Abstract
Purpose
Sorafenib is an antiangiogenic agent with activity in renal cancer. We conducted a randomized trial to investigate dynamic contrast magnetic resonance imaging (DCE-MRI) as a pharmacodynamic biomarker.
Patients and Methods
Patients were randomly assigned to placebo or 200 or 400 mg twice per day of sorafenib. DCE-MRI was performed at baseline and 4 weeks. DCE-MRI parameters, area under the contrast concentration versus time curve 90 seconds after contrast injection (IAUC90), and volume transfer constant of contrast agent (Ktrans) were calculated for a metastatic site selected in a blinded manner. Primary end point was change in Ktrans.
Results
Of the 56 assessable patients, 48 underwent two MRIs; 44 MRIs were assessable for study end points. Mean Ktrans log ratios were 0.131 (standard deviation [SD], 0.315), −0.148 (SD, 0.382), −0.271 (SD, 0.499) in placebo, 200- and 400-mg cohorts, respectively (P = .0077 for trend) corresponding to changes of +14%, −14%, and −24%. IAUC90 log ratios were 0.041 (SD, 0.197), −0.040 (SD, 0.132), −0.356 (SD, 0.411), respectively (P = .0003 for trend), corresponding to changes of +4%, −4%, and −30%. Using a log-rank test, IAUC90 and Ktrans changes were not associated with progression-free survival (PFS). Patients with high baseline Ktrans had a better PFS (P = .027).
Conclusion
IAUC90 and Ktrans are pharmacodynamic biomarkers for sorafenib, but variability is high and magnitude of effect is less than previously reported. Changes in DCE-MRI parameters after 4 weeks of sorafenib are not predictive of PFS, suggesting that these biomarkers are not surrogate end points. The value of baseline Ktrans as a prognostic or predictive biomarker requires additional study.
INTRODUCTION
Angiogenesis is essential for the growth, invasion, and metastases of tumors, and antiangiogenic drugs are standard in oncology. In renal cancer, the vascular endothelial growth factor receptor (VEGFR) inhibitors sorafenib and sunitinib and the VEGF binding agent bevacizumab improve progression-free survival (PFS) in patients with good prognosis.1-4 Numerous additional antiangiogenic agents are under study. Their development is, however, complicated by the observation that some of their dose-limiting toxicities may not be mechanism related and their antitumor effects may not lead to sufficient tumor shrinkage to qualify for a partial response by the usual criteria.2
Novel biomarkers assessing antiangiogenic effects have the potential for improved monitoring of patients treated with these agents. One potential tool for biomarker development is dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI), which assesses the kinetics of contrast inflow and egress from a tumor region of interest.5,6 Both quantitative parameters, such as the volume transfer constant of contrast agent (Ktrans) or the blood plasma volume fraction (Vp), as well as semi-quantitative measures, such as area under the contrast concentration versus time curve for 90 seconds after contrast injection (IAUC90), can be derived from DCE-MRI studies. DCE-MRI studies in animals have demonstrated that changes in Ktrans or IAUC90 correlate with antiangiogenic drug activity and standard vascular physiology measurements.7-9 In patients, some studies have suggested that DCE-MRI changes with antiangiogenic therapy may be predictive of clinical benefit, but most data suggest that DCE-MRI changes are a pharmacodynamic biomarker.10-14 In other words, they reflect the effect of an antiangiogenic drug on the host or tumor, analogous to the effect of the tubule targeting agent paclitaxel on neutrophil count.
Nevertheless, there are few controlled studies assessing the variability and value of DCE-MRI as a pharmacodynamic biomarker for a validated antiangiogenic agent. Here, we report the results of a randomized, dose-ranging trial in which the effects of sorafenib on both quantative and semiquantitative DCE-MRI parameters were evaluated at baseline and after 4 weeks of therapy. We demonstrate that changes in several DCE-MRI parameters are dose-dependent pharmacodynamic biomarkers of sorafenib and that baseline parameters may be predictive or prognostic of time to progression.
PATIENTS AND METHODS
Patients
Patients with histologically confirmed metastatic clear-cell renal cancer without prior VEGF pathway directed therapy were eligible. Other requirements included measurable disease with at least one lesion ≥ 20 mm and suitable for DCE-MRI, WHO performance status 0 to 2, blood pressure lower than 140/90, and normal organ function defined as creatinine lower than 2.8 mg/dL, AST lower than 2.5 × upper limit of normal, and total bilirubin lower than upper limit of normal. Exclusion criteria included patients with CNS metastases, uncontrolled intercurrent illness, pregnancy, and anticancer treatment within 4 weeks of study enrollment.
Study Design
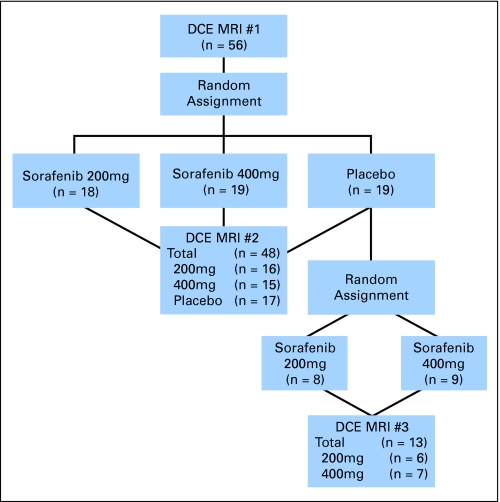
Patients were randomly assigned in a double-blind manner to placebo, 200 mg, or 400 mg twice daily of sorafenib (Nexavar, Bayer Pharmaceuticals Corporation, West Haven, CT). The study was conducted under an investigational new drug application held by the University of Chicago. After 4 weeks of treatment, patients were partially unblinded; those in the placebo group underwent rerandom assignment to treatment with 200 mg versus 400 mg twice daily of sorafenib (Fig 1). Patients were evaluated for tumor response by standard computed tomography (CT) scans performed every 12 weeks using Response Evaluation Criteria in Solid Tumors.15 On progression, patients had the option of sorafenib dose escalation to 400 and 600 mg. Patients initially treated with placebo underwent a second baseline CT scan before starting investigational therapy. Toxicity monitoring using National Cancer Institute Common Toxicity Criteria version 3.0 was performed and dose modifications made for grade 3 or 4 toxicities. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines and was approved by the University of Chicago's institutional review board.
Fig 1.
Schema of trial design. After a baseline dynamic contrast magnetic resonance imaging (DCE-MRI), patients were randomly assigned to placebo, sorafenib 200 mg twice per day, sorafenib 400 mg twice per day. After 4 weeks of therapy, patients underwent another DCE-MRI and were partially unblinded; patients initially on placebo were rerandomly assigned to a treatment arm.
DCE-MRI Methodology
All patients had baseline DCE-MRI within 14 days before and a follow-up scan day 28 ± 5 after initiating therapy. Patients initially assigned to placebo had a third DCE-MRI 28 ± 5 days after initiating sorafenib. The tumor region of interest (ROI) to be imaged sequentially was selected by the clinical radiologist (M.M.) and in general, measured at least 2 cm and was located in an area with minimal MRI artifacts or motion.
DCE-MR images were acquired on SIGNA 1.5 Tesla scanners (General Electric Medical Systems, Waukesha, WI) equipped with self-shielded (Echo Speed+) gradients using procedures consistent with recent consensus criteria.16,17 Briefly, after a scout scan to localize the lesions, two T1 weighted image slices were acquired with 2 second temporal resolution for about 8 minutes. Using a power injector (Medrad, Indianola, PA), 0.1 mmol/kg gadodiamide (Ominscan, GE Healthcare, Chalfont St Giles, United Kingdom) was injected over 10 seconds followed by 20 mL saline flush. A two-dimensional spoiled gradient echo pulse sequence was used with TR/TE = 7.8/1.7 ms, flip angle 60°, matrix size 256 × 128, typical field of view 30 to 35 cm, slice thickness 8 mm, and slice spacing 1 mm.
All analyses were conducted by investigators (O.M.H., C.Y., M.M.) blinded to patient identification, treatment group, and scan date. The ROI for analysis was modified to exclude large blood vessels and tissues affected by motion artifact. Contrast media concentration as a function of time in selected ROIs was calculated from signal intensity at each time point and literature values for the baseline T1 value in a reference tissue within the field of view. This allowed calculation of IAUC90 as previously described.18 Ktrans and Vp were calculated using the modified Tofts model and a tumor local arterial input function derived by a multiple reference tissue method.19,20 Kinetic models in which the effective Vpwas not included or for which an assumed population arterial input function was used did not reliably fit the contrast agent concentration versus time data (data not shown). Figure 2 displays a selected tumor ROI with histogram mapping in one patient.
Fig 2.
Sagittal image of a liver metastasis in a selected patient. Color map reflects the relative area under the contrast concentration versus time curve 90 seconds after contrast injection (IAUC90) value in each voxel with yellow highest and violet as the lowest demonstrating heterogeneity of the parameter within the imaged region of interest. Negative voxels arise due to low signal and accompanying noise. (A) Baseline; (B) repeat image after 4 weeks of treatment.
Statistical Analysis
The primary study end point was the change in Ktrans between baseline and week 4. A linear trend between sorafenib dose and the logarithm of the ratio of week 4 Ktrans (and IAUC90) to the baseline Ktrans (and IAUC90) was tested. Kaplan-Meier estimates and log-rank tests were computed to evaluate the association between changes in Ktrans or IAUC90 and PFS. Patients initially assigned to placebo were included in survival analyses by using changes in the noted parameters between the second and third DCE-MRI and by using tumor measurements from the second CT scan as a new baseline. After data collection, exploratory analyses of associations between dose and change in Vp as well as associations between baseline Vp and Ktrans and PFS were also conducted. PFS was additionally modeled using the Cox proportional hazards approach with each of the DCE-MRI parameters as continuous covariates with and without the inclusion of dose as an additional covariate. For placebo patients, the dose level assigned after cross-over was the value used in the Cox model. When baseline level and change in the DCE-MRI parameters were dichotomized for log-rank tests, either the median value or the zero value, respectively, was chosen as the cut point. For all patients, the initial progression date, regardless of any subsequent dose escalations, was used to calculate PFS. One placebo patient who did not cross-over to active therapy was excluded from the PFS analysis. Intrapatient variability was assessed by computing the within patient coefficients of variation for the placebo group as previously described.21
Trial size was based on phase I data with the alternative VEGFR inhibitor valatanib, that suggested a 40% reduction in Ki (equivalent to Ktrans) was necessary for clinical activity.11 A sample size of 66 patients was selected in order to provide 86% power to detect a 20% and 40% decrease in the 200-mg and 400-mg groups respectively at a significance level of .05.
RESULTS
Patient Characteristics and Response
The study was terminated early because of the US Food and Drug Administration's approval of sorafenib for metastatic renal cancer; 57 of the planned 66 patients were enrolled. The median age was 64 (range, 42 to 82) and the median time since diagnosis was 54 weeks. Prior therapies included: nephrectomy in 51 patients, immunotherapy in 32, cytotoxic chemotherapy in 10, radiation in 23, allogeneic stem-cell transplantation in two, and other (mainly investigational) therapies in 22 (Table 1). Analysis includes one patient with a nonclear cell renal cancer (papillary histology), but does not include one patient who enrolled but never received drug due to disease related complications.
Table 1.
Baseline Characteristics for All Patients Enrolled onto Trial
| Characteristic | No. of Patients (N = 57) |
|---|---|
| Sex | |
| Male | 13 |
| Female | 44 |
| Age | |
| Median | 64 |
| Range | 42-82 |
| Race/ethnicity | |
| White | 51 |
| African American | 4 |
| Hispanic | 1 |
| Asian | 1 |
| WHO performance status | |
| 0 | 32 |
| 1 | 22 |
| 2 | 3 |
| Histologic subtype | |
| Cell clear | 56 |
| Papillary | 1 |
| Prior therapy | |
| Nephrectomy | 51 |
| Immunotherapy | 32 |
| Cytotoxic chemotherapy | 10 |
| Radiation | 23 |
| Allogeneic stem cell transplant | 2 |
| Other (investigational) | 22 |
| No. of metastatic sites | |
| 1 | 9 |
| 2 | 22 |
| 3 | 19 |
| ≥ 4 | 7 |
Major treatment toxicities were similar to those previously reported, including hand-foot reaction, dermatitis, facial erythema, skin dryness, hypertension, diarrhea, and fatigue.2,22,23 Eleven patients required a dose reduction or treatment interruption due to adverse effects, four of which occurred during the first cycle, (two in the 400 mg, one in the 200 mg, one in the placebo cohorts). All but one of these underwent the second protocol specified MRI. Four patients experienced a partial response (7%), and seven and four underwent protocol defined dose escalation to 400 and 600 mg twice per day, respectively, on progression. All four patients escalated to 600 mg sorafenib experienced stable disease.
Change in DCE-MRI Parameters With Sorafenib Therapy
Of the 56 assessable patients, 48 underwent at least two DCE-MRIs. MRI was not performed due to early disease progression (n = 6), drug toxicity (n = 1), or withdrawal from study due to retrospective determination that lesion size was inadequate for DCE-MRI analysis (n = 1). Of the 48 completed DCE- MRIs, 44 were technically assessable for study end points. Reasons for nonassessablility included slice misregistration (n = 2), lack of intravenous contrast delivery (n = 1), and primary imaging data not saved for analysis (n = 1). Tumor ROIs used for analysis were located in the following locations: abdominal mass/lymphadenopathy (n = 13), liver (n = 11), renal fossa (n = 4), lumbar muscle (n = 2), lungs/chest wall (n = 5), mediastinum (n = 4), pelvic mass (n = 1), bone (n = 2), shoulder mass (n = 1), subcutaneous (n = 1).
The reduction in IAUC90, Ktrans, and Vp after 4 weeks of therapy with sorafenib is depicted in Figure 3. Mean IAUC90 log ratios in the placebo, 200- and 400-mg groups corresponded to relative changes of +4%, −4%, and −30% (P = .0003 for linear trend), Ktrans log ratios corresponded to changes of +14%, −14%, and −24% (P = .0077), and Vp log ratios corresponded to changes of +15%, −23%, and −34% (P < .0001). Intrapatient variability, as determined from changes in the placebo group, were 14%, 23%, and 29% for IAUC90, Ktrans, and Vp, respectively. There was no significant change in the extravascular extracellular volume fraction Ve.
Fig 3.
Log ratio of individual dynamic contrast magnetic resonance imaging (DCE-MRI) parameters (4 weeks/baseline) plotted by sorafenib dose (mg) demonstrating a decrease with dose. Mean log ratio and corresponding standard deviations (SD) in the placebo, 200-, and 400-mg cohorts are provided. (A) area under the contrast concentration versus time curve 90 seconds after contrast injection (IAUC90); (B) volume transfer constant of contrast agent (Ktrans); (C) blood plasma volume fraction (Vp).
As in previous studies, patient blood pressure increased with treatment.2,22,23 The change in mean arterial blood pressure calculated as the difference between the first post-treatment visit (approximately 2 to 4 weeks) and baseline was 0.8 ± 8.0 mmHg, 8.3 ± 10.4 mmHg, and 10.5 ± 11.1 mmHg for the placebo, 200-, and 400-mg groups, respectively (P = .006 for linear trend) There was no significant correlation between the change in mean arterial blood pressure and the log ratio of Ktrans or IAUC90 (4 weeks/baseline).
DCE-MRI Parameters and Clinical Outcome
To assess whether change in DCE-MRI parameters might predict clinical outcome, patients were divided into those with log ratio IAUC, Ktrans, or Vp of lower than 0 versus ≥ 0, corresponding to an absolute decrease or increase. Using Kaplan-Meier estimates and log-rank tests and including DCE-MRI parameter changes between MRI 2 and MRI 3 for the placebo patients (see Methods and Fig 1) no changes were statistically significant predictors of PFS. Furthermore, there was no detectable difference in PFS between the two doses.
As part of an unplanned, exploratory analysis, PFS was determined for patients with low versus high baseline Ktrans and Vp using the median value for each as a cut point and values from MRI 2 as the baseline value in the placebo patients. Using this dichotomous distinction and a Kaplan-Meier analysis, there is a statistically significant difference between subjects with a low versus high baseline Ktrans (P = .027) as well as a low versus high baseline Vp(P = .014; Figs 4A and B). Using Cox proportional hazards models with either log transformed baseline Ktrans or Vp analyzed as a continuous variables the former was significant (P = .036), but the latter was not (P = .087). When dose is included in the model, Ktrans retains significance (P = .025).
Fig 4.
Kaplan-Meier estimates for progression-free survival (PFS) of patients with low and high baseline volume transfer constant of contrast agent (Ktrans) and blood plasma volume fraction (Vp). (A) Ktrans (cut point defined as the median baseline Ktrans = 0.182). (B): Vp (cut point defined as the median baseline Vp = 0.072).
DISCUSSION
Results from this randomized, dose-ranging trial suggest that both semiquantitative and quantitative DCE-MRI parameters, IAUC90, Vp, and Ktrans, are pharmacodynamic biomarkers of sorafenib in metastatic renal cancer. To our knowledge, this is the first report of a placebo-controlled, randomized trial evaluating DCE-MRI based biomarkers in a prospective manner, thus providing an unbiased evaluation of their feasibility and variability. Importantly, only 44 of 56 patients had two protocol-specified technically adequate images available for analysis. Although some of this was due to rapidly progressive disease, it is clear that further technical advances, including improved determination of the arterial input function, improved kinetic models, and incorporation of image registration software, will be necessary before DCE-MRI can be recommended for routine use.
The trial did demonstrate through the use of a placebo arm that intrapatient variability of DCE-MRI parameters, 14% for IAUC90 and 23% for Ktrans, is sufficiently low in comparison to the treatment effect to be used as biomarkers. We were thus able to demonstrate the pharmacodynamic properties of IAUC90 and Ktrans in the context of sorafenib therapy. Nevertheless, the magnitude of change in this prospective study is lower than previously reported, which likely reflects the less selected population investigated here.11,12 Alternatively, technical differences or biases introduced by exclusion of patients who were unable to obtain the second DCE-MRI due to either disease progression or imaging failure may explain the lower observed changes. Whether differences in timing of successive MRI examinations or different VEGF pathway targeted drugs have a similar magnitude of effect remains to be determined. In addition, the observed effects of sorafenib on DCE-MRI parameters across large tumor regions of interest are highly variable (Fig 2). Preliminary analysis did not suggest any pattern in subregion changes, such as in voxels with a high baseline Ktrans (data not shown). It thus remains to be determined whether a more detailed voxel based analysis would demonstrate a larger effect. The high interpatient variability noted in the change of Ktrans and IAUC within a dose cohort may also be due to the previously described variability in sorafenib pharmacokinetics, which could not be analyzed in this study.24,25
A few single arm trials have reported a relationship between DCE-MRI biomarker change and clinical outcome, but these findings could not be replicated.12,26 The modest size of the study limits the power of this analysis, but it is likely that changes in Ktrans and IAUC90 are pharmocodynamic biomarkers (such as neutropenia with taxane therapy) and not predictive biomarkers (such as HER2 amplification with herceptin therapy).
An unplanned, exploratory analysis of baseline Ktrans and Vp as a predictor of time to progression or death was performed. In each case, high values, using the median value as the cutoff, were associated with a prolonged time to progression or death but only Ktrans retained significance when analyzed as a continuous variable in a Cox proportional hazards model. It is possible that inclusion of patients initially assigned to placebo and then rerandomly assigned to one of the two doses introduced a systematic bias into this analysis, but because new baseline CT measurements were used and only 4 weeks elapsed, this is considered unlikely. More importantly, even in the absence of any bias, it is unknown if these parameters are predictive of benefit to antiangiogenic agents or prognostic biomarkers of patient's disease. However, results here are similar to those reported by O'Dwyer et al12 in a trial of 12 patients with metastatic renal cancer receiving sorafenib and are similar to observations in other therapeutic areas.27 Further investigation into the potential predictive value of baseline DCE-MRI biomarkers is thus indicated.
Finally, there are several limitations to calculating even the basic DCE-MRI parameters evaluated here. The semiquantitative measure, IAUC90, is easy to calculate and has good reproducibility.18 However, its absolute value is dependent on an accurate precontrast T1 map, the temporal resolution of the data, and represents a composite of physiologic processes, which may not accurately reflect vascular changes. While Ktrans may be more physiologically meaningful, its calculation relies on the accurate determination of the arterial input function (AIF) for which the Tofts method is commonly used.28 This assumes a two-compartment model that describes contrast accumulation and washout in a tumor region; an approach limited by the observation that the concentration-time curve cannot always be fit by the model.29 Newer methods of analyzing DCE-MRI data and additional kinetic parameters for clinical analysis are continually being refined.30,31 Our group has proposed a novel approach for calculating a more accurate AIF using dynamic data from multiple reference tissues, with the underlying assumption that all of the reference tissues have a common arterial input function with different bolus arrival times, which was used in this study.20 While this method is not widely used in clinical trials, use of a more common assumed AIF did not allow reliably fit of the contrast agent concentration versus time data and would have led to an even lower rate of technically usable images for data analysis.
In addition, tumor motion registration during the image acquisition was not employed, which may have increased variability of the data, and T1 was not directly measured in every patient, which could introduce errors in the kinetic analysis. Finally, as noted previously, the analysis concentrated on a tumor ROI and ignored the inherent heterogeneity within the region. More sophisticated voxel based analyses could therefore also be envisioned and could potentially provide useful information.
In conclusion, this prospective, randomized dose ranging trial reveals that DCE-MRI derived Ktrans and IAUC90 are pharmacodynamic biomarkers of sorafenib in metastatic renal cancer. While the results do not indicate that changes in DCE-MRI parameters correlate with prolonged time to progression or death, hypothesis-generating analyses indicate that high baseline Ktrans and Vpmay be a prognostic or predictive biomarker of benefit to sorafenib. Further studies evaluating this hypothesis in prospective studies and extending it to other antiangiogenic agents is indicated, as are further studies refining the methods of DCE-MRI acquisition and analysis.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Elizabeth Manchen, Bayer (C), Onyx (C); Mark J. Ratain, Onyx (C); Walter M. Stadler, Bayer (C), Onyx (C) Stock Ownership: None Honoraria: Elizabeth Manchen, Bayer, Onyx Research Funding: Cheng Yang, Bayer; Walter M. Stadler, Bayer Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Gregory Karczmar, Theodore Karrison, Myrosia Mitchell, Mark J. Ratain, Walter M. Stadler
Financial support: Walter M. Stadler
Administrative support: Gregory Karczmar, Theodore Karrison, Mark J. Ratain, Walter M. Stadler
Provision of study materials or patients: Walter M. Stadler
Collection and assembly of data: Olwen M. Hahn, Cheng Yang, Milica Medved, Elizabeth Manchen, Myrosia Mitchell, Walter M. Stadler
Data analysis and interpretation: Olwen M. Hahn, Cheng Yang, Milica Medved, Gregory Karczmar, Emily Kistner, Myrosia Mitchell, Mark J. Ratain, Walter M. Stadler
Manuscript writing: Olwen M. Hahn, Walter M. Stadler
Final approval of manuscript: Olwen M. Hahn, Cheng Yang, Milica Medved, Gregory Karczmar, Emily Kistner, Theodore Karrison, Elizabeth Manchen, Myrosia Mitchell, Mark J. Ratain, Walter M. Stadler
Supported by Bayer Pharmaceuticals Corporation and Grant No. 1R21 CA108184-01A2 from the National Institutes of Health.
Presented at 43rd Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 1-5, 2007.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Escudier B, Koralewski P, Pluzanska A, et al: A randomized, controlled, double-blind phase III study (AVOREN) of bevacizumab/interferon-α2a vs placebo/interferon- α2a as first-line therapy in metastatic renal cell carcinoma. J Clin Oncol 25:2s, 2007. (suppl; abstr 3), [Google Scholar]
- 2.Escudier B, Eisen T, Stadler WM, et al: Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 356:125-134, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Motzer RJ, Hutson TE, Tomczak P, et al: Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 356:115-124, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Yang JC, Haworth L, Sherry RM, et al: A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 349:427-434, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Padhani AR: Dynamic contrast-enhanced MRI in clinical oncology: Current status and future directions. J Magn Reson Imaging 16:407-422, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Checkley D, Tessier JJ, Kendrew J, et al: Use of dynamic contrast-enhanced MRI to evaluate acute treatment with ZD6474, a VEGF signalling inhibitor, in PC-3 prostate tumours. Br J Cancer 89:1889-1895, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrett T, Brechbiel M, Bernardo M, et al: MRI of tumor angiogenesis. J Magn Reson Imaging 26:235-249, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Drevs J, Muller-Driver R, Wittig C, et al: PTK787/ZK 222584, a specific vascular endothelial growth factor-receptor tyrosine kinase inhibitor, affects the anatomy of the tumor vascular bed and the functional vascular properties as detected by dynamic enhanced magnetic resonance imaging. Cancer Res 62:4015-4022, 2002 [PubMed] [Google Scholar]
- 9.Kiessling F, Morgenstern B, Zhang C: Contrast agents and applications to assess tumor angiogenesis in vivo by magnetic resonance imaging. Curr Med Chem 14:77-91, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Stevenson JP, Rosen M, Sun W, et al: Phase I trial of the antivascular agent combretastatin A4 phosphate on a 5-day schedule to patients with cancer: Magnetic resonance imaging evidence for altered tumor blood flow. J Clin Oncol 21:4428-4438, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Morgan B, Thomas AL, Drevs J, et al: Dynamic contrast-enhanced magnetic resonance imaging as a biomarker for the pharmacological response of PTK787/ZK 222584, an inhibitor of the vascular endothelial growth factor receptor tyrosine kinases, in patients with advanced colorectal cancer and liver metastases: Results from two phase I studies. J Clin Oncol 21:3955-3964, 2003 [DOI] [PubMed] [Google Scholar]
- 12.O'Dwyer PJ, Gallagher M, Schwartz B, et al: Pharmacodynamic study of BAY 43-9006 in patients with metastatic renal cell carcinoma. J Clin Oncol 23:193s, 2005. (suppl; abstr 3005) [Google Scholar]
- 13.Liu G, Rugo HS, Wilding G, et al: Dynamic contrast-enhanced magnetic resonance imaging as a pharmacodynamic measure of response after acute dosing of AG-013736, an oral angiogenesis inhibitor, in patients with advanced solid tumors: Results from a phase I study. J Clin Oncol 23:5464-5473, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Mross K, Drevs J, Muller M, et al: Phase I clinical and pharmacokinetic study of PTK/ZK, a multiple VEGF receptor inhibitor, in patients with liver metastases from solid tumours. Eur J Cancer 41:1291-1299, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Therasse P, Arbuck SG, Eisenhauer EA, et al: New guidelines to evaluate the response to treatment in solid tumors: European Organisation for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205-216, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Evelhoch J, Garwood M, Vigneron D, et al: Expanding the use of magnetic resonance in the assessment of tumor response to therapy: Workshop report. Cancer Res 65:7041-7044, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Leach MO, Brindle KM, Evelhoch JL, et al: The assessment of antiangiogenic and antivascular therapies in early-stage clinical trials using magnetic resonance imaging: Issues and recommendations. Br J Cancer 92:1599-1610, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medved M, Karczmar G, Yang C, et al: Semiquantitative analysis of dynamic contrast enhanced MRI in cancer patients: Variability and changes in tumor tissue over time. J Magn Reson Imaging 20:122-128, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Tofts PS, Brix G, Buckley DL, et al: Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: Standardized quantities and symbols. J Magn Reson Imaging 10:223-232, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Yang C, Karczmar GS, Medved M, et al: Multiple reference tissue method for contrast agent arterial input function estimation. Magn Reson Med 58:1266-1275, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Bland JM, Altman DG: Measurement error proportional to the mean. BMJ 313:106, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ratain MJ, Eisen T, Stadler WM, et al: Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol 24:2505-2512, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Strumberg D, Awada A, Hirte H, et al: Pooled safety analysis of BAY 43-9006 (sorafenib) monotherapy in patients with advanced solid tumours: Is rash associated with treatment outcome? Eur J Cancer 42:548-556, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Clark JW, Eder JP, Ryan D, et al: Safety and pharmacokinetics of the dual action Raf kinase and vascular endothelial growth factor receptor inhibitor, BAY 43-9006, in patients with advanced, refractory solid tumors Clin Cancer Res 11:5472-5480, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Strumberg D, Richly H, Hilger RA, et al: Phase I clinical and pharmacokinetic study of the novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43-9006 in patients with advanced refractory solid tumors. J Clin Oncol 23:965-972, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Conrad C, Friedman H, Reardon D, et al: A phase I/II trial of single-agent PTK 787/ZK 222584 (PTK/ZK), a novel, oral angiogenesis inhibitor, in patients with recurrent glioblastoma multiforme (GBM). J Clin Oncol 22:110s, 2004. (suppl; abstr 1512) [Google Scholar]
- 27.Griebel J, Mayr NA, de Vries A, et al: Assessment of tumor microcirculation: A new role of dynamic contrast MR imaging. J Magn Reson Imaging 7:111-119, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Tofts PS, Kermode AG: Measurement of the blood-brain barrier permeability and leakage space using dynamic MR imaging: 1. Fundamental concepts. Magn Reson Med 17:357-367, 1991 [DOI] [PubMed] [Google Scholar]
- 29.Parker GJ, Tanner SF, Leach MO: Pitfalls in the measurement of tissue permeability over short-scales using a low temporal resolution blood input function. Proceedings of the 4th Annual Meeting of International Society of Magnetic Resonance in Medicine, New York, NY, April 27-May 3, 1996
- 30.Li X, Rooney WD, Springer CS Jr: A unified magnetic resonance imaging pharmacokinetic theory: Intravascular and extracellular contrast reagents. Magn Reson Med 54:1351-1359, 2005 [DOI] [PubMed] [Google Scholar]
- 31.St Lawrence KS, Lee TY: An adiabatic approximation to the tissue homogeneity model for water exchange in the brain: I. Theoretical derivation. J Cereb Blood Flow Metab 18:1365-1377, 1998 [DOI] [PubMed] [Google Scholar]