Abstract
Background
Persistent organic pollutants (POPs)—such as organochlorine pesticides (OCPs), polychlorinated biphenyls (PCBs), and polybrominated diphenyl ethers (PBDEs)—and heavy metals have been reported in sea turtles at various stages of their life cycle. These chemicals can disrupt development and function of wildlife. Furthermore, in areas such as Peninsular Malaysia, where the human consumption of sea turtle eggs is prevalent, egg contamination may also have public health implications.
Objective
In the present study we investigated conservation and human health risks associated with the chemical contamination of green turtle (Chelonia mydas) eggs in Peninsular Malaysia.
Methods
Fifty-five C. mydas eggs were collected from markets in Peninsular Malaysia and analyzed for POPs and heavy metals. We conducted screening risk assessments (SRAs) and calculated the percent of acceptable daily intake (ADI) for POPs and metals to assess conservation and human health risks associated with egg contamination.
Results
C. mydas eggs were available in 9 of the 33 markets visited. These eggs came from seven nesting areas from as far away as Borneo Malaysia. SRAs indicated a significant risk to embryonic development associated with the observed arsenic concentrations. Furthermore, the concentrations of coplanar PCBs represented 3 300 times the ADI values set by the World Health Organization.
Conclusions
The concentrations of POPs and heavy metals reported in C. mydas eggs from markets in Peninsular Malaysia pose considerable risks to sea turtle conservation and human health.
Keywords: C. mydas, heavy metals, human health, persistent organic pollutants, risk assessments
Peninsular Malaysia has historically supported large nesting populations of Chelonia mydas, predominantly in the east coast state of Terengganu (Hendrickson and Alfred 1961; Hendrickson and Balasingham 1966). However, C. mydas nesting populations in Peninsular Malaysia have declined by > 80% since the 1950s, primarily because of the collection of eggs for human consumption (Hendrickson and Alfred 1961; Ibrahim 1994; Ibrahim et al. 2003; Limpus 1993; Siow and Moll 1981). It has been estimated that prior to the late 1950s, nearly 100% of all sea turtle eggs laid on the beaches of Peninsular Malaysia were collected for human consumption at a rate of up to 2 million eggs per year (Hendrickson and Alfred 1961; Siow and Moll 1981).
Under current legislation, the majority of eggs are protected, with the remaining eggs allocated to collectors under a permit system (Ibrahim et al. 2003). In 2006, it was estimated that 90% of all C. mydas eggs laid in Peninsular Malaysia were relocated to hatcheries or protected in situ by the Department of Fisheries, Malaysia (Ibrahim K, unpublished data). However, this figure is dependent on Department of Fisheries budgetary allocations and can fluctuate from year to year. Furthermore, considering the high economic value of sea turtle eggs [~ $0.60 1.20 (US$) each], there is an incentive for permit holders to underreport egg collection, and eggs may even be harvested without permits for sale in black markets.
The consumption of sea turtle products (tissues, eggs, and blood) poses a number of public health concerns because of high lipid content and the presence of bacteria, parasites, and environmental contaminants (Aguirre et al. 2006). As a guide for consumption of foods containing environmental contaminants, the World Health Organization (WHO) and other regional organizations have determined acceptable daily intakes (ADIs) of these compounds [Food and Agriculture Organization of the United Nations (FAO)/ WHO 2007]. Persistent organic pollutants (POPs) and heavy metals have been reported in the eggs of a number of C. mydas populations (Clark and Krynitsky 1980; Godley et al. 1999; Lam et al. 2006; McKenzie et al. 1999; Podreka et al. 1998; Thompson et al. 1974). Determining the contamination of C. mydas eggs in Peninsular Malaysia and estimating the risks associated with consuming these eggs is therefore an important aspect of public health management.
The chemical contamination of C. mydas eggs may also have conservation implications. POPs and heavy metals are known to affect development, reproduction, and immune function in fish, birds, reptiles, and mammals (Chang 1996; Colborn et al. 1993; Guillette and Gunderson 2001; McKinlay et al. 2008). Correlative and in vitro studies have shown that environmentally relevant concentrations of mercury, polychlorinated biphenyls (PCBs), and 4,4′-DDE (dichloro diphenyl-dichloroethylene) can affect the immune function and health of loggerhead sea turtles, Caretta caretta (Day et al. 2007; Keller et al. 2004, 2006a, 2006b). However, the most well-documented effects of POPs in oviparous reptiles are on hatchling development. Eggs of the American alligator (Alligator mississippiensis) with high levels of p,p′-DDE, p,p′-DDD (dichlorodiphenyldichloroethane), dieldrin, and cis-chlordane experienced high egg mortality (Guillette and Crain 1996). Similarly, PCBs and organochlorine pesticides (OCPs) in the eggs of the freshwater snapping turtle (Chelydra serpentina) have been associated with increased embryonic mortalities and deformities (Bishop et al. 1991; Bryan et al. 1987; de Solla et al. 2008; Helwig and Hora 1983; Olafsson et al. 1983). Similar effects of POPs on the development of sea turtle eggs may therefore be expected.
The chemical contamination of C. mydas eggs in Peninsular Malaysia potentially has both conservation and human health consequences. The availability of C. mydas eggs in the markets of Peninsular Malaysia provides an opportunity to collect a sample of this turtle population’s eggs and assess the level of chemical contamination. This information can be used to predict the effects of these chemicals on C. mydas populations and the potential harm they may cause to the humans consuming them.
Materials and Methods
Market egg collection
In August 2006, we conducted a survey by road to identify markets in Peninsular Malaysia selling C. mydas eggs for human consumption. Over 6 days, a 1,115-km route covering ~ 730 km of coastline was taken from Kota Bharu through Kuantan, Kuala Lumpur, Johor Bahru, and back up the east coast to Kuantan. Each of the 33 markets encountered on this route was surveyed for C. mydas eggs. A random sample of 3 13 eggs was purchased from each market where eggs were being sold. In larger markets with multiple vendors, eggs were taken from a random sample of the vendors. In total, 55 eggs were collected and kept frozen ( 20°C) until analysis. At the time of collection, vendors were asked about the nesting location and the approximate date the eggs had been collected. Although we could not confirm the reliability of these locations and dates, we did not expect the reliability of information to interfere with the interpretation of the calculated risk assessments.
Chemical analysis
We analyzed egg samples for 83 PCB congeners, 23 OCPs, and 19 polybrominated diphenyl ether (PBDE) congeners using gas chromatography with tandem mass spectrometry (GC-MS/ MS), following previously described methods (van de Merwe et al. 2009). We determined that the limit of detection (LOD) was compound and sample specific, although for most compounds the LOD was < 10 pg/g. The coefficient of variation [SD ÷ (mean × 100)] of 10 replicates of pooled C. mydas egg collected from Heron Island (Queensland, Australia) in 1998 was < 20% for all compounds, although generally < 5%. We analyzed avian egg control (QC04-ERM1: common murre, Uria aalge, and thick-billed murre, Uria lomvia; National Institute of Standards and Technology, Charleston, SC, USA), finding concentrations within 60% of the reference values for all POP compounds (Vander Pol et al. 2007). Recoveries of mass-labeled internal standards ranged from 30 to 96% and were < 60% only for the higher chlorinated PCBs.
We also analyzed eggs for zinc, copper, cobalt, selenium, arsenic, cadmium, and lead using acid digestion [nitric acid (HNO3)] and inductively coupled plasma mass spectrometry, following methods modified from Scheelings (2002), Tinggi et al. (2004), and Sakao and Uchida (1999). Eggs were analyzed for mercury using acid digestion (sulfuric acid, HNO3, hydrochloric acid) and cold vapor atomic absorption spectrometry, using methods modified from Tinggi and Craven (1996). The limit of reporting was 0.05 μg/g for Cu, Zn, Se, As, and Pb, and 0.01 μg/g for Cd and Hg; the coefficient of variation of 10 replicates of pooled C. mydas egg collected from Heron Island in 1998 ranged from 0.5 to 1.9%. We analyzed DORM-I dogfish muscle standard reference material (National Research Council Canada, Ottawa, Ontario, Canada) and Queensland Health Scientific Services (Coopers Plains, Queensland, Australia) in-house seafood mix standard reference materials (QAC 180, QAC 150, and FFM 04), finding values of 88–109% of the certified values for all elements.
Screening risk assessments (SRAs)
We performed SRAs for a number of the major POPs and metals, using methods previously reported for risk assessments of metals in sea turtle eggs (Lam et al. 2006). Hazard quotients (HQs) were calculated for each compound by dividing the measured concentration (MEC) by the predicted no-effect concentration (PNEC). We generally estimated the PNECs from toxicologic studies on the effects of these compounds on sea turtles and other oviparous reptiles. However, in cases where this information was not available, PNECs were estimated from bird data [see Supplemental Material available online (doi:10.1289/ehp.0900813.S1 via http://dx.doi.org/)]. The HQs were calculated for both a best-case and worst-case scenario (Equations 1 and 2). In cases where there was limited information and a maximum and minimum PNEC could not be determined, the same value was used for both HQs.
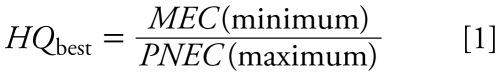 |
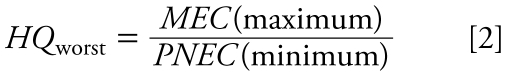 |
Human health risk assessments
The FAO/ WHO Joint Expert Committee on Food Additives (JECFA) has calculated ADIs for most POPs and heavy metals (FAO/WHO 2007; WHO 2008). The ADIs are based on human and animal experiments that investigate the no observable adverse effect levels of these chemicals, and are generally presented as micrograms per kilogram of body weight per day. Human health risk assessments generally include the percentage of people within a population with a chemical consumption level above the ADI for each compound (Van Oostdam et al. 2005). However, because the rate of C. mydas egg consumption in Peninsular Malaysia was not available and we did not investigate it in the present study, we used the maximum concentration of each of the major POP and metal compounds reported to evaluate risk by expressing the contaminant concentration as percentage of ADI in a single C. mydas egg (Equation 3). This calculation was based on a person weighing 65 kg consuming a single C. mydas egg of 35 g.
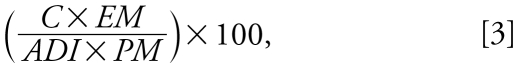 |
where C is the maximum concentration of contaminant reported in C. mydas eggs (micrograms per gram), EM is the mass of a C. mydas egg (35 g), ADI is in micrograms per kilogram of body weight per day, and PM is a person’s body weight (65 kg).
Statistical analysis
We arranged POPs into six major groups:
PCBs: 83 PCB congeners
OCPs: mirex, endosulfan I, pentachloro-benzene, dieldrin, and heptachlor epoxide
Chlordanes: oxychlordane, trans/cis-chlordane, trans/cis-nonachlor
Hexachlorocyclohexanes (HCHs): α-HCH, β-HCH, and-γ-HCH
Dichlorodiphenyltrichloroethanes (DDTs): 2,4′-DDE, 4,4′-DDE, 2,4′-DDD, 2,4′-DDT, 4,4′-DDD, and 4,4′-DDT
PBDEs: 19 PBDE congeners.
We calculated the mean concentration (± SE) of each POP group and metal and assigned all values < LOD a value of LOD/2. This produced the least amount of bias while not requiring the use of complex iteration software (Helsel 1990). We also calculated the number of individual POP compounds in each egg.
Results
Green turtle eggs in markets of Peninsular Malaysia
C. mydas eggs were found in 9 of the 33 markets visited (Table 1). All of these markets were on the east coast of Peninsular Malaysia and ranged from multiple vendors selling hundreds of eggs each (in the larger town markets) to a single vendor selling 50 100 eggs at a roadside fish market. According to the vendors, the eggs had been collected from seven different nesting sites, at times ranging from the previous night to 10 days before purchase for this study (Table 1). The nesting areas from which the eggs had been collected ranged from nearby beaches to as far away as Sabah in Borneo (Malaysia) [see Supplemental Material (doi:10.1289/ehp.0900813.S1)].
Table 1.
The nesting locations and time between collection and purchase of C. mydas eggs from markets in Peninsular Malaysia.
| Market | Egg collection location | Distance from market (km) | Days since collection |
|---|---|---|---|
| Kota Bharu | Turtle Island (Borneo) | 1,800 | 10 |
| Redang/Perhentian Islands | 80 | 7 | |
| Pasir Putih | Tioman Island | 400 | 8 |
| Penarik | Punti Bari | 2 | 1 |
| Kuala Terengganu | Redang Island | 65 | 1 |
| Turtle Island (Borneo) | 1,700 | 3 | |
| Perak | 300 | 2 | |
| Paka | Paka beach | 1 | 1 |
| Dungun | Paka beach | 1 | 3–7 |
| Mersing | Tioman Island | 55 | 1–7 |
| Cukai | Cherating | 10 | 7–10 |
| Kuantan | Turtle Island (Borneo) | 1,700 | 3–6 |
We identified OCPs, PCBs, chlordanes, and HCHs in all 55 of the eggs analyzed. Compounds most commonly identified were PCBs 18, 28/31, 52, 56, 74, 99, 118, 132/153, 138/158, 156, 170, 178, 180/193, 183, 187, 194, 196/203, and 202; dieldrin, endosulfan I, heptachlor epoxide, hexachlorobenzene, and mirex; β-HCH and γ-HCH; 4,4′-DDE and 4,4′-DDT; and PBDEs 47, 99, 100, 153, and 154. The OCPs and PCBs were the most highly concentrated POP compounds, whereas chlordanes and HCHs were generally found at trace levels. The PBDEs and DDTs were identified at trace levels in 84% of the eggs analyzed (Table 2). Percent lipids ranged from 7.3% to 13.5% (mean ± SE, 9.3 ± 0.1%).
Table 2.
Concentrations of POPs (pg/g wet mass) and metals (μg/g wet mass) detected in C. mydas eggs (n = 55) from the markets of Peninsular Malaysia.
| Compound | Mean ± SE | Range (n)a |
|---|---|---|
| POPs | ||
| ∑PCBs | 470.5 ± 83.3 | 146.6–3691.5 |
| ∑OCPs | 394.9 ± 43.1 | 169.5–2286.7 |
| ∑Chlordanes | 57.5 ± 9.4 | 24.7–514.2 |
| ∑HCHs | 68.8 ± 8.7 | 13.2–230.1 |
| ∑DDTs | 83.5 ± 18.3 | LOD–701.9 (46) |
| ∑PBDEs | 21.4 ± 6.6 | LOD–352.7 (46) |
| ∑POPs | 1096.6 ± 432.5 | 432.5– 6266.9 |
| No. of POPs | 27.0 ± 0.8 | 17–45 |
| Essential metals | ||
| Copper | 0.526 ± 0.023 | 0.056–1.073 |
| Zinc | 15.34 ± 0.93 | 1.33–39.48 |
| Selenium | 0.464 ± 0.026 | 0.049–0.836 |
| ∑Essential | 16.33 ± 0.96 | 1.44–40.92 |
| Toxic metals | ||
| Arsenic | 0.097 ± 0.011 | LOD–0.351 (36) |
| Cadmium | 0.009 ± 0.001 | LOD–0.029 (27) |
| Lead | 0.031 ± 0.003 | LOD–0.124 (6) |
| ∑Toxic | 0.138 ± 0.012 | LOD–0.380 (46) |
| Lipids (%) | 9.33 ± 0.14 | 7.3–13.54 |
sum of compounds.
Number of samples > LOD is shown in parentheses.
Of the essential metals, Zn, Cu, and Se were identified in all of the 55 eggs analyzed. However, Co was not detected in any of the eggs. Zn was the most highly concentrated element in each egg. For the toxic metals, As was the most common element with the highest concentrations, detected in 65% of the eggs sampled. Cd and Pb were less common, being detected in 49% and 11% of the eggs, respectively. Hg was not detected in any of the eggs (Table 2).
Screening risk assessments
The worst-case HQs ranged from < 0.01 to 0.51 for POPs and from 0.1 to 19.5 for heavy metals (Table 3).
Table 3.
The best and worst case HQs for metals and POPs identified in C. mydas eggs from markets in Peninsular Malaysia.
| Compound | MECmin | MECmax | PNECmin | PNECmax | HQworst | HQbest |
|---|---|---|---|---|---|---|
| POPs (ng/g) | ||||||
| DDTsa | 0 | 0.702 | 543 | 543 | < 0.01 | 0 |
| Dieldrin | 0.008 | 2.02 | 4 | 4 | 0.51 | < 0.01 |
| Tri-CBsb | 0 | 0.512 | 26 | 26 | < 0.01 | 0 |
| Tetra-CBsc | 0.060 | 2.00 | 26 | 26 | < 0.01 | < 0.01 |
| ∑ PCBsd | 0.147 | 3.69 | 26 | 26 | 0.14 | < 0.01 |
| Metals (μg/g) | ||||||
| Pb | 0 | 0.124 | 1 | 1 | 0.1 | 0 |
| Se | 0.049 | 0.836 | 0.34 | 6 | 2.5 | < 0.01 |
| Cd | 0 | 0.029 | 0.0014 | 0.013 | 0.2 | 0 |
| As | 0 | 0.351 | 0.018 | 0.018 | 19.5 | 0 |
Abbreviations, ∑, sum of compounds; max, maximum; min, minimum.
The sum of DDTs was used for MECs, but values for PNEC were derived from effects of p,p′-DDE.
The sum of tri-chlorinated biphenyls was used for MECs, but values for PNEC were derived from effects of a single trichlorinated biphenyl.
The sum of tetrachlorinated biphenyls was used for the MECs, but values for PNEC were derived from effects of a single tetrachlorinated biphenyl.
The sum of all PCBs was used for the MECs but values for PNEC were derived from effects of a single tetrachlorinated biphenyl.
Human health risk assessments
The maximum percent of ADI in one C. mydas egg ranged from < 0.1 to 30,247% for POP compounds and from 1.5 to 12.2% for metals (Table 4).
Table 4.
The maximum percentage of ADI in one C. mydas egg for the major POPs and metal compounds reported in eggs from Peninsular Malaysia.
| Compound | ADIa (μg/kg/day) | Egg concentration (ng/g wet mass) | Maximum percent of ADI in one eggb |
|---|---|---|---|
| POPs | |||
| PBDEs | 100 | < LOD–0.353 | < 0.1 |
| DDT | 20 | < LOD–0.702 | < 0.1 |
| Endosulfan | 6 | 0.104–0.271 | < 0.1 |
| PCBs | 1 | 0.147–3.69 | 0.2 |
| HCH | 0.3 | 0.013–0.231 | < 0.1 |
| Dieldrin | 0.1 | 0.008–2.02 | 1.1 |
| Mirex | 0.07 | < LOD–0.027 | < 0.1 |
| Chlordane | 0.05 | 0.025–0.514 | 0.6 |
| Coplanar PCBs | 2 × 10−6 | 0.014–1.29 | 30,247 |
| Metals | |||
| Zn | 300–1,000 | 1,330–39,480 | 9.1c |
| Cu | 50–500 | 56–1,070 | 1.5c |
| Se | 12.5 | 49–836 | 4.6 |
| Pb | 3.6 | < LOD–124 | 2.4 |
| As | 2 | < LOD–351 | 12.2 |
| Cd | 1 | < LOD–29 | 2.0 |
ADI determined by the JECFA and the International Programme on Chemical Safety (FAO/WHO 2007; WHO 2008).
Calculated based on a person weighing 65 kg consuming a single 35-g C. mydas egg.
Calculated from the most conservative ADI and maximum concentration of zinc reported (FAO/WHO 2007).
Discussion
Availability of green turtle eggs in markets of Peninsular Malaysia
The sale of C. mydas eggs was confined to towns on the east coast of Peninsular Malaysia and occurred in only 9 of the 33 markets surveyed. Furthermore, the long-distance transportation of eggs from Sabah and Perak to east coast markets may indicate that the local demand for sea turtle eggs is not currently being met because of the level of egg protection. Between 1996 and 2006, the proportion of C. mydas eggs laid in Peninsular Malaysia that were protected in hatcheries or in situ by the Department of Fisheries, Malaysia, increased from 13% to 94%. This effectively decreased the number of eggs available for collection and consumption per year from > 170,000 to < 15,000 (Department of Fisheries Malaysia, unpublished data). This drastic decline in supply could cause collection of eggs from other areas to meet the sustained demand.
The importation of C. mydas eggs from areas such as Sabah and Perak to compensate for the decline in local supply has ethical and legal implications that sea turtle managers need to consider. Conservation of C. mydas in Southeast Asia would be compromised if the protection of eggs in one area increased the collection of eggs in other areas. This would be a particular concern if egg collection increased in nesting habitats that were relatively less disturbed and more difficult to patrol. Furthermore, the transport of C. mydas eggs from Sabah to Peninsular Malaysia is illegal under the Convention on the Conservation of Migratory Species of Wild Animals (Convention on Migratory Species 2003) and Sabah state law (Sabah Ministry of Tourism, Culture and Environment 1997). Immediate conservation efforts must therefore increase patrolling and monitoring of egg transport between Borneo and Peninsular Malaysia. However, long-term solutions lie in reducing the demand for—and the consumption of—sea turtle eggs in the region.
The concentrations of POPs and metals in C. mydas eggs we found in the present study were generally lower than concentrations previously reported in loggerhead sea turtle (C . caretta) eggs (Alam and Brim 2000; Alava et al. 2006; Clark and Krynitsky 1980, 1985; Godley et al. 1999; Sakai et al. 1995; Stoneburner et al. 1980). Additionally, POP and metal concentrations in the present study were considerably different from those reported in previous studies on C. mydas eggs (Table 5). Higher concentrations in C. caretta eggs may be explained by the higher trophic level occupied by this carnivorous species. Differences in egg contamination between C. mydas from different locations may reflect varied regional use of these chemicals. However, the contamination of C. mydas is more likely caused by chemical use in the turtles’ foraging areas (Newman and Unger 2003). Therefore, because a C. mydas nesting population generally comprises turtles from a wide range of foraging areas (Cheng 2000; Godley et al. 2002; Liew et al. 1995; Seminoff et al. 2008), the precise source of egg contamination is difficult to determine.
Table 5.
Summary of the concentrations (mean ± SE or range) of common POP compounds (ng/g wet mass) and heavy metals (μg/g wet mass) reported in C. mydas eggs from previous studies.
| Location | No. of eggs | p,p′-DDE | ∑ PCBs | Cd | Pb | Reference |
|---|---|---|---|---|---|---|
| Florida (USA) | 2 | 2.0 ± 2.0 | — | — | — | Clark and Krynitsky 1980 |
| Ascension Islanda | 10 | < LOD–9 | 20–220 | — | — | Thompson et al. 1974 |
| Heron Islandb | 15 | 1.7 ± 0.3 | — | — | — | Podreka et al. 1998 |
| Hong Kong | 30 | — | — | < LOD | 0.03–0.14 | Lam et al. 2006 |
| Cyprus | 17 | — | — | 0.05–1.2 | LOD–1.61 | Godley et al. 1999 |
| Peninsular Malaysia | 55 | < LOD | 0.15–3.7 | LOD–0.03 | LOD–0.12 | Present study |
—, Not investigated.
Island in the South Atlantic Ocean.
Queensland, Australia.
Egg contamination and conservation
The SRAs indicated that the POP concentrations observed in the C. mydas eggs collected from markets in Peninsular Malaysia were unlikely to cause sex reversal in the developing embryos. Generally speaking, if the HQ of a particular compound is > 1, that compound is likely to have toxicologic effects (Suter 1993; Tannenbaum et al. 2003). Dieldrin posed the highest risk to sex reversal in the C. mydas eggs, although a worst-case HQ of 0.51 indicated that this risk was minimal. However, the metal concentrations observed in these C. mydas eggs indicated a higher risk of disruptions to embryonic development. In particular, the concentrations of As (worst-case HQ, 19.5) suggested a relatively high risk of embryonic mortality and reduced hatch success. Similarly, the HQ for Se (2.5) found in these eggs indicated that this metal would likely affect hatching success.
There are many uncertainties in the calculations of PNECs for the SRAs in the present study because of the low number of toxicologic studies specific to C. mydas eggs. Furthermore, we calculated the HQs for the toxicologic effects of single compounds acting alone. The present study is the first of its kind to analyze and identify such a large number of POP and metal compounds in C. mydas eggs. The large number of POP compounds we observed in the eggs (mean ± SE, 27.0 ± 0.8; range, 17 45) could therefore be important in terms of combined effects. As previously reported in Trachemys scripta, the combination of a trichlorinated biphenyl with a tetra-chlorinated biphenyl produced sex reversal at a lower concentration than when the PCBs were administered individually (Bergeron et al. 1994). The effect of multiple PCB compounds on sex reversal in the present study may therefore be more important than the HQs have indicated.
Egg contamination and human health
The most alarming statistic from the assessment of the human health risk associated with consuming C. mydas eggs in Peninsular Malaysia was related to coplanar PCBs.
The human health risk assessment we calculated in the present study indicated that the consumption of a single C. mydas egg can result in the intake of > 300 times the ADI of coplanar PCBs, as determined by JECFA (FAO/WHO 2007). Even though this represents the maximum risk calculated, coplanar PCBs were detected in all 55 C. mydas eggs analyzed. Even the consumption of an egg with the lowest concentration of coplanar PCBs reported would result in the intake of more than 3 times the ADI. Coplanar PCBs are particularly toxic and are often considered in the same context as dioxins and furans, some of the most toxic chemicals known (McKinlay et al. 2008). The reported concentrations of coplanar PCBs therefore create serious health risks to humans who consume C. mydas eggs in Peninsular Malaysia.
The risks associated with consuming C. mydas eggs in the present study are also likely to be an underestimation of the overall risk of these chemicals to human health in this region. Other foods consumed by the people of Peninsular Malaysia may also be contaminated with POPs and heavy metals. If these chemicals were also being consumed through other dietary intake, the contribution from C. mydas eggs would become more significant. In a recent global study on the contamination of skipjack tuna (Katsuwonus pelamis), concentrations of coplanar PCBs were highest in Southeast Asia (Ueno et al. 2005). Furthermore, high levels of other contaminants have been reported in the seafood of this region (Agusa et al. 2007). The human health risks associated with consuming contaminated food is therefore of particular concern in coastal communities of Southeast Asia. In addition, the ADIs are generally calculated on an individual compound basis (FAO/ WHO 2007). The effects of consuming foods containing multiple chemical compounds are not well understood. Despite the fact that the concentrations of coplanar PCBs indicate a high risk, the large number of chemicals found in C. mydas eggs may further increase the human health risks associated with consuming these eggs.
Incidentally, the chemical contamination found in the C. mydas eggs in Peninsular Malaysia may assist conservation efforts in the region. The health risk of consuming eggs contaminated with various chemical compounds could potentially reduce the number of eggs collected for consumption, which in turn would allow an increase in sea turtle populations in Southeast Asia. Although there are alternative, cost-effective sources of protein available to east coast communities of Peninsular Malaysia (e.g., fish, chicken, beef), it is the cultural perception that sea turtle eggs have medicinal qualities that primarily drives their consumption. One of the greatest challenges to sea turtle conservation agencies in this region is to reduce this cultural demand for sea turtle eggs. This could be achieved by awareness campaigns highlighting the health consequences of consuming sea turtle eggs containing such a large number of toxic compounds. If effective, this would presumably reduce the collection of sea turtle eggs for human consumption and contribute significantly to sea turtle conservation efforts in the region.
Footnotes
Supplemental Material is available online (doi:10.1289/ehp.0900813.S1 via http://dx.doi.org/).
Staff at the Department of Fisheries, Malaysia, provided logistical support for obtaining samples from Peninsular Malaysia. We especially thank J. Keller (National Institute of Standards and Technology, South Carolina, USA) for supply of calibrants and internal standards for POP measurements. We also thank all Queensland Health Scientific Services staff for assistance in the laboratories and R. Bak and B. Monczko (Griffith University) for assistance with setup and maintenance of the GC-MS/MS instrumentation.
This work was supported by the Australian Research Council (Linkage Grant LP0455513).
References
- Aguirre AA, Gardner SC, Marsh JC, Delgado SG, Limpus CJ, Nichols WJ. Hazards associated with the consumption of sea turtle meat and eggs: a review for health care workers and the general public. Ecohealth. 2006;3(3):141–153. [Google Scholar]
- Agusa T, Kunito T, Sudaryanto A, Monirith I, Kan-Atireklap S, Iwata H, et al. Exposure assessment for trace elements from consumption of marine fish in Southeast Asia. Environ Pollut. 2007;2007:766–777. doi: 10.1016/j.envpol.2006.04.034. [DOI] [PubMed] [Google Scholar]
- Alam SK, Brim MS. Organochlorine, PCB, PAH, and metal concentrations in eggs of loggerhead sea turtles (Caretta caretta) from northwest Florida, USA. J Environ Sci Health B. 2000;35(6):705–724. doi: 10.1080/03601230009373303. [DOI] [PubMed] [Google Scholar]
- Alava JJ, Keller JM, Kucklick JR, Wyneken J, Crowder L, Scott GI. Loggerhead sea turtle (Caretta caretta) egg yolk concentrations of persistent organic pollutants and lipid increase during the last stage of embryonic development. Sci Total Environ. 2006;367:170–181. doi: 10.1016/j.scitotenv.2006.02.029. [DOI] [PubMed] [Google Scholar]
- Bergeron JM, Crews D, McLachlan JA. PCBs as environmental estrogens: Turtle sex determination as a biomarker of environmental contamination. Environ Health Perspect. 1994;102:780–781. doi: 10.1289/ehp.94102780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop CA, Brooks RJ, Carey JH, Ng P, Norstrom RJ, Lean DRS. The case for cause-effect linkage between environmental contamination and development in eggs of the common snapping turtle (Chelydra serpentina serpentina) J Toxicol Environ Health. 1991;33(4):521–547. doi: 10.1080/15287399109531539. [DOI] [PubMed] [Google Scholar]
- Bryan AM, Olafsson PG, Stone CR. Disposition of low and high environmental concentrations of PCBs in snapping turtle tissue. Bull Environ Contam Toxicol. 1987;38:1000–1005. doi: 10.1007/BF01609087. [DOI] [PubMed] [Google Scholar]
- Chang LW. Toxicology of Metals. New York: CRC Press; 1996. [Google Scholar]
- Cheng I-J. Post-nesting migrations of green turtles (Chelonia mydas) at Wan-An Island, Penghu Archipelago, Taiwan. Mar Biol. 2000;137:747–754. [Google Scholar]
- Clark DR, Krynitsky AJ. Organochlorine residues in eggs of loggerhead turtles (Caretta caretta) and green sea turtles (Chelonia mydas) nesting at Merritt Island, Florida, USA—July and August 1976. Pestic Monit J. 1980;14(1):121–125. [PubMed] [Google Scholar]
- Clark DR, Krynitsky AJ. DDE residues and artificial incubation of loggerhead sea turtle eggs. Bull Environ Contam Toxicol. 1985;34:121–125. doi: 10.1007/BF01609712. [DOI] [PubMed] [Google Scholar]
- Colborn T, Vom Saal FS, Soto AM. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect. 1993;101:378–384. doi: 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Convention on Migratory Species. Convention on the Conservation of Migratory Species of Wild Animals. 2003. [[accessed 23 July 2009]]. Available: http://www.cms.int/pdf/convtxt/cms_convtxt_english.pdf.
- Day RD, Segars AL, Arendt MD, Lee AM, Peden-Adams MM. Relationship of blood mercury levels to health parameters in the loggerhead sea turtle (Caretta caretta) Environ Health Perspect. 2007;115:1421–1428. doi: 10.1289/ehp.9918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Solla SR, Fernie KJ, Ashpole S. Snapping turtles (Chelydra serpentina) as bioindicators in Canadian Areas of Concern in the Great Lakes Basin. II. Changes in hatching success and hatchling deformities in relation to persistent organic pollutants. Environ Pollut. 2008;153(3):529–536. doi: 10.1016/j.envpol.2007.09.017. [DOI] [PubMed] [Google Scholar]
- FAO/WHO (Food and Agriculture Organization of the United Nations/World Health Organization) Summary of Evaluations Performed by the Joint FAO/WHO Expert Committee on Food Additives (JECFA 1956–2007) 2007. [[accessed 24 July 2009]]. Available: http://jecfa.ilsi.org/
- Godley BJ, Richardson S, Broderick AC, Coyne MS, Glen F, Hays GC. Long-term satellite telemetry of the movements and habitat utilisation by green turtles in the Mediterranean. Ecography. 2002;25:352–362. [Google Scholar]
- Godley BJ, Thompson DR, Furness RW. Do heavy metal concentrations pose a threat to marine turtles from the Mediterranean Sea? Mar Pollut Bull. 1999;38(6):497–502. [Google Scholar]
- Guillette LJ, Jr, Crain DA. Endocrine-disrupting contaminants and reproductive abnormalities in reptiles. Comment Toxicol. 1996;5(4–5):381–399. [Google Scholar]
- Guillette LJ, Jr, Gunderson MP. Alterations in development of reproductive and endocrine systems of wildlife populations exposed to endocrine-disrupting contaminants. Reproduction. 2001;122:857–864. doi: 10.1530/rep.0.1220857. [DOI] [PubMed] [Google Scholar]
- Helsel DR. Less than obvious. Statistical treatment of data below the detection limit. Environ Sci Technol. 1990;24(12):1766–1774. [Google Scholar]
- Helwig DD, Hora ME. Polychlorinated biphenyl, mercury and cadmium concentrations in Minnesota snapping turtles. Bull Environ Contam Toxicol. 1983;30:186–190. doi: 10.1007/BF01610119. [DOI] [PubMed] [Google Scholar]
- Hendrickson JR, Alfred ER. Nesting populations of sea turtles on the east coast of Malaya. Bull Raffles Mus. 1961;26:190–196. [Google Scholar]
- Hendrickson JR, Balasingham E. Nesting beach preferences of Malayan sea turtles. Bull Natl Mus Singapore. 1966;33(10):69–76. [Google Scholar]
- Ibrahim K. The status of marine turtle conservation in Peninsular Malaysia. In: Nacu A, Trono R, Palma JA, Torres D, Agas F Jr, editors. Proceedings of the first ASEAN Symposium Workshop on Marine Turtle Conservation; 6–10 December 1993; Manila, Philippines . Manila, Philippines: ASEAN; 1994. pp. 87–103. [Google Scholar]
- Ibrahim K, Kassim AR, Schauble C, Hamann M. Making the most of hatchling production in Peninsular Malaysia—an urgent need to increase egg protection in marine parks. In: Seminoff JA, editor. Proceedings of the 22nd Annual Symposium on Sea Turtle Biology and Conservation; 4–7 April 2002; Miami, FL, USA. Miami, FL: NOAA Technical Memorandum; 2003. p. 116. NMFS-SEFSC-503. [Google Scholar]
- Keller JM, Kucklick JR, Stamper MA, Harms CA, McClellan-Green PD. Associations between organochlorine contaminant concentrations and clinical health parameters in loggerhead sea turtles from North Carolina, USA. Environ Health Perspect. 2004;112:1074–1079. doi: 10.1289/ehp.6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller JM, McClellan-Green PD, Kucklick JR, Keil DE, Peden-Adams MM. Effects of organochlorine contaminants on loggerhead sea turtle immunity: comparison of a correlative field study and in vitro exposure experiments. Environ Health Perspect. 2006a;114:70–76. doi: 10.1289/ehp.8143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller JM, Peden-Adams MM, Aguirre AA. Immunotoxicology and implications for reptilian health. In: Gardner SC, Oberborster E, editors. Toxicology of Reptiles. Boca Raton, FL: CRC Press; 2006b. pp. 199–240. [Google Scholar]
- Lam JCW, Tanabe S, Chan SKF, Lam MHW, Martin M, Lam PKS. Levels of trace elements in green turtle eggs collected from Hong Kong: evidence of risks due to selenium and nickel. Environ Pollut. 2006;144(3):790–801. doi: 10.1016/j.envpol.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Liew HC, Chan EH, Papi F, Luschi P. Long distance migration of green turtles from Redang Island: the need for regional cooperation in sea turtle conservation. In: Devaux B, editor. Proceedings of the International Congress of Chelonian Conservation; 6–10 July 1995; Gonfaron, France . Gonfaron, France: SOPTOM; 1995. pp. 73–75. [Google Scholar]
- Limpus CJ. Report to Department of Fisheries, Ministry of Agriculture, Malaysia. Brisbane, Australia: Queensland Department of Environment and Heritage; 1993. Recommendations for Conservation of Marine Turtles in Peninsula Malaysia. [Google Scholar]
- McKenzie C, Godley BJ, Furness RW, Wells DE. Concentrations and patterns of organochlorine contaminants in marine turtles from Mediterranean and Atlantic waters. Mar Environ Res. 1999;47:117–135. [Google Scholar]
- McKinlay R, Plant JA, Bell JNB, Voulvoulis N. Endocrine disrupting pesticides: implications for risk assessment. Environ Int. 2008;34:168–183. doi: 10.1016/j.envint.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Newman MC, Unger MA. Fundamentals of Ecotoxicology. Boca Raton, FL: Lewis Publishers; 2003. [Google Scholar]
- Olafsson PG, Bryan AM, Bush B, Stone W. Snapping turtles—a biological screen for PCBs. Chemosphere. 1983;12(11/12):1525–1532. [Google Scholar]
- Podreka S, Georges A, Maher B, Limpus CJ. The environmental contaminant DDE fails to influence the outcome of sexual differentiation in the marine turtle Chelonia mydas. Environ Health Perspect. 1998;106:185–188. doi: 10.1289/ehp.98106185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabah Ministry of Tourism, Culture and Environment. Wildlife Conservation Enactment 1997. 1997. [[accessed 23 July 2009]]. Enactment No. 6 of 1997. Available: http://www.sabahlaw.com/Wildliferegulation.htm.
- Sakai H, Ichihashi H, Suganuma H, Tatsukawa R. Heavy metal monitoring in sea turtles using eggs. Mar Pollut Bull. 1995;30(5):347–353. [Google Scholar]
- Sakao S, Uchida H. Determination of trace elements in shellfish tissue samples by inductively coupled mass spectrometry. Anal Chim Acta. 1999;382:215–223. [Google Scholar]
- Scheelings P. Determination of trace elements in foods by ICP-MS after microwave digestion. Brisbane, Australia: Queensland Health Scientific Services; 2002. [Google Scholar]
- Seminoff JA, Zarate P, Coyne MS, Foley DG, Parker D, Lyon BN, et al. Post-nesting migrations of Galapagos green turtles, Chelonia mydas, in relation to oceanographic conditions: integrating satellite telemetry with remotely sensed ocean data. Endang Species Res. 2008;4:57–72. [Google Scholar]
- Siow KT, Moll EO. Status and conservation of estuarine and sea turtles in West Malaysian waters. In: Bjorndal KA, editor. Biology and Conservation of Sea Turtles. Washington, DC: Smithsonian, Institute Press; 1981. pp. 339–347. [Google Scholar]
- Stoneburner DL, Nicora MN, Blood ER. Heavy metals in loggerhead sea turtle eggs (Caretta caretta) – evidence to support the hypothesis that demes exist in the western Atlantic population. J Herpetol. 1980;14(2):171–175. [Google Scholar]
- Suter GW. Ecological Risk Assessment. Boca Raton, FL: CRC Press; 1993. [Google Scholar]
- Tannenbaum LV, Johnson MS, Bazar M. Application of the hazard quotient method in remedial decisions: a comparison of human and ecological risk assessments. Hum Ecol Risk Assess. 2003;9(1):387–401. [Google Scholar]
- Thompson NP, Rankin PW, Johnston DW. Polychlorinated biphenyls and p,p′-DDE in green turtle eggs from Ascension Island, South Atlantic Ocean. Bull Environ Contam Toxicol. 1974;11(5):399–403. doi: 10.1007/BF01685294. [DOI] [PubMed] [Google Scholar]
- Tinggi U, Craven G. Determination of total mercury in biological materials by cold vapour atomic absorption spectrometry after microwave digestion. Microchem J. 1996;54:168–173. [Google Scholar]
- Tinggi U, Gianduzzo T, Francis R, Nicol D, Shahin M, Scheelings P. Determination of selenium in red blood cells by inductively coupled plasma mass spectrometry (ICP-MS) after microwave digestion. J Radioanal Nucl Chem. 2004;259(3):469–472. [Google Scholar]
- Ueno D, Watanabe M, Subramanian A, Tanaka H, Fillmann G, Lam PKS, et al. Global pollution monitoring of poly-chlorinated dibenzo-p-dioxins (PCDDs), furans (PCDFs) and coplanar polychlorinated biphenyls (coplanar PCBs) using skipjack tuna as bioindicator. Environ Pollut. 2005;136(2):303–313. doi: 10.1016/j.envpol.2004.12.036. [DOI] [PubMed] [Google Scholar]
- van de Merwe JP, Whittier JM, Ibrahim K, Hodge M, Lee SY. Analysing persistent organic pollutants in eggs, blood and tissue of the green sea turtle (Chelonia mydas) using gas chromatography with tandem mass spectrometry (GC- MS/MS) Anal Bioanal Chem. 2009;393(6):1719–1731. doi: 10.1007/s00216-009-2608-0. [DOI] [PubMed] [Google Scholar]
- Vander Pol SS, Ellisor MB, Pugh RS, Becker PR, Poster DL, Schantz MM, et al. Development of a murre (Uria spp.) egg control material. Anal Bioanal Chem. 2007;387(8):2357–2363. doi: 10.1007/s00216-006-0887-2. [DOI] [PubMed] [Google Scholar]
- Van Oostdam J, Donaldson SG, Feeley M, Arnold D, Ayotte P, Bondy G, et al. Human health implications of environ -mental contaminants in Arctic Canada: a review. Sci Total Environ. 2005;2005:165–246. doi: 10.1016/j.scitotenv.2005.03.034. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization) Inventory of IPCS and other WHO pesticide evaluations and summary of toxicological evaluations performed by the Joint Meeting on Pesticide Residues (JMPR) through 2008. 2008. [[accessed 12 February 2008]]. Available: http://www.who.int/ipcs/publications/jmpr/jmpr_pesticide/en/index.html.


