Abstract
Background
Fanconi anemia (FA) is an autosomal and X-linked recessive disease of chromosomal instability which results in bone marrow failure. Children with FA have been shown to have an increased risk of diabetes mellitus (DM).
Procedure
A cross-sectional study of glucose and insulin metabolism was conducted in 17 children with FA who had undergone hematopoietic cell transplantation (HCT) at the University of Minnesota. First phase insulin release (FPIR) was determined by intravenous glucose tolerance test (IVGTT). Oral glucose tolerance test (OGTT), lipid panel, blood pressure, and medical history were reviewed for additional metabolic abnormalities.
Results
Seventeen FA participants, median age 11.3 (range 5.5–17.6) years, were evaluated. IVGTT identified three separate groups: low FPIR, normal FPIR, and high FPIR. Those with low FPIR were more likely to have low BMI, but had normal glucose levels. Those with high FPIR, had high BMI, elevated lipids and body fat. One patient with normal FPIR had impaired glucose tolerance and another with normal FPIR has impaired fasting glucose. No participant was diagnosed with DM by fasting glucose, 2 hour glucose during OGTT, or hemoglobin A1c.
Conclusions
The majority of children with FA had normal glucose tolerance and normal beta-cell function after HCT. Two small subsets of patients had lower than expected and higher than expected FPIR. The clinical significance of these differences is not yet known given the normal glucose tolerance and fasting glucose levels in these two groups.
Keywords: Fanconi anemia, bone marrow transplantation, insulin resistance, insulin secretion, glucose intolerance, IVGTT, OGTT
Introduction
Fanconi anemia (FA) is a genetically and phenotypically heterogenous disorder characterized by chromosomal instability which results in bone marrow failure and an increased risk of malignancy. Congenital abnormalities of the heart, kidneys, extremities (e.g. radial aplasia), ears, eyes, head, and genitals are common [1]. Most children with FA are eventually treated with hematopoietic cell transplantation (HCT). With improved outcomes after HCT, more children with FA are living into adulthood. Consequently, long-term health implications of both FA and HCT, such as diabetes, have become important to understand.
In the general population, HCT itself has been associated with a long-term increased risk of DM [2–8]. However, patients with FA have a high likelihood of developing DM even prior to HCT [9–11]. In a study by Wajnrajch et al. 25% of the children with FA had impaired glucose tolerance by oral glucose tolerance testing (OGTT) [11]. The majority of these patients (94%) had not received HCT, arguing for a glucose/insulin defect that is intrinsic to FA. In addition, a recent study by Elder et al. found that 46% of children with FA before HCT had impaired glucose tolerance (IGT) or DM by OGTT [9], also suggesting an innate defect to FA. Giri et al. [12] found 4% (1/24) of children studied with FA had diabetes by OGTT; it is not stated whether any of these children were treated with HCT.
Both insulin resistance and impaired insulin secretion have been implicated in the development of DM in children with FA [9,11,12]. Giri etal. [12] reported that 17% (4/24) of their group of FA children had insulin resistance diagnosed by the homeostasis model assessment of insulin resistance (HOMA-IR). In the study by Wajnrajch et al. a subgroup of patients with complementation group FA-C had significantly lower peak OGTT insulin levels even though their baseline insulin levels were elevated suggesting both insulin resistance and diminished insulin secretory capacity [11]. Elder et al. found that 51% of patients with FA had insulin secretion levels (determined by insulinogenic index) lower than their non-FA control population [9]. They suggested that the relatively low insulin is pathologic and contributes to impaired weight gain and growth.
The OGTT gives only a crude estimate of β-cell secretory function. In order to more accurately assess insulin secretion [13,14] and glucose tolerance post-HCT, we performed the intravenous glucose tolerance test (IVGTT) and an OGTT. IVGTT testing has not been previously reported in patients with FA. Metabolic parameters including obesity, hypertension, and hyperlipidemia were assessed as well.
Patients and Methods
Patients
From March 2005 to May 2007, all children with FA (<18 years), who had received treatment with HCT at the University of Minnesota, were eligible for this study. Seventeen children, 12 males and 5 females, agreed to participate; 8 declined and 1 was excluded due to a technical problem during the IVGTT. This study was approved by the Institutional Review Board (IRB) of the University of Minnesota. Written informed consent was obtained before the study from the parent/guardian and assent from the child between age 8 and 17 years.
IVGTT
The IVGTT was performed according to the ICARUS protocol [15]. After an overnight fast, blood samples were collected through a peripheral intravenous catheter (or central line when available), 10 minutes and 1 minute before 0.5 g/kg (maximum 25 g) 20% dextrose was given intravenously over 3 minutes, then again 1, 3, 5, 7, and 10 minutes after the end of glucose infusion. First phase insulin release (FPIR) was calculated as the sum of the 1 and 3 minute insulin levels minus fasting insulin (FI) [15]. FPIR was compared to data by Lorini et al. evaluating healthy, pediatric, control subjects and non-FA children treated with HCT [5].
Fasting Glucose and Insulin, HOMA β-cell function, and HOMA Insulin Resistance
Plasma glucose analysis was conducted at the University of Minnesota Fairview laboratory by the glucose oxidase assay (Vitros, Rochester, NY). Fasting glucose was categorized as follows per the 2008 American Diabetes Association (ADA) diagnostic criteria [16]: fasting glucose <100 mg/dl (5.6 mmol/l) = normal, fasting glucose 100–125 mg/dl (5.6–6.9 mmol/l) = impaired fasting glucose (IFG), and fasting glucose ≥126 mg/dl (7.0 mmol/l) = diabetes.
Insulin analysis was conducted in the University of Minnesota Fairview laboratory by a chemiluminescence solid phase immunometric assay (Immulite and Immulite 2000, Diagnostic Products, Corp, Los Angeles, CA). The homeostasis model assessment of insulin resistance index (HOMA-IR) was calculated as the product of fasting insulin (μU/mL) and fasting glucose (mmol/l) divided by 22.5 [17] and used as a measure of insulin resistance [18]. HOMA β-cell function was determined by the following equation: (20 × fasting insulin (μU/mL)) divided by (fasting glucose (mmol/l) – 3.5) [17,18].
OGTT
Twelve of the 17 patients had an OGTT done within 1 year of the IVGTT. OGTT was performed after an overnight fast. Blood was sampled through peripheral intravenous catheter or central line if available at time 0, and 30, 60, 90, and 120 minutes after 1.75 g/kg (maximum 75 g) of glucose was administered as a 20% oral solution. Plasma glucose and insulin levels were determined. Patients were classified as having normal glucose tolerance (2 hour plasma glucose <140 mg/dl (7.8 mmol/l)), impaired glucose tolerance (2 hour plasma glucose 140–199 mg/dl (7.8–11.1 mmol/l)), or diabetes (2 hour plasma glucose ≥200 mg/dl (11.1 mmol/l)) per the ADA diagnostic criteria [16].
Metabolic assessments
A serum fasting lipid panel was obtained during routine clinical practice within 1 year of IVGTT in 13 patients. Lipid panels were conducted at the University of Minnesota Fairview laboratory by colorimetric reflectance spectrophotometry (cholesterol oxidase) for total cholesterol (CHL), triglycerides (TG), and high density lipoprotein (HDL). Low density lipoprotein (LDL) was calculated. A CHL < 200 mg/dl, TG < 150 mg/dl (1.7 mmol/l), HDL > 40 mg/dl (1.0 mmol/l), and LDL < 130 mg/dl were considered normal. Body mass index (BMI) was calculated by weight (kg)/height (m)2; BMI Z-scores are based on the Center for Disease Control (CDC) growth charts. Systolic blood pressure was taken as the average of up to 3 measurements within the 3 months prior to the IVGTT. Percent body fat was determined by dual energy x-ray absorptiometry (DXA) performed at University of Minnesota- Fairview (GE Lunar Prodigy).
Statistical analysis
Data distribution was assessed for skewness. Non-normally distributed data are presented with both median, first and third quartiles (Q1, Q3). Normally distributed data are presented with mean and SD. Non-normally distributed data were analyzed by non-parametric methods (Spearman correlation, Wilcoxon or Kruskal-Wallis tests), and normally distributed data were analyzed by Pearson correlation and Student’s t-test. All associations with a p value <0.05 were considered statistically significant. Statistical analysis was conducted using SAS, version 9.1; graphics were created in Microsoft Excel and R, version 2.8.1.
Results
Patients
Seventeen FA participants, median age 11.3 (range 5.5–17.6) years, were enrolled in this study (Table I). All 17 patients had undergone HCT at the University of Minnesota, between May 2001 and November 2006. The median age at HCT was 9.2 (range 4.7–17.3) years, and time since HCT 1.6 (range 0.2–4.2) years. At the time of IVGTT, 8 children were pubertal (2 Tanner II, 2 Tanner III, 4 Tanner IV), 4 were receiving treatment with steroids – 2 for chronic graft versus host disease (cGVHD), 1 for hemolytic anemia, and 1 for pre-treatment with amphotericin B lipid complex. One patient was being treated with growth hormone (GH). No child had a known diagnosis of diabetes.
Table I.
Characteristics of Study Population.
| Characteristic | All (N=17) | Low FPIR (N=4) | Normal FPIR (N=10) | High FPIR (N=3) | p-value |
|---|---|---|---|---|---|
| Age at IVGTT | 10.8 ± 3.6 | 8.7 ± 2.8 | 11.2 ± 4.2 | 12.1 ± 0.7 | ns |
| Female sex - N (%) | 5 (30) | 1 (25) | 3 (30) | 1 (33) | ns |
| Low BMI (Z-score < -2)–N (%) | 4 (24) | 3 (75) | 0 | 1 (33) | 0.01 |
| Pubertal - N (%) | 8 (47) | 1 (25) | 5 (50) | 2 (67) | ns |
| Steroid treatment at time of IVGTT - N (%) | 4 (24) | 0 | 3 (30) | 1 (33) | ns |
| GH treatment at time of IVGTT - N (%) | 1 (6) | 0 | 1 (10) | 0 | ns |
| Complementation Group† | |||||
| A - N (%) | 11 (65) | 2 (100) | 7 (78) | 2 (67) | ns |
| B - N (%) | 1 (6) | 0 | 1 (10) | 0 | ns |
| D1 - N (%) | 1 (6) | 0 | 1 (10) | 0 | ns |
| G - N (%) | 1 (6) | 0 | 0 | 1 (33) | ns |
| Age at HCT | 9.2 ± 3.7 | 7.6 ± 1.7 | 9.6 ± 4.6 | 9.8 ± 2.5 | ns |
| Time since HCT | 1.6 ± 1.3 | 1.1 ± 1.4 | 1.6 ± 1.2 | 2.3 ±1.8 | ns |
| Donor/source HCT | |||||
| Related marrow | 4 (24) | 1 (25) | 3 (30) | 0 | ns |
| Unrelated marrow - N (%) | 9 (53) | 3 (75) | 4 (40) | 2 (67) | ns |
| Cord - N (%) | 4 (23) | 0 | 3 (30) | 1 (33) | ns |
| aGVHD - N (%) | 3 (18) | 0 | 2 (20) | 1 (33) | ns |
| cGVHD - N (%) | 2 (12) | 0 | 2 (20) | 0 | ns |
| Total body irradiation – N (%) | 12 (71) | 3 (75) | 6 (60) | 3 (100) | ns |
| Fasting insulin – median (Q1, Q3) | 6.5 (4, 9.5) | 3.8 (2.3, 4.8) | 7 (4.5, 8.0) | 21 (10, 22.5) | 0.0002 |
| HOMA-IR – median (Q1, Q3) | 1.5 (0.9, 2.0) | 0.8 (0.5, 1.0) | 1.6 (0.9, 1.9) | 4.7 (2.1, 5.1) | 0.0095 |
| Glucose AUC – median (Q1, Q3)** | 1230 (975,1665) | 1125 (825, 1425) | 1305 (975, 1665) | 1230 (1095, 1920) | ns |
| HOMA β-cell function – median (Q1, Q3) | 91 (57, 130) | 57 (45, 86) | 82 (65, 122) | 265 (152, 279) | 0.0231 |
| Insulin AUC – median (Q1, Q3)** | 1245 (1215, 1800) | 728 (210, 1245) | 1335 (1215, 1335) | 1605 (1230, 8355) | ns |
| Lipids | |||||
| Triglycerides† | 106 ± 64 | 69 ± 32 | 102 ± 70 | 153 ± 56 | ns |
| Cholesterol† | 172 ± 35 | 153 ± 23 | 172 ± 41 | 192 ± 24 | ns |
| Low density lipoprotein (LDL)* | 95 ± 23 | 89 ± 10 | 86 ± 23 | 120 ± 16 | ns |
| High density lipoprotein (HDL)* | 51 ± 17 | 50 ± 19 | 58 ± 16 | 41 ±17 | ns |
| Percent body fat† | 22 ± 7.3 | 17 ± 4a | 21 ± 6a | 32 ± 2b | 0.0079 |
| Elevated systolic blood pressure – N (%) | 5 (29) | 0 | 3 (30) | 2 (67) | ns |
Plus-minus values are means ± SD. Median, first and third quartiles (Q1 and Q3), are presented for skewed variables. P-value for differences between the low, normal, and high groups. FPIR=first phase insulin release; IVGTT=intravenous glucose tolerance test; HCT=hematopoietic cell transplantation; BMI=body mass index; aGVHD=acute graft versus host disease; cGVHD=chronic graft versus host disease; GH=growth hormone; AUC=area under the curve; ns=no significant difference.
Data available for 13 participants;
Data available for 12 participants;
Data available for 11 participants.
Fasting Glucose and Insulin Levels
Fasting glucose was normal in 16 patients, impaired in only one. No child had a fasting glucose level consistent with diabetes. Fasting insulin level was less than 15 μU/ml in 15/17 subjects, which is the upper limit of normal in the laboratory performing the insulin assay. Fasting insulin was positively correlated with age at IVGTT and age at HCT. Those with higher fasting insulin levels had greater adiposity as measured by DXA, and fasting insulin levels were positively associated with TG and LDL levels. Fasting insulin was not significantly associated with gender, pubertal status, BMI, previous history of total body irradiation (TBI), HCT source, GVHD, current steroid or GH treatment, or history of small for gestational age at birth (SGA).
Intravenous glucose tolerance test (IVGTT)
In general, FPIR level was positively correlated with BMI, CHL, TG, and percent body fat, but not with age at IVGTT, age at HCT, time since HCT, TBI, gender, HCT source, GVHD, pubertal status, current steroid or GH treatment, history of SGA, or hypertension. Comparing to FPIR from healthy pediatric controls and non-FA children treated with HCT reported by Lorini et al. [5], our study sample fell into three groups (Figure 1). The majority of FA children (10/17, 59%) had completely normal FPIR. These children had normal BMI Z-scores. Four participants (24%) had lower than expected FPIR- FPIR was significantly lower than both healthy controls and children without FA who were treated with HCT (p<0.001). This group tended to be underweight, with a low BMI for age and gender, compared to other FA patients, and had lower fasting insulin levels (Table I). Three participants (17%) had significantly increased FPIR compared to other children with FA and to both healthy children and non-FA children who were treated with HCT (p<0.001). With the exception of one child with a low BMI who was receiving treatment with glucocorticoids, this group had normal BMI Z-scores, however they had a significantly higher percent body fat than the other two FA groups. In addition, they had higher fasting insulin levels, increased HOMA-IR, and increased HOMA β-cell function compared to the other two FPIR groups (Table I). Both participants with a fasting insulin level above 15 μU/ml were in this group. There were no other significant differences between the three FPIR groups for the prevalence of known or suspected risk factors for abnormal glucose and insulin metabolism such as pubertal status, steroid or GH treatment, or TBI (Table I).
Figure 1.
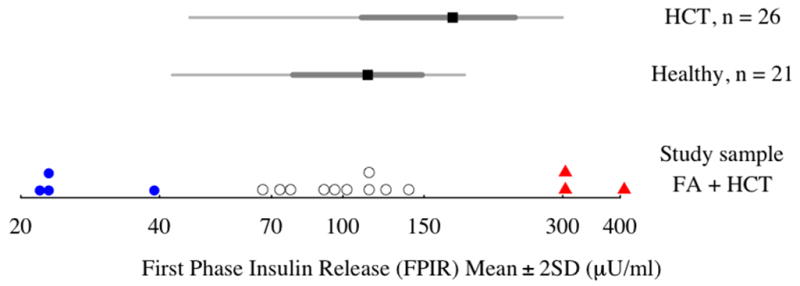
First Phase Insulin Release (FPIR, μU/ml) for the study participants (n = 17) with reference intervals (means ± 2 standard deviations) for FPIR in healthy children (n = 21) and children without FA treated with HCT (n = 26), from Lorrini et al, 1995. Three distinct groups are identified by blue dots (low FPIR), open circles (normal FPIR), and red triangles (high FPIR) in comparison to healthy controls.
HOMA Assessment of β-Cell Function and Insulin Resistance
When analyzed as a continuous variable, FPIR was positively correlated with HOMA β-cell function. Study participants with high FPIR tended to have high HOMA β-cell function, and those with low FPIR had lower HOMA β-cell function (Figure 2). HOMA β-cell function was also positively correlated with CHL, TG, LDL, and percent body fat by DXA, indicating that increased serum lipids and increased body fat were associated with higher levels of insulin secretion during OGTT.
Figure 2.
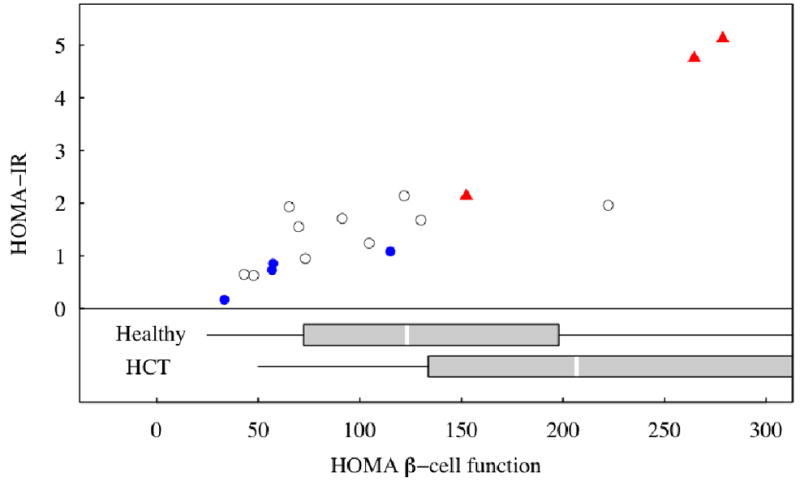
HOMA-IR and HOMA β-cell function in the study sample; those with low FPIR marked by blue dots, high FPIR by red triangles, and normal FPIR by open circles. – Reference intervals (boxplots) are from healthy children (Healthy, n = 98) and children without FA who were treated with HCT (HCT, n = 21), from d’Annunzio, 2006 [21]. The boxplots display the interval from 25th to 75th percentiles with a box, divided by a white line at the median, with lines drawn to the minimum and maximum values. The boxplot for HCT children is truncated below the 75th percentile.
HOMA-IR was positively associated with percent body fat by DXA and with FPIR. Figure 2 shows the relation between HOMA-IR and HOMA β-cell function in FA patients identified by FPIR status. HOMA β-cell function had a strong, positive correlation with HOMA-IR. Study participants with low FPIR had low HOMA-IR, suggestive of increased insulin sensitivity. In contrast, subjects with increased FPIR had higher HOMA-IR suggesting they were more insulin resistant (Figure 2). HOMA-IR differed significantly between these two groups with low and high FPIR (Table I). Neither HOMA-IR nor HOMA β-cell function was significantly associated with age at OGTT, BMI, TBI, gender, HCT source, GVHD, pubertal status, current steroid or GH treatment, or a history of SGA.
Oral glucose tolerance test (OGTT)
OGTT data were available on 12 patients (Figure 3). Glucose tolerance was completely normal in the 4 participants with lower than expected FPIR. No participant had diabetes mellitus by fasting glucose, 2-hour plasma glucose, or hemoglobin A1C (mean 5.2%±0.4, range 4.8–6.1%). One participant had IFG; she had a normal BMI, normal FPIR, was pubertal, had a normal lipid panel and blood pressure, and was not born SGA. One other participant had IGT. She had normal BMI, normal FPIR, was pubertal, had normal lipid panel and blood pressure, but was born SGA. Neither individual received TBI, was currently treated with steroids or GH, or had acute or chronic GVHD.
Figure 3.
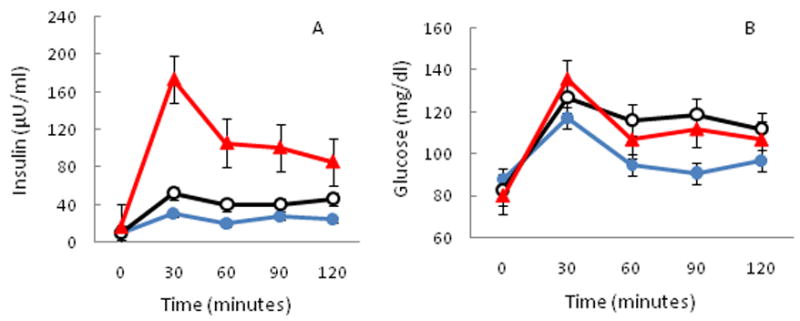
Comparison of OGTT data by low, normal, or high FPIR. Three distinct groups are identified by blue closed circles (low FPIR, n=2), black open circles (normal FPIR, n=7), and red triangles (high FPIR, n=3). Mean±SE insulin (A) and mean±SE glucose (B) data are presented.
Discussion
In contrast to previous reports of significant glucose tolerance abnormalities in children with FA prior to HCT, we found normal glucose tolerance and beta-cell function in the majority of FA children following HCT. FPIR was normal in more than half of the children, elevated in about a quarter, and reduced in about a quarter. Abnormal glucose tolerance was only found in one child and impaired fasting glucose in another child, both of whom had normal FPIR.
Two previous studies have identified low insulin levels during OGTT in children with FA [9,11], and it has been suggested that this is pathologic and may lead to poor weight gain and growth[9]. The current study more accurately assessed beta-cell function using the IVGTT and found 4 out of 17 children with lower than expected FPIR. These children were younger, less likely to have entered puberty, and more sensitive to insulin than those with greater FPIR. Importantly, they had completely normal fasting glucose levels and oral glucose tolerance. Thus it is difficult to ascribe any pathology to their relatively low insulin secretion.
Elevated FPIR was associated with insulin resistance, increased adiposity, and greater abnormalities in lipid levels in our FA participants. Previous studies have found an increased risk of hyperinsulinism and diabetes mellitus in long-term survivors of childhood HCT, even in the presence of normal BMI [2,3,8], and in particular in association with a history of irradiation [2,4–6,8]. Our data is consistent with these findings of hyperinsulinism in the face of normal BMI after HCT. However, there appears to be a synergistic effect of FA and HCT in some patients on insulin resistance, since three of our FA participants had significantly higher FPIR compared to FPIR in children without FA after HCT [5]. This group with elevated FPIR represented a minority of subjects in the current study, and we were unable to find any association with TBI history. Surprisingly, current steroid treatment did not appear to have a significant impact on insulin secretion or glucose metabolism either.
Wajnrajch reported genotype-phenotype correlations between complementation group and hyperinsulinism (FA-G, FA-A) and with insulinopenia (FA-C) [11]. It is intriguing that the mutation for FA-C lies in the same region on chromosome 9 as the gene encoding the protein fructose-1,6-bisphosphatase, which is thought to be associated with Type 2 diabetes mellitus [19]. There were no children with FA-C in this study. The children with lower FPIR were all FA-A, however two did not have mutation analysis performed. Unfortunately, our sample size is too small to determine any statistically significant genotype-phenotype correlations which could account for metabolic differences.
Elder et al. found a prevalence of abnormal glucose metabolism in 46% (n=16) of FA patients who had not been treated with HCT; 34% had impaired glucose tolerance and 11% had diabetes mellitus [9]. After HCT, we found a prevalence of abnormal glucose metabolism of 17% (n=2), and no children had diabetes mellitus. Factors contributing to this lower prevalence may be the fact that our group had lower BMI Z-scores and fewer children who were SGA at birth. However, the difference in prevalence of abnormal glucose metabolism is surprising given that our population was slightly older on average, had received HCT, and some patients were being treated with steroids, whereas this was not the case in patients studied by Elder et al. We do not fully understand the difference in the prevalence of glucose intolerance before and after HCT. It would be premature to speculate that HCT itself might have had a positive impact on glucose metabolism in these patients. To clarify these differences, more prospective studies are needed assessing IVGTT and OGTT before and after HCT.
A major limitation of this pilot study is that we did not study the participants prior to their HCT. Thus, although published data have reported a high frequency of glucose tolerance abnormalities in FA children who have not undergone HCT, we cannot say for certain that the children in the current study had worse FPIR or oral glucose tolerance before their transplant. Also, we were obligated to use control data from the literature for FPIR levels since our IRB would not allow testing on non-FA children who had undergone HCT. We did not perform euglycemic clamp studies, but instead relied upon surrogate markers of insulin resistance.
In conclusion, the majority of children with FA had normal glucose tolerance and normal beta-cell function after HCT. Two small subsets of patients had lower than expected and higher than expected FPIR. The clinical significance of these differences is not yet known given the normal glucose tolerance and fasting glucose levels in these two groups. Future studies are needed to better delineate the impact of HCT on glucose metabolism, and the clinical significance of relative insulinopenia in children with FA who are underweight. We recommend OGTT assessment a minimum of every two years after HCT in children with FA. In addition, screening should be done prior to HCT in view of the high prevalence of abnormal glucose metabolism in FA patients who have not been treated with HCT, and the possible increased risk of infection during HCT reported by Derr et al. [20].
Acknowledgments
We would like to thank Dr. Arleen Auerbach for the complementation analyses as well as the patients and families of all the children who participated in this study. This study was supported by the Fanconi Anemia Research Foundation. Additional support was provided in part by M01-RR00400 National Center for Research Resources, National Institutes of Health through the services of the University of Minnesota General Clinical Research Center.
References
- 1.Nilsson LR. Chronic pancytopenia with multiple congenital abnormalities (Fanconi’s anaemia) Acta Paediatr. 1960;49:518–529. doi: 10.1111/j.1651-2227.1960.tb07767.x. [DOI] [PubMed] [Google Scholar]
- 2.Baker KS, Ness KK, Steinberger J, et al. Diabetes, hypertension, and cardiovascular events in survivors of hematopoietic cell transplantation: a report from the bone marrow transplantation survivor study. Blood. 2007;109(4):1765–1772. doi: 10.1182/blood-2006-05-022335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonanomi S, Gaiero A, Masera N, et al. Distinctive characteristics of diabetes mellitus after hematopoietic cell transplantation during childhood. Pediatr Transplant. 2006;10(4):461–465. doi: 10.1111/j.1399-3046.2006.00498.x. [DOI] [PubMed] [Google Scholar]
- 4.Hoffmeister PA, Storer BE, Sanders JE. Diabetes mellitus in long-term survivors of pediatric hematopoietic cell transplantation. J Pediatr Hematol Oncol. 2004;26(2):81–90. doi: 10.1097/00043426-200402000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Lorini R, Cortona L, Scaramuzza A, et al. Hyperinsulinemia in children and adolescents after bone marrow transplantation. Bone Marrow Transplant. 1995;15(6):873–877. [PubMed] [Google Scholar]
- 6.Neville KA, Cohn RJ, Steinbeck KS, et al. Hyperinsulinemia, impaired glucose tolerance, and diabetes mellitus in survivors of childhood cancer: prevalence and risk factors. J Clin Endocrinol Metab. 2006;91(11):4401–4407. doi: 10.1210/jc.2006-0128. [DOI] [PubMed] [Google Scholar]
- 7.Taskinen M, Saarinen-Pihkala UM, Hovi L, et al. Impaired glucose tolerance and dyslipidaemia as late effects after bone-marrow transplantation in childhood. Lancet. 2000;356(9234):993–997. doi: 10.1016/S0140-6736(00)02717-3. [DOI] [PubMed] [Google Scholar]
- 8.Traggiai C, Stanhope R, Nussey S, et al. Diabetes mellitus after bone marrow transplantation during childhood. Med Pediatr Oncol. 2003;40(2):128–129. doi: 10.1002/mpo.10098. [DOI] [PubMed] [Google Scholar]
- 9.Elder DA, D’Alessio DA, Eyal O, et al. Abnormalities in glucose tolerance are common in children with fanconi anemia and associated with impaired insulin secretion. Pediatr Blood Cancer. 2008;51(2):256–260. doi: 10.1002/pbc.21589. [DOI] [PubMed] [Google Scholar]
- 10.Morrell D, Chase CL, Kupper LL, et al. Diabetes mellitus in ataxia-telangiectasia, Fanconi anemia, xeroderma pigmentosum, common variable immune deficiency, and severe combined immune deficiency families. Diabetes. 1986;35(2):143–147. doi: 10.2337/diab.35.2.143. [DOI] [PubMed] [Google Scholar]
- 11.Wajnrajch MP, Gertner JM, Huma Z, et al. Evaluation of growth and hormonal status in patients referred to the International Fanconi Anemia Registry. Pediatrics. 2001;107(4):744–754. doi: 10.1542/peds.107.4.744. [DOI] [PubMed] [Google Scholar]
- 12.Giri N, Batista DL, Alter BP, et al. Endocrine abnormalities in patients with Fanconi anemia. J Clin Endocrinol Metab. 2007;92(7):2624–2631. doi: 10.1210/jc.2007-0135. [DOI] [PubMed] [Google Scholar]
- 13.Chase HP, Cuthbertson DD, Dolan LM, et al. First-phase insulin release during the intravenous glucose tolerance test as a risk factor for type 1 diabetes. J Pediatr. 2001;138(2):244–249. doi: 10.1067/mpd.2001.111274. [DOI] [PubMed] [Google Scholar]
- 14.Vardi P, Crisa L, Jackson RA. Predictive value of intravenous glucose tolerance test insulin secretion less than or greater than the first percentile in islet cell antibody positive relatives of type 1 (insulin-dependent) diabetic patients. Diabetologia. 1991;34(2):93–102. doi: 10.1007/BF00500379. [DOI] [PubMed] [Google Scholar]
- 15.Bingley PJ, Colman P, Eisenbarth GS, et al. Standardization of IVGTT to predict IDDM. Diabetes Care. 1992;15(10):1313–1316. doi: 10.2337/diacare.15.10.1313. [DOI] [PubMed] [Google Scholar]
- 16.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2008;31 (Suppl 1):S55–60. doi: 10.2337/dc08-S055. [DOI] [PubMed] [Google Scholar]
- 17.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 18.Uwaifo GI, Fallon EM, Chin J, et al. Indices of insulin action, disposal, and secretion derived from fasting samples and clamps in normal glucose-tolerant black and white children. Diabetes Care. 2002;25(11):2081–2087. doi: 10.2337/diacare.25.11.2081. [DOI] [PubMed] [Google Scholar]
- 19.Rothschild CB, Freedman BI, Hodge R, et al. Fructose-1,6-bisphosphatase: genetic and physical mapping to human chromosome 9q22.3 and evaluation in non-insulin-dependent diabetes mellitus. Genomics. 1995;29(1):187–194. doi: 10.1006/geno.1995.1230. [DOI] [PubMed] [Google Scholar]
- 20.Derr RL, Hsiao VC, Saudek CD. Antecedent hyperglycemia is associated with an increased risk of neutropenic infections during bone marrow transplantation. Diabetes Care. 2008;31(10):1972–1977. doi: 10.2337/dc08-0574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.d’Annunzio G, Bonetti F, Locatelli F, et al. Insulin resistance in children and adolescents after bone marrow transplantation for malignancies. Haematologica. 2006;91(12 Suppl) ELT12; author reply ELT13. [PubMed] [Google Scholar]


