Abstract
We report the formation and N2 reactivity of novel cobalt hydride complexes supported by bulky β-diketiminate ligands (L). Addition of KHBEt3 to LCoCl gives [LCo(μ-H)]2 (1) or K2[LCoH]2 (2), depending on the amount of borohydride used. Compound 2 is the first example of a crystallographically characterized hydride complex in which a transition metal is three-coordinate. Both 1 and 2 react with N2 at room temperature to give dinuclear N2 complexes with loss of H2.
Complexes of a transition metal (defined here as a metal with a partially filled d shell) having only three bonds to the metal are interesting because the orbital energies, spin states, and reaction pathways can be different than traditional complexes.1 Isolating three-coordinate complexes invariably depends on the use of extremely bulky supporting ligands, which protect the metal center through steric effects. However, this strategy might seem to be incompatible with three-coordinate hydride complexes, because H– is the smallest possible ligand. Accordingly, no three-coordinate hydride complexes are known.2 New kinds of hydride complexes are desired because of the many roles for hydrides in organometallic chemistry and catalysis.3
Using iron complexes, we recently introduced the use of the very bulky bidentate β-diketiminate ligand L (L = 2,2,6,6-tetramethyl-3,5-bis(2,4-diisopropylphenylimido)hept-4-yl) for enabling the isolation of three-coordinate complexes in which one of the three ligands is small (e.g. halide, CH3).4 Here, we report that L can be used to stabilize the first crystallographically characterized three-coordinate hydride complex of any transition metal. We also report that this cobalt-hydride complex and another related complex react with N2 at room temperature and atmospheric pressure, through the bimetallic reductive elimination of H2.
Figure 1 shows the syntheses and structures of dimeric cobalt(II) hydride (1) and cobalt(I) hydride (2) complexes, which come from adding different amounts of KHBEt3 to toluene solutions of the three-coordinate cobalt(II) complex LCoCl under Ar.5 Addition of 1 equiv of KHBEt3 to LCoCl gives [LCo(μ-H)]2 (1) in 72% yield. Although we were not able to completely free 1 from impurities (see Supporting Information), several forms of characterization have been possible. The X-ray crystal structure of 1 reveals that it has two hydride ligands (located in the difference Fourier map) that bridge diketiminate-bound cobalt centers. The metal coordination and geometry closely resemble those for the iron(II) complex [LFe(μ-H)]26 and a dimeric β-diketiminate nickel(II) hydride complex recently reported by Limberg.7 The cobalt atoms in 1 are separated by 2.476(5) Å, which is intermediate between the metal-metal distances in the iron (2.624(2) Å) and nickel (2.3939(6) Å) analogues. The presence of the hydride ligands in 1 was confirmed through the reaction of a toluene solution of 1 with 2 equiv of cyclohexene to give the cobalt(II) cyclohexyl product LCo(C6H11), which results from [1,2]-addition of the cobalt hydride to the C-C double bond.
Figure 1.
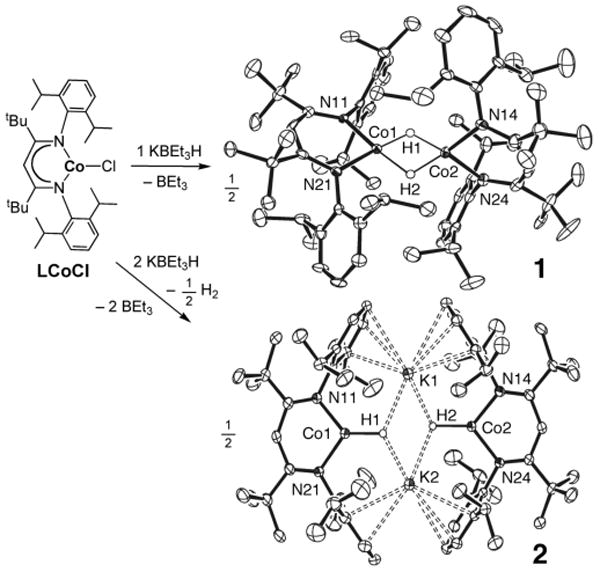
Formation and structures of unsaturated cobalt hydride complexes, using 50% thermal ellipsoids. Carbon-bound hydrogen atoms are omitted for clarity. All cobalt-bound hydrogens were refined with isotropic thermal parameters. Selected bond lengths and bond angles follow; because compound 1 has three crystallographically independent dimers, ranges are listed here (details are in the Supporting Information). 1: Co-Co 2.472-2.481 Å, Co-N 1.962-1.993 Å, N-Co-N 95.4-96.5°. For 2: Co1-Co2 5.7386(7) Å, Co1-H1 1.84(2) Å, Co1-N11 1.9162(13) Å, Co1-N21 1.9164(12) Å, N11-Co1-N21 96.73(6)°, N11-Co1-H1 128.2(7)°, N21-Co1-H1 134.1(7)°, Co2-H2 1.78(2) Å, Co2-N14 1.9106(13) Å, Co2-N24 1.9121(13) Å, N14-Co2-N24 96.59(6)°, N14-Co2-H2 133.5(7)°, N24-Co2-H2 129.4(7)°.
Reaction of LCoCl with 2 equiv of KHBEt3 under Ar gave compound 2, with presumed loss of H2, in 70% yield. Compound 2 is very unusual. Its X-ray crystal structure reveals two nearly parallel three-coordinate cobalt units related by a pseudo-inversion center. The high quality of the crystallographic data enabled refinement of the positions of the hydrogen atoms, giving Co-H distances of 1.81(3) Å. The cobalt geometry is trigonal-planar, and each H atom lies near the pseudo-mirror plane of the β-diketiminate ligand on the same cobalt atom (N-Co-H angles ranging from 128° to 134°). The two H atoms are separated by 3.02(3) Å, and the two cobalt atoms by 5.7386(7) Å, showing that there is no direct connection between the cobalt-bound atoms. Instead, the halves of the molecule are held together by K+ ions that form cation-π interactions with the aryl rings of the β-diketiminate ligands. The potassium ions are also close to the hydride ligands (K-H 2.60(2) Å and 2.67(2) Å).
The three-coordinate cobalt ions in complex 2 are high-spin cobalt(I), as shown by the paramagnetically shifted 1H NMR spectrum and the solution magnetic moment of μeff = 5.6(2) μB per dimeric molecule of 2. Complex 2 is soluble and gives similar 1H NMR spectra in cyclohexane, benzene, and THF, suggesting that the alkali metals remain bound in solution. The freezing-point depression of a solution of 2 in naphthalene indicated a molecular weight of 1140 ± 200 (3σ), supporting the dimeric formulation in solution. Seven β-diketiminate resonances are observed in its 1H NMR spectrum in C6D6 from 200-353 K (the hydrides are not observed due to fast relaxation), showing that the β-diketiminate ligands of 2 have averaged C2v symmetry in solution. Therefore, there is a low-energy mechanism that enables the molecule to reach an arrangement in which the CoN2H units are transiently coplanar, without dissociation of the molecule into halves.
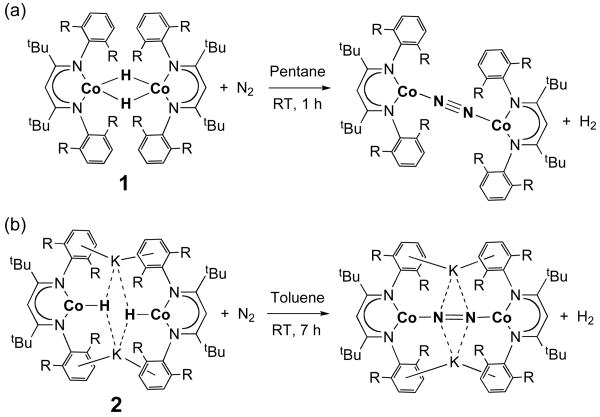
Solutions of 1 and 2 in aromatic and hydrocarbon solvents show no signs of decomposition by 1H NMR when heated to 100 °C for several days under an Ar atmosphere. On the other hand, exposure of room-temperature solutions of each compound to an atmosphere of purified N2 in pentane, diethyl ether, or toluene leads to the growth of resonances in the 1H NMR spectrum that are characteristic of the analogous bimetallic dinitrogen complexes (Scheme 1). These cobalt dinitrogen complexes have been characterized separately.5b The reaction of 1 with N2 was complete in less than 1 h, giving 88% spectroscopic yield of LCoNNCoL. H2, the other product of the reaction, was detected by gas chromatography in 80% yield. The reaction of 2 with N2 gave K2[LCoNNCoL] over several hours, in 90% spectroscopic and isolated yield. In the latter reaction, H2 was detected in 83% yield. The reactions in Scheme 1 occur at roughly the same rate in the dark as in ambient light.8
Scheme 1.
Reactions of the Low-Coordinate Cobalt Hydride Complexes with Dinitrogen (R = isopropyl)
The reactions to produce H2 are formal reductive eliminations: the dicobalt(II) complex 1 leads to the dicobalt(I) dinitrogen complex LCoNNCoL, and the dicobalt(I) complex 2 gives the formally dicobalt(0) dinitrogen complex K2[LCoNNCoL]. In the latter complex, the N-N distance is 1.22 Å, indicating that the dinitrogen ligand is best described as [N=N]2–.5b Thus, using this picture, the electrons from the reductive elimination of H2 end up in the π* orbital of bound N2. This is interesting in the context of N2 binding and activation.9 Although H2 reductive elimination from metastable hydride complexes has been used previously as a route to dinitrogen complexes,10,11,12,13 there is only one literature example of N2 binding directly from a crystallographically verified hydride complex.10c This H2-N2 exchange is of interest in the context of catalytic N2 reduction, because the formation of N2 complexes in this way avoids the use of harsh reducing agents.14
We explored the mechanism of H2 reductive elimination by treating a mixture of the isotopologues K2[LCo(μ-H)]2 (2) and K2[LCo(μ-D)]2 (2-D) with N2 for 2 days. H2 and D2 were present in the headspace, and no HD was detected. This result indicates that (a) 2 does not dissociate in solution at room temperature, and (b) the elimination of H2 from 2 is an intramolecular process. The analogous reaction of 1 and 1-D with N2 gave a significant amount of HD, probably through the pre-equilibration of isotopologues of 1 through monomer-dimer equilibrium.
In conclusion, we have crystallographically characterized the first three-coordinate transition-metal hydride complex, K2[LCo(μ-H)]2 (2). The hydride ligands in this species are labile, eliminating as H2 upon the addition of N2. It is surprising that 2 readily reacts with N2 but does not react at room temperature with THF or arenes, suggesting that the approach of additional donors to cobalt is sterically restricted by the potassium-bound diketiminate ligands.
Supplementary Material
Figure 2.
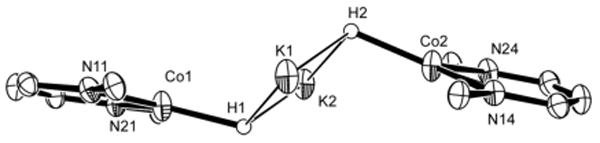
Side view of the core of 2, showing the relative orientation of the two CoN2H planes.
Acknowledgments
Funding was provided by the National Institutes of Health (GM-065313). We thank Richard Eisenberg for the use of a gas chromatograph for H2 measurements.
Footnotes
Supporting Information Available: Synthetic, spectroscopic, kinetic, and crystallographic data. This material is available free of charge via the Internet at http://pubs.acs.org.
References and Notes
- 1.(a) Cummins CC. Prog Inorg Chem. 1998;47:685–836. [Google Scholar]; (b) Alvarez S. Coord Chem Rev. 1999:193–195. 13–41. [Google Scholar]
- 2.Putative three-coordinate complexes [Pt(PtBu3)2H]+ were not crystallized, and probably have a weak fourth ligand. Goel R, Srivastava RC. J Organomet Chem. 1981;204:C13–C15.Goel R, Srivastava RC. Can J Chem. 1983;61:1352–1359.Butts MD, Scott BL, Kubas GJ. J Am Chem Soc. 1996;118:11831–11843.
- 3.Peruzzini M, Poli R, editors. Recent Advances in Hydride Chemistry. Elsevier Science Ltd.; Amsterdam; New York: 2001. [Google Scholar]
- 4.Holland PL. Acc Chem Res. 2008;41:905–914. doi: 10.1021/ar700267b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Holland PL, Cundari TR, Perez LL, Eckert NA, Lachicotte RJ. J Am Chem Soc. 2002;124:14416–14424. doi: 10.1021/ja025583m. [DOI] [PubMed] [Google Scholar]; (b) Ding K, Pierpont AW, Brennessel WW, Lukat-Rodgers G, Rodgers KR, Cundari TR, Bill E, Holland PL. J Am Chem Soc. 2009;131 doi: 10.1021/ja808783u. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Smith JM, Lachicotte RJ, Holland PL. J Am Chem Soc. 2003;125:15752–15753. doi: 10.1021/ja038152s. [DOI] [PubMed] [Google Scholar]; (b) Yu Y, Sadique AR, Smith JM, Dugan TR, Cowley RE, Brennessel WW, Flaschenriem CJ, Bill E, Cundari TR, Holland PL. J Am Chem Soc. 2008;130:6624–6638. doi: 10.1021/ja710669w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfirrmann S, Limberg C, Ziemer B. Dalton Trans. 2008:6689–6691. doi: 10.1039/b816136b. [DOI] [PubMed] [Google Scholar]
- 8.The analogous iron hydride complex loses H2 only with photolysis or the addition of stronger ligands: see ref 6b.
- 9.(a) MacKay BA, Fryzuk MD. Chem Rev. 2004;104:385–401. doi: 10.1021/cr020610c. [DOI] [PubMed] [Google Scholar]; (b) Gambarotta S, Scott J. Angew Chem, Int Ed. 2004;43:5298–5308. doi: 10.1002/anie.200301669. [DOI] [PubMed] [Google Scholar]
- 10.(a) Fryzuk MD, Johnson SA, Rettig SJ. J Am Chem Soc. 1998;120:11024–11025. [Google Scholar]; (b) Fryzuk MD, Johnson SA, Patrick BO, Albinati A, Mason SA, Koetzle TF. J Am Chem Soc. 2001;123:3960–3973. doi: 10.1021/ja0041371. [DOI] [PubMed] [Google Scholar]; (c) Akagi F, Matsuo T, Kawaguchi H. Angew Chem, Int Ed Engl. 2007;46:8778–8781. doi: 10.1002/anie.200703336. [DOI] [PubMed] [Google Scholar]
- 11.(a) Aresta M, Giannoccaro P, Rossi M, Sacco A. Inorg Chim Acta. 1971;5:203–206. [Google Scholar]; (b) Brintzinger H, Bercaw JE. J Am Chem Soc. 1971;93:2045–2046. [Google Scholar]; (c) Brintzinger H, Marvich RH. J Am Chem Soc. 1971;93:2046–2048. [Google Scholar]; (d) de Wolf JM, Blaauw R, Meetsma A, Teuben JH, Gyepes R, Varga V, Mach K, Veldman N, Spek AL. Organometallics. 1996;15:4977–4983. [Google Scholar]; (e) Chirik PJ, Henling LM, Bercaw JE. Organometallics. 2001;20:534–544. [Google Scholar]; (f) Pool JA, Lobkovsky E, Chirik PJ. J Am Chem Soc. 2003;125:2241–2251. doi: 10.1021/ja020960g. [DOI] [PubMed] [Google Scholar]; (g) Hanna TE, Bernskoetter WH, Bouwkamp MW, Lobkovsky E, Chirik PJ. Organometallics. 2007;26:2431–2438. [Google Scholar]; (h) Avenier P, Taoufik M, Lesage A, Solans-Monfort X, Baudouin A, de Mallmann A, Veyre L, Basset JM, Eisenstein O, Emsley L, Quadrelli EA. Science. 2007;317:1056–1060. doi: 10.1126/science.1143078. [DOI] [PubMed] [Google Scholar]; (i) Scott J, Vidyaratne I, Korobkov I, Gambarotta S, Budzelaar PHM. Inorg Chem. 2008;47:896–911. doi: 10.1021/ic701643d. [DOI] [PubMed] [Google Scholar]; (j) Pfirrmann S, Limberg C, Herwig C, Stösser, Ziemer B. Angew Chem Int Ed Engl. 2009;48:3357–3361. doi: 10.1002/anie.200805862. [DOI] [PubMed] [Google Scholar]
- 12.Schrock's catalytic N2 reduction can be initiated by a molybdenum-hydride species, indicating that this hydride can somehow form an N2 complex. Yandulov DV, Schrock RR. Inorg Chem. 2005;44:1103–1117. doi: 10.1021/ic040095w.
- 13.A number of metal-dihydrogen complexes react with N2 to generate N2 complexes. Selected examples: Chaudret B, Devillers J, Poilblanc R. Organometallics. 1985;4:1727–1732.Hills A, Hughes DL, Jimenez-Tenorio M, Leigh GJ. J Organomet Chem. 1990;391:C41–C44.Hills A, Hughes DL, Jimenez-Tenorio M, Leigh GJ, Rowley AT. J Chem Soc, Dalton Trans. 1993:3041–3049.Gilbertson JD, Szymczak NK, Tyler DR. J Am Chem Soc. 2005;127:10184–10185. doi: 10.1021/ja053030g.
- 14.See: Fryzuk MD. Acc Chem Res. 2009;42:127–133. doi: 10.1021/ar800061g. The necessity for strong reducing agents has prevented catalysis in some systems that perform stoichiometric N2 reduction cycles: Curley JJ, Sceats EL, Cummins CC. J Am Chem Soc. 2006;128:14036–14037. doi: 10.1021/ja066090a.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.