Abstract
Introduction
Inability to distinguish periapical cysts from granulomas prior to performing root canal treatment leads to uncertainty in treatment outcomes, because cysts have lower healing rates. Searching for differential expression of molecules within cysts or granulomas could provide information with regard to the identity of the lesion or suggest mechanistic differences that may form the basis for future therapeutic intervention. Thus, we investigated whether granulomas and cysts exhibit differential expression of extracellular matrix (ECM) molecules.
Methods
Human periapical granulomas, periapical cysts, and healthy periodontal ligament tissues were used to investigate the differential expression of ECM molecules by microarray analysis. Since matrix metalloproteinases (MMP) showed the highest differential expression in the microarray analysis, MMPs were further examined by in situ zymography and immunohistochemistry. Data were analyzed using one-way ANOVA followed by Tukey test.
Results
We observed that cysts and granulomas differentially expressed several ECM molecules, especially those from the matrix metalloproteinase (MMP) family. Compared to cysts, granulomas exhibited higher MMP enzymatic activity in areas stained for MMP-9. These areas were composed of polymorphonuclear cells (PMNs), in contrast to cysts. Similarly, MMP-13 was expressed by a greater number of cells in granulomas compared to cysts.
Conclusion
Our findings indicate that high enzymatic MMP activity in PMNs together with MMP-9 and MMP-13 stained cells could be a molecular signature of granulomas, unlike periapical cysts.
Keywords: periapical cyst, periapical granuloma, periodontal ligament, extracellular matrix components, matrix metalloproteinases
INTRODUCTION
Periapical disease represents the progression of a bacterial infection from the dental pulp to apical foramen that results in a localized inflammatory response concomitant with bone resorption (1–4). Periapical lesions include granulomas and cysts, and both are thought to represent different stages of the same inflammatory process (5–7).
Differential diagnosis of granulomas and cysts using radiographic analysis is problematic. Although preliminary studies have proposed that CT scans and ultrasound with power Doppler flowmetry can provide an additional diagnostic tool in Endodontics (8–10), it is widely accepted that histologic evaluation is necessary to confirm diagnosis (4, 11–14). However, microscopic examination can only be performed after the periapical disease is removed, which is a limitation, since in most cases root canal treatment is performed without removing the lesion. This inability to identify the status of periapical disease makes treatment outcomes unpredictable because cysts exhibit lower healing rates and generally require additional surgical treatment (6, 15–18).
Identifying extracellular matrix molecules (ECM) specific to human periapical cysts or granulomas can provide information to potentially discriminate between these lesions. Specific proteins present in the extracellular matrix and their respective receptors may offer the basis to develop novel approaches aiming to detect disease biomarkers and therefore to improve diagnosis for cysts and granulomas prior to performing root canal treatment. Possible likely candidates as biomarkers for periapical inflammation include proteases that are responsible for ECM degradation such as matrix metalloproteinases (MMPs). MMPs are a family of metal-dependent endopeptidases, which are secreted as inactive proenzymes (zymogens) and activated in tissues by cleavage of the propeptide (19–23). Although MMPs have been reported in periapical lesions (11, 24–30), a direct comparison of MMPs in cysts and granulomas has not been undertaken. Since MMPs must be activated to exert their function, it is also important to localize areas of proteinase activity within lesions. Thus, the aim of this study was to compare the expression of several ECM molecules and cell membrane receptors within different cellular components from human periapical granulomas and cysts. In addition, we sought to examine the presence and activity of MMPs in these tissues because of their reported involvement in these lesions, and because our initial data revealed differential expression of this class of proteins in these tissues.
MATERIAL AND METHODS
Collection of samples
Formalin-fixed paraffin-embedded sections from 10 periapical granulomas and 10 periapical cysts were obtained from the archives of the Oral Pathology Biopsy Service at the University of Michigan School of Dentistry after Institutional Review Board approval. Periodontal ligament (PDL) tissues obtained from the middle third of the dental root of extracted healthy teeth, after Institutional Review Board approval, were used as controls.
Histological examination of the samples
Histological examination was performed by a pathologist on hematoxylin-eosin stained slides of tissues from the apical regions of non vital teeth. Periapical cysts were selected based on the presence of granulation tissue with lining stratified squamous epithelium. Periapical granulomas were selected based on the presence of granulation tissue without lining epithelium (16, 17, 31). Fibroblast-like cells were characterized by their spindle shaped morphology, mononuclear inflammatory cells were identified as cells with a large single nucleus, and PMNs were identified by their multilobed nuclei.
RNA extraction and microarray analyses
Paraffin-embedded tissue sections were deparaffinized, exposed to protease digestion and immersed in Trizol® Reagent (Invitrogen Corporation, Carlsbad, CA) for RNA extraction. Extracted RNA was purified using the RNEasy Micro Kit (Qiagen, Valencia, CA). The quality and concentration of isolated RNA was evaluated using a capillary electrophoresis system in an RNA 6000 Pico LabChip (Agilent 2100 BioAnalyzer, Agilent Technologies Inc, Santa Clara, CA). Analysis of expression of ECM molecules (Table 1) in tissues was performed using focused cDNA microarrays (SuperArray, Bioscience Corporation, Frederick, MD). Ten micrograms of total RNA were used as a template to generate biotin-16-dUTP labeled cDNA probes. cDNA probes were denatured and hybridized at 60°C with the SuperArray membranes, which were developed using chemiluminescence. Gene expression was evaluated by densitometric analysis of the membrane spots. Comparison among groups was performed on a gene-by-gene basis after normalization by β-actin mRNA expression.
Table 1.
Genes evaluated by the Oligo GEArray Human Extracellular Matrix and Adhesion Molecules Microarray
| Extracellular Matrix Proteins |
|---|
| Basement Membrane Constituents Collagen, type IV, alpha 2 |
| Collagen, type IV, alpha 3 |
| Collagen, type IV, alpha 6 |
| Collagen, type VII, alpha 1 |
| Collagen, type VIII, alpha 2 |
| Laminin, alpha 1 |
| Laminin, alpha 2 |
| Laminin, alpha 3 |
| Laminin, alpha 4 |
| Laminin, alpha 5 |
| Laminin, beta 1 |
| Laminin, beta 2 |
| Laminin, beta 3 |
| Laminin, gamma 1 (formerly LAMB2) |
| Secreted protein, acidic, cysteine-rich (osteonectin) |
| Collagens and ECM Structural |
| Constituents Collagen, type XI, alpha 1 |
| Collagen, type XI, alpha 2 |
| Collagen, type XII, alpha 1 |
| Collagen, type XIV, alpha 1 |
| Collagen, type XV, alpha 1 |
| Collagen, type XVI, alpha 1 |
| Collagen, type XIII, alpha 1 |
| Collagen, type XIX, alpha 1 |
| Collagen, type I, alpha 1 |
| Collagen, type XXIV, alpha 1 |
| Collagen, type XXVII, alpha 1 |
| Collagen, type IV, alpha 2 |
| Collagen, type IV, alpha 3 |
| Collagen, type IV, alpha 6 |
| Collagen, type V, alpha 1 |
| Collagen, type V, alpha 3 |
| Collagen, type VI, alpha 1 |
| Collagen, type VI, alpha 2 |
| Collagen, type VI, alpha 3 |
| Collagen, type VII, alpha 1 (epidermolysis bullosa, dystrophic, dominant and recessive) |
| Collagens and ECM Structural |
| Constituents |
| Collagen, type VIII, alpha 1 |
| Collagen, type VIII, alpha 2 |
| Collagen, type IX, alpha 1 |
| Fibronectin 1 |
| Kallmann syndrome 1 sequence |
| Laminin, alpha 4 |
| ECM Proteases ADAM metallopeptidase with thrombospondin type 1 motif, 1 |
| ADAM metallopeptidase with thrombospondin type 1 motif, 13 |
| ADAM metallopeptidase with thrombospondin type 1 motif, 8 |
| Matrix metallopeptidase 1 (interstitial collagenase) |
| Matrix metallopeptidase 10 (stromelysin 2) |
| Matrix metallopeptidase 11 (stromelysin 3) |
| Matrix metallopeptidase 12 (macrophage elastase) |
| Matrix metallopeptidase 13 (collagenase 3) |
| Matrix metallopeptidase 14 (membrane-inserted) |
| Matrix metallopeptidase 15 (membrane-inserted) |
| Matrix metallopeptidase 16 (membrane-inserted) |
| Matrix metallopeptidase 17 (membrane-inserted) |
| Matrix metallopeptidase 2 (gelatinase A, 72kDa gelatinase, 72kDa type IV collagenase) |
| Matrix metallopeptidase 20 (enamelysin) |
| Matrix metallopeptidase 3 (stromelysin 1, progelatinase) |
| Matrix metallopeptidase 7 (matrilysin, uterine) |
| ECM Proteases Matrix metallopeptidase 8 (neutrophil collagenase) |
| Matrix metallopeptidase 9 (gelatinase B, 92kDa gelatinase, 92kDa type IV collagenase) |
| Matrix metallopeptidase 24 (membrane-inserted) |
| Matrix metallopeptidase 26 |
| Spastic paraplegia 7 (pure and complicated autosomal recessive) |
| TIMP metallopeptidase inhibitor 1 |
| ECM Protease Inhibitors Collagen, type IV, alpha 3 |
| Collagen, type VI, alpha 3 |
| Collagen, type VII, alpha 1 (epidermolysis bullosa, dystrophic, dominant and recessive) |
| Kallmann syndrome 1 sequence |
| Sparc/ Osteonectin, cwcv and kazal-like domains proteoglycan |
| Thrombospondin 1 |
| TIMP metallopeptidase inhibitor 1 |
| TIMP metallopeptidase inhibitor 2 |
| TIMP metallopeptidase inhibitor 3 |
| Other ECM Molecules Chondroitin sulphate proteoglycan 2 (versican) |
| Connective tissue growth factor |
| Extracellular matrix protein 1 |
| Hyaluronan synthase 1 |
| Secreted phosphoprotein 1 (osteopontin, bone sialoprotein I, early T-lymphocyte activation 1) |
| Transforming growth factor, beta-induced, 68kDa |
| Thrombospondin 2 |
| Thrombospondin 3 |
| Thrombospondin 4 |
| Laminin, beta 4 |
| Tetranectin (plasminogen binding protein) |
| Tenascin C (hexabrachion) |
| Vitronectin |
| Cell Adhesion Molecules |
| Transmembrane Molecules Cadherin 1, type 1, E-cadherin (epithelial) |
| CD44 molecule |
| Hyaluronan synthase 1 |
| Intercellular adhesion molecule 1 (CD54), human rhinovirus receptor |
| Integrin, alpha 1 |
| Integrin, alpha 2 (CD49B, alpha 2 subunit of VLA-2 receptor) |
| Integrin, alpha 2b |
| Integrin, alpha 3 (antigen CD49C, alpha 3 subunit of VLA-3 receptor) |
| Integrin, alpha 4 (antigen CD49D, alpha 4 subunit of VLA-4 receptor) |
| Integrin, alpha 5 (fibronectin receptor, alpha polypeptide) |
| Integrin, alpha 6 |
| Integrin, alpha 7 |
| Integrin, alpha 8 |
| Integrin, alpha L |
| Integrin, alpha M |
| Integrin, alpha V |
| Integrin, beta 1 |
| Integrin, beta 2 |
| Integrin, beta 3 |
| Integrin, beta 4 |
| Integrin, beta 5 |
| Integrin, beta 6 |
| Integrin, beta 7 |
| Integrin, beta 8 |
| Matrix metallopeptidase 14 (membrane-inserted) |
| Matrix metallopeptidase 15 (membrane-inserted) |
| Matrix metallopeptidase 16 (membrane-inserted) |
| Matrix metallopeptidase 17 (membrane-inserted) |
| Matrix metallopeptidase 24 (membrane-inserted) |
| Neural cell adhesion molecule 1 |
| Platelet/endothelial cell adhesion molecule (CD31 antigen) |
| Transmembrane Molecules Selectin E (endothelial adhesion molecule 1) |
| Selectin L (lymphocyte adhesion molecule 1) |
| Selectin P (granule membrane protein 140kDa, antigen CD62) |
| Sarcoglycan, epsilon |
| Spastic paraplegia 7 (pure and complicated autosomal recessive) |
| Vascular cell adhesion molecule 1 |
| Cell-Cell Adhesion CD44 molecule |
| Cadherin 1, type 1, E-cadherin (epithelial) |
| Collagen, type XI, alpha 1 |
| Collagen, type VI, alpha 2 |
| Collagen, type VIII, alpha 2 |
| Collagen, type XIX, alpha 1 |
| Catenin (cadherin-associated protein), delta 1 |
| Integrin, alpha 8 |
| Intercellular adhesion molecule 1 (CD54), human rhinovirus receptor |
| Vascular cell adhesion molecule 1 |
| Cell-Matrix Adhesion ADAM metallopeptidase with thrombospondin type 1 motif, 13 |
| CD44 molecule |
| Integrin, alpha 1 |
| Integrin, alpha 2 (CD49B, alpha 2 subunit of VLA-2 receptor) |
| Integrin, alpha 2b |
| Integrin, alpha 3 (antigen CD49C, alpha 3 subunit of VLA-3 receptor) |
| Integrin, alpha 4 (antigen CD49D, alpha 4 subunit of VLA-4 receptor) |
| Integrin, alpha 5 (fibronectin receptor, alpha polypeptide) |
| Integrin, alpha 6 |
| Integrin, alpha 7 |
| Integrin, alpha 8 |
| Integrin, alpha 9 |
| Integrin, alpha 10 |
| Integrin, alpha 11 |
| Cell-Matrix Adhesion Integrin, alpha L (antigen CD11A (p180), lymphocyte function-associated antigen 1; alpha polypeptide) |
| Integrin, alpha M (complement component 3 receptor 3 subunit) |
| Integrin, alpha V (vitronectin receptor, alpha polypeptide, antigen CD51) |
| Integrin, alpha X |
| Integrin, beta 1 (fibronectin receptor, beta polypeptide, antigen CD29 includes MDF2, MSK12) |
| Integrin, beta 2 (complement component 3 receptor 3 and 4 subunit) |
| Integrin, beta 3 (platelet glycoprotein IIIa, antigen CD61) |
| Integrin, beta 4 |
| Integrin, beta 5 |
| Integrin, beta 6 |
| Integrin, beta 7 |
| Integrin, beta 8 |
| Sarcoglycan, epsilon |
| Secreted phosphoprotein 1 (osteopontin, bone sialoprotein I, early T-lymphocyte activation 1) |
| Thrombospondin 3 |
| Other Adhesion Molecules Contactin 1 |
| Collagen, type XI, alpha 2 |
| Collagen, type XII, alpha 1 |
| Collagen, type XV, alpha 1 |
| Collagen, type XVI, alpha 1 |
| Collagen, type XVIII, alpha 1 |
| Collagen, type XXIV, alpha 1 |
| Collagen, type XXVII, alpha 1 |
| Collagen, type IV, alpha 3 |
| Collagen, type IV, alpha 6 |
| Collagen, type V, alpha 1 |
| Collagen, type V, alpha 3 |
| Collagen, type VI, alpha 1 |
| Collagen, type VI, alpha 3 |
| Collagen, type VII, alpha 1 (epidermolysis bullosa, dystrophic, dominant and recessive) |
| Other Adhesion Molecules Collagen, type VIII, alpha 1 |
| Collagen, type IX, alpha 1 |
| Chondroitin sulphate proteoglycan 2 (versican) |
| Connective tissue growth factor |
| Catenin (cadherin-associated protein), alpha 1 |
| Catenin (cadherin-associated protein), beta 1 |
| Catenin (cadherin-associated protein), delta 2 |
| Fibronectin 1 |
| Kallmann syndrome 1 sequence |
| Laminin, alpha 1 |
| Laminin, alpha 2 |
| Laminin, alpha 3 |
| Laminin, alpha 4 |
| Laminin, alpha 5 |
| Laminin, beta 1 |
| Laminin, beta 2 |
| Laminin, beta 3 |
| Laminin, gamma 1 (formerly LAMB2) |
| Sparc/ Osteonectin, cwcv and kazal-like domains proteoglycan |
| Thrombospondin 1 |
| Thrombospondin 2 |
| Thrombospondin 4 |
| Tetranectin (plasminogen binding protein) |
| Tenascin C (hexabrachion) |
| Vitronectin |
In situ zymography
Five µm-thick tissue sections were immersed in sodium borohydride (1 mg / ml) followed by incubation with a fluorescein isothiocyanate (FITC)-bound gelatin substrate (DQ™ Gelatin, Molecular Probes, Eugene, OR) dissolved in agarose (0.1 mg / ml) for 3 hr at 37°C in a humidified light-protected chamber. Nuclei were counterstained by adding 4'-6-Diamidino-2-phenylindole (DAPI; 0.5 µg / ml) to the incubation medium. Control slides were preincubated in 20 mM ethylene diamine tetraacetic acid (EDTA, Sigma, St Louis, MO) for 1 hr, and then EDTA was added to the incubation medium. Quantification of gelatinolytic activity in the sections was assessed by counting the number of spots of fluorescence in representative areas (40× magnification) and expressed as number of spots of enzymatic activity per mm2.
Immunohistochemistry
Specific MMPs were detected by immunohistochemistry. Tissue sections were quenched in a 6% H2O2 methanol solution for 30 min and boiled in 10 mM sodium citrate (pH 6.0) at 93°C for 15 min for antigen retrieval. Nonspecific binding was blocked with 1% bovine serum albumin (Sigma) for 30 min and sections were incubated for 1 hr with primary antibodies for MMP-2 (5 µg / ml; MAB3308, Chemicon, Temecula, CA), MMP-9 (5 µg / ml; MAB3309, Chemicon), and MMP-13 (5 µg / ml; M4052, Sigma). Sections were incubated with secondary antibody followed by streptavidin horseradish peroxidase and 3,3’-diaminobenzidine (DAB500 Chromogen System, Biocare Medical). Tissues were counterstained with Mayer’s Hematoxylin and mounted using standard protocols. Negative controls consisted of replacing the primary antibody with mouse or rabbit IgG. The number of positive cells was calculated for each antibody in three representative fields-of-view (100× magnification).
Statistical analysis
Data were analyzed using one-way ANOVA followed by Tukey test (α = 0.05).
RESULTS
Periapical granulomas exhibit a higher percentage of PMNs compared to cysts
Mononuclear inflammatory cells were the most prevalent cells found in both cysts and granulomas (59.8% ± 19.8 versus 43.1% ± 3.8, respectively; p > 0.05). Similar quantities of fibroblast-like cells were also observed in both lesions (24.1% ± 8.4 in cysts versus 24.6% ± 4.0 in granulomas; p > 0.05). In contrast, PMNs were more prevalent in periapical granulomas compared to cysts (32.1 ± 5.8% versus 15.9% ± 7.5 respectively; p < 0.05). Inflammatory activity, assessed as a ratio of inflammatory cellular components (PMNs plus mononuclear cells) to fibroblast-like cell count, was similar for both cysts and granulomas (3.2 and 3.3 respectively), however the composition of inflammatory activity was different between the lesions, since PMNs predominated in granulomas. Periapical cysts were surrounded by a stratified squamous epithelium and fibroblast-like cells were observed in the stroma and surrounding the lesions. Similar to cysts, fibroblast-like cells were distributed throughout granuloma lesions but no epithelial cell layer was evident.
ECM genes are differentially expressed in granulomas and cysts compared to healthy PDL
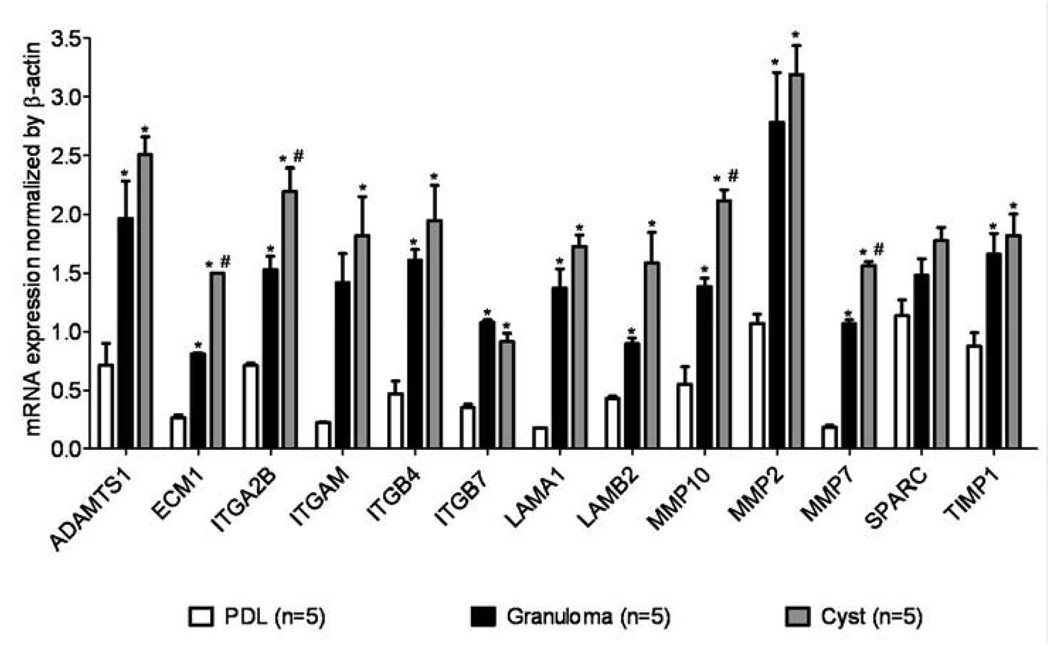
Out of 113 genes, eleven genes in periapical granulomas and 17 genes in cysts showed higher expression levels than in healthy PDL (Figure 1). Of those overexpressed, ADAM metallopeptidase-1 (ADAMTS1), integrin-β4 (ITGB4), integrin-β7 (ITGB7), laminin-α1 (LAMA1), MMP-2 and tissue inhibitor of metalloproteinase-1 (TIMP1) were similarly expressed in cysts and granulomas.
Figure 1.
mRNA expression for human extracellular matrix molecules in periapical granulomas and cysts is different from healthy PDL tissue. For comparison purposes, mRNA expression was normalized to the housekeeping gene β-actin. Graph bars depict mean and standard deviation. * p < 0.05 compared to healthy PDL tissue, #p < 0.05 compared to periapical granuloma; n = 15.
Extracellular matrix protein-1 (ECM1), integrin-α2B (ITGA2B), MMP-10, MMP-7, integrin-αM (ITGAM), and laminin-β2 (LAMB2) genes were more frequently expressed in cysts, followed by a lower expression in granulomas, and the lowest expression was detected in healthy PDL. Osteonectin (SPARC) gene expression was similar in all tissues.
Integrin-α3, integrin-α5, and integrin-β1 in cysts and transforming growth factor β-in granulomas were significantly elevated in lesions compared to healthy PDL. MMP-24 was detected in granulomas and cysts but not in healthy PDL, and ADAM metallopeptidase-13 and secreted phosphoprotein-1 were found exclusively in cysts but not in granulomas or healthy PDL. Catenin-α1, TIMP-3, and selectin-L were found exclusively in healthy PDL but not in periapical lesions, thus these genes were suppressed in the lesions (Table 2). We selected for presentation only those genes which were up or downregulated in cysts and granulomas. Relative expression for genes not modulated or genes not expressed were not reported.
Table 2.
Human extracellular matrix molecules mRNA expression in periapical cysts, granulomas, and / or in healthy PDL tissue. mRNA expression was normalized by β-actin
| Transcripts | Healthy PDL tissue | Granuloma | Cyst |
|---|---|---|---|
| ADAM metallopeptidase 13 | — | — | 1.22 ± 0.06 |
| Collagen type XVIII α1 | 0.67 ± 0.24 | — | 1.14 ± 0.01 |
| Catenin-α1 | 0.39 ± 0.11 | — | — |
| Integrin-α3 | 0.46 ± 0.08 | — | 1.67 ± 0.01* |
| Integrin-α5 | 0.79 ± 0.02 | — | 1.25 ± 0.01* |
| Integrin-β1 | 0.75 ± 0.1 | — | 2.06 ± 0.01* |
| Laminin-α5 | 0.74 ± 0.1 | — | 1.03 ± 0.01 |
| Matrix metalloproteinase-24 | — | 1.05 ± 0.32 | 1.34 ± 0.59 |
| Secreted phosphoprotein-1 | — | — | 1.38 ± 0.01 |
| Transforming growth factor-β | 0.46 ± 0.04 | 1.01 ± 0.1* | — |
| Tissue inhibitor of metalloproteinase-3 | 0.64 ± 0.21 | — | — |
| L-selectin | 0.32 ± 0.12 | — | — |
p < 0.05 compared to healthy PDL tissue
Periapical granulomas exhibit high gelatinolytic activity compared to cysts
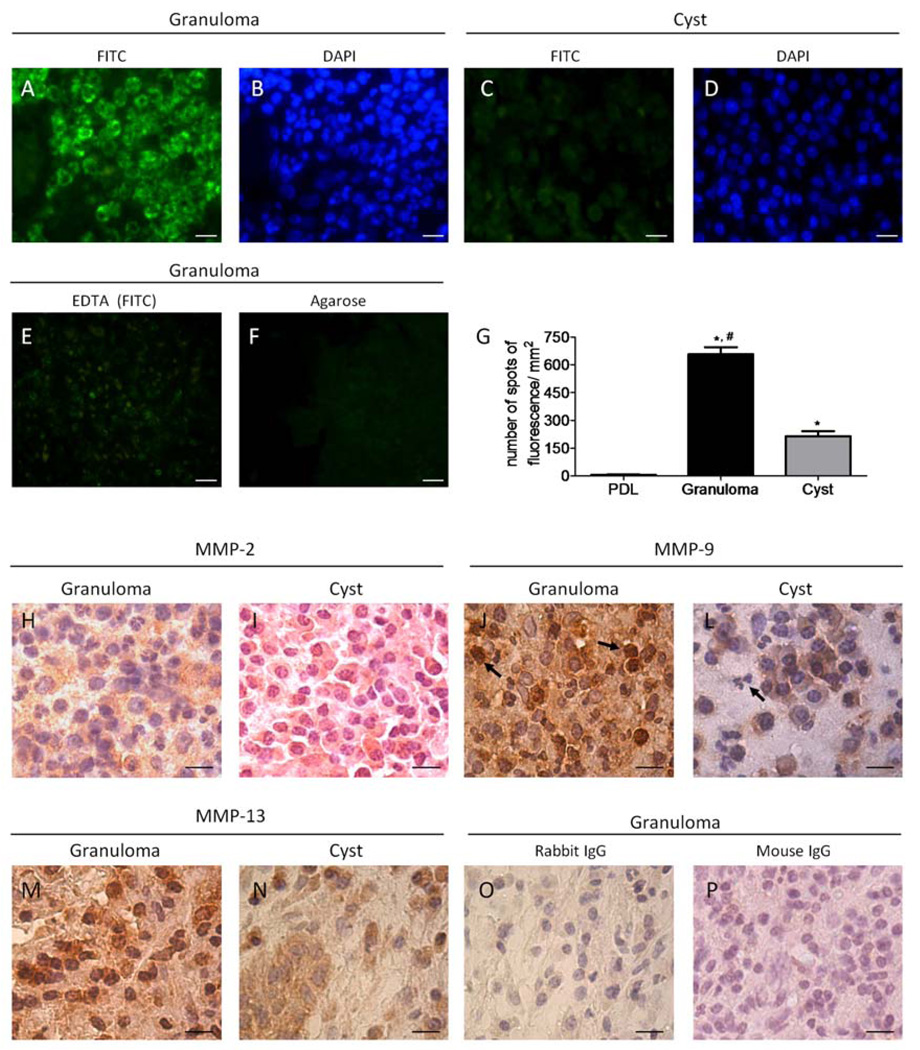
Given the high prevalence of MMPs and higher MMP-2 mRNA expression in both cysts and granulomas compared to healthy PDL, the presence of MMP activity in tissue sections was further investigated. In situ zymography revealed gelatinolytic activity in both cysts and granulomas. Gelatinolytic activity, detected in the inflammatory cell area, was more intense in granulomas (Figure 2A, 2B) than in cysts (Figure 2C, 2D). Low enzymatic activity was detected in healthy PDL (Figure 2G).
Figure 2.
Matrix metalloproteinase expression and activity are distinct in periapical granulomas and cysts. Areas of gelatinolytic activity were detected by in situ zymography and appeared as green spots of fluorescence (A, C, E, F); cellular components in the area were visualized by DAPI nuclear staining (B, D). Control slides were incubated with EDTA to confirm if gelatinolytic activity was from MMPs (E). Levels of autofluorescence in the sections were evaluated by incubating the tissue with agarose without FITC-bound gelatin (F). Graphs represent the number of spots of fluorescence per mm2 in cysts, granulomas, and healthy PDL tissue counted as described in M & M; *p < 0.05 compared to healthy PDL tissue, #p < 0.05 compared to periapical cysts (n = 25) (G). Immunostaining for MMP-2, MMP-9 and MMP-13 was performed to confirm the profile of MMPs in periapical cysts and granulomas. MMP-2 stained similarly cells in granulomas (H) and cysts (I) whereas MMP-9 expression was observed in PMN (arrows) in granulomas (J) but in not in periapical cysts (L). A wide-range of cells was strongly positive for MMP-13 in granulomas (M) unlike periapical cysts, which presented faint positive staining in mononuclear cells (N). Control staining for slides where the primary antibody was omitted and the slides were incubated with rabbit (O) or mouse IgG (P) revealed low unspecific staining; bar = 10 µm; magnification 100×.
The shape of the DAPI-stained nuclei, indicated that gelatinolytic activity was predominantly in areas with PMNs (Figure 2B). Enzymatic activity was sparse adjacent to mononuclear inflammatory cells and fibroblast-like cells, and absent in the epithelial layer in periapical cysts (data not shown). Sections incubated with EDTA confirmed that proteases responsible for gelatinolytic activity were MMPs (Figure 2E). Absence of autofluorescence was confirmed by incubating tissues with agarose lacking FITC-bound gelatin (Figure 2F).
MMP-2, MMP-9 and MMP-13 are differentially expressed by inflammatory and stromal cells in cysts and granulomas
In situ zymography revealed the presence of generic MMP activity, however it did not reveal the specific MMP profile present in these tissues. Since MMP-2, MMP-9 and MMP-13 use gelatin as a substrate (22, 32), immunostaining for these specific MMPs was performed.
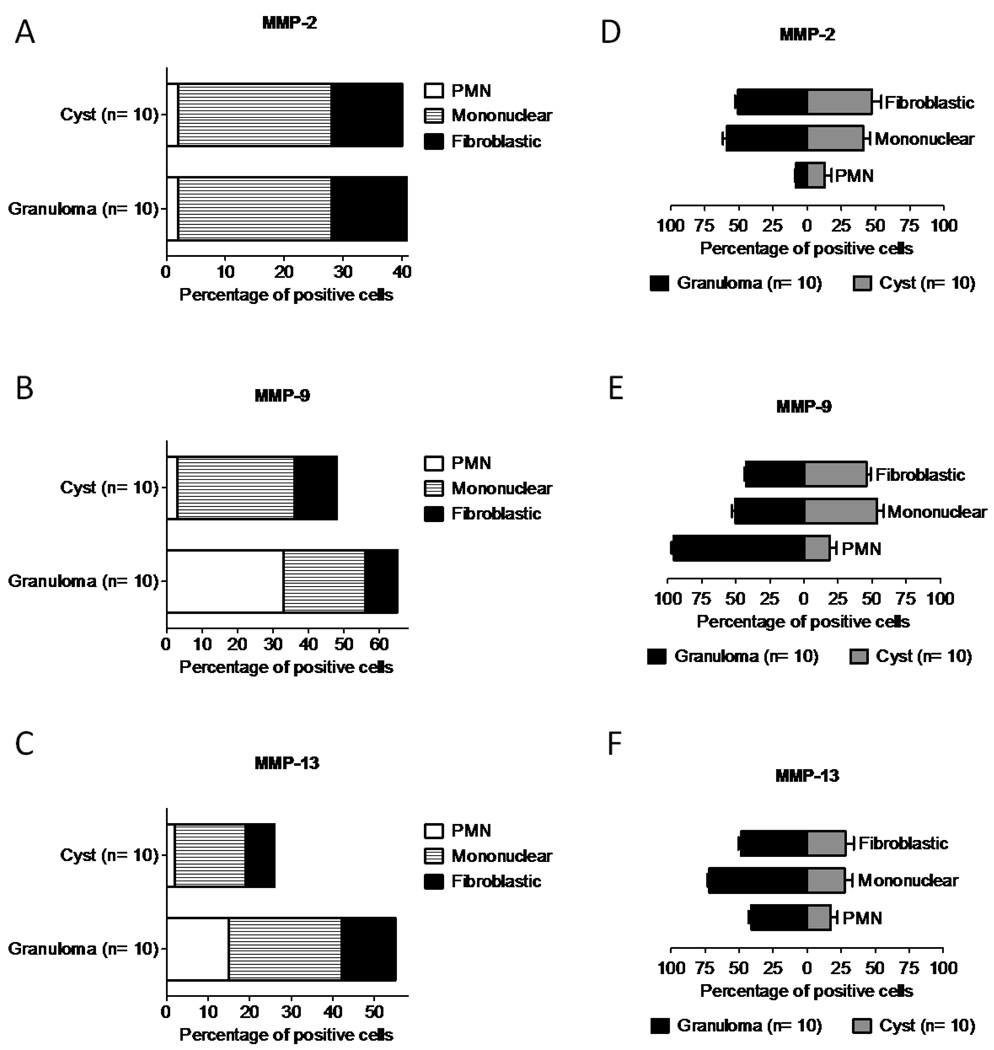
Immunopositive cytoplasmic staining for MMP-2 was observed in a higher number of stromal and mononuclear inflammatory cells compared to PMNs (p < 0.01) in both granulomas and cysts, without differences between them (Figure 2H, 2I, Figure 4A, 4D). In contrast, MMP-9 and MMP-13 were expressed by a greater number of cells in granulomas compared to cysts (p < 0.01) (Figure 2J, 2L, 2M, 2N, Figure 4B, 4C).
Figure 4.
MMPs are differently expressed by several cellular components in periapical lesions. Quantification of cell staining was performed by grouping cells into polymorphonuclear leukocytes (PMN), mononuclear or fibroblast-like cells. The percentage of cells expressing MMP-2, MMP-9 and MMP-13 was calculated in relation to the total amount of cells per field-of-view in three representative areas (A, B, C). To further estimate the amount of positive cells within the same group of cells, the percentage of PMN, mononuclear and fibroblastic expressing MMP-2 (D), MMP-9 (E) and MMP-13 (F) was calculated; n = 20.
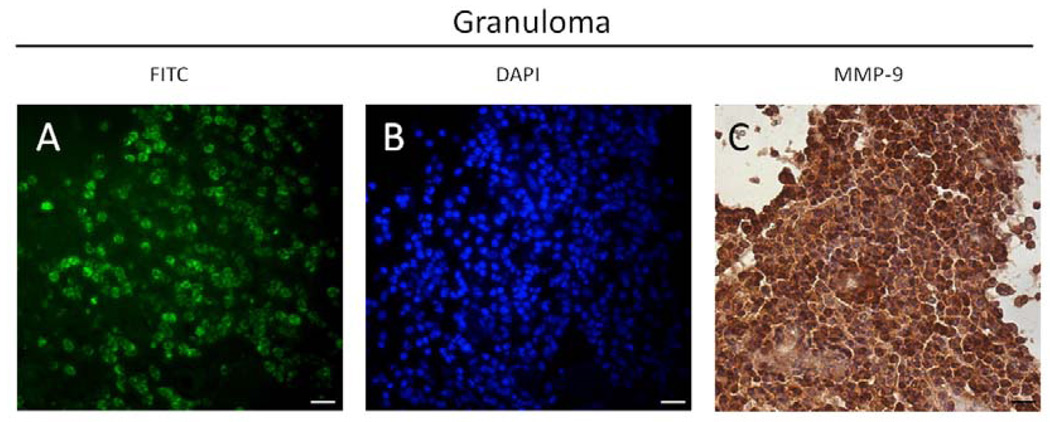
MMP-9 was expressed more intensely by inflammatory cells versus stromal cells in both granulomas and cysts. Among these, PMNs were predominantly stained in granulomas (Figure 2J), whereas mononuclear cells were stained to a higher percentage in cysts (Figure 2L). Of all PMNs in periapical granulomas, 95.6% were MMP-9 positive, unlike cysts, which exhibited fewer MMP-9 positive PMNs (Figure 4E). MMP-9 expression in granulomas was observed in areas with high levels of enzymatic activity in PMNs (Figure 3).
Figure 3.
Areas of MMP enzymatic activity (A) in PMN (B) in periapical granulomas were co-localized to MMP-9 expression detected by immunostaining (C). Bar = 10 µm.
MMP-13 was observed primarily in mononuclear cells in both lesions, and to a lesser degree in stromal cells (Figure 2M, 2N). A lower percentage of PMNs was stained in granulomas, however this was higher than that in cysts, where most PMNs were not stained (p < 0.01) (Figure 4C and 4F).
In periapical cysts, epithelial cells were positively stained for MMP-2, MMP-9, and MMP-13 but no enzymatic activity was detected (data not shown), indicating that these MMPs were inactive. IgG staining controls revealed low non-specific staining (Figure 2O and 2P).
DISCUSSION
Periapical disease pathogenesis involves degradation of several ECM components including collagen, fibronectin, laminin, and small proteoglycans (33). MMPs are likely candidates in this process since these ECM components are known MMP substrates. Using in situ zymography, we demonstrated the presence and increased levels of active MMPs in the inflammatory cell zone in periapical granulomas compared to cysts. Although MMP expression has been immunolocalized in human periapical lesions (11, 24–28, 34), this is the first study to identify areas of MMP enzymatic activity.
Different MMP immunostaining patterns were observed in cysts and granulomas. The minimal epithelial layer in cysts was positively stained for MMP-2, MMP-9, and MMP-13, although it exhibited low MMP enzymatic activity. This may result from the reduced stroma present in this thin epithelial compartment (35). However, the larger stromal compartments of both lesions, where the inflammatory cells are located, exhibited higher MMP enzymatic activity. In lesional stroma, MMP-2 expression was diffuse throughout the ECM, and cells exhibited weak cytoplasmic staining. On the other hand, MMP-9 and MMP-13 staining were localized and highly expressed in the inflammatory cell area, with strong intracellular staining. Interestingly, higher MMP-13 expression and more evident MMP-9 positive staining in PMNs were observed in granulomas compared to cysts.
Simultaneous upregulation of MMP-9 and MMP-13 in granulomas suggests coordinate MMP regulation in the dynamics of periapical disease. Interaction between MMP-9 and MMP-13 could be explained by the ability of MMP-13 to activate MMP-9 (32), thus explaining the more intense enzymatic activity observed in granulomas.
The role of PMNs in periapical disease pathogenesis is well-established (36). Differential expression of MMP-9 in PMNs from granulomas compared to cysts represents an interesting finding, since PMNs uniquely regulate MMP expression. Synthesis of MMP-9 is complete by the time PMNs enter the vasculature, and regulation of this protease is mediated by granule release rather than by transcriptional events (37). Thus, it is not surprising that microarray data did not reveal changes in mRNA expression for MMP-9. Furthermore, MMP activity in general is regulated post-transcriptionally by proteolytic processing and by tissue inhibitors of metalloproteinases, so changes in MMP activity would not necessarily be reflected at the gene level or in the microarray data for any of the MMPs. However, the mechanism involved in the recruitment of a higher percentage of PMNs positively stained for MMP-9 and the higher enzymatic activity observed in granulomas compared to cysts remains to be investigated.
Previous reports showed that MMP-1 expression is restricted to the lining epithelium, subepithelial macrophages, fibroblasts, and endothelial cells in human periapical cysts (11, 29) and to areas showing active bone resorption during periapical disease development in rats (39). However, we observed MMP-9 and MMP-13 staining more intensely in inflammatory cells in these lesions. Taken together, these findings indicate that specific MMPs are produced in different cell types in periapical lesions, suggesting specific roles for these enzymes in periapical disease pathogenesis. Indeed, recent reports have demonstrated distinct roles for MMPs in periapical disease progression, either stimulating (40) or attenuating lesion development (41). These data and our current findings underscore the importance of characterizing the profile of MMPs involved in periapical lesions prior to using pan-MMP inhibitors therapeutically that can non-specifically inhibit MMPs (23).
Using ECM-focused microarrays, we demonstrated that some MMPs are more highly expressed in cysts and granulomas than in healthy PDL. In addition, high gelatinolytic activity was present in granulomas, especially in the inflammatory cell zone, and immunostaining confirmed the presence of MMP-9 and MMP-13 in this process. Recently, gingival crevicular fluid analysis (GCF) revealed that patients with periapical lesions exhibit higher MMP-2 and MMP-9 expression compared to control subjects (29), however the identity of the disease was not further investigated. Since differential MMP-9 and MMP-13 expression was observed between cysts and granulomas in this study, these molecules may serve as molecular markers of the status of periapical disease and could be further explored in vivo in combination with GCF analysis to clinically differentiate periapical cysts from granulomas.
ACKNOWLEDGMENTS
This study was supported by Grant NIH-R01-DE013725 to YLK and a CAPES Foundation Fellowship to FWGPS (0668/07-9). No financial affiliation exists.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kawashima N, Stashenko P. Expression of bone-resorptive and regulatory cytokines in murine periapical inflammation. Arch Oral Biol. 1999;44:55–66. doi: 10.1016/s0003-9969(98)00094-6. [DOI] [PubMed] [Google Scholar]
- 2.Martón IJ, Kiss C. Protective and destructive immune reactions in apical periodontitis. Oral Microbiol Immunol. 2000;l15:139–150. doi: 10.1034/j.1399-302x.2000.150301.x. [DOI] [PubMed] [Google Scholar]
- 3.Liapatas S, Nakou M, Rontogianni D. Inflammatory infiltrate of chronic periradicular lesions: an immunohistochemical study. Int Endod J. 2003;36:464–471. doi: 10.1046/j.1365-2591.2003.00627.x. [DOI] [PubMed] [Google Scholar]
- 4.Kabak SL, Kabak YS, Anischenko SL. Light microscopic study of periapical lesions associated with asymptomatic apical periodontitis. Ann Anat. 2005;187:185–194. doi: 10.1016/j.aanat.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Orstavik D, Pitt Ford TR. Essential endodontology: Prevention and treatment of apical periodontitis. Blackwell Science: Malden. 1998 [Google Scholar]
- 6.García CC, Sempere FV, Diago MP, Bowen EM. The post-endodontic periapical lesion: histologic and etiopathogenic aspects. Med Oral Patol Oral Cir Bucal. 2007;12:E585–E590. [PubMed] [Google Scholar]
- 7.Ricucci D, Mannocci F, Pitt Ford TR. A study of periapical lesions correlating the presence of a radiopaque lamina with histological findings. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:389–394. doi: 10.1016/j.tripleo.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 8.Cotti E, Campisi G, Ambu R, Dettori C. Ultrasound real-time imaging in the differential diagnosis of periapical lesions. Int Endod J. 2003;36:556–563. doi: 10.1046/j.1365-2591.2003.00690.x. [DOI] [PubMed] [Google Scholar]
- 9.Simon JHS, Enciso E, Malfaz JM, Roges R, Bailey-Perry M, Patel A. Differential diagnosis of large periapical lesions using cone-beam computed tomography measurements and biopsy. J Endod. 2006;32:833–837. doi: 10.1016/j.joen.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Aggarwal V, Logani A, Shah N. The evaluation of computed tomography scans and ultrasounds in the differential diagnosis of periapical lesions. J Endod. 2008;34:1312–1315. doi: 10.1016/j.joen.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Lin SK, Chiang CP, Hong CY, Lin CP, Lan WH, Hsieh CC, Kuo MYP. Immunolocalization of interstitial collagenase (MMP-1) and tissue inhibitor of metalloproteinases-1 (TIMP-1) in radicular cysts. J Oral Pathol Med. 1997;26:458–463. doi: 10.1111/j.1600-0714.1997.tb00016.x. [DOI] [PubMed] [Google Scholar]
- 12.Lin LM, Huang GTJ, Rosenberg PA. Proliferation of epithelial cell rests, formation of apical cysts, and regression of apical cysts after periapical wound healing. J Endod. 2007;33:908–916. doi: 10.1016/j.joen.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Lin LM, Ricucci D, Lin J, Rosenberg PA. Nonsurgical root canal therapy of large cyst-like inflammatory periapical lesions and inflammatory apical cysts. J Endod. 2009;35:607–615. doi: 10.1016/j.joen.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Schulz M, von Arx T, Altermatt HJ, Bosshardt D. Histology of periapical lesions obtained during apical surgery. J Endod. 2009;35:634–642. doi: 10.1016/j.joen.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 15.Nair PN, Sjögren U, Schumacher E, Sundqvist G. Radicular cyst affecting a root-filled human tooth: a long-term posttreatment follow-up. Int Endod J. 1993;26:225–233. doi: 10.1111/j.1365-2591.1993.tb00563.x. [DOI] [PubMed] [Google Scholar]
- 16.Ramachandran Nair PN, Pajarola G, Schroeder HE. Types and incidence of human periapical lesions obtained with extracted teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:93–102. doi: 10.1016/s1079-2104(96)80156-9. [DOI] [PubMed] [Google Scholar]
- 17.Nair PNR. New perspectives on radicular cysts: do they heal? Int Endod J. 1998;31:155–160. doi: 10.1046/j.1365-2591.1998.00146.x. [DOI] [PubMed] [Google Scholar]
- 18.Peters E, Lau M. Histopathologic examination to confirm diagnosis of periapical lesions: a review. J Can Dent Assoc. 2003;69:598–600. [PubMed] [Google Scholar]
- 19.Woessner JF. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991:2145–2154. [PubMed] [Google Scholar]
- 20.Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- 21.Krane SM. Clinical Importance of metalloproteinases and their inhibitors. Ann NY Acad Sci. 1994;732:1–10. doi: 10.1111/j.1749-6632.1994.tb24719.x. [DOI] [PubMed] [Google Scholar]
- 22.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verma RP, Hansch C. Matrix metalloproteinases (MMPs): chemical-biological functions and (Q)SARs. Bioorg Med Chem. 2007;15:2223–2268. doi: 10.1016/j.bmc.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Teronen O, Salo T, Konttinen YT, Rifkin B, Vernillo A, Ramamurthy NS, Kjeldsen L, Borregaard N, Hietanen J, Sorsa T. Identification and characterization of gelatinase / type IV collagenase in jaw cysts. J Oral Pathol Med. 1995;24:78–84. doi: 10.1111/j.1600-0714.1995.tb01143.x. [DOI] [PubMed] [Google Scholar]
- 25.Teronen O, Salo T, Laitinen J, Törnwall J, Ylipaavalniemi P, Konttinen YT, Hietanen J, Sorsa T. Characterization of interstitial collagenases in jaw cyst wall. Eur J Oral Sci. 1995;103:141–147. doi: 10.1111/j.1600-0722.1995.tb00015.x. [DOI] [PubMed] [Google Scholar]
- 26.Shin SJ, Lee J, Baek SH, Lin SS. Tissue levels of matrix metalloproteinases in pulps and periapical lesions. J Endod. 2002;28:313–315. doi: 10.1097/00004770-200204000-00013. [DOI] [PubMed] [Google Scholar]
- 27.Wahlgren J, Salo T, Teronen O, Luoto H, Sorsa T, Tjäderhane L. Matrix metalloproteinase-8 (MMP-8) in pulpal and periapical inflammation and periapical root-canal exudates. Int Endod J. 2002;35:897–904. doi: 10.1046/j.1365-2591.2002.00587.x. [DOI] [PubMed] [Google Scholar]
- 28.Leonardi R, Caltabiano R, Loreto C. Collagenase-3 (MMP-13) is expressed in periapical lesions: na immunohistochemical study. Int Endod J. 2005;38:297–301. doi: 10.1111/j.1365-2591.2005.00943.x. [DOI] [PubMed] [Google Scholar]
- 29.Belmar MJ, Pabst C, Mart#x000ED;nez B, Hernández M. Gelatinolytic activity in gingival crevicular fluid from teeth with periapical lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:801–806. doi: 10.1016/j.tripleo.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Morimoto T, Yamasaki M, Nakata K, Tsuji M, Nakamura H. The expression of macrophage and neutrophil elastases in rat periradicular lesions. J Endod. 2008;34:1072–1076. doi: 10.1016/j.joen.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 31.Vier FV, Figueiredo JAP. Prevalence of different periapical lesions associated with human teeth and their correlation with the presence and extension of apical external root resorption. Int Endod J. 2002;35:710–719. doi: 10.1046/j.1365-2591.2002.00554.x. [DOI] [PubMed] [Google Scholar]
- 32.Leeman MF, Curran S, Murray GI. The structure, regulation, and function of human matrix metalloproteinase-13. Crit Rev Biochem Mol Biol. 2002;37:149–166. doi: 10.1080/10409230290771483. [DOI] [PubMed] [Google Scholar]
- 33.Delzangles B, Boy-Lefevre ML, Forest N. Glycoproteins expression in apical pathologic tissues: clinical incidences. J Endod. 1997;23:565–568. doi: 10.1016/S0099-2399(06)81122-2. [DOI] [PubMed] [Google Scholar]
- 34.Carneiro E, Menezes R, Garlet GP, Garcia RB, Bramante CM, Figueira R, Sogayar M, Granjeiro JM. Expression analysis of matrix metalloproteinase-9 in epithelialized and nonepithelialized apical periodontitis lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:127–132. doi: 10.1016/j.tripleo.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fu X, Kassim SY, Parks WC, Heinecke JW. Hypochlorous acid oxygenates the cysteine switch domain of pro-matrilysin (MMP-7). A mechanism for matrix metalloproteinase activation and atherosclerotic plaque rupture by myeloperoxidase. J Biol Chem. 2001;276:41279–41287. doi: 10.1074/jbc.M106958200. [DOI] [PubMed] [Google Scholar]
- 36.Yamasaki M, Kumazawa M, Kohsaka T, Nakamura H. Effect of methotrexate-induced neutropenia on rat periapical lesion. Oral Surg Oral Med Oral Pathol. 1994;77:655–661. doi: 10.1016/0030-4220(94)90330-1. [DOI] [PubMed] [Google Scholar]
- 37.Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T. Regulation of matrix metalloproteinases: an overview. Mol Cell Biochem. 2003;253:269–285. doi: 10.1023/a:1026028303196. [DOI] [PubMed] [Google Scholar]
- 38.Lin SK, Kok SH, Kuo MYP, Wang TJ, Wang JT, Yeh FTC, Hsiao M, Lan WH, Hong CY. Sequential expressions of MMP-1, TIMP-1, IL-6 and COX-2 genes in induced periapical lesions in rats. Eur J Oral Sci. 2002;110:246–253. doi: 10.1034/j.1600-0447.2002.11227.x. [DOI] [PubMed] [Google Scholar]
- 39.Hong CY, Lin SK, Kok SH, Cheng SJ, Lee MS, Wang TM, Chen CS, Lin LD, Wang JS. The role of lipopolysaccharide in infectious bone resorption of periapical lesion. J Oral Pathol Med. 2004;33:162–169. doi: 10.1111/j.0904-2512.2004.00045.x. [DOI] [PubMed] [Google Scholar]
- 40.Metzger Z, Belkin D, Kariv N, Dotan M, Kfir A. Low-dose doxycycline inhibits bone resorption associated with apical periodontitis. Int Endod J. 2008;41:303–309. doi: 10.1111/j.1365-2591.2007.01363.x. [DOI] [PubMed] [Google Scholar]
- 41.Tjäderhane L, Hotakainen T, Kinnunen S, Ahonen M, Salo T. The effect of chemical inhibition of matrix metalloproteinases on the size of experimentally induced apical periodontitis. Int Endod J. 2007;40:282–289. doi: 10.1111/j.0143-2885.2007.01223.x. [DOI] [PubMed] [Google Scholar]