Abstract
Myelination is an important process in brain development, and delays or abnormalities in this process have been associated with a number of conditions including autism, developmental delay, attention deficit disorder, and schizophrenia. Myelination can be sensitive to developmental experience; however, although the adult brain remains highly plastic, it is unknown whether myelination continues to be sensitive to experience during adulthood. Male and female rats were socially housed until four months of age, at which time they were moved into either a complex or “enriched” environment (EC) or an isolated condition (IC). Although the area of the splenium (posterior 20% of the callosum, which contains axons from visual cortical neurons) increased by about 10% following two months of EC housing, the area occupied by myelinated axons was not influenced by adult housing condition. Instead, it was the area occupied by glial cell processes and unmyelinated axons which significantly increased following EC housing. Neither the size nor the myelin content of the genu (anterior 15% of the callosum) was sensitive to manipulations of adult housing condition, but males had more area occupied by myelinated axons in both callosal regions. Finally, the inability of two months of complex environment housing during adulthood to impact the number of myelinated axons in the splenium was confirmed in a subset of animals using quantitative electron microscopy. We conclude that the sensitivity of myelination to experience is reduced in adulthood relative to development in both sexes.
Keywords: enrichment, EC, electron microscopy, sex differences, splenium, genu
1. Introduction
The brain is exquisitely responsive to experience, including learning. Since Hebb and his students first showed that housing rats in a complex environment improves their performance on numerous tests of rodent cognition (Forgays and Forgays, 1952; Hebb, 1949; Hymovitch, 1952), this so-called “enrichment” paradigm has been a favorite of researchers interested in understanding how the brain reorganizes itself to accomplish learning. Raising animals in a complex environment induces dendritic growth and synaptogenesis on neurons in many neocortical areas (Greenough and Volkmar, 1973; Markham and Greenough, 2004; Turner and Greenough, 1983; 1985). While the most dramatic effects have been seen following developmental experience, similar changes in neuronal morphology have been reported following complex environment housing or training on a learning paradigm during adulthood (Black et al., 1990; Greenough et al., 1979; Greenough et al., 1985; Juraska et al., 1980; Kleim et al., 1996; Markham and Greenough, 2004; Uylings et al., 1978). For example, rats housed in a complex environment as adults for either 30 or 60 days had significantly more synapses per neuron in the visual cortex compared to control animals of the same age (Briones et al., 2004). Animals that had experienced the complex environment for 30 days and then were placed in individual housing for a subsequent 30 days were comparable to animals subjected to the reverse sequence, reflecting the persistence of experience-induced synaptic plasticity (Briones et al., 2004). Similarly, learning-induced changes in cerebellar synapse number persisted undiminished for at least four weeks after training on a motor learning task was completed (Kleim et al., 1997).
In recent years it has become clear that experience-driven structural changes in the brain are by no means limited to neurons. For example, the degree of synaptic ensheathement by astrocytic processes is increased by complex environment housing (Jones and Greenough, 1996), and enrichment induces increases in astrocytic cell size (hypertrophy) and number (hyperplasia) (Anderson et al., 1994; Jones and Greenough, 2002; Sirevaag and Greenough, 1987; Sirevaag and Greenough, 1991). In general, morphological plasticity of astrocytes in response to complex environment housing occurs on a time scale that is comparable to the timeline for neuronal changes that occur in this paradigm (Jones and Greenough, 1996; Jones et al., 1996; Sirevaag and Greenough, 1985). In contrast to neuronal changes, however, learning-induced astrocytic hypertrophy appears to be more transient (Kleim et al., 2007).
Evidence also suggests that oligodendrocytes are responsive to experience. In the visual cortex, increases in the density and volume fraction of oligodendrocyte nuclei have been observed following developmental rearing in a complex environment (Sirevaag and Greenough, 1987; Szeligo and Leblond, 1977). The influence of developmental experience on oligodendrocytes is not limited to the visual cortex; Juraska and Kopcik (1988) found that raising rats in a complex environment increased the number of myelinated axons in the splenium (the portion of the corpus callosum that contains visual cortical axons (Kim et al., 1996)). A similar effect has also been demonstrated in rhesus monkeys—when raised in a complex environment, they have larger corpora callosa (Sanchez et al., 1998). Finally, in humans, extensive piano practicing beginning during childhood increases fractional anisotropy, a measure thought to be correlated with degree of myelination (Larvaron et al., 2006), in cerebral white matter (Bengtsson et al., 2005).
Despite studies indicating that myelination continues well into adulthood in both rodents and humans (Benes et al., 1994; Nunez et al., 2000; Yakovlev, 1967; Yates and Juraska, 2007), thequestion of whether myelination remains sensitive to experience during adulthood has remained largely unexplored. The conversion of previously unmyelinated axons to the myelinated fiber pool could be a form of plasticity with a potential comparable to the addition or strengthening of synapses, with speed rather than efficacy of communication being enhanced in this case: myelinated axons conduct action potentials at velocities that are ~50–100 times faster than unmyelinated axons (Brinley, 1980).
The present experiment was designed to determine whether myelination is responsive to experience in adulthood, using the complex housing environment paradigm (see Fig. 1 for experimental design). Animals were housed in small groups until adulthood, at which time half of the animals were moved into a complex environment (“enriched condition” or EC) and half were moved into a standard cage and housed alone (“isolated condition” or IC). After one month, half of the animals switched housing conditions, while the other half remained in their original condition for a second month, thus creating four groups of animals: IC/IC, IC/EC, EC/IC, and EC/EC (see experimental procedures for details). Comparison of IC/EC and EC/EC groups (i.e., one versus two months of EC housing) was designed to indicate whether any identified effect of adult housing on myelination was additive, and comparison of EC/IC and IC/EC groups would indicate how well an effect of complex environment housing persisted in the absence of continued exposure to the environment. Both males and females in the various conditions were examined because sex differences occur in callosal myelination (Kim et al., 1996; Kim and Juraska, 1997; Mack et al., 1995) and can vary with rearing environment (Juraska and Kopcik, 1988).
Fig. 1.
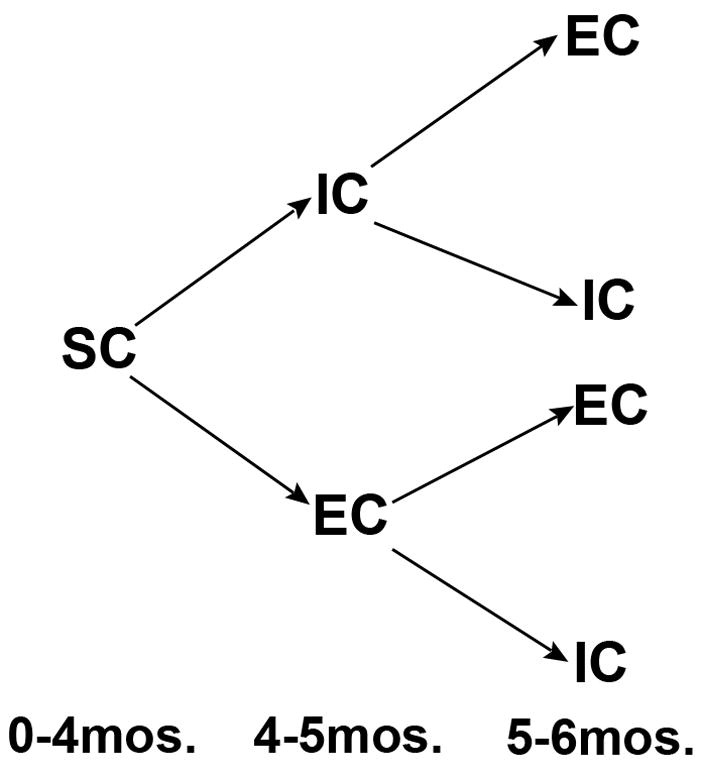
Experimental design. Animals were housed in small groups (“social condition” or SC) until age 4 months (adulthood), at which time half of the animals were moved into a complex environment (“enriched condition” or EC) and half were moved into a standard cage and housed alone (“isolated condition” or IC). After one month, half of the animals switched housing conditions, while the other half remained in their original condition. All animals were sacrificed at age 6 months.
2. Results
2.1 Callosal Length
The total anterior to posterior length of the callosum was greater in males than females (F1,49 =14.2, p<.0001; males: 7.25mm +/−0.03mm, females: 7.06mm +/−0.04mm) and was influenced by housing (F3,49=3.2, p<.04) (Fig. 2). The corpus callosum was longer in both groups of animals that were housed in the complex environment for the month prior to sacrifice, compared to animals never housed in EC (IC/IC<IC/EC, p<.02; IC/IC<EC/EC, p<.01). Two months of EC did not increase callosal length beyond that observed following one month of recent EC (EC/EC vs. IC/EC, p=.8). Furthermore, the effect of EC housing on callosal length was temporary, since EC/IC animals had shorter callosa than EC/EC animals (p<.03) and a trend towards shorter callosa than IC/EC animals (p<.06).
Fig. 2.
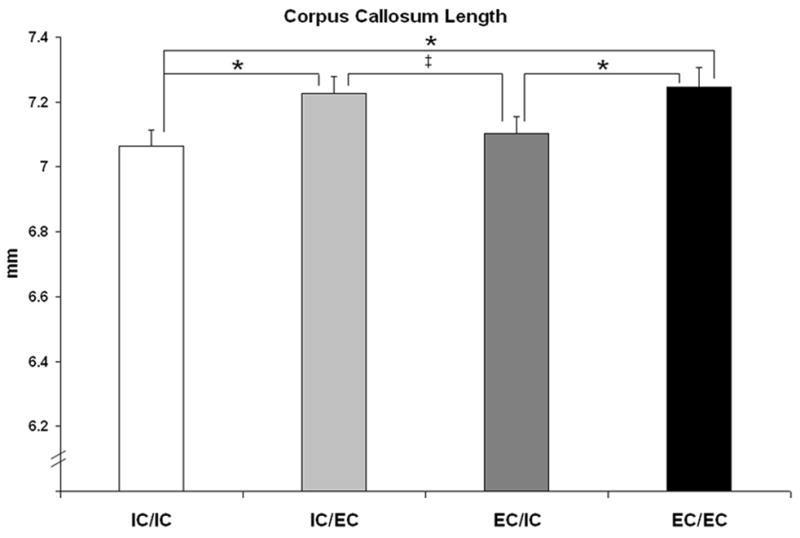
Length of the corpus callosum. Length of the callosum was greater in males than females (data not shown; see text for details) and was influenced by adult housing condition. Significant post hoc comparisons are shown, *p<.05, ‡p<.06.
2.2 Splenium
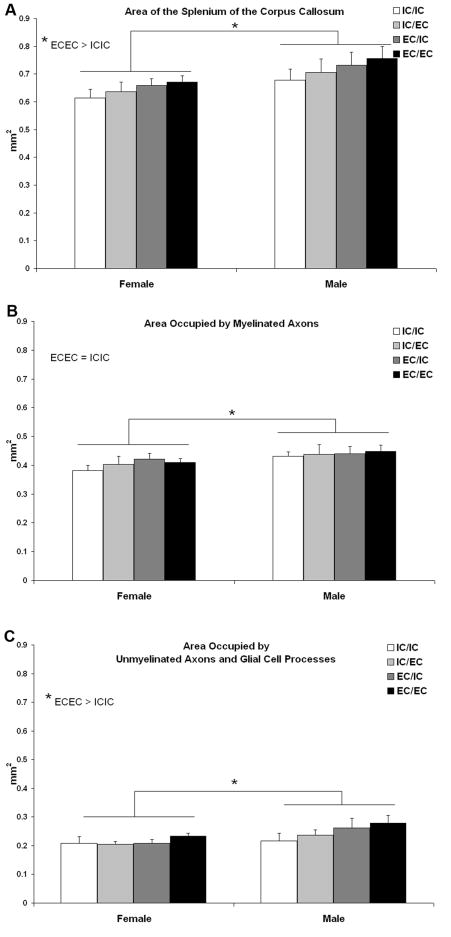
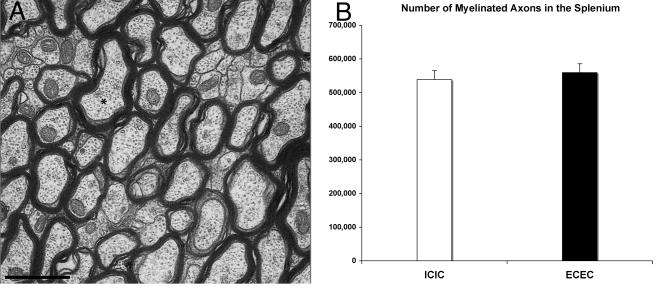
Subcellular composition of the splenium was quantified using the stereological point counting method (Fig. 3). There was no main effect of housing condition on splenial area; however, when a planned post hoc comparison was made of the two extreme groups, the animals who spent two months in a complex environment (EC/EC) during adulthood had approximately 10% larger splenia than animals never housed in a complex environment (IC/IC) (p<.05) (Fig. 4A). Housing did not significantly influence the area fraction or absolute area in mm2 occupied by myelinated axons (myelin area fraction p=.4, area occupied by myelinated axons p=.7), unmyelinated axons and glial cell processes, glial soma, or blood vessels (Table 1). When the EC/EC and IC/IC groups were directly compared, no differences in myelin content were found (p=.3, Fig. 4B), although EC/EC animals had a greater amount of area occupied by glial cell processes and unmyelinated axons (p<.03) (Fig 4C) (Table 1). In order to confirm the results obtained using stereological point counting, an electron microscopic analysis of myelinated axon number was undertaken in a subset of animals. Comparison of the two housing groups with the greatest difference in splenial area (and therefore with the greatest potential for experience-dependent differences in myelination), IC/IC and EC/EC females, confirmed that housing condition did not impact the number of myelinated axons in the splenium (IC/IC=EC/EC, p=.55) (Fig. 5).
Fig. 3.
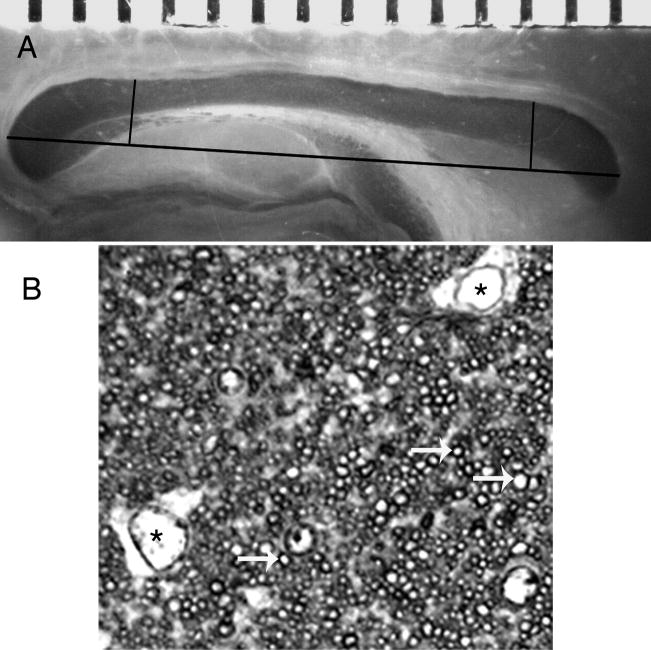
Stereological point counting technique. Length of the corpus callosum is shown on a mid-sagittal section through a corpus callosum stained with osmium tetraoxide (each tic mark on the scale at the top equal to 0.5mm) and the boundaries for the regions sampled (genu on the right, splenium on the left) are indicated by black lines (A). Toluidine blue stained section through the corpus callosum (B). The arrows (white) denote myelinated axons and the * indicate the location of two glial cell bodies. The area occupied by other material (unmyelinated axons and glial processes) is the amorphous, interstitial space surrounding the myelinated axons. Scale bar = 10 μm.
Fig. 4.
Splenium of the corpus callosum. EC/EC animals had larger splenia than IC/IC animals (A), an effect which was due to the increased area occupied by glial processes and unmyelinated axons (C) rather than myelinated axons (B). All three measures were greater in males compared to females.
Table 1.
Splenium of the corpus callosum.
| Area Fraction | Myelinated Axons | Unmyelin. Axons, Glial Processes | Glial Soma | Blood Vessels | |
|---|---|---|---|---|---|
| ♂ | IC/IC | .64 +/−0.02 | .31 +/−0.02 | .023 +/−0.002 | .024 +/−0.004 |
| IC/EC | .62 +/−0.02 | .33 +/−0.02 | .024 +/−0.001 | .020 +/−0.004 | |
| EC/IC | .60 +/−0.02 | .35 +/−0.03 | .021 +/−0.002 | .022 +/−0.005 | |
| EC/EC | .60 +/−0.02 | .37 +/−0.02 | .022 +/−0.002 | .016 +/−0.003 | |
| ♀ | IC/IC | .63 +/−0.02 | .34 +/−0.03 | .023 +/−0.002 | .017 +/−0.004 |
| IC/EC | .63 +/−0.02 | .32 +/−0.01 | .027 +/−0.004 | .020 +/−0.004 | |
| EC/IC | .64 +/−0.02 | .32 +/−0.02 | .026 +/−0.002 | .020 +/−0.004 | |
| EC/EC | .61 +/−0.01 | .35 +/−0.004 | .024 +/−0.003 | .018 +/−0.004 | |
| Area (mm2) | Myelinated Axons* | Unmyelin. Axons, Glial Processes*† | Glial Soma | Blood Vessels | |
| ♂ | IC/IC | .43 +/−0.02 | .22 +/−0.03 | .015 +/−0.001 | .016 +/−0.002 |
| IC/EC | .44 +/−0.03 | .24 +/−0.02 | .017 +/−0.002 | .014 +/−0.004 | |
| EC/IC | .44 +/−0.02 | .26 +/−0.03 | .015 +/−0.001 | .015 +/−0.003 | |
| EC/EC | .45 +/−0.02 | .28 +/−0.03 | .016 +/−0.002 | .012 +/−0.003 | |
| ♀ | IC/IC | .38 +/−0.02 | .21 +/−0.02 | .014 +/−0.001 | .010 +/−0.002 |
| IC/EC | .40 +/−0.03 | .20 +/−0.01 | .017 +/−0.002 | .012 +/−0.002 | |
| EC/IC | .42 +/−0.02 | .21 +/−0.01 | .017 +/−0.001 | .013 +/−0.002 | |
| EC/EC | .41 +/−0.01 | .23 +/−0.01 | .016 +/−0.002 | .012 +/−0.003 | |
Area fraction and area occupied (in mm2) by cellular components in the genu of the corpus callosum were unaffected by adult housing experience. Group means +/-standard error of the mean are shown.
indicates male > female.
indicates EC/EC > IC/IC. See text for details.
Fig. 5.
Electron micrograph taken in the splenium of the corpus callosum (A). *indicates myelinated axon. Scale bar = 1 μm. Total number of myelinated axons in the splenium did not differ between females housed for two months in a complex environment and those housed alone (B).
Splenial area was greater in males compared to females (F1,49 =8.2, p<.01), an effect that did not interact with housing condition (Fig. 4A). This size difference was primarily due to the greater myelin content of the male splenium (F1,49 =5.0, p<.03) as well as a greater area occupied by unmyelinated axons and glial cell processes (F1,49=5.5, p<.03) (Fig 4B,C). The sexes did not differ in area occupied by glial somata or blood vessels, or in the area fraction of any of the components (Table 1).
2.3 Genu
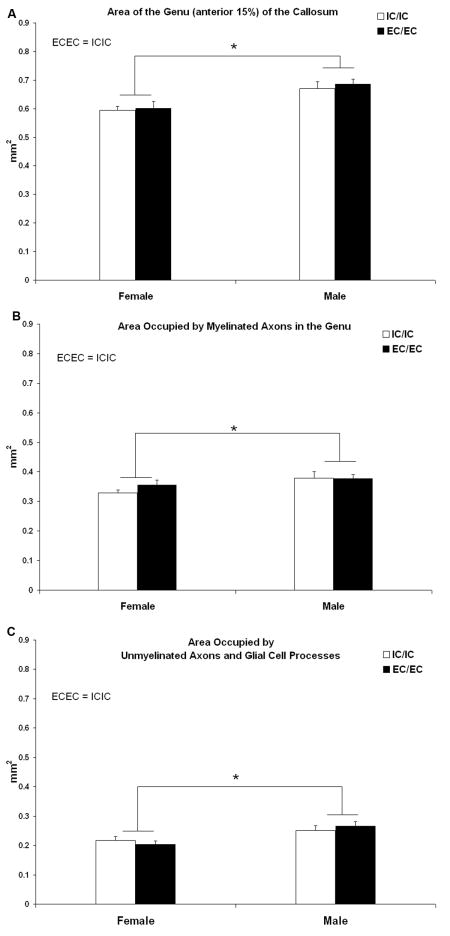
The area of the genu was larger in males than females (F1,24 =26.7, p<.0001), and a correspondingly greater area of the male genu was occupied by myelinated axons (F1,24 =6.5, p<.02) and by glial cell processes and unmyelinated axons (F1,24 =12.7, p<.01) (Fig. 6). The area occupied by glial somata and blood vessels, as well as the area fraction of each of the components, were equivalent between the sexes (Table 2). Housing condition did not influence the size (p=.38) or myelin content (p=.36) of the genu, nor did it influence any other measure (Fig. 6, Table 2).
Fig. 6.
Area of the genu of the corpus callosum. Area of the genu was greater in males than females but was not influenced by adult housing condition.
Table 2.
Genu of the corpus callosum.
| Area Fraction | Myelinated Axons | Unmyelin. Axons, Glial Processes | Glial Soma | Blood Vessels | |
|---|---|---|---|---|---|
| ♂ | IC/IC | .57 +/−0.01 | .37 +/−0.01 | .028 +/−0.003 | .033 +/−0.006 |
| EC/EC | .55 +/−0.02 | .39 +/−0.02 | .026 +/−0.003 | .038 +/−0.008 | |
| ♀ | IC/IC | .57 +/−0.02 | .37 +/−0.02 | .028 +/−0.002 | .030 +/−0.006 |
| EC/EC | .60 +/−0.02 | .34 +/−0.02 | .030 +/−0.002 | .029 +/−0.004 | |
| Area (mm2) | Myelinated Axons* | Unmyelin. Axons, Glial Processes* | Glial Soma | Blood Vessels | |
| ♂ | IC/IC | .38 +/−0.02 | .25 +/−0.01 | .019 +/−0.32 | .022 +/−0.004 |
| EC/EC | .38 +/−0.01 | .27 +/−0.02 | .018 +/−0.25 | .026 +/−0.006 | |
| ♀ | IC/IC | .33 +/−0.01 | .22 +/−0.01 | .016 +/−0.23 | .017 +/−0.004 |
| EC/EC | .36 +/−0.02 | .20 +/−0.02 | .018 +/−0.15 | .017 +/−0.002 | |
Area fraction and area occupied (in mm2) by cellular components in the genu of the corpus callosum were unaffected by adult housing experience. Group means +/-standard error of the mean are shown.
indicates male > female. See text for details.
3. Discussion
Our findings indicate that myelination in the rat corpus callosum is not readily sensitive to manipulations of housing environment during adulthood, in contrast to differences in the degree of myelination that have been observed following developmental rearing in a complex environment (Juraska and Kopcik, 1988). Although the length of the corpus callosum was increased, the area occupied by myelinated axons in both the splenium and the genu was unaltered by two months of adult housing in a complex environment. While the overall size of the splenium increased in response to two months of complex environment housing, this was due to an increase in the area occupied by glial cell processes and unmyelinated axons and not to any statistically significant change in the area occupied by myelinated axons. Estimation of myelinated axon number at the electron microscopic level in the splenia of a subset of animals provided a strong confirmation that complex environment housing did not impact the number of myelinated axons. Neither the size of the genu nor the area occupied by any of the components in this region was responsive to the manipulation of housing during adulthood. Values observed here for six month old animals of both sexes (including size of the genu and splenium as well as the area occupied by myelinated axons) fall in the range that would be expected given values obtained from standard, socially housed rats of the same strain at age four months and age 12–13 months (Yates and Juraska, 2007). Finally, several measures showed robust sex differences, with males having larger callosal regions and more area occupied by myelinated axons than females, as previously reported (Yates and Juraska, 2007).
One interpretation of the greater myelination observed in the splenium of post-weanling animals raised in a complex environment (Juraska and Kopcik, 1988) is that more axons are selected for myelination as a result of this experience because it is a time when myelination is peaking (Kim and Juraska, 1997). Many other developmental processes are still ongoing in the first month post-weaning including apoptosis of cortical neurons, which includes axon elimination (Nunez et al., 2001); (Markham et al., 2007). Because myelination and axon elimination are overlapping processes in postnatal callosal development (Kim and Juraska, 1997), it is possible that complex environment rearing is an experience which rescues cortical neurons, and thus some callosal axons (which go on to be myelinated), from cell death. Such a mechanism would be unavailable to the mature brain.
One methodological difference between the present study and Juraska and Kopcik’s (1988), although relatively minor, deserves mentioning. Whereas in the present study, animals in the isolated condition were handled daily beginning at age 4 months (since the complex environment housed animals were handled daily when toys were changed), the isolated condition animals in Juraska and Kopcik’s study of weanling-aged rats were handled weekly. Handling prior to weaning can result in a measurable, albeit temporary, effect on callosal area (Berrebi et al., 1988), although others have found that daily handling prior to weaning does not impact myelination (Sturrock et al., 1983). While an effect of handling on myelination during adulthood has never been reported, it is possible that more frequent handling of adult animals induces greater callosal myelination and that this effect minimized differences between experimental groups in the present study. This seems improbable, however, given that the values for area occupied by myelinated axons in both the genu and the splenium from animals in this study are comparable to those obtained by Yates and Juraska (2007) for adult animals handled weekly and not housed in a complex environment.
Whereas the area occupied by myelinated axons was stable following manipulations of adult housing environment, the area occupied by glial cell processes and unmyelinated axons in the splenium increased in response to two months of complex environment housing. Astrocytes comprise nearly 30% of all glial cells in the corpus callosum (Mori and Leblond, 1970). Astrocytic hypertrophy in response to complex environment housing has been observed previously in the visual cortex (e.g., Jones and Greenough, 1996; Jones et al., 1996; Sirevaag and Greenough, 1991), the cortical area from which splenial axons originate (Kim et al., 1996). Although the majority of glial cells in the callosum are oligodendrocytes, extension of oligodendrocytic processes seems an unlikely mechanism to account for the observed effect, given that myelin content remained stable across housing conditions. It is, however, possible that the diameter of unmyelinated axons was increased following adult complex environment housing, since animals reared in a complex environment show this effect (Juraska and Kopcik, 1988).
The mid-sagittal length of the corpus callosum was increased following either one or two months of complex environment housing. This effect was observed in both sexes, although the callosum of males was longer than females, an effect which emerges over development but is present by age two months (Kim and Juraska, 1997). The cellular basis for this alteration is unknown; however, it seems likely that factors outside of the callosum are influencing this measure. Specifically, it is known that the overall size of the cortex increases in response to complex environment housing (e.g., Bennett et al., 1964; Diamond et al., 1964). This effect has been observed numerous times in both young and mature animals, and it is primarily due to dendritic and glial process extension (reviewed by Markham and Greenough, 2004). While we did not measure cerebral cortical length in the present study, our interpretation of the lengthening of the callosum in response to complex environment housing observed here is that, because callosal axons are from cortical neurons, the callosum is simply stretching with the cortex in the anterior-posterior direction. Although the relative stability of complex environment-induced synaptic increases (Briones et al., 2004) could present a difficulty with this interpretation, dendritic extension is only one mechanism that contributes to synaptic number (another being synaptic density). At present, therefore, we do not know why some environment-initiated neural changes are more permanent than others.
Sex differences in the size and composition of the rat corpus callosum have been extensively studied and discussed elsewhere (Juraska and Kopcik, 1988; Kim et al., 1996; Kim and Juraska, 1997; Mack et al., 1995; Yates and Juraska, 2007). Essentially, while the sexes do not differ in total number of axons in the splenium, the number of myelinated axons is greater in males (Kim and Juraska, 1997). The larger size of myelinated axons (0.35 μm diameter (excluding the myelin sheath), as compared to 0.16 μm diameter of unmyelinated axons (Kim and Juraska, 1997)) results in a larger splenial size in males. Sex differences in myelination could be the result of a sex difference in oligodendrocyte turnover, which has been observed by others (Cerghet et al., 2006), as well as the effects of ovarian hormones (Yates and Juraska, 2008). The present study was primarily interested, however, not in sex differences per se, but in potential sex differences in plasticity of myelination following complex environment housing during adulthood. This was not the observed pattern of results; in fact, there were no interactions between sex and housing factors for any measure.
In summary, our results indicate that previously unmyelinated axons in the corpus callosum are not myelinated in response to complex environment housing during adulthood, in contrast to what has been demonstrated previously in younger animals. These results are in line with those showing correlations between childhood piano practicing and reductions in fractional anisotropy in cerebral white matter, which also suggest reduced malleability of white matter in adulthood (Bengtsson et al., 2005). Adult animals are able to flexibly respond to their environment in many ways, but altering the number of myelinated axons after the period of developmental organization of connections has been established does not appear to be a mechanism for adult experience-dependent plasticity. In contrast, both complex environment housing and training in learning paradigms during adulthood induce robust changes in other components of the nervous system, such as synaptogenesis, dendritic extension, astrocytic hypertrophy, and increased astrocytic ensheathement of synapses (reviewed by Markham and Greenough, 2004).
4. Experimental Procedure
4. 1 Subjects
Male (n=30) and female (n=27) Long-Evans hooded rats were born in-house to breeders ordered from Simonsen (Gilroy, CA). At the time of weaning (day 25), pups were marked with a distinctive ear punch pattern and socially housed in groups of two or three same-sex litter mates until four mos. of age, in standard laboratory cages. All animals were handled weekly from the time of weaning until age four months. Within a given litter, pups were randomly assigned to one of four housing conditions (see below). Animals in all conditions were maintained on a 12 hour light/dark cycle and allowed free access to food and water. Experimental procedures were approved by the University of Illinois IACUC.
4. 2 Complex Environment Housing (Fig. 1)
At four months of age, half of the animals were moved into a complex environment condition (EC; males n=15, females n=14) while the other half were moved to single housing (IC, inactive or isolated condition; males n=15, females n=13). Rats in the complex environment were same-sex group housed in a large wire mesh cage (1 cubic meter) filled with toys and other objects. Approximately one third of the toys were exchanged each day for new toys and all of the toys were rearranged daily; during this time each day, the EC animals explored a novel arrangement of toys and objects in an open field (1.2 cubic meters) for approximately 30 minutes. Rats in the isolated condition were housed in the same room as the EC rats but individually in a standard laboratory cage (50 × 27 × 36 cm) with minimal opportunity for learning or exercise. To control for handling effects, IC animals were briefly handled each day while the EC rats were in the open field. At five months of age (after one month in either EC or IC), half of the animals switched housing condition while the other half remained in the same condition for the final month, until the time of sacrifice. This resulted in four housing condition groups: animals housed for two months in isolated condition (IC/IC: males n=7, females n=7), animals housed for one month in the isolated condition followed by one month in complex environment (IC/EC: males n=8, females n=6), animals housed for one month in a complex environment followed by one month in the isolated condition (EC/IC: males n=7, females n=8), and animals housed for two months in the complex environment (EC/EC: males n=8, females n=6).
4. 3 Histology
At six months of age, animals were deeply anesthetized with a lethal dose of sodium pentobarbital and were perfused intracardially with Ringer’s solution followed by a fixative solution of 1% gluteraldehyde and 4% paraformaldehyde in 0.1M phosphate buffer. Brains were removed and post-fixed overnight in the fixative solution. The following day, brains were weighed and blocks containing the genu and splenium of the corpus callosum were dissected from the right hemisphere. Tissue blocks were first stained with 2% osmium tetroxide and then dehydrated in successively increasing concentrations of acetone prior to embedding in Eponate 12 resin (Ted Pella, Redding, CA). Seven days after perfusion, a 1mm thick section showing the mid-sagittal callosum was stained en bloc with 2% osmium tetroxide and a digital image was taken of this section next to a calibration ruler using a dissecting microscope (Zeiss SV11 stereomicroscope). This photo was used to determine the total length of the corpus callosum. Boundaries for the genu (anterior 15% (Mack et al., 1995)) and splenium (posterior 20%, which carries 100% of visual cortical axons (Kim et al., 1996)) of the callosum were determined for each animal using this photo.
One micron thick sections were taken in the sagittal plane from embedded blocks using an ultramicrotome (Leica Ultracut UCT), mounted on gelatin-coated slides, stained with Toluidine blue, and then coverslipped. An outline of the callosal region of interest (genu or splenium) was traced at 125X using a camera lucida connected to a microscope (Olympus BH2). Regional areas were obtained from the scanned drawings using ImageJ software (version 1.33u).
Callosal composition was quantified by an investigator who was blind to group identification, using the stereological point counting technique, in which area fraction of each component is multiplied by the area of the region of interest to obtain the area occupied by each component in mm2 (e.g., Weibel, 1979; Yates and Juraska, 2007). The area fraction is simply the proportion of points determined to rest over a particular component. The area fraction of myelinated axons, glial cell bodies, blood vessels, and other material (glial cell processes and unmyelinated axons, which cannot be resolved using the light microscope) was quantified under oil immersion at 1250X while simultaneously viewing a counting grid with 110 intersections (“points”) through a camera lucida. There were 12 sample sites in each of the two regions (for a total of 1320 points per animal per region).
4. 4. Electron Microscopy
To confirm the results obtained using the stereological point counting technique, myelinated axon composition of the splenium was evaluated in a subset of animals using quantitative electron microscopy. To maximize the potential for detecting any housing-related change in this measure, comparison was made between IC/IC (n=6) and EC/EC (n=6) females, as the difference in splenial area was greatest between these two groups (there were seven animals per group initially, but two animals were excluded due to difficulties that arose during sectioning). The experimenter was blind to treatment condition until data collection was complete. Silver/gold ultrathin sections (60–90 nm) were cut from embedded tissue blocks containing the splenium, using an ultramicrotome (Leica Ultracut UCT), and mounted on formvar coated grids, which were then stained with uranyl acetate and lead citrate and examined using a Philips CM200 transmission electron microscope using a sampling method we have previously described (Kim et al., 1996). Sampling was restricted to the posterior fifth of the corpus callosum, which was divided into six equally spaced columns. Between eight and fifteen photographs (taken using a Peltier-cooled Tietz 2k × 2k CCD camera mounted below the viewing chamber) were taken per column (depending on the column height) at a magnification of 6600X, such that the entire dorsal-to-ventral extent of the corpus callosum was extensively sampled with a total of 66 micrographs per animal. The number of myelinated axons was counted on each digital micrograph (the area of which was 27 μm2; total area sampled was thus 1782 μm2) using Image J software (version 1.38X) and used to determine myelinated axon density. Appropriate inclusion and exclusion lines were applied as part of the unbiased stereological estimation of myelinated axon density. Myelinated axon number was calculated by determining the product of axon density and splenial area.
4. 5 Statistical Analysis
All statistical analyses were conducted using the SPSS statistical package (version 14.0). For measurements of callosal length, splenial area, area fractions, and area occupied by cellular components (in mm2) in the splenium, a 2-way analysis of variance (ANOVA) was conducted for the effects of sex (male, female) and housing condition (IC/IC, IC/EC, EC/IC, EC/EC). Post hoc analyses, including planned pairwise comparisons of IC/IC and EC/EC groups, were conducted using the least significant differences method. Measurements in the genu (genu area, area fractions, and area occupied by cellular components (in mm2)) were collected only in IC/IC and EC/EC groups. For comparisons of axon density and number of myelinated axons in the splenium between IC/IC and EC/EC females (obtained using quantitative electron microscopy), one-way ANOVAs for the effect of treatment were conducted. For all analyses, p<.05 was considered significant.
Acknowledgments
The authors wish to thank Donghui Wei, Karlena Alonso, Melissa Yates, Tiffany Li, Nirali Shah, and the Beckman Institute Information Technology Group, especially Scott Robinson, for assistance with various aspects of data collection. This work was supported by NIMH 35321,AG10154 and AG022499. J.M. supported by NICHHD07333.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
Literature References
- Anderson BJ, Li X, Alcantara AA, Isaacs KR, Black JE, Greenough WT. Glial hypertrophy is associated with synaptogenesis following motor-skill learning, but not with angiogenesis following exercise. Glia. 1994;11:73–80. doi: 10.1002/glia.440110110. [DOI] [PubMed] [Google Scholar]
- Benes FM, Turtle M, Khan Y, Farol P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Arch Gen Psychiatry. 1994;51:477–84. doi: 10.1001/archpsyc.1994.03950060041004. [DOI] [PubMed] [Google Scholar]
- Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullen F. Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci. 2005;8:1148–50. doi: 10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- Bennett EL, Diamond MC, Krech D, Rosenzweig MR. Chemical and Anatomical Plasticity Brain. Science. 1964;146:610–9. doi: 10.1126/science.146.3644.610. [DOI] [PubMed] [Google Scholar]
- Berrebi AS, Fitch RH, Ralphe DL, Denenberg JO, Friedrich VL, Jr, Denenberg VH. Corpus callosum: region-specific effects of sex, early experience and age. Brain Res. 1988;438:216–24. doi: 10.1016/0006-8993(88)91340-6. [DOI] [PubMed] [Google Scholar]
- Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc Natl Acad Sci U S A. 1990;87:5568–72. doi: 10.1073/pnas.87.14.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinley FJ. Excitation and conduction in nerve fibers. In: Mountcastle VB, editor. Medical Physiology. Vol. 1. The C.V. Mosby Co; St. Louis: 1980. pp. 46–81. [Google Scholar]
- Briones TL, Klintsova AY, Greenough WT. Stability of synaptic plasticity in the adult rat visual cortex induced by complex environment exposure. Brain Res. 2004;1018:130–5. doi: 10.1016/j.brainres.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Cerghet M, Skoff RP, Bessert D, Zhang Z, Mullins C, Ghandour MS. Proliferation and death of oligodendrocytes and myelin proteins are differentially regulated in male and female rodents. J Neurosci. 2006;26:1439–47. doi: 10.1523/JNEUROSCI.2219-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond MC, Krech D, Rosenzweig MR. The Effects of an Enriched Environment on the Histology of the Rat Cerebral Cortex. Journal of Comparative Neurology. 1964;123:111–20. doi: 10.1002/cne.901230110. [DOI] [PubMed] [Google Scholar]
- Forgays DG, Forgays JW. The nature of the effect of free-environmental experience in the rat. Journal of Comparative and Physiological Psychology. 1952;45:322–8. doi: 10.1037/h0053731. [DOI] [PubMed] [Google Scholar]
- Greenough WT, Volkmar FR. Pattern of dendritic branching in occipital cortex of rats reared in complex environments. Exp Neurol. 1973;40:491–504. doi: 10.1016/0014-4886(73)90090-3. [DOI] [PubMed] [Google Scholar]
- Greenough WT, Juraska JM, Volkmar FR. Maze training effects on dendritic branching in occipital cortex of adult rats. Behav Neural Biol. 1979;26:287–97. doi: 10.1016/s0163-1047(79)91278-0. [DOI] [PubMed] [Google Scholar]
- Greenough WT, Larson JR, Withers GS. Effects of unilateral and bilateral training in a reaching task on dendritic branching of neurons in the rat motor-sensory forelimb cortex. Behav Neural Biol. 1985;44:301–14. doi: 10.1016/s0163-1047(85)90310-3. [DOI] [PubMed] [Google Scholar]
- Hebb DO. The Organization of Behavior. Wiley; New York: 1949. [DOI] [PubMed] [Google Scholar]
- Hymovitch B. The effects of experimental variations on problem solving in the rat. J Comp Physiol Psychol. 1952;45:313–21. doi: 10.1037/h0061535. [DOI] [PubMed] [Google Scholar]
- Jones TA, Greenough WT. Ultrastructural evidence for increased contact between astrocytes and synapses in rats reared in a complex environment. Neurobiol Learn Mem. 1996;65:48–56. doi: 10.1006/nlme.1996.0005. [DOI] [PubMed] [Google Scholar]
- Jones TA, Hawrylak N, Greenough WT. Rapid laminar-dependent changes in GFAP immunoreactive astrocytes in the visual cortex of rats reared in a complex environment. Psychoneuroendocrinology. 1996;21:189–201. doi: 10.1016/0306-4530(95)00041-0. [DOI] [PubMed] [Google Scholar]
- Jones TA, Greenough WT. Behavioural experience-dependent plasticity of glial-neuronal interactions. In: Volterra A, Magistretti P, Hayden PG, editors. The Tripartite Synapse: Glia in Synaptic Transmission. Oxford University Press; Oxford: 2002. pp. 248–265. [Google Scholar]
- Juraska JM, Greenough WT, Elliott C, Mack KJ, Berkowitz R. Plasticity in adult rat visual cortex: an examination of several cell populations after differential rearing. Behav Neural Biol. 1980;29:157–67. doi: 10.1016/s0163-1047(80)90482-3. [DOI] [PubMed] [Google Scholar]
- Juraska JM, Kopcik JR. Sex and environmental influences on the size and ultrastructure of the rat corpus callosum. Brain Res. 1988;450:1–8. doi: 10.1016/0006-8993(88)91538-7. [DOI] [PubMed] [Google Scholar]
- Kim JH, Ellman A, Juraska JM. A re-examination of sex differences in axon density and number in the splenium of the rat corpus callosum. Brain Res. 1996;740:47–56. doi: 10.1016/s0006-8993(96)00637-3. [DOI] [PubMed] [Google Scholar]
- Kim JH, Juraska JM. Sex differences in the development of axon number in the splenium of the rat corpus callosum from postnatal day 15 through 60. Brain Res Dev Brain Res. 1997;102:77–85. doi: 10.1016/s0165-3806(97)00080-1. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Lussnig E, Schwarz ER, Comery TA, Greenough WT. Synaptogenesis and Fos expression in the motor cortex of the adult rat after motor skill learning. J Neurosci. 1996;16:4529–35. doi: 10.1523/JNEUROSCI.16-14-04529.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim JA, Vij K, Ballard DH, Greenough WT. Learning-dependent synaptic modifications in the cerebellar cortex of the adult rat persist for at least four weeks. J Neurosci. 1997;17:717–21. doi: 10.1523/JNEUROSCI.17-02-00717.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim JA, Markham JA, Vij K, Freese JL, Ballard DH, Greenough WT. Motor learning induces astrocytic hypertrophy in the cerebellar cortex. Behav Brain Res. 2007;178:244–9. doi: 10.1016/j.bbr.2006.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larvaron P, Boespflug-Tanguy O, Renou JP, Bonny JM. In vivo analysis of the post-natal development of normal mouse brain by DTI. NMR Biomed. 2006 doi: 10.1002/nbm.1082. [DOI] [PubMed] [Google Scholar]
- Mack CM, Boehm GW, Berrebi AS, Denenberg VH. Sex differences in the distribution of axon types within the genu of the rat corpus callosum. Brain Res. 1995;697:152–60. doi: 10.1016/0006-8993(95)00804-y. [DOI] [PubMed] [Google Scholar]
- Markham JA, Greenough WT. Experience-driven brain plasticity: beyond the synapse. Neuron Glia Biol. 2004;1:351–363. doi: 10.1017/s1740925x05000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JA, Morris JR, Juraska JM. Neuron number decreases in the rat ventral, but not dorsal, medial prefrontal cortex between adolescence and adulthood. Neuroscience. 2007;144:961–8. doi: 10.1016/j.neuroscience.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Mori S, Leblond CP. Electron microscopic identification of three classes of oligodendrocytes and a preliminary study of their proliferative activity in the corpus callosum of young rats. J Comp Neurol. 1970;139:1–28. doi: 10.1002/cne.901390102. [DOI] [PubMed] [Google Scholar]
- Nunez JL, Nelson J, Pych JC, Kim JH, Juraska JM. Myelination in the splenium of the corpus callosum in adult male and female rats. Brain Res Dev Brain Res. 2000;120:87–90. doi: 10.1016/s0165-3806(99)00193-5. [DOI] [PubMed] [Google Scholar]
- Nunez JL, Lauschke DM, Juraska JM. Cell death in the development of the posterior cortex in male and female rats. J Comp Neurol. 2001;436:32–41. [PubMed] [Google Scholar]
- Sanchez MM, Hearn EF, Do D, Rilling JK, Herndon JG. Differential rearing affects corpus callosum size and cognitive function of rhesus monkeys. Brain Res. 1998;812:38–49. doi: 10.1016/s0006-8993(98)00857-9. [DOI] [PubMed] [Google Scholar]
- Sirevaag AM, Greenough WT. Differential rearing effects on rat visual cortex synapses II. Synaptic morphometry. Brain Res. 1985;351:215–26. doi: 10.1016/0165-3806(85)90193-2. [DOI] [PubMed] [Google Scholar]
- Sirevaag AM, Greenough WT. Differential rearing effects on rat visual cortex synapses III. Neuronal and glial nuclei, boutons, dendrites, and capillaries. Brain Res. 1987;424:320–32. doi: 10.1016/0006-8993(87)91477-6. [DOI] [PubMed] [Google Scholar]
- Sirevaag AM, Greenough WT. Plasticity of GFAP-immunoreactive astrocyte size and number in visual cortex of rats reared in complex environments. Brain Res. 1991;540:273–8. doi: 10.1016/0006-8993(91)90517-y. [DOI] [PubMed] [Google Scholar]
- Sturrock RR, Smart JL, Tricklebank MD. A quantitative neurohistological study of the long term effects in the rat brain of stimulation in infancy. J Anat. 1983;136:129–44. [PMC free article] [PubMed] [Google Scholar]
- Szeligo F, Leblond CP. Response of the three main types of glial cells of cortex and corpus callosum in rats handled during suckling or exposed to enriched, control and impoverished environments following weaning. J Comp Neurol. 1977;172:247–63. doi: 10.1002/cne.901720205. [DOI] [PubMed] [Google Scholar]
- Turner AM, Greenough WT. Synapses per neuron and synaptic dimensions in occipital cortex of rats reared in complex, social, or isolation housing. Acta Stereol. 1983;2(Suppl I):239–244. [Google Scholar]
- Turner AM, Greenough WT. Differential rearing effects on rat visual cortex synapses I. Synaptic and neuronal density and synapses per neuron. Brain Research. 1985;329:195–203. doi: 10.1016/0006-8993(85)90525-6. [DOI] [PubMed] [Google Scholar]
- Uylings HB, Kuypers K, Diamond MC, Veltman WA. Effects of differential environments on plasticity of dendrites of cortical pyramidal neurons in adult rats. Exp Neurol. 1978;62:658–77. doi: 10.1016/0014-4886(78)90276-5. [DOI] [PubMed] [Google Scholar]
- Weibel ER. Stereological Methods for Biological Morphometry. Vol. 1. Academic Press; London: 1979. [Google Scholar]
- Yakovlev PaLA. The myelinogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Retional development of the brain early in life. Blackwell Scientific Publications, Inc; Boston: 1967. pp. 3–70. [Google Scholar]
- Yates MA, Juraska JM. Increases in size and myelination of the rat corpus callosum during adulthood are maintained into old age. Brain Res. 2007;1142:13–8. doi: 10.1016/j.brainres.2007.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates MA, Juraska JM. Pubertal ovarian hormone exposure reduces the number of myelinated axons in the splenium of the rat corpus callosum. Exp Neurol. 2008;209:284–7. doi: 10.1016/j.expneurol.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]