Abstract
Recently, it has been shown that lipoxygenase (LO) products affect the substrate specificity of human 15-LO. In the current paper, we demonstrate that soybean LO-1 (sLO-1) is not affected by its own products, however, inhibitors which bind the allosteric site, oleyl sulfate (OS) and palmitoleyl sulfate (PS), not only lower catalytic activity, but also change the substrate specificity, by increasing the arachidonic acid (AA)/ linoleic acid (LA) ratio to 4.8 and 4.0, respectively. The fact that LO inhibitors can lower activity and also change the LO product ratio is a new concept in lipoxygenase inhibition, where the goal is to not only reduce the catalytic activity but also alter substrate selectivity towards a physiologically beneficial product.
Keywords: soybean lipoxygenase-1, allosteric inhibition, substrate specificity
Lipoxygenases (LO) are a family of metallo-enzymes which initiate oxylipin signaling cascades in response to metabolic needs and external stimuli. Signal initiation begins with the catalytic incorporation of molecular oxygen into unsaturated fatty acids, producing the respective hydroperoxide fatty acid products (1). There are three human LO (hLO) isozymes of pharmacological importance (5-hLO, 12-hLO, and 15-hLO) which are designated by their relative oxidation position on arachidonic acid (AA) (2). The hydroperoxide products (hydroperoxyeicosatetraenoic acids (HPETEs)) regulate pro-inflammatory (leukotrienes) and anti-inflammatory/resolution (lipoxins and resolvins) responses (3). The LO metabolites of AA, as well as linoleic acid (LA), have been implicated in a variety of inflammatory diseases and cancers, making hLO a possible target for drug therapy (4).
Our current understanding of LO biochemistry comes from extensive kinetic, structural, and mechanistic investigations of the soybean lipoxygenase-1 (sLO-1) (5–8). In plants, lipoxygenases react with the C18 polyunsaturated fatty acids, LA and α-linolenic acid (ALA), producing predominately 13-hydroperoxyoctadecadienoic acid (13-HPODE) and 13-hydroperoxyoctadecatrienoic acid (13-HPOTrE), respectively (9). The sLO-1 metabolites of these two fatty acids have many physiological effects, including the regulation of germination and senescence; with one of the most, well-characterized metabolic pathways being that of jasmonic acid synthesis, a powerful biomolecule used for plant defense against pathogens (10, 11). sLO-1 has been consistently used as a model for 15-hLO-1 due to their mechanistic similarities in AA metabolism, both producing 15-HPETE, as well as their structural similarities in both the alpha-helical (catalytic) and beta-barrel (membrane associated) domains (5, 8). In addition, both sLO-1 and 15-hLO-1 react preferentially towards AA over LA, adding substrate specificity to the list of similarities between these two LOs (12, 13).
With regards to 15-hLO, there is growing evidence that substrate specificity may be the underlying cause for the advancement of certain diseases (14–17). For example, reticulocyte 15-hLO-1 reacts preferentially with LA to produce 13-HPODE, which causes prostate carcinoma cells to undergo proliferation and differentiation, while epithelial 15-hLO-2 reacts preferentially with AA to produce 15-HPETE, which inhibits cell proliferation (18, 19). Therefore, it is proposed that 15-hLO-1 and its product, 13-HPODE, promote cancer progression, while 15-hLO-2 and its product, 15-HPETE, inhibit cancer progression. This hypothesis was recently supported by the fact that the substrate specificity of the 15-hLO isozymes is directly affected by an allosteric product-feedback mechanism (12), which could modify the ratio of LO products in the cell, and affect its carcinogenic progression. This result of 15-hLO-1 raised the question of whether LO products affected the substrate specificity of sLO-1 as well, since sLO-1 is similar in many respects to 15-hLO-1.
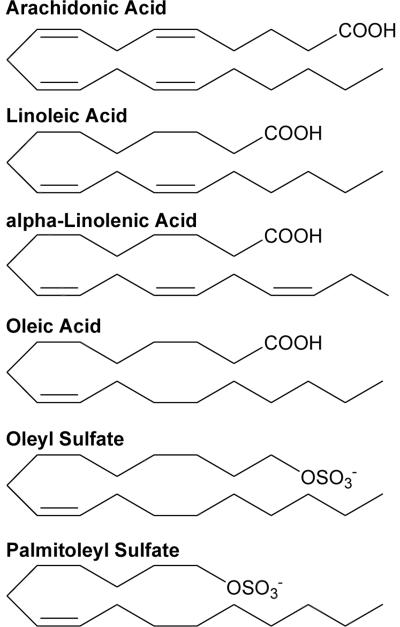
In the current work, we have investigated the allosteric effect of the reduced LO products, 13-(S)-hydroxyoctadecadienoic acid (13-HODE), 13-(S)-hydroxyoctadecatrienoic acid (13-HOTrE) and 12-(S)-hydroxyeicosatetraenoic acid (12-HETE), on sLO-1 substrate specificity with the endogenous substrate mixture, ALA:LA, and the non-endogenous substrate mixture, AA:LA. These results demonstrate that there is no observed allosteric product feedback with sLO-1's endogenous products, however, the non-endogenous product, 12-HETE, increased the substrate specificity of sLO-1 towards AA, when challenged with an LA/AA mixture. Allosteric effects on substrate specificity of sLO-1 were also probed with three well-characterized inhibitors of sLO-1 (Figure 1), oleic acid (OA, competitive inhibition), oleyl sulfate (OS, allosteric inhibition) and palmitoleyl sulfate (PS, mixed-type inhibition) on the substrate specificity of sLO-1. Intriguingly, the inhibitors which bind the allosteric site (OS and PS) displayed an effect on the substrate specificity of sLO-1, correlating to their respective binding affinities towards the allosteric site (20–23), while the competitive inhibitor, OA, had no effect. These results may have implications in the potential targeting of 15-hLO in human disease.
Figure 1.
Chemical structures of fatty acid substrates, arachidonic acid, linoleic acid, and α-linolenic acid and fatty acid inhibitors, oleic acid, oleyl sulfate, and palmitoleyl sulfate.
MATERIALS AND METHODS
Materials
All commercial fatty acids (Sigma-Aldrich Chemical Company) were re-purified using a Higgins HAIsil Semi-Preparative (5μm, 250 × 10 mm) C-18 column. Solution A was 99.9% MeOH and 0.1% acetic acid; solution B was 99.9% H2O and 0.1% acetic acid. An isocratic elution of 85% A:15% B was used to purify all fatty acids, which were stored at −80 °C for a maximum of 6 months. LO products were generated by reacting substrate with the appropriate LO isozyme (13-HPODE from sLO-1 and LA, 13-HPOTrE from sLO-1 and ALA, and 12-HPETE from 12-hLO and AA). Product generation was performed as follows. An assay of 100 mL of 50–100 μM substrate was run to completion, extracted twice with 300 mL of dichloromethane, evaporated to dryness, and reconstituted in MeOH for HPLC purification. The products were HPLC purified using an isocratic elution of 75% A:25% B, as described above for the fatty acid purification. All products were tested with enzyme to show that no residual substrate was present, as well as tested using both analytical HPLC and LC/MS/MS, demonstrating greater than 98% purity. The reduced products were purified similarly; however, prior to purification, trimethylphosphite was added to selectively reduce the peroxide to the alcohol moiety prior to purification. Purified hydroxide products were then tested with enzyme to ensure no loss of lag phase by activation from residue hydroperoxide product. Perdeuterated LA (d31-LA) (98% deuterated, Cambridge Isotope Labs) was purified as previously described (24). All other chemicals were reagent grade or better and were used without further purification.
Fatty Acid Substrate Analogues
Palmitoleyl sulfate (PS) and oleyl sulfate (OS) were prepared as previously described (21). Oleic acid (OA) was obtained from Aldrich Chemical Co., and all fatty acids were dissolved in EtOH (95%) and stored at −80 °C (Figure 1).
Overexpression and Purification of sLO-1
Overexpression and purification of sLO-1 followed a protocol outlined previously (23, 25). Briefly, following expression of the protein in BL21-DE3 (Escherichia coli), the cells were harvested by centrifugation and their membranes were disrupted by sonication. Cell debris was pelleted and the supernatant was first dialyzed against 20 mM Bis-Tris buffer (pH 6.0) and then applied to a SP-Sephadex high-flow ion exchange column (Pharmacia), which was equilibrated with the same buffer. Eluted fractions containing lipoxygenase activity were pooled, concentrated, dialyzed against 20 mM Bis-Tris buffer (pH 6.0), and applied to a Macro-Prep 25-S ion exchange column (Bio-Rad). After concentration and buffer exchange into 0.1 M Borate (pH 9.2), the isolated enzyme was estimated to be greater than 90% pure (SDS-PAGE).
Determination of Substrate Specificity using the Competitive Substrate Capture Method with Substrate Mixtures of Arachidonic Acid:Linoleic Acid, and α-Linolenic Acid:Linoleic acid
The competitive substrate capture experiment was performed as previously described (12). Briefly reaction mixtures of AA:LA and ALA:LA of known molar ratio (1:1) were initiated with sLO-1 (~1.0 nM, normalized to iron content) using the buffer conditions described above (100 mM Borate, pH 9.2, 22° C), and performed in an over-sized reaction cuvette. The ratio of the simultaneous product formation (15-HPETE/ 13-HPODE or 13-HPOTrE/ 13-HPODE) by sLO-1 was determined at 1 μM total substrate concentration (substrate-limiting conditions). The reaction was monitored at 234 nm with a Perkin-Elmer Lambda 40 and stopped with an acetic acid quench at ~10% total substrate consumption (100 nM). The acidified reaction mixture was extracted with dichloromethane, evaporated to dryness under vacuum, reconstituted in 50 μL of MeOH and injected onto a Phenomenex Luna (5 μm, 250 × 4.6 mm) C-18 column. The elution protocol consisted of 1 mL/min, isocratic mobile phase of 74.9% MeOH:25% H2O:0.1% acetic acid. The molar amount of product formation was equated to the corresponding peak areas determined by HPLC as previously described (12). The ratio of the peak areas was then used to determine the (kcat/KM)AA/(kcat/KM)LA and (kcat/KM)ALA/(kcat/KM)LA ratio, as previously published (12). Considering that the amount of enzyme is 1 nM and only 50 nM of product is produced, the ratio was subsequently shown to be unaffected by varying the substrate and enzyme concentrations.
Determining the Lipoxygenase Products Effect on the Substrate Specificity of sLO-1
The product effect on substrate specificity was determined under similar conditions as describe above. Purified d31-13-HODE, 13-HOTrE, and 12-HETE were pre-incubated (1 – 20 μM product) with sLO-1 (~1.0 – 5.0 nM, normalized to iron content) subsequent to the addition of either a mixture of AA:LA or ALA:LA (1 μM substrate). The allosteric effects on substrate specificity were determined by comparing the ratio of product turnover of the controls (no addition of product) vs. experimental (addition of product), as previously described (12).
IC50 Assays of sLO-1 with PS, OS and OA Inhibitors, with LA as substrate
The IC50 values of PS, OS and OA were determined by methods previously published (26). Briefly, enzymatic assays were initiated by the addition of sLO-1 (~1.5 nM, 5μM LA) to 2 mL of reaction mixture and stirred constantly with a magnetic stir bar at room temperature (100 mM Borate, pH 9.2, 22 °C). IC50 values were obtained by determining the change in the enzymatic rates at various inhibitor concentrations, followed by plotting the rates against inhibitor concentration. The data was fit to a saturation curve and the inhibitor concentration at 50% activity was determined (IC50).
Determining the Inhibitor Effect on the Substrate Specificity of sLO-1
Reactions were carried out using the competitive substrate capture experiment as described above (100 mM Borate, pH 9.2, 22° C) with addition of PS, OS and OA, at their determined IC50 values (11 μM, 13 μM and 1 μM for OA, PS and OS, respectively). Enzymatic reactions were initiated by the addition of sLO-1 (~1.0 – 5.0 nM, normalized to iron content) using both AA:LA and ALA:LA reaction mixtures, and the (kcat/KM)AA/(kcatKM)LA and (kcat/KM)ALA(kcat/KM)LA ratio was obtained using the HPLC method, as described above. Experiments were also performed at 5-fold the IC50 values, (55, 65 and 5 μM for OA, PS and OS, respectively), to determine the effect at saturating inhibitor concentrations.
Determination of the Saturating Effect of Inhibitors on the Substrate Specificity of sLO-1
The changes in substrate specificity of sLO-1 by OS and PS were determined by titrating varying amounts inhibitor and monitoring the changes in the (kcat/KM)AA(kcat/KM)LA ratio using the described competitive substrate capture method. The saturation curve was visualized by plotting the (kcat/KM)AA/(kcat/KM)LA ratio vs. [Inhibitor] μM, however, the half saturation point and maximum effect at the saturation point were determined by graphing the Δ(kcat/KM)AA/(kcat/KM)LA ratio vs. [Inhibitor] μM (Supporting Information).
RESULTS
Determination of Substrate Specificity using the Competitive Substrate Capture Method with Substrate Mixtures of Arachidonic Acid:Linoleic Acid, and α-Linolenic Acid:Linoleic acid
The ratio of substrate turnover for sLO-1 using either AA:LA or ALA:LA reaction mixtures (100 mM Borate, pH 9.2, 22° C) were determined by monitoring the simultaneous product formation using the HPLC method described above. The competitive substrate capture (kcat/KM)AA/(kcat/KM)LA ratio was determined to be 1.8 ± 0.2, in agreement with the steady-state kinetic data and previously published results (12, 13). The (kcat/KM)ALA/(kcat/KM)LAratio was determined to be 0.75 ± 0.5, demonstrating sLO-1 displays a slight preference towards LA over ALA.
Determining the Lipoxygenase Products Effect on the Substrate Specificity of sLO-1
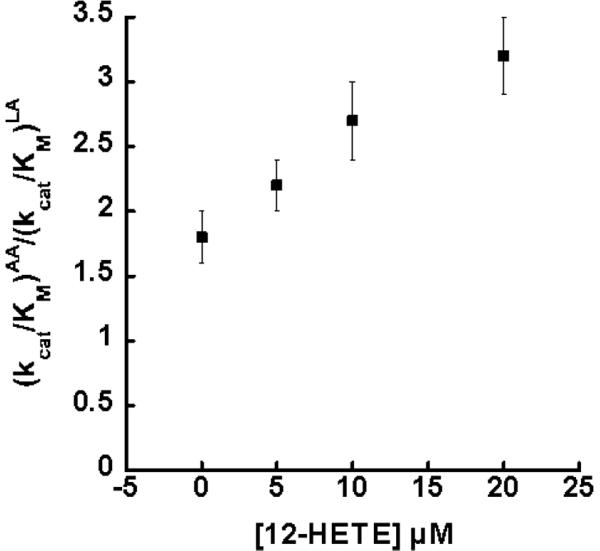
The product titration experiments with 13-HODE, 13-HOTrE and 12-HETE demonstrated no effect on the endogenous substrate mixture ratio, ALA:LA, up to 5 μM product addition. These results were anticipated since ALA and LA are structurally similar in length (19 atoms), and are only differentiated by an additional unsaturation found in ALA. Testing the product effects on the non-endogenous substrate mixture (AA:LA), it was found that 13-HODE and 13-HOTrE displayed no observable effect on the AA:LA ratio up to 5 μM product. This is different than what was observed for 15-hLO-1, where 5 μM of 13-HODE was sufficient to saturate 15-hLO-1 substrate specificity, increasing the (kcat/KM)AA/(kcatKM)LA ratio from 1.4 to 2.9 (12). In contrast, the non-endogenous, reduced product, 12-HETE, did increase the substrate specificity, with the addition of 12-HETE (5 μM) increasing the (kcat/KM)AA/(kcatKM)LA ratio from 1.8 ± 0.2 to 2.4 ± 0.2. The 12-HETE titration data (Figure 2) was fit to a saturation curve by plotting the change in the ratio (Δ(kcat/KM)AA/(kcatKM)LA versus [12-HETE] μM (Figure S1, Supporting data). The half saturation point was determined to be 28 ± 2 μM, and the maximum (kcat/KM)AA/(kcat/KM)LA ratio being 5.1 ± 0.3. It should be noted that 20 μM of 12-HETE is the maximum concentration that still allowed the observation of the LA and AA products by HPLC, so further saturation of the 12-HETE effect on substrate specificity could not be achieved. For comparison, 12-HETE increased the (kcat/KM)AA/(kcat/KM)LA ratio of 15-hLO-1 from 1.8 to 2.7, with a half saturation point of 1.1 ± 0.1 μM (12), indicating that the binding affinity of 12-HETE to sLO-1 is nearly 30-fold weaker than that of 15-hLO-1 (12). It should also be noted that saturating amounts of 12-HETE to the allosteric site inhibit sLO-1 with both AA and LA as substrate, while 12-HETE activates the 15-hLO isozymes (12), indicating possible differences in their molecular mechanisms by which the allosteric site affects catalysis.
Figure 2.
Competitive substrate capture method saturation curve of (kcat/Km)AA/(kcat/Km)LA versus [12-HETE] μM for sLO-1 (100 mM Borate, pH 9.2, 22° C). The half saturation point was determined to be 28 ± 2 μM of 12-HETE and the maximum effect at saturating conditions was calculated to be 5.1 ± 0.3, as determined from the change in the ratio versus [12-HETE] (Figure S1).
IC50 Assays of sLO-1 with OS, PS, and OA Inhibitors
IC50 enzymatic assays were performed as described above and the IC50 values, for known sLO-1 inhibitors (OS, PS, and OA), were determined from inhibition plots (data not shown) (21–23). The allosteric inhibitor, OS, had an IC50 value of 1.0 ± 0.1 μM, the mixed-type inhibitor, PS, had an IC50 value of 13 ± 2 μM, and the competitive inhibitor, OA, had an IC50 value of 11 ± 3 μM (Table 1). The IC50 values, with LA as substrate, are in agreement with previously published inhibition kinetic results (21–23). It is important to note that IC50 values are not equivalent to Ki values, and they are dependent on both Ki and concentration of the substrate used.
Table 1.
Comparison of sLO-1 IC50 data with LAa
| Substrate | OAIC50 (Competitive) | PSIC50 (Mixed-Type) | OS IC50 (Allosteric) |
|---|---|---|---|
| LA | 11 ± 3 μM | 13 ± 2 μM | 1.0 ± 0.1 μM |
Enzymatic assays were at performed at 5 μM substrate concentrations in 100 mMBorate (pH 9.2) at 22 °C.
Determining the Inhibitor Effect on the Substrate Specificity of sLO-1
The substrate specificity of sLO-1 was determined with the addition of each inhibitor in excess of their IC50 values (LA as substrate) and none were observed to affect the (kcat/KM)ALA/(kcat/KM)LA ratio for sLO-1. These results were not surprising considering there was no observable effect on the ALA:LA ratio with product addition either. However, there was an observable effect on the (kcat/KM)AA/(kcat/KM)LA ratio for sLO-1 with the addition of inhibitors which target the allosteric site (OS and PS). At their respective IC50 value, the (kcat/KM)AA/(kcat/KM)LA ratio for sLO-1 was determine to be 2.3 ± 0.2, 3.2 ± 0.4 and 2.0 ± 0.1 for the inhibitors PS, OS and OA, respectively (Table 2). The effect of each inhibitor at 5-fold their respective IC50 values were determined to be 4.0 ± 0.3, 4.8 ± 0.3 and 1.9 ± 0.1 for PS, OS and OA, respectively (Table 2).
Table 2.
Inhibition Effects on sLO-1 Substrate Specificity for AA vs. LA
| Inhibitor (Type) | OA (Competitive) | PS (Mixed-Type) | OS (Allosteric) | ||||
|---|---|---|---|---|---|---|---|
| [Inhibitor] | 0 μM | 11 μM | 55 μM | 13 μM | 65 μM | 1 μM | 5 μM |
| (kcat/KM)AA/(kcat/KM)LA | 1.8 ± 0.2 | 2.0 ± 0.2 | 1.9 ± 0.1 | 2.3 ± 0.2 | 4.0 ± 0.3 | 3.2 ± 0.4 | 4.8 ± 0.3 |
aEnzymatic assays were at performed at 1 μM total substrate concentrations in 100 mM Borate (pH 9.2) at 22 °C.
Determination of the Saturating Effect of Inhibitors on the Substrate Specificity of sLO-1
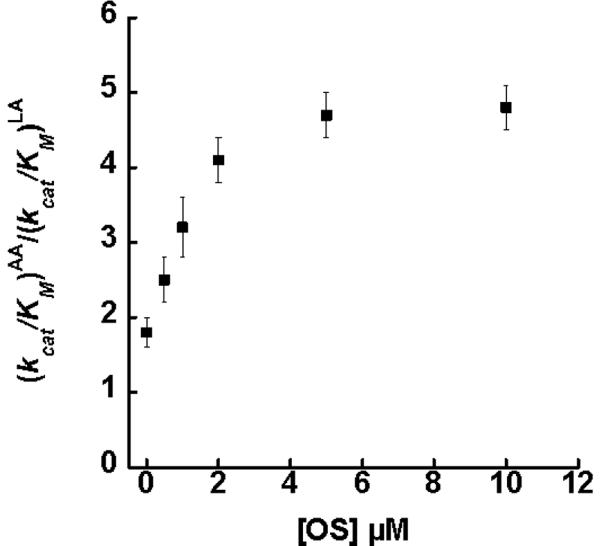
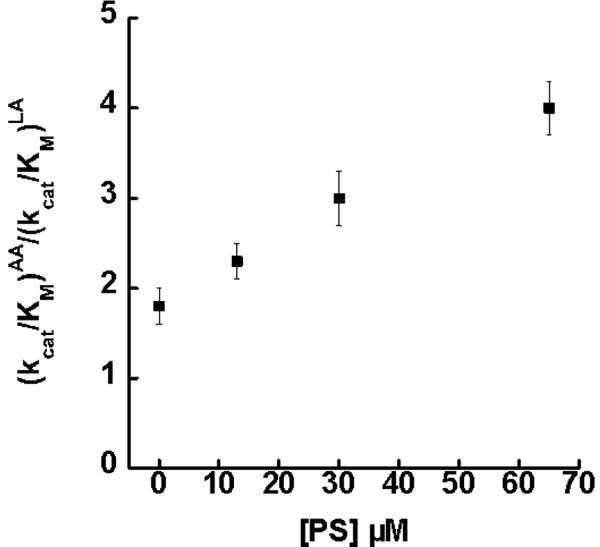
The inhibitors, OS and PS, were further investigated and shown to display a hyperbolic saturating effect on the substrate specificity for sLO-1. OS was titrated at 0.5, 1, 2, 5 and 10 μM and the (kcat/KM)AA/(kcat/KM)LA ratios were determined to be 2.5 ± 0.3, 3.2 ± 0.4, 4.7 ± 0.3 and 4.8 ± 0.3, respectively (Figure 3). The data was fit to a saturation curve by plotting Δ(kcat/KM)AA/(kcat/KM)LA versus [OS] μM (Figure S2, Supporting data). The half saturation point was determined to be 1.5 ± 0.3 μM, and at saturating conditions of OS, the maximum (kcat/KM)AA/(kcat/KM)LA ratio was calculated to be 5.4 ± 0.4. PS was titrated at 13, 30, and 65 μM and the (kcat/KM)AA/(kcat/KM)LA ratios were determined to be 2.3 ± 0.2, 3.0 ± 0.3, and 4.0 ± 0.3, respectively (Figure 4). The data was fit to a saturation curve by plotting Δ(kcat/KM)AA/(kcat/KM)LA versus [PS] μM (Figure S3, Supporting data). The half saturation point was determined to be 118 ± 35 μM, and at saturating conditions of PS, the maximum (kcat/KM)AA/(kcat/KM)LA ratio was calculated to be 7.4 ± 1.6. The half saturating point determined for both inhibitors correlated well with the previously determined binding affinities towards the allosteric site (OS Ki = 0.6 μM, PS Ki = 140 μM) (21, 23). The magnitude of the substrate specificity changes of sLO-1 with OS and PS are similar to that of 13-HODE and 12-HETE on 15-hLO-1 substrate specificity, which also confer a nearly 3-fold increase in selectivity towards AA over LA (12).
Figure 3.
Competitive substrate capture method saturation curve of (kcat/Km)AA/(kcat/Km)LA versus [OS]μM for sLO-1 (100 mM Borate, pH 9.2, 22° C). The half saturation point was determined to be 1.5 ± 0.3 μM of OS and the maximum effect at saturating conditions was calculated to be 5.4 ± 0.4, as determined from the change in the ratio versus [OS] (Figure S2).
Figure 4.
Competitive substrate capture method saturation curve of (kcat/Km)AA/(kcat/Km)LA versus [PS]μM for sLO-1 (100 mM Borate, pH 9.2, 22° C). The half saturation point was determined to be 120 ± 35 μM of OS and the maximum effect at saturating conditions was calculated to be 7.4 ± 1.6, as determined from the change in the ratio versus [PS] (Figure S3).
DISCUSSION
In our previous report, we uncovered an allosteric product-feedback mechanism that directly affected the substrate specificity of 15-hLO-1 (12). In order to probe if the substrate specificity of sLO-1 can also be manipulated by allosteric product binding, we investigated the effect of the addition of 13-HODE, 13-HOTrE and 12-HETE, on the substrate specificity for the endogenous sLO-1 substrate mixture, ALA:LA, and for the non-endogenous substrate mixture, AA:LA. The results from these experiments demonstrated that sLO-1 selectivity towards the endogenous substrate ratio (ALA:LA) was not affected by the addition of any of the LO products, indicating that the allosteric site does not affect the differentiation between ALA and LA, which have the same length, but different unsaturation on the methyl end of the fatty acid (Figure 1). Nevertheless, the addition of one of the LO products did affect the sLO-1 selectivity for the non-endogenous substrate mixture, AA:LA (12), with 12-HETE increasing the (kcat/KM)AA/(kcat/KM)LA ratio to a maximum of 5.1 ± 0.3 μM, with an AC50 of 28 ± 2 μM. As compared to 15-hLO-1, these results are similar in that both sLO-1 and 15-hLO-1 increase their selectivity towards AA over LA when their allosteric sites are bound by 12-HETE and indicate that allosteric binding affects the differentiation between substrate length and/or unsaturation on the carboxylate end of the fatty acid substrate (AA versus LA). Nevertheless, the binding affinity of 12-HETE to sLO-1 is nearly 30-fold less than that of 15-hLO-1, and unlike 15-hLO-1, 13-HODE did not display any observable effect on the (kcat/KM)AA/(kcat/KM)LA ratio of sLO-1 up to 5 μM product addition.
Given that the LO products did not effect the substrate specificity for sLO-1 with its endogenous substrates (ALA:LA), it was not surprising to observe that none of the inhibitors, OS, OA, or PS, affected sLO-1 selectivity for this substrate mixture either. However, OS did affect the non-endogenous substrate ratio (AA:LA), with a maximal ratio of 5.4 ± 0.4, with an AC50 of 1.5 ± 0.3 μM, similar in magnitude to its Ki as an allosteric inhibitor (Ki = 0.7 μM) (21). The mix-type inhibitor, PS, affected the non-endogenous substrate ratio (AA:LA), with a maximal ratio of 7.4 ± 1.6 and an AC50 of 118 ± 35 μM, which is very similar in magnitude to its secondary (allosteric) site Ki' of 140 μM (23). OA, which is a competitive inhibitor, had no effect on the ratio. These data for the three inhibitors, OS, PS, and OA, are consistent with the hypothesis that allosteric binding elicits a substrate specificity change in sLO-1.
It should be noted that our laboratory previously observed that OS (Ki = 0.7 μM) and OA (Ki = 22 μM) induced an increase in the KIE of sLO-1, suggesting that both OS and OA bound to the allosteric site, albeit with different potency (21). However, since OA does not affect substrate specificity, these results may indicate that the allosteric effects on substrate specificity are not mechanistically linked to the allosteric effects on the KIE. This is an unusual result since one would suspect the KIE for the abstraction step to be more sensitive to allosteric binding than the change in substrate specificity. Nevertheless, we have previously observed an analogous result, where the allosteric effector, 13-HODE, changed the substrate specificity of 15-hLO-1 but did not affect the observed KIE (27). We are currently investigating these differences further in the hopes of understanding the structural change which leads to these functional differences.
In summary, these data indicate that the allosteric effect of sLO-1 and 15-hLO-1 are similar in that they both increase the AA/LA ratio, however, 15-hLO-1 achieves this change in ratio by activation (12), while sLO-1 achieves this change by inhibition. This difference between the two LOs raises the question of whether the structural change of active site, which differentiates AA from LA, is similar. We have recently docked 13-HODE to a homology model of 15-hLO-2 (28) and proposed a potential location for the allosteric site, which is between the two domains of LO and is approximately 10 Å from the active site. We are currently mutating key residues of this potential allosteric site in both 15-hLO and sLO-1 in the hopes of confirming this hypothesis. The native function of the allosteric site in sLO-1, however, remains unclear since 12-HETE is an LO product that sLO-1 never encounters in nature. This may represent an evolutionary divergence of between human and soybean LOs, since sLO-1 retains the ability to allosterically regulate substrate specificity, yet does not bind its catalytic products (13-HPODE and 13-HPOTrE), as the 15-hLO isozymes do (13-HPODE and 12-HPETE) (12). Once the location of the allosteric site is established for both sLO-1 and 15-hLO-1, it will be informative to compare the amino acid conservation between the two sites and their similarities/ differences. Finally, this work demonstrates that inhibitors can be generated that not only lower activity but also change the LO product ratio. This is a new concept in lipoxygenase targeting, in which the goal is to not only reduce the catalytic activity but also alter substrate selectivity towards a physiologically beneficial substrate, such as AA in the case of 15-hLO-1 and prostate cancer. An analogous change in the product ratio is observed with the inhibition of cyclooxygenase and aspirin, where aspirin reduces the production of prostaglandins, pro-inflammatory mediators, but does not affect the production of resolvins, anti-inflammatory mediators (29). We are currently investigating inhibitors of the 15-hLO isozymes, in the hopes of increasing the AA/LA ratio of 15-hLO-1, while simultaneously inhibiting its enzymatic activity.
Supplementary Material
Acknowledgements
The authors acknowledge the Holman group members for helpful discussions and proof reading the manuscript.
This work was supported by the National Institutes of Health (GM56062, TRH)
Abbreviations
- LO
lipoxygenase
- sLO-1
soybean lipoxygenase-1
- 15-hLO-1
human reticulocyte 15-lipoxygenase
- AA
arachidonic acid
- 15-HPETE
15-(S)-hydroperoxyeicosatetraenoic acid
- 15-HETE
15-(S)-hydroxyeicosatetraenoic acid (reduced oxidation product of AA)
- 12-HPETE
12-(S)-hydroperoxyeicosatetraenoic acid
- 12-HETE
12-(S)-hydroxyeicosatetraenoic acid (reduced oxidation product of AA)
- LA
linoleic acid
- 13-HPODE
13-(S)-hydroperoxyoctadienoic acid
- `13-HODE
13-(S)-hydroxyoctadecadienoic acid (reduced oxidation product of LA)
- ALA
α-linolenic acid
- 13-HPOTrE
13-hydroperoxyoctadecatrienoic acid
- 13-HOTrE
13-(S)-hydroxyoctadecatrienoic acid (reduced oxidation product of ALA)
- kcat
the rate constant for product release
- kcat/Km
the rate constant for substrate capture
- (kcat/KM)AA/(kcat/KM)LA
substrate capture ratio between AA and LA
- OS
oleyl sulfate
- PS
palmitoleyl sulfate
- OA
oleic acid
- IC50
inhibitor concentration at 50% inhibition
- AC50
product concentration at 50% effect
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Solomon EI, Zhou J, Neese F, Pavel EG. New insights from spectroscopy into the structure/function relationships of lipoxygenases. Chem. Biol. 1997;4:795–808. doi: 10.1016/s1074-5521(97)90113-7. [DOI] [PubMed] [Google Scholar]
- 2.Yamamoto S. Mammalian lipoxygenases: molecular structures and functions. Biochim. Biophys. Acta. 1992;1128:117–131. doi: 10.1016/0005-2760(92)90297-9. [DOI] [PubMed] [Google Scholar]
- 3.Samuelsson B, Dahlen SE, Lindgren JA, Rouzer CA, Serhan CN. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237:1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- 4.Steele VE, Holmes CA, Hawk ET, Kopelovich L, Lubet RA, Crowell JA, Sigman CC, Kellof GJ. Lipoxygenase Inhibitors as Potential Cancer Chemopreventives. Cancer Epidemiology, Biomarkers & Prevention. 1999;8:467–483. [PubMed] [Google Scholar]
- 5.Prigge ST, Boyington JC, Gaffney BJ, Amzel LM. Structure conservation in lipoxygenases: structural analysis of soybean lipoxygenase-1 and modeling of human lipoxygenases. Proteins. 1996;24:275–291. doi: 10.1002/(SICI)1097-0134(199603)24:3<275::AID-PROT1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 6.Glickman MH, Klinman JP. Nature of rate-limiting steps in the soybean lipoxygenase-1 reaction. Biochemistry. 1995;34:14077–14092. doi: 10.1021/bi00043a013. [DOI] [PubMed] [Google Scholar]
- 7.Knapp MJ, Klinman JP. Kinetic studies of oxygen reactivity in soybean lipoxygenase-1. Biochemistry. 2003;42:11466–11475. doi: 10.1021/bi0300884. [DOI] [PubMed] [Google Scholar]
- 8.Minor W, Steczko J, Stec B, Otwinowski Z, Bolin JT, Walter R, Axelrod B. Crystal structure of soybean lipoxygenase L-1 at 1.4 A resolution. Biochemistry. 1996;35:10687–10701. doi: 10.1021/bi960576u. [DOI] [PubMed] [Google Scholar]
- 9.Oliw EH. Plant and fungal lipoxygenases. Prostaglandins Other Lipid Mediat. 2002;68-69:313–323. doi: 10.1016/s0090-6980(02)00037-0. [DOI] [PubMed] [Google Scholar]
- 10.Grechkin A. Recent developments in biochemistry of the plant lipoxygenase pathway. Prog. Lipid Res. 1998;37:317–352. doi: 10.1016/s0163-7827(98)00014-9. [DOI] [PubMed] [Google Scholar]
- 11.Liechti R, Farmer EE. The jasmonate biochemical pathway. Sci. STKE. 2003;2003:CM18. doi: 10.1126/stke.2003.203.cm18. [DOI] [PubMed] [Google Scholar]
- 12.Wecksler AT, Kenyon V, Deschamps JD, Holman TR. Substrate specificity changes for human reticulocyte and epithelial 15-lipoxygenases reveal allosteric product regulation. Biochemistry. 2008;47:7364–7375. doi: 10.1021/bi800550n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruddat VC, Mogul R, Chorny I, Chen C, Perrin N, Whitman S, Kenyon V, Jacobson MP, Bernasconi CF, Holman TR. Tryptophan 500 and arginine 707 define product and substrate active site binding in soybean lipoxygenase-1. Biochemistry. 2004;43:13063–13071. doi: 10.1021/bi0489098. [DOI] [PubMed] [Google Scholar]
- 14.Hsi LC, Wilson LC, Eling TE. Opposing effects of 15-lipoxygenase-1 and -2 metabolites on MAPK signaling in prostate. Alteration in peroxisome proliferator-activated receptor gamma. J. Biol. Chem. 2002;277:40549–40556. doi: 10.1074/jbc.M203522200. [DOI] [PubMed] [Google Scholar]
- 15.Hsi LC, Wilson L, Nixon J, Eling TE. 15-lipoxygenase-1 metabolites down-regulate peroxisome proliferator-activated receptor gamma via the MAPK signaling pathway. J. Biol. Chem. 2001;276:34545–34552. doi: 10.1074/jbc.M100280200. [DOI] [PubMed] [Google Scholar]
- 16.Nie D, Che M, Grignon D, Tang K, Honn KV. Role of eicosanoids in prostate cancer progression. Cancer Metastasis Rev. 2001;20:195–206. doi: 10.1023/a:1015579209850. [DOI] [PubMed] [Google Scholar]
- 17.Shureiqi I, Lippman SM. Lipoxygenase modulation to reverse carcinogenesis. Cancer Res. 2001;61:6307–6312. [PubMed] [Google Scholar]
- 18.Shappell SB, Manning S, Boeglin WE, Guan YF, Roberts RL, Davis L, Olson SJ, Jack GS, Coffey CS, Wheeler TM, Breyer MD, Brash AR. Alterations in lipoxygenase and cyclooxygenase-2 catalytic activity and mRNA expression in prostate carcinoma. Neoplasia. 2001;3:287–303. doi: 10.1038/sj.neo.7900166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Butler RM, Butler SH, Tindall DJ, Young CY. Nonapoptotic cell death associated with S-phase arrest of prostate cancer cells via the peroxisome proliferator-activated receptor gamma ligand, 15-deoxy-delta12,14-prostaglandin J2. Cell Growth Diff. 2000;11:49–61. [PubMed] [Google Scholar]
- 20.van der Heijdt LM, Schilstra MJ, Feiters MC, Nolting HF, Hermes C, Veldink GA, Vliegenthart JF. Changes in the iron coordination sphere of Fe(II) lipoxygenase-1 from soybeans upon binding of linoleate or oleate. Eur. J. Biochem. 1995;231:186–191. doi: 10.1111/j.1432-1033.1995.tb20685.x. [DOI] [PubMed] [Google Scholar]
- 21.Mogul R, Johansen E, Holman TR. Oleyl sulfate reveals allosteric inhibition of soybean lipoxygenase-1 and human 15-lipoxygenase. Biochemistry. 2000;39:4801–4807. doi: 10.1021/bi992805t. [DOI] [PubMed] [Google Scholar]
- 22.Mogul R, Holman TR. Inhibition studies of soybean and human 15-lipoxygenases with long-chain alkenyl sulfate substrates. Biochemistry. 2001;40:4391–4397. doi: 10.1021/bi002581a. [DOI] [PubMed] [Google Scholar]
- 23.Ruddat VC, Whitman S, Holman TR, Bernasconi CF. Stopped-flow kinetic investigations of the activation of soybean lipoxygenase-1 and the influence of inhibitors on the allosteric site. Biochemistry. 2003;42:4172–4178. doi: 10.1021/bi020698o. [DOI] [PubMed] [Google Scholar]
- 24.Lewis E, Johnson E, Holman T. Large Competitive Kinetic Isotope Effects in Human 15-Lipoxygenase Catalysis Measured by a Novel HPLC Method. J. Am. Chem. Soc. 1999;121:1395–1396. [Google Scholar]
- 25.Holman TR, Zhou J, Solomon EI. Spectroscopic and functional characterization of a ligand coordination mutant of soybean lipoxygenase: First coordination sphere analogue of human 15-lipoxygenase. J. Am. Chem. Soc. 1998;120:12564–12572. [Google Scholar]
- 26.Deschamps JD, Kenyon VA, Holman TR. Baicalein is a potent in vitro inhibitor against both reticulocyte 15-human and platelet 12-human lipoxygenases. Bioorg. Med. Chem. 2006;14:4295–4301. doi: 10.1016/j.bmc.2006.01.057. [DOI] [PubMed] [Google Scholar]
- 27.Wecksler AT, Jacquot C, van der Donk WA, Holman TR. Mechanistic investigations of human reticulocyte 15- and platelet 12-lipoxygenases with arachidonic acid. Biochemistry. 2009;48:6259–6267. doi: 10.1021/bi802332j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wecksler AT, Deschamps JD, Kenyon V, Garcia NK, van der Donk WA, Holman TR. Kinetic and structural investigations of the allosteric site in human epithelial 15-lipoxygenase-2. Biochemistry Manuscript in revisions. 2009 doi: 10.1021/bi9009242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.