Abstract
Background
Decreasing exposure to airborne particulates was previously associated with reduced age-related decline in lung function. However, whether the benefit from improved air quality depends on genetic background is not known. Recent evidence points to the involvement of the genes p53 and p21 and of the cell cycle control gene cyclin D1 (CCND1) in the response of bronchial cells to air pollution.
Objective
We determined in 4,326 participants of the Swiss Cohort Study on Air Pollution and Lung and Heart Diseases in Adults (SAPALDIA) whether four single-nucleotide polymorphisms in three genes [CCND1 (rs9344 [P242P], rs667515), p53 (rs1042522 [R72P]), and p21 (rs1801270 [S31R])] modified the previously observed attenuation of the decline in the forced expiratory flow between 25% and 75% of the forced vital capacity (FEF25–75) associated with improved air quality.
Methods
Subjects of the prospective population-based SAPALDIA cohort were assessed in 1991 and 2002 by spirometry, questionnaires, and biological sample collection for genotyping. We assigned spatially resolved concentrations of particulate matter with aerodynamic diameter ≤ 10 μm (PM10) to each participant’s residential history 12 months before the baseline and follow-up assessments.
Results
The effect of diminishing PM10 exposure on FEF25–75 decline appeared to be modified by p53 R72P, CCND1 P242P, and CCND1 rs667515. For example, a 10-μg/m3 decline in aver-age PM10 exposure over an 11-year period attenuated the average annual decline in FEF25–75 by 21.33 mL/year (95% confidence interval, 10.57–32.08) among participants homozygous for the CCND1 (P242P) GG genotype, by 13.72 mL/year (5.38–22.06) among GA genotypes, and by 6.00 mL/year (−4.54 to 16.54) among AA genotypes.
Conclusions
Our results suggest that cell cycle control genes may modify the degree to which improved air quality may benefit respiratory function in adults.
Keywords: air pollution, cell cycle, cohort study, genes, respiratory function tests
A large body of evidence underscores the adverse effect of long-term exposure to ambient particulate matter (PM) air pollution on respiratory health (Brunekreef and Forsberg 2005; Gotschi et al. 2008). Among adults in Switzerland, we have previously demonstrated cross-sectionally that residents of more polluted areas have lower lung function (Ackermann-Liebrich et al. 1997). More recently, we presented evidence from the same population-based cohort [Swiss Cohort Study on Air Pollution and Lung and Heart Diseases in Adults (SAPALDIA)] that decreasing exposure to airborne PM attenuated the average age-related decline in lung function. The associations were strongest for respiratory function tests reflecting small-airway function, namely, FEF25–75 [forced expiratory flow between 25% and 75% of forced vital capacity (FVC)] (Downs et al. 2007). Similar results from studies following interventions such as building bypasses for congested traffic routes (Burr et al. 2004; Hedley et al. 2002) or banning environmental tobacco smoke (ETS) exposure (Goodman et al. 2007; Menzies et al. 2006) showed that the improvements in air quality were accompanied by a decrease in cardiopulmonary mortality and an improvement in respiratory symptoms and lung function. However, it is still unknown whether all subjects benefit equally from a reduction in air pollution.
Variation in genes mediating the pathobiological effect of air pollution in the lung may codetermine the degree to which a person benefits from better air quality. Experimental evidence indicates that PM alters expression of tumor protein gene p53, cyclin-dependent kinase inhibitor 1A gene (p21), and the cyclin D1 gene (CCND1) and subsequently affects cell proliferation and apoptosis of lung fibroblasts, lymphocytes, and alveolar epithelial cells (Bayram et al. 2006; Dagher et al. 2006; Nyunoya et al. 2006; Rosas Perez et al. 2007; Soberanes et al. 2006). PM is furthermore well known to induce oxidative stress in the airways (Li et al. 2008). In fact, the expression of all three gene candidates, p53, p21, and CCND1, in bronchial epithelial cells and lung fibroblasts seems to be regulated in part by redox-dependent mechanisms (Jiao et al. 2008; Ranjan et al. 2006; Yao et al. 2008).
The tumor suppressor p53, a nuclear transcription factor, binds to response elements in the promoter region of many genes and plays a pivotal role in apoptosis. It induces up-regulation of the expression of many pro-apoptotic genes and down-regulation of anti-apoptotic genes (Oren et al. 2002). CCND1 (cyclin D1) is known to promote cell proliferation through cell cycle G1–S phase transition. The protein p21 (also known as Waf1 or Cip1) is a direct functional counterpart of CCND1 and an important downstream effector of p53 action that negatively regulates cell proliferation. CCND1, p21, and p53 all harbor polymorphisms of hypothesized functional relevance that have been extensively studied in the context of cancer (Choi et al. 2008; Lu et al. 2008; Zhou et al. 2007). In this study, we examined whether these polymorphisms modified the degree to which the age-related FEF25–75 decline was attenuated by reduced exposure to PM with aerodynamic diameter ≤ 10 μm (PM10).
Materials and Methods
SAPALDIA cohort study population
The study design and methodology of SAPALDIA have been described in detail elsewhere (Ackermann-Liebrich et al. 2005; Martin et al. 1997). In 1991, health examinations focusing on respiratory health status were conducted in 9,651 adults (18–60 years of age) randomly selected from population registries of eight environmentally diverse areas of Switzerland. Ethical approval was obtained from the Swiss Academy of Medical Sciences and the Regional Ethics Committees. Written informed consent was obtained from all participants before health examination and biological sample collection at both surveys. Nonparticipation at follow-up and missing information on covariates led to the exclusion of participants for the present study [see Supplemental Material, Figure 1 and Table 2 available online (doi:10.1289/ehp.0800430. S1 via http://dx.doi.org)]. In summary, of the 9,651 subjects who initially participated at baseline (SAPALDIA1), 1,604 had died, left Switzerland, or refused to participate at the follow-up examination (SAPALDIA2). Of 8,047 cohort participants remaining, 5,732 completed the interview questionnaire and spirometry at both the baseline and follow-up surveys. Participants were excluded from the analysis (n= 1,406) if they had lived for < 1 year at their last residential address at follow-up, could not be assigned home outdoor PM10 concentrations, or did not provide blood samples for DNA extraction or if the genetic analysis of their sample was unsuccessful. Thus, the present study sample included 4,326 participants with available blood samples and genotype data and complete data from both surveys on spirometry, smoking history, PM10 exposure, and residential history during follow-up.
Lung function assessment
Identical spirometer devices (model 2200, SensorMedics Corp., Yorba Linda, CA, USA) and protocols were used at baseline and follow-up examinations, and their comparability was assessed before the follow-up study (Künzli et al. 1995, 2005). Details of these measurements have been described elsewhere (Ackermann-Liebrich et al. 2005; Downs et al. 2005). Briefly, three to eight forced expiratory lung function maneuvers were performed by each participant to obtain a minimum of two measures of FEF, FVC, forced expiratory volume in the first second (FEV1), and FEF25–75 that were considered acceptable according to American Thoracic Society (1995) criteria. Expiratory flow measures were taken from the same flow-volume curve. For each participant, the rate of change in lung function was defined as the difference in each parameter between the two examinations (measurement at follow-up minus measurement at baseline), divided by the participant-specific follow-up time (in nontruncated years).
Selection of genetic variants
We selected the same common, well-studied, and potentially functional candidate single-nucleotide polymorphism (SNP) in CCND1 [proline-to-proline substitution at amino acid 242 (P242P), rs9344] that we evaluated in our previous research on the interaction of CCND1 with oxidative stress in breast and colon cancer (Ceschi et al. 2005; Probst-Hensch et al. 2006). We selected one additional common SNP in the CCND1 gene (rs667515) based on a pilot study involving haplotype-tagging polymorphisms in CCND1 and their association with accelerated lung function decline in a subsample of the SAPALDIA cohort (unpublished observations). In addition, we examined SNPs in two other important cell cycle control genes that have been repeatedly assessed in cancer association studies and are reported to have a functional effect: p53 [arginine-to-proline substitution at amino acid 72 (R72P), rs1042522] (Bergamaschi et al. 2006) and p21 [serine-to-arginine substitution at amino acid 31 (S31R), rs1801270](Lukas et al. 1997).
Genotpying
DNA was extracted from EDTA-buffered whole blood as previously described (Ackermann-Liebrich et al. 2005). Genotyping strategy used was fluorescent 5′-nuclease real-time polymerase chain reaction (TaqMan, Applera Europe, Rotkreuz, Switzerland) methodology using ABI Prism 7900 sequence detection system (ABI, Rotkreuz, Switzerland). The SNP-specific primers and locked nucleic acid (LNA) dual-labeled fluorogenic probes were designed by Sigma Proligo (Evry, France). SNP-specific probes and primers were as follows: rs1042522, 5′-CTGCTCCCCuC/G3CGTGGC-3′, forward 5′-ACTGAAGACCCAGGTCCA-3′, reverse 5′-GCCGGTGTAGGAGCT-3′; rs1801270, 5′-GCTGAGuC/A3CGCGAC-3′, forward 5′-TGCCGCCGCCTCTT-3′, reverse 5′-GATGCAGCCCGCCATTAG-3′; rs667515, 5′-AGCTCCCTTGCuG/C3 CCC-3′, forward 5′-TGGCTTCAT_ C A G A T G A C A A C -3′, reverse 5′-AACCTGGGCTTCTCCAA-3′; rs9344, 5′-TGTGACCCuA/G3GTAAGTGA-3′, forward 5′-ACGCTTCCTCTCCAGAG_3′, reverse 5′-CAAGGCTGCCTGG-3′. A 10% random sample of all DNA samples was regenotyped, and all geno types were confirmed. The genotype call rate was > 99%.
Individual residential PM10 exposure assessment
Air pollution exposure assessment has been described in detail elsewhere (Downs et al. 2007; Liu et al. 2007). We estimated PM10 exposure for each participant according to residential history using a hybrid exposure model incorporating Gaussian dispersion (from PolluMap model, version 2.0; Liu et al. 2007) and geoinformation based on data on seasonal, meteorologic, and geographic annual emission characteristics from various source categories (e.g., traffic, industrial, regional, and agricultural activities) (Liu et al. 2007). Hourly concentrations of PM10 were simulated with a spatial resolution of 200 × 200 m grid cells for 1990 and 2002. Annual average PM10 exposure concentrations during follow-up were estimated for each residential address with the help of an algorithm that allowed interpolation of modeled values on the basis of historical trends in central-site measurements between 1990 and 2002. Evaluation of dispersion model predictions using a total of 57 PM10 Swiss central-site monitors has been described elsewhere (Liu et al. 2007). Based on the validated dispersion model, each subject was assigned an annual PM10 concentration every year between 1990 and 2002. For that purpose, modeled PM10 concentrations were averaged over the year to obtain annual averages for each grid cell. Each participant’s residential history was coded using the geographic information system data. Assignment of individual PM10 exposure was performed by matching address codes with annual concentrations derived from the grid cells generated by the dispersion model.
The exposure variable used for the present study was the difference in the annual average PM10 exposure between 2002 and 1991. It was calculated for each participant as follows: average home outdoor PM10 concentration in the 12 months before the baseline examination was subtracted from the corresponding average concentration in the 12 months before the follow-up examination, and this difference was divided by the mean time of follow-up (in nontruncated years). In our previous study (Downs et al. 2007), we used two exposure indexes: a) the difference in the annual average exposure between 2002 and 1991 (the index used in the present study ) and b) the “interval exposure,” defined as the sum of the annual exposures for each subject for each year of follow-up between their examinations. Because of the high correlation (R2 > 0.9) between these two exposure variables, we were unable to single out the superior measure in our previous report. Both indices showed similar associations with change in lung function (Downs et al. 2007).
Collection of data on covariates
Information on relevant covariates known or likely to determine lung function decline, such as age, height, smoking history, ETS exposure, occupational exposure, and education level, was gathered in a computer-assisted, individually administered interview based on the European Community Respiratory Health Survey questionnaire (Burney et al. 1994). Current and past smoking habits, exposure to ETS, and occupational exposure to dust and fumes were assessed with the same questions at both surveys. Participants who reported smoking < 20 packs of cigarettes and using < 360 g of tobacco in their lifetime at both time points were defined as never-smokers. Cumulative cigarette exposure of participants was assessed by pack-years smoked before the first examination and pack-years smoked during follow-up. Participants were asked not to smoke in the hour before the examination, and smoking was validated by measuring the carbon monoxide concentration in exhaled breath using an EC 50 Micro-Smokerlizer (Bedfont Scientific, Rochester, UK). Atopic status was assessed at baseline using skin prick tests (Phazet; Pharmacia, Uppsala, Sweden) for eight common inhalant allergens: dog epithelium; cat fur; pollen of timothy grass, Parietaria, and birch; the house dust mite Dermatophagoides pteronyssinus; and the molds Alternaria tenuis and Cladosporium herbarum (Martin et al. 1997; Wuthrich et al. 1996). Atopy was defined by an adjusted mean wheal diameter ≥ 3 mm to at least one allergen.
Statistical analysis
We inferred CCND1 haplotypes from unphased genotype data using PHASE 2.1 algorithm software [see Supplemental Material, Table 1 (doi:10.1289/ehp.0800430.S1)] (Stephens et al. 2001). Hardy-Weinberg equilibrium was tested using STATA gtab command for global κ-statistic testing (STATA version 10; StataCorp, College Station, TX, USA). All four SNPs were in Hardy-Weinberg equilibrium. We obtained Lewtonin’s linkage disequilibrium (LD) metric D′using STATA command pwld for pairwise LD. Descriptive analyses of the lung function parameters, PM10, smoking, socioeconomic variables, and other relevant covariates have been described previously and in detail in different SAPALDIA cohort study reports (Ackermann-Liebrich et al. 2005; Downs et al. 2007; Imboden et al. 2007). We compared baseline characteristics of the cohort participants included in this study (n = 4,326) with subjects participating only at baseline as well as with cohort participants excluded from this analysis because information on genotype or covariate data was missing.
Results are presented as the estimated effect of a 10-μg/m3 decrease in the annual average PM10 exposure over the follow-up period (ΔPM10) on the average attenuation of the annual decline in FEF25–75.
The association between ΔPM10 and the average annual rate of lung function decline had been previously assessed using mixed linear model analysis, and selection of relevant covariates was based on this previous investigation (Downs et al. 2007): age at baseline (SAPALDIA1), age2, sex, height, parental smoking during childhood reported at baseline, sine and cosine function of day of examination to control for seasonal effects, level of education at baseline, change in level of education, Swiss nationality, self-reported occupational exposure to dust and occupational exposure to fumes at SAPALDIA1 and SAPALDIA2 (yes/no), smoking status at follow-up (never, former, or current), pack-years up to SAPALDIA1, pack-years between SAPALDIA1 and -2, cigarettes per day at SAPALDIA1 and -2, atopy, body mass index (BMI) at SAPALDIA1, change in BMI, interaction between the two BMI variables, and baseline PM10 exposure. Random effects were included to adjust for clustering of residuals within area and were assumed to be independent between the areas and to have an exchangeable correlation structure. To estimate main effects of gene variants of CCND1, p21, and p53 and rate of lung function decline, we used the same mixed model with random area effects additionally adjusted for ΔPM10. To estimate modification of the ΔPM10 effect on average lung function decline by genotype, we introduced interaction terms between ΔPM10 and genotypes into the above-described covariate-adjusted mixed linear models independently for each SNP. Also, for each SNP we evaluated three different genetic models (codominant, dominant, and recessive) because previous cancer association studies were not conclusive about the underlying genetic model of the SNP effects. The codominant model assumes that the gene effect depends on the number of alleles in a dose-dependent manner, the dominant model assumes that the gene effect depends on the presence of at least one of two high-risk alleles, and the recessive model assumes that the gene effect depends on the presence of both high-risk alleles. The three genetic models required different coding of genotypes. For each SNP we present the genetic model with the smallest interaction term p-value. We obtained effect estimates for the association between ΔPM10 and average annual lung function decline in genotype subgroups by creating genotype- specific PM10 exposures variables that we introduced into separate covariate-adjusted mixed linear models. Analyses were conducted using SAS release 9.1 (SAS Institute Inc., Cary, NC, USA) and STATA version 10. p-Values < 0.05 were interpreted as statistically significant for main and interaction effects. p-Values presented as main p-values in the tables are not corrected for multiple testing. Nevertheless, Bonferroni-corrected significance level (α = 0.05 divided by the number of tests) is indicated in tables and figures as appropriate.
Results
Population characterization
Table 1 describes the general characteristics of the study population (n = 4,326), including relevant predictors of lung function. This is a population consisting mostly of Caucasians. At follow-up participants were more likely to be women and less likely to be smokers than were non-participants. Participants tended to gain weight, give up smoking, and reduce exposure to dust or fumes at work or to ETS [for details, see Supplemental Material, Table 2 (doi:10.1289/ehp.0800430.S1)].
Table 1.
Characteristics of the study population: SAPALDIA cohort.
| Characteristic | Participants (n = 4,326) |
|---|---|
| Female (%) | 53.0 |
| Swiss nationality (%) | 87.7 |
| Educational level in 2002 (professional education or higher, %) | 27.9 |
| Increase in educational level between surveys (%) | 17.9 |
| Age in 1991 [mean ± SD (years)] | 41.3 ± 11.2 |
| Height [mean ± SD (cm)] | 169.3 ± 8.8 |
| BMI in 1991 [mean ± SD (kg/m2)] | 23.7 ± 3.6 |
| BMI change [mean ± SD (kg/m2)] | 2.1 ± 2.2 |
| Smoking | |
| Never-smokers in 1991 (%) | 48.4 |
| Never-smokers in 2002 (%) | 49.3 |
| Smoking quitters during follow-up (%) | 8.1 |
| Current smokers in 1991 (%) | 29.3 |
| Current smokers in 2002 (%) | 21.9 |
| No. of pack-years for current smokers in 2002 [median (IQR)] | 26.7 (14.0–42.6) |
| Cigarettes per day for current smokers in 1991 [median (IQR)] | 20 (10–25) |
| Cigarettes per day for current smokers in 2002 [median (IQR)] | 15 (7–20) |
| Passive smoking and occupational exposure (%) | |
| ETS exposure in never-smokers in 1991 | 13.1 |
| ETS exposure in never-smokers in 2002 | 7.7 |
| Father or mother smoked during childhood | 56.1 |
| Workplace exposure to dust and fumes in 1991 | 30.3 |
| Workplace exposure to dust and fumes in 2002 | 13.2 |
| Atopy in 1991 | 22.3 |
| Change in average individual home outdoor PM10 exposurea | |
| All areas (n = 4,326) | −5.8 (−7.3 to −4.2) |
| Basel area (n = 486) | −8.0 (−9.1 to −6.9) |
| Wald area (n = 889) | −4.5 (−4.8 to −3.9) |
| Davos area (n = 318) | −3.0 (−3.1 to −2.8) |
| Lugano area (n = 568) | −12.1 (−13.5 to −10.8) |
| Montana area (n = 431) | −4.0 (−4.2 to −3.7) |
| Payerne area (n = 595) | −5.0 (−5.3 to −4.6) |
| Aarau area (n = 689) | −6.4 (−6.8 to −5.8) |
| Geneva area (n = 350) | −6.2 (−7.3 to −5.7) |
| Lung function in 1991 [mean ± SD (L)]b | |
| FVC in women | 3.82 ± 0.61 |
| FVC in men | 5.30 ± 0.82 |
| FEV1 in women | 3.07 ± 0.55 |
| FEV1 in men | 4.11 ± 0.72 |
| FEF25–75 in women | 3.07 ± 1.00 |
| FEF25–75 in men | 3.79 ± 1.29 |
| Annual change [mean ± SD) (mL/year)]b | |
| FVC in women | −20.79 ± 34.11 |
| FVC in men | −29.17 ± 45.47 |
| FEV1 in women | −31.88 ± 25.72 |
| FEV1 in men | −39.77 ± 32.91 |
| FEF25–75 in women | −68.89 ± 58.88 |
| FEF25–75 in men | −74.11 ± 70.24 |
| Genotype distribution | |
| p53 P72R, rs1042522 | |
| GG | 2,407 |
| CG | 1,637 |
| CC | 282 |
| p21 S31R, rs1801270 | |
| CC | 3,701 |
| CA or AAc | 625 |
| CCND1 P242P, rs9344 | |
| GG | 1,211 |
| GA | 2,140 |
| AA | 975 |
| CCND1 −7006G>C, rs667515 | |
| GG | 1,628 |
| GC | 2,058 |
| CC | 640 |
| Diplotype distributiond | |
| CCND1 haplotype 1 (rs667515, rs9344) | |
| −/− | 3,125 |
| GG/− | 1,088 |
| GG/GG | 113 |
| CCND1 haplotype 2 (rs667515, rs9344) | |
| −/− | 1,251 |
| GA/− | 2,150 |
| GA/GA | 925 |
| CCND1 haplotype 3 (rs667515, rs9344) | |
| −/− | 1,678 |
| CG/− | 2,048 |
| CG/CG | 600 |
| CCND1 haplotype 4 (rs667515, rs9344) | |
| −/− | 4,239 |
| CA/− | 84 |
| CA/CA | 3 |
IQR, interquartile range.
PM10 (μg/m3) in the year before SAPALDIA1 minus PM10 in the year before SAPALDIA2.
Women, n = 2,293; men, n = 2,033.
Genotype distribution: p21 CA, n = 594; p21 AA, n = 31.
Diplo-type distribution is labeled as follows: −/−, none of the specific haplotype present; (rs667515, rs9344)/− (e.g., GG/−), one of the specific haplotypes present; (rs667515, rs9344)/(rs667515, rs9344) (e.g., GG/GG), for two of the specific haplotypes present.
In 2002, 87% of the participants were living in the same area as in 1991, and 54% had the same address. In general, individual home outdoor concentrations of PM10 declined during the follow-up period (Table 1), as previously described in detail (Downs et al. 2007; Liu et al. 2007). The median decline between examinations for subjects included in this analysis was 5.8 μg/m3 (interquartile range, 4.2–7.3 μg/m3). Mean decline was greatest for participants living in urban areas and lowest in alpine areas.
Mean lung function for the cohort was within the range of predicted values for the general population and declined during the follow-up period (Table 1). The mean ± SD annual change in mid FEF25–75 during the 11-year follow-up period was −74.1 ± 70.3 mL/year in men and −68.9 ± 58.9 mL/year in women included in this analysis.
The main effects of the investigated genetic variants on change in lung function are described in more detail in Supplemental Material, Table 3 (doi:10.1289/ehp.0800430. S1). Briefly, we observed no statistically significant associations of the polymorphisms either with change in FEF25–75 or with FEV1 or FVC.
Modification of the ΔPM10 effect on aver-age decline in lung function by genotype
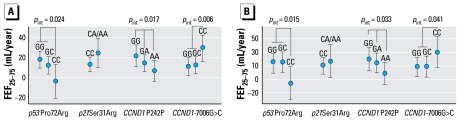
As previously reported (Downs et al. 2007), a 10-μg/m3 decline in average annual home outdoor PM10 concentration over an 11-year period (ΔPM10) reduced the annual rate of decline in FEF25–75 on average by 11.2 mL/year or 16%. Here we report statistically signifi cant modifications of this association of ΔPM10 with FEF25–75 decline by three of the four genetic variants investigated: CCND1 P242P [p-value for interaction (pint) = 0.017], CCND1 rs667515 (pint = 0.006), and p53 R72P (pint = 0.016). Figure 1A and Table 2 present results for the association of ΔPM10 with the aver-age annual change in FEF25–75 within genotype and diplotype strata for the entire study population; for data for never-smokers, see Figure 1B and Supplemental Material, Table 4 (doi:10.1289/ehp.0800430.S1). Equivalent to the previously reported main effect of ΔPM10 (Downs et al. 2007), the genotype-specific results we observed in the entire study popu lation were comparable to those in never-smokers. The A allele for CCND1 P242P reduced the attenuating effect estimate of ΔPM10 on FEF25–75 decline in a codominant manner. A 10 μg/m3 decline in PM10 over an 11-year period was associated with an average attenuation of annual decline in FEF25–75 of 6.0 mL/year [95% confidence interval (CI), −4.54 to 16.54] in participants with an AA genotype, 13.7 mL/year (5.38–22.06) in heterozygous AG participants, and 21.3 mL/year (10.57–32.08) in participants with a GG genotype.
Figure 1.
Attenuation of average annual FEF25–75 decline associated with a 10-μg/m3 decrease in average home outdoor PM10 exposure between 1991 and 2002, by genotype status, in all study participants (A) and in never-smokers only (B). A positive value for FEF25–75 on the y-axes represents the average attenuation in lung function decline associated with an average 10-μg/m3 PM10 decrease during follow-up period. Bonferroni significance level for four comparisons p = 0.013.
Table 2.
Effect modification by genotypes: associationa of change in average home outdoor PM10 (per decrease of 10 μg/m3 between 1991 and 2002) with average annual decline in FEF25–75 (mL/year), by genotype status.
| Genotype | No. | Average annual FEF25–75 declineb | 95% CI | p-Value | pintc |
|---|---|---|---|---|---|
| p53 R72P; rs1042522 | |||||
| GG | 2,407 | 17.37 | 8.95 to 25.78 | < 0.001 | 0.016(codominant) |
| CG | 1,637 | 11.63 | 2.65 to 20.61 | 0.011 | |
| CC | 282 | −4.33 | −21.37 to 12.7 | 0.618 | |
| p21 S31R; rs1801270 | |||||
| CC | 3,701 | 12.72 | 5.35 to 20.1 | 0.001 | 0.115(dominant) |
| CA or AAd | 625 | 23.88 | 9.25 to 38.51 | 0.001 | |
| CCND1 P242P; rs9344 | |||||
| GG | 1,211 | 21.33 | 10.57 to 32.08 | < 0.001 | 0.017(codominant) |
| AG | 2,140 | 13.72 | 5.38 to 22.06 | 0.001 | |
| AA | 975 | 6.00 | −4.54 to 16.54 | 0.265 | |
| CCND1 −7006G>C; rs667515 | |||||
| GG | 1,628 | 10.39 | 1.32 to 19.47 | 0.025 | 0.006(recessive) |
| CG | 2,058 | 11.83 | 3.24 to 20.42 | 0.007 | |
| CC | 640 | 28.83 | 15.6 to 42.07 | < 0.001 | |
| CCND1 haplotype 1 (rs667515, rs9344)e | |||||
| −/− | 3,125 | 13.67 | 5.99 to 21.34 | < 0.001 | 0.156(recessive) |
| GG/− | 1,088 | 11.1 | 0.26 to 21.94 | 0.045 | |
| GG/GG | 113 | 37.77 | 3.24 to 72.3 | 0.032 | |
| CCND1 haplotype 2 (rs667515, rs9344)e | |||||
| −/− | 1,251 | 20.66 | 10.07 to 31.24 | < 0.001 | 0.022(codominant) |
| GA/− | 2,150 | 13.75 | 5.34 to 22.15 | 0.001 | |
| GA/GA | 925 | 6.08 | −4.5 to 16.67 | 0.26 | |
| CCND1 haplotype 3 (rs667515, rs9344)e | |||||
| −/− | 1,678 | 10.28 | 1.22 to 19.35 | 0.026 | 0.003(recessive) |
| CG/− | 2,048 | 11.92 | 3.4 to 20.44 | 0.006 | |
| CG/CG | 600 | 30.9 | 17.21 to 44.58 | < 0.001 | |
| CCND1 haplotype 4 (rs667515, rs9344)e | |||||
| −/− | 4,239 | 13.52 | 6.22 to 20.82 | < 0.001 | 0.434(codominant) |
| CA/− | 84 | −1.4 | −39.67 to 36.88 | 0.943 | |
| CA/CA | 3 | −11.8 | −468.22 to 444.62 | 0.96 |
Covariates were age, age2, sex, height, parental smoking, sine and cosine function of day of examination to control for seasonal effects, level of education at SAPALDIA1, change in level of education, Swiss nationality, self-reported occupational exposure to dust and occupational exposure to fumes at SAPALDIA1 and SAPALDIA2 (yes/no), smoking status at SAPALDIA2 (never, former, or current), pack-years up to SAPALDIA1, pack-years between SAPALDIA1 and -2, cigarettes per day at SAPALDIA1 and -2, atopy, BMI at SAPALDIA1, change in BMI, interaction between the two BMI variables, and baseline PM10 exposure.
Positive estimates indicate attenuation of lung function decline associated with PM10 decrease. Negative estimates indicate acceleration of lung function decline with PM10 decrease.
p-Value for interaction between change in home outdoor exposure of PM10 and genotype parameterized in three different genetic models. The pint values presented here represent the most significant (lowest) p-value obtained from the three different genetic models. Bonferroni significance level for 12 comparisons [three respiratory function tests (FVC, FEV1, FEF25–75) × times four association tests], p = 0.00417.
Genotype distribution: p21 CA, n = 594; p21 AA, n = 31.
Diplotype distribution is labeled as follows: −/−, none of the specific haplotype present; (rs667515, rs9344)/− (e.g., GG/−), one of the specific haplotypes present; (rs667515, rs9344)/(rs667515, rs9344) (e.g., GG/GG), for two of the specific haplotypes present.
For the CCND1 rs667515 SNP, the beneficial ΔPM10 effect was most pronounced in participants homozygous for the minor allele (CC genotype) and attenuated annual FEF25–75 decline in this subgroup by 28.83 mL/year (95% CI, 15.60–42.07) compared with 10.39 (3.24–20.47) and 11.83 (1.32–19.47) among GG and GC genotypes, respectively. This effect modification thus followed a recessive genetic model.
For the p53 R72P SNP, the observed effect modification followed a codominant model. ΔPM10 was associated with an aver-age attenuation of annual FEF25–75 decline by an average of 17.4 mL/year (95% CI, 8.95–25.78) in GG genotypes (Pro/Pro) and by an average of 11.6 mL/year (2.65–20.61) in CG genotypes (Arg/Pro) but no attenuation in CC genotypes (Arg/Arg).
CCND1 haplotypes 2 and 3, but not 1 and 4, were also statistically significant modifiers of the ΔPM10 association with FEF25–75 decline. In participants exhibiting the CCND1 haplotype 3 (CG/CG) on both alleles (representing the combination of the single SNP alleles associated with the greatest lung function attenuation), the effect estimate for attenuation of FEF25–75 decline associated with ΔPM10 was 30.9 mL/year (95% CI, 17.21–44.58) compared with 11.92 (3.40–20.44) and 10.28 (1.22–19.35) among subjects with the CG haplotype in one or none of their alleles (Table 2).
Genotype-specific ΔPM10 effect estimates for the attenuation of the average annual decline in FEV1 and FVC are presented in Supplemental Material, Table 5 (doi:10.1289/ehp.0800430.S1). For FEV1 decline, effect modification by genotypes was comparable to that seen for FEF25–75 decline. Again, effect modification was strongest for the CCND1 rs667515 SNP and for CCND1 haplo-type3 [see Supplemental Material, Table 5 (doi:10.1289/ehp.0800430.S1)] We observed no modification of the ΔPM10 effect on FVC decline for any of the SNPs or haplotypes.
Discussion
Understanding the pathways by which PM10 damages lung structures and identifying susceptible subjects are important steps in managing the public health challenges of anthropogenic ambient air pollution. In this study, we present novel evidence that genetic polymorphisms in the cell fate controlling genes, p53, p21, and CCND1 may modify the degree to which adults benefit from improved air quality. If confirmed by independent studies, the results are of public health relevance. The observed difference between genotype subgroups with regard to ΔPM10 effects on average lung function decline ranged between 11 mL/year and 21 mL/year for FEF25–75. This difference compares with the observed excess mean annual decline in FEF25–75 of 18 mL and 14 mL per pack per day smoked during follow-up by, respectively, male and female SAPALDIA participants who smoked at baseline (modeled according to Downs et al. 2005). This difference in lung function decline may seem small in absolute terms and from an individual perspective, but even slight shifts in the population distribution of lung function can substantially increase the prevalence of subjects exhibiting respiratory function below clinical thresholds. In addition, lung function is known to be a strong and independent predictor of overall mortality (Künzli et al. 2000a, 2000b).
The mechanisms by which air pollutants induce harmful changes in bronchial tissue and ultimately lead to respiratory symptoms and clinically relevant respiratory dysfunction have been investigated. It seems well established that PM directly increases oxidative stress (Donaldson et al. 2003), activates the expression of proinflammatory cytokines (e.g., tumor necrosis factor α, nuclear factor-κβ, and interleukin-8) (Rahman et al. 2002) and induces the homing of inflammatory cells such as neutrophils, alveolar macrophages, and dendritic cells, thus resulting in inflammation of the lung (Behndig et al. 2006; Bosson et al. 2008; Li et al. 1996,1997; Nightingale et al. 2000). The toxic effects of PM are further enhanced by bronchial tissue injuries and increased epithelial permeability (Morrison et al. 1999).
Our results are in line with more recent in vitro experiments with different types of bronchial cell lines demonstrating that PM induces cell proliferation arrest and apoptosis in exposed cells (Bayram et al. 2006; Dagher et al. 2006; Rosas Perez et al. 2007; Soberanes et al. 2006; Upadhyay et al. 2003; Zhang et al. 2007). Air pollutants or cigarette smoke, both exhibiting oxidative properties, specifically alter the expression levels of tumor suppressor genes such as p53 and p21 and of the cell cycle control gene CCND1 in human and rodent in vitro bronchial cell culture models. Although p53 and p21 expression has been consistently shown to be increased upon PM exposure (Bayram et al. 2006; Dagher et al. 2006; Jiao et al. 2008; Nyunoya et al. 2006; Palozza et al. 2006; Rosas Perez et al. 2007; Soberanes et al. 2006; Yao et al. 2008; Zhang et al. 2007), CCND1 expression has been reported to be either inhibited (via p21 activation) (Palozza et al. 2006; Zhang et al. 2007) or increased [p53-driven N-terminal C-Jun kinase (cJUN) activation] (Bayram et al. 2006; Dagher et al. 2007; Jiao et al. 2008). Such variations in the expression of cell cycle genes mediate the adaptive cellular response to changes in environmental exposure. They might well be the starting point of PM10-induced pathologic changes in morphology and in the type and number of lung fibroblasts and bronchial epithelial cells. Activation of cell proliferation processes may help to maintain intact airways even in the presence of environmental toxins, as suggested by genetic ablation of p21 in mice, which conferred protection against cigarette-smoke–induced lung inflammation and injury (Yao et al. 2008). Aspects of bronchial tissue remodeling represent key features of pathologic changes in the etiology of most airway disorders. However, how proliferation and apoptosis relate to airway remodeling in general remains poorly understood and is likely to be disease specific.
Three of the four polymorphisms investigated here have been studied before, mainly in cancer studies, and allelic functional differences have been proposed. The functional consequence of p53 R72P polymorphism was studied in detail. The Arg72-allele (G-allele) of p53 is more active at inducing apoptosis than is the p53 Pro72-allele. In fact, Pro72 is part of a PXXP motif known to be critical in binding of a p53-specific inhibitory protein, iASPP (inhibitory member of the apoptosis- stimulating protein phosphatase family) (Bergamaschi et al. 2006). Along the line of the proposed functional consequence of this genetic variant, meta-analysis results have recently shown that the homozygous Pro/Pro genotype showed a proproliferative effect and was associated with increased gastric cancer risk (Zhou et al. 2007). In our association study, we observed the most pronounced benefits of ΔPM10 with regard to FEF25–75 decline in subjects with one or two Arg72-alleles, which are expected to induce apoptosis more potently than the Pro72-allele. In contrast, homozygous carriers of the Pro72-allele did not appear to benefit from the improvement in air quality.
The functional studies of the common CCND1 polymorphism P242P showed a modification of the alternative splicing events in exon 4 because the G-to-A substitution alters the consensus sequence of the splicing donor site (Betticher et al. 1995; Howe and Lynas 2001). As recently reported by three meta-analyses, the homozygous AA genotype was associated with increased risk of various types of cancer (Lu et al. 2008; Pabalan et al. 2008; Tan et al. 2008). The A-allele of the CCND1 870G>A (P242P) polymorphism, a proposed genetic risk factor for lung cancer, was previously associated with impaired cell cycle regulation and accumulation of DNA damage in the airway (Buch et al. 2005; Munnia et al. 2006). In our association analysis, we observed that homo- or heterozygous G-allele carriers benefited more from the decrease in PM10 exposure than did homozygous A-allele carriers. The association of the second CCND1 (−7006G>C) SNP with cancer or any other outcome has not been investigated previously. Its functional consequence, if any, might be regulatory because it is situated upstream of the CCND1 gene. But it is also possible that this SNP is in high linkage with a yet unknown functional CCND1 variant, just as it is also in high LD with the CCND1 P242P variant.
The polymorphism S31R in the p21 gene was shown to be located in the DNA-binding zinc finger motif and thus has been thought to alter the function of the p21 protein (Lukas et al. 1997). However, results of cancer studies for this SNP have been inconsistent with regard to the size and direction of relative risk estimates (Choi et al. 2008), and we observed no interaction between this SNP and ΔPM10 on lung function decline.
Thus, the cancer-promoting p53 and CCND1 alleles seem to reduce the benefit of improved air quality on respiratory function. Only limited epidemiological data exist on the association of p53, p21, or CCND1 SNPs with respiratory diseases such as asthma, emphysema, and chronic obstructive pulmonary disease (COPD). The polymorphisms we evaluated in p53 and p21 have previously been associated with smoking-related COPD. Compared with healthy smokers, the cancer risk allele of p53 (Pro72-allele), and the p21 Arg31-allele were overrepresented in COPD patients (Lee et al. 2006). The extrapolation of observed associations with cancer to expected associations with lung function is not straightforward. The present findings and our interpretation of them should be considered as exploratory in nature and need confirmation in independent studies. The relative impacts of cell proliferation and apoptosis on different cell types in the lung and on respiratory function must be further investigated by experimental studies.
The strengths of the present study are its prospective design, its rather large sample size, and detailed characterization of the study participants, as well as the availability of individual air pollution exposure history since 1990. We were thus able to adjust lung function decline for most of the potential confounders. Nonetheless, information on some relevant confounders such as dietary antioxidant intake and physical activity was collected only at follow-up. Lacking data on the degree of genetic admixture of the Swiss general population is an additional drawback. However, we expect little bias due to genetic admixture, because the prevalence of several polymorphisms studied in the cohort did not vary across the three major Swiss language groups. We assessed a very limited number of carefully selected candidate polymorphisms; for some of the SNPs and haplotypes, and especially for the p21 SNP and CCND1 haplotype 4, statistical power to detect effect estimation was very limited. A more comprehensive assessment of gene variants in the apoptosis pathway and genes regulating apoptosis in lung tissue needs to be addressed in future larger studies. Nevertheless, even for the small number of genetic variants investigated, multiple testing represents a limitation in the interpretation of the present results. Consistency of the associations across genes involved in cell cycle control, as well as across various lung function outcomes, diminishes the likelihood of chance findings, as does the strength of the observed pint values.
Finally, participation at follow-up and in this present study was not complete, so participation bias cannot be excluded. However, because we observed comparable results in all subjects and in never-smokers and in the absence of different effects between men and women, the lower participation rate in men and smokers is not likely to have caused bias.
In conclusion, our results suggest that even at low to moderate levels of air pollution, such as in Switzerland, some but not all persons benefit from improved air quality. Given the novelty of the finding and the limitations inherent to this study, the results need independent confirmation. Future studies must address additional issues. First, gene variants relevant to cell cycle control must be studied more comprehensively. Second, it is of interest to know whether this novel candidate pathway might determine susceptibility to both improvements and declines in air quality.
Appendix 1.
The SAPALDIA Team.
| Member | Specialty |
|---|---|
| Study directorate
| |
| U. Ackermann-Liebrich | Epidemiology |
| J.M. Gaspoz | Cardiology |
| P. Leuenberger | Pneumology |
| L.J.S. Liu | Exposure |
| N.M. Probst Hensch | Epidemiology/genetic and molecular biology |
| C. Schindler | Statistics |
| T. Rochat | Pneumology |
| Scientific team
| |
| J.C. Barthélémy | Cardiology |
| W. Berger | Genetic and molecular biology |
| R. Bettschart | Pneumology |
| A. Bircher | Allergology |
| G. Bolognini | Pneumology |
| O. Brändli | Pneumology |
| M. Brutsche | Pneumology |
| L. Burdet | Pneumology |
| M. Frey | Pneumology |
| M.W. Gerbase | Pneumology |
| D. Gold | Epidemiology/cardiology/pneumology |
| W. Karrer | Pneumology |
| R. Keller | Pneumology |
| B. Knöpfli | Pneumology |
| N. Künzli | Epidemiology/exposure |
| U. Neu | Exposure |
| L. Nicod | Pneumology |
| M. Pons | Pneumology |
| E. Russi | Pneumology |
| P. Schmid-Grendelmeyer | Allergology |
| J. Schwartz | Epidemiology |
| P. Straehl | Exposure |
| J.M. Tschopp | Pneumology |
| A. von Eckardstein | Clinical chemistry |
| J.P. Zellweger | Pneumology |
| E. Zemp Stutz | Epidemiology |
| Scientific team at coordinating centers
| |
| P.O. Bridevaux | Pneumology |
| I. Curjuric | Epidemiology |
| J. Dratva | Epidemiology |
| D. Felber Dietrich | Cardiology |
| D. Keidel | Statistics |
| M. Imboden | Genetic and molecular biology |
| H. Phuleria | Exposure |
| E. Schaffner | Statistics |
| G.A. Thun | Genetic and molecular biology |
Footnotes
Supplemental Material is available online (doi:10.1289/ehp.0800430.S1 via http://dx.doi.org/)
The study could not have been done without the help of the study participants, technical and administrative support, and the medical teams and fieldworkers at the local study sites: local field-workers M. Broglie, M. Bünter, D. Gashi (Aarau); R. Armbruster, T. Damm, U. Egermann, M. Gut, L. Maier, A. Vögelin, L. Walter (Basel); D. Jud, N. Lutz (Davos); M. Ares, M. Bennour, B. Galobardes, E. Namer (Geneva); B. Baumberger, S. Boccia Soldati, E. Gehrig-Van Essen, S. Ronchetto (Lugano); C. Bonvin, C. Burrus (Montana); S. Blanc, A.V. Ebinger, M.L. Fragnière, J. Jordan (Payerne); and R. Gimmi, N. Kourkoulos, U. Schafroth (Wald); and administrative staff N. Bauer, D. Baehler, C. Gabriel, and R. Nilly.
Research support was provided by the Swiss National Science Foundation (grants 4026-28099, 3347CO-108796, 3247BO-104283, 3247BO-104288, 3247BO-104284, 32-65896.01, 32-59302.99, 32-52720.97, and 32-4253.94); Swiss Federal Office for Forest, Environment, and Landscape; Swiss Federal Office of Public Health; Swiss Federal Office of Roads and Transport; canton governments of Aargau, Basel-Stadt, Basel-Land, Geneva, Luzern, Ticino, and Zurich; Swiss Lung League; canton’s Lung League of Basel Stadt/Basel Landschaft, Geneva, Ticino, and Zurich; and the Walter-Honegger Foundation.
References
- Ackermann-Liebrich U, Kuna-Dibbert B, Probst-Hensch N, Schindler C, Felber Dietrich D, Zemp Stutz E, et al. Follow-up of the Swiss Cohort Study on Air Pollution and Lung Diseases in Adults (SAPALDIA 2) 1991–2003: methods and characterization of participants. Soz Praventiv Med. 2005;50:245–263. doi: 10.1007/s00038-005-4075-5. [DOI] [PubMed] [Google Scholar]
- Ackermann-Liebrich U, Leuenberger P, Schwartz J, Schindler C, Monn C, Bolognini G, et al. Lung function and long term exposure to air pollutants in Switzerland. Study on Air Pollution and Lung Diseases in Adults (SAPALDIA) Team. Am J Respir Crit Care Med. 1997;155(1):122–129. doi: 10.1164/ajrccm.155.1.9001300. [DOI] [PubMed] [Google Scholar]
- American Thoracic Society. Standardization of spirometry, 1994 Update. Am J Respir Crit Care Med. 1995;152(3):1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- Bayram H, Ito K, Issa R, Ito M, Sukkar M, Chung KF. Regulation of human lung epithelial cell numbers by diesel exhaust particles. Eur Respir J. 2006;27(4):705–713. doi: 10.1183/09031936.06.00012805. [DOI] [PubMed] [Google Scholar]
- Behndig AF, Mudway IS, Brown JL, Stenfors N, Helleday R, Duggan ST, et al. Airway antioxidant and inflammatory responses to diesel exhaust exposure in healthy humans. Eur Respir J. 2006;27(2):359–365. doi: 10.1183/09031936.06.00136904. [DOI] [PubMed] [Google Scholar]
- Bergamaschi D, Samuels Y, Sullivan A, Zvelebil M, Breyssens H, Bisso A, et al. iASPP preferentially binds p53 proline-rich region and modulates apoptotic function of codon 72-polymorphic p53. Nat Genet. 2006;38(10):1133–1141. doi: 10.1038/ng1879. [DOI] [PubMed] [Google Scholar]
- Betticher DC, Thatcher N, Altermatt HJ, Hoban P, Ryder WD, Heighway J. Alternate splicing produces a novel cyclin D1 transcript. Oncogene. 1995;11(5):1005–1011. [PubMed] [Google Scholar]
- Bosson J, Barath S, Pourazar J, Behndig AF, Sandstrom T, Blomberg A, et al. Diesel exhaust exposure enhances the ozone induced airway inflammation in healthy humans. Eur Respir J. 2008;31(6):1234–1240. doi: 10.1183/09031936.00078407. [DOI] [PubMed] [Google Scholar]
- Brunekreef B, Forsberg B. Epidemiological evidence of effects of coarse airborne particles on health. Eur Respir J. 2005;26(2):309–318. doi: 10.1183/09031936.05.00001805. [DOI] [PubMed] [Google Scholar]
- Buch S, Zhu B, Davis AG, Odom D, Siegfried JM, Grandis JR, et al. Association of polymorphisms in the cyclin D1 and XPD genes and susceptibility to cancers of the upper aero-digestive tract. Mol Carcinog. 2005;42(4):222–228. doi: 10.1002/mc.20086. [DOI] [PubMed] [Google Scholar]
- Burney PG, Luczynska C, Chinn S, Jarvis D. The European Community Respiratory Health Survey. Eur Respir J. 1994;7(5):954–960. doi: 10.1183/09031936.94.07050954. [DOI] [PubMed] [Google Scholar]
- Burr ML, Karani G, Davies B, Holmes BA, Williams KL. Effects on respiratory health of a reduction in air pollution from vehicle exhaust emissions. Occup Environ Med. 2004;61(3):212–218. doi: 10.1136/oem.2002.003244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceschi M, Sun CL, Van Den Berg D, Koh WP, Yu MC, Probst-Hensch N. The effect of cyclin D1 (CCND1) G870A-polymorphism on breast cancer risk is modified by oxidative stress among Chinese women in Singapore. Carcinogenesis. 2005;26(8):1457–1464. doi: 10.1093/carcin/bgi093. [DOI] [PubMed] [Google Scholar]
- Choi YY, Kang HK, Choi JE, Jang JS, Kim EJ, Cha SI, et al. Comprehensive assessment of P21 polymorphisms and lung cancer risk. J Hum Genet. 2008;53(1):87–95. doi: 10.1007/s10038-007-0222-6. [DOI] [PubMed] [Google Scholar]
- Dagher Z, Garcon G, Billet S, Gosset P, Ledoux F, Courcot D, et al. Activation of different pathways of apoptosis by air pollution particulate matter (PM2.5) in human epithelial lung cells (L132) in culture. Toxicology. 2006;225(1):12–24. doi: 10.1016/j.tox.2006.04.038. [DOI] [PubMed] [Google Scholar]
- Dagher Z, Garcon G, Billet S, Verdin A, Ledoux F, Courcot D, et al. Role of nuclear factor-kappa B activation in the adverse effects induced by air pollution particulate matter (PM2.5) in human epithelial lung cells (L132) in culture. J Appl Toxicol. 2007;27(3):284–290. doi: 10.1002/jat.1211. [DOI] [PubMed] [Google Scholar]
- Donaldson K, Stone V, Borm PJ, Jimenez LA, Gilmour PS, Schins RP, et al. Oxidative stress and calcium signaling in the adverse effects of environmental particles (PM10) Free Radic Biol Med. 2003;34(11):1369–1382. doi: 10.1016/s0891-5849(03)00150-3. [DOI] [PubMed] [Google Scholar]
- Downs S, Brandli O, Zellweger JP, Schindler C, Künzli N, Gerbase M, et al. Accelerated decline in lung function in smoking women with airway obstruction: SAPALDIA 2 cohort study. Respir Res. 2005;6(1):45. doi: 10.1186/1465-9921-6-45. [Online 26 May 2005] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs SH, Schindler C, Liu LJ, Keidel D, Bayer-Oglesby L, Brutsche MH, et al. Reduced exposure to PM10 and attenuated age-related decline in lung function. N Engl J Med. 2007;357(23):2338–2347. doi: 10.1056/NEJMoa073625. [DOI] [PubMed] [Google Scholar]
- Goodman P, Agnew M, McCaffrey M, Paul G, Clancy L. Effects of the Irish smoking ban on respiratory health of bar workers and air quality in Dublin pubs. Am J Respir Crit Care Med. 2007;175(8):840–845. doi: 10.1164/rccm.200608-1085OC. [DOI] [PubMed] [Google Scholar]
- Gotschi T, Heinrich J, Sunyer J, Künzli N. Long-term effects of ambient air pollution on lung function: a review. Epidemiology. 2008;19(5):690–701. doi: 10.1097/EDE.0b013e318181650f. [DOI] [PubMed] [Google Scholar]
- Hedley AJ, Wong CM, Thach TQ, Ma S, Lam TH, Anderson HR. Cardiorespiratory and all-cause mortality after restrictions on sulphur content of fuel in Hong Kong: an intervention study. Lancet. 2002;360(9346):1646–1652. doi: 10.1016/s0140-6736(02)11612-6. [DOI] [PubMed] [Google Scholar]
- Howe D, Lynas C. The cyclin D1 alternative transcripts [a] and [b] are expressed in normal and malignant lymphocytes and their relative levels are influenced by the polymorphism at codon 241. Haematologica. 2001;86(6):563–569. [PubMed] [Google Scholar]
- Imboden M, Downs SH, Senn O, Matyas G, Brandli O, Russi EW, et al. Glutathione S-transferase genotypes modify lung function decline in the general population: SAPALDIA cohort study. Respir Res. 2007;8:2. doi: 10.1186/1465-9921-8-2. [Online 11 January 2007] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao S, Liu B, Gao A, Ye M, Jia X, Zhang F, et al. Benzo(a) pyrene-caused increased G(1)-S transition requires the activation of c-Jun through p53-dependent PI-3K/Akt/ERK pathway in human embryo lung fibroblasts. Toxicol Lett. 2008;178(3):167–175. doi: 10.1016/j.toxlet.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Künzli N, Ackermann-Liebrich U, Brandli O, Tschopp JM, Schindler C, Leuenberger P. Clinically “small” effects of air pollution on FVC have a large public health impact. Swiss Study on Air Pollution and Lung Disease in Adults (SAPALDIA) team. Eur Respir J. 2000a;15(1):131–136. doi: 10.1183/09031936.00.15113100. [DOI] [PubMed] [Google Scholar]
- Künzli N, Ackermann-Liebrich U, Keller R, Perruchoud AP, Schindler C. Variability of FVC and FEV1 due to technician, team, device and subject in an eight centre study: three quality control studies in SAPALDIA. Swiss Study on Air Pollution and Lung Disease in Adults. Eur Respir J. 1995;8(3):371–376. doi: 10.1183/09031936.95.08030371. [DOI] [PubMed] [Google Scholar]
- Künzli N, Kaiser R, Medina S, Studnicka M, Chanel O, Filliger P, et al. Public-health impact of outdoor and traffic-related air pollution: a European assessment. Lancet. 2000b;356(9232):795–801. doi: 10.1016/S0140-6736(00)02653-2. [DOI] [PubMed] [Google Scholar]
- Künzli N, Kuna-Dibbert B, Keidel D, Keller R, Brändli O, Schindler C, et al. Longitudinal validity of spirometers—a challenge in lung function follow-up studies. Swiss Med Wkly. 2005;135(33–34):503–508. doi: 10.4414/smw.2005.11050. [DOI] [PubMed] [Google Scholar]
- Lee YL, Chen W, Tsai WK, Lee JC, Chiou HL, Shih CM, et al. Polymorphisms of p53 and p21 genes in chronic obstructive pulmonary disease. J Lab Clin Med. 2006;147(5):228–233. doi: 10.1016/j.lab.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Li N, Xia T, Nel AE. The role of oxidative stress in ambient particulate matter-induced lung diseases and its implications in the toxicity of engineered nanoparticles. Free Radic Biol Med. 2008;44(9):1689–1699. doi: 10.1016/j.freeradbiomed.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XY, Gilmour PS, Donaldson K, MacNee W. Free radical activity and pro-inflammatory effects of particulate air pollution (PM10) in vivo and in vitro. Thorax. 1996;51(12):1216–1222. doi: 10.1136/thx.51.12.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XY, Gilmour PS, Donaldson K, MacNee W. In vivo and in vitro proinflammatory effects of particulate air pollution (PM10) Environ Health Perspect. 1997;105(suppl 5):1279–1283. doi: 10.1289/ehp.97105s51279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LJ, Curjuric I, Keidel D, Heldstab J, Künzli N, Bayer-Oglesby L, et al. Characterization of source-specific air pollution exposure for a large population-based Swiss cohort (SAPALDIA) Environ Health Perspect. 2007;115:1638–1645. doi: 10.1289/ehp.10177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Dong J, Ma H, Jin G, Hu Z, Peng Y, et al. CCND1 G870A polymorphism contributes to breast cancer susceptibility: a meta-analysis. Breast Cancer Res Treat. 2008;116(3):571–575. doi: 10.1007/s10549-008-0195-y. [DOI] [PubMed] [Google Scholar]
- Lukas J, Groshen S, Saffari B, Niu N, Reles A, Wen WH, et al. WAF1/Cip1 gene polymorphism and expression in carcinomas of the breast, ovary, and endometrium. Am J Pathol. 1997;150(1):167–175. [PMC free article] [PubMed] [Google Scholar]
- Martin BW, Ackermann-Liebrich U, Leuenberger P, Künzli N, Stutz EZ, Keller R, et al. SAPALDIA: methods and participation in the cross-sectional part of the Swiss Study on Air Pollution and Lung Diseases in Adults. Soz Praventivmed. 1997;42(2):67–84. doi: 10.1007/BF01318136. [DOI] [PubMed] [Google Scholar]
- Menzies D, Nair A, Williamson PA, Schembri S, Al-Khairalla MZ, Barnes M, et al. Respiratory symptoms, pulmonary function, and markers of inflammation among bar workers before and after a legislative ban on smoking in public places. JAMA. 2006;296(14):1742–1748. doi: 10.1001/jama.296.14.1742. [DOI] [PubMed] [Google Scholar]
- Morrison D, Rahman I, Lannan S, MacNee W. Epithelial permeability, inflammation, and oxidant stress in the air spaces of smokers. Am J Respir Crit Care Med. 1999;159(2):473–479. doi: 10.1164/ajrccm.159.2.9804080. [DOI] [PubMed] [Google Scholar]
- Munnia A, Bonassi S, Verna A, Quaglia R, Pelucco D, Ceppi M, et al. Bronchial malondialdehyde DNA adducts, tobacco smoking, and lung cancer. Free Radic Biol Med. 2006;41(9):1499–1505. doi: 10.1016/j.freeradbiomed.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Nightingale JA, Maggs R, Cullinan P, Donnelly LE, Rogers DF, Kinnersley R, et al. Airway inflammation after controlled exposure to diesel exhaust particulates. Am J Respir Crit Care Med. 2000;162(1):161–166. doi: 10.1164/ajrccm.162.1.9908092. [DOI] [PubMed] [Google Scholar]
- Nyunoya T, Monick MM, Klingelhutz A, Yarovinsky TO, Cagley JR, Hunninghake GW. Cigarette smoke induces cellular senescence. Am J Respir Cell Mol Biol. 2006;35(6):681–688. doi: 10.1165/rcmb.2006-0169OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren M, Damalas A, Gottlieb T, Michael D, Taplick J, Leal JF, et al. Regulation of p53: intricate loops and delicate balances. Biochem Pharmacol. 2002;64(5–6):865–871. doi: 10.1016/s0006-2952(02)01149-8. [DOI] [PubMed] [Google Scholar]
- Pabalan N, Bapat B, Sung L, Jarjanazi H, Francisco-Pabalan O, Ozcelik H. Cyclin D1 Pro241Pro (CCND1-G870A) polymorphism is associated with increased cancer risk in human populations: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2008;17(10):2773–2781. doi: 10.1158/1055-9965.EPI-08-0169. [DOI] [PubMed] [Google Scholar]
- Palozza P, Serini S, Curro D, Calviello G, Igarashi K, Mancuso C. beta-Carotene and cigarette smoke condensate regu late heme oxygenase-1 and its repressor factor Bach1: relationship with cell growth. Antioxid Redox Signal. 2006;8(5–6):1069–1080. doi: 10.1089/ars.2006.8.1069. [DOI] [PubMed] [Google Scholar]
- Probst-Hensch NM, Sun CL, Van Den Berg D, Ceschi M, Koh WP, Yu MC. The effect of the cyclin D1 (CCND1) A870G polymorphism on colorectal cancer risk is modified by glutathione-S-transferase polymorphisms and isothiocyanate intake in the Singapore Chinese Health Study. Carcinogenesis. 2006;27(12):2475–2482. doi: 10.1093/carcin/bgl116. [DOI] [PubMed] [Google Scholar]
- Rahman I, Gilmour PS, Jimenez LA, MacNee W. Oxidative stress and TNF-alpha induce histone acetylation and NF-kappaB/AP-1 activation in alveolar epithelial cells: potential mechanism in gene transcription in lung inflammation. Mol Cell Biochem. 2002:234–235. 1–2, 239–248. [PubMed] [Google Scholar]
- Ranjan P, Anathy V, Burch PM, Weirather K, Lambeth JD, Heintz NH. Redox-dependent expression of cyclin D1 and cell proliferation by Nox1 in mouse lung epithelial cells. Antioxid Redox Signal. 2006;8(9–10):1447–1459. doi: 10.1089/ars.2006.8.1447. [DOI] [PubMed] [Google Scholar]
- Rosas Perez I, Serrano J, Alfaro-Moreno E, Baumgardner D, Garcia-Cuellar C, Martin Del Campo JM, et al. Relations between PM10 composition and cell toxicity: a multivariate and graphical approach. Chemosphere. 2007;67(6):1218–1228. doi: 10.1016/j.chemosphere.2006.10.078. [DOI] [PubMed] [Google Scholar]
- Soberanes S, Panduri V, Mutlu GM, Ghio A, Bundinger GR, Kamp DW. p53 mediates particulate matter-induced alveolar epithelial cell mitochondria-regulated apoptosis. Am J Respir Crit Care Med. 2006;174(11):1229–1238. doi: 10.1164/rccm.200602-203OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001;68(4):978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan XL, Nieters A, Kropp S, Hoffmeister M, Brenner H, Chang-Claude J. The association of cyclin D1 G870A and E-cadherin C-160A polymorphisms with the risk of colorectal cancer in a case control study and meta-analysis. Int J Cancer. 2008;122(11):2573–2580. doi: 10.1002/ijc.23363. [DOI] [PubMed] [Google Scholar]
- Upadhyay D, Panduri V, Ghio A, Kamp DW. Particulate matter induces alveolar epithelial cell DNA damage and apoptosis: role of free radicals and the mitochondria. Am J Respir Cell Mol Biol. 2003;29(2):180–187. doi: 10.1165/rcmb.2002-0269OC. [DOI] [PubMed] [Google Scholar]
- Wuthrich B, Schindler C, Medici TC, Zellweger JP, Leuenberger P. IgE levels, atopy markers and hay fever in relation to age, sex and smoking status in a normal adult Swiss population. SAPALDIA (Swiss Study on Air Pollution and Lung Diseases in Adults) Team. Int Arch Allergy Immunol. 1996;111(4):396–402. doi: 10.1159/000237398. [DOI] [PubMed] [Google Scholar]
- Yao H, Edirisinghe I, Yang SR, Rajendrasozhan S, Kode A, Caito S, et al. Genetic ablation of NADPH oxidase enhances susceptibility to cigarette smoke-induced lung inflammation and emphysema in mice. Am J Pathol. 2008;172(5):1222–1237. doi: 10.2353/ajpath.2008.070765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ghio AJ, Gao M, Wei K, Rosen GD, Upadhyay D. Ambient particulate matter induces alveolar epithelial cell cycle arrest: role of G1 cyclins. FEBS Lett. 2007;581(27):5315–5320. doi: 10.1016/j.febslet.2007.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Li N, Zhuang W, Liu GJ, Wu TX, Yao X, et al. P53 codon 72 polymorphism and gastric cancer: a meta-analysis of the literature. Int J Cancer. 2007;121(7):1481–1486. doi: 10.1002/ijc.22833. [DOI] [PubMed] [Google Scholar]