Abstract
Body dysmorphic disorder (BDD) is a severe psychiatric condition in which individuals are preoccupied with perceived defects in their appearance. Little is known of the pathophysiology or neurobiology of BDD. Recent evidence from a functional MRI study examining visual processing of faces demonstrated abnormal activation patterns in regions including left-sided inferior frontal gyrus (IFG) and amygdala. To investigate morphometric abnormalities we compared brain volumes from high-resolution T1 magnetic resonance images of twelve unmedicated subjects with BDD to twelve matched controls using voxel-based morphometry (VBM). In addition, we compared volumes in specific regions of interest including the IFG, amygdala, caudate, and total grey and white matter and examined correlations with symptom severity. VBM revealed no statistically significant volumetric differences, nor were there significant differences in any of the regions of interest. However, there were significant positive correlations between scores on the BDD version of the Yale-Brown Obsessive-Compulsive Disorder Scale (BDD-YBOCS) and volumes of the left IFG (r=0.69) and the right amygdala (r=0.54). These findings of correlations between BDD symptom severity and volumes of the left IFG and right amygdala are in concordance with the involvement of these regions in pathological face processing, which may contribute to the primary symptomatology.
Keywords: voxel-based morphometry, imaging, magnetic resonance, morphometric, amygdala, inferior frontal gyrus
1. Introduction
Body dysmorphic disorder (BDD) is a severe psychiatric condition in which patients are preoccupied with perceived defects in their appearance. This causes them to believe they are disfigured and ugly, and causes significant suffering and functional impairment. Most patients with BDD have poor insight, and 36–38% are classified as delusional (Eisen et al., 2004; Phillips et al., 2006). BDD affects 1–2% of the population (Faravelli et al., 1997; Otto et al., 2001; Rief et al., 2006), and is associated with high rates of psychiatric hospitalization (48%) (Phillips and Diaz, 1997) and suicide attempts (22–27.5%) (Veale et al., 1996; Phillips and Diaz, 1997; Phillips et al., 2005).
Despite the prevalence and severity of this disorder, very little is known of the pathophysiology or neurobiology of BDD. Only one previous morphometric magnetic resonance imaging (MRI) study in BDD has been published. In this study eight females, some of whom were medicated, demonstrated greater total white matter compared to controls and a leftward shift in caudate asymmetry (Rauch et al., 2003a), which the authors interpreted as suggesting similar striatal pathophysiology to obsessive-compulsive disorder (OCD) (Saxena et al., 2001). A small functional imaging study of six BDD patients, using single photon emission computed tomography (SPECT), showed variable, discrepant findings including relative perfusion deficits in bilateral anterior-medial temporal and occipital regions and asymmetric perfusion in parietal lobes (Carey et al., 2004). This study, however, had no control or comparison group. We recently performed a functional magnetic resonance imaging (fMRI) study in BDD that examined visual processing of faces (Feusner et al., 2007). Individuals with BDD as compared to healthy controls demonstrated abnormal activation patterns that included greater left hemisphere activity in regions including the inferior frontal gyrus, as well as abnormal amygdala activation (R>L). No other neuroimaging studies of BDD have been published.
Given these previous findings and the paucity of data on the neurobiology of BDD, the objective of this study was to further investigate regional brain volumes in BDD as compared to healthy controls. Based on our fMRI findings (Feusner et al., 2007), we selected the inferior frontal gyrus (IFG) and the amygdala as regions of interest. Based on the previous morphometric MRI study’s findings (Rauch et al., 2003a), we also examined total grey matter (GM), white matter (WM), and the caudate as a region-of-interest, and calculated laterality quotients. We also tested whether brain volumes in the regions of interest were correlated with symptom severity. In addition, we performed voxel-based morphometry for regional whole-brain analysis to detect any other brain volume differences. We hypothesized that in the BDD group there would be abnormal caudate asymmetry and greater total WM. Based on findings from our previous fMRI study in the same cohort of BDD patients, we also hypothesized that they would demonstrate greater volumes of the amygdalae and left IFG, given the previously-found hyperactivity in these regions. In addition, we hypothesized that symptom severity would positively correlate with size of the left IFG and bilateral amygdalae. A better understanding of patterns of brain morphometry in BDD could assist in understanding the pathophysiology underlying the clinical symptoms, as well as how it relates to other disorders with similar features.
2. Methods
2.1. Subjects
The UCLA Institutional Review Board approved the protocol for the study. We obtained informed consent from 12 subjects with BDD and 12 healthy controls, ages 18 to 54 (mean 28.7±10), recruited from the community. The BDD group and controls were matched by gender, age, and level of education, and all were right-handed, as determined by the Edinburgh Handedness Inventory (Oldfield, 1971). All BDD subjects met DSM-IV criteria for body dysmorphic disorder, as determined by the first author (Dr. Feusner), who has clinical expertise with this population. Diagnoses were made using the Body Dysmorphic Disorder Module (Phillips, 1995), a reliable diagnostic module modeled after the Structured Clinical Interview for DSM. In addition, we screened them for comorbid psychiatric disorders with the Mini International Neuropsychiatric Inventory (MINI) (Sheehan et al., 1998). All BDD subjects were required to have a BDD version of the Yale-Brown Obsessive-Compulsive Disorder Scale (BDD-YBOCS) (Phillips et al., 1997) score of ≥ 18. We allowed subjects with delusional beliefs.
Exclusion criteria for subjects and controls included: active substance abuse, current neurological disorder, pregnancy, and any current medical disorder that may affect cerebral metabolism. We excluded subjects with any concurrent Axis I disorder besides dysthymia, major depressive disorder, or generalized anxiety disorder. As depression and anxiety are so frequently comorbid in this population, we believed it would not be a representative sample to exclude these. We included subjects with a 17-item Hamilton Depression Rating Scale (HAM-D) (Hamilton, 1960) score of <20 and subjects whom the investigator (JF) judged were not actively suicidal. In addition to the BDD-YBOCS and the HAM-D, we also administered the Hamilton Anxiety Scale (HAM-A) (Hamilton, 1969) to all subjects. All participants were free from psychoactive medications for at least three weeks prior to entering the study, and free of fluoxetine for at least five weeks. Subjects were not receiving any cognitive-behavioral therapy.
2.2. MRI
We obtained high-resolution T1-weighted three-dimensional magnetic resonance images on a 3-Tesla Allegra (Siemens, Munich, Germany) MRI scanner with 1mm3 voxel size for each subject to provide detailed brain anatomy. Magnetization-prepared rapid gradient echo (MP-RAGE) sequences were used, with the parameters: TE=2.83 ms, TR=2300 ms, TI= 1100 ms, flip angle=9.00, field of view =240 × 256, matrix =240 × 256, slice thickness 1mm, 160 slices interleaved.
2.3. Segmentation Processing and Data Analysis
We used FMRIB’s Automated Segmentation Tool (part of the Oxford Centre for Functional Magnetic Resonance Imaging of the Brain Software Library – FSL) (Ashburner and Friston, 2000), to acquire total GM and WM volumes. Regions of interest volumes in the caudate and amygdala were acquired from hand-traced coronal slices. The IFG and right and left hemisphere total GM and WM were acquired from hand-traced axial slices. For hand-traced regions of interest we followed UCLA Laboratory of Neuro Imaging (LONI) protocols for the volumetric parcellation of cortical and sub-cortical regions of interest http://cms.loni.ucla.edu/ncrr/protocols.aspx?id=1482, blinded to all subject demographics. Interrater reliability was 0.94, which we established between investigators (HM and JF) on a training set of five brains. Volumes for each structure were calculated by multiplying the number of voxels by the voxel size (1×1×1 mm). We normalized values for individual regions of interest (caudate, amygdala, and IFG) to individual total intracranial volumes: raw volume/total intracranial volume *106. Total intracranial volume consisted of total white matter, grey matter, and CSF, excluding the brain stem and cerebellum. Laterality quotients, as an index of regional asymmetry by hemisphere, were calculated as (left − right)/(left+right)(0.5). Additionally, we performed a subanalysis of the females-only, given the previous morphometric study in BDD included only females and given gender differences in regional brain volumes (Good et al., 2001; Mechelli et al., 2005a). We used two-tailed t-tests to compare mean volumes between groups, with a threshold of P < 0.05, uncorrected for multiple comparisons. (Bokde et al., 2005)
2.4. Correlation analyses
In this step we tested the hypothesis that symptom severity is proportional to volumes of the left IFG and the amygdalae, regions found to be hyperactive in the previous fMRI study. We tested correlations between normalized volumes in these regions and scores on the BDD version of the Yale-Brown Obsessive-Compulsive Disorder Scale (BDD-YBOCS) and the Hamilton Rating Scale for Depression (HAM-D) using Pearson product-moment correlations, one-tailed.
2.5. Voxel-Based Morphometry Analyses
Structural data was analyzed with FSL-VBM, a voxel-based morphometry style analysis (Ashburner and Friston, 2000). Structural images were brain-extracted and tissue segmented (Zhang et al., 2001; Smith, 2002; Smith et al., 2004). GM partial volume images were aligned to MNI152 standard space using nonlinear registration. Resulting images were averaged to create a study-specific template, to which the native GM images were then non-linearly re-registered. These images were then modulated (to correct for local expansion or contraction) by dividing by the Jacobian of the warp field. The modulated segmented images were then smoothed with a Gaussian 4mm kernel. Finally, voxel-wise general linear model (GLM) was applied using permutation-based non-parametric testing, forming clusters at Z > 2.3 and testing clusters for significance at P < 0.05, corrected for multiple comparisons across space.
3. Results
3.1. Characteristics of the subject group
Table 1 summarizes the demographic and psychometric data for both groups. The average BDD-YBOCS score was 28.7 ± 7.0. One BDD subject had comorbid major depressive disorder, one had dysthymic disorder, two had generalized anxiety disorder, and two had both major depressive disorder and generalized anxiety disorder. The BDD symptoms were the primary concern in every subject. Typical of this population, all 12 subjects had preoccupations with perceived facial defects.
Table 1.
Demographics and psychometric scoresa
| BDD group (N=12) | Control group (N=12) | P valueb | |
|---|---|---|---|
| Age | 28.7±10.0 | 31.2±11.8 | 0.57 |
| Gender (F/M) | 10/2 | 10/2 | 1 |
| Handedness | 12R | 12R | 1 |
| Years of education | 15.5±2.9 | 15.9±1.4 | 0.66 |
| BDD-YBOCS score | 28.7±7.0 | 0.5±1.0 | <0.001 |
| HAM-D score | 8.6±5.7 | 0.83±1.7 | <0.001 |
| HAM-A score | 12.7±9.8 | 1.6±1.6 | 0.002 |
Abbreviations: BDD: body dysmorphic disorder; BDD-YBOCS: BDD version of the Yale-Brown Obsessive-Compulsive Scale; HAM-D: Hamilton Depression Rating Scale; HAM-A: Hamilton Anxiety Rating Scale
Data are given as mean±SD unless otherwise indicated.
t-test for all comparisons except gender and handedness (X2 Test)
3.2. Volumetric and region of interest analyses
There were no significant differences in total WM or GM between groups as determined by automated segmentation (see Table 2). There were also no significant differences in normalized volumes between groups for the right or left IFG, amygdala, caudate, or for the laterality quotients for any of these regions.
Table 2.
Regional brain volumes and laterality quotients.
| CONTROLS (raw values) |
CONTROLS (normalized values)* |
CONTROLS (raw values) |
BDD (normalized values)* |
t | df | P | ||
|---|---|---|---|---|---|---|---|---|
| Grey Matter | Total GM | 597443.26 ± 58526.71 | 583858.95 ± 45838.09 | −0.63 | 22 | 0.53 | ||
| Total GM (females) | 591192.03 ± 54231.54 | 576970.20 ± 47379.35 | 0.62 | 18 | 0.54 | |||
|
| ||||||||
| White Matter | Total WM | 460415.71 ± 62583.11 | 466145.58 ± 42547.26 | −0.26 | 22 | 0.80 | ||
| Total WM (females) | 450312.66 ± 64057.46 | 458688.79 ± 42376.90 | −0.34 | 18 | 0.73 | |||
|
| ||||||||
| Caudate | Left | 4138.42 ± 390.48 | 3380.10 ± 481.89 | 4054.25 ± 509.47 | 3292.42 ± 362.09 | −0.5 | 22 | 0.31 |
| Left (females) | 4068.20 ± 385.38 | 3381.71 ± 532.62 | 3957.50 ± 493.04 | 3248.33 ± 368.51 | −0.65 | 18 | 0.26 | |
| Right | 4222.17 ± 538.08 | 3450.31 ± 477.23 | 4064.08 ± 530.09 | 3306.17 ± 422.88 | −0.78 | 22 | 0.22 | |
| Right (females) | 4139.60 ± 332.59 | 3443.71 ± 526.78 | 3955.90 ± 449.23 | 3253.79 ± 390.44 | −0.92 | 18 | 0.19 | |
| laterality quotient | −0.0162 ± 0.0326 | −0.00267 ± 0.0682 | 0.83 | 22 | 0.21 | |||
| laterality quotient (females) | −0.0184 ± 0.0341 | −0.00138 ± 0.0686 | 0.7 | 18 | 0.25 | |||
|
| ||||||||
| Amygdala | Left | 1167.67 ± 252.45 | 950.63 ± 215.40 | 1164.92 ± 252.11 | 944.36 ± 179.08 | −0.08 | 22 | 0.47 |
| Left (females) | 1109.00 ± 233.12 | 921.30 ± 225.73 | 1117.50 ± 246.69 | 916.53 ± 182.64 | −0.05 | 18 | 0.48 | |
| Right | 1106.42 ± 197.15 | 901.33 ± 176.11 | 1163.42 ± 156.40 | 946.59 ± 127.04 | 1.02 | 22 | 0.16 | |
| Right (females) | 1062.30 ± 174.93 | 882.61 ± 185.36 | 1150.10 ± 167.46 | 945.50 ± 137.92 | 0.86 | 18 | 0.20 | |
| laterality quotient | −0.0465 ± 0.272 | −0.0116 ± 0.147 | −0.65 | 22 | 0.26 | |||
| laterality quotient (females) | 0.0358 ± 0.298 | −0.0398 ± 0.136 | −0.73 | 18 | 0.24 | |||
|
| ||||||||
| Inferior Frontal Gyrus | Left | 14606.50 ± 2764.65 | 11884.47 ± 2242.52 | 14640.93 ± 2226.31 | 11949.31 ± 2157.70 | 0.07 | 22 | 0.47 |
| Left (females) | 13716.49 ± 1985.57 | 11399.11 ± 2137.59 | 14838.81 ± 2276.86 | 12230.77 ± 2208.04 | 0.86 | 18 | 0.20 | |
| Right | 14081.33 ± 2364.53 | 11395.23 ± 1501.78 | 13647.67 ± 2239.35 | 11099.47 ± 1757.16 | −0.44 | 22 | 0.33 | |
| Right (females) | 13955.79 ± 2347.46 | 11453.53 ± 1341.51 | 13489.51 ± 1943.59 | 11092.42 ± 1621.75 | −0.54 | 18 | 0.30 | |
| laterality quotient | 0.0332 ± 0.250 | 0.0704 ± 0.164 | 0.43 | 22 | 0.34 | |||
| laterality quotient (females) | −0.0135 ± 0.230 | 0.0923 ± 0.165 | 1.18 | 18 | 0.13 | |||
Values normalized to total intracranial volumes: raw volume/total intracranial volume *106.
3.6. Correlation analyses
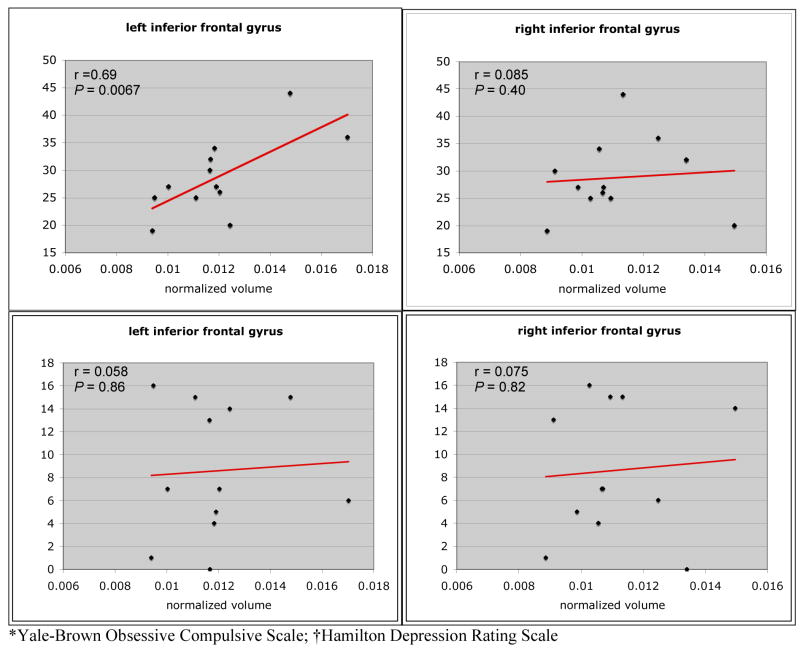
To test the hypotheses that regional brain volumes varied in proportion to severity of symptoms, we performed correlation analyses for the regions of interest. There was a significant correlation between BDD-YBOCS scores and normalized volume of the left IFG (r=0.69, P=0.0067) (Fig. 1). The right IFG volume was not significantly correlated with the BDD-YBOCS (r=0.09, P=0.4). Likewise, there were no significant correlations between the right or left IFG and HAM-D scores (r=0.075, P=0.82; and r=0.058, P=0.86, respectively).
Fig 1.
Correlations between inferior frontal gyrus volumes and BDD-YBOCS* and HAM-D† scores.
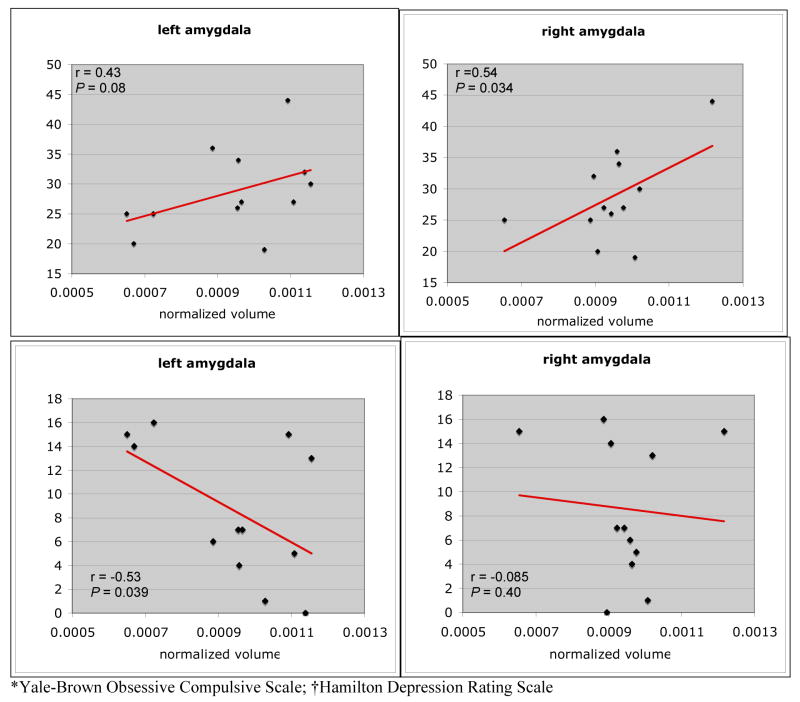
There was a significant positive correlation between normalized volume of the right amygdala and BDD-YBOCS scores (r=0.54, P=0.034) (Fig. 2). There was a trend for a positive correlation between BDD-YBOCS scores and normalized volume of the left amygdala (r=0.43, P=0.08). There was a significant negative correlation between normalized left amygdala volume and HAM-D scores (r=−0.53, P=0.039). The right amygdala was not significantly correlated with HAM-D scores (r=0.08, P=0.4).
Fig 2.
Correlations between amygdala volumes and BDD-YBOCS* and HAM-D† scores.
There were no significant correlations between volume in the right or the left caudate and the BDD-YBOCS (r=−0.13, P=0.69; r=−0.13, P=0.70, respectively) or the HAM-D (r=−0.19, P=0.54; r=−0.27, P=0.40, respectively).
3.3. Subgroup analysis of females
To better compare results of this study with the only previous morphometric MRI study in BDD, we analyzed the females-only subgroups of BDD subjects and controls with respect to the total grey and white matter and regions of interest. There were no significant differences between the female groups for any of the analyses (see Table 2).
3.4. Voxel-based morphometry (VBM) analysis
We performed this step to investigate whole-brain differences in regional brain volumes on a voxel-wise basis. There were no statistically significant differences between patient and control groups. This was true using a range of Gaussian kernels (2.5–4mm), lowering the Z value to 1.7, and investigating at (subthreshold) P<0.1.
3.5. Subgroup analysis of non-depressed subjects
The current study contained 4 subjects with a depressive disorder, while the previous morphometric MRI study in BDD (Rauch et al., 2003a) had only one of eight subjects with comorbid MDD. To further compare to the significant regions in the previous study, we reanalyzed our data after removing the subjects with comorbid MDD or dysthymia (N=4). There were still no significant differences between means for the caudate laterality quotient (−0.0032±0.071 BDD vs. −0.016±0.033 controls, t=0.75, df=18, P=0.46) or for the total WM (474781±43537 mm3 BDD vs. 460416±62583 mm3 controls, t=0.58, df=18, P=0.58).
4. Discussion
The principle finding in this study is that BDD symptom severity correlates significantly with the size of the left IFG and the right amygdala. These were the regions of interest selected based on abnormal hyperactivity from the previous fMRI study in the same cohort (Feusner et al., 2007). As we did not detect significant volumetric differences from the voxel-based morphometry or segmented analyses, the nature of the morphometric findings appears not to be in gross morphometry at the group level, but rather the size of these regions in relationship to symptom severity.
Clinical observation and neuropsychological testing indicate that BDD patients hone in on details of certain appearance features, usually their face, at the expense of global or configural aspects (Deckersbach et al., 2000). BDD patients most often perceive “defects” of their face and head areas (Phillips, 2005). They tend to frequently check their appearance in mirrors, and often scrutinize others’ faces to compare to their own (Phillips, 2005). Greater left-hemisphere activations suggest that BDD subjects may rely more on extraction and processing of details. BDD patients may process faces in a piecemeal manner, while healthy controls’ perception of faces may be more configural and holistic.
In the previous fMRI study of the same BDD cohort we investigated visual processing of faces using high, low, and normal spatial frequency face images to probe abnormalities relative to matched, healthy controls. The BDD cohort demonstrated greater activity than controls in the left hemisphere, including the IFG and lateral temporal and parietal regions. This left-sided activity suggests detailed, analytic processing of all face types in the BDD group, a pattern that was evident in healthy controls only for the high spatial frequency (high-detail) faces. Individuals with BDD may therefore have a bias for local, or detail, processing over global processing of faces. The BDD group also demonstrated hyperactive amygdalae for high and low spatial frequency faces, which was significant on the right and at a trend level on the left.
Thus, taken together with the results of the current study, left IFG and right amygdala in individuals with BDD demonstrate functional hyperactivity for processing faces, and their size correlates with symptom severity. The concordance of structure and function in this cohort supports these regions as being involved in pathological face processing that may be integral to their core symptoms.
The IFG, as part of a network of prefrontal, temporal, and occipital regions, appears to be involved in higher-order visual processing of faces (Haxby et al., 1994; Rajah et al., 1999; Pourtois et al., 2005). Moreover, functional neuroimaging studies have shown a shift in activity from right to left IFG with increasing task difficulty, which is thought to reflect a shift from a perceptually-based processing strategy to one based more on elaboration and analytic encoding (Haxby, 1995; Grady, 1996; McIntosh et al., 1996; Bokde et al., 2005). It has also been found to be involved in perception of facial attractiveness (Nakamura et al., 1998). (Other well-established functions of the left IFG include its involvement in working memory, semantic memory retrieval, and written and spoken language and lexical decisions (Cabeza and Nyberg, 2000)). The observation of the left IFG varying with severity of BDD symptoms could be a reflection of the degree of detailed, analytic encoding that occurs on a day-to-day basis when viewing others and themselves, and that likely underlies their symptoms. To our knowledge this is the first study to report on the morphometry of the IFG in BDD.
The amygdala is well known to have a role in face processing for emotional and even neutral expressions, as well as mediating emotional responses in general. Hyperactive amygdalae in response to emotional and neutral faces have been found in social phobia, particularly on the right side (Etkin et al., 2007). Social phobia is characterized by self-consciousness and heightened sensitivity to social situations, analogous to what individuals with BDD experience. It is conceivable, then that BDD may also be associated with heightened amygdala reactivity, as both disorders may pathologically engage fear circuitry in response to perceived social threat from face stimuli. To our knowledge, no study has reported on amygdala volume in social phobia. Although showing heightened amygdala activity, the previous fMRI experiment in BDD was not specifically designed to test amygdala reactivity; future studies using emotionally-valenced face stimuli could better assess this. Nevertheless, amygdala hyperactivity in BDD may also be related to right amygdala volume, as it varied in proportion to symptom severity in the current study.
Similar to our findings, the previous morphometric MRI study in BDD did not find group differences in amygdala volumes compared to controls (Rauch et al., 2003a). That study, however, did not test the relationship between symptom severity and region brain volumes. Amygdala brain volumes have been tested more extensively in obsessive-compulsive disorder (OCD). Szeszko et al. (2004) examined amygdala volumes in a pediatric cohort and found abnormal asymmetry (L>R) compared to healthy controls, as well as reduction in left amygdala volume after treatment (Szeszko et al., 2004). Kwon et al. (2003) found enlarged left amygdalae in a population of 22 adult subjects with OCD. However, Szeszko et al. (1999) found reduced right amygdala volume in 26 adults with OCD, relative to matched controls (Szeszko et al., 1999). Reduced right amygdala volumes were also evident in a subgroup of individuals with OCD who had prominent aggressive obsessions and checking compulsions, using a voxel-wise analysis (Pujol et al., 2004). They did not find any relationship between regional brain volumes and symptom severity. These discrepancies in the OCD literature of amygdala as well as other volume abnormalities have yet to be resolved. It is therefore difficult to draw any conclusions about the relationship between BDD and OCD based on amygdala or any other regional volumes.
This morphometric MRI study did not demonstrate significant volumetric differences in individuals with BDD relative to controls. This was the case for the whole-brain (voxel-wise analysis), total WM and GM, and for the caudate, IFG, and amygdala regions of interest. There were also no significant laterality differences from controls in any of these brain regions. This study therefore did not replicate the only previous morphometric MRI study published in BDD, which found greater total white matter and a leftward shift in caudate asymmetry (Rauch et al., 2003a). Although there was a leftward shift in caudate asymmetry in our BDD sample, it did not reach statistical significance relative to controls. The failure to replicate the previous study held true even for the analysis of the females-only and for the non-depressed subjects, which we performed to account for the gender and comorbidity differences between the studies.
Some differences in this study from the previous one may have accounted for the divergent results. One important difference in the current study is a younger mean age (28.7±10.0 vs. 36.7±10.3). An older cohort would likely have had a longer duration of illness. This may result in the emergence of volumetric abnormalities over time that would not be observable in a younger cohort. Another difference is a larger sample size (12 vs. 8 in each group) and all medication-naive patients in this study. The previous study included two subjects with past histories of treatment with serotonin-reuptake inhibitors (SRIs) and two patients currently being treated with SRIs. Although no study has specifically addressed SRI treatment effects on regional brain volumes in BDD, treatment with an SRI has been demonstrated to result in changes in thalamic (Gilbert et al., 2000) and amygdala (Szeszko et al., 2004) volumes in pediatric patients with OCD, as well as amygdala size in depression (Hamilton et al., 2008). There were also differences in comorbidities; the previous study included 3 subjects with social phobia (one of whom also had major depressive disorder) while the current study included one with major depressive disorder, one with dysthymia, two with generalized anxiety disorder, and two with major depressive disorder and generalized anxiety disorder. Other factors that may account for discrepant findings are differences in the strength of the magnet, image acquisition parameters, and higher resolution images in the current study.
BDD is currently classified in the DSM-IV-TR as a somatoform disorder (American Psychiatric Association., 2000). Yet many researchers and clinicians consider it to be an “obsessive-compulsive spectrum disorder,” based on similarities to OCD in phenomenology, epidemiology, comorbidity, familial aggregation, and response to treatment (Hollander, 1993). However, there are also significant differences between BDD and OCD. (Phillips et al., 1998; Saxena and Feusner, 2006; Feusner et al., 2008) The previous morphometric MRI study in BDD suggested that the abnormal caudate asymmetry and greater total white matter were consistent with conceptualization of BDD as an obsessive-compulsive spectrum disorder. Although the current study did not replicate these findings, the implication from this study for such a conceptualization is still unclear, as previous structural MRI results in OCD have also been inconsistent (Menzies et al., 2008). Future morphometric studies that include direct comparisons of BDD and OCD (and perhaps social phobia given the amygdala findings) using the same scanner, acquisition parameters, inclusion and exclusion criteria for comorbidities and medication use, and matched ages will be necessary to more accurately compare neurobiological features that can help classify these disorders. One of the strengths of the current morphometric study is that the data set included the same subjects as the fMRI study, allowing for more meaningful interpretations of the results.
There are several limitations of this study. Although larger than the previous study, it was still a relatively small sample size. With a sample size of 12 in each group, the power to detect even large effect sizes is still relatively low. This may have resulted in inadequate power to detect regional volumetric differences. This may have explained the lack of significance for caudate laterality and the right amygdala, for which there were small group differences in means.
In addition, the fact that several BDD subjects had comorbid major depressive disorder and/or generalized anxiety disorder conceivably could have masked or diluted the effects of volumetric differences associated with BDD alone. Complicating the picture is that there also appears to be a reciprocal clinical relationship between MDD and BDD symptoms, in which patients with comorbid MDD have greater severity of BDD, and more severe BDD independently predicts current MDD (Phillips et al., 2007). Previous research has found depression to be associated with morphometric differences in multiple cortical and subcortical brain regions (Sheline et al. 1998) (Drevets et al., 2008). Particularly relevant to the current study, previous studies in depression have found reduced amygdala volume, most consistently in patients with a chronic or intermittent course (Drevets et al., 2008) and who are unmedicated (Hamilton et al., 2008). Although these cross-sectional studies are mixed with respect to laterality, a recent prospective study found reduced amygdala volume specifically on the left in non-remitted depressed patients (Frodl et al., 2008). On the other hand, in certain anxiety disorders the right amygdala appears to show greater hyperactivity to perceived threatening stimuli (Rauch et al., 2003b), although data is lacking with regards to volume. Comorbid anxiety and depression in the current study may therefore account for the laterality of the findings in the amygdala; the left being negatively correlated with depression scores and the right positively correlated with BDD concerns (which, like anxiety states, appear to involve enhanced vigilance and threat perception).
Other limitations of the study include inherent limitations of VBM analysis due to differences in gyrification patterns, contrast, or problems with registration (Mechelli et al., 2005b). Future studies looking at cortical thickness, sulcal depth, or folding patterns could avoid some of these limitations, as well as investigate other possible morphometric abnormalities aside from regional brain volumes. We limited the ROI investigations to regions for which we had clear a priori hypotheses such as the IFG and amygdala (because of the functional difference from our previous fMRI study) and the caudate (in attempt to replicate the previous MRI study). Although limiting ROIs reduces type I error, there may have been volumetric differences in other regions that were not detected by the relatively conservative VBM statistical analysis. Given that this and the previous fMRI study are cross-sectional, the cause or effect nature of these findings is unclear. Future studies of unaffected siblings could help clarify the cause or effect nature of these findings. It is also possible that the relationship between the volumes in these regions and symptom severity is related to other, as-yet unknown pathological processes in BDD unrelated to face processing.
Nevertheless, results from this study provide insights into the neurobiology of BDD. The findings of left IFG and right amygdala size correlating with BDD symptom severity further support the functional neuroimaging findings of abnormal hyperactivity in these regions for processing faces. As abnormalities in face processing appear to be an integral component of the clinical symptoms in BDD, this has relevance to our understanding of the underlying pathophysiology. How these regions function in relationship to each other and other regions involved in BDD will need to be explored with future studies using different (or combined) imaging, electrophysiological, and psychophysical techniques.
Acknowledgments
Funding for this study was provided by the Saban Family Foundation and an NIMH grant K23MH079212-02 (Dr. Feusner).
Footnotes
Presented at the 14th Annual Meeting of the Organization for Human Brain Mapping, June 15-19, 2008, Melbourne, Australia.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. 4. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11(6 Pt 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Bokde AL, Dong W, Born C, Leinsinger G, Meindl T, Teipel SJ, Reiser M, Hampel H. Task difficulty in a simultaneous face matching task modulates activity in face fusiform area. Brain research. Cognitive brain research. 2005;25(3):701–710. doi: 10.1016/j.cogbrainres.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. The Journal of Cognitive Neuroscience. 2000;12(1):1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Carey P, Seedat S, Warwick J, van Heerden B, Stein DJ. SPECT imaging of body dysmorphic disorder. Journal of Neuropsychiatry and Clinical Neurosciences. 2004;16(3):357–359. doi: 10.1176/jnp.16.3.357. [DOI] [PubMed] [Google Scholar]
- Deckersbach T, Savage C, Phillips K, Wilhelm S, Buhlmann U, Rauch S, Baer L, Jenike M. Characteristics of memory dysfunction in body dysmorphic disorder. Journal of the International Neuropsychological Society. 2000;6(6):673–681. doi: 10.1017/s1355617700666055. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213(1–2):93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen JL, Phillips KA, Coles ME, Rasmussen SA. Insight in obsessive compulsive disorder and body dysmorphic disorder. Comprehensive Psychiatry. 2004;45(1):10–15. doi: 10.1016/j.comppsych.2003.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faravelli C, Salvatori S, Galassi F, Aiazzi L, Drei C, Cabras P. Epidemiology of somatoform disorders: a community survey in Florence. Social Psychiatry and Psychiatric Epidemiology. 1997;32(1):24–29. doi: 10.1007/BF00800664. [DOI] [PubMed] [Google Scholar]
- Feusner JD, Townsend J, Bystritsky A, Bookheimer S. Visual information processing of faces in body dysmorphic disorder. Archives of General Psychiatry. 2007;64(12):1417–1425. doi: 10.1001/archpsyc.64.12.1417. [DOI] [PubMed] [Google Scholar]
- Feusner JD, Yaryura-Tobias J, Saxena S. The pathophysiology of body dysmorphic disorder. Body Image. 2008;5(1):3–12. doi: 10.1016/j.bodyim.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl TS, Koutsouleris N, Bottlender R, Born C, Jager M, Scupin I, Reiser M, Moller HJ, Meisenzahl EM. Depression-related variation in brain morphology over 3 years: effects of stress? Arch Gen Psychiatry. 2008;65(10):1156–1165. doi: 10.1001/archpsyc.65.10.1156. [DOI] [PubMed] [Google Scholar]
- Gilbert AR, Moore GJ, Keshavan MS, Paulson LA, Narula V, Mac Master FP, Stewart CM, Rosenberg DR. Decrease in thalamic volumes of pediatric patients with obsessive-compulsive disorder who are taking paroxetine. Archives of General Psychiatry. 2000;57(5):449–456. doi: 10.1001/archpsyc.57.5.449. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude I, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage. 2001;14(3):685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- Grady CL, Horwitz B, Pietrini MJ, Mentis LG, Ungerleider L, Rapoport S, Haxby J. Effect of task difficulty on cerebral blood flow during perceptual matching of faces. Human Brain Mapping. 1996;4(4):227–239. doi: 10.1002/(SICI)1097-0193(1996)4:4<227::AID-HBM1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Siemer M, Gotlib IH. Amygdala volume in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Molecular Psychiatry. 2008 doi: 10.1038/mp.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. Diagnosis and rating of anxiety. British Journal of Psychiatry. 1969;3:76–79. [Google Scholar]
- Haxby J, Ungerleider L, Horwitz B, Rapoport S, Grady C. Hemispheric differences in neural systems for face working memory: a PET-rCBF study. Human Brain Mapping. 1995;3(2):68–82. [Google Scholar]
- Haxby JV, Horwitz B, Ungerleider LG, Maisog JM, Pietrini P, Grady CL. The functional organization of human extrastriate cortex: a PET-rCBF study of selective attention to faces and locations. The Journal of Neuroscience. 1994;14(11 Pt 1):6336–6353. doi: 10.1523/JNEUROSCI.14-11-06336.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander E. Obsessive compulsive spectrum disorders: an overview. Psychiatric Annals. 1993;23(7):355–358. [Google Scholar]
- McIntosh AR, Grady CL, Haxby JV, Ungerleider LG, Horwitz B. Changes in limbic and prefrontal functional interactions in a working memory task for faces. Cerebral Cortex. 1996;6(4):571–584. doi: 10.1093/cercor/6.4.571. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Friston KJ, Frackowiak RS, Price CJ. Structural covariance in the human cortex. The Journal of Neuroscience. 2005a;25(36):8303–8310. doi: 10.1523/JNEUROSCI.0357-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A, Price CJ, Friston KJ, Ashburner J. Voxel-based morphometry of the human brain: methods and applications. Current medical imaging reviews. 2005b;1:105–113. [Google Scholar]
- Menzies L, Chamberlain SR, Laird AR, Thelen SM, Sahakian BJ, Bullmore ET. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited. Neuroscience and Biobehavioral Reviews. 2008;32(3):525–549. doi: 10.1016/j.neubiorev.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Kawashima R, Nagumo S, Ito K, Sugiura M, Kato T, Nakamura A, Hatano K, Kubota K, Fukuda H, Kojima S. Neuroanatomical correlates of the assessment of facial attractiveness. Neuroreport. 1998;9(4):753–757. doi: 10.1097/00001756-199803090-00035. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Otto MW, Wilhelm S, Cohen LS, Harlow BL. Prevalence of body dysmorphic disorder in a community sample of women. The American Journal of Psychiatry. 2001;158(12):2061–2063. doi: 10.1176/appi.ajp.158.12.2061. [DOI] [PubMed] [Google Scholar]
- Phillips KA. The Broken Mirror. Oxford University Press; New York: 2005. [Google Scholar]
- Phillips KA, Atala K, Pope H. Diagnostic instruments for body dysmorphic disorder. Paper presented at the In: New Research Program and Abstracts. American Psychiatric Association 148th Annual Meeting; Miami. 1995. [Google Scholar]
- Phillips KA, Coles ME, Menard W, Yen S, Fay C, Weisberg RB. Suicidal ideation and suicide attempts in body dysmorphic disorder. Journal of Clinical Psychiatry. 2005;66(6):717–725. doi: 10.4088/jcp.v66n0607. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Diaz SF. Gender differences in body dysmorphic disorder. Journal of Nervous and Mental Disease. 1997;185(9):570–577. doi: 10.1097/00005053-199709000-00006. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Didie ER, Menard W. Clinical features and correlates of major depressive disorder in individuals with body dysmorphic disorder. J Affect Disord. 2007;97(1–3):129–135. doi: 10.1016/j.jad.2006.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KA, Gunderson CG, Mallya G, McElroy SL, Carter W. A comparison study of body dysmorphic disorder and obsessive-compulsive disorder. Journal of Clinical Psychiatry. 1998;59(11):568–575. doi: 10.4088/jcp.v59n1102. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Hollander E, Rasmussen SA, Aronowitz BR, DeCaria C, Goodman WK. A severity rating scale for body dysmorphic disorder: development, reliability, and validity of a modified version of the Yale-Brown Obsessive Compulsive Scale. Psychopharmacological Bulletin. 1997;33(1):17–22. [PubMed] [Google Scholar]
- Phillips KA, Menard W, Pagano ME, Fay C, Stout RL. Delusional versus nondelusional body dysmorphic disorder: clinical features and course of illness. Journal of Psychiatric Research. 2006;40(2):95–104. doi: 10.1016/j.jpsychires.2005.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourtois G, Schwartz S, Seghier ML, Lazeyras F, Vuilleumier P. View-independent coding of face identity in frontal and temporal cortices is modulated by familiarity: an event-related fMRI study. Neuroimage. 2005;24(4):1214–1224. doi: 10.1016/j.neuroimage.2004.10.038. [DOI] [PubMed] [Google Scholar]
- Pujol J, Soriano-Mas C, Alonso P, Cardoner N, Menchon JM, Deus J, Vallejo J. Mapping structural brain alterations in obsessive-compulsive disorder. Archives of General Psychiatry. 2004;61(7):720–730. doi: 10.1001/archpsyc.61.7.720. [DOI] [PubMed] [Google Scholar]
- Rajah MN, McIntosh AR, Grady CL. Frontotemporal interactions in face encoding and recognition. Brain Research. Cognitive Brain Research. 1999;8(3):259–269. doi: 10.1016/s0926-6410(99)00030-0. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Phillips KA, Segal E, Makris N, Shin LM, Whalen PJ, Jenike MA, Caviness VS, Jr, Kennedy DN. A preliminary morphometric magnetic resonance imaging study of regional brain volumes in body dysmorphic disorder. Psychiatry Research. 2003a;122(1):13–19. doi: 10.1016/s0925-4927(02)00117-8. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Wright CI. Neuroimaging studies of amygdala function in anxiety disorders. Ann N Y Acad Sci. 2003b;985:389–410. doi: 10.1111/j.1749-6632.2003.tb07096.x. [DOI] [PubMed] [Google Scholar]
- Rief W, Buhlmann U, Wilhelm S, Borkenhagen A, Brahler E. The prevalence of body dysmorphic disorder: a population-based survey. Psychological Medicine. 2006;36(6):877–885. doi: 10.1017/S0033291706007264. [DOI] [PubMed] [Google Scholar]
- Saxena S, Bota R, Brody A. Brain-behavior relationships in obsessive-compulsive disorder. Seminars in Clinical Neuropsychiatry. 2001;6:82–101. doi: 10.1053/scnp.2001.21833. [DOI] [PubMed] [Google Scholar]
- Saxena S, Feusner JD. Toward a neurobiology of body dysmorphic disorder. Primary Psychiatry. 2006;13:41–48. [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, MacMillan S, McMeniman M, Lorch E, Madden R, Ivey J, Banerjee SP, Moore GJ, Rosenberg DR. Amygdala volume reductions in pediatric patients with obsessive-compulsive disorder treated with paroxetine: preliminary findings. Neuropsychopharmacology. 2004;29(4):826–832. doi: 10.1038/sj.npp.1300399. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Robinson D, Alvir JM, Bilder RM, Lencz T, Ashtari M, Wu H, Bogerts B. Orbital frontal and amygdala volume reductions in obsessive-compulsive disorder. Archives of General Psychiatry. 1999;56(10):913–919. doi: 10.1001/archpsyc.56.10.913. [DOI] [PubMed] [Google Scholar]
- Veale D, Boocock A, Gournay K, Dryden W, Shah F, Willson R, Walburn J. Body dysmorphic disorder. A survey of fifty cases. British Journal of Psychiatry. 1996;169(2):196–201. doi: 10.1192/bjp.169.2.196. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Transactions on Medical Imaging. 2001;20(1):45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]