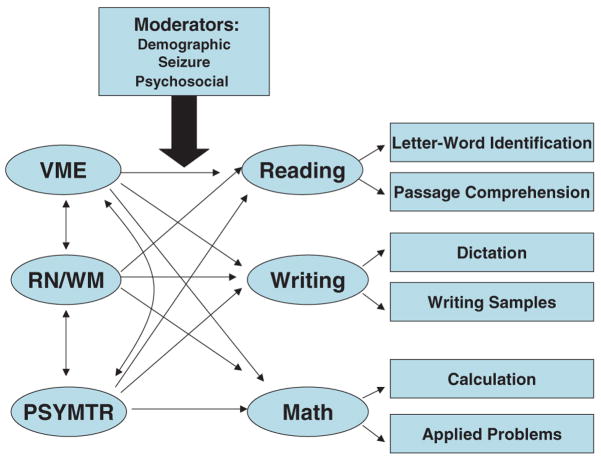
FIG. 2.
Structural model. VME, Verbal, Memory, Executive/Attention; RN/WM, Rapid Naming and Working Memory; PSYMTR, Psychomotor.
For examining direct effects, α was set to p ≤ 0.05, with p ≤ 0.10 considered a trend. Because of the large number of significance tests required to test the models examining moderation, α was set more conservatively (p ≤ 0.01), and findings at p ≤ 0.05 were considered trends and were interpreted with caution in those models.
RESULTS
The standardized scores for the sample are presented in Table 3. Normative values for each test also are provided for reference.
Step 1: Measurement model
Exploratory factor analysis
The initial factor structures among 16 neuropsychological variables were explored by using EFA. The EFA results showed that three latent factors might be underlying the neuropsychological variables. About 56% of the total variance was explained by the three factors. The number of factors was determined based on the scree plot and interpretability. All three factors had eigenvalues >1.0, at least four variables per factor, and reasonable clinical interpretations. Factor 1, “Verbal/Memory/Executive” (VME), consisted of measures of verbal ability, memory, and executive/attention skills. Factor 2, “Rapid Naming/Working Memory” (RN/WM), consisted of measures of rapid naming and auditory–verbal working memory. Factor 3, Psychomotor (PM), consisted of psychomotor tasks. No cross-loading was found except for WRAML Design Memory, which is theoretically related to both VME and PM; therefore these cross-loadings were specified in the model.
Modification through confirmatory factor analysis
CFA was used to examine further the initial EFA factor structure. Through examination of the modification indices and residual matrix, we found that the independent correlation assumptions might have been violated for the three Stroop variables (Word, Color, and Color-Word Trials), the two manual skill variables (Grooved Pegboard Dominant Hand and Non-Dominant Hand Trials), and the two Trail-Making Test variables (TMT-A and TMT-B). Therefore we revised the measurement model by adding correlation parameters among those variables to obtain a better model fit. Efforts to isolate the “executive” variance in the Stroop Color-Word Trial (e.g., a difference score comparing the Color-Word Trial to the Color Trial) and in Trail-Making Part B (e.g., a difference score comparing the TMT-B with TMT-A) did not change the measurement model (i.e., even the Stroop and TMT difference scores loaded on the RN/WM and PM factors, respectively).
Standard goodness-of-fit statistical criteria (38) showed that the revised measurement model had a good fit. Root mean square error of approximation (RMSEA) was 0.06 (90% CI, 0.04–0.08). Expected cross-validation index (ECVI) was 1.54, with 90% CI from 1.35 to 1.79. The standardized root mean square residual (RMR) was 0.056. The non-normed fit index (NNFI) and comparative fit index (CFI) both displayed values >0.9. All those criteria indicated the model had an acceptable fit. Therefore we accepted the revised model as a “final” measurement model to fit the entire SEM model further.
Raw and standardized factor loadings for the neuropsychological variables together with standard errors and squared multiple correlations (SMCs) are presented in Table 4. We used the ratio of the unstandardized loading to its corresponding standard error to assess its significance. A value of 1.96 or higher (and −1.96 or lower) for the ratio indicates two-sided significance at the level of 0.05. Table 4 shows that all loadings except the loading of WRAML Design Memory to VME are significant. The loading of WRAML Design Memory to VME was retained because it fits equally well with the other memory and learning subtests on VME. Squared multiple correlation (SMC) is a measure of strength of a linear relation between an observed variable and its underlying factor. In practice, it gives a measure of how much variance of the variable is explained by the underlying factor. In Table 4, it can be seen that the values of SMC range from 0.22 to 0.73, indicating the model has an acceptable fit, given that 56% of the total variance is explained by the three factors.
TABLE 4.
Factor loadings for neuropsychological variables (standard errors) and squared multiple correlations (SMCs)
| Unstandardized loadings | Standardized loadings | SMC | |||||
|---|---|---|---|---|---|---|---|
| VME | RN/WM | PM | VME | RN/WM | PM | ||
| WRAML Story Memory | 1.00a | 0.64 | 0.41 | ||||
| WJR Picture Vocabulary | 3.75 (0.63) | 0.57 | 0.33 | ||||
| Tokens Test | 2.31 (0.34) | 0.67 | 0.45 | ||||
| WRAML Verbal Learning | 1.17 (0.16) | 0.78 | 0.61 | ||||
| WRAML Design Copy | 1.73 (0.35) | 0.46 | 0.22 | ||||
| Children’s Category Test | 1.88 (0.38) | 0.46 | 0.22 | ||||
| CPT Hit RTSE | −3.85 (0.70) | −0.52 | 0.27 | ||||
| WRAML Design Memoryb | 0.17 (0.26) | −1.47(0.65) | 0.13 | −0.51 | 0.37 | ||
| Stroop Word Trial | 1.00a | 0.80 | 0.64 | ||||
| Stroop Color Trial | 0.84 (0.08) | 0.67 | 0.44 | ||||
| Stroop Color-Word Trial | 0.69 (0.10) | 0.55 | 0.31 | ||||
| Attentional Capacity Test | 0.17 (0.02) | 0.85 | 0.73 | ||||
| Trail Making Part A (Time) | 1.00a | 0.73 | 0.53 | ||||
| Trail Making Part B (Time) | 1.05 (0.13) | 0.58 | 0.34 | ||||
| Grooved Pegboard, DH (Time) | 1.32 (0.23) | 0.58 | 0.33 | ||||
| Grooved Pegboard, NDH (Time) | 2.55 (0.48) | 0.52 | 0.27 | ||||
WJR, Woodcock-Johnson Psychoeducational Test Battery Revised; WRAML, Wide Range Assessment of Memory and Learning; CPT, Conners’ Continuous Performance Test; DH, dominant hand; NDH, nondominant hand, VME, Verbal Memory, Executive/Attention; RN/WM, Rapid Naming and Working Memory; PM, Psychomotor.
By convention, we fix the first loading as 1 for each factor.
Indicates the variable with cross-loading.
In summary, we identified three factors underlying neuropsychological performance in this sample (Fig. 1). The factors were Verbal/Memory/Executive (VME), Rapid Naming/Working Memory (RN/WM), and Psychomotor (PM). These factors were used in subsequent analyses testing the broader theoretical model.
Step 2: Structural model
The theoretical models for the causal relations between neuropsychological factors and academic achievement are presented in Figure 2. It was hypothesized that all three neuropsychological factors would predict academic achievement, with each factor predicting each area of achievement to different degrees. We fit separate SEM models for each domain of academic achievement; that is, we fit one model for reading, another model for math, and a third model for writing. It was further hypothesized that demographic, seizure, and psychosocial variables would moderate the prediction process in the structural model (Fig. 2).
To determine whether our theoretical models were supported, we examined goodness-of-fit indices for the SEM models (Table 5). The table shows that all models have a GFI of 0.87, CFI >0.95, and RMSEA and RMR values ~0.06. Although goodness-of-fit of the models is rejected based on the χ2 criterion, it is known that the χ2 test statistic is very sensitive to sample size and departures from multivariate normality. In practice, it is recommended that the model χ2 test be used only for model comparisons, with smaller values having better fit. Combined, these results indicated the models have acceptable fit.
TABLE 5.
Goodness-of-fit statistics for reading, math, and writing models
| Model | df | χ2 | p | RMSEA (90% CI) | SRMR | GFI | AGFI | CFI |
|---|---|---|---|---|---|---|---|---|
| Reading | 123 | 211.85 | <0.0001 | 0.066 (0.05–0.08) | 0.058 | 0.87 | 0.82 | 0.97 |
| Math | 123 | 204.10 | <0.0001 | 0.063 (0.05–0.08) | 0.055 | 0.87 | 0.82 | 0.97 |
| Writing | 123 | 223.39 | <0.0001 | 0.066 (0.05–0.08) | 0.058 | 0.87 | 0.82 | 0.96 |
RMSEA, root-mean-square error of approximation; SRMR, standardized root mean residual; GFI, goodness-of-fit index; AGFI, adjusted goodness-of-fit index; CFI, comparative fit index.
Direct effects
Table 6 displays the estimated structural path coefficients for the hypothesized paths from the neuropsychological factors to each domain of academic achievement. VME and RN/WM significantly predicted reading, math, and writing; PM significantly predicted writing skills only. A marginally significant relation was found between PM and reading ability. From the standardized coefficients, we can see the importance of each neuropsychological factor related to academic achievement. VME is the most important neuropsychological factor because it has the highest standardized coefficients for the three models (0.69, 0.57, and 0.86 for reading, math, and writing models, respectively). RN/WM is the next most important factor because its standard coefficients are lower than those for VME but higher than those for PM. PM has the smallest standardized coefficients in each model; this pattern is consistent with what was shown by formal significance tests.
TABLE 6.
Estimated coefficients (standard error), standardized coefficients, and significance tests for neuropsychological factors in reading, math, and writing models
| Neuropsychological factor |
|||
|---|---|---|---|
| Model | VME | RN/WM | PM |
| Reading | |||
| Coefficient (SE) | 4.13 (1.08) | 1.01 (0.24) | 4.50 (2.53) |
| Std coefficient | 0.69 | 0.54 | 0.32 |
| t-value | 3.84a | 4.16a | 1.77b |
| Math | |||
| Coefficient (SE) | 4.00 (1.25) | 0.94 (0.27) | 1.30 (2.49) |
| Std coefficient | 0.57 | 0.42 | 0.08 |
| t-value | 3.21a | 3.45a | 0.52 |
| Writing | |||
| Coefficient (SE) | 3.95 (1.05) | 0.84 (0.23) | 5.44 (2.38) |
| Std coefficient | 0.86 | 0.56 | 0.51 |
| t-value | 3.75a | 3.72a | 2.28a |
VME, Verbal, Memory, Executive/Attention; RN/WM, Rapid Naming and Working Memory; PM, Psychomotor.
p ≤ 0.05
p ± 0.10, two-tailed (trend).
Moderating effects
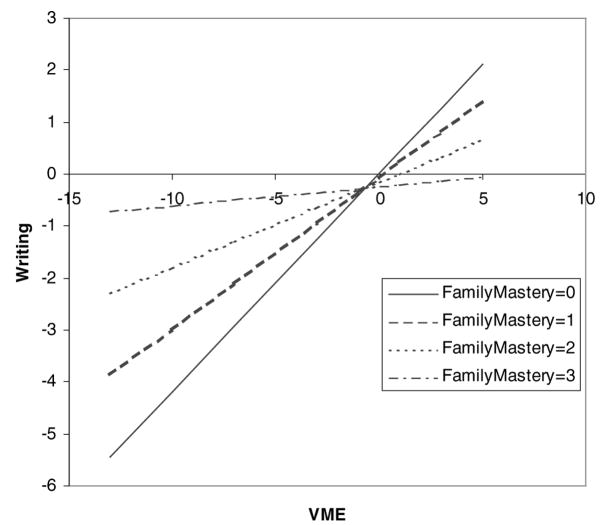
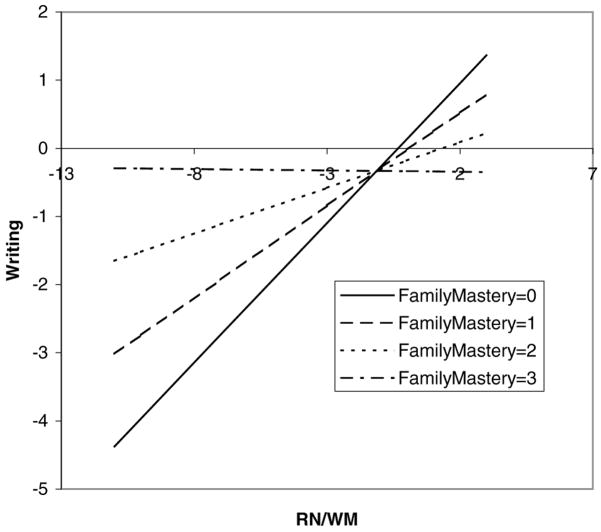
Only Family Mastery (FM) was found to have significant moderating effects. The results are graphically presented in Figures 3 to 5. For writing achievement, each model (VME, RN/WM, and FM main effects and their interactions with FM) explained 56% of the total variance. The FM main effect and FM × VME interaction (p = 0.004) accounted for 3.7% of the total variance (or 6.6% of the explained variance); the FM main effect and FM × RN/WM interaction (p = 0.01) accounted for 2.9% of the total variance (or 5.2% of the explained variance). It can be seen from Figures 3 and 4 that the relation between neuropsychological functioning and writing achievement varied depending on FM level; VME and RN/WM were strongly related to writing achievement in those children with less FM (i.e., with disorganization and little support at home), but neuropsychological deficits had little or no detrimental impact on writing achievement for children with greater FM (i.e., with organization and strong support at home). The pattern was nearly identical for both interactions.
FIG. 3.
The moderating effect of family mastery (FM) on the relation between verbal, memory, executive/attention (VME) functioning and writing. The plot shows how the unit change on FM (continuous; range, 0–3) affects the relation between VME and reading. The regression equation is Writing = 0.017 – 0.085 FM +0.42 VME − 0.128 FM × VME + others.
FIG. 5.
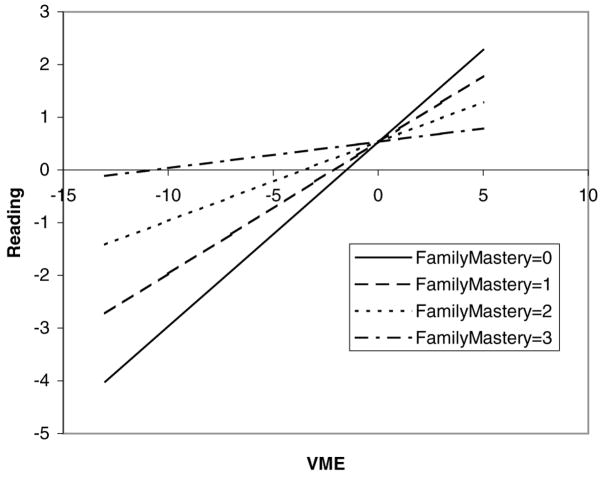
The moderating effect of family mastery (FM) on the relation between verbal, memory, executive/attention (VME) functioning and reading. The plot shows how the unit change in FM (continuous; range, 0–3) affects the relation between VME and reading. The regression equation is Reading = 0.53 + 0.002 FM +0.35 VME − 0.01 FM × VME + others.
FIG. 4.
The moderating effect of family mastery (FM) on the relation between rapid naming and working memory (RN/WM) and writing. The plot shows how the unit change in FM (continuous; range, 0–3) affects the relation between RN/WM and writing. The regression equation is Writing =0.135 – 0.157 FM +0.41 RN/WM −0.138 FM × RN/WM + others.
For reading achievement, a trend was noted for an interaction between FM and VME (p = 0.05). The model (VME, RN/WM, and FM main effects and the FM ×VME interaction) explained 54% of the total variance in reading. The FM main effect and FM ×VME interaction accounted for 2.2% of the total variance (or 4.1% of the explained variance). As can be seen in Figure 5, the pattern of this interaction was nearly identical to the previous two interactions between neuropsychological functioning and FM. Because this trend follows the same pattern of two more-robust findings and because it is theoretically defensible, this trend was considered to be interpretable, albeit subject to replication.
A trend was found for an interaction between seizure type and VME in predicting math ability (p = 0.02). The linear relation between VME and math ability was weakest for the absence seizure type and strongest for GTC and partial seizure types. Because this finding did not fit any other statistically significant pattern in the results and because it was not theoretically interpretable, this finding was more likely obtained by chance.
In summary, we confirmed direct effects between all neuropsychological factors and academic achievement, with different neuropsychological profiles emerging for each of the three areas of achievement. Of the moderating variables tested, FM moderated the relations between neuropsychological functioning (particularly VME and RN/WM) and academic achievement in writing and possibly in reading.
DISCUSSION
Past studies focusing on seizure and psychosocial predictors of achievement in childhood epilepsy have been limited in their measurement of academic achievement and of neuropsychological functioning. More important, previous studies examined only direct effects without attempting to model complex moderating relations. The present study built on past studies by recruiting a large sample of school-age children with diverse seizure types; by using a more comprehensive battery of neuropsychological and achievement tests; and by testing a complex model that incorporates demographic, seizure, and psychosocial variables as moderators of the relation between neuropsychological functioning and academic achievement. We first discuss the reduction of neuropsychological data into composites or factors (measurement model). Then we discuss the moderating relations (structural model) and their implications for intervention.
Measurement model
Our three-factor model compares very favorably with a study by Seidenberg et al. (29). In a very similar sample (147 children with epilepsy, ages 9–14 years) they extracted six factors (in order of strength): Verbal Skills/Verbal Learning; Spatial Skills/Executive; Attention; Visual Memory/Learning; Fine Motor/Construction; and Visual-Motor Speed. Our primary factor (VME) consisted of the same skills represented in their first three factors (verbal skills, verbal memory/learning, spatial skills/executive, attention). Our third factor (PM) was similar to their last three factors (fine motor skills, visual-motor speed, and visual-spatial memory); spatial skills, attention, visual-spatial memory, motor, and visual-motor constructs separated into unique factors in the earlier study most likely because many more measures were in each domain than were in the present study. Consequently, our study appears to cross-validate this measurement model with regard to these neuropsychological constructs.
By contrast, however, we identified an additional factor that was not observed by Seidenberg et al. (29). The RN/WM factor observed here was unique because the earlier study did not include specific measures of rapid naming or auditory-verbal working memory. These constructs have become increasingly salient with regard to academic achievement (particularly for reading) since the time of the study by Seidenberg et al. (e.g., 40–42). Their emergence as a coherent factor (independent of general verbal skills) supports their unique role in the developing cognitive repertoire of the school-aged child.
Structural model
Direct effects
VME and achievement
VME was strongly related to all three academic outcomes. Inasmuch as this factor represents broader cognitive abilities (somewhat akin to global intelligence), this is not surprising. Verbal skills have been shown to be strongly predictive of general academic achievement in children with epilepsy (29,43) and to vocational outcomes in high school students with epilepsy (8). Seidenberg et al. (29) showed that in addition to verbal abilities, attention strongly discriminated between successful and unsuccessful achievers.
RN/WM and achievement
RN/WM was strongly related to all three academic outcomes. Rapid naming and working memory play a prominent role in vocabulary development and reading (40–42,44). It is reasonable to expect that these skills would also play a role in writing (e.g., drawing on the same internal lexicon). A longitudinal study supported the relation between reading and writing in the age range of the current sample (45), and some direct empirical support exists for the shared role of phonologic processes and working memory in particular (46,47). The role of RN/WM has been appreciated in math as well (e.g., 48,49).
PM and writing
Psychomotor skills were strongly related to writing achievement. It is important to note in this regard that our writing achievement measures were not scored on the basis of handwriting or neatness. Instead, those measures reflect spelling, punctuation, grammar, and syntactical expression. The relation between visual-motor skills and writing development is well established, and a recent longitudinal study demonstrates their critical role in later writing mechanics and not just handwriting (45).
Moderating effects
Academic achievement was less affected by neuropsychological deficits when children’s homes were more organized and supportive. However, in homes that were disorganized, disruptive, and unsupportive, neuropsychological deficiencies were related to much lower achievement.
One other study showed that the family environment is important for children with epilepsy. Mitchell et al. (4) assessed socioeconomic factors, home environment, and seizure/treatment variables in a sample of 78 children with epilepsy (ages 5–13 years). After adjusting achievement scores for IQ, family environment (e.g., emotional climate, stimulation, parental involvement) accounted for a significant amount of variance in reading comprehension, general knowledge, and basic reading (word-identification/word-attack skills). Family environment did not account for any unique variance in math computations or spelling; math reasoning and writing composition were not assessed in that study. Seizure variables were unrelated to IQ-adjusted achievement in most domains, with the exception of a very subtle effect of duration of disorder with computational math.
Similar findings have been demonstrated in pediatric traumatic brain injury (TBI). Taylor et al. (50) found recovery of math skills only for children whose families reported less stress, thus demonstrating the protective role of a supportive, stable family environment in academic achievement in a neurologic population. Family factors were not associated with changes in neuropsychological functioning in that sample (51); this suggests that the impact of neuropsychological deficiencies on academic achievement is moderated by the family environment in TBI, which parallels the findings in the present study in children with epilepsy.
Several possible explanations exist for how the family environment exerts its influence. Organization, support, planning, and order at home can help the child with cognitive limitations to complete homework. In addition, involving the parents in the learning process has dramatic effects on achievement, even compared with additional formal reading training (52–54). In addition to educational interventions with the family, psychotherapy with the family can be very beneficial for children with special needs (53) and for individuals with epilepsy, in particular (55). These influences could be expected to benefit any child with special needs, including children with epilepsy.
A disorder-specific benefit for children with epilepsy might exist, inasmuch as a more organized family could possibly promote better sleep habits and medication adherence, both of which can contribute to better seizure control (e.g., 56–58). Studies by Dahl et al. (59,60) document the lasting impact of psychosocial interventions on seizure control, which might be one mechanism by which family environment improves academic achievement in children with cognitive deficits.
None of the other moderating variables was significant. Learned helplessness might not have entered the model because of low reliability. Self-concept was measured by using the Happiness-Satisfaction Subscale rather than the academic self-concept, because the latter would be expected to be affected by achievement. This might have been too global a construct. Even though other moderator variables showed direct relations with academic achievement in past research, as described in the introduction to this article, they do not appear to moderate the impact of neuropsychological deficits on achievement.
Limitations
Some possible limitations exist for this study. First, the modest reliability of the CASQ might have limited its sensitivity. Second, the hypothesized direct and moderating relations were construed theoretically to be causal and occurring over time in the structural model; however, the design of the study was correlational and cross-sectional in nature, precluding conclusions of causation. In addition, conventional regression modeling does not take into account measurement error but rather assumes the composite variables to be accurate measures for the latent ability; thus the moderating relations are preliminary and must be further confirmed by SEM. Finally, the findings regarding the family environment might not be unique to children with epilepsy; a control group in future studies could help to delineate the extent to which those findings and other aspects of the model differ for children with epilepsy compared with healthy children or children with nonneurologic chronic health conditions.
CONCLUSION
The present study suggests that a subgroup of children with epilepsy (those with neuropsychological deficits and disorganized unsupportive home environments) are particularly at risk for adverse academic outcomes. These outcomes might be ameliorated with aggressive family intervention to increase structure, stability, and emotional support in the home. Further research would help to develop and test such interventions.
Acknowledgments
This research was supported by grant PHS R01 NR04536 from the National Institute of Nursing Research to J.K.A. We acknowledge assistance from B. Garg, O. Markand, as well as the Epilepsy and Pediatric Neurology Clinics at Riley Hospital, Indiana University Medical Center, Indianapolis, Indiana. We thank Cheryl P. Shore, J. I. Koop, S. E. Woodrome, P. A. Taylor-Cooke, B. C. LeJeune, J. M. Fairbanks, and N. C. Cunningham for coordinating data collection; the many other staff who assisted in all phases of the study; and the many children and families who gave so generously of their time, energy, and patience to this research endeavor. Finally, we acknowledge IUPUI University Information Technology Services for providing access to the LISREL software.
References
- 1.Austin JK, Huberty TJ, Huster GA, et al. Academic achievement in children with epilepsy. Dev Med Child Neurol. 1998;40:248–55. doi: 10.1111/j.1469-8749.1998.tb15457.x. [DOI] [PubMed] [Google Scholar]
- 2.Farwell JR, Dodrill CB, Batzel LW. Neuropsychological abilities of children with epilepsy. Epilepsia. 1985;26:395–400. doi: 10.1111/j.1528-1157.1985.tb05670.x. [DOI] [PubMed] [Google Scholar]
- 3.Fowler MG, Johnson MP, Atkinson SS. School achievement and absence in children with chronic health conditions. J Pediatr. 1985;106:683–7. doi: 10.1016/s0022-3476(85)80103-7. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell WG, Chavez JM, Lee H, et al. Academic underachievement in children with epilepsy. J Child Neurol. 1991;6:65–72. doi: 10.1177/088307389100600114. [DOI] [PubMed] [Google Scholar]
- 5.Seidenberg M, Beck N, Geisser M, et al. Academic achievement of children with epilepsy. Epilepsia. 1986;27:753–9. doi: 10.1111/j.1528-1157.1986.tb03606.x. [DOI] [PubMed] [Google Scholar]
- 6.Sillanpaa M, Jalava M, Kaleva O, et al. Long-term prognosis of seizures with onset in childhood. N Engl J Med. 1998;338:1715–22. doi: 10.1056/NEJM199806113382402. [DOI] [PubMed] [Google Scholar]
- 7.Westbrook LE, Silver EJ, Coupey SM, et al. Social characteristics of adolescents with idiopathic epilepsy: a comparison to chronically ill and nonchronically ill peers. J Epilepsy. 1991;4:87–94. [Google Scholar]
- 8.Dodrill CB, Clemmons D. Use of neuropsychological tests to identify high school students with epilepsy who later demonstrate inadequate performances in life. J Consult Clin Psychol. 1984;52:520–7. doi: 10.1037//0022-006x.52.4.520. [DOI] [PubMed] [Google Scholar]
- 9.Fastenau PS, Dunn DW, Austin JK. Pediatric epilepsy. In: Rizzo M, Eslinger PJ, editors. Principles and practice of behavioral neurology and neuropsychology. New York: Saunders/Churchill Living-stone/Mosby; 2003. pp. 965–82. [Google Scholar]
- 10.Dodrill CB, Wilkus RJ. Neuropsychological correlates of the electroencephalogram in epileptics, III: generalized nonepileptiform abnormalities. Epilepsia. 1978;19:453–62. doi: 10.1111/j.1528-1157.1978.tb05172.x. [DOI] [PubMed] [Google Scholar]
- 11.Kasteleijn-Nolst Trenité DGA, Bakker DJ, Binnie CD, et al. Psychological effects of subclinical epileptiform discharges: scholastic skills. Epilepsy Res. 1988;2:111–6. doi: 10.1016/0920-1211(88)90027-7. [DOI] [PubMed] [Google Scholar]
- 12.Koop JI, Fastenau PS, Austin JK, et al. Neuropsychological correlates of electroencephalograms in children with epilepsy. J Intl Neuropsychol Soc. 2000;6:227. doi: 10.1016/j.eplepsyres.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Siebelink BM, Bakker DJ, Binnie CD, et al. Psychological effects of subclinical epileptiform discharges: general intelligence tests. Epilepsy Res. 1988;2:117–21. doi: 10.1016/0920-1211(88)90028-9. [DOI] [PubMed] [Google Scholar]
- 14.Wilkus RJ, Dodrill CB. Neuropsychological correlates of the electroencephalogram in epileptics, I: topographic distribution and average rate of epileptiform activity. Epilepsia. 1976;17:89–100. doi: 10.1111/j.1528-1157.1976.tb03387.x. [DOI] [PubMed] [Google Scholar]
- 15.Wirrell EC, Camfield CS, Camfield PR, et al. Long-term psychosocial outcome in typical absence epilepsy: sometimes a wolf in sheeps’ clothing. Arch Pediatr Adolesc Med. 1997;151:152–8. doi: 10.1001/archpedi.1997.02170390042008. [DOI] [PubMed] [Google Scholar]
- 16.Camfield C, Camfield P, Smith B, et al. Biologic factors as predictors of social outcome of epilepsy in intellectually normal children: a population-based study. J Pediatr. 1993;122:869–73. doi: 10.1016/s0022-3476(09)90009-9. [DOI] [PubMed] [Google Scholar]
- 17.Mandelbaum DE, Burack GD. The effect of seizure type and medication on cognitive and behavioral functioning in children with idiopathic epilepsy. Dev Med Child Neurol. 1997;39:731–5. doi: 10.1111/j.1469-8749.1997.tb07374.x. [DOI] [PubMed] [Google Scholar]
- 18.Sturniolo MG, Galletti F. Idiopathic epilepsy and school achievement. Arch Dis Child. 1994;70:424–8. doi: 10.1136/adc.70.5.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams J, Phillips T, Griebel ML, et al. Factors associated with academic achievement in children with controlled epilepsy. Epilepsy Behav. 2001;2:217–23. doi: 10.1006/ebeh.2001.0166. [DOI] [PubMed] [Google Scholar]
- 20.Bulteau C, Jambaque I, Viguier D, et al. Epileptic syndromes, cognitive assessment and school placement: a study of 251 children. Dev Med Child Neurol. 2000;42:319–27. doi: 10.1017/s0012162200000566. [DOI] [PubMed] [Google Scholar]
- 21.O’Leary DS, Seidenberg M, Berent S, et al. Effects of age of onset of tonic-clonic seizures on neuropsychological performance in children. Epilepsia. 1981;22:197–204. doi: 10.1111/j.1528-1157.1981.tb04102.x. [DOI] [PubMed] [Google Scholar]
- 22.Schoenfeld J, Seidenberg M, Woodard A, et al. Neuropsychological and behavioral status of children with partial complex seizures. Dev Med Child Neurol. 1999;41:724–31. doi: 10.1017/s0012162299001486. [DOI] [PubMed] [Google Scholar]
- 23.Bailet LL, Turk WR. The impact of childhood epilepsy on neurocognitive and behavioral performance: a prospective longitudinal study. Epilepsia. 2000;41:426–31. doi: 10.1111/j.1528-1157.2000.tb00184.x. [DOI] [PubMed] [Google Scholar]
- 24.Howe GW, Feinstein C, Reiss D, et al. Adolescent adjustment to chronic physical disorders, 1: comparing neurological and non-neurological conditions. J Child Psychol Psychiatry. 1993;34:1153–71. doi: 10.1111/j.1469-7610.1993.tb01780.x. [DOI] [PubMed] [Google Scholar]
- 25.Hermann BP, Trenerry MR, Colligan RC. Learned helplessness, attributional style, and depression in epilepsy: Bozeman Epilepsy Surgery Consortium. Epilepsia. 1996;37:680–6. doi: 10.1111/j.1528-1157.1996.tb00633.x. [DOI] [PubMed] [Google Scholar]
- 26.Dunn DW, Austin JK, Huster GA. Symptoms of depression in adolescents with epilepsy. J Am Acad Child Adolesc Psychiatry. 1999;38:1132–8. doi: 10.1097/00004583-199909000-00017. [DOI] [PubMed] [Google Scholar]
- 27.Peterson C, Maier SF, Seligman MEP. Learned helplessness: a theory for the age of personal control. New York: Oxford University Press; 1993. [Google Scholar]
- 28.Townes BD, Trupin EW, Martin DC, et al. Neuropsychological correlates of academic success among elementary school children. J Consult Clin Psychol. 1980;48:675–84. doi: 10.1037//0022-006x.48.6.675. [DOI] [PubMed] [Google Scholar]
- 29.Seidenberg M, Beck N, Geisser M, et al. Neuropsychological correlates of academic achievement in children with epilepsy. J Epilepsy. 1987;1:23–30. [Google Scholar]
- 30.Aicardi J. Epilepsy in children. 2. Philadelphia: Lippincott-Raven; 1996. [Google Scholar]
- 31.Austin JK, Huberty TJ. Development of the Child Attitude Toward Illness Scale. J Pediatr Psychol. 1993;18:467–80. doi: 10.1093/jpepsy/18.4.467. [DOI] [PubMed] [Google Scholar]
- 32.Heimlich TE, Westbrook LE, Austin JK, et al. Brief report: adolescents’ attitudes toward epilepsy: further validation of the Child Attitude Toward Illness Scale (CATIS) J Pediatr Psychol. 2000;25:339–45. doi: 10.1093/jpepsy/25.5.339. [DOI] [PubMed] [Google Scholar]
- 33.Piers EV. Piers-Harris Children’s Self-Concept Scale: revised manual. Los Angeles: Western Psychological Services; 1984. [Google Scholar]
- 34.Austin JK, Smith MS, Risinger MW, et al. Childhood epilepsy and asthma: comparison of quality of life. Epilepsia. 1994;35:608–15. doi: 10.1111/j.1528-1157.1994.tb02481.x. [DOI] [PubMed] [Google Scholar]
- 35.Seligman MEP, Kaslow NJ, Alloy LB, et al. Attributional style and depressive symptoms among children. J Abnorm Psychol. 1984;93:235–8. doi: 10.1037//0021-843x.93.2.235. [DOI] [PubMed] [Google Scholar]
- 36.Kuttner MJ, Delamater AM, Santiago JV. Learned helplessness in diabetic youths. J Pediatr Psychol. 1990;15:581–94. doi: 10.1093/jpepsy/15.5.581. [DOI] [PubMed] [Google Scholar]
- 37.McCubbin HI, Thompson AI, McCubbin MA. Family assessment: resiliency, coping and adaptation: inventories for research and practice. Madison, WI: University of Wisconsin Publishers; 1996. p. 38. [Google Scholar]
- Jöreskog KG, Sörbom D. LISREL 8: user’s reference guide. Chicago: Scientific Software International; 1996. [Google Scholar]
- 39.SAS Institute. SAS Propriety Software Version 8.2. Cary, NC: Author; 2001. [Google Scholar]
- 40.Baddeley A, Gathercole S, Papagno C. The phonological loop as a language learning device. Psychol Rev. 1998;105:158–73. doi: 10.1037/0033-295x.105.1.158. [DOI] [PubMed] [Google Scholar]
- 41.Frost R. Toward a strong phonological theory of visual word recognition: true issues and false trials. Psychol Bull. 1998;123:71–99. doi: 10.1037/0033-2909.123.1.71. [DOI] [PubMed] [Google Scholar]
- 42.Wagner RK, Torgesen JK. The nature of phonological processing and its causal role in the acquisition of reading skills. Psychol Bull. 1987;101:192–212. [Google Scholar]
- 43.Townes BD, Trupin EW, Martin DC, et al. Neuropsychological correlates of academic success among elementary school children. J Consult Clin Psychol. 1980;48:675–84. doi: 10.1037//0022-006x.48.6.675. [DOI] [PubMed] [Google Scholar]
- 44.Just MA, Carpenter PA. A capacity theory of comprehension: individual differences in working memory. Psychol Rev. 1992;99:122–49. doi: 10.1037/0033-295x.99.1.122. [DOI] [PubMed] [Google Scholar]
- 45.Maki H, Voeten M, Vauras M, et al. Predicting writing skill development with word recognition and preschool readiness skills. Reading and writing: an interdisciplinary journal. 2001;14:643–72. [Google Scholar]
- 46.Berninger VW, Cartwright AC, Yates CM, et al. Developmental skills related to writing and reading acquisition in the intermediate grades: shared and unique functional systems. Reading Writing. 1994;6:161–96. [Google Scholar]
- 47.Swanson H, Berninger V. Individual differences in children’s working memory and writing skill. J Exp Child Psychol. 1996;63:358–85. doi: 10.1006/jecp.1996.0054. [DOI] [PubMed] [Google Scholar]
- 48.Hecht SA, Torgesen JK, Wagner RK, et al. The relations between phonological processing abilities and emerging individual differences in mathematical computational skills: a longitudinal study from second to fifth grades. J Exp Child Psychol. 2001;79:192–227. doi: 10.1006/jecp.2000.2586. [DOI] [PubMed] [Google Scholar]
- 49.Hitch GJ, McAuley E. Working memory in children with specific arithmetical learning difficulties. Br J Psychol. 1991;82:375–86. doi: 10.1111/j.2044-8295.1991.tb02406.x. [DOI] [PubMed] [Google Scholar]
- 50.Taylor HG, Yeates KO, Wade SL, et al. A prospective study of short- and long-term outcomes after traumatic brain injury in children: behavior and achievement. Neuropsychology. 2002;16:15–27. doi: 10.1037//0894-4105.16.1.15. [DOI] [PubMed] [Google Scholar]
- 51.Yeates KO, Taylor HG, Wade SL, et al. A prospective study of short- and long-term neuropsychological outcomes after pediatric traumatic brain injury. Neuropsychology. 2002;16:514–23. doi: 10.1037//0894-4105.16.4.514. [DOI] [PubMed] [Google Scholar]
- 52.Christenson SL, Buerkle K. Families as educational partners for children’s school success: suggestions for school psychologists. In: CR Reynolds, TB Gutkin., editors. The handbook of school psychology. 3. New York: John Wiley & Sons; 1999. pp. 709–44. [Google Scholar]
- 53.Fish MC. Best practices in working with parents of children with disabilities. In: A Thomas, J Grimes., editors. Best practices in school psychology III. Washington, DC: National Association of School Psychologists; 1995. pp. 1061–70. [Google Scholar]
- 54.Tizard J, Schofield WN, Hewison J. Collaboration between teachers and parents in assisting children’s reading. Br J Educ Psychol. 1982;52:1–15. [Google Scholar]
- 55.Ferrari M, Verbanac A, Kane V. Family systems theory: an approach to therapy for families of patients with epilepsy. In: McConnell HW, Snyder PJ, editors. Psychiatric comorbidity in epilepsy: basic mechanisms, diagnosis, and treatment. Washington, DC: American Psychiatric Press; 1998. pp. 363–81. [Google Scholar]
- 56.Cockerell OC, Johnson AL, Sander JW, et al. Prognosis of epilepsy: a review and further analysis of the first nine years of the British national general practice study of epilepsy, a prospective population-based study. Epilepsia. 1997;38:31–46. doi: 10.1111/j.1528-1157.1997.tb01075.x. [DOI] [PubMed] [Google Scholar]
- 57.Herman ST, Walczak TS. Effects of sleep on seizures. In: CW Bazil, BA Malow, MR Sammaritano., editors. Sleep and epilepsy: the clinical spectrum. Amsterdam: Elsevier; 2002. pp. 165–80. [Google Scholar]
- 58.Sammaritano MR, Malow BA. Effects of sleep and sleep deprivation on interictal epileptiform discharges. In: Bazil CW, Malow BA, Sammaritano MR, editors. Sleep and epilepsy: the clinical spectrum. Amsterdam: Elsevier; 2002. pp. 157–164. [Google Scholar]
- 59.Dahl J, Melin L, Brorson LO, et al. Effects of a broad-spectrum behavior modification treatment program on children with refractory epileptic seizures. Epilepsia. 1985;26:303–9. doi: 10.1111/j.1528-1157.1985.tb05654.x. [DOI] [PubMed] [Google Scholar]
- 60.Dahl J, Brorson LO, Melin L. Effects of a broad-spectrum behavioral medicine treatment program on children with refractory epileptic seizures: an 8-year follow-up. Epilepsia. 1992;33:98–102. doi: 10.1111/j.1528-1157.1992.tb02289.x. [DOI] [PubMed] [Google Scholar]
- 61.DiSimoni F. The Token Test for Children manual. Austin, TX: Pro-Ed; 1978. [Google Scholar]
- 62.Golden CJ. Stroop Color and Word Test. Chicago: Stoelting; 1978. [Google Scholar]
- 63.Boll T. Children’s Category Test. San Antonio, TX: Psychological Corp; 1993. [Google Scholar]
- 64.Kaufman AS, Kaufman NL. K-BIT: Kaufman Brief Intelligence Test. Circle Pines, MN: American Guidance Service; 1983. [Google Scholar]
- 65.Adams W, Sheslow D. Wide Range Assessment of Memory and Learning. Wilmington, DE: Jastak Associates; 1990. [Google Scholar]
- 66.Conners CK. Conners’ Continuous Performance Test , v 3.0 computer program. N. Tonawanda, NY: MHS; 1995. [Google Scholar]
- 67.Weber AM. A new clinical measure of attention: the Attentional Capacity Test. Neuropsychology. 1988;2:59–71. [Google Scholar]
- 68.Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: theory and clinical interpretation. 2. Tuscon, AZ: Neuropsychology Press; 1993. [Google Scholar]
- 69.Knights RM, Moule AD. Normative data on the Motor Steadiness Battery for children. Percept Mot Skills. 1968;26:643–50. doi: 10.2466/pms.1968.26.2.643. [DOI] [PubMed] [Google Scholar]
- 70.Woodcock RW, Johnson MB. Woodcock-Johnson Psycho-Educational Test Battery revised. Allen, TX: DLM Teaching Resources; 1989. [Google Scholar]