Abstract
The HPV-16 E7 oncoprotein has previously been reported to stimulate DNA damage and to activate host cell DNA damage checkpoints. How HPV-16 E7 maintains proliferation despite activated DNA damage checkpoints is incompletely understood. Here, we provide evidence that cells expressing the HPV-16 E7 oncoprotein can enter mitosis in the presence of DNA damage. We show that this activity of HPV-16 E7 involves attenuation of DNA damage checkpoint control by accelerating the proteolytic turnover of claspin. Claspin mediates the activation of CHK1 by ATR in response to replication stress and its degradation plays a critical role in DNA damage checkpoint recovery. Expression of a non-degradable mutant of claspin was shown to inhibit mitotic entry in HPV-16 E7-expressing cells. Multiple components of the SCFβ-TrCP-based claspin degradation machinery were found deregulated in the presence of HPV-16 E7, including cullin 1 (CUL1), β-TrCP, Aurora A and Polo-like kinase-1 (PLK1). In contrast, no difference in the expression level of the claspin deubiquitinating enzyme USP7 was detected. Levels of Aurora A and PLK1 as well as phosphorylated PLK1 at threonine 210, a prerequisite for DNA damage checkpoint recovery, remained detectable following replication stress in HPV-16 E7-expressing cells but not in control cells. In summary, our results suggest that the HPV-16 E7 oncoprotein alleviates DNA damage checkpoint responses and promotes mitotic entry by accelerating claspin degradation through a mechanism that involves deregulation of components of the SCFβ-TrCP-based claspin degradation machinery.
Keywords: HPV-16 E7, DNA damage checkpoint, claspin
Introduction
Infection with high-risk human papillomaviruses (HPVs), such as HPV-16, is intimately associated with squamous cell carcinomas (SCCs) of the anogenital tract as well as a subset of oropharyngeal cancers (1). HPV-16 encodes two oncoproteins, E6 and E7, which play central roles in the viral life cycle and are commonly found overexpressed in high-risk HPV-associated tumors (2). The HPV-16 E7 oncoprotein is a multi-functional protein that binds and degrades the retinoblastoma tumor suppressor protein (pRB) as well as the related proteins p107 and p130 (3). The HPV-16 E7 oncoprotein further disrupts G1/S cell cycle checkpoint control by inhibiting the cyclin dependent kinase inhibitors p21Cip1 and p27Kip1 as well as various other activities (4–7). The cooperating HPV-16 E6 oncoprotein induces the degradation of the p53 tumor suppressor protein, stimulates hTERT expression and has several other functions that promote proliferation (8, 9). Together, the high-risk HPV oncoproteins relax G1/S checkpoint control in order to induce unscheduled entry into S phase and promote an S phase-like milieu conducive for viral genome replication in differentiated human keratinocytes (7, 10).
Several studies have suggested that deregulated S phase entry is associated with DNA replication stress (11–13). In line with this notion, the HPV-16 E7 oncoprotein has been shown to activate the Fanconi Anemia (FA) pathway (14), a DNA damage response pathway that responds primarily to replication stress and stalled DNA replication forks (15).
Replication stress can ultimately lead to DNA breakage and several lines of evidence suggest that expression of the high-risk HPV-16 E7 oncoprotein triggers host cell DNA damage. HPV-16 E7 has been reported to induce structural chromosomal changes (16, 17), as well as promote the integration of foreign DNA, an event that requires the formation of DNA double strand breaks (DSBs) (18). HPV-16 E7 expression has also been shown to stimulate an increase of nuclear foci that contain γ-H2AX, a marker of DSBs (17).
Despite signs of DNA damage and checkpoint activation, HPV-16 E7-expressing cells remain proliferative. Although several reports suggest that the ability of HPV-16 E7-expressing cells to maintain proliferation following DNA damage is associated with manipulation of cell cycle checkpoints (19, 20), it is not known in detail whether the HPV-16 E7 oncoprotein can also interfere more directly with the host cell DNA damage checkpoint response in order to overcome anti-proliferative stimuli.
Here, we show that HPV-16 E7-expressing cells can enter mitosis in the presence of DNA damage. We provide evidence that this is not simply a reflection of increased overall DNA damage, but that HPV-16 E7-expressing cells can relax DNA damage checkpoint control when challenged with DNA replication stress. We found that the HPV-16 E7 oncoprotein attenuates the DNA damage checkpoint response by accelerating the proteolytic turnover of claspin, a critical regulator of the ATR/CHK1 signaling axis and DNA damage checkpoint recovery in the G2 phase of the cell division cycle. This accelerated degradation occurred despite increased baseline levels of claspin in HPV-16 E7-expressing cells. A non-degradable mutant of claspin was found to inhibit mitotic entry in HPV-16 E7-expressing cells, suggesting a reinforced G2/M checkpoint. Several components of the SCFβ-TrCP-mediated claspin degradation machinery, including CUL1, β-TrCP, Aurora A and PLK1, were deregulated in the presence of HPV-16 E7. We show that Aurora A and PLK1 protein levels, as well as Aurora A-mediated phosphorylation of PLK1, a prerequisite for DNA damage checkpoint recovery, are maintained in the presence of replication stress in HPV-16 E7-expressing cells but not in control cells. Since claspin itself as well as components of its degradation machinery are E2F-responsive, our results suggest a model in which HPV-16 E7-mediated disruption of the pRB/E2F signaling axis upregulates claspin to promote efficient DNA replication in S phase, but also leads to accelerated degradation of claspin as cells approach the G2/M transition of the cell division cycle. Our results highlight the astonishing ability of the HPV-16 E7 oncoprotein to promote DNA replication and cell division despite a DNA damage response-activated, anti-proliferative host cell environment.
Materials and Methods
Cell culture, treatments and transfections
Primary human foreskin keratinocytes (HFKs) were harvested from foreskins and maintained in serum-free keratinocyte growth media (Epilife, Cascade Biologics/Invitrogen, Carlsbad, CA) supplemented with human keratinocyte growth supplement (HKGS; Invitrogen), 50 U/ml penicillin (Cambrex, East Rutherford, NJ), 50 µg/ml streptomycin (Cambrex) and fungizone (Invitrogen). Human foreskin fibrobasts (BJ; ATCC, Manassas, VA) and C33A cells (ATCC) were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Cambrex) supplemented with 10% fetal bovine serum (Mediatech, Herndon, VA) and antibiotics as indicated above. CaSki cells (ATCC) were maintained in RPMI media (Biowhittaker/Lonza, Allendale, NJ) supplemented with 1% L-glutamine (Invitrogen) and antibiotics as indicated above. To assess protein stability cells were treated with 30 µg/ml cycloheximide (Calbiochem, San Diego, CA) or dH2O for the indicated times. The proteasome was inhibited by treating cells with 1 µM Z-Leu-Leu-Leu-Vinyl Sulfone (Z-L3VS; BioMol International/Enzo Life Sciences, Plymouth Meeting, PA) or DMSO for 24 h. Stalled replication forks were induced by treating cells with 1 mM HU (Calbiochem) or dH2O for 24 h.
Primary HFKs were transduced with an LXSN-based high-risk HPV-16 E7 construct or LXSN empty vector followed by selection in G418-supplemented media. Expression was confirmed through immunoblot analysis. Transient transfections of primary HFKs stably expressing HPV-16 E7 was done using nucleofection (Lonza) with 2 µg of plasmids encoding a HA-tagged mutant claspin S30/34A (kindly provided by Michele Pagano, New York University School of Medicine, New York, NY) or empty vector control (neo-HA) in combination with 0.5 µg of dsRED as transfection marker. Tissue culture media was replaced 24 h after transfection and cells were fixed and mitotic index was assessed 48 h after transfection. BJ fibroblasts were stably transfected using nucleofection (Lonza) with 2 µg of HA-tagged HPV-16 E7, mutant HPV-16 E7 Δ21-24 or empty vector control (neo-HA) plasmids kindly provided by Karl Münger (Channing Laboratory, Brigham & Women’s Hospital, Boston, MA). Cells stably transfected with HPV-16 E7-HA or empty vector control were selected using 0.5 mg/ml G418 for approximately 7 days. Cells stably transfected with HPV-16 E7 Δ21-24-HA (Δ21-24-HA) were selected using 0.125 mg/ml G418 for 2 days. Plasmid expression was verified by determining pRB protein destabilization (data not shown).
Immunological Methods
Whole cell lysates were prepared and immunoblot analysis was performed as previously described (14). Quantification of band intensities was performed using NIH Image J software.
Immunofluorescence analysis of cells grown on coverslips was performed as previously described (17). Briefly, cells were fixed in 4% paraformaldehyde for 10 min at room temperature, washed in PBS and permeabilized using 1% Triton-× 100 for 15 min at room temperature. Following a PBS wash, cells were blocked with 10% normal donkey serum (Jackson Immunoresearch, West Grove, PA) in distilled water for 15 min at room temperature. Cells were then incubated in primary antibodies overnight at 4°C and again for at least another 2 h at 37°C in a humidified chamber. Following primary antibody incubation, cells were washed in PBS, incubated with FITC- or Rhodamine Red- conjugated anti-mouse or anti-rabbit secondary antibodies (Jackson Immunoresearch) for at least 2 h at 37°C, washed in PBS and counterstained with 4’,6’-diamidino-2-phenylinodole (DAPI, Vector Laboratories, Burlingame, CA). Cells were analyzed using an Olympus AX70 epifluorescence microscope equipped with a SpotRT digital camera.
Paraffin-embedded tissue samples were retrieved from the archives of the Department of Pathology, University of Pittsburgh School of Medicine (IRB # 0505181) and HPV typed using the Rembrandt/PanPath in situ hybridization kit (Invitrogen). Immunofluorescence microscopic analysis of paraffin-embedded tissue samples was performed as previously described (14), with the exception that slides were not subjected to pepsin digest. Briefly, slides were deparaffinized by baking and xylene treatment, followed by dehydration in 100% ethanol. After rehydration in a graded ethanol series (90%, 70%, 50%), slides were washed twice in dH2O and microwave treated in 0.01 M Citrate buffer, pH 6.0 for 30 min. Slides were allowed to cool and washed once in dH2O and twice in PBS. Slides were then blocked in 10% normal donkey serum for 30 min at room temperature and primary and secondary antibodies were applied as described above, with the exception that primary antibody solution was applied for 2–3 nights at 4°C, followed by several hours at 37°C. Slides were incubated in secondary overnight at 4°C, followed by 2–3 hours at 37°C, followed by counterstain using DAPI.
Primary antibodies used for immunoblotting, and immunofluorescence were 53BP1 (Novus Biologicals, Littleton, CO), actin (Sigma, Saint Louis, Missouri), phosphorylated ATM at Serine 1981 (Gene Tex, San Antonio, TX), Aurora A (Cell Signaling Technology, Danvers, MA), claspin (antibody kindly provided by Raimundo Freire, Unidad de Investigacion, Hospital Universitario de Canarias, Tenerife, Spain), CUL-1 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), cyclin A (Novocastra Laboratories Ltd, Newcastle-upon-Tyne, England), phosphorylated H2AX at serine 139 (Upstate, Temecula, CA), HPV-16 E7 (Santa Cruz Biotechnology, Inc), phosphorylated PLK1 at threonine 210 (BD Pharmingen, San Diego, CA), PLK1 (Santa Cruz Biotechnology, Inc.) and β-TrCP (Zymed/Invitrogen).
Statistical Analysis
Student’s two-tailed t test for independent samples was used to assess statistical significance.
Results
HPV-16 E7-expressing cells enter mitosis despite DNA damage
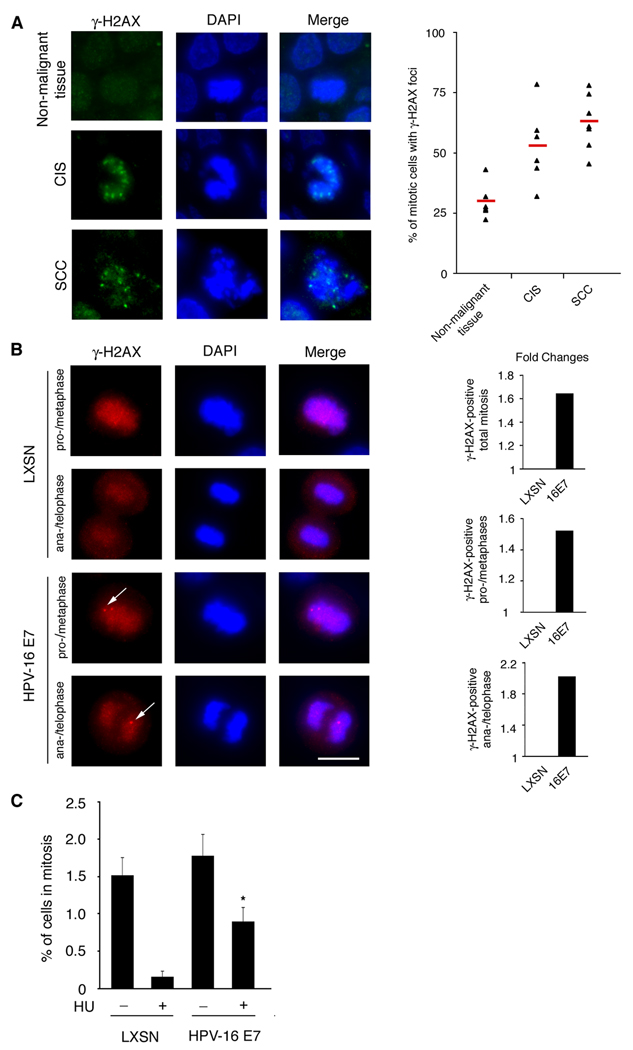
To determine the frequency of DNA damage in mitosis, an immunofluorescence microscopic analysis for the DNA damage maker γ-H2AX was performed in tissue samples obtained from high-risk HPV-positive (types 16/18 and/or 31/33) anal neoplasms or non-malignant tissue. A total of six carcinomas in situ (CIS) samples, seven squamous cell carcinomas (SCCs) and six non-malignant controls (hemorrhoids) were analyzed for the presence of γ-H2AX foci in mitotic cells. Microscopic analysis revealed that high-risk HPV-positive tissue samples displayed a statistically significant increase in the percentage of γ-H2AX-positive mitotic cells (Fig. 1A, left panel), from 29.5% in non-malignant tissue to 52.6% in CIS and 62.4% in SCC tissue (p≤0.01 and p≤0.0001, respectively; Fig. 1A, right panel). Since DNA damage checkpoints are crucial to prevent mitotic entry in the presence of unrepaired DNA damage (21), these results suggest that high-risk HPV oncoproteins can attenuate DNA damage checkpoint control that would normally lead to cell cycle arrest in G2/M.
Figure 1. The HPV-16 E7 oncoprotein promotes mitotic entry despite the presence of DNA damage.
(A) Immunofluorescence microscopic analysis of γ̃H2AX foci in biopsy samples obtained from high-risk HPV-positive anal carcinomas in situ (CIS), squamous cell carcinomas (SCCs) or non-malignant tissue samples (hemorrhoids). Nuclei stained with DAPI. Scale bar indicates 10 µm (left panel). Quantification of the proportion of mitotic cells with γ̃H2AX foci in CIS, SCC or non-malignant tissue samples. The red bar indicates the mean percentage of γ̃H2AX-postitive mitotic cells for each category. At least six cases and a total of at least 350 mitotic cells were analyzed for each category (right panel).
(B) Immunofluorescence microscopic analysis of γ̃H2AX foci in primary HFKs stably expressing the HPV-16 E7 oncoprotein or empty vector control (LXSN). Nuclei stained with DAPI. Panels depict γ̃H2AX staining in cells found in pro-/metaphase or ana-/telophase. Arrows point to γ̃H2AX foci. Scale bar indicates 10 µm (left panel). Quantification of fold changes of the percentage of total mitotic cells (top), pro-/metaphase cells (middle) or ana-/telophase cells (bottom) that contain γ̃H2AX foci. Five counts of at least 50 cells were analyzed from a representative experiment (right panel).
(C) Quantification of the percentage of mitotic cells detected in primary HFKs stably expressing HPV-16 E7 or empty vector control (LXSN) following treatment with either 1 mM HU or dH2O for 24 h. Mean values and standard error of two independent experiments with triplicate quantification of a minimum of 50 cells are shown. Asterisk indicates statistically significant differences in comparison to controls.
The HPV-16 E7 oncoprotein stimulates DNA damage in a cell cycle-dependent manner (Supplemental Fig. 1) and activates DNA damage checkpoints associated with DNA replication stress (14). Since HPV-16 E7-expressing cells continue to proliferate and enter mitosis, we asked whether HPV-16 E7 plays a role in overcoming DNA damage checkpoint control. We performed an immunofluorescence microscopic analysis for γ-H2AX in primary human foreskin keratinocytes (HFKs) stably transduced with HPV-16 E7 or empty vector control (LXSN). We first analyzed interphase cells and found a statistically significant 1.8-fold increase of the percentage of cells with γ-H2AX foci in HPV-16 E7-expressing cells in comparison to empty vector controls (53.1% and 28.3%, respectively; p≤0.001) similar to what has previously been reported (17). We then specifically analyzed mitotic cells and found that a significant proportion of HPV-16 E7-expressing cells display γ-H2AX foci in pro-/metaphase as well as later stages of mitosis (ana-/telophase; Figure 2B, left panel). A statistically significant 1.6-fold increase in the percentage of total γ-H2AX-positive mitotic cells was observed in HPV-16 E7-expressing cells (72.3%) compared to controls (44%, Fig. 1B, right panel/top position; p≤0.005). Furthermore, a significant 1.5-fold increase of γ-H2AX-positive pro-/metaphase was observed in HPV-16 E7-expressing cells (75.2%) compared to controls (49.5%, Fig. 1B, right panel/middle position; p≤0.005) and a significant 2.0-fold increase of γ-H2AX-positive ana-/telophases in HPV-16 E7-expressing cells (68%) compared to controls (33.7%, Fig. 1B, right panel/bottom position; p≤0.005). Together, these results underscore that the HPV-16 E7 oncoprotein stimulates DNA damage and furthermore suggest that HPV-16 E7-expressing cells can enter mitosis despite DNA damage.
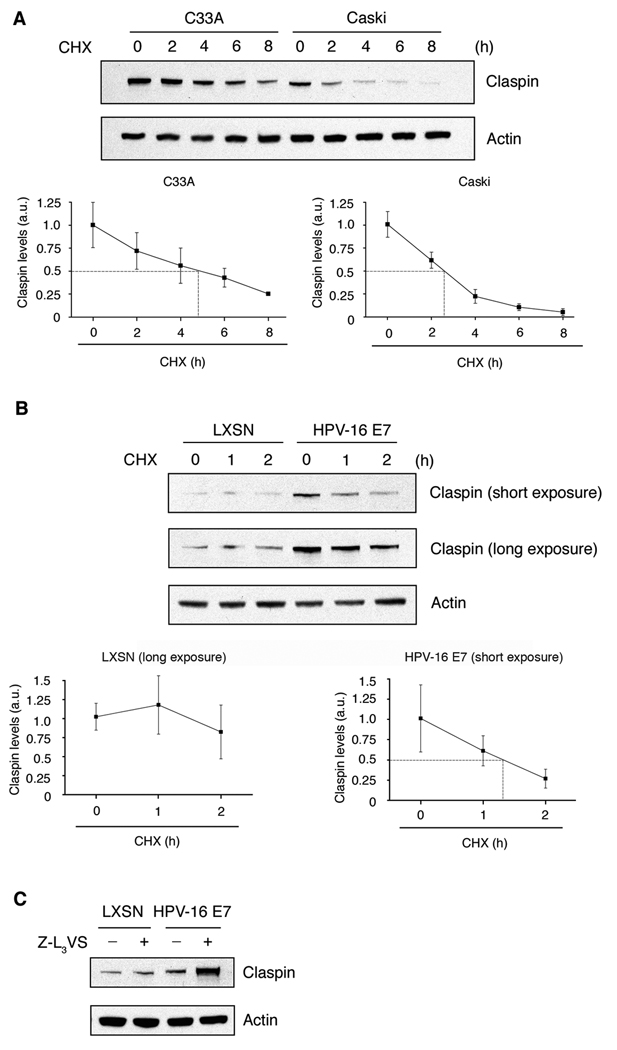
Figure 2. The proteolytic turnover of claspin is accelerated in HPV-positive cells and involves the HPV-16 E7 oncoprotein.
(A) Immunoblot analysis of C33A (HPV-negative) and CaSki (HPV-16-positive) cells treated with cycloheximide (CHX) for the indicated time intervals. Immunoblot for actin was used to demonstrate protein loading (upper panel). Densitometric analyses of claspin protein levels were quantified from three experiments using NIH ImageJ. Claspin band intensities were calculated using arbitrary units (a.u.) by subtracting the background and normalizing to actin levels. For each cell line, the mean value of claspin levels at time 0 was set to 1 and all sequential values were calculated accordingly. The mean value and standard error for each time point are shown and the half-life is indicated with a dotted line (lower panels).
(B) Immunoblot analysis of primary human keratinocytes stably expressing HPV-16 E7 or empty vector control (LXSN) following treatment with CHX for the indicated time intervals. Immunoblot for actin was used to demonstrate protein loading (upper panel). Densitometric analyses of claspin protein levels was quantified from two independent experiments as described above. The mean value and standard error for each time point are graphed and the half-life is indicated with a dotted line (lower panels).
(C) Immunoblot analysis of primary human keratinocytes stably expressing HPV-16 E7 or empty vector control following treatment with Z-L3VS or DMSO for 24 h. Immunoblot for actin was used to demonstrate protein loading.
HPV-16 E7 attenuates DNA damage checkpoint control and promotes mitotic entry in the presence of replication stress
We next sought to rule out that the increase of mitotic cells with γ-H2AX foci in HPV-16 E7 cell populations is not simply a result of an overall increase of cells with DNA breakage. HPV-16 E7-expressing or control primary HFKs were treated for 24 hours with hydroxyurea (HU), a ribonucleotide reductase inhibitor that causes replication forks to stall by depleting the intracellular deoxynucleotide pool, followed by quantification of the percentage of cells in mitosis. HU was used because it mimics certain aspects of the DNA damage response observed in HPV-16 E7-expressing cells, such as activation of the FA pathway (14). No significant difference in the mitotic index of HPV-16 E7-expressing cells (1.8%) compared to control cells (1.5%, p>0.05) was observed following control treatment (Fig. 1C). In contrast, a statistically significant 4.5-fold increase of HPV-16 E7-expressing cells were found in mitosis (0.9%) following HU treatment compared to control cells (0.2%, p≤0.001; Fig. 1C). These results underscore that the HPV-16 E7 oncoprotein not only stimulates DNA damage but also attenuates DNA damage checkpoint responses that would normally prevent mitotic entry following DNA replication stress.
HPV-positive cells lines show altered claspin protein stability
Claspin facilitates replication stress-associated ATR phosphorylation of CHK1 (22, 23) and its degradation promotes recovery from DNA damage checkpoint arrest (24–26). Therefore, we next asked whether claspin expression is altered in the presence of high-risk HPV oncoproteins. HPV-16-positive cancer cells (CaSki) or HPV-negative cancer cells (C33A) were treated with cycloheximide (CHX) for up to 8 hours and claspin protein levels were assessed by immunoblot analysis (Fig. 2A, upper panel). CaSki cells overexpress HPV-16 E6 and E7 and hence resemble high-risk HPV-associated lesions. As expected from previous studies in cancer cell lines (27), both C33A and CaSki cells contain elevated baseline claspin levels. However, calculation of claspin protein half-life revealed a half-life of 4–6 h in C33A cells whereas the claspin half-life was significantly reduced and close to only 2 h in HPV-16-positive CaSki cells (Fig. 2A). These results suggest that claspin protein stability is decreased in the presence of high-risk HPV oncoproteins.
The HPV-16 E7 oncoprotein accelerates the proteolytic turnover of claspin
To determine the individual contributions of HPV-16 oncoproteins, we analyzed claspin protein stability in BJ fibroblasts stably expressing empty vector, HPV-16 E7 alone or HPV-16 E7 in combination with HPV-16 E6. Co-expression of HPV-16 E7 with HPV-16 E6 was not associated with any significant changes in claspin protein stability compared to cells expressing HPV-16 E7 alone (Suppl. Fig. 2). These results suggest that the reduced claspin stability in HPV-16-positive cells is primarily due to expression of the HPV-16 E7 oncoprotein. To further corroborate these results, we analyzed claspin protein stability in primary HFKs stably expressing HPV-16 E7 or control cells (Fig. 2B). Immunoblot analysis showed increased base-line levels of claspin in HPV-16 E7-expressing cells in comparison to control cells. The differences in baseline levels of claspin between BJ fibroblasts and primary HFKs are very likely due to the different cellular background. Importantly, however, half-life calculation revealed a claspin half-life of 1–2 h (closer to 1 h than 2 h) in HPV-16 E7-expressing HFKs whereas no significant drop of claspin protein levels was detected in empty vector controls within the time interval analyzed (Fig. 2B). These results are in agreement with the shortened claspin half-life in HPV-16 E7-expressing BJ fibroblasts (Suppl. Fig. 2). We also analyzed mutant HPV-16 E7 Δ21-24 for both claspin protein stability and mitotic entry and no significant differences were detected in comparison to empty vector controls (Suppl. Fig. 2). These results indicate that, despite the overall increase of claspin protein levels in HPV-16 E7-expressing cells, the proteolytic turnover of claspin is accelerated in the presence of the HPV-16 E7 oncoprotein. Our mutational analysis furthermore suggests that disruption of the pRB/E2F signaling axis by HPV-16 E7 may contribute to accelerated claspin degradation.
In order to further corroborate the role of HPV-16 E7 in claspin degradation, we next tested whether inhibition of the proteasome lead to stabilization of claspin protein levels. Treatment of primary HFKs stably expressing HPV-16 E7 or control cells with the proteasome inhibitor Z-L3VS for 24 hours led to a significant increase of claspin protein levels in HPV-16 E7-expressing cells compared to control cells (Fig. 2C). These results underscore that the HPV-16 E7 oncoprotein destabilizes claspin protein through a mechanism that involves the ubiquitin-proteasome pathway.
Claspin degradation is required for mitotic entry in HPV-16 E7-expressing cells
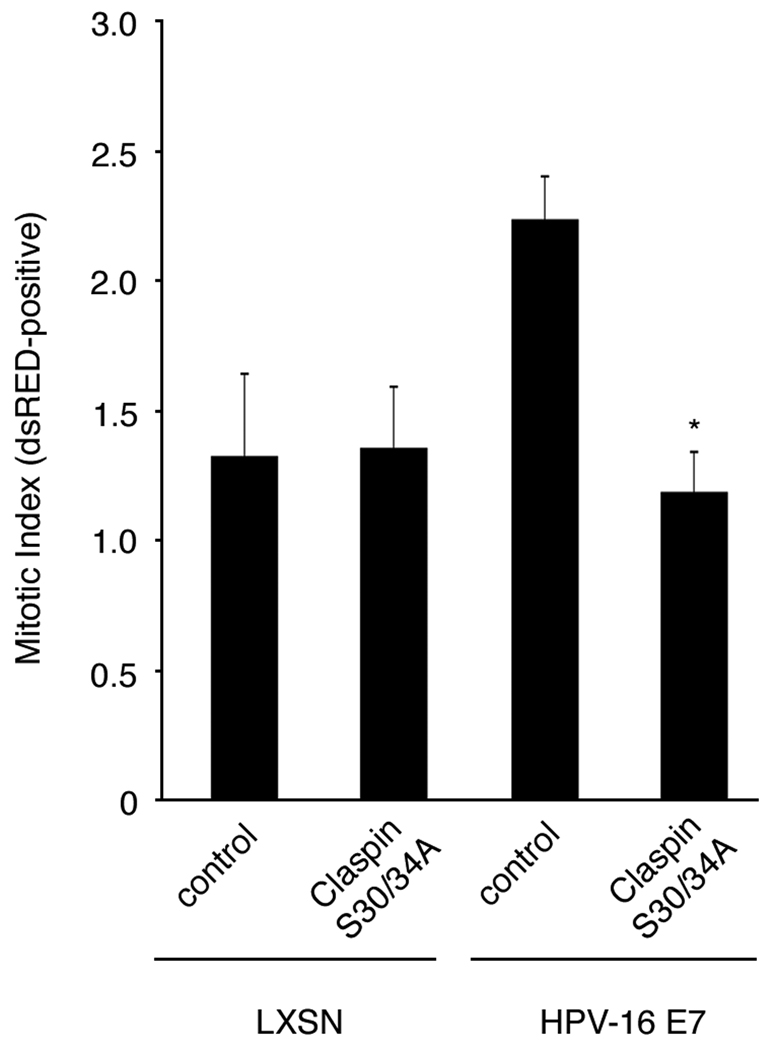
In order for cells to enter mitosis following DNA damage checkpoint responses, claspin must be degraded, thereby relieving further ATR-mediated CHK1-associated cell cycle arrest (24–26). To directly test whether claspin degradation facilitates entry into mitosis in HPV-16 E7-expressing cells, we transiently transfected empty vector-transduced HFKs or HPV-16 E7-expressing HFKs with a non-degradable mutant of claspin, in which two conserved serine residues at amino acid position 30 and 34 within the phospho-degron motif of claspin have been mutated to alanines (S30/34A; kindly provided by M. Pagano, New York University School of Medicine, New York, NY) or vector control and monitored the mitotic index 48 hours following transfection (Fig. 3). No effect of degradation-impaired claspin on mitotic entry was detected in control HFKs, which is in line with the notion that non-degradable claspin does not significantly affect entry into mitosis in the absence of DNA damage as suggested previously (24). However, a significant 1.9-fold decrease in the percentage of mitotic cells was observed in HPV-16 E7-expressing primary HFKs transiently transfected with the phospho-degron mutant claspin S30/34A (1.2%) compared to cells transfected with empty vector controls (2.2%, p≤0.0001). These findings underscore not only that HPV-16 E7 induces DNA damage but also that acceleration of claspin degradation by the HPV-16 E7 oncoprotein is necessary to facilitate entry into mitosis. Furthermore, they suggest that accumulation of claspin in HPV-16 E7-expressing cells can reinforce the G2/M checkpoint.
Figure 3. A non-degradable mutant of claspin inhibits mitotic entry in HPV-16 E7-expressing cells.
The mitotic index was assessed in HPV-16 E7-expressing or empty vector control (LXSN) primary human keratinocytes 48 h after transient transfection with either a phospho-degron mutant of claspin (claspin S30/34A) or empty vector (control) using dsRED as a transfection marker. Mean values and standard error for LXSN cells were generated from a representative experiment with at least 100 cells counted in triplicate. Mean values and standard error for HPV-16 E7-expressing cells were generated from three independent experiments with at least 100 cells counted in triplicate. Asterisk indicates statistically significant differences in comparison to cells transiently transfected with empty vector (control).
HPV-16 E7-induced claspin degradation involves deregulation of multiple components of the SCFβ-TrCP-based claspin degradation machinery
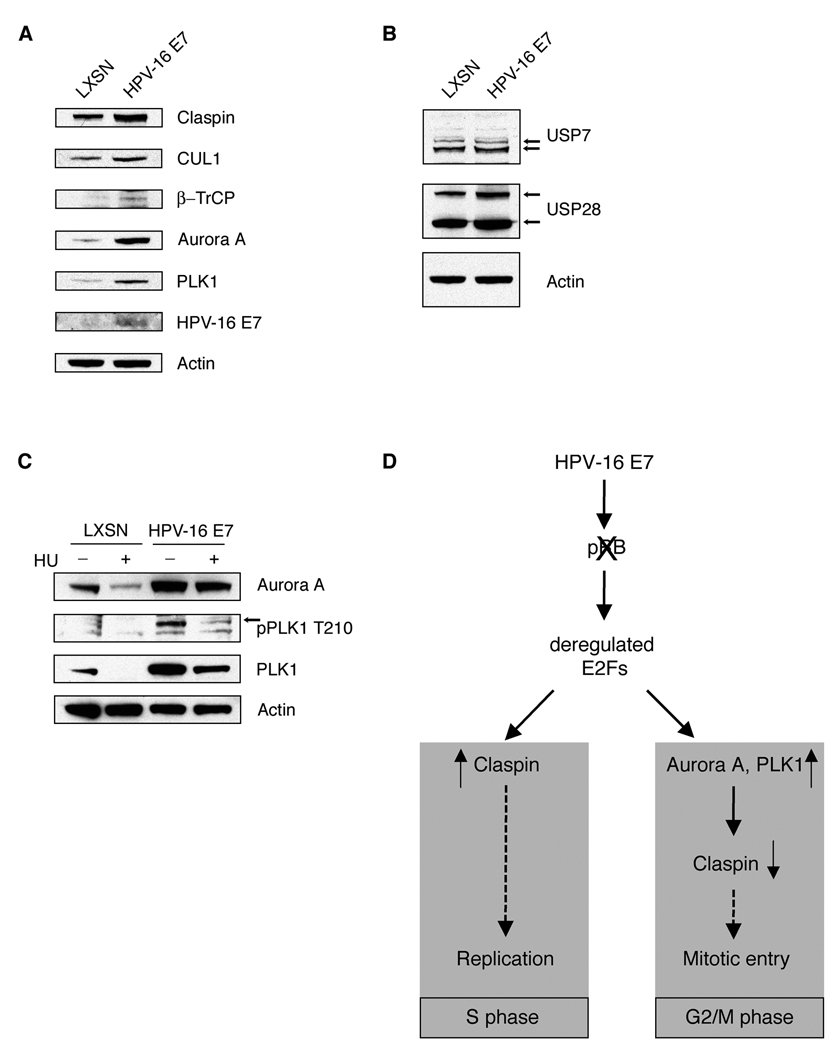
DNA damage checkpoint recovery is facilitated, in part, by the activities of the mitotic kinases Aurora A and Polo-like kinase 1 (PLK1), which promote SCFβ-TrCP-mediated claspin proteolysis (25, 26, 28, 29). In order to test whether the HPV-16 E7 oncoprotein affects claspin protein stability by deregulating SCFβ-TrCP-based claspin degradation machinery, we examined the expression levels of various proteins involved in claspin ubiquitination in primary HFKs stably expressing HPV-16 E7 or control. Multiple proteins implicated in SCFβ-TrCP-mediated claspin degradation were upregulated in HPV-16 E7-expressing primary HFKs, including cullin 1 (CUL1), β-TrCP, Aurora A and PLK1 (Fig. 4A). Claspin protein stability, however, is also regulated by the deubiquitinating enzymes (DUBs), USP7 and USP28 (28, 30, 31). No significant difference in the expression level of USP7, which primarily counteracts SCFβ-TrCP-based claspin ubiquitination in G2/M (28), was detected in HPV-16 E7-expressing cells compared to control cells (Fig. 4B). A slight increase of USP28, which opposes APC/CCdh1-based claspin degradation in G1 (28, 30), was observed in HPV-16 E7-expressing cells compared to controls. Together, these findings suggest that HPV-16 E7-associated acceleration of claspin degradation involves the deregulation of components of the SCFβ-TrCP-based claspin degradation machinery, but not the deregulation of the DUB that thwarts SCFβ-TrCP-mediated claspin degradation.
Figure 4. The HPV-16 E7 oncoprotein deregulates multiple components of the claspin degradation machinery.
(A) Immunoblot analysis of primary human keratinocytes stably expressing HPV-16 E7 or empty vector control (LXSN) for proteins involved in claspin degradation. Immunoblot for HPV-16 E7 was used to verify stable plasmid expression. Immunoblot for actin was used to demonstrate protein loading.
(B) Immunoblot analysis of primary human keratinocytes stably expressing HPV-16 E7 or empty vector control (LXSN) for claspin deubiquitinating enzymes. Immunoblot for actin was used to demonstrate protein loading. Arrows point to bands corresponding to USP7 or USP28.
(C) Immunoblot analysis of primary human keratinocytes stably expressing HPV-16 E7 or empty vector control (LXSN) following treatment with either 1 mM HU or dH2O for 24 h. Immunoblot for actin was used to demonstrate protein loading. Arrow points to the band corresponding to pPLK1 T210.
(D) Proposed model of HPV-16 E7-induced deregulation of claspin protein stability. HPV-16 E7-associated pRB/E2F disruption leads to increased levels of claspin in S phase in order to promote DNA replication. However, deregulated E2F gene transcription in G2/M may stimulate an increase of PLK1 and Aurora A expression levels, which accelerates SCFβ-TrCP-mediated claspin proteolysis, thereby facilitating aberrant entry into mitosis in the presence of DNA damage.
The activation of PLK1 prior to DNA damage checkpoint recovery is mediated by the Aurora A kinase (29). We therefore, analyzed Aurora A and PLK1 expression as well as PLK1 activation in primary HFKs stably expressing HPV-16 E7 or control cells following DNA replication stress. We found that HPV-16 E7-expressing cells maintain high expression levels of Aurora A in response to HU-induced replication stress compared to control cells (Fig. 4C). In addition, HPV-16 E7-expressing cells also showed higher PLK1 protein levels and PLK1 phosphorylation at threonine 210 (T210). Phosphorylation at this residue has been shown to be a prerequisite for PLK1 to promote mitotic entry after DNA damage checkpoint arrest (29). Together, these results show that multiple layers of control involved in recovery from the DNA damage checkpoint are disrupted in HPV-16 E7-expressing cells.
Discussion
In the present report, we show that the HPV-16 E7 oncoprotein can promote mitotic entry in the presence of DNA damage. We provide evidence that attenuation of DNA damage checkpoint control is mediated by HPV-16 E7-associated accelerated proteolysis of claspin, a critical mediator of the ATR/CHK1 signaling axis (22, 23). Several previous reports have shown that claspin degradation by the SCFβ-TrCP-based ubiquitin ligase in G2/M is linked to recovery from DNA damage checkpoint activation (24–26). We found that several proteins involved in this process are upregulated in HPV-16 E7-expressing cells thus creating a cellular environment that promotes aberrant mitotic entry.
Although our results clearly show that the HPV-16 E7 oncoprotein attenuates DNA damage checkpoint control (Fig. 1C), it is noteworthy that we discovered γ-H2AX-positive mitotic cells in non-malignant tissue samples as well as control keratinocytes. It is possible that genotoxic stress from unfavorable growth conditions contributes to this damage. Remarkably, a number of studies suggest that cells with DNA damage avoid a prolonged cell cycle arrest and enter mitosis through checkpoint adaptation (32–36). It is hence possible that HPV-16 E7 manipulates a process that, to a certain degree, represents a physiological response to excessive DNA damage.
Our finding that HPV-16 E7-expressing cells show an accelerated proteolytic turnover of claspin provides an explanation for aberrant mitotic entry in the presence of DNA damage. While we assume that claspin degradation occurs at the G2/M-transition, the technical difficulties to efficiently synchronize HPV-16 E7-expressing keratinocyte populations prevented us from directly testing this hypothesis.
Since claspin is involved in the ATR/CHK1 signaling cascade (22, 23), it is important to mention that phosphorylation of CHK1 was observed in HPV-16 E7-expressing cells (data not shown). These results suggest that claspin remains functional within the ATR pathway in HPV-16 E7-expressing cells, but that it is more efficiently degraded during the G2 phase of the cell cycle in order to attenuate DNA damage checkpoint responses and promote mitotic entry. This notion is supported by our finding that a non-degradable mutant of claspin inhibits mitotic entry in HPV-16 E7-expressing cells, thus underscoring that claspin accumulation reinforces G2/M checkpoint control in HPV-16 E7-expressing cells.
We found that several proteins involved in SCFβ-TrCP-mediated claspin degradation are upregulated in HPV-16 E7-expressing cells, including CUL1, β-TrCP, PLK1 and Aurora A. Claspin protein stability is also regulated by the deubiquitinating enzymes (DUBs) USP7 and USP28, which counteract SCFβ-TrCP- and APC/CCdh1-mediated claspin degradation, respectively (28, 30, 31). Despite the slight upregulation of USP28 levels, no such increase in USP7 protein expression, which opposes SCFβ-TrCP-based claspin degradation in G2 (28), was detected in HPV-16 E7 keratinocyte populations compared to controls.
The degradation of claspin during DNA damage checkpoint recovery involves its phosphorylation by PLK1 which creates a recognition motif for the SCFβ-TrCP ubiquitin ligase (24–26). PLK1 is activated prior to DNA damage checkpoint recovery by Aurora A-mediated phosphorylation of PLK1 at T210 (29). Here, we show that HPV-16 E7-expressing cells maintain high levels of Aurora A and PLK1 when compared to control cells in the presence of HU-induced replication stress. Furthermore, we found that the level of phosphorylated PLK1 at T210 also remains detectable in HPV-16 E7-expressing cells treated with HU. The finding that HU-treated control cells show significantly decreased Aurora A and PLK1 protein levels could be explained by the fact that these cells are more efficiently arrested by HU treatment in early S phase, when PLK1 and Aurora A levels are still low (37–39). In contrast, the higher levels of Aurora A and PLK1 found in HPV-16 E7-expressing cells treated with HU may suggest that cells do not arrest properly following HU and/or that the regulation of these two kinases is disrupted by HPV-16 E7.
Together, our findings indicate that the HPV-16 E7 oncoprotein increases claspin degradation by disrupting the balance between positive and negative regulators involved in SCFβ-TrCP-mediated claspin protein stability during DNA damage checkpoint recovery. However, we cannot rule out the possibility that the HPV-16 E7 oncoprotein may accelerate the proteolytic turnover of claspin in a more direct manner.
Claspin, PLK1 and Aurora A have been found to harbor E2F-responsive promoter elements (40–42). Therefore, it is likely that degradation of pRB by HPV-16 E7 and the associated increase in E2F-mediated gene transcription may contribute to claspin expression levels in both a positive and negative manner. Deregulation of E2F-mediated gene transcription is likely to stimulate high levels of claspin in S phase, which may help to promote efficient DNA replication. This is consistent with the increased baseline levels of claspin protein expression observed in HPV-16 E7-expressing HFKs (Fig. 2B). However, enhanced E2F-mediated gene transcription would also upregulate components of the SCFβ-TrCP-based claspin degradation pathway leading to accelerated and/or premature degradation of claspin and hence, attenuated DNA damage checkpoint control and aberrant entry into mitosis (25) (Fig. 4D). Our finding that pRB-degradation-deficient mutant HPV-16 E7 Δ21-24 does not significantly alter claspin stability supports this hypothesis. The fact that inhibition of claspin degradation prevents mitotic entry in HPV-16 E7-expressing cells suggests that Aurora A or PLK1 inhibitors may have therapeutic potential in high-risk HPV-associated neoplasms and potentially in cancers in which deregulated claspin degradation is observed.
Supplementary Material
Acknowledgements
We are grateful to Michele Pagano, Raimundo Freire and Karl Munger for sharing important reagents and to Shih-Fan Kuan and Anna Chin for helping with retrieving tissue samples and performing HPV typing. This work was supported by NIH/NCI grant R01 CA112598 and a Research Scholar Grant from the American Cancer Society (to S. Duensing).
References
- 1.zur Hausen H. Papillomaviruses in the causation of human cancers - a brief historical account. Virology. 2009;384:260–265. doi: 10.1016/j.virol.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 2.Munger K, Howley PM. Human papillomavirus immortalization and transformation functions. Virus Res. 2002;89:213–228. doi: 10.1016/s0168-1702(02)00190-9. [DOI] [PubMed] [Google Scholar]
- 3.Munger K, Basile JR, Duensing S, et al. Biological activities and molecular targets of the human papillomavirus E7 oncoprotein. Oncogene. 2001;20:7888–7898. doi: 10.1038/sj.onc.1204860. [DOI] [PubMed] [Google Scholar]
- 4.Jones DL, Alani RM, Munger K. The human papillomavirus E7 oncoprotein can uncouple cellular differentiation and proliferation in human keratinocytes by abrogating p21Cip1-mediated inhibition of cdk2. Genes Dev. 1997;11:2101–2111. doi: 10.1101/gad.11.16.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Funk JO, Waga S, Harry JB, Espling E, Stillman B, Galloway DA. Inhibition of CDK activity and PCNA-dependent DNA replication by p21 is blocked by interaction with the HPV-16 E7 oncoprotein. Genes Dev. 1997;11:2090–2100. doi: 10.1101/gad.11.16.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zerfass-Thome K, Zwerschke W, Mannhardt B, Tindle R, Botz JW, Jansen-Durr P. Inactivation of the cdk inhibitor p27KIP1 by the human papillomavirus type 16 E7 oncoprotein. Oncogene. 1996;13:2323–2330. [PubMed] [Google Scholar]
- 7.Longworth MS, Laimins LA. Pathogenesis of human papillomaviruses in differentiating epithelia. Microbiol Mol Biol Rev. 2004;68:362–372. doi: 10.1128/MMBR.68.2.362-372.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klingelhutz AJ, Foster SA, McDougall JK. Telomerase activation by the E6 gene product of human papillomavirus type 16. Nature. 1996;380:79–82. doi: 10.1038/380079a0. [DOI] [PubMed] [Google Scholar]
- 9.Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 10.DiMaio D, Liao JB. Human papillomaviruses and cervical cancer. Adv Virus Res. 2006;66:125–159. doi: 10.1016/S0065-3527(06)66003-X. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka S, Diffley JF. Deregulated G1-cyclin expression induces genomic instability by preventing efficient pre-RC formation. Genes Dev. 2002;16:2639–2649. doi: 10.1101/gad.1011002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartkova J, Horejsi Z, Koed K, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–870. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- 13.Ekholm-Reed S, Mendez J, Tedesco D, Zetterberg A, Stillman B, Reed SI. Deregulation of cyclin E in human cells interferes with prereplication complex assembly. J Cell Biol. 2004;165:789–800. doi: 10.1083/jcb.200404092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spardy N, Duensing A, Charles D, et al. The human papillomavirus type 16 E7 oncoprotein activates the Fanconi anemia (FA) pathway and causes accelerated chromosomal instability in FA cells. J Virol. 2007;81:13265–13270. doi: 10.1128/JVI.01121-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howlett NG, Taniguchi T, Durkin SG, D'Andrea AD, Glover TW. The Fanconi anemia pathway is required for the DNA replication stress response and for the regulation of common fragile site stability. Hum Mol Genet. 2005;14:693–701. doi: 10.1093/hmg/ddi065. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Han S, Baluda MA, Park NH. HPV-16 oncogenes E6 and E7 are mutagenic in normal human oral keratinocytes. Oncogene. 1997;14:2347–2353. doi: 10.1038/sj.onc.1201078. [DOI] [PubMed] [Google Scholar]
- 17.Duensing S, Munger K. The human papillomavirus type 16 E6 and E7 oncoproteins independently induce numerical and structural chromosome instability. Cancer Res. 2002;62:7075–7082. [PubMed] [Google Scholar]
- 18.Kessis TD, Connolly DC, Hedrick L, Cho KR. Expression of HPV16 E6 or E7 increases integration of foreign DNA. Oncogene. 1996;13:427–431. [PubMed] [Google Scholar]
- 19.Song S, Gulliver GA, Lambert PF. Human papillomavirus type 16 E6 and E7 oncogenes abrogate radiation-induced DNA damage responses in vivo through p53-dependent and p53-independent pathways. Proc Natl Acad Sci U S A. 1998;95:2290–2295. doi: 10.1073/pnas.95.5.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Helt AM, Galloway DA. Destabilization of the retinoblastoma tumor suppressor by human papillomavirus type 16 E7 is not sufficient to overcome cell cycle arrest in human keratinocytes. J Virol. 2001;75:6737–6747. doi: 10.1128/JVI.75.15.6737-6747.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Connell MJ, Walworth NC, Carr AM. The G2-phase DNA-damage checkpoint. Trends Cell Biol. 2000;10:296–303. doi: 10.1016/s0962-8924(00)01773-6. [DOI] [PubMed] [Google Scholar]
- 22.Kumagai A, Dunphy WG. Claspin, a novel protein required for the activation of Chk1 during a DNA replication checkpoint response in Xenopus egg extracts. Mol Cell. 2000;6:839–849. doi: 10.1016/s1097-2765(05)00092-4. [DOI] [PubMed] [Google Scholar]
- 23.Chini CC, Chen J. Claspin, a regulator of Chk1 in DNA replication stress pathway. DNA Repair (Amst) 2004;3:1033–1037. doi: 10.1016/j.dnarep.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Mailand N, Bekker-Jensen S, Bartek J, Lukas J. Destruction of Claspin by SCFbetaTrCP restrains Chk1 activation and facilitates recovery from genotoxic stress. Mol Cell. 2006;23:307–318. doi: 10.1016/j.molcel.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 25.Peschiaroli A, Dorrello NV, Guardavaccaro D, et al. SCFbetaTrCP-mediated degradation of Claspin regulates recovery from the DNA replication checkpoint response. Mol Cell. 2006;23:319–329. doi: 10.1016/j.molcel.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 26.Mamely I, van Vugt MA, Smits VA, et al. Polo-like kinase-1 controls proteasome-dependent degradation of Claspin during checkpoint recovery. Curr Biol. 2006;16:1950–1955. doi: 10.1016/j.cub.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 27.Lin SY, Li K, Stewart GS, Elledge SJ. Human Claspin works with BRCA1 to both positively and negatively regulate cell proliferation. Proc Natl Acad Sci U S A. 2004;101:6484–6489. doi: 10.1073/pnas.0401847101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faustrup H, Bekker-Jensen S, Bartek J, Lukas J, Mailand N. USP7 counteracts SCF{beta}TrCP- but not APCCdh1-mediated proteolysis of Claspin. J Cell Biol. 2009 doi: 10.1083/jcb.200807137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macurek L, Lindqvist A, Lim D, et al. Polo-like kinase-1 is activated by aurora A to promote checkpoint recovery. Nature. 2008;455:119–123. doi: 10.1038/nature07185. [DOI] [PubMed] [Google Scholar]
- 30.Bassermann F, Frescas D, Guardavaccaro D, Busino L, Peschiaroli A, Pagano M. The Cdc14B-Cdh1-Plk1 axis controls the G2 DNA-damage-response checkpoint. Cell. 2008;134:256–267. doi: 10.1016/j.cell.2008.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang D, Zaugg K, Mak TW, Elledge SJ. A role for the deubiquitinating enzyme USP28 in control of the DNA-damage response. Cell. 2006;126:529–542. doi: 10.1016/j.cell.2006.06.039. [DOI] [PubMed] [Google Scholar]
- 32.Lupardus PJ, Cimprich KA. Checkpoint adaptation; molecular mechanisms uncovered. Cell. 2004;117:555–556. doi: 10.1016/j.cell.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 33.Yoo HY, Kumagai A, Shevchenko A, Dunphy WG. Adaptation of a DNA replication checkpoint response depends upon inactivation of Claspin by the Polo-like kinase. Cell. 2004;117:575–588. doi: 10.1016/s0092-8674(04)00417-9. [DOI] [PubMed] [Google Scholar]
- 34.Toczyski DP, Galgoczy DJ, Hartwell LH. CDC5 and CKII control adaptation to the yeast DNA damage checkpoint. Cell. 1997;90:1097–1106. doi: 10.1016/s0092-8674(00)80375-x. [DOI] [PubMed] [Google Scholar]
- 35.Sandell LL, Zakian VA. Loss of a yeast telomere: arrest, recovery, and chromosome loss. Cell. 1993;75:729–739. doi: 10.1016/0092-8674(93)90493-a. [DOI] [PubMed] [Google Scholar]
- 36.Syljuasen RG, Jensen S, Bartek J, Lukas J. Adaptation to the ionizing radiation-induced G2 checkpoint occurs in human cells and depends on checkpoint kinase 1 and Polo-like kinase 1 kinases. Cancer Res. 2006;66:10253–10257. doi: 10.1158/0008-5472.CAN-06-2144. [DOI] [PubMed] [Google Scholar]
- 37.Lindon C, Pines J. Ordered proteolysis in anaphase inactivates Plk1 to contribute to proper mitotic exit in human cells. J Cell Biol. 2004;164:233–241. doi: 10.1083/jcb.200309035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uchiumi T, Longo DL, Ferris DK. Cell cycle regulation of the human polo-like kinase (PLK) promoter. J Biol Chem. 1997;272:9166–9174. doi: 10.1074/jbc.272.14.9166. [DOI] [PubMed] [Google Scholar]
- 39.Kimura M, Kotani S, Hattori T, et al. Cell cycle-dependent expression and spindle pole localization of a novel human protein kinase, Aik, related to Aurora of Drosophila and yeast Ipl1. J Biol Chem. 1997;272:13766–13771. doi: 10.1074/jbc.272.21.13766. [DOI] [PubMed] [Google Scholar]
- 40.Iwanaga R, Komori H, Ishida S, et al. Identification of novel E2F1 target genes regulated in cell cycle-dependent and independent manners. Oncogene. 2006;25:1786–1798. doi: 10.1038/sj.onc.1209210. [DOI] [PubMed] [Google Scholar]
- 41.He L, Yang H, Ma Y, Pledger WJ, Cress WD, Cheng JQ. Identification of Aurora-A as a direct target of E2F3 during G2/M cell cycle progression. J Biol Chem. 2008;283:31012–31020. doi: 10.1074/jbc.M803547200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Ishida S, Huang E, Zuzan H, et al. Role for E2F in control of both DNA replication and mitotic functions as revealed from DNA microarray analysis. Mol Cell Biol. 2001;21:4684–4699. doi: 10.1128/MCB.21.14.4684-4699.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.