Abstract
Interleukin (IL)-10, a prototypical anti-inflammatory cytokine, has been shown to provide beneficial effects in neuronal injury in vivo but the full range of actions has not been established. In order to understand the neuronal mechanisms underlying IL-10 mediated neuroprotection, we examined the effect of IL-10 on primary neurons in culture. We found that IL-10 exerts a direct trophic influence on spinal cord neurons, and that activation of the neuronal IL-10 receptor provides trophic support and survival cues to overcome the neurotoxic effects of glutamate in vitro. IL-10 treatment resulted in activation of Jak-Stat3 and PI3K-AKT pathways in neurons to enhance expression of Bcl-2 and Bcl-xL; under stress conditions IL-10 blocks cytochrome c release and caspase cleavage. IL-10 activation of the canonical NF-κB pathway enhanced translocation of p50 and p65 and enhanced their binding to κB DNA sequences, with p50 playing a more prominent role in neuronal survival. These data indicate that in addition to known anti-inflammatory effects through astroglia in other inflammatory cells, IL-10 has direct neuronal effects with important implications for development and neuroprotection.
Keywords: IL-10, cytokine, neurotrophin, apoptosis, development
Introduction
IL-10 is a prototypical anti-inflammatory cytokine originally identified as a Th2 secreted counter-regulatory factor that inhibits Th1 cell proliferation and cytokine production. IL-10 inhibits the synthesis and release of TNF-α, IL-1β, IL-6, IL-8 and IL-12 pro-inflammatory cytokines and suppresses cellular immunity by regulating the expression of MHC class II and co-stimulatory molecules on antigen presenting cells (Howard et al. 1992; Moore et al. 2001). In the nervous system, IL-10 receptor mRNA and protein have been found in microglia, astrocytes and oligodendrocytes; in those cells IL-10 down-regulates expression and secretion of pro-inflammatory cytokines and growth factors (Frei et al. 1994; Jander et al. 1998). Treatment with IL-10 has been shown to improve outcome in models of ischemic neuronal damage (Dietrich et al. 1999), spinal cord trauma (Bethea et al. 1999) or excitotoxic injury (Brewer et al. 1999). The beneficial effects are due in part to IL-10 mediated down-regulation of the inflammatory response and cytokine production that accompanies tissue injury, an interpretation supported by in vitro studies demonstrating that IL-10 blocks the inhibitory effect of IL-1β on long-term potentiation (Kelly et al. 2001). In addition to its anti-inflammatory effects, IL-10 has been shown to promote cell survival of cells of hematopoietic lineage (Weber-Nordt et al. 1996), survival of retinal ganglion cells challenged by serum deprivation (Boyd et al. 2003), and of cerebellar granule cells exposed to toxic concentrations of glutamate (Bachis et al. 2001).
In non-neuronal cells the effects of IL-10 appear to be mediated by the Stat and NF-κB families of transcription factors (Moore et al. 2001). NF-κB transcription factors function as hetero- or homodimers that bind to κB consensus sequences in nuclear DNA. NF-κB proteins are maintained in the cytoplasm through interaction with constitutively expressed repressors. The most common neuronal species are the p50/p50 homodimer and the p50/p65 heterodimer. The canonical pathway of NF-κB activation involves the engagement of the IκB kinase (IKK) complex (Israel 2000), p50/p65 dimers are released from the binding inhibitor IκBα, allowing p50 and p65 to translocate to the nucleus while the phosphorylated IκBα is ubiquitinated and targeted to the proteasome for degradation (Hoffmann and Baltimore 2006). Within the nucleus NF-κB proteins act as transcriptional regulators to drive expression of gene products involved in physiologic processes including differentiation, neurite extension, dendritic plasticity, and survival responses (Mattson 2005). The profile of gene expression and functional outcome after NF-κB activation depends critically on cell type, the nature of the stimulus, and other coincidental temporal signaling events (Perkins and Gilmore 2006). In the central nervous system, NF-κB activation can support either pro-inflammatory or anti-inflammatory responses, and pro-apoptotic or anti-apoptotic outcomes depending on the cell type involved and the nature of the stimulus (Massa et al. 2006).
Here, we report that IL-10 provides trophic and survival influences directly to spinal cord neurons through the IL-10 receptor localized on the neuronal plasma membrane and that those effects are neuroprotective against excitotoxicity in vitro that are distinct from the anti-inflammatory effects of the cytokine. The activation of the IL-10 receptor leads to signaling through two principal pathways: Jak-Stat3 and PI3K-AKT. Although signaling through both of these pathways can enhance expression of the antiapoptotic proteins Bcl-2 and Bcl-xL, we found that PI3K-AKT activation of the canonical NF-κB pathway mediated the neuroprotective effect of IL-10, and that IL-10-mediated increase in nuclear p50 NF-κB is a key determinant in preventing neuronal apoptosis. In the setting of neuronal injury IL-10 blocked cytochrome c release from mitochondria and caspase 3 cleavage.
Materials and Methods
Cell Culture
Spinal cord from 17-day-old rat embryos were cultured in Neurobasal Medium containing B27, Glutamax I, Albumax I, and penicillin/streptomycin (Gibco-BRL). After 10 days in culture, the cells were transfected with either QHIL10 or QHLacZ at a multiplicity of infection (MOI) of 1 for 2 h. Fresh medium was replaced and collected 48 h later for determination of IL-10 by enzyme-linked immunosorbent assay (ELISA). Spinal cord neurons were also examined for expression of IL-10 protein by immunocytochemistry and Western blot. For MTT assay and LDH assay experiments, spinal cord neurons were treated with recombinant IL-10 (R&D Systems, Minneapolis, MN) for 12 h, and the neurons then exposed to 100 μg/ml glutamate (Sigma, St. Louis, MO) for 3 h. NF-κB inhibitors (Calbiochem, Gibbstown, NJ) were added 12 h prior to MTT assay: SN50, a mimetic of the nuclear localization signal of p50, acts as a competitive inhibitor of p50 nuclear translocation (maximal translocation inhibition at 18 μM in murine endothelial cells) (Lin et al. 1995); 11q inhibitor of NF-κB transcriptional activation (IC50 11 nM in Jurkat cells and 7 nM in splenocytes) (Tobe et al. 2003); JHS-23, a selective inhibitor of nuclear translocation of NF-κB p65 (IC50 = 7.1 μM) (Shin et al. 2004).
Enzyme-Linked ImmunoSorbent Assay
The amount of TNFα and IL-1β released in response to 100 μM glutamate was determined by ELISA (R&D System). Each of the experiments was repeated 4 times.
MTT assay and LDH assay
Cell viability was determined using MTT assay kit (Roche, Penzberg, Germany) according to the manufacture's protocol. Measurement of necrotic cell death was determined by measuring the release of cytosolic lactate dehydrogenase (LDH) after necrosis of cells using LDH assay kit (Takara, Madison, WI) according to the manufacture's recommended protocol. All of the values were calculated from at least 4 independent experiments, with each experiment containing at least 8 replicates under each experimental condition.
NF-κB DNA binding assay
DNA binding activity of NF-κB subunits p50 and p65 from purified nuclear sample obtained from spinal cord neurons were determined using a chemiluminescent based assay (Chemicon, Temecula, CA ). Briefly, 5 μg nuclear extract and NF-κB capture probe were added to the binding reaction mixture for 2 hrs at room temperature, and after washing 100 μl of diluted primary antibody was added to each assay well for 50 minutes, followed by the secondary antibody for 30 minutes after which the chemiluminescent detection reagent was added. This non radioactive method combines the principle of the traditional electromobility shift assay (EMSA) with the 96-well based enzyme-lined immunosorbent assay (ELISA) and rapidly detects activated NF-κB complex binding with a detection limit of < 0.5 μg nuclear extract. TNFα-treated HeLa whole cell extract was used as a positive control. To demonstrate binding specificity an unlabeled specific NF-κB competitor control oligoneucleotide was used as a competitor; a TFA negative probe was used as a negative control. The experiment was repeated 4 times.
Western blot
Proteins from nuclear and cytosolic extracts were separated on 12% SDS-PAGE gels and then transferred onto a polyvinylidene diflouride membrane (Millipore, Medford, MA). Immunoblots were probed with primary antibody to anti-caspase 3 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-cytochrome c (Santa Cruz Biotechnology), anti-Bcl-2 (Santa Cruz Biotechnology), anti-Bcl-xL (Santa Cruz Biotechnology), anti-p65 (Santa Cruz Biotechnology), anti-p50 (Santa Cruz Biotechnology), anti-IL10R (Santa Cruz Biotechnology), anti-HA (Sigma), or anti-β-actin (Sigma), then incubated with HRP-conjugated secondary antibody, followed by enhanced chemiluminescence detection (Amersham Biosciences, Arlington Heights, IL). Chemiluminescence detection values were used to quantitate the Western blot results. A ratio of each band of interest to the appropriate internal control was obtained and the statistical significance of the difference between control and experimental groups determined. Each in vitro experiment was repeated 4 times and each animal experiment represents the results of samples from 5 different animals. Data presented as mean ± SEM.
Semiquantitative RT-PCR analysis
Total RNA was isolated from cells via TRIzol (Invitrogen). cDNA prepared from mRNA isolated from spinal cord neurons or rat spinal cord was amplified using following primer sets: ß-actin-forward (5'-CAG TTC GCC ATG GAT GAC GAT ATC-3') and β-actin-reverse (5'-CAC GCT CGG TCA GGA TCT TCA TG-3') for β-actin, IL-10R1-forward (5’-CAT TCC TCG TCT CGA TCT CCA G-3’) and IL-10R1-reverse (5’-CCA GAT TAG TGC CAA GGC TAT C-3’) for IL-10R1. The levels of Bcl-2 and Bcl-xL were determined by their specific primers: Bcl-2 forward (5’-AAG CCG GGA GAA CAG GGT ATG-3’) and Bcl-2-reverse (5’-ACT TGT GGC CCA GGT ATG CAC-3’) for Bcl-2. Bcl-xL forward (5’-AAT GGA CTG GTT GAG CCC ATC-3’) and Bcl-xL-reverse (5’-CAG TGT CTG GTC ACT TCC GAC-3’). All reactions involved initial denaturation at 94°C for 5 min followed by 28 cycles (94°C for 30 sec, 68°C for 3 min and 1 cycle 68°C for 8 min using a GeneAmp PCR 2700 (Applied Biosystems, Foster City, CA). Each in vitro experiment was repeated 4 times and each animal experiment represents the results of samples from 5 different animals. Data presented as mean ± SEM.
Results
Expression of IL-10 receptor in spinal cord neurons in vitro and in adult spinal cord neurons in vivo
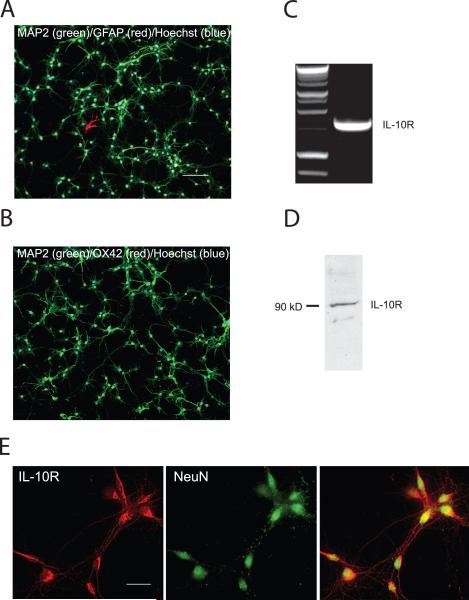
The IL-10 receptor is composed of two subunits R1 and R2. IL-10R1 binds IL-10 with high affinity and is expressed in all IL-10 responsive cells; specific antibodies to R1 block IL-10 effects, supporting the importance of IL-10R1 in mediating the cellular response to IL-10 (Ho et al. 1993; Liu et al. 1994). In order to determine whether IL-10 might have effects on neurons independent of those mediated through microglia and astrocytes, we examined the expression of IL-10R1 subunit mRNA and protein in spinal cord neurons in vitro. E17 rat spinal cord neurons grown in defined medium were studied at DIV 10. These cultures are made up almost exclusively of neurons assessed by NeuN (data not shown) and MAP immunostaining (Fig. 1A,B). There were fewer than 20 GFAP positive astrocytes per well plated with 105 cells and no OX-42 positive cells. IL-10R1 mRNA in these cultures was detected by RT-PCR using specific primers to amplify the IL-10R1 subunit (Fig. 1C). Western blot of spinal cord cell lysates demonstrated a 90 kDa band corresponding to the IL-10R1 protein (Fig. 1D). Neurons identified by their characteristic morphology and by NeuN immunostaining co-stained by the antibody IL-10R1 (Fig. 1E).
Figure 1.
Cultures of primary E17 spinal cord in defined medium (10 DIV) contain > 99% neurons assessed by MAP2 (green), Hoechst (blue), GFAP (A, red) and OX-42 (B, red). Bar = 100 μm. IL-10 receptor mRNA and protein were found in E17 spinal cord neurons cultured (10 DIV) in defined medium: (C) RT-PCR; (D) Western blot; (E) immunocytochemistry; scale bar = 10 μm.
The effect of IL-10 on neurons is mediated through Jak-Stat3 and PI3K/AKT
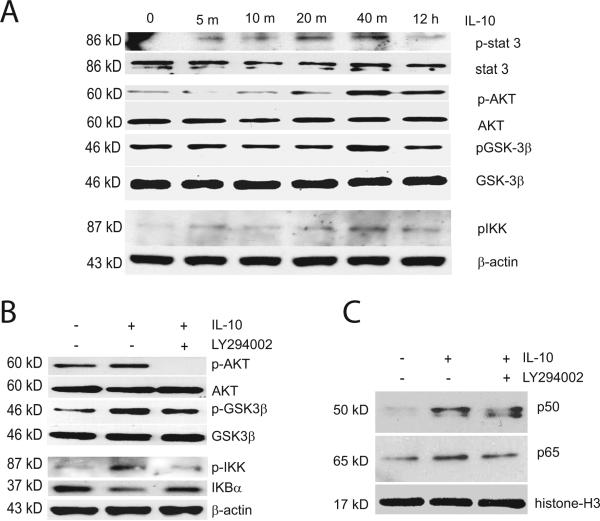
Cytokines signal via the ubiquitous Jak-Stat pathway (O'Shea and Murray 2008). Members of a family of peptides related to the cytokine interleukin-6 activate a similar signaling cascade to produce a trophic effect on motor neurons and PC12 cells (Wu and Bradshaw 2000). In order to explore the possibility that IL-10 may also have neurotrophic and prosurvival properties separate from its anti-inflammatory effects, we studied the response of spinal cord neurons in vitro to treatment with recombinant IL-10 protein (10 ng/ml) over time ranging from 5 minutes to 12 hours. We observed rapid phosphorylation of Stat3 at Tyr705 beginning at 5 minutes after IL-10 (Fig. 2A). These results demonstrate that spinal cord neurons respond directly to IL-10 stimulation by activating the Jak-Stat3 signaling pathway. IL-10 also engaged the PI3K–AKT pathway in spinal cord neurons and induced the phosphorylation of AKT (S473) with peak activation at 40 minutes following IL-10 treatment (Fig. 2A). In addition, we found phosphorylation (inactivation) of GSK-3β (S9) a kinase important in the organization and maintenance of neuronal cytoskeleton and synaptic plasticity, at similar time following IL-10 stimulation (Fig. 2A). The effect of IL-10 on GSK-3β was blocked by pre-treatment of spinal cord neurons with the PI3K inhibitor LY294002 (Fig. 2B) confirming that inactivation of GSK-3β is downstream of AKT.
Figure 2.
The effect of IL-10 on NF-kB activation is mediated through PI3K and AKT. (A) Addition of recombinant IL-10 protein to cultured spinal cord neurons in vitro induced phosphorylation of Stat3, AKT (S473), IKK (S180/181) and GSK3β (S9). Western blot of cultured spinal cord neuron lysates after 5, 10, 20, 40 min and 12 h of IL-10 treatment (10 ng/ml). (B) The effects of IL-10 on phosphorylation of AKT, IKK and GSK3β and on IκBα levels were blocked by LY294002. (C) Increases in p50 and p65 NF-κB in the nuclear fraction of cultured spinal cord neurons induced by IL-10 was partially blocked by LY294002. Histone 3 is used as an internal control for the nuclear fraction. Cultured cells were treated with 50 μM LY294002 for 30 min, followed by exposure to IL-10 (10 ng/ml) for 40 min (B) and 12 h (C). Quantitative analysis of the blot results is presented in Supplementary Figures 2 and 3.
Activation of canonical pathway of NF-κB by IL-10 induces expression of anti-apoptotic peptides Bcl-2 and Bcl-xL
Previous work has implicated AKT in phosphorylating and activating IKK upstream of the NF-κB complex (Romashkova and Makarov 1999). In spinal cord neurons, IL-10 induced phosphorylation of IKK (S180/181), maximal at 40 minutes after treatment (Fig. 2A) and IKK phosphorylation was prevented by pre-treatment with the PI3K inhibitor LY294002 (Fig. 2B). The phosphorylation and activation of IKK by IL-10 suggests the possible engagement of the canonical pathway of NF-κB signaling and transcription regulation by IL-10, in which activation of IKKα causes IKBα phosphorylation, disassembly from NF-κB p50 and p65 complex and shuttling of IKBα into the proteosome for degradation while NF-κB p50 and p65 translocate from cytosol to nucleus. In our studies, IL-10 treatment of spinal cord neurons caused a reduction in IKBα levels at the time of IKK activation (Fig. 2B) followed by enhanced translocation of NF-κB p50 and p65 into the nuclear compartment (Fig. 2C). IKBα levels in the cytosol and p50 and p65 levels in the nuclear fraction were restored when neurons were pretreated with the PI3K inhibitor.
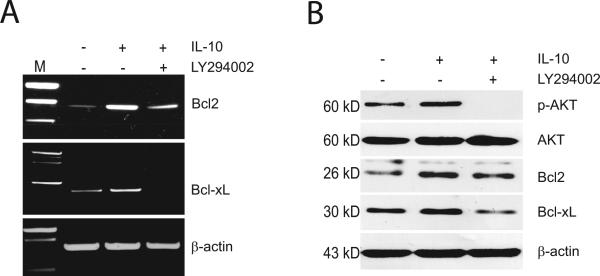
To determine whether activation of the canonical pathway of NF-κB in spinal cord neurons by IL-10 leads to enhanced transcription, we examined the effect of IL-10 on the expression of the prosurvival proteins Bcl-2 and Bcl-xL, well-known targets of NF-κB transcriptional regulation. Addition of IL-10 enhanced the expression of Bcl-2 and Bcl-xL mRNA and protein (Fig 3A,B). These effects were blocked by the PI3K inhibitor LY294002 (Fig 3A,B) which not only completely blocked the activation of transcription of Bcl-xL by IL-10, but reduced the mRNA and protein to below baseline levels.
Figure 3.
The effect of IL-10 on expression of Bcl-2 and Bcl-xL mRNA (A) and protein (B) was blocked by LY294002. Cultured cells were treated with 50 μM LY294002 for 30 min followed by exposure to IL-10 (10 ng/ml) for 12 h. Quantitative analysis of the blot results is presented in Supplementary Figures 4 and 5.
IL-10 protects embryonic spinal cord neurons against glutamate-induced apoptosis in vitro
Excess glutamate plays a role in the pathogenesis of a number of diseases of the nervous system (Choi 1988) and glutamate concentration is elevated following spinal cord trauma in rodents. Although the mechanisms underlying its neurotoxicity are complex, it has been established that glutamate-triggered neuronal injury correlates with calcium influx that results in activation of second messenger systems and mitochondrial dysfunction leading to apoptosis.
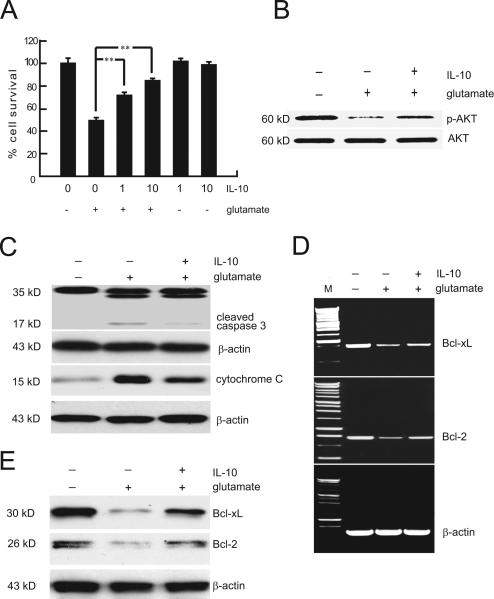
In order to study the neuroprotective effects of IL-10, we used the well established model of glutamate induced apoptosis in vitro. Exposure of spinal cord neurons in culture to 100 μM glutamate resulted in progressive loss of viable cells over 24 hour exposure using MTT reduction assay that uses as cofactors nucleotides generated by mitochondrial activity. The loss of cell viability induced by glutamate appears to be largely apoptotic, as LDH release from neurons exposed to the same concentration of glutamate remained at a very low level over a similar time period and only reached 4% at 3 hrs of glutamate exposure (Suppl Fig 1). There was no release of either TNFα or IL-1β into the medium after exposure to glutamate (ELISA, data not shown) consistent with the paucity of glial cells in these cultures. We chose to study the adverse effects of 3 hrs of exposure to glutamate (50% on cell viability by MTT) and neuronal toxicity was substantially prevented by the addition of recombinant IL-10 to the glutamate-containing medium (Fig. 4A). IL-10 at concentration of 10 ng/ml, preserved neuronal viability up to 80% of control; substantial protective effects were observed at concentrations of IL-10 as low as 1 ng/ml. In the absence of glutamate, IL-10 had no effect on cell survival within the short time course of this experiment. In neurons in vitro glutamate treatment resulted in reduction of AKT phosphorylation; the level of phosphorylated AKT was restored by exposure to IL-10, suggesting that engaging activation of PI3K-AKT signaling by IL-10 promotes its neuroprotective effects in the setting of excitotoxicity (Fig. 4B).
Figure 4.
IL-10 protects embryonic spinal cord neurons against glutamate-induced apoptosis. (A) Spinal cord neurons in vitro treated with IL-10 (10 ng/ml) for 12 h before exposure to 100 μM glutamate for 3 h were protected from apoptotic cell death, measured by MTT assay. Data, expressed as percentage of control, are the mean ± SME of five separate experiments. **P < 0.01. (B) Treatment with IL-10 prevented the reduction in AKT phosphorylation caused by exposure to glutamate. (C) Cleaved (activated) caspase-3 (~17 kDa) induced by glutamate exposure was markedly decreased in spinal cord neurons pretreated with IL-10. Western blot of cytosol fraction of spinal cord neurons demonstrates increase cytochrome c after glutamate exposure; pretreatment with IL-10 inhibited translocation of cytochrome c from mitochondria. (D,E) Bcl-2 and Bcl-xL mRNA levels determined by RT-PCR (D) and protein levels determined by Western blot (E) decreased after exposure to glutamate; these decrease were prevented by pretreatment with IL-10. In all experiments IL-10 treatment was 12 h followed by 100 μM glutamate exposure for 3 h in vitro. Quantitation of the blot result is presented in Supplementary Figures 6 and 7.
IL-10 blocks cytochrome c release from mitochondria and cleavage of caspase 3 in neurons in vitro
Overactivation of the NMDA receptor results in a sustained rise in intracellular calcium and a time-dependent activation of caspase 3 (Du et al. 1997). In our studies spinal cord neurons exposed to 100 μM glutamate for 3 hrs show cleavage of caspase 3 into an intermediate fragment and the active product of 17 kD (Fig. 4C), a step which precedes the activation of DNase and fragmentation of DNA, and activation of caspase 3 induced by glutamate was prevented in spinal cord neurons pre-treated with IL-10 recombinant protein (Fig. 4C). In traumatic injury and in NMDA toxicity, apoptosis is initiated by release of mitochondrial cytochrome c, and the released cytochrome c associates with apoptosis activating factor-1 to activate caspase 3 (Li et al. 1997). To determine whether IL-10 can block caspase 3 cleavage through inhibition of cytochrome c release from mitochondria, we examined the change in distribution of cytochrome c after exposure to glutamate. Western blot of subcellular fractions revealed that glutamate increased cytochrome c release into cytosol and pretreatment with IL-10 partially blocked that release from mitochondria (Fig. 4C). These results suggest that IL-10 activation of the neuronal IL-10 receptor results in stabilization of the outer mitochondrial membrane to prevent apoptosis.
In glutamate induced neurotoxicity IL-10 restores expression of anti-apoptotic peptides Bcl-2 and Bcl-xL
Bcl-2 family proteins regulate the electrochemical gradient across the mitochondrial membrane (Kluck et al. 1997; Yang et al. 1997). While the pro-apoptotic members of the Bcl-2 family (e.g., Bid, Bax, and Bak) are known to permeabilize the outer mitochondrial membrane, the anti-apoptotic members (e.g., Bcl-2 and Bcl-xL), render the cells resistant to apoptosis. To determine whether IL-10 inhibits cytochrome c release from mitochondria through regulation of Bcl-2 and Bcl-xL levels, we evaluated the mRNA and protein in the glutamate neurotoxicity model in vitro. We found that exposure to glutamate markedly reduced the levels of the anti-apoptotic Bcl-2 and Bcl-xL mRNA and protein (Fig. 4D,E) and these changes were prevented by pre-treatment of spinal cord neurons with IL-10 (Fig. 4D,E), confirming that IL-10 neuroprotective effects in neurons are in part due to enhancing expression of Bcl-2 and Bcl-xL, restoring the equilibrium between pro- and anti-apoptotic peptides, preventing release of cytochrome c to the cytosol and thus blocking the caspase-dependent cell death pathway.
IL-10 enhances p50 and p65 NF-κB nuclear localization and binding activity to DNA in spinal cord neurons
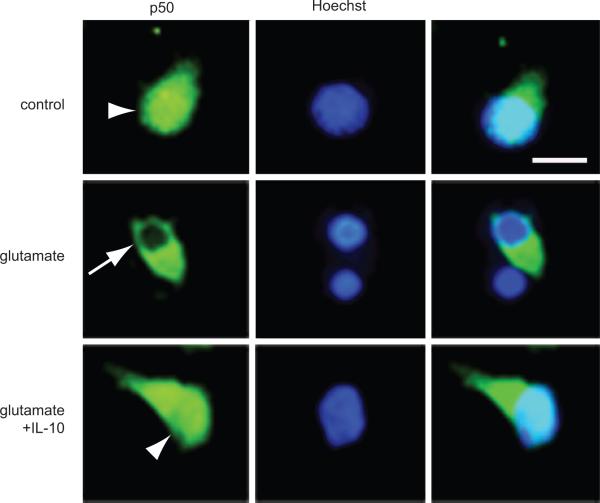
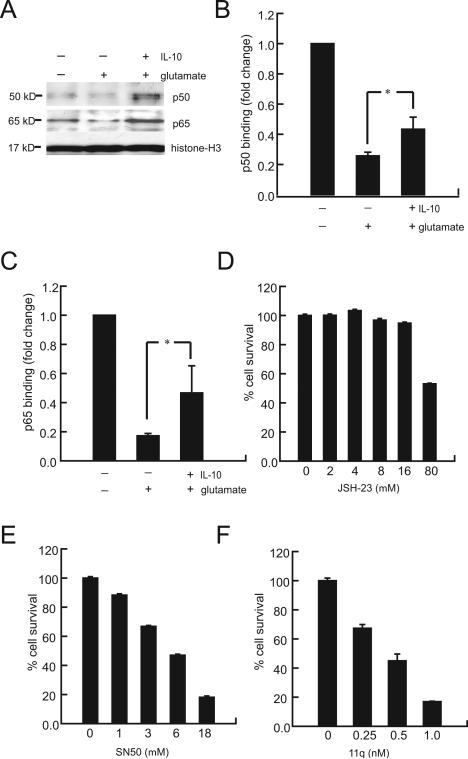
In order to determine whether IL-10 restores Bcl-2 and Bcl-xL expression in the presence of glutamate toxicity through activation of the NF-κB pathway, we examined the translocation of the p50 and p65 subunits of NF-κB from the cytoplasm to the nucleus in response to 3 hrs exposure to 100 μM glutamate. In primary spinal cord neurons in culture, p50 immunoreactivity was visible in the cytoplasm and nucleus. After 3 hrs exposure to glutamate, p50 was absent from the nucleus. Pretreatment with IL-10 prevented the disappearance of p50 from the nucleus (Fig. 5). These changes in distribution were confirmed by Western blot analysis of isolated nuclear fractions (Fig. 6A), which clearly demonstrated that IL-10 restored nuclear p50 and p65 to levels above those observed in control neurons (Fig. 6A); the increase was more marked for p50.
Figure 5.
IL-10 enhances nuclear translocation and DNA binding activity of p50 and p65 NF-κB. Neurons exposed to 100 μM glutamate for 3 h show decreased p50 in the nucleus (arrow), an effect that was prevented in cells pretreated with IL-10 (10 ng/ml) for 12 h (arrowhead); scale bar = 2 μm. A low-power view containing a field of cells is shown in Supplementary Figure 8.
Figure 6.
(A) Treatment with IL-10 increased p50 and p60 in the nuclear fraction after exposure to glutamate (Western blot). (B, C) p50 and p65 NF-κB activity was reduced in cells exposed to glutamate; pretreatment with IL-10 prevented this reduction in part. Spinal cord neurons were treated with NF-κB inhibitors (JSH-23, SN50 and 11q.) for 12 h. Neuronal viability measured by MTT assay was markedly reduced by exposure to the NF-κB inhibitors (D) JSH-23, (E) SN50 and (F) 11q. Data expressed as percentage of control, the mean ± SEM of four separate experiments. *P < 0.05.
Quantitation of DNA binding activity of p50 and p65 to specific nucleotide sequences was substantially reduced by exposure to glutamate, and IL-10 treatment resulted in a significant reversal of this reduction (Fig. 6B,C). Taken together the results suggest that the neuroprotective effects of IL-10 in the presence of glutamate toxicity are mediated by the activation of NF-κB dependent transcription in neurons by preventing the loss of nuclear p50 and p65 induced by glutamate.
In order to further investigate the protective role of NF-κB, and because our results were different from those in a previous report (Bachis et al. 2001) we examined the effect of 3 different NF-κB inhibitors on spinal cord neurons in culture. Application of JSH-23 or SN50, cell-permeable inhibitors of p65 and p50 nuclear translocation respectively (Fig. 6D,E), or of 11q (6-amino-4-(4-phenoxyphenylethylamino) quinazoline, Fig. 6F), an inhibitor of NF-κB transcriptional activation (Tobe et al. 2003) resulted in death of primary spinal cord neurons in culture in the absence of glutamate (Fig. 6D-F). Spinal cord neurons were highly sensitive to inhibition of NF-κB transcription by 11q; apoptosis was induced in 50% of cells at concentrations of 11q 14-fold lower than those reported to inhibit NF-κB transcriptional activation in non-neuronal cells (Tobe et al. 2003). The concentration range through which SN50, a mimetic of the p50 nuclear localizing signal, causes neuronal apoptosis parallels the concentrations reported to selectively inhibit p50 nuclear translocation (Maggirwar et al. 1998). While spinal cord neurons in vitro were much more resistant to inhibition by JSH-23 where 50% apoptosis was induced at concentrations ten fold higher than those reported to inhibit p65 nuclear translocation (Shin et al. 2004). These results suggest that while NF-κB transcriptional activation and translocation is essential for neuronal survival, spinal cord neurons in vitro are more vulnerable to inhibition of p50 nuclear translocation and IL-10 neuroprotective effects may result from the particular enhancement of p50 nuclear levels.
Discussion
There are three principal findings from these experiments: (1) the IL-10 receptor is found in spinal cord neurons; (2) IL-10 binding activates the Jak-Stat3 and PI3K/AKT pathways, the latter activating the canonical NF-κB pathway and inactivating GSK-3β; and, (3) in the face of glutamate toxicity IL-10 provides a neuroprotective effect by enhancing nuclear p50 and p65 with consequent transcription of Bcl-2 and Bcl-xL preventing cytochrome c release and caspase 3 activation.
This is the first report documenting wide distribution of IL-10 receptor in embryonic spinal cord neurons. The presence of the IL-10 receptor in primary spinal cord neurons suggests that IL-10 may play a role in neuronal development in addition to the anti-apoptotic effects. The IL-10 receptor has previously been observed in retinal ganglion cells but has not otherwise been reported in neurons.
Investigating the mechanisms through which the neuronal IL-10 receptor functions we observed that IL-10 binding to its receptor activates several second messengers including the Jak/Stat3 and PI3K/AKT pathways. The latter resulted in phosphorylation of GSK-3β. This phosphorylation promotes GSK-3β binding to the 14-3-3 chaperone protein resulting in inactivation. The combined engagement of these signaling pathways has been associated with cytokine and growth factor mediated pro-survival responses (Rodgers and Theibert 2002), axon polarity and neurite growth (Yoshimura et al. 2005) and modulation of long term potentiation and depression (Peineau et al. 2007) raising the possibility that IL-10 may provide not only trophic but regenerative and plastic cues to neurons.
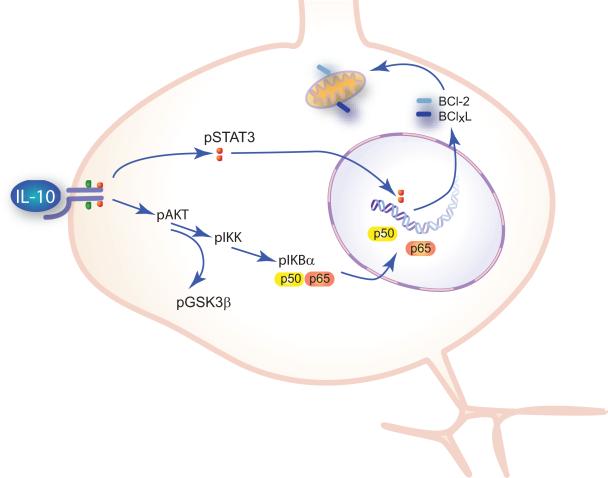
We focused our studies on the PI3K/AKT pathway. We found that in spinal cord neurons, phosphorylation of AKT by activation of the IL-10 receptor resulted in activation of the canonical NF-κB pathway, increased nuclear p50 and p65 and enhanced Bcl-2 and Bcl-xL transcription. The effects of IL-10 on Bcl-2 and Bcl-xL levels occurred in normal spinal cord neurons in culture, further supporting the interpretation that IL-10 plays a physiologic role in modulating neuronal function in a pro-survival direction (Fig. 7).
Figure 7.
Schematic. IL-10 binding to IL-10–R1 activates Jak-Stat3 and PI3K-AKT. AKT activates the canonical NF-κB pathway enhancing transcription of Bcl-2 and Bcl-xL. Bcl-2 and Bcl-xL render neurons resistant to glutamate toxicity and traumatic injury through stabilization of the mitochondrial membrane to prevent the release of cytochrome c and downstream activation of caspase 3. IL-10 activation of PI3K-AKT also results in phosphorylation of GSK-3β to regulate cytoskeleton organization and synaptic plasticity.
Although Bcl-2 and Bcl-xL transcription can also be enhanced by activation of Stat3 (Grad et al. 2000), in our experiments inhibiting PI3K with LY294002 not only blocked IL-10 induced phosphorylation of AKT and IKK but prevented the degradation of IκBα and the induction of Bcl-2 and Bcl-xL expression, indicating a major role for PI3K/AKT in modulating Bcl-2 and Bcl-xL levels. The extent of inhibition by LY294002 was greater for Bcl- xL than Bcl-2 mRNA and protein suggesting that in spinal cord neurons IL-10 activation of transcription of Bcl-xL is mainly PI3K/AKT dependent while activation of transcription of Bcl-2 may also require Stat3-dependent signaling similar to that observed previously in hematopoietic cells (Weber-Nordt et al. 1996).
Previous studies of IL-10 neuroprotection by Boyd and co-workers demonstrated that IL-10 can protect a retinal ganglion cell line from cell death induced by serum deprivation (Boyd et al. 2003). Our work agrees with and extends those studies, demonstrating that IL-10 in vitro can prevent the release of cytochrome c from mitochondria and cleavage of caspase-3, the final steps prior to DNA fragmentation in apoptotic cell death (Namura et al. 1998). This is the first demonstration that IL-10 treatment stabilizes the mitochondrial membrane and prevents of release of cytochrome c, effects that are likely due to the increased expression of Bcl-2 and Bcl-xL thus altering the equilibrium between pro- and anti-apoptotic peptides at the mitochondrial membrane. In addition, activation of AKT by IL-10 may have additional effects on apoptosis through inactivation by phosphorylation of the pro-apoptotic molecules Bax and of pro-caspase 9 protease (Parcellier et al. 2008) leading to the prevention of caspase-3 cleavage that we observed in vitro.
The beneficial effects of IL-10 appear to be derived from its ability to enhance NF-κB DNA binding activity of p50 and p65 leading to the increase in Bcl-2 and Bcl-xL transcription. In cerebellar granule cells in vitro, Bachis et al. observed that IL-10 prevented glutamate-induced apoptosis by blocking the activation of NF-κB p52 and p65 by glutamate (Bachis et al. 2001). However cerebellar granule cells are unique among neurons in requiring high extracellular potassium concentrations for survival in vitro, conditions that result in high intracellular calcium concentrations and may account for the atypical response to glutamate observed in that system. Our observation that exposure of spinal cord neurons to glutamate resulted in inhibition of NF-κB p50 and p65 activity is in agreement with other previous reports (Mao et al. 1999).
IL-10 not only enhanced nuclear p50 and p65 in normal neurons but also prevented the loss caused by glutamate and restored the levels of p50 and p65 in the nucleus above baseline levels. This effect on nuclear translocation was more marked for p50, an observation which is in agreement with studies of non-neuronal cells where IL-10 has been shown to increase levels and enhance nuclear translocation of p50 to a greater extent than p65 (Li et al. 1997). The differential effects of IL-10 on p50 compared to p65 may reflect differential rates of turnover and/or nuclear transport of these two components of NF-κB. Our results also show that spinal cord neurons are more sensitive to inhibitors of p50 translocation and NF-κB binding activity than to inhibition of p65 translocation, and suggest that p50 transcription is essential for neuronal survival. These results complement reports showing that p50 knockout mice are substantially more vulnerable to excitotoxicity and ischemia (Yu et al. 1999; Kassed et al. 2002).
Taken together, the results of the studies reported herein are consistent with the notion that endogenous IL-10 may provide trophic and pro-survival cues to neurons during development, and raise the possibility that in the setting of injury or disease exogenously administered IL-10 may have direct beneficial effects on neurons in addition to the well-studied effects of modulating the inflammatory response associated with injury.
Supplementary Material
Supplementary Figure 1. Spinal cord neurons exposed to 100 μM glutamate for 0-24 h show a reduction in cell survival over time determined by MTT assay. Cell death, assessed by LDH release was insignificant through 6 h of glutamate exposure.
Supplementary Figure 2. Quantitative analysis of the blot results shown in Figure 2A. The experiment was repeated 3 times and the data are presented as mean ± SEM of the ratios indicated.
Supplementary Figure 3. Quantitative analysis of the blot results shown in Figure 2B. The experiment was repeated 3 times and the data are presented as mean ± SEM of the ratios indicated. * P < 0.05; ** P < 0.01
Supplementary Figure 4. Quantitative analysis of the blot results shown in Figure 3A. The experiment was repeated 3 times and the data are presented as mean ± SEM of the ratios indicated. ** P < 0.01
Supplementary Figure 5. Quantitative analysis of the blot results shown in Figure 3B. The experiment was repeated 3 times and the data are presented as mean ± SEM of the ratios indicated. * P < 0.05; ** P < 0.01
Supplementary Figure 6. Quantitative analysis of the blot results shown in Figure 4B and C. The experiment was repeated 3 times and the data are presented as mean ± SEM of the ratios indicated. * P < 0.05; ** P < 0.01
Supplementary Figure 7. Quantitative analysis of the blot results shown in Figure 4D and E. The experiment was repeated 3 times and the data are presented as mean ± SEM of the ratios indicated. * P < 0.05; ** P < 0.01
Supplementary Figure 8. Low power view of IL-10 mediated nuclear translocation of p50 and p65 NF-κB. The cells shown in high power in Figure 5 are outlined by white boxes; scale bar = 20 μm.
Acknowledgments
The authors wish to acknowledge the excellent technical assistance of Vikram Thakur Singh in vector propagation, Shue Liu in tissue culture, and Carrissia Holloway in animal care. This work was supported by grants from the US Department of Veterans Affairs and the National Institutes of Health to MM and DJF.
References
- Bachis A, Colangelo AM, Vicini S, Doe PP, De Bernardi MA, Brooker G, Mocchetti I. Interleukin-10 prevents glutamate-mediated cerebellar granule cell death by blocking caspase-3-like activity. J Neurosci. 2001;21:3104–3112. doi: 10.1523/JNEUROSCI.21-09-03104.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethea JR, Nagashima H, Acosta MC, Briceno C, Gomez F, Marcillo AE, Loor K, Green J, Dietrich WD. Systemically administered interleukin-10 reduces tumor necrosis factor-alpha production and significantly improves functional recovery following traumatic spinal cord injury in rats. J Neurotrauma. 1999;16:851–863. doi: 10.1089/neu.1999.16.851. [DOI] [PubMed] [Google Scholar]
- Boyd ZS, Kriatchko A, Yang J, Agarwal N, Wax MB, Patil RV. Interleukin-10 receptor signaling through STAT-3 regulates the apoptosis of retinal ganglion cells in response to stress. Invest Ophthalmol Vis Sci. 2003;44:5206–5211. doi: 10.1167/iovs.03-0534. [DOI] [PubMed] [Google Scholar]
- Brewer KL, Bethea JR, Yezierski RP. Neuroprotective effects of interleukin-10 following excitotoxic spinal cord injury. Exp Neurol. 1999;159:484–493. doi: 10.1006/exnr.1999.7173. [DOI] [PubMed] [Google Scholar]
- Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1:623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Dietrich WD, Busto R, Bethea JR. Postischemic hypothermia and IL-10 treatment provide long-lasting neuroprotection of CA1 hippocampus following transient global ischemia in rats. Exp Neurol. 1999;158:444–450. doi: 10.1006/exnr.1999.7115. [DOI] [PubMed] [Google Scholar]
- Du Y, Bales KR, Dodel RC, Hamilton-Byrd E, Horn JW, Czilli DL, Simmons LK, Ni B, Paul SM. Activation of a caspase 3-related cysteine protease is required for glutamate-mediated apoptosis of cultured cerebellar granule neurons. Proc Natl Acad Sci U S A. 1997;94:11657–11662. doi: 10.1073/pnas.94.21.11657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei K, Lins H, Schwerdel C, Fontana A. Antigen presentation in the central nervous system. The inhibitory effect of IL-10 on MHC class II expression and production of cytokines depends on the inducing signals and the type of cell analyzed. J Immunol. 1994;152:2720–2728. [PubMed] [Google Scholar]
- Grad JM, Zeng XR, Boise LH. Regulation of Bcl-xL: a little bit of this and a little bit of STAT. Curr Opin Oncol. 2000;12:543–549. doi: 10.1097/00001622-200011000-00006. [DOI] [PubMed] [Google Scholar]
- Ho AS, Liu Y, Khan TA, Hsu DH, Bazan JF, Moore KW. A receptor for interleukin 10 is related to interferon receptors. Proc Natl Acad Sci U S A. 1993;90:11267–11271. doi: 10.1073/pnas.90.23.11267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann A, Baltimore D. Circuitry of nuclear factor kappaB signaling. Immunol Rev. 2006;210:171–186. doi: 10.1111/j.0105-2896.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- Howard M, O'Garra A, Ishida H, de Waal Malefyt R, de Vries J. Biological properties of interleukin 10. J Clin Immunol. 1992;12:239–247. doi: 10.1007/BF00918147. [DOI] [PubMed] [Google Scholar]
- Israel A. The IKK complex: an integrator of all signals that activate NF-kappaB? Trends Cell Biol. 2000;10:129–133. doi: 10.1016/s0962-8924(00)01729-3. [DOI] [PubMed] [Google Scholar]
- Jander S, Pohl J, D'Urso D, Gillen C, Stoll G. Time course and cellular localization of interleukin-10 mRNA and protein expression in autoimmune inflammation of the rat central nervous system. Am J Pathol. 1998;152:975–982. [PMC free article] [PubMed] [Google Scholar]
- Kassed CA, Willing AE, Garbuzova-Davis S, Sanberg PR, Pennypacker KR. Lack of NF-kappaB p50 exacerbates degeneration of hippocampal neurons after chemical exposure and impairs learning. Exp Neurol. 2002;176:277–288. doi: 10.1006/exnr.2002.7967. [DOI] [PubMed] [Google Scholar]
- Kelly A, Lynch A, Vereker E, Nolan Y, Queenan P, Whittaker E, O'Neill LA, Lynch MA. The anti-inflammatory cytokine, interleukin (IL)-10, blocks the inhibitory effect of IL-1 beta on long term potentiation. A role for JNK. J Biol Chem. 2001;276:45564–45572. doi: 10.1074/jbc.M108757200. [DOI] [PubMed] [Google Scholar]
- Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- Lin YZ, Yao SY, Veach RA, Torgerson TR, Hawiger J. Inhibition of nuclear translocation of transcription factor NF-kappa B by a synthetic peptide containing a cell membrane-permeable motif and nuclear localization sequence. J Biol Chem. 1995;270:14255–14258. doi: 10.1074/jbc.270.24.14255. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wei SH, Ho AS, de Waal Malefyt R, Moore KW. Expression cloning and characterization of a human IL-10 receptor. J Immunol. 1994;152:1821–1829. [PubMed] [Google Scholar]
- Maggirwar SB, Sarmiere PD, Dewhurst S, Freeman RS. Nerve growth factor-dependent activation of NF-kappaB contributes to survival of sympathetic neurons. J Neurosci. 1998;18:10356–10365. doi: 10.1523/JNEUROSCI.18-24-10356.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X, Moerman AM, Lucas MM, Barger SW. Inhibition of the activity of a neuronal kappaB-binding factor by glutamate. J Neurochem. 1999;73:1851–1858. [PubMed] [Google Scholar]
- Massa PT, Aleyasin H, Park DS, Mao X, Barger SW. NFkappaB in neurons? The uncertainty principle in neurobiology. J Neurochem. 2006;97:607–618. doi: 10.1111/j.1471-4159.2006.03810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. NF-kappaB in the survival and plasticity of neurons. Neurochem Res. 2005;30:883–893. doi: 10.1007/s11064-005-6961-x. [DOI] [PubMed] [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Namura S, Zhu J, Fink K, Endres M, Srinivasan A, Tomaselli KJ, Yuan J, Moskowitz MA. Activation and cleavage of caspase-3 in apoptosis induced by experimental cerebral ischemia. J Neurosci. 1998;18:3659–3668. doi: 10.1523/JNEUROSCI.18-10-03659.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea JJ, Murray PJ. Cytokine signaling modules in inflammatory responses. Immunity. 2008;28:477–487. doi: 10.1016/j.immuni.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parcellier A, Tintignac LA, Zhuravleva E, Hemmings BA. PKB and the mitochondria: AKTing on apoptosis. Cell Signal. 2008;20:21–30. doi: 10.1016/j.cellsig.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Peineau S, Taghibiglou C, Bradley C, Wong TP, Liu L, Lu J, Lo E, Wu D, Saule E, Bouschet T, Matthews P, Isaac JT, Bortolotto ZA, Wang YT, Collingridge GL. LTP inhibits LTD in the hippocampus via regulation of GSK3beta. Neuron. 2007;53:703–717. doi: 10.1016/j.neuron.2007.01.029. [DOI] [PubMed] [Google Scholar]
- Perkins ND, Gilmore TD. Good cop, bad cop: the different faces of NF-kappaB. Cell Death Differ. 2006;13:759–772. doi: 10.1038/sj.cdd.4401838. [DOI] [PubMed] [Google Scholar]
- Rodgers EE, Theibert AB. Functions of PI 3-kinase in development of the nervous system. Int J Dev Neurosci. 2002;20:187–197. doi: 10.1016/s0736-5748(02)00047-3. [DOI] [PubMed] [Google Scholar]
- Romashkova JA, Makarov SS. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- Shin HM, Kim MH, Kim BH, Jung SH, Kim YS, Park HJ, Hong JT, Min KR, Kim Y. Inhibitory action of novel aromatic diamine compound on lipopolysaccharide-induced nuclear translocation of NF-kappaB without affecting IkappaB degradation. FEBS Lett. 2004;571:50–54. doi: 10.1016/j.febslet.2004.06.056. [DOI] [PubMed] [Google Scholar]
- Tobe M, Isobe Y, Tomizawa H, Nagasaki T, Takahashi H, Fukazawa T, Hayashi H. Discovery of quinazolines as a novel structural class of potent inhibitors of NF-kappa B activation. Bioorg Med Chem. 2003;11:383–391. doi: 10.1016/s0968-0896(02)00440-6. [DOI] [PubMed] [Google Scholar]
- Weber-Nordt RM, Henschler R, Schott E, Wehinger J, Behringer D, Mertelsmann R, Finke J. Interleukin-10 increases Bcl-2 expression and survival in primary human CD34+ hematopoietic progenitor cells. Blood. 1996;88:2549–2558. [PubMed] [Google Scholar]
- Wu YY, Bradshaw RA. Activation of the Stat3 signaling pathway is required for differentiation by interleukin-6 in PC12-E2 cells. J Biol Chem. 2000;275:2147–2156. doi: 10.1074/jbc.275.3.2147. [DOI] [PubMed] [Google Scholar]
- Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- Yoshimura T, Kawano Y, Arimura N, Kawabata S, Kikuchi A, Kaibuchi K. GSK-3beta regulates phosphorylation of CRMP-2 and neuronal polarity. Cell. 2005;120:137–149. doi: 10.1016/j.cell.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Yu Z, Zhou D, Bruce-Keller AJ, Kindy MS, Mattson MP. Lack of the p50 subunit of nuclear factor-kappaB increases the vulnerability of hippocampal neurons to excitotoxic injury. J Neurosci. 1999;19:8856–8865. doi: 10.1523/JNEUROSCI.19-20-08856.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Spinal cord neurons exposed to 100 μM glutamate for 0-24 h show a reduction in cell survival over time determined by MTT assay. Cell death, assessed by LDH release was insignificant through 6 h of glutamate exposure.
Supplementary Figure 2. Quantitative analysis of the blot results shown in Figure 2A. The experiment was repeated 3 times and the data are presented as mean ± SEM of the ratios indicated.
Supplementary Figure 3. Quantitative analysis of the blot results shown in Figure 2B. The experiment was repeated 3 times and the data are presented as mean ± SEM of the ratios indicated. * P < 0.05; ** P < 0.01
Supplementary Figure 4. Quantitative analysis of the blot results shown in Figure 3A. The experiment was repeated 3 times and the data are presented as mean ± SEM of the ratios indicated. ** P < 0.01
Supplementary Figure 5. Quantitative analysis of the blot results shown in Figure 3B. The experiment was repeated 3 times and the data are presented as mean ± SEM of the ratios indicated. * P < 0.05; ** P < 0.01
Supplementary Figure 6. Quantitative analysis of the blot results shown in Figure 4B and C. The experiment was repeated 3 times and the data are presented as mean ± SEM of the ratios indicated. * P < 0.05; ** P < 0.01
Supplementary Figure 7. Quantitative analysis of the blot results shown in Figure 4D and E. The experiment was repeated 3 times and the data are presented as mean ± SEM of the ratios indicated. * P < 0.05; ** P < 0.01
Supplementary Figure 8. Low power view of IL-10 mediated nuclear translocation of p50 and p65 NF-κB. The cells shown in high power in Figure 5 are outlined by white boxes; scale bar = 20 μm.