Abstract
Over the past three decades there has been a substantial increase in the amount of fructose consumed by North Americans. Recent evidence from rodents indicates that hippocampal insulin signaling facilitates memory and excessive fructose consumption produces hippocampal insulin resistance. Based on this evidence, the present study tested the hypothesis that a high fructose diet would impair hippocampal-dependent memory. Adult male Sprague-Dawley rats (postnatal day 61) were fed either a control (0 % fructose) or high fructose diet (60 % of calories). Food intake and body mass were measured regularly. After 19 weeks, the rats were given 3 days of training (8 trials/day) in a spatial version of the water maze task, and retention performance was probed 48 h later. The high fructose diet did not affect acquisition of the task, but did impair performance on the retention test. Specifically, rats fed a high fructose diet displayed significantly longer latencies to reach the area where the platform had been located, made significantly fewer approaches to that area, and spent significantly less time in the target quadrant than did control diet rats. There was no difference in swim speed between the two groups. The retention deficits correlated significantly with fructose-induced elevations of plasma triglyceride concentrations. Consequently, the impaired spatial water maze retention performance seen with the high fructose diet may have been attributable, at least in part, to fructose-induced increases in plasma triglycerides.
Keywords: water maze, triglycerides
Introduction
Over the past three decades there has been a substantial increase in the amount of fructose found in the North American diet. Several factors have contributed to the increase in the availability and per capita consumption of fructose (Hein, Storey, White, and Lineback, 2005; Sigman-Grant and Morita, 2003); most notably, technological advances in the late 1960s led to the development of a cost-effective method for producing large amounts of extremely sweet corn-based syrups containing high concentrations of fructose (high fructose corn syrup, HFCS; either 42 or 55 % fructose; Hanover & White, 1993). Between 1970 and 1990, the consumption of HFCS increased by 20-40 %, surpassing consumption increases in any other foods, (Bray, Nielsen, and Popkin, 2004; Havel, 2005), and by the year 2000, 42 % of added sweeteners were corn syrups (Putnam and Allshouse, 1999). In addition, fructose is added to food in the form of fruit juice concentrates (over 60 % of calories in apple juice), crystalline fructose (almost 100 % fructose), and sucrose (50 % fructose; Hanover & White, 1993). Fructose, in many forms, is added to countless foods including carbonated beverages, fruit products, baked goods, cereals, and dairy products (Hanover and White, 1993). Indeed, North Americans would be greatly challenged to purchase processed foods not containing some form of fructose.
A high fructose diet causes numerous pathological changes, including oxidative stress, glucose intolerance, insulin resistance, type 2 diabetes, liver disease, hypertension, and cardiovascular disease (Busserolles, Gueux, Rock, Mazur, and Rayssiguier, 2002; Elliott, Keim, Stern, Teff, and Havel, 2002; Hwang, Ho, Hoffman, and Reaven, 1987; Montonen, Jarvinen, Knekt, Heliovaara, and Reunanen, 2007; Nandhini, Thirunavukkarasu, Ravichandran, and Anuradha, 2005; Zavaroni, Sander, Scott, and Reaven, 1980). Furthermore, a study from one of the present investigators showed that the damaging effects of a high fructose diet extend directly to the brain (Mielke, Taghibiglou, Liu, Zhang, Jia, Adeli, and Wang, 2005). Specifically, placing male Syrian hamsters on a 60 % fructose diet for 6 weeks produced hippocampal insulin resistance. This finding is particularly significant given that the hippocampus is integral to many forms of learning and memory (Ergorul and Eichenbaum, 2004) and that converging lines of evidence indicate that neural insulin signaling facilitates hippocampal-dependent memory (Park, 2001). For instance, extensive evidence suggests that peripheral insulin resistance and type 2 diabetes are associated with deficits in hippocampal-dependent declarative memory (Convit, 2005; Messier, 2005; Stewart and Liolitsa, 1999; Strachan, Deary, Ewing, and Frier, 1997; Zhao, Chen, Xu, Moore, Meiri, Quon, and Alkon, 1999). Moreover, learning and memory of a spatial water maze experience are correlated with activation of the hippocampal insulin signaling pathway (Dou, Chen, Dufour, Alkon, and Zhao, 2005; Zhao et al., 1999). Most importantly, direct infusions of insulin into the hippocampus enhance performance in a variety of memory tasks, and the memory-enhancing effects of hippocampal insulin administration are not observed in diabetic rats (Babri, Gholamipour, Rad, and Khameneh, 2006; McNay, Herzog, McCrimmon, and Sherwin, 2005; Moosavi, Naghdi, Maghsoudi, and Zahedi Asl, 2006).
Given that fructose is preferentially metabolized by the liver into lipids (Havel, 2005; Topping and Mayes, 1971) and produces large increases in plasma triglyceride (TG) concentrations (Basciano, Federico, and Adeli, 2005; Havel, 2005; Kelley, Allan, and Azhar, 2004; Le, Faeh, Stettler, Ith, Kreis, Vermathen, Boesch, Ravussin, and Tappy, 2006; Park, Cesar, Faix, Wu, Shackleton, and Hellerstein, 1992), a high fructose diet is analogous to a high fat diet in many metabolic ways. Importantly, rats fed a diet high in saturated fatty acids exhibit impaired performance on a number of hippocampal-dependent memory tasks (Greenwood and Winocur, 1990; 1996; McNay et al., 2005). Moreover, high fat diets produce insulin resistance in the brain (Banas, Rouch, Kassis, Markaki, and Gerozissis, 2008), and injecting TGs directly into the brain ventricles impairs memory (Farr, Yamada, Butterfield, Abdul, Xu, Miller, Banks, and Morley, 2008). Collectively, the reviewed evidence led us to hypothesize that a high fructose diet would impair hippocampal-dependent memory, and that the deficits would be attributable, at least in part, to fructose-induced increases in plasma TGs. Consequently, the present experiment tested the effects of feeding rats a high fructose diet on hippocampal-dependent spatial water maze learning and memory, and sought to determine whether any deficits would be correlated with fructose-induced increases in plasma TGs.
Materials and Methods
Animals
Male Sprague-Dawley rats (Charles River, Wilmington, MA) aged 53 days upon arrival were used. Rats are an excellent animal model to study the effects of fructose intake because their metabolism of fructose closely resembles that of humans (Bar-On and Stein, 1968; Mayes, 1993; Van Den Berg, 1986). The present research focused on male rats, given that men are the greatest consumers of fructose (French, Lin, and Guthrie, 2003; Park and Yetley, 1993; Vos, Kimmons, Gillespie, Welsh, and Blanck, 2008).
The rats were weighed the day they arrived and again during each of the 3 days before the diet change, which occurred one week after their arrival. Rats were matched on absolute body mass and percent body mass change during the habituation week and assigned to either the control (0 % fructose; n = 14) or fructose-fed (60 % fructose; n = 15) group. In order to measure food intake, the animals were housed in suspended cages with wire mesh bottoms (Hazelton Systems, Aberdeen, MD). All procedures were approved by the Georgia State University Institutional Animal Care and Use Committee and are in accordance with PHS guidelines.
Diets
The fructose-fed group was provided ad libitum with a diet that consisted of 60 % fructose (Research Diets, New Brunswick, NJ). The 60 % fructose concentration was chosen because this amount produces hippocampal insulin resistance in hamsters (Mielke et al., 2005), leads to peripheral pathology in rats similar to the pathology associated with fructose consumption in humans (Elliott et al., 2002; Montonen et al., 2007), and is the amount used most extensively in rodent studies (de Moura, Ribeiro, de Oliveira, Stevanato, and de Mello, 2008; Kelley et al., 2004; Shapiro, Mu, Roncal, Cheng, Johnson, and Scarpace, 2008; Suga, Hirano, Kageyama, Osaka, Namba, Tsuji, Miura, Adachi, and Inoue, 2000; Taghibiglou, Rashid-Kolvear, Van Iderstine, Le-Tien, Fantus, Lewis, and Adeli, 2002; Tobey, Mondon, Zavaroni, and Reaven, 1982). The control group was fed a diet of standard rat chow (60 % vegetable starch; Research Diets, New Brunswick, NJ) ad libitum. Both diets contained equal percentages of carbohydrates (70 %), proteins (20 %), and lipids (10 %), and both diets were also isocaloric on a weight basis (kcal/gm). The rats were fed the diets for 18 weeks, and behavioral testing was performed during the nineteenth week.
Body Mass and Food Intake
Rat body mass and food intake were recorded for 1 week out of every 3 weeks until behavioral tests were performed. To measure food intake, pellets in each hopper and dried spillage from under each cage were weighed and then subtracted from the amount placed in the hopper the previous day. Average daily kcal consumption was calculated by multiplying the average grams of food consumed daily by kcal per gram of food.
Spatial Water Maze
The spatial water maze task was used to assess learning and memory for several reasons. First, the task is dependent on the integrity of the hippocampus for successful performance (Bolhuis, Stewart, and Forrest, 1994; Clark, Broadbent, and Squire, 2005; Korol, Abel, Church, Barnes, and McNaughton, 1993; Martin, de Hoz, and Morris, 2005; Morris, Garrud, Rawlins, and O’Keefe, 1982; Mumby, Astur, Weisend, and Sutherland, 1999; Sutherland, Weisend, Mumby, Astur, Hanlon, Koerner, Thomas, Wu, Moses, Cole, Hamilton, and Hoesing, 2001). Secondly, spatial water maze training increases hippocampal insulin signaling (Zhao et al., 1999). Third, hippocampal infusions of insulin enhance spatial water maze performance (Choopani, Moosavi, and Naghdi, 2008; Moosavi, Naghdi, and Choopani, 2007; Moosavi et al., 2006; Zhao et al., 1999).
For water maze acquisition, the rats were trained to locate a submerged platform (26 cm in height and 10 cm in diameter) in a circular pool (0.46 m in depth and 1.35 m in diameter). Acquisition consisted of 8 training trials per day for 3 consecutive days. Immediately before the first training trial of each day, rats were placed on the platform for 30 s and were then placed in the water facing the wall of the pool in one of three randomly determined quadrants. The fourth quadrant contained the platform and was referred to as the target quadrant. If the rats did not reach the platform within 60 s, then they were guided by hand to the platform. Rats were allowed to remain on the platform for 15 s at the end of each trial and were then placed in an empty cage for a 30 s inter-trial interval. Latency to reach the platform was used as the measure of acquisition. Retention of the training was tested 48 h after the last training day. Rats were placed in the pool facing the wall in a randomly determined quadrant and allowed to swim for 20 s. The platform was not present, and retention measures during the probe test included: 1) time spent in the target quadrant, 2) latency to cross the platform location (target), and 3) number of target approaches. Swim speed was also measured.
Postmortem Measures
Two to three days after the retention test, the rats were fasted for 4 h then anesthetized with isoflurane gas (5 % in 95 % oxygen) and euthanized by decapitation. Trunk blood was collected immediately in heparinized tubes and centrifuged to collect plasma, which was then stored at -80° C until the assays were performed. Given that the liver is the primary site of fructose metabolism (Havel, 2005; Topping and Mayes, 1971), the liver was also extracted and weighed.
Using spectrophotometry, plasma samples were assayed for TGs (Sigma, St. Louis, MO), free fatty acids (FFA; Wako Chemicals, Richmond, VA), leptin (ELISA, St. Charles, MO), and insulin (ELISA, St. Charles, MO). Glucose was measured using an Accu-Chek glucose meter (Roche, Indianapolis, IN). Samples were run in duplicate. All assays were performed according to the manufacturers’ instructions.
Data Analysis
The data were stored and analyzed using Microsoft Excel, Version 5.0 and Statistical Package for the Social Sciences (SPSS), Version 15.0. A two-tailed Student’s t-test was performed to determine whether there were differences between the means of the control and fructose-fed rats for percent change in body mass, kcal consumed, plasma assays (TG, FFA, leptin, insulin, glucose), liver mass, time spent in the target quadrant, and swim speed. Latency to cross the target and the number of target approaches were not normally distributed. As a result, a Mann-Whitney U-test was used to analyze these scores. A mixed analysis of variance (ANOVA) was performed to determine whether there were differences between control and fructose-fed rats (between factor) in time to reach the platform across water maze acquisition trials (within factor). To determine if there was an association between the peripheral and cognitive effects of the high fructose diet, Pearson correlation coefficients were computed for the plasma and liver measures and any of the behavioral scores that were significantly different between the two groups. Differences among groups were considered statistically significant if p < 0.05. Exact probabilities and test values have been omitted for simplification and clarity of the presentation of the results.
Results
Chronic, High Fructose Consumption did not Alter Body Mass
Average daily kcal consumption was slightly, but significantly, greater in fructose-fed rats than in control rats [p < 0.05; Figure 1A]; however, the groups did not significantly differ in percent change in body mass [Figure 1B].
Figure 1.
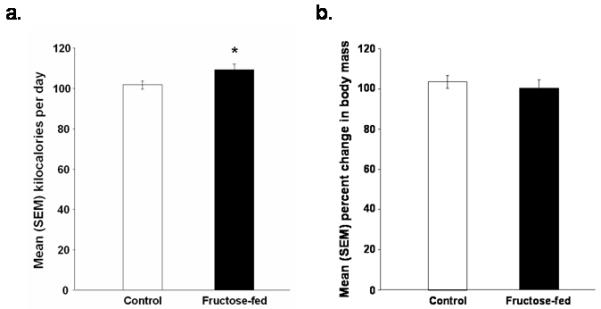
Mean (+/-) SEM (a) kilocalories of food consumed per day and (b) percent change in body mass of rats fed a control or high fructose (60% of calories) diet for 138 days (*p < 0.05 vs. control rats).
The High Fructose Diet Impaired Retention Performance in a Spatial Water Maze
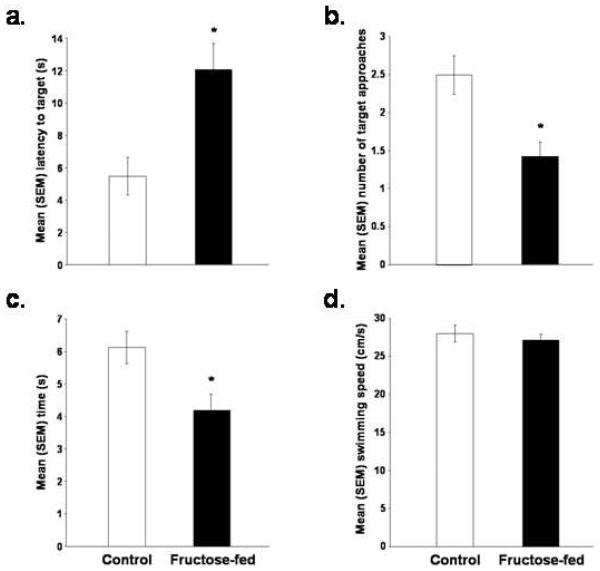
The high fructose diet did not affect water maze acquisition, but did impair retention tested 48 h after the last training trial. During acquisition the latency to reach the platform was significantly decreased [p < 0.05; Figure 2] and was comparable in both control and fructose-fed rats. Fructose-fed rats, however, displayed significantly longer latencies to reach the target on the retention test [p < 0.05; Figure 3A], made significantly fewer target approaches [p < 0.05; Figure 3B], and spent significantly less time in the target quadrant [p < 0.05; Figure 3C] than did control rats. Swimming speed did not differ significantly between the two groups on the probe test [Figure 3D].
Figure 2.
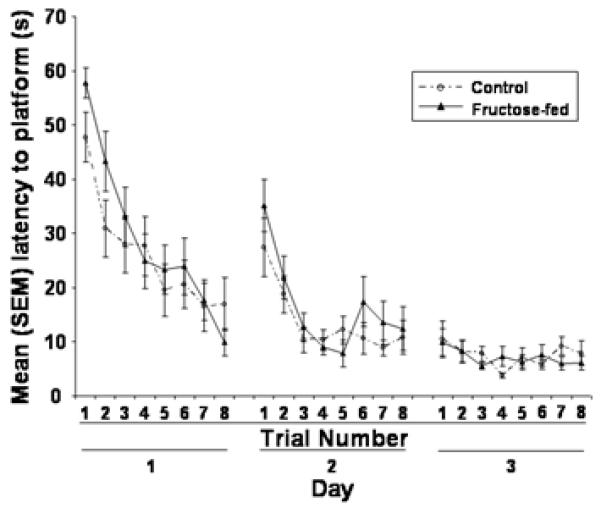
The effects of eating a control or high fructose (60%) diet for 138 days on the mean (+/-) SEM latency to reach the platform during spatial water maze training.
Figure 3.
The effects of eating a control or high fructose (60%) diet for 138 days on the mean (+/-) SEM (a) latency to reach the target, (b) number of target approaches, (c) amount of time spent in the target quadrant and (d) swimming speed during the spatial water maze retention test (*p < 0.05 vs. control rats).
High Fructose Consumption Caused Hepatomegaly and Elevated Plasma Triglycerides
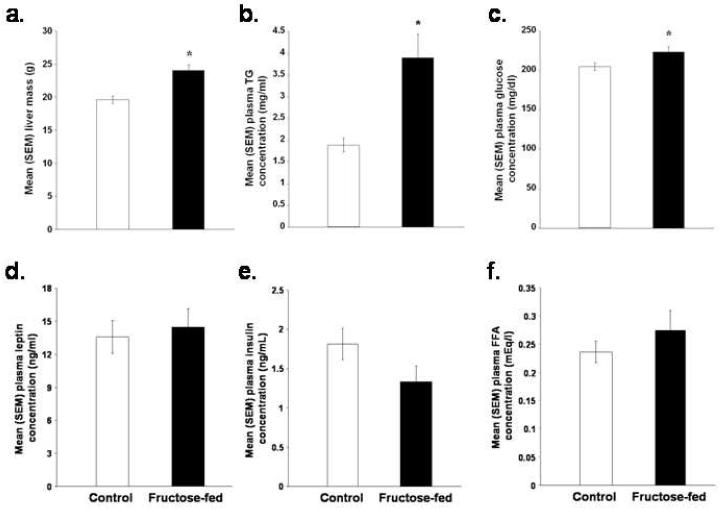
The high fructose diet significantly increased liver mass [p < 0.05; Figure 4A], circulating TGs [p < 0.05; Figure 4B], and glucose concentrations [p < 0.05; Figure 4C]. Plasma leptin, insulin, and FFA concentrations did not significantly differ between the two groups [p > 0.05; Figures 4D, 4E and 4F].
Figure 4.
Mean (+/-) SEM (a) liver mass, (b) plasma TG concentrations, (c) plasma glucose concentrations, (d) plasma leptin concentrations, (e) plasma insulin concentrations and (f) plasma FFA concentrations of rats fed a control or high fructose (60%) diet for 138 days (*p < 0.05 vs. control rats).
Spatial Memory Impairments are Correlated with Altered Liver Function
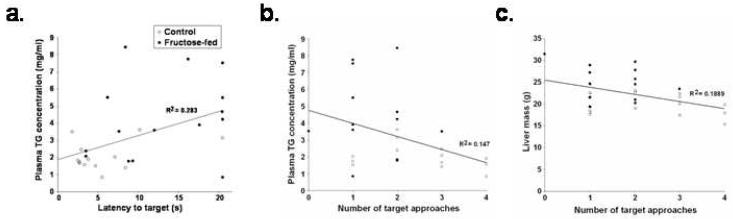
The effects of fructose on plasma TG concentrations were associated with the fructose-induced retention deficits in the spatial water maze task. Specifically, plasma TG concentrations were positively correlated with latencies to reach the target [r (28) = 0.53, p < 0.05; Figure 5A] and negatively correlated with target approaches [r (28) = -0.34, p < 0.05; Figure 5B]. Target approaches also were negatively correlated with liver mass [r (33) = -0.44, p < 0.05; Figure 5C].
Figure 5.
Scatterplots illustrating the association between (a) postmortem plasma TG concentrations and latency to reach the target and (b) the number of target approaches and (c) postmortem liver mass and the number of target approaches during spatial water maze retention (*p < 0.05 vs. control rats).
Discussion
The present study demonstrates for the first time that a high fructose diet impairs hippocampal-dependent memory in rats. Our results show that consuming a 60 % fructose diet for 19 weeks impairs retention performance in a spatial water maze probe test. Specifically, elevated dietary fructose increased latency to reach the target and decreased time spent in the target quadrant and the number of target approaches. The diet did not impair acquisition performance during training, which suggests that the fructose diet did not influence navigational ability and that the rats were able to learn and retain the location of the platform for short periods of time. Deficits were observed exclusively on the retention test given 48 h after training, which indicates that the diet specifically impaired long term storage and/or retrieval. Accordingly, one would expect a deficit on the first trial of the second and third training days, given the 24 hr interval between training days. It is likely that a deficit was not observed, however, because the rats were placed on the platform for 30 sec before training on all of the three training days.
The present findings are inconsistent with a previous report showing that consuming fructose enhances performance in an operant learning task in C57BL/6 mice (Messier, Whately, Liang, Du, and Puissant, 2007). It is difficult to interpret what these contrasting findings may mean because of key differences in dietary protocol, cognitive task, and species. For instance, the fructose concentration was lower (15%) and delivered in water (although actual amount consumed was not verified), and the cognitive measure (lever press for food on a continuous reinforcement schedule) does not likely depend as much on the hippocampus. In addition, they also examined the effects of a high fat diet and contrary to previous findings the high fat diet did not impair learning and memory.
Our findings provide indirect support for the hypothesis that the retention deficits produced by the fructose diet are mediated through a process involving hepatic metabolism of fructose into TGs. The terminal measures indicated that high dietary fructose significantly increased plasma TGs and glucose concentrations along with liver mass, which is consistent with previous reports (Ackerman, Oron-Herman, Grozovski, Rosenthal, Pappo, Link, and Sela, 2005; Cave, Deaciuc, Mendez, Song, Joshi-Barve, Barve, and McClain, 2007; Zavaroni et al., 1980). Furthermore, the fructose-induced retention deficits were significantly correlated with fructose-induced increases in liver mass and, more directly, circulating TG concentrations. Specifically, as TG concentrations increased, the latency to reach the target increased and the number of target approaches decreased. The number of target approaches also varied inversely with liver mass. Moreover, the retention deficits were not correlated with plasma concentrations of insulin, glucose, FFA, or leptin.
Our working hypothesis is that fructose, via increases in TGs, impairs memory by producing hippocampal insulin resistance. Supporting our hypothesis are previous studies showing that application of TGs to liver cells decreases the ability of insulin to activate its signaling cascade (Kim, Jeong, Kim, Kim, Chae, and Chae, 2007) and TGs can penetrate the blood brain barrier (Drew, Smith, and Thomas, 1998). Furthermore, diets high in either fructose or fat produce insulin resistance in the brain (Banas et al., 2008; McNay et al., 2005; Mielke et al., 2005; Posey, Clegg, Printz, Byun, Morton, Vivekanandan-Giri, Pennathur, Baskin, Heinecke, Woods, Schwartz, and Niswender, 2009; but see also Mielke, Nicolitch, Avellaneda, Earlam, Ahuja, Mealing, and Messier, 2006 wherein a high fat diet did not affect brain insulin signaling, perhaps because the effects were measured in mice at a time point (12 months of age) when the insulin system was likely to have been diminished by aging). Moreover, high fat diets impair memory (Greenwood and Winocur, 1990; 1996; McNay et al., 2005) and administration of TGs directly into the ventricles produces hippocampal-dependent memory deficits (Farr et al., 2008). Another possibility is that leptin resistance plays a part in the effects of fructose and TGs on brain function and behavior. For instance, TGs interfere with leptin transport across the blood brain barrier (Banks, Coon, Robinson, Moinuddin, Shultz, Nakaoke, and Morley, 2004). Peripheral and central administration of leptin enhances memory (Paz-Filho, Esposito, Hurwitz, Sharma, Dong, Andreev, Delibasi, Erol, Ayala, Wong, and Licinio, 2008), including hippocampal-dependent memory (Farr, Banks, and Morley, 2006; Oomura, Hori, Shiraishi, Fukunaga, Takeda, Tsuji, Matsumiya, Ishibashi, Aou, Li, Kohno, Uramura, Sougawa, Yada, Wayner, and Sasaki, 2006). Moreover, leptin receptors are typically found on the same neurons that express high densities of insulin receptors (Hakansson, Brown, Ghilardi, Skoda, and Meister, 1998; Mercer, Hoggard, Williams, Lawrence, Hannah, and Trayhurn, 1996; Shioda, Funahashi, Nakajo, Yada, Maruta, and Nakai, 1998), and leptin and insulin often have common effects on brain function (Baskin, Figlewicz Lattemann, Seeley, Woods, Porte, and Schwartz, 1999; Paulus, Schulz, and Lehnert, 2005; Shanley, Irving, and Harvey, 2001). To test the hypothesis that the effects of fructose are mediated by TGs, it would be interesting to determine whether combining fructose with a treatment that lowers lipid levels (e.g., gembifrozil) also would prevent the memory impairments induced by fructose.
Although the correlations between plasma TG concentrations and memory are significant, the correlational data are scattered, there is not a clear relation between the correlates, and we have accounted for only a small proportion of the variance. This suggests that other effects of fructose also contribute to the diet-induced changes in brain function. One possibility is that fructose directly influences neural tissue. Unfortunately, whether fructose can penetrate the BBB is still not known definitively (Funari, Crandall, and Tolan, 2007). Some early studies suggested that fructose cannot penetrate the blood brain barrier in any appreciable amount (Klein, Hurwitz, and Olsen, 1946; Thurston, Levy, Warren, and Jones, 1972). In contrast, evidence is accumulating that neuronal cells can metabolize fructose (Funari et al., 2007) and that fructose-feeding increases the expression of fructose sensitive glucose transporters in the hippocampus (i.e., glut5; Shu, Isenberg, Cormier, Benz, and Zorumski, 2006). Thus, it is possible that fructose or one of its brain metabolites directly induced the memory deficits that were observed here.
Although deriving 60 % of calories from fructose produces pathology in rodents that is similar to that experienced by humans, the level consumed is outside the current range of the human diet (Vos et al., 2008; Wells and Buzby, 2008). Notably, determining what concentration would be comparable between humans and rats is difficult, given that a rat is expected to metabolize fructose at a different rate than a human (Truswell, 1994) and because rats typically require higher doses of drugs than humans to observe an effect. The 60 % fructose concentration, however, produces hippocampal insulin resistance in hamsters (Mielke et al., 2005) and is the amount that is used most extensively in current rodent studies (Behr-Roussel, Oudot, Compagnie, Gorny, Le Coz, Bernabe, Wayman, Alexandre, and Giuliano, 2008; de Moura et al., 2008; Tsai, Wu, and Hwang, 2008), which greatly facilitates comparison across studies.
In summary, the present findings indicate that feeding male rats a high fructose diet impairs hippocampal-dependent spatial water maze retention performance, but does not affect acquisition. The pattern of behavioral deficits produced by fructose suggests a specific effect on long term storage and/or retrieval processes. Moreover, the retention deficits produced by fructose are correlated with fructose-induced increases in circulating TG concentrations and liver mass, which raises the possibility that fructose may influence brain function, at least in part, via its effects on TGs.
Acknowledgments
We would like to thank Dr. Ruth Harris for her advice and loan of the water maze and Dr. Nilton Brito, Dr. Marcia Brito, and Adebimpe Kasumu for their technical assistance.
This research was supported in part by the STC Program of the National Science Foundation under Agreement No. IBN-9876754 and the Georgia State University Brains and Behavior Program and National Institutes of Health Research Grant DK-35254 to TJB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackerman Z, Oron-Herman M, Grozovski M, Rosenthal T, Pappo O, Link G, Sela BA. Fructose-induced fatty liver disease: hepatic effects of blood pressure and plasma triglyceride reduction. Hypertension. 2005;45:1012–1018. doi: 10.1161/01.HYP.0000164570.20420.67. [DOI] [PubMed] [Google Scholar]
- Babri S, Gholamipour H, Rad SN, Khameneh S. Long-Term Potentiation: Forty Unforgettable Years. Atlanta, GA: 2006. Evaluation of insulin intrahippocampal injection on memory consolidation in normal and diabetic male rats. [Google Scholar]
- Banas SM, Rouch C, Kassis N, Markaki EM, Gerozissis K. A Dietary Fat Excess Alters Metabolic and Neuroendocrine Responses Before the Onset of Metabolic Diseases. Cell Mol Neurobiol. 2008 doi: 10.1007/s10571-008-9307-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Coon AB, Robinson SM, Moinuddin A, Shultz JM, Nakaoke R, Morley JE. Triglycerides induce leptin resistance at the blood-brain barrier. Diabetes. 2004;53:1253–1260. doi: 10.2337/diabetes.53.5.1253. [DOI] [PubMed] [Google Scholar]
- Bar-On H, Stein Y. Effect of glucose and fructose administration on lipid metabolism in the rat. J Nutr. 1968;94:95–105. doi: 10.1093/jn/94.1.95. [DOI] [PubMed] [Google Scholar]
- Basciano H, Federico L, Adeli K. Fructose, insulin resistance, and metabolic dyslipidemia. Nutr Metab (Lond) 2005;2:5. doi: 10.1186/1743-7075-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin DG, Figlewicz Lattemann D, Seeley RJ, Woods SC, Porte D, Jr., Schwartz MW. Insulin and leptin: dual adiposity signals to the brain for the regulation of food intake and body weight. Brain Res. 1999;848:114–123. doi: 10.1016/s0006-8993(99)01974-5. [DOI] [PubMed] [Google Scholar]
- Behr-Roussel D, Oudot A, Compagnie S, Gorny D, Le Coz O, Bernabe J, Wayman C, Alexandre L, Giuliano F. Impact of a long-term sildenafil treatment on pressor response in conscious rats with insulin resistance and hypertriglyceridemia. American Journal of Hypertension. 2008;21:1258–1263. doi: 10.1038/ajh.2008.273. [DOI] [PubMed] [Google Scholar]
- Bolhuis JJ, Stewart CA, Forrest EM. Retrograde amnesia and memory reactivation in rats with ibotenate lesions to the hippocampus or subiculum. Q J Exp Psychol B. 1994;47:129–150. [PubMed] [Google Scholar]
- Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79:537–543. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- Busserolles J, Gueux E, Rock E, Mazur A, Rayssiguier Y. Substituting honey for refined carbohydrates protects rats from hypertriglyceridemic and prooxidative effects of fructose. J Nutr. 2002;132:3379–3382. doi: 10.1093/jn/132.11.3379. [DOI] [PubMed] [Google Scholar]
- Cave M, Deaciuc I, Mendez C, Song Z, Joshi-Barve S, Barve S, McClain C. Nonalcoholic fatty liver disease: predisposing factors and the role of nutrition. J Nutr Biochem. 2007;18:184–195. doi: 10.1016/j.jnutbio.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Choopani S, Moosavi M, Naghdi N. Involvement of nitric oxide in insulin induced memory improvement. Peptides. 2008;29:898–903. doi: 10.1016/j.peptides.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Clark RE, Broadbent NJ, Squire LR. Hippocampus and remote spatial memory in rats. Hippocampus. 2005;15:260–272. doi: 10.1002/hipo.20056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Convit A. Links between cognitive impairment in insulin resistance: an explanatory model. Neurobiol Aging. 2005;26(Suppl 1):31–35. doi: 10.1016/j.neurobiolaging.2005.09.018. [DOI] [PubMed] [Google Scholar]
- de Moura RF, Ribeiro C, de Oliveira JA, Stevanato E, de Mello MA. Metabolic syndrome signs in Wistar rats submitted to different high-fructose ingestion protocols. Br J Nutr. 2008:1–7. doi: 10.1017/S0007114508066774. [DOI] [PubMed] [Google Scholar]
- Dou JT, Chen M, Dufour F, Alkon DL, Zhao WQ. Insulin receptor signaling in long-term memory consolidation following spatial learning. Learn Mem. 2005;12:646–655. doi: 10.1101/lm.88005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew PA, Smith E, Thomas PD. Fat distribution and changes in the blood brain barrier in a rat model of cerebral arterial fat embolism. J Neurol Sci. 1998;156:138–143. doi: 10.1016/s0022-510x(98)00039-2. [DOI] [PubMed] [Google Scholar]
- Elliott SS, Keim NL, Stern JS, Teff K, Havel PJ. Fructose, weight gain, and the insulin resistance syndrome. Am J Clin Nutr. 2002;76:911–922. doi: 10.1093/ajcn/76.5.911. [DOI] [PubMed] [Google Scholar]
- Ergorul C, Eichenbaum H. The hippocampus and memory for “what,” “where,” and “when”. Learn Mem. 2004;11:397–405. doi: 10.1101/lm.73304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr SA, Banks WA, Morley JE. Effects of leptin on memory processing. Peptides. 2006;27:1420–1425. doi: 10.1016/j.peptides.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Farr SA, Yamada KA, Butterfield DA, Abdul HM, Xu L, Miller NE, Banks WA, Morley JE. Obesity and hypertriglyceridemia produce cognitive impairment. Endocrinology. 2008;149:2628–2636. doi: 10.1210/en.2007-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French SA, Lin BH, Guthrie JF. National trends in soft drink consumption among children and adolescents age 6 to 17 years: prevalence, amounts, and sources, 1977/1978 to 1994/1998. J Am Diet Assoc. 2003;103:1326–1331. doi: 10.1016/s0002-8223(03)01076-9. [DOI] [PubMed] [Google Scholar]
- Funari VA, Crandall JE, Tolan DR. Fructose metabolism in the cerebellum. Cerebellum. 2007;6:130–140. doi: 10.1080/14734220601064759. [DOI] [PubMed] [Google Scholar]
- Greenwood CE, Winocur G. Learning and memory impairment in rats fed a high saturated fat diet. Behav Neural Biol. 1990;53:74–87. doi: 10.1016/0163-1047(90)90831-p. [DOI] [PubMed] [Google Scholar]
- Greenwood CE, Winocur G. Cognitive impairment in rats fed high-fat diets: A specific effect of saturated fatty-acid intake. Behavioral Neuroscience. 1996;110:451–459. doi: 10.1037//0735-7044.110.3.451. [DOI] [PubMed] [Google Scholar]
- Hakansson ML, Brown H, Ghilardi N, Skoda RC, Meister B. Leptin receptor immunoreactivity in chemically defined target neurons of the hypothalamus. J Neurosci. 1998;18:559–572. doi: 10.1523/JNEUROSCI.18-01-00559.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanover LM, White JS. Manufacturing, composition, and applications of fructose. Am J Clin Nutr. 1993;58:724S–732S. doi: 10.1093/ajcn/58.5.724S. [DOI] [PubMed] [Google Scholar]
- Havel PJ. Dietary fructose: implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. Nutr Rev. 2005;63:133–157. doi: 10.1301/nr.2005.may.133-157. [DOI] [PubMed] [Google Scholar]
- Hein GL, Storey ML, White JS, Lineback DR. Highs and lows of high fructose corn syrup. Nutrition Today. 2005;40:253–256. [Google Scholar]
- Hwang I, Ho H, Hoffman B, Reaven GM. Fructose-induced insulin resistance and hypertension in rats. Hypertension. 1987;10:512–516. doi: 10.1161/01.hyp.10.5.512. [DOI] [PubMed] [Google Scholar]
- Kelley GL, Allan G, Azhar S. High dietary fructose induces a hepatic stress response resulting in cholesterol and lipid dysregulation. Endocrinology. 2004;145:548–555. doi: 10.1210/en.2003-1167. [DOI] [PubMed] [Google Scholar]
- Kim DS, Jeong SK, Kim HR, Kim DS, Chae SW, Chae HJ. Effects of triglyceride on ER stress and insulin resistance. Biochem Biophys Res Commun. 2007;363:140–145. doi: 10.1016/j.bbrc.2007.08.151. [DOI] [PubMed] [Google Scholar]
- Klein J, Hurwitz R, Olsen N. Distribution of intravenously injected fructose and glucose between blood and brain. Journal of Biological Chemistry. 1946;164:509–512. [PubMed] [Google Scholar]
- Korol DL, Abel TW, Church LT, Barnes CA, McNaughton BL. Hippocampal synaptic enhancement and spatial learning in the Morris swim task. Hippocampus. 1993;3:127–132. doi: 10.1002/hipo.450030204. [DOI] [PubMed] [Google Scholar]
- Le KA, Faeh D, Stettler R, Ith M, Kreis R, Vermathen P, Boesch C, Ravussin E, Tappy L. A 4-wk high-fructose diet alters lipid metabolism without affecting insulin sensitivity or ectopic lipids in healthy humans. Am J Clin Nutr. 2006;84:1374–1379. doi: 10.1093/ajcn/84.6.1374. [DOI] [PubMed] [Google Scholar]
- Martin SJ, de Hoz L, Morris RG. Retrograde amnesia: neither partial nor complete hippocampal lesions in rats result in preferential sparing of remote spatial memory, even after reminding. Neuropsychologia. 2005;43:609–624. doi: 10.1016/j.neuropsychologia.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Mayes PA. Intermediary metabolism of fructose. Am J Clin Nutr. 1993;58:754S–765S. doi: 10.1093/ajcn/58.5.754S. [DOI] [PubMed] [Google Scholar]
- McNay EC, Herzog RI, McCrimmon RJ, Sherwin RS. Intrahippocampal insulin administration: Cognitive and metabolic effects. Society for Neuroscience; Washington, D.C.: 2005. [Google Scholar]
- Mercer JG, Hoggard N, Williams LM, Lawrence CB, Hannah LT, Trayhurn P. Localization of leptin receptor mRNA and the long form splice variant (Ob-Rb) in mouse hypothalamus and adjacent brain regions by in situ hybridization. FEBS Lett. 1996;387:113–116. doi: 10.1016/0014-5793(96)00473-5. [DOI] [PubMed] [Google Scholar]
- Messier C. Impact of impaired glucose tolerance and type 2 diabetes on cognitive aging. Neurobiol Aging. 2005;26(Suppl 1):26–30. doi: 10.1016/j.neurobiolaging.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Messier C, Whately K, Liang J, Du L, Puissant D. The effects of a high-fat, high-fructose, and combination diet on learning, weight, and glucose regulation in C57BL/6 mice. Behav Brain Res. 2007;178:139–145. doi: 10.1016/j.bbr.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Mielke J, Taghibiglou C, Liu L, Zhang Y, Jia Z, Adeli K, Wang Y. A biochemical and functional characterization of diet-induced brain insulin resistance. Journal of Neurochemistry. 2005;93:1568–1578. doi: 10.1111/j.1471-4159.2005.03155.x. [DOI] [PubMed] [Google Scholar]
- Mielke JG, Nicolitch K, Avellaneda V, Earlam K, Ahuja T, Mealing G, Messier C. Longitudinal study of the effects of a high-fat diet on glucose regulation, hippocampal function, and cerebral insulin sensitivity in C57BL/6 mice. Behav Brain Res. 2006;175:374–382. doi: 10.1016/j.bbr.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Montonen J, Jarvinen R, Knekt P, Heliovaara M, Reunanen A. Consumption of sweetened beverages and intakes of fructose and glucose predict type 2 diabetes occurrence. J Nutr. 2007;137:1447–1454. doi: 10.1093/jn/137.6.1447. [DOI] [PubMed] [Google Scholar]
- Moosavi M, Naghdi N, Choopani S. Intra CA1 insulin microinjection improves memory consolidation and retrieval. Peptides. 2007;28:1029–1034. doi: 10.1016/j.peptides.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Moosavi M, Naghdi N, Maghsoudi N, Zahedi Asl S. The effect of intrahippocampal insulin microinjection on spatial learning and memory. Horm Behav. 2006;50:748–752. doi: 10.1016/j.yhbeh.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Mumby DG, Astur RS, Weisend MP, Sutherland RJ. Retrograde amnesia and selective damage to the hippocampal formation: memory for places and object discriminations. Behav Brain Res. 1999;106:97–107. doi: 10.1016/s0166-4328(99)00097-2. [DOI] [PubMed] [Google Scholar]
- Nandhini AT, Thirunavukkarasu V, Ravichandran MK, Anuradha CV. Effect of taurine on biomarkers of oxidative stress in tissues of fructose-fed insulin-resistant rats. Singapore Med J. 2005;46:82–87. [PubMed] [Google Scholar]
- Oomura Y, Hori N, Shiraishi T, Fukunaga K, Takeda H, Tsuji M, Matsumiya T, Ishibashi M, Aou S, Li XL, Kohno D, Uramura K, Sougawa H, Yada T, Wayner MJ, Sasaki K. Leptin facilitates learning and memory performance and enhances hippocampal CA1 long-term potentiation and CaMK II phosphorylation in rats. Peptides. 2006;27:2738–2749. doi: 10.1016/j.peptides.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Park CR. Cognitive effects of insulin in the central nervous system. Neurosci Biobehav Rev. 2001;25:311–323. doi: 10.1016/s0149-7634(01)00016-1. [DOI] [PubMed] [Google Scholar]
- Park OJ, Cesar D, Faix D, Wu K, Shackleton CH, Hellerstein MK. Mechanisms of fructose-induced hypertriglyceridaemia in the rat. Activation of hepatic pyruvate dehydrogenase through inhibition of pyruvate dehydrogenase kinase. Biochem J. 1992;282(Pt 3):753–757. doi: 10.1042/bj2820753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YK, Yetley EA. Intakes and food sources of fructose in the United States. Am J Clin Nutr. 1993;58:737S–747S. doi: 10.1093/ajcn/58.5.737S. [DOI] [PubMed] [Google Scholar]
- Paulus K, Schulz C, Lehnert H. Central nervous effects of leptin and insulin on hippocampal leptin and insulin receptor expression following a learning task in Wistar rats. Neuropsychobiology. 2005;51:100–106. doi: 10.1159/000084167. [DOI] [PubMed] [Google Scholar]
- Paz-Filho G, Esposito K, Hurwitz B, Sharma A, Dong C, Andreev V, Delibasi T, Erol H, Ayala A, Wong ML, Licinio J. Changes in insulin sensitivity during leptin replacement therapy in leptin-deficient patients. Am J Physiol Endocrinol Metab. 2008;295:E1401–1408. doi: 10.1152/ajpendo.90450.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posey KA, Clegg DJ, Printz RL, Byun J, Morton GJ, Vivekanandan-Giri A, Pennathur S, Baskin DG, Heinecke JW, Woods SC, Schwartz MW, Niswender KD. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am J Physiol Endocrinol Metab. 2009;296:E1003–1012. doi: 10.1152/ajpendo.90377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam JJ, Allshouse JE. Food consumption, prices and expeditures, 1970-97. US Government Printing Office; Washington, DC: 1999. [Google Scholar]
- Shanley LJ, Irving AJ, Harvey J. Leptin enhances NMDA receptor function and modulates hippocampal synaptic plasticity. J Neurosci. 2001;21:RC186. doi: 10.1523/JNEUROSCI.21-24-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro A, Mu W, Roncal C, Cheng KY, Johnson RJ, Scarpace PJ. Fructose-induced leptin resistance exacerbates weight gain in response to subsequent high-fat feeding. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1370–1375. doi: 10.1152/ajpregu.00195.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioda S, Funahashi H, Nakajo S, Yada T, Maruta O, Nakai Y. Immunohistochemical localization of leptin receptor in the rat brain. Neurosci Lett. 1998;243:41–44. doi: 10.1016/s0304-3940(98)00082-2. [DOI] [PubMed] [Google Scholar]
- Shu HJ, Isenberg K, Cormier RJ, Benz A, Zorumski CF. Expression of fructose sensitive glucose transporter in the brains of fructose-fed rats. Neuroscience. 2006;140:889–895. doi: 10.1016/j.neuroscience.2006.02.071. [DOI] [PubMed] [Google Scholar]
- Sigman-Grant M, Morita J. Defining and interpreting intakes of sugars. Am J Clin Nutr. 2003;78:815S–826S. doi: 10.1093/ajcn/78.4.815S. [DOI] [PubMed] [Google Scholar]
- Stewart R, Liolitsa D. Type 2 diabetes mellitus, cognitive impairment and dementia. Diabet Med. 1999;16:93–112. doi: 10.1046/j.1464-5491.1999.00027.x. [DOI] [PubMed] [Google Scholar]
- Strachan MW, Deary IJ, Ewing FM, Frier BM. Is type II diabetes associated with an increased risk of cognitive dysfunction? A critical review of published studies. Diabetes Care. 1997;20:438–445. doi: 10.2337/diacare.20.3.438. [DOI] [PubMed] [Google Scholar]
- Suga A, Hirano T, Kageyama H, Osaka T, Namba Y, Tsuji M, Miura M, Adachi M, Inoue S. Effects of fructose and glucose on plasma leptin, insulin, and insulin resistance in lean and VMH-lesioned obese rats. Am J Physiol Endocrinol Metab. 2000;278:E677–683. doi: 10.1152/ajpendo.2000.278.4.E677. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, Weisend MP, Mumby D, Astur RS, Hanlon FM, Koerner A, Thomas MJ, Wu Y, Moses SN, Cole C, Hamilton DA, Hoesing JM. Retrograde amnesia after hippocampal damage: recent vs. remote memories in two tasks. Hippocampus. 2001;11:27–42. doi: 10.1002/1098-1063(2001)11:1<27::AID-HIPO1017>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Taghibiglou C, Rashid-Kolvear F, Van Iderstine S, Le-Tien H, Fantus I, Lewis G, Adeli K. Hepatic very low density lipoprotein-ApoB overproduction is associated with attenuated hepatic insulin signaling and overexpression of protein-tyrosine phosphatase 1B in a fructose-fed hamster model of insulin resistance. The Journal of Biological Chemistry. 2002;1:793–803. doi: 10.1074/jbc.M106737200. [DOI] [PubMed] [Google Scholar]
- Thurston JH, Levy CA, Warren SK, Jones EM. Permeability of the blood-brain barrier to fructose and the anaerobic use of fructose in the brains of young mice. J Neurochem. 1972;19:1685–1696. doi: 10.1111/j.1471-4159.1972.tb06213.x. [DOI] [PubMed] [Google Scholar]
- Tobey T, Mondon C, Zavaroni I, Reaven G. Mechanism of insulin resistance in fructose-fed rats. Metabolism. 1982;31:608–612. doi: 10.1016/0026-0495(82)90100-7. [DOI] [PubMed] [Google Scholar]
- Topping DDL, Mayes PPA. The concentration of fructose, glucose and lactate in the splanchnic blood vessels of rats absorbing fructose. Nutrition and metabolism. 1971;13:331–338. doi: 10.1159/000175352. [DOI] [PubMed] [Google Scholar]
- Truswell AS. Dietary recommendations, goals and guidelines. S Afr Med J. 1994;(Suppl):54–57. [PubMed] [Google Scholar]
- Tsai HY, Wu LY, Hwang LS. Effect of a proanthocyanidin-rich extract from longan flower on markers of metabolic syndrome in fructose-fed rats. J Agric Food Chem. 2008;56:11018–11024. doi: 10.1021/jf801966y. [DOI] [PubMed] [Google Scholar]
- Van Den Berg G. Fructose: Metabolism and short-term effects on carbohydrate and purine metabolic pathways. Progress in Biochemical Pharmacology. 1986;21:1–32. [PubMed] [Google Scholar]
- Vos MB, Kimmons JE, Gillespie C, Welsh J, Blanck HM. Dietary fructose consumption among US children and adults: the Third National Health and Nutrition Examination Survey. Medscape J Med. 2008;10:160. [PMC free article] [PubMed] [Google Scholar]
- Wells H, Buzby J. Dietary Assessment of Major Trends in U.S. Food Consumption, 1970-2005. Economic Information Bulletin. 2008;33 [Google Scholar]
- Zavaroni I, Sander S, Scott S, Reaven GM. Effect of fructose feeding on insulin secretion and insulin action in the rat. Metabolism. 1980;29:970–973. doi: 10.1016/0026-0495(80)90041-4. [DOI] [PubMed] [Google Scholar]
- Zhao W, Chen H, Xu H, Moore E, Meiri N, Quon MJ, Alkon DL. Brain insulin receptors and spatial memory. Correlated changes in gene expression, tyrosine phosphorylation, and signaling molecules in the hippocampus of water maze trained rats. The Journal of Biological Chemistry. 1999;274:34893–34902. doi: 10.1074/jbc.274.49.34893. [DOI] [PubMed] [Google Scholar]