Abstract
Past mining activities in northern Mexico left a legacy of delerict landscapes devoid of vegetation and seasonal formation of salt efflorescence. Metal content was measured in mine tailings, efflorescent salts, soils, road dust and residential soils to investigate contamination. Climatic effects such as heavy wind and rainfall events can have great impact on the dispersion of metals in semi-arid areas, since soils are typically sparsely vegetated. Geochemical analysis of this site revealed that even though total metal content in mine tailings was relatively low (e.g. Cu = 1000 mg kg-1), metals including Mn, Ba, Zn, and Cu were all found at significantly higher levels in efflorescence salts formed by evaporation on the tailings impoundment surface following the rainy season (e.g. Cu=68000 mg kg-1). Such efflorescent fine-grained salts are susceptible to wind erosion resulting in increased metal spread to nearby residential soils. Our results highlight the importance of seasonally dependent salt-formation and wind erosion in determining risk levels associated with potential inhalation or ingestion of airborne particulates originating from contaminated sites such as tailings impoundments. In low metal-content mine tailings located in arid and semi-arid environments, efflorescence salts could represent a human health risk and a challenge for plant establishment in mine tailings.
Keywords: efflorescence salts, dust, metals, wind-dispersion
Introduction
Past mining activities have generated large amounts of unconfined waste in Mexico. The environmental impact of such waste, specifically mine tailings, generally results from their low pH, high metal content and the high incidence of wind- and water-driven erosion events. The impact of mining activities in arid and semi-arid regions of Mexico has received less attention than temperate regions (Ramos-Arroyo and Siebe-Grabach, 2006). Yet erosion of mine tailings in arid environments, such as found in northern Mexico, poses risk to surrounding neighborhoods in two ways: i) the stability of the tailings may be seriously affected by intensive erosion processes or meteorological situations that can, in some cases result in partial or total collapse of the tailings structure; and ii) airborne release of metal-laden particulates from tailings sites (Mendez and Maier, 2008).
In recent decades, there has been growing concern for the potential contribution of dust as a vehicle for metal ingestion by humans. While some trace metals (such as copper and zinc) are harmless when ingested in small amounts, other metals, notably lead and cadmium, are toxic and are potential cofactors, initiators or promoters in many diseases including cardiovascular disease and cancer even at extremely low concentrations (Nriagu, 1988). Young children are particularly vulnerable to heavy metal poisoning for two reasons. Firstly, young children are more likely to ingest non-food objects and repetitive hand/finger sucking. Secondly, children have a much higher absorption rate of heavy metals from digestion system and higher hemoglobin sensitivity to metals than adults (Nriagu, 1988).
Despite the environmental significance of airborne particulates generated from mining sites, there is a lack of exposure information for children residing in neighborhoods adjacent to such sites. This includes information concerning typical particulate composition and size as well as information concerning the deposition of particulates into soils surrounding mining sites. The latter is of particular concern for urban playgrounds and residential areas where children spend significant amounts of time. In fact, the ingestion of dust and soil is widely regarded as one of the key pathways by which children are exposed to heavy metals and metalloids from a variety of sources (De Miguel et al., 1997; Rasmussen et al., 2001; Madrid et al., 2002).
The old Nacozari mining district hosts the most important copper deposits in Mexico and some of the major Cu mines in the world. The district contains a variety of ore deposits (porphyry copper, breccia pipe and veins) with Cu, Mo, Au, Ag and Zn deposits. Some of the most important are: La Caridad, Pilares Breccia, El Batamote, Los Alisos, La Gloria and San Nicolás.
The Nacozari tailings impoundments have been breached by sporadic but concentrated surface water flows (from heavy rainfall events) that have eroded the tailings from their original location and transported the materials downstream to residential areas. In addition, the upper flat surfaces of the remaining tailings impoundments have dried and are susceptible to wind erosion. While erosional processes are driven by both water and wind, most studies of soil erosion in nonagricultural settings have focused on water-driven rather than wind-driven processes. However, recent data estimating the relative rates of water- and wind-driven erosion and transport suggest that wind-driven processes have similar, and in many cases greater, impact on loss and local redistribution of soil in semiarid grassland, and forest ecosystems (Breshears et al., 2003). Wind erosion is also important in determining risks levels associated with potential inhalation of airborne particulates from contaminated soils (Larney et al., 1999). Thus, the objective of this study was to compare metal concentrations in mine tailings, road dust, and residential soils and to evaluate transport mechanisms for metals in the Nacozari mine site area.
Site description
Nacozari de García, Sonora is a mining town located near the U.S-Mexico border. It hosts a population of 14,000. The growth of the town of Nacozari de García has encroached around the mine tailings which cover an area of 19 ha and an amount of about 3 million tons (Fig. 1). This mine site contains multiple large tailings impoundments, old mill building foundations, a former copper refinery, and other mine structures. Historic records indicate that copper may have been discovered at this site as early as 1880. The Moctezuma Copper Company exploited the former Pilares mine located 10 km east of Nacozari. The Pilares mine produced 3000 tons of copper per day and approximately 40 million tons of copper during its operations from 1900 to 1949. Mine tailings were deposited over Tertiary age volcanic rocks (andesites, ignimbrites and rhyolites) and also over the Baucarit Formation made of conglomerate interlayered with tuff and sandstones. According to historical records, Cu values in the Pilares deposit were of 0.7 to 1.2%, this deposit was characterized by low metal sulfide content including pyrite (FeS), galena (PbS) and sphalerite (ZnS).
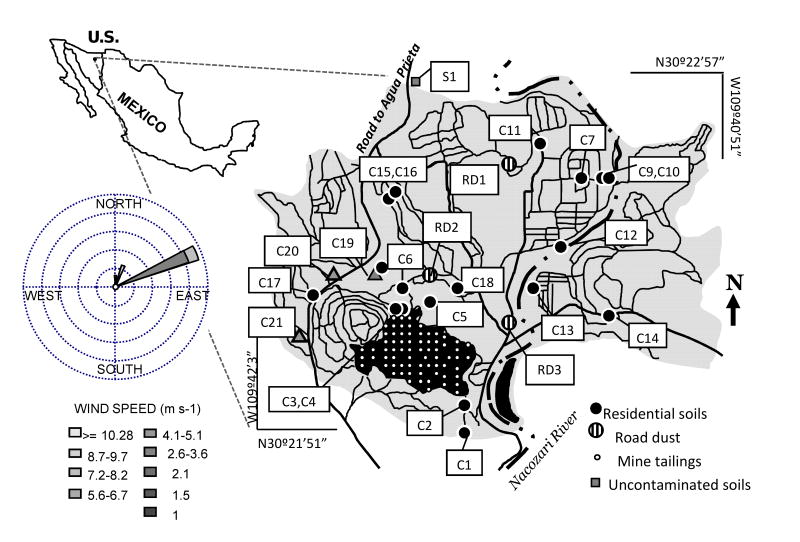
Figure 1.
A map showing the location of sampling sites used in this study. Sample labels are: C, residential sites; S, non-contaminated soils; RD road dust sites. Soil samples S2 -6 are not shown in figure, they are located 6-15 kilometers to the north of the town. On the left is a wind rose showing that the preferential wind direction in the site is from SSW to NNE.
The study area has a semi-arid climate with an annual average precipitation of 490 mm, an annual average temperature of 19.6 °C with maximum monthly average temperature of 28.4 °C and minimum 10.8 °C. Maximum temperatures occur in July and August along with maximum precipitation. Evaporation exceeds 2 times the precipitation according to mean annual balances (maximum average monthly evapotranspiration is 151 mm in July and 115 mm in August). Surface run-off is very high, since 46% of the annual precipitation can occur in 60 days. Therefore, the main mechanisms of contaminant mobilization from primary sources are run-off and mechanical dispersion caused by wind. Dominant wind directions are S to N and SWW to NNE oriented with average wind speed of 5.36 m s-1, and 8.33% of calm winds (Fig. 1).
Materials and methods
Sampling and analysis
Mine tailings (70 samples), efflorescence salts (7 samples), soils (S1-S6), residential soils (C1-C21), and road dust (RD1-RD3), were collected during a year period (2007), results are shown in Tables 1 and 2. Bulk tailings samples were taken from one historical tailings pile for metal analyses by hand-excavation as deep as 0.3 m (5-10 kg per sample) and immediately stored in plastic bags to avoid moisture loss and to minimize exposure to atmospheric gases. Bulk tailings samples were sieved (<2 mm) prior to analysis. Efflorescence salt samples forming at the tailings surface were collected at seven main areas of salt accumulation within a surface area of 3 hectares at the southern side of the tailings pile. Each salt sample was approximately 100 g. Sampling was performed according to standard methods for contaminated soils (USEPA 1991). Samples of natural soils were collected from six points (<20 cm depth) within an uncontaminated control area 20 km north of the mine tailings area. Residential soils were collected with a Teflon knife after scraping away the top 0.5 cm. The soil samples were placed in plastic bags. Three composite roadway dust samples were taken after a dry period in 2007, at different points, along a main street with moderate traffic density. The road dust samples were collected by gently sweeping the road with a soft plastic brush. A total of 1.5 kg was collected for each sample and stored in plastic bags. Road dust samples were dried at 30°C for 48 h and passed through a 30-mesh sieve.
Table 1.
Average concentration (mg kg-1) of metals in mine tailings from Nacozari, Sonora, México.
| Mine tailings | Efflorescence salts | |
|---|---|---|
| Number of analyzed samples | 70 bulk | 7 |
| pH | 3.87± 0.3 | 4± 0.2 |
| EC μS/cm | 340.1 ± 2 | n.a. |
| Ti | 1508 ± 365 | <150 |
| Cr | <25 | 219.3 ± 68.6 |
| Mn | 158.5 ± 10.5 | 31084 ± 458 |
| Fe | 31739 ± 381.9 | 9298 ± 230 |
| Ni | 69.5 ± 15.5 | 204 ± 34.7 |
| Cu | 400.5 ± 15.8 | 68751 ± 865 |
| Zn | 78.8 ± 6.5 | 17858 ± 238.5 |
| As | 29.3 ± 4 | 23.8 ± 5 |
| Se | 5.9 ± 1.6 | 13.5 ± 1.8 |
| Rb | 298.4 ± 5.6 | 175 ± 5.5 |
| Sr | 68.6 ± 2.6 | 8 ± 3 |
| Zr | 49.8 ± 3.7 | <6 |
| Mo | 58.3 ± 3.5 | 10.8 ± 4 |
| Ag | 47.9 ± 2.3 | 321 ± 16 |
| Ba | 423.1 ± 140 | 1671 ± 139 |
| Hg | 28.1 ± 2.8 | <11 |
| Pb | 39 ± 4.2 | 12 ± 5 |
| Cd | <40 | <40 |
| Sn | <80 | <80 |
| Tl | <15 | <15 |
Table 2.
Metal contents analyzed in Nacozari urban soils. Concentrations are in mg kg-1.
| Sample | Type | Ti | Mn | Fe | Ni | Cu | Zn | As | Se | Rb | Sr | Zr | Mo | Ag | Ba | Hg | Pb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RD1 | R | 52 | 55 | 41 | 78 | 3 | 2 | 20 | <L | 1 | 35 | 66 | 36 | <L | 40 | <L | 7 |
| D | 90 | 4 | 67 | 5 | 7 | O | 1 | 1 | O | 7 | O | 1 | |||||
| 5 | 4 | 7 | D | 8 | D | D | |||||||||||
| RD2 | R | 45 | 37 | 27 | <L | 1 | 1 | 11 | 10 | 1 | 50 | 12 | <L | <L | 49 | <L | 5 |
| D | 13 | 4 | 02 | O | 6 | 4 | 1 | 4 | 6 | O | O | 1 | O | 0 | |||
| 6 | D | 7 | 3 | 0 | D | D | D | ||||||||||
| RD3 | R | 30 | 34 | 22 | <L | 3 | 1 | 14 | <L | 1 | 44 | 71 | <L | <L | 27 | <L | 3 |
| D | 50 | 6 | 77 | O | 1 | 3 | O | 1 | 5 | O | O | 8 | O | 4 | |||
| 4 | D | 3 | 4 | D | 0 | D | D | D | |||||||||
| C1 | RS | 31 | 32 | 23 | 76 | 4 | 5 | 27 | <L | 8 | 30 | 77 | <L | <L | 43 | <L | 2 |
| 60 | 2 | 89 | 8 | 3 | O | 8 | 7 | O | O | 7 | O | 3 | |||||
| 9 | D | D | D | D | |||||||||||||
| C2 | RS | 22 | 23 | 26 | <L | 9 | 8 | <L | <L | 1 | 33 | 58 | <L | <L | 44 | <L | 2 |
| 74 | 5 | 00 | O | 5 | 3 | O | O | 2 | 5 | O | O | 3 | O | 4 | |||
| 4 | D | D | D | 4 | D | D | D | ||||||||||
| C3 | RS | 23 | 23 | 27 | <L | 2 | 1 | 16 | <L | 1 | 44 | 22 | <L | <L | 41 | 21 | 2 |
| 01 | 5 | 15 | O | 1 | 3 | O | 7 | 7 | O | O | 1 | 5 | |||||
| 1 | D | 3 | 3 | D | 9 | D | D | ||||||||||
| C4 | RS | 38 | 27 | 27 | 75 | 2 | 8 | 31 | <L | 2 | 32 | 47 | 12 | <L | 47 | 18 | 3 |
| 22 | 0 | 42 | 9 | 0 | O | 3 | 1 | O | 6 | 4 | |||||||
| 2 | 6 | D | 8 | D | |||||||||||||
| C5 | RS | 29 | 47 | 22 | <L | 4 | 3 | 17 | 10 | 1 | 38 | 76 | 27 | <L | 48 | <L | 4 |
| 41 | 8 | 31 | O | 5 | 4 | 9 | 3 | O | 3 | O | 4 | ||||||
| 9 | D | 0 | 8 | 3 | D | D | |||||||||||
| C6 | RS | 49 | 65 | 32 | <L | 3 | 3 | 26 | <L | 1 | 47 | 59 | <L | <L | 50 | <L | 4 |
| 85 | 6 | 76 | O | 1 | 3 | O | 4 | 6 | O | O | 2 | O | 7 | ||||
| 6 | D | 6 | 0 | D | 0 | D | D | D | |||||||||
| C7 | RS | 32 | 56 | 27 | <L | 1 | 1 | 23 | <L | 1 | 29 | 50 | <L | <L | 55 | <L | 7 |
| 45 | 8 | 29 | O | 7 | 7 | O | 3 | 4 | O | O | 9 | O | 1 | ||||
| 1 | D | 7 | 5 | D | 2 | D | D | D | |||||||||
| C9 | RS | 36 | 54 | 27 | 71 | 1 | 1 | 41 | <L | 1 | 38 | 35 | <L | <L | 37 | <L | 3 |
| 17 | 6 | 49 | 4 | 1 | O | 1 | 4 | O | O | 6 | O | 2 | |||||
| 0 | 9 | 4 | D | 6 | D | D | D | ||||||||||
| C10 | RS | 37 | 59 | 31 | 76 | 2 | 1 | 28 | <L | 9 | 40 | 36 | <L | <L | 41 | <L | 5 |
| 75 | 7 | 40 | 5 | 8 | O | 5 | 7 | O | O | 8 | O | 4 | |||||
| 4 | 2 | 0 | D | D | D | D | |||||||||||
| C11 | RS | 44 | 42 | 27 | 79 | 2 | 3 | 17 | <L | 1 | 32 | 44 | <L | <L | 58 | <L | 3 |
| 10 | 7 | 15 | 0 | 3 | O | 1 | 0 | O | O | 9 | O | 5 | |||||
| 4 | 7 | 9 | D | 6 | D | D | D | ||||||||||
| C12 | RS | 29 | 35 | 17 | <L | 1 | 5 | 32 | <L | 1 | 38 | 18 | 12 | <L | 36 | <L | 1 |
| 26 | 3 | 78 | O | 7 | 2 | O | 1 | 0 | O | 7 | O | 4 | |||||
| 6 | D | 6 | 3 | D | 0 | D | D | 9 | |||||||||
| C13 | RS | 26 | 28 | 19 | <L | 3 | 1 | 15 | 11 | 1 | 28 | 66 | 27 | <L | 42 | <L | 4 |
| 48 | 9 | 31 | O | 6 | 8 | 4 | 0 | O | 8 | O | 1 | ||||||
| 2 | D | 1 | 1 | 0 | D | D | |||||||||||
| C14 | RS | 30 | 49 | 26 | 70 | 1 | 8 | 18 | 11 | 1 | 56 | 41 | <L | <L | 60 | <L | 3 |
| 34 | 1 | 77 | 3 | 4 | 2 | 5 | O | O | 9 | O | 4 | ||||||
| 8 | 2 | 6 | D | D | D | ||||||||||||
| C15 | RS | 30 | 53 | 23 | <L | 1 | 1 | 15 | <L | 1 | 97 | <L | <L | <L | 44 | <L | 2 |
| 39 | 6 | 35 | O | 3 | 4 | O | 1 | 9 | O | O | O | 2 | O | 6 | |||
| 4 | D | 1 | 4 | D | 2 | D | D | D | D | ||||||||
| C16 | RS | 65 | 69 | 43 | 95 | 8 | 1 | 15 | 11 | 8 | 14 | <L | <L | <L | 70 | <L | 2 |
| 59 | 0 | 56 | 4 | 0 | 1 | 45 | O | O | O | 6 | O | 3 | |||||
| 6 | 4 | D | D | D | D | ||||||||||||
| C17 | RS | 35 | 38 | 25 | <L | 1 | 1 | 25 | <L | 1 | 50 | 59 | 21 | <L | 38 | <L | 3 |
| 00 | 3 | 09 | O | 7 | 5 | O | 2 | 2 | O | 3 | O | 1 | |||||
| 5 | D | 8 | 0 | D | 5 | D | D | ||||||||||
| C18 | RS | 40 | 39 | 31 | <L | 2 | 1 | 25 | <L | 1 | 39 | 45 | 28 | <L | <L | <L | 3 |
| 51 | 8 | 76 | O | 3 | 7 | O | 3 | 2 | O | O | O | 5 | |||||
| 5 | D | 2 | 0 | D | 4 | D | D | D | |||||||||
| C19 | RS | 46 | 43 | 27 | 73 | 7 | 1 | <L | 11 | 1 | 67 | 21 | <L | <L | 47 | 15 | 3 |
| 09 | 6 | 24 | 3 | 0 | O | 0 | 1 | O | O | 0 | |||||||
| 4 | 6 | D | 7 | D | D | ||||||||||||
| C20 | RS | 28 | 39 | 25 | <L | 8 | 1 | <L | <L | 1 | 60 | <L | <L | <L | 60 | 17 | 7 |
| 50 | 4 | 51 | O | 5 | 2 | O | O | 0 | 9 | O | O | O | 0 | 2 | |||
| 0 | D | 9 | D | D | 7 | D | D | D | |||||||||
| C21 | RS | 52 | 38 | 26 | 77 | 1 | 1 | <L | <L | 9 | 60 | 18 | <L | <L | 54 | <L | 6 |
| 92 | 7 | 69 | 2 | 9 | O | O | 4 | 4 | O | O | 1 | O | 8 | ||||
| 6 | 4 | 4 | D | D | D | D | D | ||||||||||
| S-1 | S | 38 | 13 | 29 | <L | 4 | 5 | 11 | <L | 5 | 59 | 12 | <L | <L | 42 | <L | 2 |
| 95 | 82 | 35 | O | 7 | 5 | O | 7 | 3 | O | O | 9 | O | 2 | ||||
| 0 | D | D | D | D | D | ||||||||||||
| S-2 | S | 32 | 55 | 31 | 75 | 9 | 1 | 14 | <L | 1 | 42 | 57 | <L | <L | 53 | <L | 4 |
| 09 | 8 | 23 | 6 | 2 | O | 1 | 1 | O | O | 7 | O | 6 | |||||
| 8 | 5 | D | 6 | D | D | D | |||||||||||
| S-3 | S | 66 | 79 | 50 | 10 | 1 | 8 | 12 | 5 | 3 | 14 | <L | <L | <L | 67 | <L | 1 |
| 48 | 4 | 91 | 5 | 0 | 5 | 4 | 37 | O | O | O | 5 | O | 4 | ||||
| 3 | 8 | D | D | D | D | ||||||||||||
| S-4 | S | 37 | 39 | 26 | <L | 4 | 1 | 17 | <L | 1 | 46 | 53 | <L | <L | 39 | 16 | 4 |
| 52 | 2 | 75 | O | 7 | 4 | O | 2 | 3 | O | O | 5 | 3 | |||||
| 9 | D | 3 | 8 | D | 9 | D | D | ||||||||||
| S-5 | S | 43 | 49 | 32 | 96 | 1 | 1 | 15 | 4 | 1 | 66 | 30 | <L | <L | 53 | 16 | 4 |
| 20 | 2 | 06 | 6 | 2 | 0 | 8 | O | O | 0 | 6 | |||||||
| 7 | 2 | 1 | 5 | D | D | ||||||||||||
| S-6 | S | 43 | 76 | 38 | <L | 4 | 1 | 14 | <L | 4 | 52 | 33 | <L | <L | 44 | <L | 1 |
| 21 | 7 | 17 | O | 1 | 0 | O | 6 | 2 | O | O | 9 | O | 9 | ||||
| 6 | D | 1 | 9 | D | D | D | D |
Abbreviations are: <LOD: below limit of detection; RD: road dust; RS: residential soils; SP: school playgrounds; S: soil background.
All samples were analyzed after sieving using an Innov-XXT400 portable X-ray fluorescence (XRF) analyzer with a miniature, rugged x-ray tube excitation source. The XT400 XRF analyzer utilizes a Hewlett-Packard (HP) iPAQ personal data assistant for data storage. The XT400 analyzer can analyze elements from potassium to uranium in suites of 25 elements simultaneously. The certified standard NIST SRM-2702 (Inorganics in marine sediments) was also analyzed by the XT400 XRF with recoveries ranging from 90-110%. An estimated limit of detection was determined for each metal (in mg kg-1unless otherwise specified) based on a 99.7% confidence level using a 120 second test. Limits of detection were: Ba (240), Ti (400), Cr (45), Mn (80), Fe (100), Co (200), Ni (70), Cu (50), Zn (30), As (10), Se (9), Rb (11), Sr (13), Zr (10), Mo (10), Ag (75), Cd (50), Sn (100), Hg (14), Tl (18), Pb (16). The chemical composition of samples is presented in Tables 1 and 2.
In addition, to verify the XRF analysis, 10% of the samples were analyzed using acid digestion coupled with plasma-atomic emission spectrometry (ICP-AES), in accordance with EPA Method 3050B/6010B. ICP-AES analyses were performed using a Perkin-Elmer 4200 DV ICP, coupled with an ultrasonic nebulizer. When compared, the XRF results were within 10% agreement with the ICP-AES results.
Electric conductivity and pH were determined using samples that were dried at 30°C for 48 h, and then sieved. Further, the mineralogy of the tailings, efflorescence salt, and road dust samples was obtained by X-ray powder diffraction (XRD) using a D8 Advance X-Ray Diffractometer Bruker AXS at the University of Sonora.
Statistical analysis
Principal component analysis (PCA) and cluster analysis (CA) were used for data analysis in this study (Mendiguchía et al., 2004) using JMP4 software for Windows. Since sample metal content varied by up to several orders of magnitude, a correlation matrix was used for PCE analysis and each variable was normalized to unit variance to allow equal contributions (Loska and Wiechula, 2003; Farnham et al., 2003).
To make the results more easily interpretable, PCA with VARIMAX normalized rotation was also applied, which can maximize the variances of the factor loadings across variables for each factor. Factor loadings >0.71 are typically regarded as excellent and <0.32 poor (García et al., 2004). In this study, all principal factors extracted from the variables were retained with eigenvalues >1.0. When PCA with VARIMAX normalized rotation is performed, each PC score contains information on all of the metal elements combined into a single number, while the loadings indicate the relative contribution that each element makes to the score. The PC loadings are plotted and the plot is inspected for similarities observed as clusters in the PC loading plot.
Cluster analysis (CA) was performed to further classify elements of different sources on the basis of the similarities of their chemical properties. Hierarchical cluster analysis, used in this study, assisted in identifying relatively homogeneous groups of variables, using an algorithm that starts with each variable in a separate cluster and combines clusters until only one is left. As the variables have large differences in scaling, standardization was performed before computing proximities, which can be done automatically by the hierarchical cluster analysis procedure. A dendrogram was constructed to assess the cohesiveness of the clusters formed in which correlations among elements can readily be seen.
Enrichment factor (EF)
Because of the lack of guidelines for levels of contamination in residential soils and road dust for the majority of the elements analyzed in this study, enrichment factors were calculated by the modified formula suggested by Buat-Menard and Chesselet (1979): EF =[Cn(sample)/Cref(sample)]/[Bn(baseline)/Bref(baseline)] where Cn(sample) is the concentration of the examined element in the studied residential soils and road dust, Cref(sample) is the concentration of the reference element in the studied residential soils and road dust, Bn(baseline) is the content of the examined element in Nacozari soils (here Nacozari background values were selected as the baseline, average of samples S1-S6), Bref(baseline) is the content of the reference element in Nacozari soils. Fe was chosen as the reference element.
EFs can give an insight into differentiating an anthropogenic source from a natural origin. EFs close to 1 point to a crustal origin while those greater than 10 are considered to have a non-crustal source (Nolting et al., 1999, Mendiguchia et al., 2004). Further, EFs can also assist the determination of the degree of metal contamination. Five contamination categories are recognized on the basis of the enrichment factor : EF<2, deficiency to minimal enrichment; EF 2-5, moderate enrichment; EF 5-20, significant enrichment; EF 20-40, very high enrichment; EF>40, extremely high enrichment (Sutherland, 2000; Loska and Wiechula, 2003).
Geoaccumulation index Igeo
The geoaccumulation index (Igeo) has been used since the late 1960s, and has been widely employed in European trace metal studies (Yaqin, et al., 2008). Originally used for bottom sediments (Müller, 1969), it has been successfully applied to the measurement of soil contamination (Loska et al., 2003). This index is expressed by Igeo=log2[Cn/1.5Bn], where Cn is the total concentration of metal n in the studied residential soil, Bn is the geochemical background value of element n, and 1.5 is a correction factor due to lithogenic effects. The geochemical background value of element n was taken from Nacozari soils (S1-S2). Müller (1969) assessed the degree of metal pollution by means of seven different grades based on the numerical values of the Igeo. According to the mentioned index values <0, 1-2, 3-4 and >5 can be interpreted as “Uncontaminated”, “Moderately polluted”, “Highly polluted” and “Very seriously polluted”, respectively (Muniz et al., 2004).
Results and discussion
Mine tailings
The Pilares ore deposit contains a variety of minerals including: pyrite, chalcopyrite, molibdenite, sphalerite, galena, chalcosine, malachite, azurite, cubanite, lollinginite. Mineralogical analysis by XRD showed that the mine tailings are mainly composed of quartz, orthoclase and muscovite. Granulometric analysis showed that the mine tailings are mostly coarse to medium-grained size (>80% of sample). Most of the tailings are characterized by a sandy texture, low water holding capacity, and are well aerated. A small area (3 hectares) located on the southern side of the studied mine tailings is composed of bulk-flotation tailings, which are fine-grained, have a clayey texture, low permeability, and are not well aerated, constituting zones where water accumulates during the rainy season. Stratification, layers of different thickness, desiccation cracks, and efflorescence salts are commonly observed in this side of the tailings. High evaporation rates seasonally accumulate salts on soil surface creating a very hostile environment for plant growth and increasing soil's erodability.
The 70 bulk tailings samples analyzed showed generally low levels of Cr, Mn, Ni, Zn As, Se, Mo, Ba and Pb and higher levels of Ag, Cu and Hg (Table 1). The relative distribution in size fraction of the tailings varied in the site as a function of weathering and topographical position. For example, samples taken from sloping areas had a coarser, less dense texture since they are subjected to more runoff erosion which washes away the fine-textured material.
Metal content in surface efflorescence salt samples from the tailings show a significant accumulation of metals, e.g., Cu increased from 400 mg kg-1in bulk tailings to 69,000 mg kg-1in efflorescent salts, an increase of 170-fold. The metals with the highest concentrations in salts were Zn, Mn, and Cu which were concentrated 230-fold, 200-fold, and 170-fold respectively in the salt crust (Table 1).
The formation of efflorescent salts is climate dependent. In humid climates, following sulfide oxidation and neutralization reactions liberated metals are transported downward until they encounter a reducing environment, such as saturated conditions. This can result in retention of these metals by pH-controlled sorption processes and/or precipitation of secondary minerals (e.g. Fe-oxides and Cu-sulfides). In contrast, in arid climates, upward migration to more oxidizing conditions can occur via capillary forces. Dold and Fontboté (2001; 2002) showed that in strongly acidic oxidation zones (paste pH 1.7- 4), the upward transport of metals leads to the formation of water-soluble sulfate minerals at the surface of porphyry copper tailings. The pH of the Nacozari mine tailings ranges from 3.7 to 4.2 and so this process would be expected to occur (Table 1). This can be contrasted to carbonate-rich tailings that might be found in arid environments, where the acidity produced by sulfide oxidation is neutralized and pH values are near neutral (e.g. Blowes et al., 1998). In this case, sorption processes would strongly limit the upward mobility of the bivalent metal cations.
The influence of climate conditions on element mobility is a major issue in arid environments. Strong evapotranspiration occurs during the hottest months of the year along with major precipitation. Since the mobilization of contaminants in soils is controlled by pH and moisture conditions in addition to other factors, soluble sulfate minerals may precipitate during dry periods, especially at the interface between saturated and unsaturated zones, at which evaporation leads to an accumulation of dissolved species (Bigham and Nordstrom, 2000). The rate of evaporation in Nacozari is high since the highest temperatures occur during the rainy season (two months). Thus, evaporation of water from the tailings induces salt transport to the tailings surface. The accumulation of salts at the surface generates a thin crust of very fine-grained material susceptible to wind erosion and transport to more sensitive environments. In addition, high trace element levels in salts, mainly in bioavailable form, could impede the revegetation to stabilize the tailings.
Residential soils
Nacozari's mine tailings are surrounded by residential areas. Backyards from houses located adjacent to mine tailings are visibly impacted; covered by yellowish sand and silt material from the tailings. Samples were collected from residential soils (C1-C21): and from road dust (RD1-RD3) at a range of distances from the tailings (Fig. 2). Background samples were collected from six non-contaminated sites outside the urban area (S1-S6). The residential soils nearest to the mine tailings exhibited the lowest clay content and were nearly barren of vegetation.
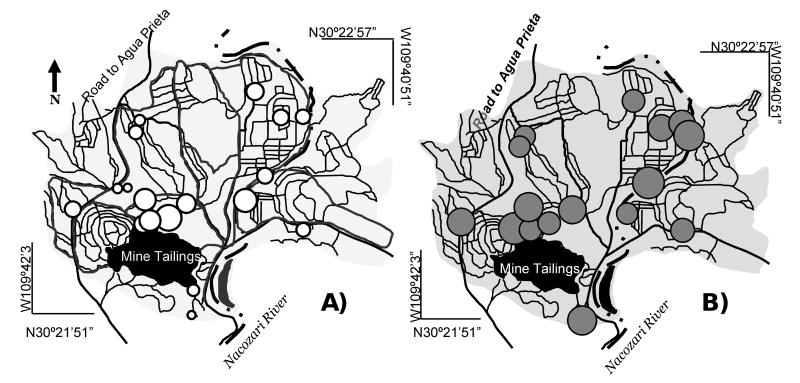
Figure 2.
The spatial distribution of Cu (A) and As (B) in residential soils. The size of the circles shows the relative concentration of the metal(loid), the larger the circle, the higher the metal(loid) concentration.
The Cu and As concentration maps in residential soils (Fig. 2A and B) show that these metal(loid)s are in highest concentration adjacent to the tailings. This is likely due to seasonal erosion due to rainfall events. As and Cu contents in the residential soils is significant despite the low total As and Cu concentrations in the mine tailings (Table 2). Naturally occurring arsenic in surface soils typically range from 1 to 50 mg kg-1; concentrations above 10 mg kg-1 are considered as potentially phytotoxic (Bowen, 1979). Concentrations of arsenic greater than 0.39 mg kg-1 may cause carcinogenic effects in humans, and concentrations above 22 mg kg-1 may result in adverse noncarcinogenic effects. According to Mexican legislation maximum permissible level (MCL) for arsenic content in residential soils is 22 mg kg-1, 45% of residential soil samples from this study exceed the MCL. Cu contents from Nacozari's background soils range from 48 to 96 mg kg-1, whereas residential soils contain higher Cu-values of 200 mg kg-1 average (Table 2).
Road dust
The mineralogical characterization of the three road dust samples revealed that quartz, andesine, muscovite and orthoclase are the main minerals, thus indicating the presence of materials derived not just from the mine tailings but also from the surrounding geological formations. Bismuth and lead antimony oxides as well as lead oxides were identified in both composite street dust samples by XRD (Fig. 3) reflecting an anthropogenic input possibly derived from the abrasion of tires and brake linings (Dietl et al., 1997; Varrica et al., 2003). Lead, copper, and zinc show higher contents in street dust samples than in background soil samples, reflecting influence of traffic sources. These data suggest that the Cu in residential soils could have a traffic-derived component in addition to a mine tailings provenance.
Figure 3.
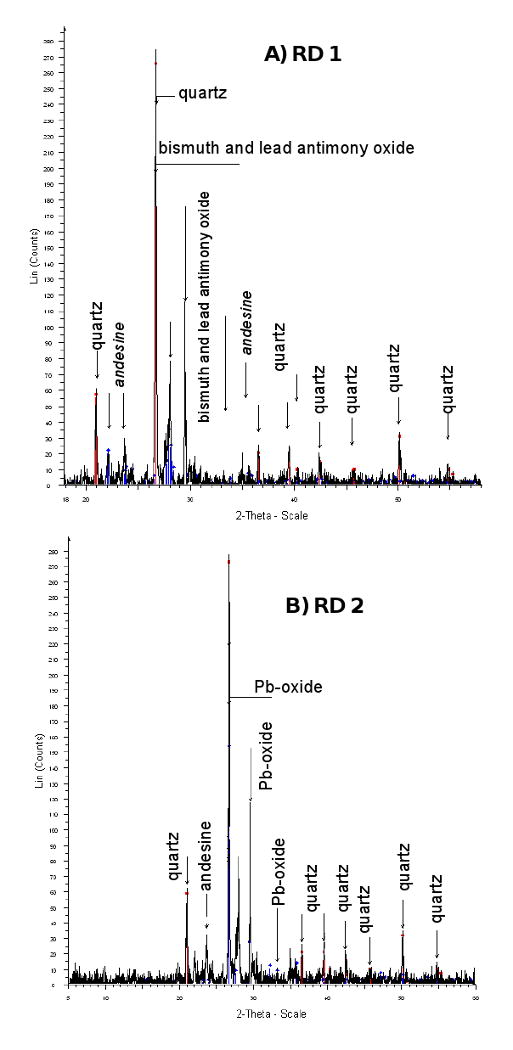
X-ray diffractograms showing main minerals in road dust from Nacozari.
Cluster analysis and spatial distribution of heavy metals
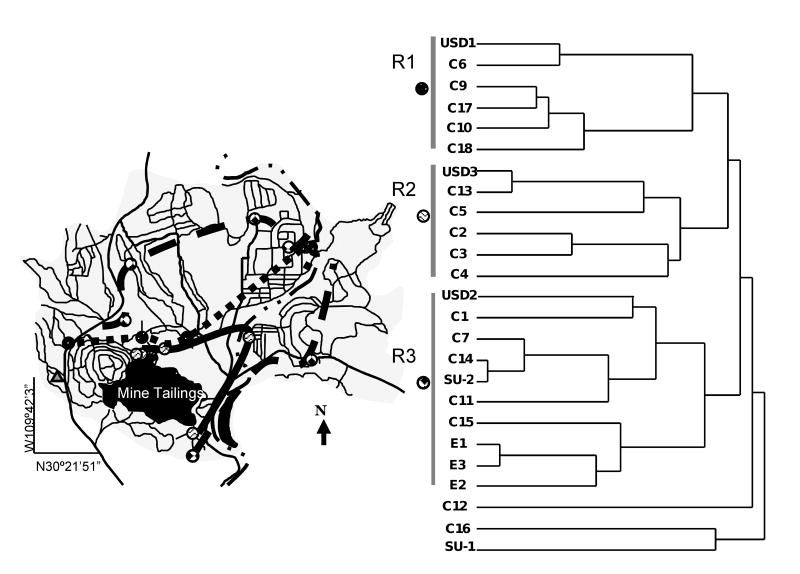
Multivariate statistical methods were applied to detect interactions between metal(loid)s and their relationship to the sample site locations. Cluster analysis was applied to the standardized bulk concentration data and samples using Ward's method, with Euclidian distances as the criterion for forming clusters of metal(loid)s. In general, this form of CA is regarded as very efficient, although it tends to create small clusters. The data set contained all 25 soil samples as cases and 11 metal(loid)s as variables. Cluster analysis of the soil samples revealed a dendrogram showing that 92% of the samples were distributed into three clusters, R1, R2, and R3 (Fig. 4). These three clusters were then plotted according to the location of the samples (Fig. 4). This plot reveals a SW-NE distribution pattern for each cluster which is coincident with prevalent wind direction at the time of sampling. Analysis of the total metal concentrations showed a significant correlation between metals in all the sampling points. This suggests that there is a common origin, the mine tailings site, for all the metals analyzed. Thus, we conclude that the mine tailings site is the main source for the Cu-enrichment in town soils in the area.
Figure 4.
A) Contour map based on cluster analysis. The short dashed line connects samples sites from Cluster R1, the solid line connects sample sites from Cluster R2, and the long dashed line connects sample sites from Cluster R3. B) Dendrogram resulting from the Ward's method of hierarchical cluster analysis for the 25 soil samples and the 11 metal(loid)s. Three major clusters (R1, R2, and R3) were identified and labeled with a symbol that corresponds to their location on the map.
Metal dispersion from the Nacozari mine tailings can occur via two mechanisms. First, the physicochemical properties of the tailings, e.g., low pH, low water retention capacity, low cation exchange capacity (and hence ability to retain metals), may result in soluble metals that are transported to surrounding areas by means of surface runoff. Second, wind transport of dust from the tailings. There is evidence for both mechanisms from the metal(loid) distribution maps generated for the studied area.
The distribution map (Fig. 4) suggests a role both for surface runoff and for wind transport of the tailings. Immediately adjacent to the tailings metal concentrations remain high in areas to the N and NE. These areas are dominated by coarse grained materials. This distribution is consistent with the direction of flow of the surface water streams that follow the slope of the area. Further away from the tailings, metal concentrations are highest along a SW to NE transect which follows the predominant SW-NE wind patterns observed at the site. It seems reasonable to suggest that this distribution is primarily due to wind transport of fine-grained efflorescence salts from the tailings surface to the NE.
Enrichment factors and geoaccumulation index
An enrichment factor (EF) can be used to differentiate between the metals originating from human activities and those from natural provenance, to help assess the degree of anthropogenic influence. One such technique that has often been applied is normalization of a tested element against a reference one (Quevauviller et al., 1989; Isakson et al., 1997; Bergamaschi et al., 2002; Conrad and Chisholm-Brause, 2004).
Table 3 shows enrichment factors calculated for road dusts, residential soils, efflorescence salts and soils. Residential soils and road dusts show significant enrichment for Cu and Hg, and moderately enrichment for As. Efflorescence salts show extremely high enrichment of Cu, As, Mn and Hg, and very high enrichment of As. This group of elements is the same enriched group shown in residential soils. This suggests a dispersion of salts by wind erosion. Background soils show deficiency to minimal enrichment for most analyzed elements, with the exception of Hg and Cu, possible due to the influence of mineralized rocks in soil forming.
Table 3.
Enrichment factors of analyzed samples from Nacozari.
| Sample type | Enrichment factors (range) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mn | Ni | Cu | Zn | As | Rb | Sr | Zr | Ba | Hg | Pb | |
| Road dust | 0.5-0.6 | 0.1-1.2 | 8.2-12 | 2.3-3 | 2.7-4 | 0.1-1.3 | 0.02-3 | 0.2-0.5 | 0.8-1.5 | 160 | 1.7-2 |
| Residential soils | 0.5-0.8 | 1.7-3.2 | 4-27 | 1-4.5 | 4-10 | 1-2.3 | 1.7-4 | 0.1-0.3 | 1.2-1.5 | 360-516 | 1-3 |
| Efflorescence salts | 132-135 | 17.7-18.6 | 97413-9982 | 844-864 | 13.8-20.1 | 5-5.03 | 0.09-0.2 | 0.06 | 13.5-15.2 | 789 | 0.9-2 |
| Soils | 0.5-1.4 | 2.5-2.8 | 2.3-5.5 | 0.9-1.7 | 2.6-2.7 | 0.3-0.9 | 2.8-6 | 0.1-0.4 | 1.2-1.4 | 279 | 0.6-1.3 |
The mean EF values for residential soils decreased in the order of Hg>Cu>As>Zn>Pb>Sr>Ni>Ba>Rb>Mn>Zr. This is also the expected order of their overall anthropogenic to lithogenic contribution at this site (Table 3). Values for Ba, Rb, Sr, Mn, Pb, and Zr were < 5 and so these elements are considered to originate primarily from natural sources (Table 3). The EF values for Cu were higher than the values for the background soil samples (S1-S6). Sample C5, located near the mine tailings, showed the highest EF for Cu (very high enrichment). This backyard soil sample, given its location adjacent to the tailings is likely influenced by runoff water transport. Enrichment of As, Cu and Zn in these soils which are further away from the tailings may be more influenced by wind-dispersion of efflorescence salts from the mine tailings.
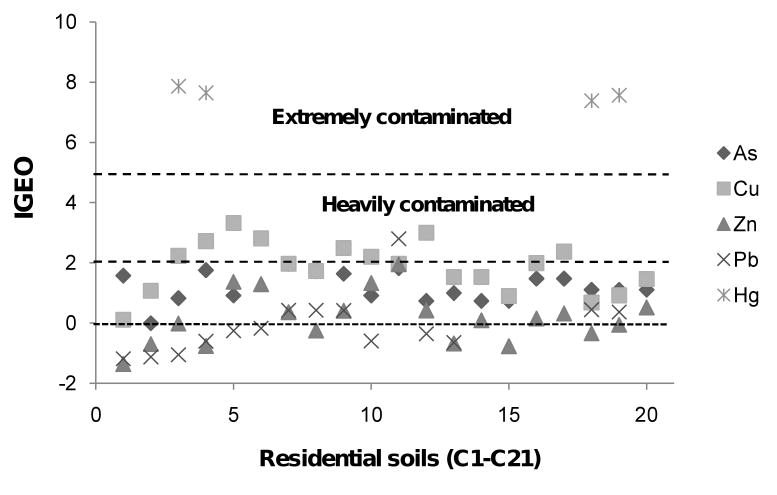
The Igeo values of As, Cu, Zn, Pb, and Hg are shown in Figure 5. Applying the classification system devised by Müller (1969), the elements identified in the Nacozari residential soils may be divided into the following groups: practically uncontaminated (class 0) for Pb and Zn, moderately contaminated (class 2) for As and Pb, heavily contaminated (class 4) mainly for Cu and As, and extremely contaminated (class 6) for Hg. The As and Cu Igeo values ranged from 0 to 4 indicating class 3 to 4 contamination. The accumulation of metals in soils is a long-term process and it is difficult to remove these substances in a short period of time.
Figure 5.
Values of the Geoaccumulation Index (Igeo) for As, Cu, Zn, Pb and Hg of the 20 sampling sites for residential soils.
Conclusion
This study focused on the Nacozari tailings site which is characterized by generally low concentrations of metal(loid)s. Results show that metal(loid)s in the tailings are dispersed both downstream and downslope via two mechanisms, surface runoff and wind-dispersion of efflorescent salts. Dispersion resulted in elevated levels of metal(loid)s in nearby residential areas in patterns that were dependent on climatic parameters (wind direction, rainfall, etc.). The pattern of elevated concentrations and of Cu and As found in residential soils indicates that dispersion of both their soluble and particulate forms is important. Further, results suggest that the processes governing metal(loid) transfer from the tailings zone to residential soils are strongly influenced by the semi-arid climate in Nacozari. In this semi-arid region, there is little likelihood of metal transport to groundwater. Instead, transport occurred via surface runoff which impacted immediately adjacent areas most strongly. Perhaps more importantly, transport of fine efflorescence salts that are created at the tailings surface following evaporation during the short rainy seasons, occur over longer distances via wind dispersion. These efflorescence salts are of concern because they can concentrate metal(loid)s, e.g., Cu, from the tailings at levels exceeding 200-fold. We suggest that one way to prevent erosion processes on site is to create a vegetative cap. It will be necessary to conduct studies to identify native plants that would be suitable candidates to tolerate growth in the tailings are create a viable vegetative cap. Measurements of air concentrations are suggested as future work in order to quantify the influence of efflorescence salts in air quality.
Acknowledgments
The research was supported by EPA-STAG grant to U.S-Mexico Binational Center for Toxicology and Environmental Sciences (R. Maier and D. Meza-Figueroa), by grant 2 P42 ES04940-11 from the National Institute of Environmental Health Sciences Superfund Basic Research Program, NIH (R. Maier), and by a Technical Assistance Contract to Universidad de Sonora by BECC and EPA Region 9 (D. Meza-Figueroa). Opinions in the paper do not constitute an endorsement or approval by the funding agencies and only reflect the personal views of the authors. The authors are grateful for the comments of two anonymous reviewers. Ana María Pérez provided invaluable technical assistance with acid digestion of samples. Victor del Castillo provided field assistance. The authors also thank Dr. Edward Surbbrugg from Tetra Tech Co. for his assistance in XRF-metal analysis in field. Finally, the authors thank the residents of Nacozari and surrounding areas for their gracious assistance during sample collection for this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Breshears DD, Whicker JJ, Johansen MP, Pinder JE., III Wind and water erosion and transport in semiarid shrubland, grassland, and forest ecosystems. Quantifying dominance of horizontal wind-driven transport. Earth Surf Proc Land. 2003;28:1189–1209. [Google Scholar]
- Bergamaschi L, Rizzio E, Valcuvia MG, Verza G, Profumo A, Gallorini M. Determination of trace elements and evaluation of their enrichment factors in Himalayan lichens. Environ Pollut. 2002;120:137–144. doi: 10.1016/s0269-7491(02)00138-0. [DOI] [PubMed] [Google Scholar]
- Bigham JM, Nordstrom DK. Iron and aluminum hydroxysulfates from acid sulfate waters. In: Alpers CN, Jambor JL, Nordstrom DK, editors. Sulfate minerals-Crystallography, Geochemistry and Environmental Significance. Rev Mineral Geochem. Vol. 40. 2000. pp. 351–403. [Google Scholar]
- Blowes DW, Jamber JL, Hanton-Fong CJ, Lortie L, Gould WD. Geochemical mineralogical and microbiological characterization of a sulphide-bearing carbonate-rich gold-mine tailings impoundment, Joutel, Quebec. Appl Geochem. 1998;13:687–705. [Google Scholar]
- Bowen HJM. Environmental Chemistry of the Elements. Academic Press; London: 1979. [Google Scholar]
- Buat-Ménard P, Chesselet R. Variable influence of the atmospheric flux on the trace metal chemistry of oceanic suspended matter. Earth Planet Sc Lett. 1979;42:399–411. [Google Scholar]
- Conrad CF, Chisholm-Brause CJ. Spatial survey of trace metal contaminants in the sediments of the Elizabeth River, Virginia. Mar Pollut Bull. 2004;49:319–324. doi: 10.1016/j.marpolbul.2004.02.019. [DOI] [PubMed] [Google Scholar]
- DeMiguel E, Llamas JF, Chacon E, Berg T, Larssen S, Royset O, Vadset M. Origin and patterns of distribution of trace elements in street dust unleaded petrol and urban lead. Atmos Environ. 1997;31:2733–2740. [Google Scholar]
- Dietl C, Reinfenhauser W, Peichl L. Association of antimony with traffic-occurrence in airborne dust, deposition and accumulation in standardized grass cultures. Sci Total Environ. 1997;205:235–244. [Google Scholar]
- Dold B, Fontboté L. Element cycling and secondary mineralogy in porphyry copper tailings as a function of climate, primary mineralogy, and mineral processing. Special Issue: geochemical studies of mining and the environment. J Geochem Explor. 2001;74:3–55. [Google Scholar]
- Dold B, Fontboté L. A mineralogical and geochemical study of element mobility in sulfide mine tailings of Fe oxide Cu-Au deposits from the Punta del Cobre belt, northern Chile. Chem Geol. 2002;189:135–163. [Google Scholar]
- Farnham IM, Johannesson KH, Singh AK, Hodge VF, Stetzenback KJ. Factor analytical approaches for evaluating groundwater trace element chemistry data. Anal Chim Acta. 2003;490:123–138. [Google Scholar]
- García JH, Li WW, Arimoto R, Okransinski R, Greenlee J, Walton J, Schloesslin C, Sage S. Characterization and implication of potential fugitive dust sources in the Paso del Norte region. Sci Total Environ. 2004;325:95–112. doi: 10.1016/j.scitotenv.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Isakson J, Oblad M, Lindgren ES, Fridell MD, Pacyna JM, Makinen M. Perturbation of background aerosol at rural sites in the Nordic countries. Atmos Environ. 1997;31:3077–3086. [Google Scholar]
- Larney FJ, Cessna AJ, Bullock MS. Herbicide transport on wind-eroded sediment. J Environ Qual. 1999;28:1412–1421. [Google Scholar]
- Loska K, Wiechula D. Application of principal component analysis for the estimation of source of heavy metal contamination in surface sediments from the Rybnik Reservoir. Chemosphere. 2003;51:723–733. doi: 10.1016/S0045-6535(03)00187-5. [DOI] [PubMed] [Google Scholar]
- Loska K, Wiechula D, Barska B, Cebula E, Chojnecka A. Assessment of Arsenic enrichment of cultivated soils in Southern Poland. Polish Journal of Environmental Studies. 2003;2:187–192. [Google Scholar]
- Madrid L, Díaz-Barrientos E, Madrid F. Distribution of heavy metal contents of urban soils in parks of Seville. Chemosphere. 2002;49:1301–1308. doi: 10.1016/s0045-6535(02)00530-1. [DOI] [PubMed] [Google Scholar]
- Mendiguchia C, Moreno C, Galindo RMD, García-Vargas M. Using chemometric tools to assess anthropogenic effects in river water. A case study: Guadalquivir River, Spain. Anal Chim Acta. 2004;515:143–149. [Google Scholar]
- Mendez MO, Maier RM. Phytoremediation of mine tailings in temperate and arid environments. Rev Environ Sci Biotechnol. 2008;7:47–59. [Google Scholar]
- Müller G. Index of geoaccumulation in sediments of the Rhine River. Geojournal. 1969;2:108–118. [Google Scholar]
- Muniz P, Venturini N, Gómez-Erache N. Spatial distribution of chromium and lead in the benthic environmental coastal areas of the Río de la Plata estuary (Montevideo, Uruguay) Brazilian Journal of Biology. 2004;64(1):103–116. doi: 10.1590/s1519-69842004000100012. [DOI] [PubMed] [Google Scholar]
- Nriagu JO. A silent epidemic of environmental metal poisoning. Environ Pollut. 1988;50:139–161. doi: 10.1016/0269-7491(88)90189-3. [DOI] [PubMed] [Google Scholar]
- Nolting RF, Ramkema A, Everaats JM. The geochemistry of Cu, Cd, Zn, Ni and Pb in sediment cores from the continental slope of the Banc d'Arguin (Mauritania) Cont Shelf Res. 1999;19:665–691. [Google Scholar]
- Quevauviller P, Lavigne R, Cortez L. Impact of industrial and mine drainage wastes on the heavy-metal distribution basin and estuary of the Sado River (Portugal) Environ Pollut. 1989;59:267–286. doi: 10.1016/0269-7491(89)90155-3. [DOI] [PubMed] [Google Scholar]
- Ramos-Arroyo YR, Siebe-Grabach CD. Estrategia para identificar jales con potencial de riesgo ambiental en un distrito minero: estudio de caso en el Distrito de Guanajuato, Mexico. Rev Mex Cienc Geol. 2006;23:54–74. [Google Scholar]
- Rasmussen PE, Subramanian SK, Jessiman BJ. A multi-element profile of housedust in relation to exterior dust and soils in the city of Ottawa, Canada. Sci Total Environ. 2001;267:125–140. doi: 10.1016/s0048-9697(00)00775-0. [DOI] [PubMed] [Google Scholar]
- Sutherland RA. Bed sediment-associated trace metals in an urban stream, Oahu, Hawaii. Environ Geol. 2000;39:611–27. [Google Scholar]
- Varrica D, Dongarrá G, Sabatino G, Monna F. Inorganic geochemistry of roadway dust from the metropolitan area of Palermo, Italy. Environ Geol. 2003;40:222–230. [Google Scholar]
- Yaqin JI, Yinchang F, Jianhui W, Tan Z, Zhipeng B, Chiqing D. Using geoaccumulation index to study source profiles of soil dust in China. Journal of Environmental Sciences. 2008;20:571–578. doi: 10.1016/s1001-0742(08)62096-3. [DOI] [PubMed] [Google Scholar]