Abstract
Peroxisome proliferator-activated receptors (PPARs) belong to the nuclear hormone-receptor superfamily. Originally cloned in 1990, PPARs were found to be mediators of pharmacologic agents that induce hepatocyte peroxisome proliferation. PPARs also are expressed in cells of the cardiovascular system. PPARγ appears to be highly expressed during atherosclerotic lesion formation, suggesting that increased PPARγ expression may be a vascular compensatory response. Also, ligand-activated PPARγ decreases the inflammatory response in cardiovascular cells, particularly in endothelial cells. PPARα, similar to PPARγ, also has pleiotropic effects in the cardiovascular system, including antiinflammatory and antiatherosclerotic properties. PPARα activation inhibits vascular smooth muscle proinflammatory responses, attenuating the development of atherosclerosis. However, PPARδ overexpression may lead to elevated macrophage inflammation and atherosclerosis. Conversely, PPARδ ligands are shown to attenuate the pathogenesis of atherosclerosis by improving endothelial cell proliferation and survival while decreasing endothelial cell inflammation and vascular smooth muscle cell proliferation. Furthermore, the administration of PPAR ligands in the form of TZDs and fibrates has been disappointing in terms of markedly reducing cardiovascular events in the clinical setting. Therefore, a better understanding of PPAR-dependent and -independent signaling will provide the foundation for future research on the role of PPARs in human cardiovascular biology. Antioxid. Redox Signal. 11, 1415–1452.
I. Introduction
Peroxisomes are organelles that participate in fatty acid metabolism. Clofibrate analogues, hypolipidemic agents that control plasma cholesterol and triglyceride levels, can induce proliferation of liver cell peroxisomes (300, 301). In addition, two lipid-lowering compounds structurally different from clofibrate, [4-chloro-6-(2,3-xylidino)-2-pyrimidinylthio]acetic acid (Wy-14,643) and 2-chloro-5-(3,5-dimethylpiperidino-sulfonyl)benzoic acid (tibric acid), also were found to stimulate hepatocyte peroxisome proliferation (302). Although hypolipidemic drugs were demonstrated to activate peroxisome proliferation, these studies did not establish a mechanism. Subsequent studies identified a protein whereby peroxisome proliferators bind with affinity (196, 197), and this protein was later identified as a member of the nuclear hormone-receptor superfamily that includes steroid, retinoid, and thyroid hormone receptors (104). The name peroxisome proliferator-activated receptor took origin from the cloning by Issemann et al. (172) to identify possible endogenous mediators of peroxisome proliferation–induced gene transcription in rodent livers. The peroxisome proliferator–activated receptors (PPARs) consist of three related transcription factors: PPARalpha (PPARα), PPARbeta/delta (PPARβ/δ), and PPARgamma (PPARγ), encoded by the genes PPARA, PPARD, and PPARG, respectively (96). In addition to the role in peroxisome proliferation, these nuclear transcription factors are involved in numerous cellular functions, including insulin sensitivity, glucose homeostasis, fatty acid oxidation, cytokine production, and vasculoprotection.
II. PPAR and the Mechanism of Action
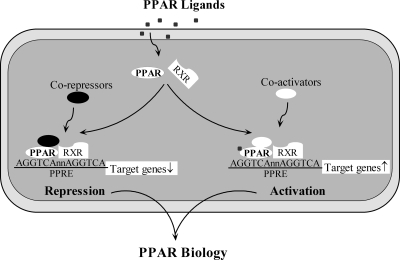
PPARs were initially shown to recognize and bind a DNA sequence upstream of the PPAR target gene. This sequence was termed the peroxisome proliferator response element (PPRE) (251, 362) (Fig. 1). Acyl-CoA oxidase is a peroxisomal enzyme involved in fatty acid oxidation. The promoter of this enzyme was found to contain a DNA sequence that was responsive to stimulation by Wy-14,643, and this stimulatory response was mediated by PPAR. Of great importance, PPAR was shown to bind to this 5' flanking portion, or peroxisome proliferator response element of the acyl-CoA oxidase gene (362). PPARs, on activation, heterodimerize with the retinoic X receptor (RXR)-α (22, 121, 182, 190), and this is followed by coactivator recruitment, which eventually leads to transcriptional regulation of gene expression (85, 312) (Fig. 1). Besides being involved in transactivation, PPARs also participate in the negative regulation of certain genes by recruiting co-repressors (233) (Fig. 1). In addition, other molecular mechanisms are found by which PPARs can inhibit gene expression. First, transrepression can be caused by physical interaction with other transcription factors, including nuclear factor-kappa B (NF-κB), Smad-3, activator protein-1 (AP-1), and signal transducers and activators of transcription (STAT) proteins (80, 114, 217, 307). Second, PPARs can modulate transrepression through the mitogen-activated protein kinase (MAPK) pathway (157). Coactivators and co-repressors, in addition to regulating transcriptional activation, are critical for the repression of certain genes (85, 305, 312). Third, PPARs recruit coactivator proteins and often compete with NF-κB and AP-1 for binding to these co-regulators (305). Thus, NF-κB and AP-1 target gene expression is attenuated because of competition with PPARs for coactivator binding.
FIG. 1.
Schematic view of PPAR action. After a ligand binds to PPAR, PPAR heterodimerizes with the retinoid X receptor (RXR) and then binds to the PPRE. Recruiting coactivators and co-repressors leads to activation and repression of PPAR target genes, respectively.
Finally, PPARs can contribute to transrepression by either inhibiting clearance of co-repressor complexes (123, 287) or releasing co-repressors, which could allow co-repressor binding to NF-κB, eventually inhibiting NF-κB target gene expression (305).
The phosphorylation of PPARs is critical to regulating many of the biologic functions of these nuclear receptors. Initially, insulin-induced phosphorylation of PPARα was shown to increase transcriptional activity (322). Also, stress-activated p38 MAPK has been shown to phosphorylate PPARα and enhance target gene expression in myocardiocytes (24). Several studies demonstrate that MAPK phosphorylation deactivates PPARγ and reduces basal and ligand-dependent transcriptional activity (5, 51, 52, 157). However, one study shows that PPARγ is activated by ERK5 in endothelial cells (ECs), and this particular MAPK does not phosphorylate PPARγ (7). A recent report demonstrates that PPARγ is under the control of Bcr, a serine/threonine kinase that phosphorylates PPARγ and prevents transcriptional activity (9). PPARδ is also considered to be a phosphoprotein because protein kinase A (PKA)-induced phosphorylation of PPARδ, similar to PPARα and PPARγ, has a stimulatory effect on transcription (200). These are just a few of many examples that demonstrate how PPAR signaling may be affected because of phosphorylation by protein kinases.
PPARγ is most abundantly expressed in adipose tissue, with less expression in the colon and immune system. PPARγ has been shown to facilitate differentiation of fibroblasts into adipocytes (59). PPARγ is also involved in the regulation of lipid metabolism, as ligand-dependent activation leads to an increase in genes that regulate fatty acid uptake and storage (320). Furthermore, PPARγ plays a role in glucose homeostasis and insulin sensitivity (110). Although PPARγ was initially found to be critical for adipocyte differentiation and function, over time, PPARγ was discovered to play an important role in the cardiovascular system. As well as in adipocytes and T cells, PPARγ is also expressed in endothelial cells, vascular smooth muscle cells (VSMCs), and macrophages.
III. PPARγ Ligands
PPARs possess varying degrees of responsiveness to certain peroxisome proliferating agents (188). Although several compounds were demonstrated to activate PPARs, initially no reports confirmed direct binding to this receptor. However, in 1995, evidence was provided that thiazolidinediones (TZDs), a class of antidiabetic drugs that improve insulin sensitivity, bind to and activate PPARγ with high affinity (209) (Fig. 2). Furthermore, PPARγ was shown to be the major target of these insulin-sensitizing agents (110).
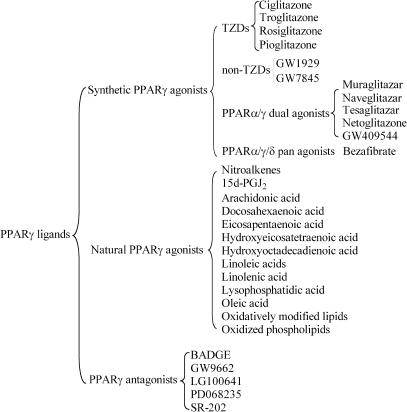
FIG. 2.
PPARγ ligands. Natural and synthetic agonists bind and activate PPARγ. Natural PPARγ agonists include 15d-PGJ2, fatty acids, oxidatively modified lipids, hydroxyeicosatetraenoic acid, hydroxyoctadecadienoic acid, oxidized phospholipids, lysophosphatidic acid, and nitroalkenes. Synthetic PPARγ agonists include TZDs, GW1929, GW7845, PPARα/γ dual agonists, and PPARα/γ/δ pan agonists. Examples of PPARγ antagonists include BADGE, GW9662, LG100641, PD068235, and SR-202.
Troglitazone (Rezulin), the first FDA-approved TZD used in the clinical setting, was discontinued from the market in 2000 because of reports of liver toxicity (125, 206, 259). Rosiglitazone (Avandia) and pioglitazone (Actos), subsequent TZD agents currently approved for clinical use, are not associated with severe hepatotoxicity (357), although weight gain and edema have been reported as side effects (263). Also, rosiglitazone has been reported to be associated with increased risks of myocardial infarction and mortality due to cardiovascular complications (265); however, the results are controversial (155, 369). Clinical data from the PROactive study found that pioglitazone reduces the risk of secondary end points, including all-cause mortality, nonfatal myocardial infarction, and stroke in diabetic patients but nonsignificantly decreases the composite primary end-point risk (95). However, a recent meta-analysis that included 19 clinical trials found that pioglitazone reduces primary end-point components, including risk of death, myocardial infarction, and stroke (225).
GW1929 and GW7845 are examples of non-TZD high-affinity ligands for PPARγ (39, 344) (Fig. 2). In addition, PPARα/γ dual and PPARα/γ/δ pan agonists have been developed to promote synergistic antidiabetic and cardiovascular protective effects. Muraglitazar, naveglitazar, tesaglitazar, and netoglitazone are several examples of PPARα/γ dual agonists (296) (Fig. 2). GW409544 has been shown to be a potent activator of both PPARα and PPARγ (390) (Fig. 2). Bezafibrate, a lipid-lowering drug that reduces the risk of myocardial infarction in patients with metabolic syndrome, is a PPARα/γ/δ pan agonist (353) (Fig. 2).
Several natural PPARγ ligands have been identified and can be classified into two major groups of compounds, fatty acids and phospholipids. PPARγ ligands consist of polyunsaturated fatty acids, including linoleic acids (36), linolenic acid (175), arachidonic acid (192), and eicosapentaenoic acid (159) (Fig. 2). Monounsaturated fatty acid compounds that bind PPARγ include oleic acid (317) (Fig. 2). Oxidatively modified lipids also bind PPARγ (Fig. 2). 15-Deoxy-δ 12,14-prostaglandin J2 (15d-PGJ2) and other J2 series prostaglandins were identified as natural ligands for PPARγ (110, 189) (Fig. 2). TZDs were demonstrated to be synthetic analogues of 15d-PGJ2 (110). Other natural PPARγ ligands include 12- and 15-hydroxyeicosatetraenoic acid (HETE) (159) and 9- and 13-hydroxyoctadecadienoic acid (HODE) (254) (Fig. 2), oxidized metabolites of arachidonic and linoleic acids, respectively. 1-O-hexadecyl-2-azelaoyl-sn-glycero-3-phosphocholine (azPC), an oxidized phospholipid, is also a PPARγ ligand (78) (Fig. 2). In addition, lysophosphatidic acid (LPA) and its naturally occurring analogue, 1-O-octadecenyl-2-hydroxy-sn-glycero-3-phosphate (AGP) also have affinity for PPARγ (361, 406) (Fig. 2).
Finally, our research group identified nitroalkenes 9-, 10-, 12-, and 13-nitro-9,12-cis-octadecadienoic acid (LNO2) (319) and 9- and 10-nitro-9-cis-octadecenoic acid (OA-NO2) (19) as natural PPARγ ligands (Fig. 2). We recently reported the crystal structure of the PPARγ ligand-binding domain bound to LNO2 and found that LNO2 promotes PPARγ interaction with coactivator motifs of transcriptional coactivators (218). The two charged residues R288 and E343 of PPARγ that make specific contacts with the NO2 are not conserved in PPARα and PPARδ (218), explaining why LNO2 preferentially activates PPARγ rather than the other two PPAR subtypes (319). LNO2 isomers bind to the two electrostatic regions of the ligand-binding pocket, and these electrostatic clusters allow binding of different ligands at the same time (218, 258). Our studies provide further evidence regarding the interaction between PPARγ and LNO2 and serve as a basis for the development of novel PPARγ ligands that could not only mimic the interactions of LNO2 on PPARγ but also extend beyond the current TZD-induced PPARγ-mediated effects in the cardiovascular system.
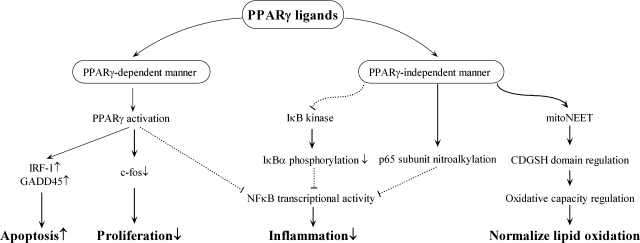
PPARγ ligands can also participate in signaling independent of PPARγ. Several studies have shown that PPARγ ligands can directly interact and inhibit transcription factors in a PPARγ-independent manner. First, although we have shown that nitroalkenes are PPARγ ligands, nitroalkene-induced inhibition of macrophage proinflammatory cytokine secretion is regulated through nitroalkylation of the p65 subunit, repressing NF-κB transcriptional activity (76) (Fig. 3).
FIG. 3.
Schematic view of PPARγ-dependent and -independent signaling pathways. PPARγ ligands can exert their effects in cardiovascular cells through PPARγ-dependent and -independent mechanisms. PPARγ-mediated increases in IRF-1 and GADD45 result in greater VSMC apoptosis. PPARγ-dependent decreases in c-fos expression attenuate VSMC proliferation. Ligand-activated PPARγ inhibits NF-κB transcriptional activity and inflammation in cardiovascular cells. PPARγ ligand–independent signaling can decrease IκB kinase activity, leading to decreased IκBα phosphorylation, NF-κB transcriptional activity, and inflammation. Another example of PPARγ ligand signaling that occurs independent of PPARγ involves nitroalkylation of the p65 subunit and eventual reduction in NF-κB activity and inflammation. Pioglitazone can regulate mitochondrial oxidative capacity and normalize lipid oxidation through direct binding to the mitoNEET protein, independent of PPARγ.
Second, 15d-PGJ2 inhibits NF-κB transcriptional activity by inhibiting IκB kinase (IKK) (54, 314, 342) and the DNA binding domains of NF-κB (342). In all likelihood, the effects of 15d-PGJ2 on IKK activity result in the inhibition of IKK-induced Ser32 and Ser36 phosphorylation of IkappaB-α (IκBα) (54) (Fig. 3). Compound G, a non-TZD agonist, also inhibits NF-κB activation by decreasing IKK activity (55). Furthermore, the administration of TZD at higher concentrations attenuates NF-κB target-gene expression in macrophages lacking PPARγ (56, 249).
Pioglitazone can bind to mitoNEET, an integral protein located in the outer mitochondrial membrane that regulates oxidative capacity (71) (Fig. 3). MitoNEET received its name because of the Asn-Glu-Glu-Thr (NEET) sequence located in the carboxyl-terminal domain. Isolated mitochondria from the hearts of mitoNEET-null mice display an overall worsening of complex 1–dependent oxygen consumption (384). Because mitoNEET is an iron-sulfur cluster containing protein, and pioglitazone has been shown to increase mitoNEET 2Fe-2S stability (279), it is possible that pioglitazone could regulate the redox potential or function of the mitoNEET iron-binding CDGSH domain [C-X-C-X(2)-(S/T)-X(3)-P-X-C-D-G-(S/A/T)-H] (385).
PPARγ antagonists are also ligands that can be used as important tools in determining PPARγ signaling and function in basic science. The safety concerns and adverse side effects of TZDs have spurred an increased effort to study possible therapeutic benefits of administering PPARγ antagonists in the clinical setting. Bisphenol A diglycidyl ether (BADGE) is often considered to be the first PPARγ ligand known to inhibit transcriptional activity (386). A potent PPARγ antagonist is GW9662, a compound that covalently modifies the Cys286 residue of the ligand-binding domain (207). Other examples of PPARγ antagonists include LG100641 (252), PD068235 (50), and SR-202 (308).
The use of different methods for studying and screening novel PPAR modulators is an important concept of drug discovery. Several examples are known by which cell-free assays can be used for PPAR-modulator screening. A cell-free competition radioreceptor assay uses recombinant PPAR along with a radioisotope-labeled ligand and competitor ligands (110, 400). The premise of coactivator-dependent receptor ligand assays (CARLAs) includes coactivator recruitment and the use of a pull-down approach to determine the amount of ligand-bound PPAR-coactivator complex. The practice of radioactive labeling is not a requirement in CARLAs, allowing a large, quantitative screening of PPAR compounds (68, 192). The scintillation proximity assays (SPAs) measure receptor–ligand interaction. Beta emission from the radioactively labeled ligand is measured, and this is advantageous because of high sensitivity, high reliability, and the lack of a required separation step (100, 262).
The use of radioisotope-free assays is an alternative approach to previous cell-free methods. Surface plasmon resonance (SPR) techniques can be beneficial for detecting ligand–nuclear receptor interactions (401) and ligand-binding effects on nuclear-receptor dimerization (402), as well as screening for ligands from ligand-bound nuclear receptor–coactivator interactions (116). Fluorescence resonance energy transfer (FRET) is a radioisotope-free assay that is used to detect and quantitate PPAR ligand binding. A ligand-induced PPAR conformational change results in coactivator recruitment, allowing the fluorescence donor indirectly linked to PPAR and the fluorescence acceptor indirectly linked to the coactivator to draw into close proximity as the excited fluorescence donor transfers energy to the acceptor (68, 411). A simple ELISA has been developed in which unliganded PPAR weakly binds to the coactivator LXXLL motifs, while ligand-bound PPAR strongly binds to these LXXLL peptides. This radioisotope-free assay uses a specific anti-PPAR antibody to detect PPAR binding (69).
IV. PPARγ and Endothelial Cells
The first evidence of PPARγ expression in endothelial cells (34, 179, 235, 387) came from several studies examining the interaction of PPARγ and plasminogen activator inhibitor type-1 (PAI-1). The expression of PAI-1 in both endothelial cells and adipoctyes is involved in limiting fibrinolysis in humans. Elevated PAI-1 has been associated with myocardial ischemia and thrombosis in mice (228). PPARγ agonists are generally found to increase PAI-I expression in endothelial cells (235, 387), although one study suggests the opposite (179). A later study provided evidence that PPARγ1 and not PPARγ2 mRNA is present in human umbilical vein endothelial cells (HUVECs) (198).
A. PPARγ and the regulation of EC inflammatory response
Adhesion molecules can bind to inflammatory cells involved in signaling and regulation on the surface of endothelial cells. These adhesion molecules include vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1), platelet–endothelial cell adhesion molecule (PECAM-1), E-selectin, and integrins. Along with monocyte chemoattractant protein-1 (MCP-1) and other chemoattractant molecules, adhesion molecules are responsible for attachment of immune cells to the endothelial layer, followed by eventual immune cell migration across the endothelium (313).
Much evidence demonstrates PPARγ inhibitory and antiinflammatory effects in endothelial cells. Several studies have reported that activation of PPARγ inhibits expression of cellular adhesion molecules, including VCAM-1, ICAM-1, PECAM, and E-selectin, in addition to inflammatory cell migration and adhesion to atherosclerotic plaques (87, 173, 241, 257, 286, 378) (Fig. 4). NF-κB plays an important role in regulating leukocyte adhesion molecule expression. Cytokines activate NF-κB in endothelial cells, thereby allowing NF-κB binding to promoters of adhesion molecule genes. Through NF-κB binding, cytokine-induced gene expression of ICAM-1, VCAM-1, and E-selectin occur in the endothelium (73). Constitutively active PPARγ inhibits NF-κB– and AP-1–regulated gene expression and binding activity in ECs, and PPARγ activation inhibits adhesion molecule expression by inhibiting NF-κB and AP-1 signaling, considered the most important transcription factors in endothelial cell signaling (378). Another mechanism that may suppress endothelial cell inflammatory signaling is the inhibition of the diacylglycerol-protein kinase C (PKC) pathway (368). A study examined the effects of PPAR-γ ligands on chemokine expression that is induced by interferon-gamma (IFN-γ) in cultured human endothelial cells. PPARγ activators decrease IFN-inducible protein of 10 kDa (IP-10), monokine induced by IFN-γ (Mig), and IFN-inducible T-cell α-chemoattractant (I-TAC) expression through the likely inhibition of NF-κB (237) (Fig. 4). However, expression of MCP-1 is not changed in this study (237), in contrast to a previous report showing that TZDs inhibit tumor necrosis factor-alpha (TNF-α)- and interleuken-1beta (IL-1β)-induced MCP-1 mRNA expression and secretion (253).
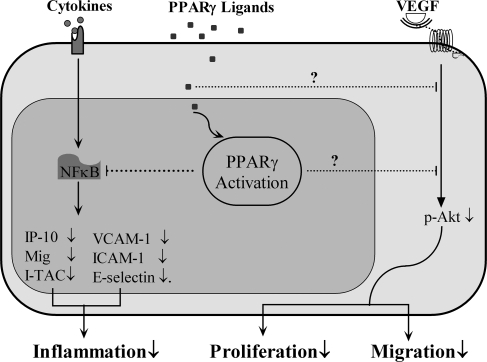
FIG. 4.
Schematic view of PPARγ activation in ECs. Natural or synthetic PPARγ ligands attenuate VEGF-induced Akt phosphorylation, inhibiting EC proliferation and migration. Ligand-activated PPARγ exerts its antiinflammatory effects by inhibiting cytokine-induced NF-κB activation in ECs.
In cultured endothelial cells, TZDs may reduce superoxide production and inflammation (162, 244) by suppressing expression of NAD(P)H oxidase subunits that are critical for superoxide generation (162). Furthermore, a recent study found that mice expressing a dominant negative PPARγ mutation show elevated oxidative stress and impaired endothelial function in cerebral arteries (32). Next, in cultured endothelial cells, TZDs, along with 15d-PGJ2, attenuate IFNγ-induced major histocompatibility complex class II (MHC-II), a protein involved in regulating immune responses and T-cell activation (194). Finally, in HUVECs, TZDs promote expression of heme-oxygenase 1 (HO-1), a PPARγ target gene with antiinflammatory properties (193).
B. PPARγ and the regulation of vascular tone
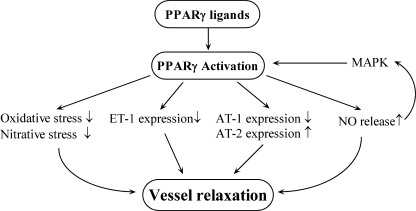
Endothelin-1 (ET-1) is a vasoconstrictive protein that can also regulate VSMC proliferation. PPARγ ligands attenuate both ET-1 expression and secretion in endothelial cells by blocking AP-1 signaling (83, 118, 163, 234, 318) (Fig. 5). Angiotensin II (AngII) is also a potent vasoconstrictor that increases angiotensin II type 1 (AT1) receptor expression, leading to narrowing of blood vessels and elevations in oxidative stress. In Sprague–Dawley rats, rosiglitazone and pioglitazone blunt AngII-induced increases in blood pressure by downregulating AT1 receptors and increasing angiotensin II type 2 receptor (AT2) expression (87) (Fig. 5). Both TZDs improve AngII-induced endothelial dysfunction (87). Subsequently, another study reported that in male apoE-knockout (apoE−/−) mice, endothelial dysfunction occurs after AngII treatment in association with decreased PPARγ gene and protein expression (355). Because human PPARγ dominant negative mutations are associated with hypertension (27), TZD-induced PPARγ activation may be one method of treatment for the effects of elevated blood pressure.
FIG. 5.
Schematic view of PPARγ activation in vascular tone regulation. PPARγ ligands decrease ET-1 and AT-1R expression and increase AT-2R expression. PPARγ ligands stimulate NO release, and NO can activate endothelial cell PPARγ through MAPK. TZDs can also decrease oxidative and nitrative stress.
Conversely, endothelial cell–derived nitric oxide (NO) is a molecule that is a key participant in vasodilatory activity (280). In 1998, it was found that troglitazone causes vasodilation in humans (117). Subsequent studies showed that PPARγ ligands increase NO production and release (49, 67, 294) (Fig. 5), although it appears that PPARγ ligands may stimulate production of endothelial cell NO through different pathways (294). Ligand-activated PPARγ was found to be critical to heat-shock protein 90/endothelial nitric oxide synthase (eNOS) interaction and eNOS phosphorylation in HUVECs (294). Furthermore, NO was recently reported to activate PPARγ in endothelial cells through a p38 MAPK signaling pathway (297) (Fig. 5). TZDs possess vasculoprotective effects through the attenuation of oxidative and nitrative stresses (Fig. 5), and elevated NO levels. One study in male hypercholesterolemic rabbits suggests that rosiglitazone protects the endothelium by inhibiting superoxide, peroxynitrite, and excess NO production (351). Similar to adipocytes and VSMCs (94, 165), TZD-induced reduction in elevated NO levels may be the result of inducible nitric oxide synthase (iNOS) inhibition in endothelial cells (351).
C. PPARγ and VEGF
PPARγ activators have been shown to modulate in vivo vascular endothelial growth factor (VEGF)-induced angiogenesis and also in vitro differentiation of endothelial cells into tubelike structures. In addition, VEGF is known to play a role in endothelial cell proliferation, migration, vascular permeability, and atherosclerosis. Several studies demonstrated that PPARγ agonist inhibition of VEGF-induced angiogenesis may be PPARγ dependent (Fig. 4), part of which includes the inhibition of VEGF receptors and urokinase plasminogen activator expression along with increased PAI-1 expression, NO, and apoptosis (185, 387). Rosiglitazone has been shown to decrease VEGF secretion and indirectly to inhibit angiogenesis in tumor endothelial cells (282). However, a recent study found that administration of GW1929, through PPARγ-mediated signaling, increases in vitro endothelial cell tube formation and in vivo neovascularization that is associated with elevated VEGF (33).
D. PPARγ and EC migration
PPARγ ligands are also involved in antimigratory actions of endothelial cells. VEGF-induced migration of HUVECs is inhibited by troglitazone and ciglitazone, providing evidence of PPARγ ligand antimigratory effects on endothelial cells. Moreover, the effects of PPARγ ligands on EC migration include inhibition of Akt phosphorylation (129) (Fig. 4). Leptin, through endothelial ob receptor activation, has been shown to promote endothelial cell proliferation, survival, and vascular angiogenesis (38, 331). In addition, leptin can regulate endothelial cell Akt phosphorylation (366) and migration (128). The administration of PPARγ ligands inhibits leptin-stimulated Akt phosphorylation and EC migration (128). The tumor-suppressor phosphatase and tensin homologue (PTEN), a modulator of the PI3K/Akt signaling pathway, has been reported to attenuate VEGF-induced EC migration through the inhibition of Akt phosphorylation (158), and PTEN levels were found to be elevated after administration of PPARγ ligands, suggesting the possibility that PTEN plays a role in the inhibitory actions of TZDs on VEGF- and leptin-induced Akt phosphorylation and endothelial cell migration (128) (Fig. 4). Another study, by using scrape-wound and chemotactic assays, found that troglitazone inhibits endothelial cell migration in high-glucose media (146). Troglitazone was shown to accelerate endothelial cell coverage and repair after rat aortic balloon injury. However, the in vivo data suggest that endothelial repair may have occurred as a result of troglitazone-induced suppression of endothelial cell apoptosis rather than a reduction in endothelial cell migration (146). A PPARγ-mediated mechanism for TZD-induced migratory activity is not suggested in this study. Moreover, further evidence suggests that the effects of TZD treatment pertaining to endothelial cell migration might occur through PPARγ-independent signaling (204).
E. PPARγ and EC apoptosis
Previous studies suggest that 15d-PGJ2 and ciglitazone may induce endothelial cell apoptosis through a PPARγ-mediated signaling pathway (34, 213). Our laboratory found that administering a PPARγ antagonist did not block 15d-PGJ2–induced inhibition of platelet-derived growth factor (PDGF), providing evidence that 15d-PGJ2 apoptotic and antiproliferative effects may be PPARγ independent in endothelial cells (409). However, PPARγ1 was reported to induce apoptotic genes in HUVECs (169), and a study with PPARγ gain- and loss-of-function techniques found PPARγ to be critical to endothelial cell apoptosis (10). Rosiglitazone was shown to inhibit angiogenesis through a PPARγ-dependent proapoptotic pathway in HUVECs (185). The induction of apoptosis is possibly beneficial, because activated cells may produce cytokines. In cases of severe pulmonary hypertension, lung arterioles consist of phenotypically altered endothelial cells that reduce blood flow and elevate blood pressure. PPARγ-mediated EC apoptosis could be beneficial in alleviating lumen-obliterating endothelial cell growth (10).
F. PPARγ and endothelial progenitor cells
Endothelial progenitor cells (EPCs) are circulating vascular progenitor cells that have been shown to stimulate reendothelialization and decrease neointima formation (376). In vitro and in vivo studies demonstrated that rosiglitazone stimulates angiogenic progenitor cell (APC) differentiation to endothelial cells to promote reendothelialization and vascular protection against injury (377). Rosiglitazone was shown to improve impaired EPC function in diabetic individuals (292). In EPCs isolated from male subjects, rosiglitazone and 15d-PGJ2 prevented C-reactive protein–induced EPC dysfunction and promoted angiogenesis (367). Rosiglitazone returns migratory activity to baseline in cultured EPCs from diabetic individuals, which may improve impaired EPC function associated with diabetes (291). Pioglitazone has been shown to increase migratory activity of cultured EPCs from patients with coronary artery disease through PPARγ-dependent signaling (383), as well as to enhance circulating and bone marrow EPC migratory activity (122). Rosiglitazone may also reduce NAD(P)H oxidase and the resultant increase in oxidative stress while enhancing EPC reendothelialization, promoting vessel repair, and improving vascular function (338). Rosiglitazone and pioglitazone, in addition to improving EPC-induced angiogenesis, can attenuate EPC apoptosis (122, 367). A reduction in EPC apoptosis may be of great benefit to individuals with vascular disease (122). PPARγ inhibition of EPC apoptosis may have significant clinical relevance because previous studies showed that different types of EPCs have different morphology, proliferation rates, survival rates, and gene-expression profiles that contribute to different functions in neovasculogenesis (160, 398). Finally, it has been suggested that many of the beneficial cardiovascular effects from TZD treatment in patients may be due to the positive effects on EPCs (367). The proapoptotic data in ECs and antiapoptotic data in EPCs may be due to different PPARγ functions in these cells. The role of PPARγ-independent effects on apoptosis in these cells is a possibility and also should be considered.
V. PPARγ and Vascular Smooth Muscle Cells
In 1998, three investigative groups reported evidence of PPARγ expression in rat aortic and human VSMCs (164, 239, 340). Similarly, a later study observed that PPARγ expression is present in early human vascular lesions and is upregulated in rat aortic smooth muscle cells after balloon injury (198). Another study reported that both human coronary artery and aortic VSMCs express PPARγ1 and PPARγ2 isoforms (29). PPARγ mRNA levels were reported to increase in mesenteric arteries of both young and adult spontaneously hypertensive rats (SHRs), suggesting that PPARγ expression is differentially regulated in SHRs (88). Similar data regarding mRNA expression in SHRs were reported from our laboratory. However, we found PPARγ protein expression and function from SHR vascular smooth muscle cells to be lower compared with those in Wistar–Kyoto rats. It is likely that the suppressed PPARγ function is a result of decreased protein expression, which could explain the increased VSMC proliferative activity in SHRs (388).
A. PPARγ and VSMC proliferation
TZDs were reported to attenuate VSMC proliferation and regulate vascular tone well before being identified as PPARγ ligands (98, 337, 407). Troglitazone was initially found to suppress basic fibroblast growth factor (bFGF)-induced vascular smooth muscle cell growth, preventing rat aortic neointima formation after endothelial injury (199) (Fig. 6). Further studies also confirmed the antiproliferative activity of troglitazone on human VSMCs (29, 250). However, these initial studies did not examine whether the vasculoprotective effects of troglitazone were PPARγ mediated. A later study with a balloon-injury model confirmed that the inhibitory effect of troglitazone on VSMC proliferation occurs through a PPARγ-mediated pathway (198). TZDs (troglitazone, rosiglitazone, and pioglitazone) inhibit VSMC proliferation in several human vascular cell beds. The particular TZD administered rather than the vascular source is critical for the potential suppression of VSMC proliferation (79).
FIG. 6.
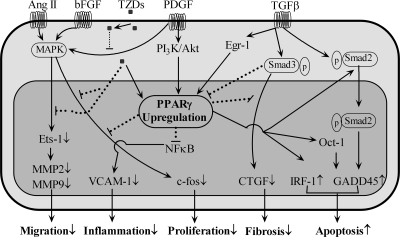
Schematic view of PPARγ activation in VSMCs. In VSMCs, TZDs attenuate growth factor–induced (e.g., AngII) cell migration, proliferation, and fibrosis in either a PPARγ-dependent or -independent manner by interfering with growth factor–stimulated signaling pathways. PPARγ activation exerts antiinflammatory roles by inhibiting the NF-κB pathway; PPARγ activation promotes apoptosis via inducing IRF-1 or GADD45 expression.
C-fos is involved in the MAPK pathway, which plays a role in cell proliferation. Troglitazone attenuates bFGF-induced c-fos expression in cultured VSMCs by inhibiting the MAPK signaling pathway (199) (Fig. 6). A later study also found troglitazone to inhibit PDGF-induced c-fos mRNA expression (29) (Fig. 6). Finally, a recent report demonstrated that rosiglitazone and PPARγ overexpression suppress bFGF-induced c-fos mRNA expression (Fig. 6). Moreover, PPARγ dominant negative gene transfer attenuates rosiglitazone-induced inhibition of c-fos mRNA expression (223).
Connective tissue growth factor (CTGF) has the ability to regulate many transforming growth factor-beta (TGF-β) responses in VSMCs, including proliferation, migration, and fibrosis. Data from our laboratory demonstrated that PPARγ interrupts the Smad3 signaling pathway, inhibiting TGF-β–stimulated CTGF expression in human aortic smooth muscle cells (HASMCs) (114) and suggesting crosstalk between PPARγ and TGF-β pathways (Fig. 6). We found that TGF-β induces early PPARγ stimulation and late PPARγ inhibition of gene expression and that growth factor– and cytokine-induced PPARγ expression is inhibited by TGF-β. Early activation of TGF-β–induced PPARγ is mediated by early growth response-1 (Egr-1) signaling, whereas inhibition of PPARγ by TGF-β is mediated by Smad3, AP-1, and Nab2 (112) (Fig. 6).
Studies from our laboratory also provided the first evidence that the PI3-kinase/Akt-dependent pathway is a regulator of PPARγ1 gene expression in VSMCs. We reported that PPARγ1 is upregulated by PDGF via PI3-kinase/Akt signaling (115) (Fig. 6). Dominant negative overexpression of the p85 subunit from PI3-kinase or Akt proteins also suppresses PDGF-induced PPARγ expression (115). We also found Egr-1 to be the transcriptional regulator of both growth factor– and cytokine-induced VSMC PPARγ1 gene expression. Our results demonstrate that PPARγ is involved in a feedback mechanism that negatively controls VSMC activation (111).
Angiotensin II plays a crucial role in controlling the proliferation and migration of VSMCs. Troglitazone blocks AngII-induced MAPK activation of VSMCs (140) (Fig. 6). One possible mechanism includes the attenuation of PKC nuclear activity and PKC-mediated extracellular signal regulated kinase 1/2 (ERK 1/2) translocation to the nucleus (132). Another mechanism of AngII-induced VSMC proliferation involves the upregulation of AT1 receptors. PPARγ ligands have been reported to be responsible for the inhibition of AT1 expression in VSMCs (343, 349). Further, it was suggested that ligand-activated PPARγ inhibits AT1 transcription by blocking Sp1, leading to the suppression of AT1-receptor expression (343). Finally, telmisartan, an AT1-receptor antagonist with partial PPARγ activator properties, inhibits AT1-receptor expression. Conversely, administration of the PPARγ antagonist GW9662 attenuates telmisartan-induced inhibition of AT1, confirming a participatory role for PPARγ in this signaling cascade (167). Both 15d-PGJ2 and rosiglitazone were shown to decrease AngII-stimulated eukaryotic initiation factor 4E-binding protein 1 (4E-BP1) and Src homology (SH) 2–containing inositol phosphatase 2 (SHIP2) phosphorylation, suppressing Ang II–induced VSMC growth (28). Rosiglitazone may directly decrease SHIP2 activity (28). A recent study suggests that pioglitazone and rosiglitazone inhibit AngII-induced Rho kinase, a known modulator of VSMC tonicity and proliferation. This may be accomplished through increased cytosolic Src homology region 2–containing protein tyrosine phosphatase-2 (SHP-2) expression and reduced Vav phosphorylation (372). However, the effects of PPARγ activators on AngII cell signaling and growth are still unclear.
One of the most important mechanisms in preventing VSMC growth involves suppression of cell-cycle signaling. In PDGF- or insulin-stimulated cultured rat VSMCs, PPARγ ligands prevent proliferation by inhibiting the G1/S phase, a rate-determining step in cell-cycle progression (373). Cell-cycle suppression likely occurs through decreased phosphorylation of the retinoblastoma protein (Rb) (373), a mediator of G1/S progression (327). Moreover, PPARγ agonists prevent mitogen-induced p27(Kip1) degradation (373), a known inhibitor of cdk and Rb phosphorylation (328). A non-TZD partial PPARγ agonist can attenuate mitogen-induced downregulation of p27(Kip1) and proliferation in rat aortic vascular smooth muscle cells. Furthermore, functional PPARγ is necessary to obtain maximal antiproliferative effects in VSMCs (42). PPARγ ligands also attenuate PDGF-induced p21(Cip1) expression through the likely inhibition of PKC-δ phosphorylation and activity in cultured rat aortic smooth muscle cells (374). p21(Cip1) promotes activation of the cyclin/cdk complex that eventually results in G1/S phase progression (195, 328). Repression of p21(Cip1) may be another mechanism by which PPARγ attenuates VSMC proliferation. Minichromosome maintenance proteins (MCMs) 6 and 7 participate in the initial stages of DNA replication (231) and are considered to be E2F target genes (272). On retinoblastoma phosphorylation, E2F dissociates from Rb and is released for transactivation of DNA synthesis target genes (151). PPARγ ligands attenuate MCM 6 and 7 expression in VSMCs through the prevention of E2F release from Rb transactivation, further demonstrating that PPARγ agonists inhibit G1/S cell-cycle progression, in this case by curtailing pRb/E2F/MCM signaling (43).
Telomerase is important for many cellular functions, including VSMC proliferation. PPARγ ligand administration was shown to downregulate telomerase activity in cultured VSMCs, because of likely inhibition of telomerase reverse transcriptase (TERT) expression, the catalytic subunit of telomerase. Overexpression of TERT abolishes PPARγ-ligand inhibition of VSMC proliferation. In addition, the Ets-1 transcriptional factor regulates TERT, and PPARγ agonists inhibit both Ets-1 mRNA expression and binding to the TERT promoter. Thus, it is likely that PPARγ ligands target TERT for downregulation to counteract the proliferative properties of vascular smooth muscle cells (269).
Another mechanism suggests that PPARγ ligands inhibit insulin-induced mitogenic signaling by preventing phosphorylation of the Elk-1 transcription factor (130). A recent in vitro study showed that troglitazone attenuates LDL-induced VSMC proliferation and production of superoxide, a contributor to proliferation of VSMCs (153). Finally, PPARγ has also been shown to induce a differentiated phenotype in proliferative VSMCs. PPARγ-dependent signaling increases smooth muscle α-actin (SM-α-actin) and smooth muscle myosin heavy chain (SM-MHC), markers of differentiated VSMCs. Moreover, the effects of PPARγ on VSMC differentiation appear to be mediated by the GATA-6 transcription factor (4).
B. PPARγ and VSMC migration
Troglitazone has been shown to inhibit PDGF-induced vascular smooth muscle cell migration (29, 199). In addition to troglitazone, 15d-PGJ2 (198, 239) and rosiglitazone (198) attenuate PDGF-induced VSMC migration. CTGF is known to be involved in VSMC migration, and data from our laboratory provide evidence that PPARγ inhibits CTGF expression (114). These studies provide strong support for the involvement of activated PPARγ in the prevention of VSMC migration that leads to subsequent neointima formation.
Angiotensin II is involved in the control of VSMC proliferation and migration. Troglitazone can block AngII-induced MAPK activation of VSMCs, resulting in the inhibition of VSMC migration (140) (Fig. 6). PPARγ activators can also inhibit PDGF-, thrombin-, and insulin-like growth factor-1 (IGF-1)-induced VSMC migration through MAPK and downstream nuclear signaling (133). Furthermore, PPARγ ligands were reported to inhibit PDGF-induced Ets-1 nuclear expression in cultured VSMCs (Fig. 6) or from rat aortic balloon injury. Ets-1 is a transcription factor that is part of ERK/MAPK cell migratory signaling. Moreover, Ets-1 is involved in the transcriptional regulation of matrix metalloproteinase-2 (MMP-2) and matrix metalloproteinase-9 (MMP-9), facilitators of VSMC migration (131) (Fig. 6). PPARγ activators decrease MMP-9 mRNA and protein expression, along with activity, whereas PPARγ inactivation through phosphorylation reverses agonist-induced inhibition of MMP-9 expression (239).
C. PPARγ and VSMC apoptosis
In VSMCs, PPARγ can signal both growth inhibition (405) and apoptosis (44, 148). PPARγ activation increases GADD45 expression and caspase-mediated apoptosis (Fig. 6). The Oct-1 protein, a transcription factor regulated by PPARγ, is critical for PPARγ-induced GADD45 protein expression (44) (Fig. 6). PPARγ ligand administration and PPARγ overexpression have been reported to upregulate interferon regulatory factor (IRF-1) expression, mediating PPARγ-induced apoptosis in VSMCs (Fig. 6). Further evidence of proapoptotic effects is provided by using an anti-sense approach to suppress IRF-1 expression in VSMCs (224). Pioglitazone is shown to increase apoptosis through PPARγ-dependent TGF-β release in cultured VSMCs, likely facilitating phosphorylated Smad2 nuclear translocation (303) (Fig. 6). TGF-β–induced apoptosis is mediated, in part, by Smad-dependent GADD45 expression, providing further evidence that GADD45 mediates VSMC apoptosis (397) (Fig. 6). Pioglitazone is also reported to induce apoptosis through Smad2 phosphorylation in cultured VSMCs from both nondiabetic and diabetic patients, usually resistant to induced apoptosis (315). Furthermore, troglitazone can induce apoptosis by activating GADD45 and p53 expression independent of PPARγ activation (275). Rosiglitazone at high concentrations can more potently induce apoptosis in intimal compared with medial smooth muscle cells (35).
D. PPARγ and the regulation of VSMC inflammatory response
CCAAT/enhancer-binding proteins (C/EBPs) are involved in transcriptional regulation of inflammatory cytokines and other proteins. PPARγ ligands attenuate C/EBPδ expression, and C/EBPδ overexpression reverses PPARγ ligand inhibition of cytokine gene expression (346). Interestingly, elevations in C/EBPδ levels due to inflammation increase PPARγ expression and strengthen its antiinflammatory effect in VSMCs (347). In addition, PPARγ ligands suppress C/EBPδ mRNA and protein levels by dephosphorylating STAT-3 (347), suggesting that PPARγ and C/EBPδ participate in negative autoregulation feedback. Moreover, PPARγ overexpression decreases C/EBPδ promoter activity, further indicating the presence of receptor-dependent signaling in C/EBPδ expression (347). This mechanism is likely involved in the suppression of inflammatory cytokines during atherosclerosis (347). Other antiinflammatory responses involving PPARγ activation include the suppression of TNF-α–induced expression of VCAM-1 (Fig. 6), MCP-1, and fractalkine (CX3CL1) in cultured VSMCs through inhibition of NF-κB (283).
VI. PPARγ and Monocytes/Macrophages
PPARγ expression is present in murine macrophages (8, 307), neointimal lesions (198), macrophage-derived foam cells in both early and advanced stages of atherosclerotic lesions (240, 306), and differentiated human monocyte–derived macrophages (64). However, PPARγ expression, critical for macrophage lipid metabolism, is not a determinant for macrophage differentiation in vivo or in vitro (56, 249). PPARγ is also found in other inflammatory cells, including human peripheral blood T cells (395), human CD4+ T cells (236), and mature dendritic cells from the spleen (106). PPARγ expression is also confirmed in mouse T-helper cells (70). The PPARγ1 isoform is found in THP-1 and RAW 264.7 cells (306).
A. PPARγ and monocyte/macrophage inflammatory signaling
Macrophages are often considered to be heterogeneous and respond to various signaling cascades (365). Different cytokines determine the type of stimulatory or inhibitory response on inflammatory signaling by inducing either a “classic” or “alternative” activation pathway in macrophages. Th1 cytokines, including lipopolysaccharide (LPS), IFN-γ, and IL-1β, tend to be involved in “classic” activation, whereas Th2 cytokines, including IL-4 and IL-13, likely activate the “alternative” pathway. M1 macrophages are involved in pro-inflammatory cytokine expression and oxidative stress, whereas M2 macrophages play a role in apoptotic cell phagocytosis, sequestering of pathogens, and wound healing (267, 341). Moreover, macrophages demonstrate functional plasticity because they have the ability to switch between M1 and M2 states of activation (295).
PPARγ was shown to be necessary for monocyte-derived M2 macrophage phenotype expression (37). PPARγ is also upregulated during M1 switching to an M2 phenotype, which is critical for increased expression of CD36 (31), arginase I (267), and the mannose receptor (37). PPARγ has been shown to regulate M1/M2 switching, in part by reducing inflammatory cytokine expression normally associated with an M1 phenotype, such as TNF-α, IL-1β, and IL-6 (174), and suppressing in vitro macrophage activation (307). The suggestion that PPARγ is an inflammatory regulator is further illustrated by the belief that PPARγ may reverse suppression of cytotoxic T lymphocytes, normally a function of M2 activation (364). In addition, specific genes from both M1 and M2 macrophages were found to be unaltered when administering TZD (56, 154, 382).
PPARγ participates in antiinflammatory signaling to protect against atherosclerotic lesion formation, in part, through negative regulation of macrophage transcriptional activity. PPARγ ligands, in a PPARγ-dependent manner, attenuate monocyte and macrophage MMP-9 expression and secretion (186, 240, 307, 330), iNOS, and scavenger receptor-A (SR-A) through the likely inhibition of AP-1, STAT, and NF-κB transcription factor signaling (307). In addition, PPARγ negatively regulates a specific population of pro-inflammatory genes controlled by these transcription factors (307, 330) (Fig. 7). PPARγ activation also inhibits macrophage osteopontin (OPN) expression by interfering with nuclear factor binding to the homeobox-like A/T rich region of the OPN promoter, providing another example of PPARγ inhibition of macrophage gene expression (277, 278). Similarly, PPARγ ligands were shown to inhibit proinflammatory cytokine (IL-6, IL-1β, TNF-α) expression in monocytes (174) (Fig. 7). However, PPARγ may not be required for IFN-α– or LPS-induced pro-inflammatory cytokine secretion in macrophages (56, 249). Moreover, it is possible that PPARγ ligands can upregulate antiinflammatory cytokines (Fig. 7), such as the IL-1–receptor antagonist (IL-1Ra), suggesting another way by which PPARγ can suppress proinflammatory activity (245). PPARγ also regulates inflammatory signaling in cells other than monocytes and macrophages. PPARγ activators can suppress IL-2 (70, 236, 395, 396), IFN-γ (236), and TNF-α (236) in human and animal lymphocytes. PPARγ ligands also decrease CD40-induced IL-12 secretion in dendritic cells (106).
FIG. 7.
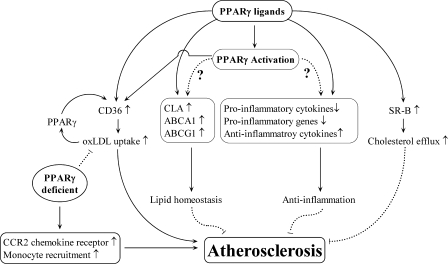
Schematic view of PPARγ roles in atherosclerosis. PPARγ ligands increase CLA, ABCA1, and ABCG1 expression, leading to improved lipid homeostasis. PPARγ agonists also decrease proinflammatory cytokine and gene expression and increase antiinflammatory cytokine expression. PPARγ ligands increase SR-B expression, which promotes cholesterol efflux. Conversely, PPARγ activation upregulates CD36 expression, resulting in increased oxLDL uptake. Increased oxLDL levels further stimulate PPARγ expression, which leads to increased CD36 expression. Finally, loss of PPARγ increases CCR2 expression and monocyte recruitment.
B. PPARγ and monocyte/macrophage migration and apoptosis
In addition to antiinflammatory properties, PPARγ ligands inhibit monocyte/macrophage migration. Troglitazone or rosiglitazone administration results in the inhibition of MCP-1–induced monocyte migration (186). Furthermore, oxidized low-density lipoproteins (oxLDLs) may attenuate MCP-1–dependent monocyte migration by inhibiting chemokine receptor 2 (CCR2) expression (145). Both 9-HODE and 13-HODE, components of oxLDL that stimulate monocyte differentiation to macrophages, inhibit macrophage migration and enhance macrophage adhesion to VSMCs by upregulating CX3CR1 and decreasing CCR2 expression through a PPARγ pathway (26), suggesting a proinflammatory role for macrophage PPARγ that may lead to the development of atherosclerosis. Moreover, a recent study showed that PPARγ-dependent signaling increases CXCR2 receptor expression in primary human macrophages, providing further evidence that PPARγ can also have proinflammatory properties (309). Next, PPARγ ligands can also induce apoptotic activity by blocking the NF-κB antiapoptotic signaling cascade in human macrophages (64). Finally, PPARγ activation during differentiation of human monocytes to macrophages decreases the ability to engulf apoptotic neutrophils (232).
C. PPARγ and monocyte/macrophage iNOS expression
Studies have shown that the ability of PPARγ to repress iNOS expression (159, 217, 307) may occur through direct interaction with the CREB-binding protein (CBP) (217). Furthermore, a recent provocative report suggested another mechanism by which PPARγ represses iNOS and other proinflammatory genes in murine macrophages. SUMO-1 covalently modifies several transcription factors, including PPARγ (271). SUMOylation of PPARγ results in binding to the nuclear-receptor co-repressor (N-CoR)-histone deacetylase-3 (HDAC-3) complex, repressing proinflammatory signaling, particularly NF-κB target genes (270, 287). Furthermore, PPARγ and the glucocorticoid receptor were found to inhibit iNOS expression through at least two different signaling pathways (270).
D. PPARγ and monocyte/macrophage CD36 expression
CD36 is a scavenger receptor that promotes uptake of oxLDL (101). Ligand-dependent PPARγ has been shown to increase CD36 expression through various signaling pathways in both cultured monocytes and macrophages (159, 254, 358). By using embryonic stem cell–derived macrophages, two studies reported that PPARγ is required for ligand-activated CD36 gene regulation (56, 249). Macrophages from PPARγ conditional knockout mice are shown to have decreased CD36 expression compared with wild-type macrophages (8). However, although CD36 is a PPARγ target gene, PPARγ is not mandatory for oxLDL uptake in differentiated macrophages (56). Moreover, an in vivo study showed that TZDs decrease macrophage CD36 protein expression in ob/ob mouse models that display characteristics of insulin resistance, diabetes, and obesity, all of which are risk factors for atherosclerosis (219). TGF-β phosphorylation of PPARγ has been suggested as an inhibitory mechanism of action regarding PPARγ-mediated CD36 expression (143).
E. PPARγ and monocyte/macrophage lipid homeostasis
A role for PPARγ activation in macrophage cholesterol homeostasis has been established. CLA-1 is a high-density lipoprotein (HDL) receptor involved in cellular cholesterol removal. CLA-1 was shown to be upregulated by PPARγ ligands in differentiated human macrophages (63) (Fig. 7). PPARγ ligands also demonstrate a role in reverse-cholesterol transport by upregulating expression of ATP-binding cassette (ABC) transporters ABCA1 (11, 57, 65) and ABCG1 (8, 11) in monocytes and macrophages (Fig. 7), possibly through an LXR-α–mediated transcriptional signaling pathway (57) that may include caveolin-1 (227). This is important, because an atheroprotective role for granulocyte–macrophage colony-stimulating factor (GM-CSF) may involve PPARγ and ABCA1 signaling (92). Providing further evidence, a PPARγ conditional knockout mouse model displays a reduction in macrophage cholesterol efflux, although this study found that troglitazone attenuates cholesterol efflux and ABCA1 expression in macrophages from both PPARγ knockout and wild-type mice, suggesting some PPARγ-independent effects (8). Finally, although PPARγ is not required for oxLDL uptake in differentiated macrophages (56), oxLDL uptake is worsened in PPARγ-deficient macrophages (249). This finding further indicates an important role for PPARγ in oxLDL lipid trafficking.
VII. PPARγ and Atherosclerosis
Diabetes has been estimated to increase the risk of developing atherosclerosis by twofold (178). Increasing evidence suggests that failure to maintain normal glycemic control influences the development of atherosclerosis (142, 356). As previously mentioned, PPARγ is expressed in atherosclerotic lesions (240, 306). Monocytes differentiate into macrophages on migration into the vessel wall. In macrophages, oxLDL uptake occurs through scavenger receptors, promoting the expression of foam cells (127, 254). Initially, PPARγ was thought to be proatherosclerotic. PPARγ ligand administration, combined with an RXR agonist, upregulates oxLDL uptake through increased CD36-receptor expression (Fig. 7). Furthermore, oxLDL exposure increases SR-A and CD36 mRNA expression through a PPARγ-dependent mechanism, signaling further oxLDL cellular uptake (254) (Fig. 7). Moreover, PPARγ is highly expressed in foam cells (358). PPARγ also is found to be highly expressed in cultured CD36+ HASMCs, and troglitazone treatment upregulates CD36 expression only in CD36+ smooth muscle cells, suggesting that VSMCs may be able to obtain a macrophage-like phenotype and differentiate into foam cells (242). Furthermore, LPA, a PPARγ ligand synthesized during mild oxidation of LDL (332), and other PPARγ agonists were also shown to increase neointima formation in rats (406). Collectively, these studies suggest that PPARγ is involved in the development of atherosclerosis. Another study found that oxLDL uptake was decreased in PPARγ-deficient macrophages, partly due to loss of CD36 expression. However, troglitazone treatment had no effect on intracellular oxLDL levels (249). A likely explanation is that troglitazone stimulates CD36 while suppressing SR-A expression (249). It is likely that TZD increases neither macrophage intracellular cholesterol levels nor foam cell formation.
However, the majority of studies suggest an atheroprotective role for TZDs and PPARγ. PPARγ-ligand treatment increases scavenger receptor B (SR-B) expression in atherosclerotic lesion macrophages of ApoE−/− mice, potentially facilitating cholesterol efflux (63) (Fig. 7). Treatment with rosiglitazone and GW7845 inhibits atherosclerosis in male low-density lipoprotein receptor knockout (LDL-R−/−) mice although CD36 expression is increased. Interestingly, PPARγ ligand treatment did not reduce atherosclerosis in female mice. Hormonal differences could be an explanation for the dissimilar outcome between genders (215). In male LDL-R−/− mice fed either a high-fructose or high-fat diet, troglitazone can suppress atherosclerotic lesion formation (72). Next, rosiglitazone reduces aortic atherosclerotic lesions in both diabetic and nondiabetic apoE−/− male mice (212). Finally, rosiglitazone treatment is associated with increased ABCA1 gene expression (Fig. 7) and decreased macrophage accumulation in diabetic mice, providing further evidence of an antiatherosclerotic role (48).
LDL-R−/− mice given transplants with bone marrow deficient in PPARγ demonstrate an overall worsening of atherosclerosis (57). Next, bone marrow generated from macrophage PPARγ knockout (MacPPARγ KO) mice was transplanted to LDL-R−/− and wild-type mice. Mice reconstituted with macrophage PPARγ knockout bone marrow display increased lesion formation in both strains compared with respective controls. In cases of mild or severe hypercholesterolemia, loss of PPARγ results in increased atherosclerosis, possibly due to increased CCR2 chemokine receptor expression and monocyte recruitment (18) (Fig. 7).
In vitro studies show that functional PPARγ is more prevalent in intimal VSMCs compared with medial smooth muscle cells. Therefore, intimal vascular smooth muscle cells are a likely target for PPARγ in regulating antiatherosclerotic effects (35). Another study showed that transfer of the PPARγ wild-type gene in a rat carotid artery balloon injury model results in decreased neointima formation and that rosiglitazone-induced inhibition of VSMC proliferation and migration is blunted by PPARγ-dominant negative gene transfer. However, the effects of rosiglitazone primarily, but not entirely, occur through PPARγ-mediated signaling (223). In human atherosclerotic plaques, PPARγ is associated with M2 macrophage marker expression, although PPARγ activation does not switch M1 macrophages, foam cells, or already differentiated resting macrophages in vitro or atherosclerotic plaque macrophages in vivo to an M2 phenotype (37).
PPARγ ligands may also reduce atherosclerotic development by inhibiting IFN-γ–induced increases in MHC-II expression that normally activate T lymphocytes and control immune responses (194). Increased expression of iNOS has been shown in coronary atherosclerotic plaques of patients with unstable angina (84). Troglitazone and 15d-PGJ2 are found to suppress IL-1β–induced iNOS production and cytokine-induced NO synthesis in vascular smooth muscle cells. NF-κB, critical for iNOS transactivation, is downregulated by both PPARγ activators in VSMCs (165). Finally, osteoprotegrin (OPG) is involved in the regulation of atherosclerotic lesion calcification. In our laboratory, we demonstrated that PPARγ ligands or PPARγ overexpression inhibits OPG expression in human aortic smooth muscle cells (113). The role of PPARγ in atherosclerosis is controversial, with much of the literature providing the rationale that PPARγ plays a regulatory role against the development of atherosclerosis. However, several considerations must be taken into account. Pioglitazone binds with less affinity to PPARγ compared with rosiglitazone, yet has been shown to be more effective at improving patient lipid profiles (135). Many of the beneficial effects of TZD-induced activation of PPARγ-mediated transcription are still unclear, particularly because the effects of TZDs on PPARγ-mediated transcriptional activity are tissue specific. Moreover, the biologic effects of PPAR target genes remain largely unestablished, and because PPAR agonists tend to participate in both gene activation and repression, the known biologic effects of PPAR target genes tend to be rather complex. Thus, a need exists for further research regarding the role of PPARγ and its ligands in atherosclerotic plaque formation, although the literature provides compelling evidence that PPARγ activation is important for the attenuation of atherosclerosis.
VIII. PPARγ and the Heart
The role of PPARγ in the heart is controversial and often paradoxical. First, myocardial PPARγ expression seems to vary between studies (16, 124, 392, 394). Next, although several reports have demonstrated beneficial effects of PPARγ agonist administration on the heart (3, 16, 136, 394) (Fig. 8), the effects of TZDs on cardiac function are in question, particularly in humans. A recent study reported that patients who receive rosiglitazone display an increased risk for myocardial infarction and possibly death from cardiovascular events (265) (Fig. 8). In vivo administration of TZDs appears to decrease PPAR target gene expression (47, 336). Nonetheless, it is likely PPARγ agonists exert an indirect action on the heart because PPARγ has minimal effects on cardiac fatty acid oxidation or PPAR gene expression in cultured myocytes (124). However, a direct role for PPARγ must be considered because ciglitazone increases insulin-induced glucose transport in cardiomyocytes. Moreover, phosphorylation of Akt residues, Thr308 and Ser473, is required for insulin stimulation of glucose transport and is decreased in insulin-resistant cardiomyocytes (248). In particular, because active Akt has been shown to be necessary for glucose transporter 4 (GLUT4) fusion with adipocyte plasma membranes (191), this may support a role for PPARγ ligand–induced Akt phosphorylation in cardiomyocytes. The possible discrepancy found in endothelial cells (128, 129) and cardiomyocytes may be explained by the use of different stimuli.
FIG. 8.
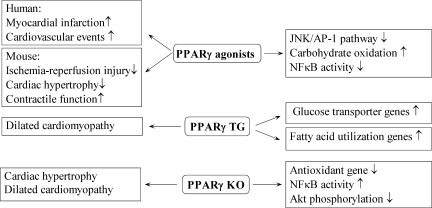
Schematic view of PPARγ roles in the heart. PPARγ agonists are associated with increased myocardial infarction and cardiovascular events in humans. PPARγ agonists decrease ischemia/reperfusion injury and cardiac hypertrophy while increasing contractile function in mice. Administration of PPARγ agonists decreases JNK/AP-1 and NF-κB signaling pathways and increases carbohydrate oxidation in mice. Mice with cardiac-specific PPARγ overexpression show a dilated cardiomyopathy phenotype. Moreover, these mice have increased expression of genes involved in glucose transport and fatty acid utilization. Myocardial PPARγ-knockout mice display characteristics of cardiac hypertrophy and dilated cardiomyopathy along with increased NF-κB activity, decreased Akt phosphorylation, and decreased antioxidant gene expression.
Although ciglitazone enhances insulin-stimulated glucose transport, ciglitazone does not improve insulin-stimulated GLUT4 expression in neonatal rat cardiomyocytes (363), adult rat cardiomyocytes (248), or cardiomyoblasts (124). One possible explanation for increased glucose transport is that elevated glucose transporter 1 (GLUT1) expression, not usually seen with insulin-induced glucose uptake, may be a contributing factor, although the mechanisms remain unclear. The cardiomyocyte microtubule network may be important in regulating insulin signaling. Disruption of the microtubule network may prevent the convergence of insulin signaling and GLUT4 vesicle trafficking (248). Conversely, ligand-independent PPARγ represses GLUT4 gene expression in adipocytes, and rosiglitazone not only alleviates PPARγ-induced repression of GLUT4, but also facilitates transcription (15). Similarly, PPARγ1 and PPARγ2 have been shown to repress GLUT4 expression in cardiomyocytes, and this is enhanced by hyperlipidemia, as free fatty acids bind to PPARγ and further repress GLUT4 transcription (12). Overall, these results suggest that the regulation of glucose transport by insulin may involve PPARγ-dependent and -independent signaling pathways.
Another proposed mechanism of action involving insulin signaling and PPARs in the cardiovascular system may include the forkhead-box class O (FOXO) family of transcription factors. FOXO1 is highly expressed in adipocytes and may enhance insulin sensitivity (13, 14) through inhibition of PPARγ1 and PPARγ2 (13). Insulin signaling results in phosphorylation of FOXO1 by Akt (360). FOXO phosphorylation may repress PPARγ1 and PPARγ promoter activity, directly or indirectly leading to increased GLUT4 expression and subsequent improved insulin sensitivity in adipocytes and cardiomyocytes (14).
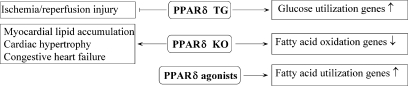
Transgenic mice overexpressing PPARγ (MHC-PPARγ) in the heart were recently generated and characterized (336). However, cardiomyopathy is present at 2 months of age in these mice, with 100% mortality occurring at 5 months. Subsequently, a new transgenic line was generated to circumvent the problem, as these mice display characteristics suggestive of milder cardiomyopathy (Fig. 8). PPARγ transgenic mice show increases in expression of fatty acid–utilization genes (Fig. 8), similar to MHC-PPARα mice. Conversely, similar to MHC-PPARδ mice, glucose-transporter expression is increased in the PPARγ transgenic model (Fig. 8). Thus, it is possible that combined elevations in cardiac lipid and glucose levels may further potentiate the development of cardiomyopathy (399).
Whole-body PPARγ deletion is embryo-lethal in murine models (21). To study the function of PPARγ in the heart, two cardiac-specific PPARγ-knockout murine models were generated (91, 97); however, these two lines manifest different phenotypes. The first mouse line shows evidence of mild ventricular hypertrophy (Fig. 8) that is further increased by rosiglitazone treatment, suggesting off-target TZD effects on hypertrophy. Systolic function does not seem to be impaired in these cardiac-specific PPARγ-null mice. NF-κB activity is increased, and surprisingly, Akt phosphorylation is decreased despite the presence of a hypertrophic phenotype (97) (Fig. 8). The second cardiac-specific murine knockout model demonstrates progressive dilated cardiomyopathy (Fig. 8) in association with mitochondrial oxidative damage and a reduction in the mitochondrial antioxidant, manganese superoxide dismutase (91) (Fig. 8). These models suggest a likely role for PPARγ in cardiac function as well as in maintaining a proper oxidation/reduction balance.
IX. PPARα
PPARα is highly expressed in the liver, with expression in other tissues including heart, kidney, skeletal muscle, small intestine, and brown adipose tissue. Similar to PPARγ, PPARα is also expressed in the cardiovascular cells. PPARα is involved in the expression of genes involved in lipid metabolism, including fatty acid uptake and oxidation. Moreover, PPARα, similar to PPARγ, can play a role in transcriptional repression of certain genes by inhibiting signaling pathways of other transcription factors. The attenuation of proinflammatory signaling is accomplished through this method by downregulating expression of genes involved in promoting the inflammatory response.
In rodent models, PPARα was shown to be activated by fibrates, hypolipidemic drugs that are involved in peroxisome proliferation and fatty acid oxidation (172). Fibrates include clofibrate, bezafibrate, fenofibrate, and gemfibrozil. Wy-14,643, nafenopin, and clofibric acid are other hypolipidemic compounds that are PPARα-activating agents. Warfarin, an anticoagulant, and trichloroacetic acid were also initially described to be stimulators of PPARα (96). Fatty acids, including linoleic acid and arachidonic acid, were also shown to activate PPARα and to regulate gene function (138).
A. PPARα ligands
However, these studies did not demonstrate whether fibrates or fatty acid compounds could directly bind to PPARα. A ligand-binding assay found that fibrates and certain fatty acids do indeed have binding affinity for PPARα (109). In addition, GW7647 (40), GW9578 (41), and LY-518674 (393) are known to be PPARα ligands. PPARα antagonists are limited in number and include GW6471 (391) and the N-acylsulfonamide compounds A and B (325).
B. PPARα and endothelial cells
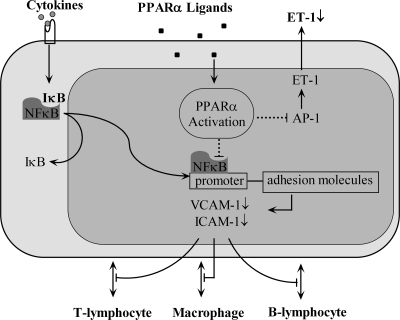
PPARα is expressed in human endothelial cells (83, 170, 240). Moreover, PPARα activators are involved in several endothelial cell functions. For example, PPARα agonists can prevent leukocyte recruitment and adhesion to endothelial cells, in part by decreasing VCAM-1 (6, 173, 241, 321), along with ICAM-1 and E-selectin expression (321) (Fig. 9). Downregulation of adhesion molecules by PPARα activators is likely through inhibition of NF-κB (241, 321) (Fig. 9). In addition to decreased adhesion molecule expression, PPARα activators impair leukocyte binding to endothelial cells (6, 173, 241, 321).
FIG. 9.
Schematic view of PPARα activation in ECs. PPARα activation attenuates NF-κB signaling and transcription in ECs, leading to decreased adhesion-molecule expression and inhibition of leukocyte interaction with ECs. PPARα ligands inhibit ET-1 synthesis by negatively regulating AP-1.
PPARα has been demonstrated to play a role in vascular function. PPARα ligands inhibit ET-1 synthesis and secretion in endothelial cells through negative regulation of AP-1 (83) (Fig. 9). A possible explanation is that PPARα activators may suppress, at least in part, PKC activity involved in endothelial cell ET-1 secretion (234). In DOCA-salt rats, fenofibrate prevents increased ET-1 synthesis in mesenteric arteries (163). PPARα ligands stimulate eNOS expression by PPARα-mediated signaling (139).
PPARα has been shown to be involved in endothelial cell inflammatory signaling. One mechanism for endothelial cell PPARα participation in antiinflammatory pathways may include oxLDL and a phospholipase A2 (PLA2)-dependent-pathway, potentially stimulating fatty acid transport protein-1 (FATP-1) expression (81). Another antiinflammatory mechanism suggests that PPARα ligands may decrease VEGFR2 expression through direct PPARα/Sp1 interaction in endothelial cells (246). Finally, bezafibrate increases the CuZn superoxide dismutase antioxidant and decreases NAD(P)H oxidase subunit expression in endothelial cells (168). PPARα ligands have also been shown to attenuate MCP-1 and IL-8 expression in endothelial cells, possibly by PPARα suppressing NF-κB (247, 285). Conversely, another study suggests that PPARα ligands increase MCP-1 and IL-8 expression through a PPARα-dependent signaling cascade in human aortic endothelial cells (203). Overall, these studies suggest that PPARα is primarily involved in antiinflammatory signaling, although it is likely that PPARα may also exert proinflammatory effects.
C. PPARα and VSMCs
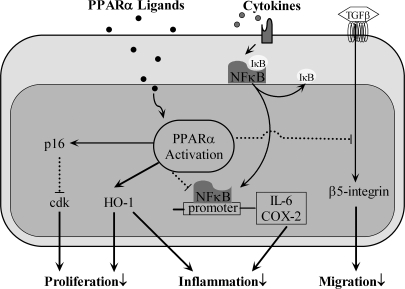
PPARα is also expressed in human vascular smooth muscle cells (239, 340). As in endothelial cells, PPARα has an antiinflammatory role in VSMCs. PPARα activators suppress IL-6 (80, 340), 6-keto-PGF1α (340), along with COX-2 protein and mRNA expression by negatively regulating NF-κB signaling (340) (Fig. 10). PPARα agonists may increase VSMC IκBα, an inhibitory protein that suppresses NF-κB nuclear translocation (82). HO-1, a PPARα target gene, is upregulated by PPARα and contributes to the antiinflammatory effects in VSMCs (193) (Fig. 10). Group IIA secretory phospholipase A2 (sPLA2-II2) is a proinflammatory mediator of atherosclerosis. PPARα has been shown to repress IL-1β–induced sPLA2-IIA expression in VSMCs (298).
FIG. 10.
Schematic view of PPARα activation in VSMCs. PPARα activation in VSMCs inhibits proliferation and migration by interfering with cdk and β5-integrin signaling pathways. PPARα activation also exerts antiinflammatory roles via inhibiting NF-κB mediated–inflammatory factor release.
In vitro studies have shown that PPARα ligands inhibit VSMC proliferation (264, 404). One possible mechanism may involve PPARα activation of p16INK4a (Fig. 10), a cdk inhibitor that blocks phosphorylation of the retinoblastoma protein and subsequent G1/S cell-cycle progression (126). Next, epoxide hydrolase inhibitors activate PPARα and suppress PDGF-induced VSMC proliferation through negative regulation of cyclin D1 expression (261). Finally, HO-1, in addition to antiinflammatory signaling, also has a role in VSMC antiproliferation (193) (Fig. 10). PPARα also has been shown to regulate VSMC migration. Integrins are critical for VSMC migration in atherosclerosis. PPARα may interact with Smad4 and inhibit TGF-β–induced beta5 integrin expression in VSMCs (187) (Fig. 10). In addition, docosahexaenoic acid may regulate VSMC apoptosis through PPARα-dependent p38 MAPK signaling (89).
D. PPARα and monocytes/macrophages
PPARα is expressed in differentiated human macrophages (64) and atherosclerotic lesion macrophages (63). This is important because differentiated macrophages play an important role in inflammation and plaque formation. The first evidence for a role of PPARα in inflammatory control demonstrated that PPARα-null mice display a prolonged response to inflammatory stimuli. Leukotriene B4 (LTB4) binding to PPARα results in activation of fatty acid oxidation (FAO) enzymes that degrade fatty acid and disrupt inflammatory signaling (86).
Further evidence that PPARα plays a protective role against the inflammatory response is shown from experiments using RAW 264.7 mouse macrophages, whereby Wy14,643 reduces nitrate accumulation in association with decreased iNOS and elevated HO-1 (Fig. 11). Interestingly, natural PPARα ligands, such as LTB4 and 8(S)-HETE, increase nitrate accumulation, an indication of proinflammatory activity. This difference may be due to variable selectivity to PPARα (74). Other studies in monocytes/macrophages provide evidence of PPARα-dependent antiinflammatory signaling. Fenofibrate suppresses LPS-induced MMP-9 secretion in monocytes (330) (Fig. 11). PPARα also has been shown to downregulate the platelet-activating factor (PAF) receptor, possibly regulating monocyte and macrophage inflammatory responses and cellular apoptosis (156).
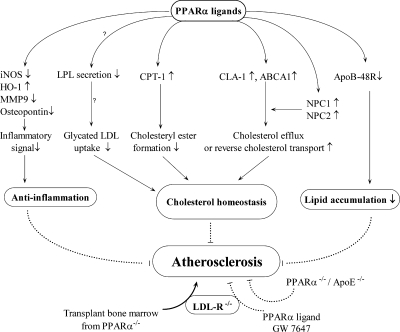
FIG. 11.
Schematic view of PPARα ligand roles in macrophages and atherosclerosis. PPARα ligands may prevent atherosclerosis by improving cholesterol homeostasis, decreasing lipid accumulation, and participating in antiinflammatory signaling in macrophages. LDL-R−/− mice transplanted with bone marrow from PPARα−/− mice have increased atherosclerosis, whereas GW7647 decreases lesion development in LDL-R−/− mice. However, PPARα−/−/apoE−/− mice are protected against the development of atherosclerosis.
Treatment with Wy-14,643 and bezafibrate inhibits osteopontin expression in human macrophages through AP-1 inhibition (Fig. 11). Moreover, osteopontin expression is not suppressed in macrophages lacking PPARα expression (255). Another antiinflammatory mechanism points to simvastatin inhibiting PKC-induced phosphorylation of PPARα that may result in reduced iNOS and IL-6 expression in macrophages (288). PPARα ligands inhibit IFNγ, TNF-α, and IL-2 proinflammatory cytokine expression in human T cells (236). Conversely, ligand-activated PPARα can increase ROS production in mouse and human macrophages (352).
ApoB-48R is involved in macrophage lipid accumulation. Wy-14,643 attenuates apoB-48R expression in both monocytes and macrophages (147) (Fig. 11). Lipoprotein lipase (LPL) hydrolyzes the lipids of lipoproteins and is generally considered to be expressed in cells of atherosclerotic plaques, including macrophage-derived foam cells (266). Although several studies demonstrated that PPARα activators increase LPL mRNA in macrophages (120, 216, 412), conflicting evidence exists regarding LPL secretion. Decreased LPL secretion due to PPARα activators may reduce glycated LDL uptake witnessed in macrophages (120) (Fig. 11). Conversely, increased LPL secretion could stimulate PPARα target gene expression in macrophages or provide an antiinflammatory role by reducing VCAM-1 expression in endothelial cells (412). PPARα ligands are involved in intracellular cholesterol homeostasis and have been shown to reduce cholesteryl ester formation in human macrophages and foam cells, possibly through upregulation of carnitine palmitoyltransferase type 1 (CPT-1), an enzyme involved in fatty acid degradation (66) (Fig. 11). PPARα ligands can also regulate reverse cholesterol transport or cholesterol efflux. PPARα ligands increase CLA-1 expression in differentiated human macrophages (63) (Fig. 11). Furthermore, Wy-14,643 was found to elevate ABCA1 expression in macrophages to facilitate apoAI-induced reverse cholesterol transport (65) (Fig. 11). Niemann-Pick type C1 and C2 (NPC1 and NPC2) proteins control intracellular cholesterol mobilization to the plasma membrane for extracellular transport. PPARα agonists were found to upregulate NPC1 and NPC2 expression in human macrophages (Fig. 11). In addition, NPC1 and NPC2 inhibition has been shown to prevent ABCA1-mediated extracellular cholesterol transport (62) (Fig. 11). Overall, these studies suggest that PPARα ligands are actively involved in macrophage cholesterol efflux (Fig. 11). Furthermore, PPARα ligands have been demonstrated to be regulators of cholesterol homeostasis in both normal and atherosclerotic lesion macrophages (Fig. 11).
E. PPARα and atherosclerosis
A role for PPARα has been identified in atherosclerotic lesion formation involving several cell types. As mentioned earlier, PPARα ligands are critical in controlling macrophage cholesterol homeostasis, and PPARα has been shown to inhibit VSMC proliferation and migration, important steps in the prevention of atherosclerosis. Wy-14,643 induces SR-B1 expression in atherosclerotic lesions (63). PPARα also may play a role in atherosclerotic thrombosis by inhibiting tissue factor (TF) mRNA and activity in human monocytes and macrophages (238, 260).
Although much evidence suggests that PPARα ligands protect against atherosclerosis, murine animal models have yielded conflicting results. The loss of PPARα is shown to protect against atherosclerosis in apoE−/− mice (359) (Fig. 11). Conversely, fenofibrate attenuates the development of atherosclerotic lesions, with a more pronounced decrease observed in apoE−/− mice that express the human apoA-I transgene (99). Another study showed that GW7647 decreases lesion formation in LDL-R−/− mice (214) (Fig. 11). Furthermore, lesion size is deceased in human apoE2 knockin mice administered fenofibrate (152). Finally, male and female LDL-R−/− mice transplanted with bone marrow from PPARα−/− mice display increased aortic atherosclerosis (Fig. 11), along with decreased peritoneal macrophage cholesterol efflux (17). Thus, from these studies, the role of PPARα in atherosclerotic lesion formation is controversial; however, much of the data tends to suggest an atheroprotective effect of PPARα.
Possible explanations for decreased atherosclerotic development witnessed with the removal of PPARα in apoE−/− mice may involve systemic or vessel wall effects. Systemic effects may include decreased glucose levels and insulin resistance, lower blood pressure, and the loss of liver PPARα target genes that lead to atherosclerotic development. Furthermore, the absence of PPARα may attenuate LPL activity in the subendothelial space of the vessel wall and decrease atherosclerosis. Systemic effects can alter gene expression in vessel walls, making it difficult to confirm the role of vascular wall PPARα in atherosclerosis (359).
F. PPARα and the heart
The use of both gain- and loss-of-function techniques has proven useful in evaluating PPARα and its effects on cardiac energy metabolism. Cultured myocyte treatment with PPARα ligands or adenoviral overexpression of PPARα induces several genes involved in fatty acid metabolism (23, 124, 161). However, the effects of PPARα ligands on myocardial target genes in vivo have been disappointing (75). PPARα ligands decrease cardiac FAO rates in diabetic mice (1, 2). PPARα, similar to fatty acid metabolism, may also display direct effects on the heart by inhibiting inflammation and collagen deposition resulting from AngII-induced hypertension. Clinically, PPARα activation may provide a cardioprotective effect against hypertension and hyperlipidemia. Furthermore, fenofibrate activation of PPARα may decrease hypertension-induced changes in mechanical overload that lead to ventricular hypertrophy (103).
PPARα ligands are known to have direct effects on mitochondrial function (180, 181). PPARα activators can differentially inhibit cardiac mitochondrial respiration. The attenuation of cardiac mitochondrial respiratory function is greater with the administration of fenofibrate compared with Wy-14,643 (413). This suggests a possible PPARα-independent effect because the Wy-14,643 compound has a higher PPARα affinity than does fenofibrate.
PPARα is regulated by hypoxia, as shown by the reduction in PPARα-dependent transcriptional activity of muscle carnitine palmitoyltransferase I, an enzyme involved in mitochondrial FAO. The DNA-binding activity of the PPARα:RXR heterodimer is reduced in hypoxic cardiomyocytes (161). Furthermore, myocardial hypoxia can decrease PPARα-dependent gene expression in two in vivo rat models (299). The level of PPARα mRNA, along with its target gene, medium-chain acyl-CoA dehydrogenase, is decreased after 7 days in a model of hypoxia-induced right ventricular hypertrophy. However, these levels are upregulated at day 14, suggesting a compensatory response by the heart due to increased load. It is likely that part of the transcriptional response to hypoxia-induced right ventricular hypertrophy involves the regulation of PPARα by hypoxia in the early stages and, in the later stages, by increased load (323).
PPARα cardiac-specific transgenic mice were developed to discern between PPARα-induced cardiac effects and ligand-induced systemic effects (107, 108, 149, 284, 316). PPARα overexpression induces genes involved in cardiac fatty acid metabolism and utilization (108) while suppressing genes known to participate in glucose uptake and utilization (108, 284). Of great interest, these abnormalities are more prominent in mice that are insulin resistant or fed a high-fat diet (107), both of which are capable of elevating circulating lipids. It is likely that increased reliance of fatty acid utilization, along with the concomitant decrease in glucose utilization by the heart, may aggressively promote remodeling, leading to eventual cardiomyopathy. However, the mechanisms whereby altered fatty acid and glucose utilization result in cardiac remodeling are still unclear.
Although a cardiac-specific PPARα-knockout mouse model has yet to be characterized, murine models with generalized PPARα-ablated gene expression have been developed and are often used in examining PPARα function in cardiac energy metabolism and utilization (53, 93, 183, 205, 211, 229, 281, 381). Malonyl-CoA decarboxylase is an important regulator of cardiac fatty acid oxidation (77), and PPARα knockout mice have decreased malonyl-CoA decarboxylase gene expression (53). PPARα-null mice show decreased fatty acid oxidation rates (53, 93, 211, 381) along with increased glucose metabolism and oxidation (53, 281). As a result, it is possible that the alterations pertaining to the dependence on each fuel source in PPARα-deficient mice make it difficult for the heart to adapt to increase workloads (53, 229). Furthermore, increased ventricular afterload is improved in PPARα-knockout mice with GLUT1 overexpression (229), suggesting that glucose ATP production in PPARα-null mice may not be sufficient to meet the demands of greater cardiac workload. Moreover, because chronic pressure overload deactivates PPARα (134, 208), this model may be suitable for studies in cardiac metabolic dysfunction. PPARα activation can reduce cardiomyocyte hypertrophy, as fenofibrate decreases ET-1–induced neonatal rat cardiomyocyte enlargement (171, 220). A recent investigation with PPARα-knockout mice demonstrated greater cardiac hypertrophy after pressure overload in association with enhanced inflammatory marker expression (335), and the follow-up study asserts that PPARα and PPARδ inhibit inflammation and cardiac hypertrophy by suppressing NF-κB signaling (334).
XV. PPARδ
PPARδ is distributed ubiquitously in almost all tissues, including liver, fat, skeletal muscle, and skin, and differs from the other two PPAR isotypes. Several studies show that PPARδ has important roles in cell growth, differentiation, placenta growth, colon tumorigenesis, and wound healing (20, 289, 350). Recent studies focused on the effects of PPARδ regarding lipid metabolism and insulin sensitivity. PPARδ is expressed in the vascular system and displays essential regulatory roles in vascular biology.
PPARs, liver X receptors (LXRs), farnesoid X receptor (FXR), and krüpple-like factor (KLF) are transcription factors controlling lipid and glucose metabolism, as well as the inflammatory response. These transcription factors interact with each other and synergistically regulate gene expression. PPARδ overexpression influences the activity of PPARα and PPARγ in 3T3 fibroblasts and nontransformed monkey kidney CV-1 cells (329). PPARδ inhibits PPARγ activity by interfering with PPARγ DNA-binding activity and not PPARγ gene expression in colon cancer cells, which is identified by PPARδ knockout and gain-of-function approaches (414). LXR can bind to all three PPAR subtypes, and PPAR ligands can regulate LXR/PPAR interaction, as studied by SPR technology (402). LXR induces fatty acid synthesis, whereas PPARδ induces fatty acid oxidation. Moreover, the diverging effects of PPARδ and LXR on metabolic gene regulation are apparent because PPARδ represses the expression of the LXR target gene angpt13, and L-165041 enhances the inhibitory effect. The likely mechanism is that PPARδ competes with LXR for binding to RXR, and L-165041 increases the affinity between PPARδ and RXR (243). KLF5, a member of the KLF superfamily, is critical for regulation of adipocyte differentiation and energy metabolism (274). KLF5+/− heterozygous mice are not prone to high-fat diet–induced obesity, insulin resistance, and hypercholesterolemia. Under basal conditions, SUMOylated KLF5, unliganded PPARδ, and co-repressors form a transcription-repressor complex. Once PPARδ agonists activate PPARδ, KLF5 is deSUMOylated and associates with the transcription activation complex composed of liganded PPARδ and the CREB binding protein (273).
A. PPARδ ligands
PPARδ, on activation by ligands, regulates gene expression. For several years, highly selective PPARδ ligands were not known, and as a result, the progress in PPARδ research was hampered. Natural ligands, such as unsaturated fatty acids, eicosanoid derivatives, and prostaglandins, have binding affinity for PPARδ, although natural ligand selectivity tends to be low (389). cPGI activates both PPARδ and PPARα (221), whereas retinoid acid activates both RAR and PPARδ without activating PPARα and PPARγ (324). As a result, synthetic ligands were developed to widen this research scope. L-796449, L-165461, and L-783483 have high affinity for PPARδ, but also to PPARγ, whereas L-165041 has a high affinity for only PPARδ (30). Both GW501516 and GW0742 are widely used and are 1,000 times more selective for PPARδ compared with PPARα and PPARγ. The EC50 of PPARδ transactivation is 1∼2 nM (345). PPARδ and RXR form an obligatory heterodimer and recruit co-repressors such as BCL-6 and SMART to form a transcription complex that binds to the gene-promoter PPRE. Once ligand activated, co-repressors dissociate from the complex, and coactivators such as p300 and SRC-1 bind to the complex, transactivating target gene expression. Recently, Shearer et al. (326) identified GSK0660 as a potent antagonist of PPARδ with a binding assay IC50 of ∼160 nM. However, GSK0660 is inactive on PPARα and PPARγ, with IC50 levels above ∼10 μM. This antagonist will be useful for elucidating the biologic roles of PPARδ (326).
B. PPARδ and endothelial cells
Endothelial dysfunction is characterized by endothelial proinflammatory, procoagulant, and profibrotic states. Impaired endothelial cell permeability, together with the previous clinical entities, is a marker of early-stage atherosclerosis. Endothelial activation is induced by several risk factors, including LDL/oxLDL, hypercholesterolemia, hyperglycemia, and cytokines (TNF-α, IL-1β), which promote increased adhesion molecule expression and ensuing leukocyte–endothelial adhesion. L-165041 inhibits TNF-α–induced MCP-1 secretion and VCAM-1 expression in the EAhy926 cell line (311). Both GW0742 and GW501516 have potent antiinflammatory effects in endothelial cells (Fig. 12), inhibiting inflammatory cytokine (TNF-α and IL-1β)-induced adhesion molecule expression and ensuing leukocyte–endothelial adhesion in primary HUVECs. The mechanisms of PPARδ antiinflammatory effects involve the attenuation of oxidative stress through the upregulation of antioxidant genes catalase, CuZn superoxide dismutase, and thioredoxin, as well as control of BCL-6 co-repressor translocation to proinflammatory genes (105).
FIG. 12.
Schematic view of PPARδ roles in atherosclerosis. PPARδ ligands are beneficial against the development of atherosclerosis by regulating lipid homeostasis in humans. PPARδ ligands attenuate the development of atherosclerosis in mice by decreasing inflammatory gene expression and macrophage migration while increasing plasma HDL. Inhibition of EC and macrophage inflammatory gene expression by PPARδ ligands prevents the development of atherosclerosis. However, PPARδ overexpression stimulates VSMC proliferation and macrophage release of inflammatory factors, which may promote atherosclerotic development.
In endothelial cells, ligand activation of PPARδ increases human endothelial cell proliferation and angiogenesis via upregulating VEGF expression and release (290). Next, PPARδ activation by either PGI2 or L-165041 inhibits H2O2-induced EC apoptosis via upregulation of 14-3-3 epsilon (226). L-165041 and GW501516 activate the 14-3-3 gene YWHAE promoter, increasing 14-3-3 expression in a C/EBP-dependent manner, and not in a PPRE-dependent fashion. PPARδ regulates expression of C/EBP and forms a transcriptional complex with C/EBP in ECs (45). PPARδ activation stimulates proliferation and attenuates apoptosis in EPCs through phosphorylated Akt-dependent signaling. These effects promote enhanced vasculogenesis and may be therapeutically beneficial in the treatment of ischemic cardiovascular disease (144).
C. PPARδ and VSMCs
PDGF, a neointimal stimulator, induces PPARδ expression via the PI3-kinase/Akt pathway in VSMCs (410). In vivo data show that PPARδ is upregulated during the development of vascular lesion formation (410). Overexpression of PPARδ in VSMCs increases post-confluent cell proliferation (Fig. 12) by modulating cell-cycle checkpoint genes including cyclin A, cdk2, and p57(Kip2) (410). The suppression of PPARδ expression may mediate the inhibitory effects of prostacyclin synthase on neointimal formation (166). However, the role of PPARδ in VSMCs is not yet agreed on. Recently, Lim et al. (222) reported that L-165041 suppresses rat VSMC proliferation by inhibiting phosphorylation of the retinoblastoma protein and cell-cycle progression. In vivo data show that L-165041 attenuates neointima formation in the carotid artery balloon injury model. GW501516 also dose-dependently suppresses TNF-α–induced VSMC proliferation (184). PPARδ receptors and agonists may play different roles in VSMC proliferation, accounting for the seemingly inconsistent results. TGF-β1, known as a potent regulator in the pathogenesis of atherosclerosis and restenosis, is upregulated by PPARδ in VSMCs as a target gene. GW501516 inhibits IL-1β–induced MCP-1 expression, which is mediated by TGF-β1 and its effector, Smad3. The expression of TGF-β1 is upregulated, and proinflammatory genes are suppressed in the thoracic aorta prepared from GW501516-treated mice. Thus, it is apparent the PPARδ/TGF-β/MCP-1 pathway stimulates PPARδ antiinflammatory signaling mechanisms (184).
D. PPARδ and monocytes/macrophages
Macrophage inflammation and lipid dysfunction are involved in the pathogenesis of atherosclerosis. PPARδ regulates lipid metabolism in macrophages. VLDL activates expression of genes involved in β-oxidation, thermogenesis, lipid mobilization, and carnitine biosynthesis through PPARδ-dependent signaling. Knocking out PPARδ has the same effect as PPARδ agonists on fatty acid utilization in macrophages, indicating that the endogenous unliganded PPARδ receptor has an inhibitory effect on lipid oxidation (202). GW501516 increases ABCA1 expression and induces apolipoprotein A1–specific cholesterol efflux in macrophages (276). However, different results are achieved in primary human macrophages and THP-1 human monocytes with compound F. Compound F upregulates genes related to lipid accumulation and downregulates genes involved in lipid efflux and metabolism. Both compound F and PPARδ overexpression promote lipid accumulation in macrophages (371). Alternatively, activated macrophages are believed to improve the metabolic syndrome although the mechanism of modulating alternative activation of tissue macrophages is still unclear. Adipocyte-derived Th2 cytokines IL-13 and IL-4 induce macrophage PPARδ expression. Both adipose tissue and liver-resident macrophages are activated to the alternative phenotype by PPARδ, and this switch is beneficial for fatty acid metabolism and improves insulin sensitivity (177, 268).
The removal of PPARδ leads to downregulation of MCP-1 and IL-1β expression, attenuating macrophage proinflammatory responses. Overexpression of PPARδ enhances the inflammatory response, suggesting that endogenous PPARδ has a proinflammatory effect in macrophages (Fig. 12). However, similar to endothelial cells, PPARδ agonists have a potent inhibitory effect on macrophage inflammation (Fig. 12). GW0742 inhibits LPS-induced expression of inflammatory genes iNOS and COX-2 in macrophages (382). Ligand-activated PPARδ regulates the translocation of nuclear repressor BCL-6 to inflammatory genes and controls the inflammatory switch in a ligand-dependent manner (201). Graham et al. (141) reported that GW0742X decreases TNF-α expression in peritoneal macrophages and adipose tissue.
Foam cell and subsequent fatty-streak formation play critical roles in atherogenesis. LDL/oxLDL induces macrophage differentiation into foam cells, in which many genes likely modulate the transformation process. One such example may include the regulation of scavenger-receptor expression by the PPAR family. Both compound F administration and overexpression of PPARδ stimulate PMA-induced macrophage differentiation (370).
E. PPARδ and atherosclerosis
A deteriorated plasma lipoprotein profile directly affects vascular function. Elevated plasma levels of low-density lipoproteins (LDLs) increase the risk of atherosclerosis. Conversely, the increase of HDLs has a cardiovascular protective effect. Very low density lipoproteins (VLDLs) and their triglyceride components regulate gene expression via activation of PPARδ in macrophages (58). Accumulating evidence demonstrates that PPARδ regulates lipid metabolism in metabolically active tissues. Adipose tissue–specific activated PPARδ protects against obesity and induces expression of genes required for fatty acid oxidation and energy uncoupling. Adipose-specific PPARδ transgenic mice also show improved overall lipid profiles and reduced plasma triglyceride levels (379), demonstrating a possible atheroprotective effect. GW501516 increases HDL levels and decreases small dense LDL, triglycerides, and insulin in insulin-resistant middle-aged obese rhesus monkeys (276). In St. Kitts vervet atherosclerotic primate models, GW501516 increases plasma HDL-C, apoA-I, and apoA-II concentrations, demonstrating protective effects of PPARδ on the cardiovascular system (375). However, considerably less is known about the function of PPARδ on lipid homeostasis in humans.
In vivo results also were observed in human subjects. A clinical study performed in healthy white normolipidemic male subjects showed that plasma triglyceride and LDL levels significantly decline, whereas HDL-C levels are enhanced after 2 weeks of GW501516 administration (339) (Fig. 12). Consistently, Riserus et al. (310) reported that GW501516 treatment significantly reduces plasma triglycerides, apoB, and LDL cholesterol in healthy moderately overweight subjects (Fig. 12). Presently, laboratory and clinical studies indicate that lowering lipid levels can be achieved by administering PPARδ agonists, resulting in improved lipid homeostasis (Fig. 12). With regard to its genetic basis, the lipid-regulating function of PPARδ is associated with gene polymorphisms (333). Plasma HDL-C levels are elevated in the PPARδ exon 4 + 15 C/C and exon 7 + 65 G/G genotypes of healthy white subjects with exposure to endurance training compared with those with other genotypes (150).
The role of PPARδ in atherosclerosis has been identified in an atherosclerotic animal model. PPARδ−/− bone marrow transplanted into γ-irradiated LDL-R−/− mice significantly reduced atherosclerosis lesions, likely as a result of the attenuated inflammatory status of macrophages (201). Li et al. (214) reported that PPARδ agonist GW0742 has no effect on atherosclerotic lesions, whereas PPARα and PPARγ agonists strongly inhibit atherosclerosis in hypercholesterolemic diet–fed LDL-R−/− mice. However, PPARδ agonists inhibit inflammatory gene expression (Fig. 12), including IFN-γ, TNF-α, MCP-1, VCAM-1, and ICAM-1 in atherosclerotic lesions (214). It is likely that the antiinflammatory effect of PPARδ may not reverse the proatherogenic impact of extreme hypercholesterolemia in this animal model. Treatment with GW0742X reduces atherosclerotic lesions in LDL-R–null mice and decreases MCP-1 and ICAM-1 expression in the aorta (141). In the apoE−/− mouse atherosclerotic model, treatment with GW501516 attenuates atherosclerotic lesion formation through multiple pathways, which may include increases in plasma HDL levels, potent antiinflammatory effects, and suppression of macrophage transmigration (Fig. 12). PPARδ inhibits the chemokines-receptor signaling pathway by increasing the expression of regulator of G-protein signaling (RGS) genes (25). In the AngII-accelerated atherosclerotic model, GW0742 attenuates AngII-induced atherosclerotic lesion formation. GW0742 increases the expression of BCL-6, RGS4, and RGS5 in the vascular wall, which inhibits inflammatory and atherogenic gene expression (348). In agreement with several in vitro studies, these in vivo data support an atheroprotective role of PPARδ agonists (Fig. 12).
F. PPARδ and the heart
PPARδ activation by GW0742 increases palmitate oxidation in neonatal and adult cardiomyocytes, meanwhile upregulating the expression of fatty acid oxidation genes (61) (Fig. 13). Consistently, the expression of key fatty acid oxidation genes (mCPT1, ACOX1, UCP3) and also basal myocardial FAO rates decrease in cardiomyocyte-specific PPARδ-knockout mice (60) (Fig. 13). Cardiac-specific overexpression of PPARδ increases the expression of GLUT4 and phosphofructokinase, a glycolytic gene, promoting myocardial glucose utilization, which may contribute to reduced myocardial injury after ischemia/reperfusion (46) (Fig. 13). GW610742X increases fatty acid oxidation after myocardial infarction in both left and right ventricles, along with the upregulation of PPARδ metabolic target gene expression, such as CD36, CPT1, and UCP3 (176). These studies suggest PPARδ increases fatty acid oxidation and related gene expression, providing a physiological benefit for metabolic-related heart disease.
FIG. 13.
Schematic view of PPARδ roles in the heart. PPARδ ligands increase myocardial fatty acid utilization genes. Cardiac-specific PPARδ-knockout mice have decreased myocardial expression of fatty acid oxidation genes along with increased myocardial lipid accumulation, cardiac hypertrophy, and congestive heart failure. Myocardial PPARδ overexpression in mice increases expression of genes involved in glucose utilization, which may prevent further injury after ischemia/reperfusion.
Inflammatory responses are involved in the pathophysiologic processes of ischemia/reperfusion, hypertrophy, and fibrosis. Much evidence suggests that PPARα and PPARγ suppress myocardial inflammatory responses. PPARδ attenuates LPS-induced expression of TNF-α through inhibition of NF-κB in cultured cardiomyocytes (90). PPARδ interacts with the p65 NF-κB subunit, inhibiting the LPS-induced NF-κB signaling pathway and decreasing MCP-1 expression in rat cardiomyocytes (293). Furthermore, GW0742 reduces cardiac expression of IL-6, IL-8, MCP-1, and ICAM-1, which are induced by ischemia/reperfusion (403).
Progressive myocardial lipid accumulation and hypertrophy occur in cardiomyocyte-specific PPARδ-knockout mice (Fig. 13). The function of the PPARδ-null heart is impaired, characterized by a decrease in rates of contraction and relaxation, decreased cardiac output, and increased left ventricular end-diastolic pressure (60) (Fig. 13). GW0742X reduces right ventricle hypertrophy and lung congestion (176). Furthermore, PPARδ activation by L-165041 inhibits phenylephrine-induced protein synthesis and increases carnitine palmitoyltransferase and pyruvate dehydrogenase kinase 4 expression in cultured rat cardiomyocytes (293).
GW501516 inhibits proliferation of cardiac fibroblasts and myofibroblasts and also suppresses differentiation of fibroblasts into myofibroblasts (354). Collagen accumulation is involved in myocardial fibrosis, and GW501516 attenuates AngII-stimulated collagen synthesis in cardiac fibroblasts (354, 408).
PPARδ is critical for maintaining normal fatty acid oxidation and energy balance in the heart (60), suggesting that PPARδ and its ligands may be important for cardiac function, distribution of muscle fiber type, and endurance performance (60, 150). PPARδ overexpression or activation may be a contributing factor to increasing endurance and may mimic the effects of exercise on muscle metabolism (102, 119). PPARδ and its ligands have been shown to improve exercise performance and regulate physical endurance and training in skeletal muscle (380). Conversely, exercise has been shown to promote skeletal muscle PPARδ accumulation in murine animal models (230). The possibility exists whereby increased exercise may activate PPARδ by facilitating the internalization of certain fatty acids that act as ligands (380). Another possibility is that exercise increases PPARγ coactivator-1α (PGC-1α) expression (137), and PGC-1α binding to PPARδ can potently activate this transcription factor, irrespective of the presence of ligands (379). Furthermore, plasma HDL-C levels are higher in the PPARδ exon 4 + 15 C/C and exon 7 + 65 G/G healthy white genotypes with endurance training compared with other genotypes (150), and PPARδ agonist administration increases plasma HDL-C concentrations in various animal models (210, 276, 375). One explanation may be that increased availability of free fatty acids due to exercise activates PPARδ and promotes reverse cholesterol transport (150). Finally, a recent study demonstrated that GW501516 and exercise training work synergistically to increase running endurance (256). These studies have important cardiovascular significance because running performance in humans appears to be linked more to cardiovascular performance and not to muscle fiber–type distribution (304).
In summary, although all three PPAR isotypes are involved in the metabolic syndrome and cardiovascular disease, evidence suggests that PPARδ is different from the other two subtypes. The PPARδ receptor and agonists can sometimes show distinct modes of action. PPARδ can repress both PPARγ and PPARα target gene activity, and PPARδ repression is likely PPRE dependent (329). PPARδ improves the metabolic syndrome and cardiovascular activity through potent antiinflammatory effects and regulation of lipid and glucose metabolism. To date, several studies indicate that PPARδ is a potential therapeutic target for treatment of the metabolic syndrome and cardiovascular diseases, including atherosclerosis and cardiac hypertrophy. PPARδ appears to act as a “housekeeper” because of its near-ubiquitous expression. Therefore, it is critical for PPARδ to be further examined regarding its effects on metabolism and the various tissues related to metabolic function.
XVI. Perspective
PPARs have now been firmly entrenched as key players in the cardiovascular system. During the past decade, considerable evidence has been accumulated regarding the role of peroxisome proliferator–activated receptors in cardiovascular diseases and clinical complications related to cardiovascular abnormalities. PPARs regulate several cell-signaling mechanisms related to cardiovascular health and disease. A continuing need exists for basic science and clinical investigations to understand fully the role of PPAR in the physiology and pathology of cardiovascular-related diseases. Thus, it is important to gain a better understanding of the regulatory role of PPARs in vascular cells and the heart.
TZDs and fibrates are pharmacologic agents that have pleiotropic effects, many of which are beneficial in alleviating cardiovascular abnormalities in animal models. However, this has not necessarily translated into markedly improved clinical cardiovascular outcomes. This may be because of differences in both uptake and effects on target pathways between various animal species and humans. In addition, increasing evidence shows that several beneficial PPAR agonist effects are not from direct participation of PPAR-signaling pathways. No definitive evidence indicates that activated PPARγ pathways are critical for the beneficial effects of TZDs in the cardiovascular system. Moreover, greater evidence exists that ligand-activated PPAR signaling may play a role in the witnessed pharmacologic side effects of TZDs.
Hence, dual PPAR agonists were generated to circumvent this problem and simultaneously to activate two PPAR isoforms. However, the administration of dual PPAR agonists in the clinical setting has been somewhat disappointing because of increased risks for cardiovascular events. Selective PPAR modulators (SPPARMs) were developed to find newer, safer, and more effective agonists and have been shown to improve the overall clinical profile. The possibility that cardiovascular diseases in patients may be the result of depleted endogenous PPAR ligand concentrations must also be considered. Furthermore, a need exists to conduct a greater number of studies on the role of PPAR antagonists in the cardiovascular system.
The development of animal model systems specifically for studying PPARs and PPAR agonists has led to greater increases in information regarding the mechanisms of these nuclear transcription factors in the cardiovascular system. Because global deletion of PPAR is embryo-lethal, the use of conditional knockout mice (e.g., ECs, VSMCs, macrophages) has been critical to understanding the development of human cardiovascular diseases. Nonetheless, limitations are found in using the mouse model. Genetically modified mice often do not show characteristics evident of the human phenotype. Thus, we need more suitable animal models that may correct for many, if not all, of these characteristics. The use of genetically modified rabbits, pigs, or monkeys may be more appropriate for studying the effects of PPARs and their agonists in the cardiovascular system and for providing a clearer understanding of the pathophysiology of cardiovascular diseases (Fig. 14).
FIG. 14.
Perspective view of PPARs and PPAR ligands in the cardiovascular system. The use of mouse models has shown that PPAR ligands have many beneficial effects in the cardiovascular system. However, PPAR ligand administration (e.g., rosiglitazone) in the clinical setting has not necessarily translated into markedly improved cardiovascular outcomes. Furthermore, some question exists as to whether the beneficial effects of PPAR agonists involve PPAR-dependent signaling. Although the development of animal model systems specifically for studying PPAR agonists and PPAR gain- and loss-of-function has elucidated important findings regarding the molecular mechanisms of cardiovascular disease, certain limitations pertain to the use of mouse models. In many cases, genetically altered murine models do not display characteristics similar to those of humans. Hence, a need exists for using genetically modified animals, such as rabbits, pigs, or monkeys, that have a closer phenotypic resemblance to humans and therefore may be more appropriate for studying PPARs and PPAR agonists in the cardiovascular system.
Finally, although previous studies have successfully targeted PPAR for deletion in cardiovascular cells, the possibility of PPAR cell–cell crosstalk should not be overlooked in the cardiovascular system. For example, does VSMC PPARγ affect function in PPARγ-null ECs and vice versa? The ability to gain a better understanding of PPARs and agonists in the cardiovascular system will enable us to address the controversy regarding the subsequent administration of pharmacologic agents that not only activate PPAR pathways, but may also have PPAR-independent effects.
Footnotes
Reviewing Editors: John Bright, Adnan Erol, M. Faadiel Essop, Shou Wei Han, and Do-Young Yoon
Acknowledgments
Dr. Chen's laboratory is funded by National Institutes of Health (HL68878, HL89544, HL75397, and HL92421). M.H. is supported by a postdoctoral fellowship from the National Institutes of Health (T32 HL007853). L.C. and J.Z. are supported by American Heart Association Midwest Affiliate Fellowship (0625705Z) and National Career Development Grant (0835237N), respectively. Y.E.C. is an established investigator of American Heart Association.
Abbreviations
4E-BP1, 4E-binding protein 1; 15d-PGJ2, 15-deoxy-δ 12,14-prostaglandin J2; ABC, ATP-binding cassette; AGP, 1-O-octadecenyl-2-hydroxy-sn-glycero-3-phosphate; AngII, angiotensin II; AP-1, activator protein-1; APC, angiogenic progenitor cell; apoE−/−, apo E knockout; AT1, angiotensin II type 1 receptor; AT2, angiotensin II type 2 receptor; azPC, 1-O-hexadecyl-2-azelaoyl-sn-glycero-3-phosphocholine; BADGE, bisphenol A diglycidyl ether; bFGF, basic fibroblast growth factor; CARLA, coactivator-dependent receptor ligand assay; CBP, CREB-binding protein; CCR2, chemokine receptor 2; C/EBP, CCAAT/enhancer-binding protein; CPT-1, carnitine palmitoyltransferase type 1; CTGF, connective tissue growth factor; ECs, endothelial cells; Egr-1, early growth response-1; eNOS, endothelial nitric oxide synthase; EPC, endothelial progenitor cell; ERK 1/2, extracellular signal regulated kinase 1/2; ET-1, endothelin-1; FAO, fatty acid oxidation; FATP-1, fatty acid transport protein-1; FRET, fluorescence resonance energy transfer; FXR, farnesoid X receptor; FOXO, forkhead-box class O; GLUT1, glucose transporter 1; GLUT4, glucose transporter 4; GM-CSF, granulocyte–macrophage colony-stimulating factor; HASMCs, human aortic smooth muscle cells; HDAC-3, histone deacetylase-3; HDL, high-density lipoprotein; HETE, hydroxyeicosatetraenoic acid; HO-1, heme-oxygenase 1; HODE, hydroxyoctadecadienoic acid; HUVECs, human umbilical vein endothelial cells; ICAM-1, intercellular adhesion molecule-1; IFN, interferon; IFN-γ, interferon-gamma; IGF, insulin-like growth factor; IκBα, IkappaB-alpha; IKK, IkappaB kinase; IL, interleukin; IL-1β, interleukin-1beta; IL-1Ra, IL-1 receptor antagonist; iNOS, inducible nitric oxide synthase; IP-10, IFN-inducible protein of 10 kDa; IRF-1, interferon regulatory factor; I-TAC, IFN-inducible T-cell α-chemoattractant; KLF, krüpple-like factor; LDL, low-density lipoprotein; LDL-R−/−, low-density lipoprotein receptor knockout; LNO2, nitro-9,12-cis-octadecadienoic acid; LPA, lysophosphatidic acid; LPL, lipoprotein lipase; LPS, lipopolysaccharide; LTB4, leukotriene B4; LXR, liver X receptor; MAPK, mitogen-activated protein kinase; MCM, minichromosome maintenance protein; MCP-1, monocyte chemoattractant protein-1; MHC-II, major histocompatibility complex class II; Mig, monokine induced by IFN-γ; MMP-2, matrix metalloproteinase-2; MMP-9, matrix metalloproteinase-9; N-CoR, nuclear receptor co-repressor; NF-κB, nuclear factor-kappa B; NO, nitric oxide; NPC, Niemann-Pick, type C; OA-NO2, nitro-9-cis-octadecenoic acid; OPG, osteoprotegrin; OPN, osteopontin; oxLDL, oxidized LDL; PAF, platelet-activating factor; PAI-1, plasminogen activator inhibitor type-1; PDGF, platelet-derived growth factor; PECAM-1, platelet–endothelial cell adhesion molecule; PGC-1α, PPARγ coactivator-1α; PKC, protein kinase C; PLA, phospholipase A2; PPAR, peroxisome proliferator-activated receptor; PPARα, PPARalpha; PPARβ/δ, PPARbeta/delta; PPARγ, PPARgamma; PPARγ E null, endothelial cell PPARgamma knockout; PPRE, peroxisome proliferator response element; PTEN, phosphatase and tensin homologue; Rb, retinoblastoma protein; RGS, regulator of G-protein signaling; RXR, retinoic X receptor; SHIP2, Src homology (SH) 2–containing inositol phosphatase 2; SHP-2, Src homology region 2–containing protein tyrosine phosphatase-2; SHRs, spontaneously hypertensive rats; SM-α-actin, smooth muscle alpha-actin; SM-MHC, smooth muscle myosin heavy chain; SPA, scintillation proximity assay; sPLA2-II2, secretory phospholipase A2; SPPARMs, selective PPAR modulators; SPR, surface plasmon resonance; SR-A, scavenger receptor A; SR-B, scavenger receptor B; STAT, signal transduction and activator of transcription; TERT, telomerase reverse transcriptase; TF, tissue factor; TGF-β, transforming growth factor-beta; TNF-α, tumor necrosis factor-alpha; TZD, thiazolidinedione; VCAM-1, vascular cell adhesion molecule-1; VEGF, vascular endothelial growth factor; VLDLs, very low density lipoproteins; VSMCs, vascular smooth muscle cells.
References
- 1.Aasum E. Belke DD. Severson DL. Riemersma RA. Cooper M. Andreassen M. Larsen TS. Cardiac function and metabolism in type 2 diabetic mice after treatment with BM 17.0744, a novel PPAR-alpha activator. Am J Physiol Heart Circ Physiol. 2002;283:H949–H957. doi: 10.1152/ajpheart.00226.2001. [DOI] [PubMed] [Google Scholar]
- 2.Aasum E. Cooper M. Severson DL. Larsen TS. Effect of BM 17.0744, a PPARalpha ligand, on the metabolism of perfused hearts from control and diabetic mice. Can J Physiol Pharmacol. 2005;83:183–190. doi: 10.1139/y04-139. [DOI] [PubMed] [Google Scholar]
- 3.Abdelrahman M. Sivarajah A. Thiemermann C. Beneficial effects of PPAR-gamma ligands in ischemia-reperfusion injury, inflammation and shock. Cardiovasc Res. 2005;65:772–781. doi: 10.1016/j.cardiores.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Abe M. Hasegawa K. Wada H. Morimoto T. Yanazume T. Kawamura T. Hirai M. Furukawa Y. Kita T. GATA-6 is involved in PPARgamma-mediated activation of differentiated phenotype in human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2003;23:404–410. doi: 10.1161/01.ATV.0000059405.51042.A0. [DOI] [PubMed] [Google Scholar]
- 5.Adams M. Reginato MJ. Shao D. Lazar MA. Chatterjee VK. Transcriptional activation by peroxisome proliferator-activated receptor gamma is inhibited by phosphorylation at a consensus mitogen-activated protein kinase site. J Biol Chem. 1997;272:5128–5132. doi: 10.1074/jbc.272.8.5128. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed W. Orasanu G. Nehra V. Asatryan L. Rader DJ. Ziouzenkova O. Plutzky J. High-density lipoprotein hydrolysis by endothelial lipase activates PPARalpha: a candidate mechanism for high-density lipoprotein-mediated repression of leukocyte adhesion. Circ Res. 2006;98:490–498. doi: 10.1161/01.RES.0000205846.46812.be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akaike M. Che W. Marmarosh NL. Ohta S. Osawa M. Ding B. Berk BC. Yan C. Abe J. The hinge-helix 1 region of peroxisome proliferator-activated receptor gamma1 (PPARgamma1) mediates interaction with extracellular signal-regulated kinase 5 and PPARgamma1 transcriptional activation: involvement in flow-induced PPARgamma activation in endothelial cells. Mol Cell Biol. 2004;24:8691–8704. doi: 10.1128/MCB.24.19.8691-8704.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akiyama TE. Sakai S. Lambert G. Nicol CJ. Matsusue K. Pimprale S. Lee YH. Ricote M. Glass CK. Brewer HB., Jr. Gonzalez FJ. Conditional disruption of the peroxisome proliferator-activated receptor gamma gene in mice results in lowered expression of ABCA1, ABCG1, and apoE in macrophages and reduced cholesterol efflux. Mol Cell Biol. 2002;22:2607–2619. doi: 10.1128/MCB.22.8.2607-2619.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexis JD. Wang N. Che W. Lerner-Marmarosh N. Sahni A. Korshunov VA. Zou Y. Ding B. Yan C. Berk BC. Abe JI. Bcr kinase activation by angiotensin II inhibits peroxisome proliferator-activated receptor {gamma} transcriptional activity in vascular smooth muscle cells. Circ Res. 2009;1:69–78. doi: 10.1161/CIRCRESAHA.108.188409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ameshima S. Golpon H. Cool CD. Chan D. Vandivier RW. Gardai SJ. Wick M. Nemenoff RA. Geraci MW. Voelkel NF. Peroxisome proliferator-activated receptor gamma (PPARgamma) expression is decreased in pulmonary hypertension and affects endothelial cell growth. Circ Res. 2003;92:1162–1169. doi: 10.1161/01.RES.0000073585.50092.14. [DOI] [PubMed] [Google Scholar]
- 11.Argmann CA. Sawyez CG. McNeil CJ. Hegele RA. Huff MW. Activation of peroxisome proliferator-activated receptor gamma and retinoid X receptor results in net depletion of cellular cholesteryl esters in macrophages exposed to oxidized lipoproteins. Arterioscler Thromb Vasc Biol. 2003;23:475–482. doi: 10.1161/01.ATV.0000058860.62870.6E. [DOI] [PubMed] [Google Scholar]
- 12.Armoni M. Harel C. Bar-Yoseph F. Milo S. Karnieli E. Free fatty acids repress the GLUT4 gene expression in cardiac muscle via novel response elements. J Biol Chem. 2005;280:34786–34795. doi: 10.1074/jbc.M502740200. [DOI] [PubMed] [Google Scholar]
- 13.Armoni M. Harel C. Karni S. Chen H. Bar-Yoseph F. Ver MR. Quon MJ. Karnieli E. FOXO1 represses peroxisome proliferator-activated receptor-gamma1 and -gamma2 gene promoters in primary adipocytes: a novel paradigm to increase insulin sensitivity. J Biol Chem. 2006;281:19881–19891. doi: 10.1074/jbc.M600320200. [DOI] [PubMed] [Google Scholar]
- 14.Armoni M. Harel C. Karnieli E. Transcriptional regulation of the GLUT4 gene: from PPAR-gamma and FOXO1 to FFA and inflammation. Trends Endocrinol Metab: TEM. 2007;18:100–107. doi: 10.1016/j.tem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Armoni M. Kritz N. Harel C. Bar-Yoseph F. Chen H. Quon MJ. Karnieli E. Peroxisome proliferator-activated receptor-gamma represses GLUT4 promoter activity in primary adipocytes, and rosiglitazone alleviates this effect. J Biol Chem. 2003;278:30614–30623. doi: 10.1074/jbc.M304654200. [DOI] [PubMed] [Google Scholar]
- 16.Asakawa M. Takano H. Nagai T. Uozumi H. Hasegawa H. Kubota N. Saito T. Masuda Y. Kadowaki T. Komuro I. Peroxisome proliferator-activated receptor gamma plays a critical role in inhibition of cardiac hypertrophy in vitro and in vivo. Circulation. 2002;105:1240–1246. doi: 10.1161/hc1002.105225. [DOI] [PubMed] [Google Scholar]
- 17.Babaev VR. Ishiguro H. Ding L. Yancey PG. Dove DE. Kovacs WJ. Semenkovich CF. Fazio S. Linton MF. Macrophage expression of peroxisome proliferator-activated receptor-alpha reduces atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 2007;116:1404–1412. doi: 10.1161/CIRCULATIONAHA.106.684704. [DOI] [PubMed] [Google Scholar]
- 18.Babaev VR. Yancey PG. Ryzhov SV. Kon V. Breyer MD. Magnuson MA. Fazio S. Linton MF. Conditional knockout of macrophage PPARgamma increases atherosclerosis in C57BL/6 and low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2005;25:1647–1653. doi: 10.1161/01.ATV.0000173413.31789.1a. [DOI] [PubMed] [Google Scholar]
- 19.Baker PR. Lin Y. Schopfer FJ. Woodcock SR. Groeger AL. Batthyany C. Sweeney S. Long MH. Iles KE. Baker LM. Branchaud BP. Chen YE. Freeman BA. Fatty acid transduction of nitric oxide signaling: multiple nitrated unsaturated fatty acid derivatives exist in human blood and urine and serve as endogenous peroxisome proliferator-activated receptor ligands. J Biol Chem. 2005;280:42464–42475. doi: 10.1074/jbc.M504212200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barak Y. Liao D. He W. Ong ES. Nelson MC. Olefsky JM. Boland R. Evans RM. Effects of peroxisome proliferator-activated receptor delta on placentation, adiposity, and colorectal cancer. Proc Natl Acad Sci U S A. 2002;99:303–308. doi: 10.1073/pnas.012610299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barak Y. Nelson MC. Ong ES. Jones YZ. Ruiz-Lozano P. Chien KR. Koder A. Evans RM. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999;4:585–595. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- 22.Bardot O. Aldridge TC. Latruffe N. Green S. PPAR-RXR heterodimer activates a peroxisome proliferator response element upstream of the bifunctional enzyme gene. Biochem Biophys Res Commun. 1993;192:37–45. doi: 10.1006/bbrc.1993.1378. [DOI] [PubMed] [Google Scholar]
- 23.Barger PM. Brandt JM. Leone TC. Weinheimer CJ. Kelly DP. Deactivation of peroxisome proliferator-activated receptor-alpha during cardiac hypertrophic growth. J Clin Invest. 2000;105:1723–1730. doi: 10.1172/JCI9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barger PM. Browning AC. Garner AN. Kelly DP. p38 mitogen-activated protein kinase activates peroxisome proliferator-activated receptor alpha: a potential role in the cardiac metabolic stress response. J Biol Chem. 2001;276:44495–44501. doi: 10.1074/jbc.M105945200. [DOI] [PubMed] [Google Scholar]
- 25.Barish GD. Atkins AR. Downes M. Olson P. Chong LW. Nelson M. Zou Y. Hwang H. Kang H. Curtiss L. Evans RM. Lee CH. PPARdelta regulates multiple proinflammatory pathways to suppress atherosclerosis. Proc Natl Acad Sci U S A. 2008;105:4271–4276. doi: 10.1073/pnas.0711875105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barlic J. Zhang Y. Foley JF. Murphy PM. Oxidized lipid-driven chemokine receptor switch, CCR2 to CX3CR1, mediates adhesion of human macrophages to coronary artery smooth muscle cells through a peroxisome proliferator-activated receptor gamma-dependent pathway. Circulation. 2006;114:807–819. doi: 10.1161/CIRCULATIONAHA.105.602359. [DOI] [PubMed] [Google Scholar]
- 27.Barroso I. Gurnell M. Crowley VE. Agostini M. Schwabe JW. Soos MA. Maslen GL. Williams TD. Lewis H. Schafer AJ. Chatterjee VK. O'Rahilly S. Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402:880–883. doi: 10.1038/47254. [DOI] [PubMed] [Google Scholar]
- 28.Benkirane K. Amiri F. Diep QN. El Mabrouk M. Schiffrin EL. PPAR-gamma inhibits ANG II-induced cell growth via SHIP2 and 4E-BP1. Am J Physiol Heart Circ Physiol. 2006;290:H390–H397. doi: 10.1152/ajpheart.00662.2005. [DOI] [PubMed] [Google Scholar]
- 29.Benson S. Wu J. Padmanabhan S. Kurtz TW. Pershadsingh HA. Peroxisome proliferator-activated receptor (PPAR)-gamma expression in human vascular smooth muscle cells: inhibition of growth, migration, and c-fos expression by the peroxisome proliferator-activated receptor (PPAR)-gamma activator troglitazone. Am J Hypertens. 2000;13:74–82. doi: 10.1016/s0895-7061(99)00148-x. [DOI] [PubMed] [Google Scholar]
- 30.Berger J. Leibowitz MD. Doebber TW. Elbrecht A. Zhang B. Zhou G. Biswas C. Cullinan CA. Hayes NS. Li Y. Tanen M. Ventre J. Wu MS. Berger GD. Mosley R. Marquis R. Santini C. Sahoo SP. Tolman RL. Smith RG. Moller DE. Novel peroxisome proliferator-activated receptor (PPAR) gamma and PPARdelta ligands produce distinct biological effects. J Biol Chem. 1999;274:6718–6725. doi: 10.1074/jbc.274.10.6718. [DOI] [PubMed] [Google Scholar]
- 31.Berry A. Balard P. Coste A. Olagnier D. Lagane C. Authier H. Benoit-Vical F. Lepert JC. Seguela JP. Magnaval JF. Chambon P. Metzger D. Desvergne B. Wahli W. Auwerx J. Pipy B. IL-13 induces expression of CD36 in human monocytes through PPARgamma activation. Eur J Immunol. 2007;37:1642–1652. doi: 10.1002/eji.200636625. [DOI] [PubMed] [Google Scholar]
- 32.Beyer AM. Baumbach GL. Halabi CM. Modrick ML. Lynch CM. Gerhold TD. Ghoneim SM. de Lange WJ. Keen HL. Tsai YS. Maeda N. Sigmund CD. Faraci FM. Interference with PPARgamma signaling causes cerebral vascular dysfunction, hypertrophy, and remodeling. Hypertension. 2008;51:867–871. doi: 10.1161/HYPERTENSIONAHA.107.103648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biscetti F. Gaetani E. Flex A. Aprahamian T. Hopkins T. Straface G. Pecorini G. Stigliano E. Smith RC. Angelini F. Castellot JJ Jr. Pola R. Selective activation of peroxisome proliferator-activated receptor (PPAR)alpha and PPAR gamma induces neoangiogenesis through a vascular endothelial growth factor-dependent mechanism. Diabetes. 2008;57:1394–1404. doi: 10.2337/db07-0765. [DOI] [PubMed] [Google Scholar]
- 34.Bishop-Bailey D. Hla T. Endothelial cell apoptosis induced by the peroxisome proliferator-activated receptor (PPAR) ligand 15-deoxy-delta12, 14-prostaglandin J2. J Bioll Chem. 1999;274:17042–17048. doi: 10.1074/jbc.274.24.17042. [DOI] [PubMed] [Google Scholar]
- 35.Bishop-Bailey D. Hla T. Warner TD. Intimal smooth muscle cells as a target for peroxisome proliferator-activated receptor-gamma ligand therapy. Circulation research. 2002;91:210–217. doi: 10.1161/01.res.0000029080.15742.85. [DOI] [PubMed] [Google Scholar]
- 36.Bocos C. Gottlicher M. Gearing K. Banner C. Enmark E. Teboul M. Crickmore A. Gustafsson JA. Fatty acid activation of peroxisome proliferator-activated receptor (PPAR) J Steroid Biochem Mol Biol. 1995;53:467–473. doi: 10.1016/0960-0760(95)00093-f. [DOI] [PubMed] [Google Scholar]
- 37.Bouhlel MA. Derudas B. Rigamonti E. Dievart R. Brozek J. Haulon S. Zawadzki C. Jude B. Torpier G. Marx N. Staels B. Chinetti-Gbaguidi G. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007;6:137–143. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 38.Bouloumie A. Drexler HC. Lafontan M. Busse R. Leptin, the product of Ob gene, promotes angiogenesis. Circ Res. 1998;83:1059–1066. doi: 10.1161/01.res.83.10.1059. [DOI] [PubMed] [Google Scholar]
- 39.Brown KK. Henke BR. Blanchard SG. Cobb JE. Mook R. Kaldor I. Kliewer SA. Lehmann JM. Lenhard JM. Harrington WW. Novak PJ. Faison W. Binz JG. Hashim MA. Oliver WO. Brown HR. Parks DJ. Plunket KD. Tong WQ. Menius JA. Adkison K. Noble SA. Willson TM. A novel N-aryl tyrosine activator of peroxisome proliferator-activated receptor-gamma reverses the diabetic phenotype of the Zucker diabetic fatty rat. Diabetes. 1999;48:1415–1424. doi: 10.2337/diabetes.48.7.1415. [DOI] [PubMed] [Google Scholar]
- 40.Brown PJ. Stuart LW. Hurley KP. Lewis MC. Winegar DA. Wilson JG. Wilkison WO. Ittoop OR. Willson TM. Identification of a subtype selective human PPARalpha agonist through parallel-array synthesis. Bioorg Med Chem Lett. 2001;11:1225–1227. doi: 10.1016/s0960-894x(01)00188-3. [DOI] [PubMed] [Google Scholar]
- 41.Brown PJ. Winegar DA. Plunket KD. Moore LB. Lewis MC. Wilson JG. Sundseth SS. Koble CS. Wu Z. Chapman JM. Lehmann JM. Kliewer SA. Willson TM. A ureido-thioisobutyric acid (GW9578) is a subtype-selective PPARalpha agonist with potent lipid-lowering activity. J Med Chem. 1999;42:3785–3788. doi: 10.1021/jm9903601. [DOI] [PubMed] [Google Scholar]
- 42.Bruemmer D. Berger JP. Liu J. Kintscher U. Wakino S. Fleck E. Moller DE. Law RE. A non-thiazolidinedione partial peroxisome proliferator-activated receptor gamma ligand inhibits vascular smooth muscle cell growth. Eur J Pharmacol. 2003;466:225–234. doi: 10.1016/s0014-2999(03)01556-5. [DOI] [PubMed] [Google Scholar]
- 43.Bruemmer D. Yin F. Liu J. Berger JP. Kiyono T. Chen J. Fleck E. Van Herle AJ. Forman BM. Law RE. Peroxisome proliferator-activated receptor gamma inhibits expression of minichromosome maintenance proteins in vascular smooth muscle cells. Mol Endocrinol (Baltimore) 2003;17:1005–1018. doi: 10.1210/me.2002-0410. [DOI] [PubMed] [Google Scholar]
- 44.Bruemmer D. Yin F. Liu J. Berger JP. Sakai T. Blaschke F. Fleck E. Van Herle AJ. Forman BM. Law RE. Regulation of the growth arrest and DNA damage-inducible gene 45 (GADD45) by peroxisome proliferator-activated receptor gamma in vascular smooth muscle cells. Circ Res. 2003;93:e38–e47. doi: 10.1161/01.RES.0000088344.15288.E6. [DOI] [PubMed] [Google Scholar]
- 45.Brunelli L. Cieslik KA. Alcorn JL. Vatta M. Baldini A. Peroxisome proliferator-activated receptor-delta upregulates 14-3-3 epsilon in human endothelial cells via CCAAT/enhancer binding protein-beta. Circ Res. 2007;100:e59–e71. doi: 10.1161/01.RES.0000260805.99076.22. [DOI] [PubMed] [Google Scholar]
- 46.Burkart EM. Sambandam N. Han X. Gross RW. Courtois M. Gierasch CM. Shoghi K. Welch MJ. Kelly DP. Nuclear receptors PPARbeta/delta and PPARalpha direct distinct metabolic regulatory programs in the mouse heart. J Clin Invest. 2007;117:3930–3939. doi: 10.1172/JCI32578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cabrero A. Jove M. Planavila A. Merlos M. Laguna JC. Vazquez-Carrera M. Down-regulation of acyl-CoA oxidase gene expression in heart of troglitazone-treated mice through a mechanism involving chicken ovalbumin upstream promoter transcription factor II. Mol Pharmacol. 2003;64:764–772. doi: 10.1124/mol.64.3.764. [DOI] [PubMed] [Google Scholar]
- 48.Calkin AC. Forbes JM. Smith CM. Lassila M. Cooper ME. Jandeleit-Dahm KA. Allen TJ. Rosiglitazone attenuates atherosclerosis in a model of insulin insufficiency independent of its metabolic effects. Arterioscler, Thromb Vasc Biol. 2005;25:1903–1909. doi: 10.1161/01.ATV.0000177813.99577.6b. [DOI] [PubMed] [Google Scholar]
- 49.Calnek DS. Mazzella L. Roser S. Roman J. Hart CM. Peroxisome proliferator-activated receptor gamma ligands increase release of nitric oxide from endothelial cells. Arterioscler Thromb Vasc Biol. 2003;23:52–57. doi: 10.1161/01.atv.0000044461.01844.c9. [DOI] [PubMed] [Google Scholar]
- 50.Camp HS. Chaudhry A. Leff T. A novel potent antagonist of peroxisome proliferator-activated receptor gamma blocks adipocyte differentiation but does not revert the phenotype of terminally differentiated adipocytes. Endocrinology. 2001;142:3207–3213. doi: 10.1210/endo.142.7.8254. [DOI] [PubMed] [Google Scholar]
- 51.Camp HS. Tafuri SR. Regulation of peroxisome proliferator-activated receptor gamma activity by mitogen-activated protein kinase. J Biol Chem. 1997;272:10811–10816. doi: 10.1074/jbc.272.16.10811. [DOI] [PubMed] [Google Scholar]
- 52.Camp HS. Tafuri SR. Leff T. c-Jun N-terminal kinase phosphorylates peroxisome proliferator-activated receptor-gamma1 and negatively regulates its transcriptional activity. Endocrinology. 1999;140:392–397. doi: 10.1210/endo.140.1.6457. [DOI] [PubMed] [Google Scholar]
- 53.Campbell FM. Kozak R. Wagner A. Altarejos JY. Dyck JR. Belke DD. Severson DL. Kelly DP. Lopaschuk GD. A role for peroxisome proliferator-activated receptor alpha (PPARalpha) in the control of cardiac malonyl-CoA levels: reduced fatty acid oxidation rates and increased glucose oxidation rates in the hearts of mice lacking PPARalpha are associated with higher concentrations of malonyl-CoA and reduced expression of malonyl-CoA decarboxylase. J Biol Chem. 2002;277:4098–4103. doi: 10.1074/jbc.M106054200. [DOI] [PubMed] [Google Scholar]
- 54.Castrillo A. Diaz-Guerra MJ. Hortelano S. Martin-Sanz P. Bosca L. Inhibition of IkappaB kinase and IkappaB phosphorylation by 15-deoxy-delta(12,14)-prostaglandin J(2) in activated murine macrophages. Mol Cell Biol. 2000;20:1692–1698. doi: 10.1128/mcb.20.5.1692-1698.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Castrillo A. Mojena M. Hortelano S. Bosca L. Peroxisome proliferator-activated receptor-gamma-independent inhibition of macrophage activation by the non-thiazolidinedione agonist L-796,449: comparison with the effects of 15-deoxy-delta(12,14)-prostaglandin J(2) J Biol Chem. 2001;276:34082–34088. doi: 10.1074/jbc.M102472200. [DOI] [PubMed] [Google Scholar]
- 56.Chawla A. Barak Y. Nagy L. Liao D. Tontonoz P. Evans RM. PPAR-gamma dependent and independent effects on macrophage-gene expression in lipid metabolism and inflammation. Nat Med. 2001;7:48–52. doi: 10.1038/83336. [DOI] [PubMed] [Google Scholar]
- 57.Chawla A. Boisvert WA. Lee CH. Laffitte BA. Barak Y. Joseph SB. Liao D. Nagy L. Edwards PA. Curtiss LK. Evans RM. Tontonoz P. A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol Cell. 2001;7:161–171. doi: 10.1016/s1097-2765(01)00164-2. [DOI] [PubMed] [Google Scholar]
- 58.Chawla A. Lee CH. Barak Y. He W. Rosenfeld J. Liao D. Han J. Kang H. Evans RM. PPARdelta is a very low-density lipoprotein sensor in macrophages. Proc Natl Acad Sci U S A. 2003;100:1268–1273. doi: 10.1073/pnas.0337331100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chawla A. Schwarz EJ. Dimaculangan DD. Lazar MA. Peroxisome proliferator-activated receptor (PPAR) gamma: adipose-predominant expression and induction early in adipocyte differentiation. Endocrinology. 1994;135:798–800. doi: 10.1210/endo.135.2.8033830. [DOI] [PubMed] [Google Scholar]
- 60.Cheng L. Ding G. Qin Q. Huang Y. Lewis W. He N. Evans RM. Schneider MD. Brako FA. Xiao Y. Chen YE. Yang Q. Cardiomyocyte-restricted peroxisome proliferator-activated receptor-delta deletion perturbs myocardial fatty acid oxidation and leads to cardiomyopathy. Nat Med. 2004;10:1245–1250. doi: 10.1038/nm1116. [DOI] [PubMed] [Google Scholar]
- 61.Cheng L. Ding G. Qin Q. Xiao Y. Woods D. Chen YE. Yang Q. Peroxisome proliferator-activated receptor delta activates fatty acid oxidation in cultured neonatal and adult cardiomyocytes. Biochem Biophys Res Commun. 2004;313:277–286. doi: 10.1016/j.bbrc.2003.11.127. [DOI] [PubMed] [Google Scholar]
- 62.Chinetti-Gbaguidi G. Rigamonti E. Helin L. Mutka AL. Lepore M. Fruchart JC. Clavey V. Ikonen E. Lestavel S. Staels B. Peroxisome proliferator-activated receptor alpha controls cellular cholesterol trafficking in macrophages. J Lipid Res. 2005;46:2717–2725. doi: 10.1194/jlr.M500326-JLR200. [DOI] [PubMed] [Google Scholar]
- 63.Chinetti G. Gbaguidi FG. Griglio S. Mallat Z. Antonucci M. Poulain P. Chapman J. Fruchart JC. Tedgui A. Najib-Fruchart J. Staels B. CLA-1/SR-BI is expressed in atherosclerotic lesion macrophages and regulated by activators of peroxisome proliferator-activated receptors. Circulation. 2000;101:2411–2417. doi: 10.1161/01.cir.101.20.2411. [DOI] [PubMed] [Google Scholar]
- 64.Chinetti G. Griglio S. Antonucci M. Torra IP. Delerive P. Majd Z. Fruchart JC. Chapman J. Najib J. Staels B. Activation of proliferator-activated receptors alpha and gamma induces apoptosis of human monocyte-derived macrophages. J Biol Chem. 1998;273:25573–25580. doi: 10.1074/jbc.273.40.25573. [DOI] [PubMed] [Google Scholar]
- 65.Chinetti G. Lestavel S. Bocher V. Remaley AT. Neve B. Torra IP. Teissier E. Minnich A. Jaye M. Duverger N. Brewer HB. Fruchart JC. Clavey V. Staels B. PPAR-alpha and PPAR-gamma activators induce cholesterol removal from human macrophage foam cells through stimulation of the ABCA1 pathway. Nat Med. 2001;7:53–58. doi: 10.1038/83348. [DOI] [PubMed] [Google Scholar]
- 66.Chinetti G. Lestavel S. Fruchart JC. Clavey V. Staels B. Peroxisome proliferator-activated receptor alpha reduces cholesterol esterification in macrophages. Circ Res. 2003;92:212–217. doi: 10.1161/01.res.0000053386.46813.e9. [DOI] [PubMed] [Google Scholar]
- 67.Cho DH. Choi YJ. Jo SA. Jo I. Nitric oxide production and regulation of endothelial nitric-oxide synthase phosphorylation by prolonged treatment with troglitazone: evidence for involvement of peroxisome proliferator-activated receptor (PPAR) gamma-dependent and PPARgamma-independent signaling pathways. J Biol Chem. 2004;279:2499–2506. doi: 10.1074/jbc.M309451200. [DOI] [PubMed] [Google Scholar]
- 68.Cho MC. Lee K. Paik SG. Yoon DY. Peroxisome proliferators-activated receptor (PPAR) modulators and metabolic disorders. PPAR Res. 2008;2008:679137. doi: 10.1155/2008/679137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cho MC. Yoon HE. Kang JW. Park SW. Yang Y. Hong JT. Song EY. Paik SG. Kim SH. Yoon DY. A simple method to screen ligands of peroxisome proliferator-activated receptor delta. Eur J Pharm Sci. 2006;29:355–360. doi: 10.1016/j.ejps.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 70.Clark RB. Bishop-Bailey D. Estrada-Hernandez T. Hla T. Puddington L. Padula SJ. The nuclear receptor PPAR gamma and immunoregulation: PPAR gamma mediates inhibition of helper T cell responses. J Immunol. 2000;164:1364–1371. doi: 10.4049/jimmunol.164.3.1364. [DOI] [PubMed] [Google Scholar]
- 71.Colca JR. McDonald WG. Waldon DJ. Leone JW. Lull JM. Bannow CA. Lund ET. Mathews WR. Identification of a novel mitochondrial protein ("mitoNEET") cross-linked specifically by a thiazolidinedione photoprobe. Am J Physiol. 2004;286:E252–E260. doi: 10.1152/ajpendo.00424.2003. [DOI] [PubMed] [Google Scholar]
- 72.Collins AR. Meehan WP. Kintscher U. Jackson S. Wakino S. Noh G. Palinski W. Hsueh WA. Law RE. Troglitazone inhibits formation of early atherosclerotic lesions in diabetic and nondiabetic low density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:365–371. doi: 10.1161/01.atv.21.3.365. [DOI] [PubMed] [Google Scholar]
- 73.Collins T. Read MA. Neish AS. Whitley MZ. Thanos D. Maniatis T. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. FASEB J. 1995;9:899–909. [PubMed] [Google Scholar]
- 74.Colville-Nash PR. Qureshi SS. Willis D. Willoughby DA. Inhibition of inducible nitric oxide synthase by peroxisome proliferator-activated receptor agonists: correlation with induction of heme oxygenase 1. J Immunol. 1998;161:978–984. [PubMed] [Google Scholar]
- 75.Cook WS. Yeldandi AV. Rao MS. Hashimoto T. Reddy JK. Less extrahepatic induction of fatty acid beta-oxidation enzymes by PPAR alpha. Biochchem Biophys Res Commun. 2000;278:250–257. doi: 10.1006/bbrc.2000.3739. [DOI] [PubMed] [Google Scholar]
- 76.Cui T. Schopfer FJ. Zhang J. Chen K. Ichikawa T. Baker PR. Batthyany C. Chacko BK. Feng X. Patel RP. Agarwal A. Freeman BA. Chen YE. Nitrated fatty acids: endogenous anti-inflammatory signaling mediators. J Biol Chem. 2006;281:35686–35698. doi: 10.1074/jbc.M603357200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cuthbert KD. Dyck JR. Malonyl-CoA decarboxylase is a major regulator of myocardial fatty acid oxidation. Curr Hypertens Rep. 2005;7:407–411. doi: 10.1007/s11906-005-0034-z. [DOI] [PubMed] [Google Scholar]
- 78.Davies SS. Pontsler AV. Marathe GK. Harrison KA. Murphy RC. Hinshaw JC. Prestwich GD. Hilaire AS. Prescott SM. Zimmerman GA. McIntyre TM. Oxidized alkyl phospholipids are specific, high affinity peroxisome proliferator-activated receptor gamma ligands and agonists. J Biol Chem. 2001;276:16015–16023. doi: 10.1074/jbc.M100878200. [DOI] [PubMed] [Google Scholar]
- 79.de Dios ST. Bruemmer D. Dilley RJ. Ivey ME. Jennings GL. Law RE. Little PJ. Inhibitory activity of clinical thiazolidinedione peroxisome proliferator activating receptor-gamma ligands toward internal mammary artery, radial artery, and saphenous vein smooth muscle cell proliferation. Circulation. 2003;107:2548–2550. doi: 10.1161/01.CIR.0000074040.31731.96. [DOI] [PubMed] [Google Scholar]
- 80.Delerive P. De Bosscher K. Besnard S. Vanden Berghe W. Peters JM. Gonzalez FJ. Fruchart JC. Tedgui A. Haegeman G. Staels B. Peroxisome proliferator-activated receptor alpha negatively regulates the vascular inflammatory gene response by negative cross-talk with transcription factors NF-kappaB and AP-1. J Biol Chem. 1999;274:32048–32054. doi: 10.1074/jbc.274.45.32048. [DOI] [PubMed] [Google Scholar]
- 81.Delerive P. Furman C. Teissier E. Fruchart J. Duriez P. Staels B. Oxidized phospholipids activate PPARalpha in a phospholipase A2-dependent manner. FEBS Lett. 2000;471:34–38. doi: 10.1016/s0014-5793(00)01364-8. [DOI] [PubMed] [Google Scholar]
- 82.Delerive P. Gervois P. Fruchart JC. Staels B. Induction of IkappaBalpha expression as a mechanism contributing to the anti-inflammatory activities of peroxisome proliferator-activated receptor-alpha activators. J Biol Chem. 2000;275:36703–36707. doi: 10.1074/jbc.M004045200. [DOI] [PubMed] [Google Scholar]
- 83.Delerive P. Martin-Nizard F. Chinetti G. Trottein F. Fruchart JC. Najib J. Duriez P. Staels B. Peroxisome proliferator-activated receptor activators inhibit thrombin-induced endothelin-1 production in human vascular endothelial cells by inhibiting the activator protein-1 signaling pathway. Circ Res. 1999;85:394–402. doi: 10.1161/01.res.85.5.394. [DOI] [PubMed] [Google Scholar]
- 84.Depre C. Havaux X. Renkin J. Vanoverschelde JL. Wijns W. Expression of inducible nitric oxide synthase in human coronary atherosclerotic plaque. Cardiovasc Res. 1999;41:465–472. doi: 10.1016/s0008-6363(98)00304-6. [DOI] [PubMed] [Google Scholar]
- 85.Desvergne B. Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev. 1999;20:649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- 86.Devchand PR. Keller H. Peters JM. Vazquez M. Gonzalez FJ. Wahli W. The PPARalpha-leukotriene B4 pathway to inflammation control. Nature. 1996;384:39–43. doi: 10.1038/384039a0. [DOI] [PubMed] [Google Scholar]
- 87.Diep QN. El Mabrouk M. Cohn JS. Endemann D. Amiri F. Virdis A. Neves MF. Schiffrin EL. Structure, endothelial function, cell growth, and inflammation in blood vessels of angiotensin II-infused rats: role of peroxisome proliferator-activated receptor-gamma. Circulation. 2002;105:2296–2302. doi: 10.1161/01.cir.0000016049.86468.23. [DOI] [PubMed] [Google Scholar]
- 88.Diep QN. Schiffrin EL. Increased expression of peroxisome proliferator-activated receptor-alpha and -gamma in blood vessels of spontaneously hypertensive rats. Hypertension. 2001;38:249–254. doi: 10.1161/01.hyp.38.2.249. [DOI] [PubMed] [Google Scholar]
- 89.Diep QN. Touyz RM. Schiffrin EL. Docosahexaenoic acid, a peroxisome proliferator-activated receptor-alpha ligand, induces apoptosis in vascular smooth muscle cells by stimulation of p38 mitogen-activated protein kinase. Hypertension. 2000;36:851–855. doi: 10.1161/01.hyp.36.5.851. [DOI] [PubMed] [Google Scholar]
- 90.Ding G. Cheng L. Qin Q. Frontin S. Yang Q. PPARdelta modulates lipopolysaccharide-induced TNFalpha inflammation signaling in cultured cardiomyocytes. J Mol Cell Cardiol. 2006;40:821–828. doi: 10.1016/j.yjmcc.2006.03.422. [DOI] [PubMed] [Google Scholar]
- 91.Ding G. Fu M. Qin Q. Lewis W. Kim HW. Fukai T. Bacanamwo M. Chen YE. Schneider MD. Mangelsdorf DJ. Evans RM. Yang Q. Cardiac peroxisome proliferator-activated receptor gamma is essential in protecting cardiomyocytes from oxidative damage. Cardiovasc Res. 2007;76:269–279. doi: 10.1016/j.cardiores.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 92.Ditiatkovski M. Toh BH. Bobik A. GM-CSF deficiency reduces macrophage PPAR-gamma expression and aggravates atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2006;26:2337–2344. doi: 10.1161/01.ATV.0000238357.60338.90. [DOI] [PubMed] [Google Scholar]
- 93.Djouadi F. Weinheimer CJ. Saffitz JE. Pitchford C. Bastin J. Gonzalez FJ. Kelly DP. A gender-related defect in lipid metabolism and glucose homeostasis in peroxisome proliferator-activated receptor alpha-deficient mice. J Clin Invest. 1998;102:1083–1091. doi: 10.1172/JCI3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dobashi K. Asayama K. Nakane T. Kodera K. Hayashibe H. Nakazawa S. Troglitazone inhibits the expression of inducible nitric oxide synthase in adipocytes in vitro and in vivo study in 3T3-L1 cells and Otsuka Long-Evans Tokushima fatty rats. Life Sci. 2000;67:2093–2101. doi: 10.1016/s0024-3205(00)00796-7. [DOI] [PubMed] [Google Scholar]
- 95.Dormandy JA. Charbonnel B. Eckland DJ. Erdmann E. Massi-Benedetti M. Moules IK. Skene AM. Tan MH. Lefebvre PJ. Murray GD. Standl E. Wilcox RG. Wilhelmsen L. Betteridge J. Birkeland K. Golay A. Heine RJ. Koranyi L. Laakso M. Mokan M. Norkus A. Pirags V. Podar T. Scheen A. Scherbaum W. Schernthaner G. Schmitz O. Skrha J. Smith U. Taton J. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive study (PROspective pioglitAzone clinical trial in macrovascular events): a randomised controlled trial. Lancet. 2005;366:1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 96.Dreyer C. Krey G. Keller H. Givel F. Helftenbein G. Wahli W. Control of the peroxisomal beta-oxidation pathway by a novel family of nuclear hormone receptors. Cell. 1992;68:879–887. doi: 10.1016/0092-8674(92)90031-7. [DOI] [PubMed] [Google Scholar]
- 97.Duan SZ. Ivashchenko CY. Russell MW. Milstone DS. Mortensen RM. Cardiomyocyte-specific knockout and agonist of peroxisome proliferator-activated receptor-gamma both induce cardiac hypertrophy in mice. Circ Res. 2005;97:372–379. doi: 10.1161/01.RES.0000179226.34112.6d. [DOI] [PubMed] [Google Scholar]
- 98.Dubey RK. Zhang HY. Reddy SR. Boegehold MA. Kotchen TA. Pioglitazone attenuates hypertension and inhibits growth of renal arteriolar smooth muscle in rats. Am J Physiol. 1993;265:R726–R732. doi: 10.1152/ajpregu.1993.265.4.R726. [DOI] [PubMed] [Google Scholar]
- 99.Duez H. Chao YS. Hernandez M. Torpier G. Poulain P. Mundt S. Mallat Z. Teissier E. Burton CA. Tedgui A. Fruchart JC. Fievet C. Wright SD. Staels B. Reduction of atherosclerosis by the peroxisome proliferator-activated receptor alpha agonist fenofibrate in mice. J Biol Chem. 2002;277:48051–48057. doi: 10.1074/jbc.M206966200. [DOI] [PubMed] [Google Scholar]
- 100.Elbrecht A. Chen Y. Adams A. Berger J. Griffin P. Klatt T. Zhang B. Menke J. Zhou G. Smith RG. Moller DE. L-764406 is a partial agonist of human peroxisome proliferator-activated receptor gamma: the role of Cys313 in ligand binding. J Biol Chem. 1999;274:7913–7922. doi: 10.1074/jbc.274.12.7913. [DOI] [PubMed] [Google Scholar]
- 101.Endemann G. Stanton LW. Madden KS. Bryant CM. White RT. Protter AA. CD36 is a receptor for oxidized low density lipoprotein. J Biol Chem. 1993;268:11811–11816. [PubMed] [Google Scholar]
- 102.Erol A. The functions of PPARs in aging and longevity. PPAR Res. 2007;2007:39654. doi: 10.1155/2007/39654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Erol A. PPARalpha activators may play role for the regression of ventricular hypertrophy in hypertensive and hyperlipidemic patients. Med Hypoth. 2006;66:1044–1045. doi: 10.1016/j.mehy.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 104.Evans RM. The steroid and thyroid hormone receptor superfamily. Science (New York) 1988;240:889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fan Y. Wang Y. Tang Z. Zhang H. Qin X. Zhu Y. Guan Y. Wang X. Staels B. Chien S. Wang N. Suppression of pro-inflammatory adhesion molecules by PPAR-delta in human vascular endothelial cells. Arterioscler, Thromb Vasc Biol. 2008;28:315–321. doi: 10.1161/ATVBAHA.107.149815. [DOI] [PubMed] [Google Scholar]
- 106.Faveeuw C. Fougeray S. Angeli V. Fontaine J. Chinetti G. Gosset P. Delerive P. Maliszewski C. Capron M. Staels B. Moser M. Trottein F. Peroxisome proliferator-activated receptor gamma activators inhibit interleukin-12 production in murine dendritic cells. FEBS Lett. 2000;486:261–266. doi: 10.1016/s0014-5793(00)02319-x. [DOI] [PubMed] [Google Scholar]
- 107.Finck BN. Han X. Courtois M. Aimond F. Nerbonne JM. Kovacs A. Gross RW. Kelly DP. A critical role for PPARalpha-mediated lipotoxicity in the pathogenesis of diabetic cardiomyopathy: modulation by dietary fat content. Proc Natl Acad Sci U S A. 2003;100:1226–1231. doi: 10.1073/pnas.0336724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Finck BN. Lehman JJ. Leone TC. Welch MJ. Bennett MJ. Kovacs A. Han X. Gross RW. Kozak R. Lopaschuk GD. Kelly DP. The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J Clin Invest. 2002;109:121–130. doi: 10.1172/JCI14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Forman BM. Chen J. Evans RM. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc Natl Acad Sci U S A. 1997;94:4312–4317. doi: 10.1073/pnas.94.9.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Forman BM. Tontonoz P. Chen J. Brun RP. Spiegelman BM. Evans RM. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 111.Fu M. Zhang J. Lin Y. Zhu X. Ehrengruber MU. Chen YE. Early growth response factor-1 is a critical transcriptional mediator of peroxisome proliferator-activated receptor-gamma 1 gene expression in human aortic smooth muscle cells. J Biol Chem. 2002;277:26808–26814. doi: 10.1074/jbc.M203748200. [DOI] [PubMed] [Google Scholar]
- 112.Fu M. Zhang J. Lin Y. Zhu X. Zhao L. Ahmad M. Ehrengruber MU. Chen YE. Early stimulation and late inhibition of peroxisome proliferator-activated receptor gamma (PPAR gamma) gene expression by transforming growth factor beta in human aortic smooth muscle cells: role of early growth-response factor-1 (Egr-1), activator protein 1 (AP1) and Smads. Biochem J. 2003;370:1019–1025. doi: 10.1042/BJ20021503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fu M. Zhang J. Lin Yg Y. Zhu X. Willson TM. Chen YE. Activation of peroxisome proliferator-activated receptor gamma inhibits osteoprotegerin gene expression in human aortic smooth muscle cells. Biochem Biophys Res Commun. 2002;294:597–601. doi: 10.1016/S0006-291X(02)00533-8. [DOI] [PubMed] [Google Scholar]
- 114.Fu M. Zhang J. Zhu X. Myles DE. Willson TM. Liu X. Chen YE. Peroxisome proliferator-activated receptor gamma inhibits transforming growth factor beta-induced connective tissue growth factor expression in human aortic smooth muscle cells by interfering with Smad3. J Biol Chem. 2001;276:45888–45894. doi: 10.1074/jbc.M105490200. [DOI] [PubMed] [Google Scholar]
- 115.Fu M. Zhu X. Wang Q. Zhang J. Song Q. Zheng H. Ogawa W. Du J. Chen YE. Platelet-derived growth factor promotes the expression of peroxisome proliferator-activated receptor gamma in vascular smooth muscle cells by a phosphatidylinositol 3-kinase/Akt signaling pathway. Circ Res. 2001;89:1058–1064. doi: 10.1161/hh2301.099642. [DOI] [PubMed] [Google Scholar]
- 116.Fujino T. Sato Y. Une M. Kanayasu-Toyoda T. Yamaguchi T. Shudo K. Inoue K. Nishimaki-Mogami T. In vitro farnesoid X receptor ligand sensor assay using surface plasmon resonance and based on ligand-induced coactivator association. J Steroid Biochem Mol Biol. 2003;87:247–252. doi: 10.1016/j.jsbmb.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 117.Fujishima S. Ohya Y. Nakamura Y. Onaka U. Abe I. Fujishima M. Troglitazone, an insulin sensitizer, increases forearm blood flow in humans. Am J Hypertens. 1998;11:1134–1137. doi: 10.1016/s0895-7061(98)00130-7. [DOI] [PubMed] [Google Scholar]
- 118.Fukunaga Y. Itoh H. Doi K. Tanaka T. Yamashita J. Chun TH. Inoue M. Masatsugu K. Sawada N. Saito T. Hosoda K. Kook H. Ueda M. Nakao K. Thiazolidinediones, peroxisome proliferator-activated receptor gamma agonists, regulate endothelial cell growth and secretion of vasoactive peptides. Atherosclerosis. 2001;158:113–119. doi: 10.1016/s0021-9150(01)00430-0. [DOI] [PubMed] [Google Scholar]
- 119.Gaudel C. Grimaldi PA. Metabolic functions of peroxisome proliferator-activated receptor beta/delta in skeletal muscle. PPAR Res. 2007;2007:86394. doi: 10.1155/2007/86394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gbaguidi FG. Chinetti G. Milosavljevic D. Teissier E. Chapman J. Olivecrona G. Fruchart JC. Griglio S. Fruchart-Najib J. Staels B. Peroxisome proliferator-activated receptor (PPAR) agonists decrease lipoprotein lipase secretion and glycated LDL uptake by human macrophages. FEBS Lett. 2002;512:85–90. doi: 10.1016/s0014-5793(02)02223-8. [DOI] [PubMed] [Google Scholar]
- 121.Gearing KL. Gottlicher M. Teboul M. Widmark E. Gustafsson JA. Interaction of the peroxisome-proliferator-activated receptor and retinoid X receptor. Proc Natl Acad Sci U S A. 1993;90:1440–1444. doi: 10.1073/pnas.90.4.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gensch C. Clever YP. Werner C. Hanhoun M. Bohm M. Laufs U. The PPAR-gamma agonist pioglitazone increases neoangiogenesis and prevents apoptosis of endothelial progenitor cells. Atherosclerosis. 2007;192:67–74. doi: 10.1016/j.atherosclerosis.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 123.Ghisletti S. Huang W. Ogawa S. Pascual G. Lin ME. Willson TM. Rosenfeld MG. Glass CK. Parallel SUMOylation-dependent pathways mediate gene- and signal-specific transrepression by LXRs and PPARgamma. Mol Cell. 2007;25:57–70. doi: 10.1016/j.molcel.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gilde AJ. van der Lee KA. Willemsen PH. Chinetti G. van der Leij FR. van der Vusse GJ. Staels B. van Bilsen M. Peroxisome proliferator-activated receptor (PPAR) alpha and PPARbeta/delta, but not PPARgamma, modulate the expression of genes involved in cardiac lipid metabolism. Circ Res. 2003;92:518–524. doi: 10.1161/01.RES.0000060700.55247.7C. [DOI] [PubMed] [Google Scholar]
- 125.Gitlin N. Julie NL. Spurr CL. Lim KN. Juarbe HM. Two cases of severe clinical and histologic hepatotoxicity associated with troglitazone. Ann Intern Med. 1998;129:36–38. doi: 10.7326/0003-4819-129-1-199807010-00008. [DOI] [PubMed] [Google Scholar]
- 126.Gizard F. Amant C. Barbier O. Bellosta S. Robillard R. Percevault F. Sevestre H. Krimpenfort P. Corsini A. Rochette J. Glineur C. Fruchart JC. Torpier G. Staels B. PPAR alpha inhibits vascular smooth muscle cell proliferation underlying intimal hyperplasia by inducing the tumor suppressor p16INK4a. J Clin Invest. 2005;115:3228–3238. doi: 10.1172/JCI22756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Glass CK. Witztum JL. Atherosclerosis: the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 128.Goetze S. Bungenstock A. Czupalla C. Eilers F. Stawowy P. Kintscher U. Spencer-Hansch C. Graf K. Nurnberg B. Law RE. Fleck E. Grafe M. Leptin induces endothelial cell migration through Akt, which is inhibited by PPARgamma-ligands. Hypertension. 2002;40:748–754. doi: 10.1161/01.hyp.0000035522.63647.d3. [DOI] [PubMed] [Google Scholar]
- 129.Goetze S. Eilers F. Bungenstock A. Kintscher U. Stawowy P. Blaschke F. Graf K. Law RE. Fleck E. Grafe M. PPAR activators inhibit endothelial cell migration by targeting Akt. Biochem Biophys Res Commun. 2002;293:1431–1437. doi: 10.1016/S0006-291X(02)00385-6. [DOI] [PubMed] [Google Scholar]
- 130.Goetze S. Kim S. Xi XP. Graf K. Yang DC. Fleck E. Meehan WP. Hsueh WA. Law RE. Troglitazone inhibits mitogenic signaling by insulin in vascular smooth muscle cells. J Cardiovasc Pharmacol. 2000;35:749–757. doi: 10.1097/00005344-200005000-00011. [DOI] [PubMed] [Google Scholar]
- 131.Goetze S. Kintscher U. Kim S. Meehan WP. Kaneshiro K. Collins AR. Fleck E. Hsueh WA. Law RE. Peroxisome proliferator-activated receptor-gamma ligands inhibit nuclear but not cytosolic extracellular signal-regulated kinase/mitogen-activated protein kinase-regulated steps in vascular smooth muscle cell migration. J Cardiovasc Pharmacol. 2001;38:909–921. doi: 10.1097/00005344-200112000-00013. [DOI] [PubMed] [Google Scholar]
- 132.Goetze S. Xi XP. Graf K. Fleck E. Hsueh WA. Law RE. Troglitazone inhibits angiotensin II-induced extracellular signal-regulated kinase 1/2 nuclear translocation and activation in vascular smooth muscle cells. FEBS Lett. 1999;452:277–282. doi: 10.1016/s0014-5793(99)00624-9. [DOI] [PubMed] [Google Scholar]
- 133.Goetze S. Xi XP. Kawano H. Gotlibowski T. Fleck E. Hsueh WA. Law RE. PPAR gamma-ligands inhibit migration mediated by multiple chemoattractants in vascular smooth muscle cells. J Cardiovasc Pharmacol. 1999;33:798–806. doi: 10.1097/00005344-199905000-00018. [DOI] [PubMed] [Google Scholar]
- 134.Goikoetxea MJ. Beaumont J. Diez J. Peroxisome proliferator-activated receptor alpha and hypertensive heart disease. Drugs. 2004;64(suppl 2):9–18. doi: 10.2165/00003495-200464002-00003. [DOI] [PubMed] [Google Scholar]
- 135.Goldberg RB. Kendall DM. Deeg MA. Buse JB. Zagar AJ. Pinaire JA. Tan MH. Khan MA. Perez AT. Jacober SJ. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care. 2005;28:1547–1554. doi: 10.2337/diacare.28.7.1547. [DOI] [PubMed] [Google Scholar]
- 136.Golfman LS. Wilson CR. Sharma S. Burgmaier M. Young ME. Guthrie PH. Van Arsdall M. Adrogue JV. Brown KK. Taegtmeyer H. Activation of PPARgamma enhances myocardial glucose oxidation and improves contractile function in isolated working hearts of ZDF rats. Am J Physiol. 2005;289:E328–E336. doi: 10.1152/ajpendo.00055.2005. [DOI] [PubMed] [Google Scholar]
- 137.Goto M. Terada S. Kato M. Katoh M. Yokozeki T. Tabata I. Shimokawa T. cDNA Cloning and mRNA analysis of PGC-1 in epitrochlearis muscle in swimming-exercised rats. Biochem Biophys Res Commun. 2000;274:350–354. doi: 10.1006/bbrc.2000.3134. [DOI] [PubMed] [Google Scholar]
- 138.Gottlicher M. Widmark E. Li Q. Gustafsson JA. Fatty acids activate a chimera of the clofibric acid-activated receptor and the glucocorticoid receptor. Proc Natl Acad Sci U S A. 1992;89:4653–4657. doi: 10.1073/pnas.89.10.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Goya K. Sumitani S. Xu X. Kitamura T. Yamamoto H. Kurebayashi S. Saito H. Kouhara H. Kasayama S. Kawase I. Peroxisome proliferator-activated receptor alpha agonists increase nitric oxide synthase expression in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2004;24:658–663. doi: 10.1161/01.ATV.0000118682.58708.78. [DOI] [PubMed] [Google Scholar]
- 140.Graf K. Xi XP. Hsueh WA. Law RE. Troglitazone inhibits angiotensin II-induced DNA synthesis and migration in vascular smooth muscle cells. FEBS Lett. 1997;400:119–121. doi: 10.1016/s0014-5793(96)01371-3. [DOI] [PubMed] [Google Scholar]
- 141.Graham TL. Mookherjee C. Suckling KE. Palmer CN. Patel L. The PPARdelta agonist GW0742X reduces atherosclerosis in LDLR(−/−) mice. Atherosclerosis. 2005;181:29–37. doi: 10.1016/j.atherosclerosis.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 142.Greenland P. Knoll MD. Stamler J. Neaton JD. Dyer AR. Garside DB. Wilson PW. Major risk factors as antecedents of fatal and nonfatal coronary heart disease events. JAMA. 2003;290:891–897. doi: 10.1001/jama.290.7.891. [DOI] [PubMed] [Google Scholar]
- 143.Han J. Hajjar DP. Tauras JM. Feng J. Gotto AM Jr. Nicholson AC. Transforming growth factor-beta1 (TGF-beta1) and TGF-beta2 decrease expression of CD36, the type B scavenger receptor, through mitogen-activated protein kinase phosphorylation of peroxisome proliferator-activated receptor-gamma. J Biol Chem. 2000;275:1241–1246. doi: 10.1074/jbc.275.2.1241. [DOI] [PubMed] [Google Scholar]
- 144.Han JK. Lee HS. Yang HM. Hur J. Jun SI. Kim JY. Cho CH. Koh GY. Peters JM. Park KW. Cho HJ. Lee HY. Kang HJ. Oh BH. Park YB. Kim HS. Peroxisome proliferator-activated receptor-delta agonist enhances vasculogenesis by regulating endothelial progenitor cells through genomic and nongenomic activations of the phosphatidylinositol 3-kinase/Akt pathway. Circulation. 2008;118:1021–1033. doi: 10.1161/CIRCULATIONAHA.108.777169. [DOI] [PubMed] [Google Scholar]
- 145.Han KH. Chang MK. Boullier A. Green SR. Li A. Glass CK. Quehenberger O. Oxidized LDL reduces monocyte CCR2 expression through pathways involving peroxisome proliferator-activated receptor gamma. J Clin Invest. 2000;106:793–802. doi: 10.1172/JCI10052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hannan KM. Dilley RJ. de Dios ST. Little PJ. Troglitazone stimulates repair of the endothelium and inhibits neointimal formation in denuded rat aorta. Arterioscler Thromb Vasc Biol. 2003;23:762–768. doi: 10.1161/01.ATV.0000069210.46539.0D. [DOI] [PubMed] [Google Scholar]
- 147.Haraguchi G. Kobayashi Y. Brown ML. Tanaka A. Isobe M. Gianturco SH. Bradley WA. PPAR(alpha) and PPAR(gamma) activators suppress the monocyte-macrophage apoB-48 receptor. J Lipid Res. 2003;44:1224–1231. doi: 10.1194/jlr.M300077-JLR200. [DOI] [PubMed] [Google Scholar]
- 148.Harkin DP. Bean JM. Miklos D. Song YH. Truong VB. Englert C. Christians FC. Ellisen LW. Maheswaran S. Oliner JD. Haber DA. Induction of GADD45 and JNK/SAPK-dependent apoptosis following inducible expression of BRCA1. Cell. 1999;97:575–586. doi: 10.1016/s0092-8674(00)80769-2. [DOI] [PubMed] [Google Scholar]
- 149.Harris IS. Treskov I. Rowley MW. Heximer S. Kaltenbronn K. Finck BN. Gross RW. Kelly DP. Blumer KJ. Muslin AJ. G-protein signaling participates in the development of diabetic cardiomyopathy. Diabetes. 2004;53:3082–3090. doi: 10.2337/diabetes.53.12.3082. [DOI] [PubMed] [Google Scholar]
- 150.Hautala AJ. Leon AS. Skinner JS. Rao DC. Bouchard C. Rankinen T. Peroxisome proliferator-activated receptor-delta polymorphisms are associated with physical performance and plasma lipids: the HERITAGE Family Study. Am J Physiol Heart Circ Physiol. 2007;292:H2498–H2505. doi: 10.1152/ajpheart.01092.2006. [DOI] [PubMed] [Google Scholar]
- 151.Helin K. Regulation of cell proliferation by the E2F transcription factors. Curr Opin Genet Dev. 1998;8:28–35. doi: 10.1016/s0959-437x(98)80058-0. [DOI] [PubMed] [Google Scholar]
- 152.Hennuyer N. Tailleux A. Torpier G. Mezdour H. Fruchart JC. Staels B. Fievet C. PPARalpha, but not PPARgamma, activators decrease macrophage-laden atherosclerotic lesions in a nondiabetic mouse model of mixed dyslipidemia. Arterioscler Thromb Vasc Biol. 2005;25:1897–1902. doi: 10.1161/01.ATV.0000175756.56818.ee. [DOI] [PubMed] [Google Scholar]
- 153.Heo KS. Kim DU. Ryoo S. Nam M. Baek ST. Kim L. Park SK. Myung CS. Hoe KL. PPARgamma activation abolishes LDL-induced proliferation of human aortic smooth muscle cells via SOD-mediated down-regulation of superoxide. Biochem Biophys Res Commun. 2007;359:1017–1023. doi: 10.1016/j.bbrc.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 154.Hevener AL. Olefsky JM. Reichart D. Nguyen MT. Bandyopadyhay G. Leung HY. Watt MJ. Benner C. Febbraio MA. Nguyen AK. Folian B. Subramaniam S. Gonzalez FJ. Glass CK. Ricote M. Macrophage PPAR gamma is required for normal skeletal muscle and hepatic insulin sensitivity and full antidiabetic effects of thiazolidinediones. J Clin Invest. 2007;117:1658–1669. doi: 10.1172/JCI31561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Home PD. Pocock SJ. Beck-Nielsen H. Gomis R. Hanefeld M. Jones NP. Komajda M. McMurray JJ. Rosiglitazone evaluated for cardiovascular outcomes: an interim analysis. N Engl J Med. 2007;357:28–38. doi: 10.1056/NEJMoa073394. [DOI] [PubMed] [Google Scholar]
- 156.Hourton D. Delerive P. Stankova J. Staels B. Chapman MJ. Ninio E. Oxidized low-density lipoprotein and peroxisome-proliferator-activated receptor alpha down-regulate platelet-activating-factor receptor expression in human macrophages. Biochem J. 2001;354:225–232. doi: 10.1042/0264-6021:3540225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hu E. Kim JB. Sarraf P. Spiegelman BM. Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARgamma. Science (New York) 1996;274:2100–2103. doi: 10.1126/science.274.5295.2100. [DOI] [PubMed] [Google Scholar]
- 158.Huang J. Kontos CD. PTEN modulates vascular endothelial growth factor-mediated signaling and angiogenic effects. J Biol Chem. 2002;277:10760–10766. doi: 10.1074/jbc.M110219200. [DOI] [PubMed] [Google Scholar]
- 159.Huang JT. Welch JS. Ricote M. Binder CJ. Willson TM. Kelly C. Witztum JL. Funk CD. Conrad D. Glass CK. Interleukin-4-dependent production of PPAR-gamma ligands in macrophages by 12/15-lipoxygenase. Nature. 1999;400:378–382. doi: 10.1038/22572. [DOI] [PubMed] [Google Scholar]
- 160.Hur J. Yoon CH. Kim HS. Choi JH. Kang HJ. Hwang KK. Oh BH. Lee MM. Park YB. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24:288–293. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- 161.Huss JM. Levy FH. Kelly DP. Hypoxia inhibits the peroxisome proliferator-activated receptor alpha/retinoid X receptor gene regulatory pathway in cardiac myocytes: a mechanism for O2-dependent modulation of mitochondrial fatty acid oxidation. J Biol Chem. 2001;276:27605–27612. doi: 10.1074/jbc.M100277200. [DOI] [PubMed] [Google Scholar]
- 162.Hwang J. Kleinhenz DJ. Lassegue B. Griendling KK. Dikalov S. Hart CM. Peroxisome proliferator-activated receptor-gamma ligands regulate endothelial membrane superoxide production. Am J Physiol Cell Physiol. 2005;288:C899–C905. doi: 10.1152/ajpcell.00474.2004. [DOI] [PubMed] [Google Scholar]
- 163.Iglarz M. Touyz RM. Amiri F. Lavoie MF. Diep QN. Schiffrin EL. Effect of peroxisome proliferator-activated receptor-alpha and -gamma activators on vascular remodeling in endothelin-dependent hypertension. Arterioscler Thromb Vasc Biol. 2003;23:45–51. doi: 10.1161/01.atv.0000047447.67827.cd. [DOI] [PubMed] [Google Scholar]
- 164.Iijima K. Yoshizumi M. Ako J. Eto M. Kim S. Hashimoto M. Sugimoto N. Liang YQ. Sudoh N. Toba K. Ouchi Y. Expression of peroxisome proliferator-activated receptor gamma (PPARgamma) in rat aortic smooth muscle cells. Biochem Biophys Res Commun. 1998;247:353–356. doi: 10.1006/bbrc.1998.8794. [DOI] [PubMed] [Google Scholar]
- 165.Ikeda U. Shimpo M. Murakami Y. Shimada K. Peroxisome proliferator-activated receptor-gamma ligands inhibit nitric oxide synthesis in vascular smooth muscle cells. Hypertension. 2000;35:1232–1236. doi: 10.1161/01.hyp.35.6.1232. [DOI] [PubMed] [Google Scholar]
- 166.Imai H. Numaguchi Y. Ishii M. Kubota R. Yokouchi K. Ogawa Y. Kondo T. Okumura K. Murohara T. Prostacyclin synthase gene transfer inhibits neointimal formation by suppressing PPAR delta expression. Atherosclerosis. 2007;195:322–332. doi: 10.1016/j.atherosclerosis.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 167.Imayama I. Ichiki T. Inanaga K. Ohtsubo H. Fukuyama K. Ono H. Hashiguchi Y. Sunagawa K. Telmisartan downregulates angiotensin II type 1 receptor through activation of peroxisome proliferator-activated receptor gamma. Cardiovasc Res. 2006;72:184–190. doi: 10.1016/j.cardiores.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 168.Inoue I. Goto S. Matsunaga T. Nakajima T. Awata T. Hokari S. Komoda T. Katayama S. The ligands/activators for peroxisome proliferator-activated receptor alpha (PPARalpha) and PPARgamma increase Cu2+,Zn2+-superoxide dismutase and decrease p22phox message expressions in primary endothelial cells. Metabolism. 2001;50:3–11. doi: 10.1053/meta.2001.19415. [DOI] [PubMed] [Google Scholar]
- 169.Inoue I. Hayashi K. Yagasaki F. Nakamura K. Matsunaga T. Xu H. Inukai K. Awata T. Komoda T. Katayama S. Apoptosis of endothelial cells may be mediated by genes of peroxisome proliferator-activated receptor gamma 1 (PPARgamma 1) and PPARalpha genes. J Atheroscler Thromb. 2003;10:99–108. doi: 10.5551/jat.10.99. [DOI] [PubMed] [Google Scholar]
- 170.Inoue I. Shino K. Noji S. Awata T. Katayama S. Expression of peroxisome proliferator-activated receptor alpha (PPAR alpha) in primary cultures of human vascular endothelial cells. Biochem Biophys Res Commun. 1998;246:370–374. doi: 10.1006/bbrc.1998.8622. [DOI] [PubMed] [Google Scholar]
- 171.Irukayama-Tomobe Y. Miyauchi T. Sakai S. Kasuya Y. Ogata T. Takanashi M. Iemitsu M. Sudo T. Goto K. Yamaguchi I. Endothelin-1-induced cardiac hypertrophy is inhibited by activation of peroxisome proliferator-activated receptor-alpha partly via blockade of c-Jun NH2-terminal kinase pathway. Circulation. 2004;109:904–910. doi: 10.1161/01.CIR.0000112596.06954.00. [DOI] [PubMed] [Google Scholar]
- 172.Issemann I. Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347:645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- 173.Jackson SM. Parhami F. Xi XP. Berliner JA. Hsueh WA. Law RE. Demer LL. Peroxisome proliferator-activated receptor activators target human endothelial cells to inhibit leukocyte-endothelial cell interaction. Arterioscler Thromb Vasc Biol. 1999;19:2094–2104. doi: 10.1161/01.atv.19.9.2094. [DOI] [PubMed] [Google Scholar]
- 174.Jiang C. Ting AT. Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 175.Jiang WG. Redfern A. Bryce RP. Mansel RE. Peroxisome proliferator activated receptor-gamma (PPAR-gamma) mediates the action of gamma linolenic acid in breast cancer cells. Prostaglandins Leukot Essent Fatty Acids. 2000;62:119–127. doi: 10.1054/plef.1999.0131. [DOI] [PubMed] [Google Scholar]
- 176.Jucker BM. Doe CP. Schnackenberg CG. Olzinski AR. Maniscalco K. Williams C. Hu TC. Lenhard SC. Costell M. Bernard R. Sarov-Blat L. Steplewski K. Willette RN. PPARdelta activation normalizes cardiac substrate metabolism and reduces right ventricular hypertrophy in congestive heart failure. J Cardiovasc Pharmacol. 2007;50:25–34. doi: 10.1097/FJC.0b013e31804b4163. [DOI] [PubMed] [Google Scholar]
- 177.Kang K. Reilly SM. Karabacak V. Gangl MR. Fitzgerald K. Hatano B. Lee CH. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell Metab. 2008;7:485–495. doi: 10.1016/j.cmet.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kannel WB. McGee DL. Diabetes and cardiovascular disease: the Framingham study. JAMA. 1979;241:2035–2038. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- 179.Kato K. Satoh H. Endo Y. Yamada D. Midorikawa S. Sato W. Mizuno K. Fujita T. Tsukamoto K. Watanabe T. Thiazolidinediones down-regulate plasminogen activator inhibitor type 1 expression in human vascular endothelial cells: a possible role for PPARgamma in endothelial function. Biochem Biophys Res Commun. 1999;258:431–435. doi: 10.1006/bbrc.1999.0648. [DOI] [PubMed] [Google Scholar]
- 180.Keller BJ. Bradford BU. Marsman DS. Cattley RC. Popp JA. Bojes HK. Thurman RG. The nongenotoxic hepatocarcinogen Wy-14,643 is an uncoupler of oxidative phosphorylation in vivo. Toxicol Appl Pharmacol. 1993;119:52–58. doi: 10.1006/taap.1993.1043. [DOI] [PubMed] [Google Scholar]
- 181.Keller BJ. Marsman DS. Popp JA. Thurman RG. Several nongenotoxic carcinogens uncouple mitochondrial oxidative phosphorylation. Biochim Biophys Acta. 1992;1102:237–244. doi: 10.1016/0005-2728(92)90105-b. [DOI] [PubMed] [Google Scholar]
- 182.Keller H. Dreyer C. Medin J. Mahfoudi A. Ozato K. Wahli W. Fatty acids and retinoids control lipid metabolism through activation of peroxisome proliferator-activated receptor-retinoid X receptor heterodimers. Proc Natl Acad Sci U S A. 1993;90:2160–2164. doi: 10.1073/pnas.90.6.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kersten S. Seydoux J. Peters JM. Gonzalez FJ. Desvergne B. Wahli W. Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. J Clin Invest. 1999;103:1489–1498. doi: 10.1172/JCI6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kim HJ. Ham SA. Kim SU. Hwang JY. Kim JH. Chang KC. Yabe-Nishimura C. Kim JH. Seo HG. Transforming growth factor-beta1 is a molecular target for the peroxisome proliferator-activated receptor delta. Circ Res. 2008;102:193–200. doi: 10.1161/CIRCRESAHA.107.158477. [DOI] [PubMed] [Google Scholar]
- 185.Kim KY. Cheon HG. Antiangiogenic effect of rosiglitazone is mediated via peroxisome proliferator-activated receptor gamma-activated maxi-K channel opening in human umbilical vein endothelial cells. J Biol Chem. 2006;281:13503–13512. doi: 10.1074/jbc.M510357200. [DOI] [PubMed] [Google Scholar]
- 186.Kintscher U. Goetze S. Wakino S. Kim S. Nagpal S. Chandraratna RA. Graf K. Fleck E. Hsueh WA. Law RE. Peroxisome proliferator-activated receptor and retinoid X receptor ligands inhibit monocyte chemotactic protein-1-directed migration of monocytes. Eur J Pharmacol. 2000;401:259–270. doi: 10.1016/s0014-2999(00)00461-1. [DOI] [PubMed] [Google Scholar]
- 187.Kintscher U. Lyon C. Wakino S. Bruemmer D. Feng X. Goetze S. Graf K. Moustakas A. Staels B. Fleck E. Hsueh WA. Law RE. PPARalpha inhibits TGF-beta-induced beta5 integrin transcription in vascular smooth muscle cells by interacting with Smad4. Circ Res. 2002;91:e35–e44. doi: 10.1161/01.res.0000046017.96083.34. [DOI] [PubMed] [Google Scholar]
- 188.Kliewer SA. Forman BM. Blumberg B. Ong ES. Borgmeyer U. Mangelsdorf DJ. Umesono K. Evans RM. Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proc Natl Acad Sci U S A. 1994;91:7355–7359. doi: 10.1073/pnas.91.15.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kliewer SA. Lenhard JM. Willson TM. Patel I. Morris DC. Lehmann JM. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation. Cell. 1995;83:813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 190.Kliewer SA. Umesono K. Noonan DJ. Heyman RA. Evans RM. Convergence of 9-cis retinoic acid and peroxisome proliferator signalling pathways through heterodimer formation of their receptors. Nature. 1992;358:771–774. doi: 10.1038/358771a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Koumanov F. Jin B. Yang J. Holman GD. Insulin signaling meets vesicle traffic of GLUT4 at a plasma-membrane-activated fusion step. Cell Metab. 2005;2:179–189. doi: 10.1016/j.cmet.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 192.Krey G. Braissant O. L'Horset F. Kalkhoven E. Perroud M. Parker MG. Wahli W. Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Mol Endocrinol (Baltimore) 1997;11:779–791. doi: 10.1210/mend.11.6.0007. [DOI] [PubMed] [Google Scholar]
- 193.Kronke G. Kadl A. Ikonomu E. Bluml S. Furnkranz A. Sarembock IJ. Bochkov VN. Exner M. Binder BR. Leitinger N. Expression of heme oxygenase-1 in human vascular cells is regulated by peroxisome proliferator-activated receptors. Arterioscler Thromb Vasc Biol. 2007;27:1276–1282. doi: 10.1161/ATVBAHA.107.142638. [DOI] [PubMed] [Google Scholar]
- 194.Kwak BR. Myit S. Mulhaupt F. Veillard N. Rufer N. Roosnek E. Mach F. PPARgamma but not PPARalpha ligands are potent repressors of major histocompatibility complex class II induction in atheroma-associated cells. Circ Res. 2002;90:356–362. doi: 10.1161/hh0302.104924. [DOI] [PubMed] [Google Scholar]
- 195.LaBaer J. Garrett MD. Stevenson LF. Slingerland JM. Sandhu C. Chou HS. Fattaey A. Harlow E. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 1997;11:847–862. doi: 10.1101/gad.11.7.847. [DOI] [PubMed] [Google Scholar]
- 196.Lalwani ND. Alvares K. Reddy MK. Reddy MN. Parikh I. Reddy JK. Peroxisome proliferator-binding protein: identification and partial characterization of nafenopin-, clofibric acid-, and ciprofibrate-binding proteins from rat liver. Proc Natl Acad Sci U S A. 1987;84:5242–5246. doi: 10.1073/pnas.84.15.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lalwani ND. Fahl WE. Reddy JK. Detection of a nafenopin-binding protein in rat liver cytosol associated with the induction of peroxisome proliferation by hypolipidemic compounds. Biochem Biophys Res Commun. 1983;116:388–393. doi: 10.1016/0006-291x(83)90534-x. [DOI] [PubMed] [Google Scholar]
- 198.Law RE. Goetze S. Xi XP. Jackson S. Kawano Y. Demer L. Fishbein MC. Meehan WP. Hsueh WA. Expression and function of PPARgamma in rat and human vascular smooth muscle cells. Circulation. 2000;101:1311–1318. doi: 10.1161/01.cir.101.11.1311. [DOI] [PubMed] [Google Scholar]
- 199.Law RE. Meehan WP. Xi XP. Graf K. Wuthrich DA. Coats W. Faxon D. Hsueh WA. Troglitazone inhibits vascular smooth muscle cell growth and intimal hyperplasia. J Clin Invest. 1996;98:1897–1905. doi: 10.1172/JCI118991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lazennec G. Canaple L. Saugy D. Wahli W. Activation of peroxisome proliferator-activated receptors (PPARs) by their ligands and protein kinase A activators. Mol Endocr (Baltimore) 2000;14:1962–1975. doi: 10.1210/mend.14.12.0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lee CH. Chawla A. Urbiztondo N. Liao D. Boisvert WA. Evans RM. Curtiss LK. Transcriptional repression of atherogenic inflammation: modulation by PPARdelta. Science (New York) 2003;302:453–457. doi: 10.1126/science.1087344. [DOI] [PubMed] [Google Scholar]
- 202.Lee CH. Olson P. Hevener A. Mehl I. Chong LW. Olefsky JM. Gonzalez FJ. Ham J. Kang H. Peters JM. Evans RM. PPARdelta regulates glucose metabolism and insulin sensitivity. Proc Natl Acad Sci U S A. 2006;103:3444–3449. doi: 10.1073/pnas.0511253103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lee H. Shi W. Tontonoz P. Wang S. Subbanagounder G. Hedrick CC. Hama S. Borromeo C. Evans RM. Berliner JA. Nagy L. Role for peroxisome proliferator-activated receptor alpha in oxidized phospholipid-induced synthesis of monocyte chemotactic protein-1 and interleukin-8 by endothelial cells. Circ Res. 2000;87:516–521. doi: 10.1161/01.res.87.6.516. [DOI] [PubMed] [Google Scholar]
- 204.Lee KS. Park JH. Lee S. Lim HJ. Jang Y. Park HY. Troglitazone inhibits endothelial cell proliferation through suppression of casein kinase 2 activity. Biochem Biophys Res Commun. 2006;346:83–88. doi: 10.1016/j.bbrc.2006.05.069. [DOI] [PubMed] [Google Scholar]
- 205.Lee SS. Pineau T. Drago J. Lee EJ. Owens JW. Kroetz DL. Fernandez-Salguero PM. Westphal H. Gonzalez FJ. Targeted disruption of the alpha isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol Cell Biol. 1995;15:3012–3022. doi: 10.1128/mcb.15.6.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lee WM. Drug-induced hepatotoxicity. N Engl J Med. 2003;349:474–485. doi: 10.1056/NEJMra021844. [DOI] [PubMed] [Google Scholar]
- 207.Leesnitzer LM. Parks DJ. Bledsoe RK. Cobb JE. Collins JL. Consler TG. Davis RG. Hull-Ryde EA. Lenhard JM. Patel L. Plunket KD. Shenk JL. Stimmel JB. Therapontos C. Willson TM. Blanchard SG. Functional consequences of cysteine modification in the ligand binding sites of peroxisome proliferator activated receptors by GW9662. Biochemistry. 2002;41:6640–6650. doi: 10.1021/bi0159581. [DOI] [PubMed] [Google Scholar]
- 208.Lehman JJ. Kelly DP. Gene regulatory mechanisms governing energy metabolism during cardiac hypertrophic growth. Heart Fail Rev. 2002;7:175–185. doi: 10.1023/a:1015332726303. [DOI] [PubMed] [Google Scholar]
- 209.Lehmann JM. Moore LB. Smith-Oliver TA. Wilkison WO. Willson TM. Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma) J Biol Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 210.Leibowitz MD. Fievet C. Hennuyer N. Peinado-Onsurbe J. Duez H. Bergera J. Cullinan CA. Sparrow CP. Baffic J. Berger GD. Santini C. Marquis RW. Tolman RL. Smith RG. Moller DE. Auwerx J. Activation of PPARdelta alters lipid metabolism in db/db mice. FEBS Lett. 2000;473:333–336. doi: 10.1016/s0014-5793(00)01554-4. [DOI] [PubMed] [Google Scholar]
- 211.Leone TC. Weinheimer CJ. Kelly DP. A critical role for the peroxisome proliferator-activated receptor alpha (PPARalpha) in the cellular fasting response: the PPARalpha-null mouse as a model of fatty acid oxidation disorders. Proc Natl Acad Sci U S A. 1999;96:7473–7478. doi: 10.1073/pnas.96.13.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Levi Z. Shaish A. Yacov N. Levkovitz H. Trestman S. Gerber Y. Cohen H. Dvir A. Rhachmani R. Ravid M. Harats D. Rosiglitazone (PPARgamma-agonist) attenuates atherogenesis with no effect on hyperglycaemia in a combined diabetes-atherosclerosis mouse model. Diabetes Obes Metab. 2003;5:45–50. doi: 10.1046/j.1463-1326.2003.00240.x. [DOI] [PubMed] [Google Scholar]
- 213.Levonen AL. Dickinson DA. Moellering DR. Mulcahy RT. Forman HJ. Darley-Usmar VM. Biphasic effects of 15-deoxy-delta(12,14)-prostaglandin J(2) on glutathione induction and apoptosis in human endothelial cells. Arterioscler Thromb Vasc Biol. 2001;21:1846–1851. doi: 10.1161/hq1101.098488. [DOI] [PubMed] [Google Scholar]
- 214.Li AC. Binder CJ. Gutierrez A. Brown KK. Plotkin CR. Pattison JW. Valledor AF. Davis RA. Willson TM. Witztum JL. Palinski W. Glass CK. Differential inhibition of macrophage foam-cell formation and atherosclerosis in mice by PPARalpha, beta/delta, and gamma. J Clin Invest. 2004;114:1564–1576. doi: 10.1172/JCI18730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Li AC. Brown KK. Silvestre MJ. Willson TM. Palinski W. Glass CK. Peroxisome proliferator-activated receptor gamma ligands inhibit development of atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2000;106:523–531. doi: 10.1172/JCI10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Li L. Beauchamp MC. Renier G. Peroxisome proliferator-activated receptor alpha and gamma agonists upregulate human macrophage lipoprotein lipase expression. Atherosclerosis. 2002;165:101–110. doi: 10.1016/s0021-9150(02)00203-4. [DOI] [PubMed] [Google Scholar]
- 217.Li M. Pascual G. Glass CK. Peroxisome proliferator-activated receptor gamma-dependent repression of the inducible nitric oxide synthase gene. Mol Cell Biol. 2000;20:4699–4707. doi: 10.1128/mcb.20.13.4699-4707.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Li Y. Zhang J. Schopfer FJ. Martynowski D. Garcia-Barrio MT. Kovach A. Suino-Powell K. Baker PR. Freeman BA. Chen YE. Xu HE. Molecular recognition of nitrated fatty acids by PPAR gamma. Nat Struct Mol Biol. 2008;15:865–867. doi: 10.1038/nsmb.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Liang CP. Han S. Okamoto H. Carnemolla R. Tabas I. Accili D. Tall AR. Increased CD36 protein as a response to defective insulin signaling in macrophages. J Clin Invest. 2004;113:764–773. doi: 10.1172/JCI19528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Liang F. Wang F. Zhang S. Gardner DG. Peroxisome proliferator activated receptor (PPAR)alpha agonists inhibit hypertrophy of neonatal rat cardiac myocytes. Endocrinology. 2003;144:4187–4194. doi: 10.1210/en.2002-0217. [DOI] [PubMed] [Google Scholar]
- 221.Lim H. Gupta RA. Ma WG. Paria BC. Moller DE. Morrow JD. DuBois RN. Trzaskos JM. Dey SK. Cyclo-oxygenase-2-derived prostacyclin mediates embryo implantation in the mouse via PPARdelta. Genes Dev. 1999;13:1561–1574. doi: 10.1101/gad.13.12.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Lim HJ. Lee S. Park JH. Lee KS. Choi HE. Chung KS. Lee HH. Park HY. PPARdelta agonist L-165041 inhibits rat vascular smooth muscle cell proliferation and migration via inhibition of cell cycle. Atherosclerosis. 2009 doi: 10.1016/j.atherosclerosis.2008.05.023. (in press). [DOI] [PubMed] [Google Scholar]
- 223.Lim S. Jin CJ. Kim M. Chung SS. Park HS. Lee IK. Lee CT. Cho YM. Lee HK. Park KS. PPARgamma gene transfer sustains apoptosis, inhibits vascular smooth muscle cell proliferation, and reduces neointima formation after balloon injury in rats. Arterioscler Thromb Vasc Biol. 2006;26:808–813. doi: 10.1161/01.ATV.0000204634.26163.a7. [DOI] [PubMed] [Google Scholar]
- 224.Lin Y. Zhu X. McLntee FL. Xiao H. Zhang J. Fu M. Chen YE. Interferon regulatory factor-1 mediates PPARgamma-induced apoptosis in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24:257–263. doi: 10.1161/01.ATV.0000109170.43400.2f. [DOI] [PubMed] [Google Scholar]
- 225.Lincoff AM. Wolski K. Nicholls SJ. Nissen SE. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA. 2007;298:1180–1188. doi: 10.1001/jama.298.10.1180. [DOI] [PubMed] [Google Scholar]
- 226.Liou JY. Lee S. Ghelani D. Matijevic-Aleksic N. Wu KK. Protection of endothelial survival by peroxisome proliferator-activated receptor-delta mediated 14-3-3 upregulation. Arterioscler Thromb Vasc Biol. 2006;26:1481–1487. doi: 10.1161/01.ATV.0000223875.14120.93. [DOI] [PubMed] [Google Scholar]
- 227.Llaverias G. Vazquez-Carrera M. Sanchez RM. Noe V. Ciudad CJ. Laguna JC. Alegret M. Rosiglitazone upregulates caveolin-1 expression in THP-1 cells through a PPAR-dependent mechanism. J Lipid Res. 2004;45:2015–2024. doi: 10.1194/jlr.M400049-JLR200. [DOI] [PubMed] [Google Scholar]
- 228.Loskutoff DJ. Samad F. The adipocyte and hemostatic balance in obesity: studies of PAI-1. Arterioscler Thromb Vasc Biol. 1998;18:1–6. doi: 10.1161/01.atv.18.1.1. [DOI] [PubMed] [Google Scholar]
- 229.Luptak I. Balschi JA. Xing Y. Leone TC. Kelly DP. Tian R. Decreased contractile and metabolic reserve in peroxisome proliferator-activated receptor-alpha-null hearts can be rescued by increasing glucose transport and utilization. Circulation. 2005;112:2339–2346. doi: 10.1161/CIRCULATIONAHA.105.534594. [DOI] [PubMed] [Google Scholar]
- 230.Luquet S. Lopez-Soriano J. Holst D. Fredenrich A. Melki J. Rassoulzadegan M. Grimaldi PA. Peroxisome proliferator-activated receptor delta controls muscle development and oxidative capability. FASEB J. 2003;17:2299–2301. doi: 10.1096/fj.03-0269fje. [DOI] [PubMed] [Google Scholar]
- 231.Maiorano D. Lutzmann M. Mechali M. MCM proteins and DNA replication. Curr Opin Cell Biol. 2006;18:130–136. doi: 10.1016/j.ceb.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 232.Majai G. Sarang Z. Csomos K. Zahuczky G. Fesus L. PPARgamma-dependent regulation of human macrophages in phagocytosis of apoptotic cells. Eur J Immunol. 2007;37:1343–1354. doi: 10.1002/eji.200636398. [DOI] [PubMed] [Google Scholar]
- 233.Marcus SL. Miyata KS. Zhang B. Subramani S. Rachubinski RA. Capone JP. Diverse peroxisome proliferator-activated receptors bind to the peroxisome proliferator-responsive elements of the rat hydratase/dehydrogenase and fatty acyl-CoA oxidase genes but differentially induce expression. Proc Natl Acad Sci U S A. 1993;90:5723–5727. doi: 10.1073/pnas.90.12.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Martin-Nizard F. Furman C. Delerive P. Kandoussi A. Fruchart JC. Staels B. Duriez P. Peroxisome proliferator-activated receptor activators inhibit oxidized low-density lipoprotein-induced endothelin-1 secretion in endothelial cells. J Cardiovasc Pharmacol. 2002;40:822–831. doi: 10.1097/00005344-200212000-00003. [DOI] [PubMed] [Google Scholar]
- 235.Marx N. Bourcier T. Sukhova GK. Libby P. Plutzky J. PPARgamma activation in human endothelial cells increases plasminogen activator inhibitor type-1 expression: PPARgamma as a potential mediator in vascular disease. Arterioscler Thromb Vasc Biol. 1999;19:546–551. doi: 10.1161/01.atv.19.3.546. [DOI] [PubMed] [Google Scholar]
- 236.Marx N. Kehrle B. Kohlhammer K. Grub M. Koenig W. Hombach V. Libby P. Plutzky J. PPAR activators as antiinflammatory mediators in human T lymphocytes: implications for atherosclerosis and transplantation-associated arteriosclerosis. Circ Res. 2002;90:703–710. doi: 10.1161/01.res.0000014225.20727.8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Marx N. Mach F. Sauty A. Leung JH. Sarafi MN. Ransohoff RM. Libby P. Plutzky J. Luster AD. Peroxisome proliferator-activated receptor-gamma activators inhibit IFN-gamma-induced expression of the T cell-active CXC chemokines IP-10, Mig, and I-TAC in human endothelial cells. J Immunol. 2000;164:6503–6508. doi: 10.4049/jimmunol.164.12.6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Marx N. Mackman N. Schonbeck U. Yilmaz N. Hombach V. Libby P. Plutzky J. PPARalpha activators inhibit tissue factor expression and activity in human monocytes. Circulation. 2001;103:213–219. doi: 10.1161/01.cir.103.2.213. [DOI] [PubMed] [Google Scholar]
- 239.Marx N. Schonbeck U. Lazar MA. Libby P. Plutzky J. Peroxisome proliferator-activated receptor gamma activators inhibit gene expression and migration in human vascular smooth muscle cells. Circ Res. 1998;83:1097–1103. doi: 10.1161/01.res.83.11.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Marx N. Sukhova G. Murphy C. Libby P. Plutzky J. Macrophages in human atheroma contain PPARgamma: differentiation-dependent peroxisomal proliferator-activated receptor gamma(PPARgamma) expression and reduction of MMP-9 activity through PPARgamma activation in mononuclear phagocytes in vitro. Am J Pathol. 1998;153:17–23. doi: 10.1016/s0002-9440(10)65540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Marx N. Sukhova GK. Collins T. Libby P. Plutzky J. PPARalpha activators inhibit cytokine-induced vascular cell adhesion molecule-1 expression in human endothelial cells. Circulation. 1999;99:3125–3131. doi: 10.1161/01.cir.99.24.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Matsumoto K. Hirano K. Nozaki S. Takamoto A. Nishida M. Nakagawa-Toyama Y. Janabi MY. Ohya T. Yamashita S. Matsuzawa Y. Expression of macrophage (Mphi) scavenger receptor, CD36, in cultured human aortic smooth muscle cells in association with expression of peroxisome proliferator activated receptor-gamma, which regulates gain of Mphi-like phenotype in vitro, and its implication in atherogenesis. Arterioscler Thromb Vasc Biol. 2000;20:1027–1032. doi: 10.1161/01.atv.20.4.1027. [DOI] [PubMed] [Google Scholar]
- 243.Matsusue K. Miyoshi A. Yamano S. Gonzalez FJ. Ligand-activated PPARbeta efficiently represses the induction of LXR-dependent promoter activity through competition with RXR. Mol Cell Endocrinol. 2006;256:23–33. doi: 10.1016/j.mce.2006.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Mehta JL. Hu B. Chen J. Li D. Pioglitazone inhibits LOX-1 expression in human coronary artery endothelial cells by reducing intracellular superoxide radical generation. Arterioscler Thromb Vasc Biol. 2003;23:2203–2208. doi: 10.1161/01.ATV.0000094411.98127.5F. [DOI] [PubMed] [Google Scholar]
- 245.Meier CA. Chicheportiche R. Juge-Aubry CE. Dreyer MG. Dayer JM. Regulation of the interleukin-1 receptor antagonist in THP-1 cells by ligands of the peroxisome proliferator-activated receptor gamma. Cytokine. 2002;18:320–328. doi: 10.1006/cyto.2002.1945. [DOI] [PubMed] [Google Scholar]
- 246.Meissner M. Stein M. Urbich C. Reisinger K. Suske G. Staels B. Kaufmann R. Gille J. PPARalpha activators inhibit vascular endothelial growth factor receptor-2 expression by repressing Sp1-dependent DNA binding and transactivation. Circ Res. 2004;94:324–332. doi: 10.1161/01.RES.0000113781.08139.81. [DOI] [PubMed] [Google Scholar]
- 247.Mishra A. Chaudhary A. Sethi S. Oxidized omega-3 fatty acids inhibit NF-kappaB activation via a PPARalpha-dependent pathway. Arterioscler Thromb Vasc Biol. 2004;24:1621–1627. doi: 10.1161/01.ATV.0000137191.02577.86. [DOI] [PubMed] [Google Scholar]
- 248.Montessuit C. Papageorgiou I. Lerch R. Nuclear receptor agonists improve insulin responsiveness in cultured cardiomyocytes through enhanced signaling and preserved cytoskeletal architecture. Endocrinology. 2008;149:1064–1074. doi: 10.1210/en.2007-0656. [DOI] [PubMed] [Google Scholar]
- 249.Moore KJ. Rosen ED. Fitzgerald ML. Randow F. Andersson LP. Altshuler D. Milstone DS. Mortensen RM. Spiegelman BM. Freeman MW. The role of PPAR-gamma in macrophage differentiation and cholesterol uptake. Nat Med. 2001;7:41–47. doi: 10.1038/83328. [DOI] [PubMed] [Google Scholar]
- 250.Morikang E. Benson SC. Kurtz TW. Pershadsingh HA. Effects of thiazolidinediones on growth and differentiation of human aorta and coronary myocytes. Am J Hypertens. 1997;10:440–446. [PubMed] [Google Scholar]
- 251.Muerhoff AS. Griffin KJ. Johnson EF. The peroxisome proliferator-activated receptor mediates the induction of CYP4A6, a cytochrome P450 fatty acid omega-hydroxylase, by clofibric acid. J Biol Chem. 1992;267:19051–19053. [PubMed] [Google Scholar]
- 252.Mukherjee R. Hoener PA. Jow L. Bilakovics J. Klausing K. Mais DE. Faulkner A. Croston GE. Paterniti JR., Jr A selective peroxisome proliferator-activated receptor-gamma (PPARgamma) modulator blocks adipocyte differentiation but stimulates glucose uptake in 3T3-L1 adipocytes. Mol Endocr (Baltimore) 2000;14:1425–1433. doi: 10.1210/mend.14.9.0528. [DOI] [PubMed] [Google Scholar]
- 253.Murao K. Imachi H. Momoi A. Sayo Y. Hosokawa H. Sato M. Ishida T. Takahara J. Thiazolidinedione inhibits the production of monocyte chemoattractant protein-1 in cytokine-treated human vascular endothelial cells. FEBS Lett. 1999;454:27–30. doi: 10.1016/s0014-5793(99)00765-6. [DOI] [PubMed] [Google Scholar]
- 254.Nagy L. Tontonoz P. Alvarez JG. Chen H. Evans RM. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell. 1998;93:229–240. doi: 10.1016/s0092-8674(00)81574-3. [DOI] [PubMed] [Google Scholar]
- 255.Nakamachi T. Nomiyama T. Gizard F. Heywood EB. Jones KL. Zhao Y. Fuentes L. Takebayashi K. Aso Y. Staels B. Inukai T. Bruemmer D. PPARalpha agonists suppress osteopontin expression in macrophages and decrease plasma levels in patients with type 2 diabetes. Diabetes. 2007;56:1662–1670. doi: 10.2337/db06-1177. [DOI] [PubMed] [Google Scholar]
- 256.Narkar VA. Downes M. Yu RT. Embler E. Wang YX. Banayo E. Mihaylova MM. Nelson MC. Zou Y. Juguilon H. Kang H. Shaw RJ. Evans RM. AMPK and PPARdelta agonists are exercise mimetics. Cell. 2008;134:405–415. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Nawa T. Nawa MT. Cai Y. Zhang C. Uchimura I. Narumi S. Numano F. Kitajima S. Repression of TNF-alpha-induced E-selectin expression by PPAR activators: involvement of transcriptional repressor LRF-1/ATF3. Biochem Biophys Res Commun. 2000;275:406–411. doi: 10.1006/bbrc.2000.3332. [DOI] [PubMed] [Google Scholar]
- 258.Nettles KW. Insights into PPARgamma from structures with endogenous and covalently bound ligands. Nat Struct Mol Biol. 2008;15:893–895. doi: 10.1038/nsmb0908-893. [DOI] [PubMed] [Google Scholar]
- 259.Neuschwander-Tetri BA. Isley WL. Oki JC. Ramrakhiani S. Quiason SG. Phillips NJ. Brunt EM. Troglitazone-induced hepatic failure leading to liver transplantation: a case report. Ann Intern Med. 1998;129:38–41. doi: 10.7326/0003-4819-129-1-199807010-00009. [DOI] [PubMed] [Google Scholar]
- 260.Neve BP. Corseaux D. Chinetti G. Zawadzki C. Fruchart JC. Duriez P. Staels B. Jude B. PPARalpha agonists inhibit tissue factor expression in human monocytes and macrophages. Circulation. 2001;103:207–212. doi: 10.1161/01.cir.103.2.207. [DOI] [PubMed] [Google Scholar]
- 261.Ng VY. Morisseau C. Falck JR. Hammock BD. Kroetz DL. Inhibition of smooth muscle proliferation by urea-based alkanoic acids via peroxisome proliferator-activated receptor alpha-dependent repression of cyclin D1. Arterioscler Thromb Vasc Biol. 2006;26:2462–2468. doi: 10.1161/01.ATV.0000242013.29441.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Nichols JS. Parks DJ. Consler TG. Blanchard SG. Development of a scintillation proximity assay for peroxisome proliferator-activated receptor gamma ligand binding domain. Anal Biochem. 1998;257:112–119. doi: 10.1006/abio.1997.2557. [DOI] [PubMed] [Google Scholar]
- 263.Niemeyer NV. Janney LM. Thiazolidinedione-induced edema. Pharmacotherapy. 2002;22:924–929. doi: 10.1592/phco.22.11.924.33626. [DOI] [PubMed] [Google Scholar]
- 264.Nigro J. Dilley RJ. Little PJ. Differential effects of gemfibrozil on migration, proliferation and proteoglycan production in human vascular smooth muscle cells. Atherosclerosis. 2002;162:119–129. doi: 10.1016/s0021-9150(01)00704-3. [DOI] [PubMed] [Google Scholar]
- 265.Nissen SE. Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 266.O'Brien KD. Gordon D. Deeb S. Ferguson M. Chait A. Lipoprotein lipase is synthesized by macrophage-derived foam cells in human coronary atherosclerotic plaques. J Clin Invest. 1992;89:1544–1550. doi: 10.1172/JCI115747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Odegaard JI. Ricardo-Gonzalez RR. Goforth MH. Morel CR. Subramanian V. Mukundan L. Eagle AR. Vats D. Brombacher F. Ferrante AW. Chawla A. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Odegaard JI. Ricardo-Gonzalez RR. Red Eagle A. Vats D. Morel CR. Goforth MH. Subramanian V. Mukundan L. Ferrante AW. Chawla A. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metabol. 2008;7:496–507. doi: 10.1016/j.cmet.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Ogawa D. Nomiyama T. Nakamachi T. Heywood EB. Stone JF. Berger JP. Law RE. Bruemmer D. Activation of peroxisome proliferator-activated receptor gamma suppresses telomerase activity in vascular smooth muscle cells. Circ Res. 2006;98:e50–e59. doi: 10.1161/01.RES.0000218271.93076.c3. [DOI] [PubMed] [Google Scholar]
- 270.Ogawa S. Lozach J. Benner C. Pascual G. Tangirala RK. Westin S. Hoffmann A. Subramaniam S. David M. Rosenfeld MG. Glass CK. Molecular determinants of crosstalk between nuclear receptors and toll-like receptors. Cell. 2005;122:707–721. doi: 10.1016/j.cell.2005.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Ohshima T. Koga H. Shimotohno K. Transcriptional activity of peroxisome proliferator-activated receptor gamma is modulated by SUMO-1 modification. J Biol Chem. 2004;279:29551–29557. doi: 10.1074/jbc.M403866200. [DOI] [PubMed] [Google Scholar]
- 272.Ohtani K. Iwanaga R. Nakamura M. Ikeda M. Yabuta N. Tsuruga H. Nojima H. Cell growth-regulated expression of mammalian MCM5 and MCM6 genes mediated by the transcription factor E2F. Oncogene. 1999;18:2299–2309. doi: 10.1038/sj.onc.1202544. [DOI] [PubMed] [Google Scholar]
- 273.Oishi Y. Manabe I. Tobe K. Ohsugi M. Kubota T. Fujiu K. Maemura K. Kubota N. Kadowaki T. Nagai R. SUMOylation of Kruppel-like transcription factor 5 acts as a molecular switch in transcriptional programs of lipid metabolism involving PPAR-delta. Nat Med. 2008;14:656–666. doi: 10.1038/nm1756. [DOI] [PubMed] [Google Scholar]
- 274.Oishi Y. Manabe I. Tobe K. Tsushima K. Shindo T. Fujiu K. Nishimura G. Maemura K. Yamauchi T. Kubota N. Suzuki R. Kitamura T. Akira S. Kadowaki T. Nagai R. Kruppel-like transcription factor KLF5 is a key regulator of adipocyte differentiation. Cell Metab. 2005;1:27–39. doi: 10.1016/j.cmet.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 275.Okura T. Nakamura M. Takata Y. Watanabe S. Kitami Y. Hiwada K. Troglitazone induces apoptosis via the p53 and Gadd45 pathway in vascular smooth muscle cells. Eur J Pharmacol. 2000;407:227–235. doi: 10.1016/s0014-2999(00)00758-5. [DOI] [PubMed] [Google Scholar]
- 276.Oliver WR., Jr Shenk JL. Snaith MR. Russell CS. Plunket KD. Bodkin NL. Lewis MC. Winegar DA. Sznaidman ML. Lambert MH. Xu HE. Sternbach DD. Kliewer SA. Hansen BC. Willson TM. A selective peroxisome proliferator-activated receptor delta agonist promotes reverse cholesterol transport. Proc Natl Acad Sci U S A. 2001;98:5306–5311. doi: 10.1073/pnas.091021198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Oyama Y. Akuzawa N. Nagai R. Kurabayashi M. PPARgamma ligand inhibits osteopontin gene expression through interference with binding of nuclear factors to A/T-rich sequence in THP-1 cells. Circ Res. 2002;90:348–355. doi: 10.1161/hh0302.105098. [DOI] [PubMed] [Google Scholar]
- 278.Oyama Y. Kurabayashi M. Akuzawa N. Nagai R. Troglitazone, a PPARgamma ligand, inhibits osteopontin gene expression in human monocytes/macrophage THP-1 cells. J Atheroscler Thromb. 2000;7:77–82. doi: 10.5551/jat1994.7.77. [DOI] [PubMed] [Google Scholar]
- 279.Paddock ML. Wiley SE. Axelrod HL. Cohen AE. Roy M. Abresch EC. Capraro D. Murphy AN. Nechushtai R. Dixon JE. Jennings PA. MitoNEET is a uniquely folded 2Fe 2S outer mitochondrial membrane protein stabilized by pioglitazone. Proc Natl Acad Sci U S A. 2007;104:14342–14347. doi: 10.1073/pnas.0707189104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Palmer RM. Ferrige AG. Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 281.Panagia M. Gibbons GF. Radda GK. Clarke K. PPAR-alpha activation required for decreased glucose uptake and increased susceptibility to injury during ischemia. Am J Physiol Heart Circ Physiol. 2005;288:H2677–H2683. doi: 10.1152/ajpheart.00200.2004. [DOI] [PubMed] [Google Scholar]
- 282.Panigrahy D. Singer S. Shen LQ. Butterfield CE. Freedman DA. Chen EJ. Moses MA. Kilroy S. Duensing S. Fletcher C. Fletcher JA. Hlatky L. Hahnfeldt P. Folkman J. Kaipainen A. PPARgamma ligands inhibit primary tumor growth and metastasis by inhibiting angiogenesis. J Clin Invest. 2002;110:923–932. doi: 10.1172/JCI15634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Park KG. Lee KM. Chang YC. Magae J. Ando K. Kim KB. Kim YN. Kim HS. Park JY. Lee KU. Lee IK. The ascochlorin derivative, AS-6, inhibits TNF-alpha-induced adhesion molecule and chemokine expression in rat vascular smooth muscle cells. Life Sci. 2006;80:120–126. doi: 10.1016/j.lfs.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 284.Park SY. Cho YR. Finck BN. Kim HJ. Higashimori T. Hong EG. Lee MK. Danton C. Deshmukh S. Cline GW. Wu JJ. Bennett AM. Rothermel B. Kalinowski A. Russell KS. Kim YB. Kelly DP. Kim JK. Cardiac-specific overexpression of peroxisome proliferator-activated receptor-alpha causes insulin resistance in heart and liver. Diabetes. 2005;54:2514–2524. doi: 10.2337/diabetes.54.9.2514. [DOI] [PubMed] [Google Scholar]
- 285.Pasceri V. Cheng JS. Willerson JT. Yeh ET. Modulation of C-reactive protein-mediated monocyte chemoattractant protein-1 induction in human endothelial cells by anti-atherosclerosis drugs. Circulation. 2001;103:2531–2534. doi: 10.1161/01.cir.103.21.2531. [DOI] [PubMed] [Google Scholar]
- 286.Pasceri V. Wu HD. Willerson JT. Yeh ET. Modulation of vascular inflammation in vitro and in vivo by peroxisome proliferator-activated receptor-gamma activators. Circulation. 2000;101:235–238. doi: 10.1161/01.cir.101.3.235. [DOI] [PubMed] [Google Scholar]
- 287.Pascual G. Fong AL. Ogawa S. Gamliel A. Li AC. Perissi V. Rose DW. Willson TM. Rosenfeld MG. Glass CK. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature. 2005;437:759–763. doi: 10.1038/nature03988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Paumelle R. Blanquart C. Briand O. Barbier O. Duhem C. Woerly G. Percevault F. Fruchart JC. Dombrowicz D. Glineur C. Staels B. Acute antiinflammatory properties of statins involve peroxisome proliferator-activated receptor-alpha via inhibition of the protein kinase C signaling pathway. Circ Res. 2006;98:361–369. doi: 10.1161/01.RES.0000202706.70992.95. [DOI] [PubMed] [Google Scholar]
- 289.Peters JM. Lee SS. Li W. Ward JM. Gavrilova O. Everett C. Reitman ML. Hudson LD. Gonzalez FJ. Growth, adipose, brain, and skin alterations resulting from targeted disruption of the mouse peroxisome proliferator-activated receptor beta(delta) Mol Cell Biol. 2000;20:5119–5128. doi: 10.1128/mcb.20.14.5119-5128.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Piqueras L. Reynolds AR. Hodivala-Dilke KM. Alfranca A. Redondo JM. Hatae T. Tanabe T. Warner TD. Bishop-Bailey D. Activation of PPARbeta/delta induces endothelial cell proliferation and angiogenesis. Arterioscler Thromb Vasc Biol. 2007;27:63–69. doi: 10.1161/01.ATV.0000250972.83623.61. [DOI] [PubMed] [Google Scholar]
- 291.Pistrosch F. Herbrig K. Oelschlaegel U. Richter S. Passauer J. Fischer S. Gross P. PPARgamma-agonist rosiglitazone increases number and migratory activity of cultured endothelial progenitor cells. Atherosclerosis. 2005;183:163–167. doi: 10.1016/j.atherosclerosis.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 292.Pistrosch F. Passauer J. Fischer S. Fuecker K. Hanefeld M. Gross P. In type 2 diabetes, rosiglitazone therapy for insulin resistance ameliorates endothelial dysfunction independent of glucose control. Diabetes Care. 2004;27:484–490. doi: 10.2337/diacare.27.2.484. [DOI] [PubMed] [Google Scholar]
- 293.Planavila A. Rodriguez-Calvo R. Jove M. Michalik L. Wahli W. Laguna JC. Vazquez-Carrera M. Peroxisome proliferator-activated receptor beta/delta activation inhibits hypertrophy in neonatal rat cardiomyocytes. Cardiovasc Res. 2005;65:832–841. doi: 10.1016/j.cardiores.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 294.Polikandriotis JA. Mazzella LJ. Rupnow HL. Hart CM. Peroxisome proliferator-activated receptor gamma ligands stimulate endothelial nitric oxide production through distinct peroxisome proliferator-activated receptor gamma-dependent mechanisms. Arterioscler Thromb Vasc Biol. 2005;25:1810–1816. doi: 10.1161/01.ATV.0000177805.65864.d4. [DOI] [PubMed] [Google Scholar]
- 295.Porcheray F. Viaud S. Rimaniol AC. Leone C. Samah B. Dereuddre-Bosquet N. Dormont D. Gras G. Macrophage activation switching: an asset for the resolution of inflammation. Clin Exp Immunol. 2005;142:481–489. doi: 10.1111/j.1365-2249.2005.02934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Pourcet B. Fruchart JC. Staels B. Glineur C. Selective PPAR modulators, dual and pan PPAR agonists: multimodal drugs for the treatment of type 2 diabetes and atherosclerosis. Expert Opin Emerg Drugs. 2006;11:379–401. doi: 10.1517/14728214.11.3.379. [DOI] [PubMed] [Google Scholar]
- 297.Ptasinska A. Wang S. Zhang J. Wesley RA. Danner RL. Nitric oxide activation of peroxisome proliferator-activated receptor gamma through a p38 MAPK signaling pathway. FASEB J. 2007;21:950–961. doi: 10.1096/fj.06-6822com. [DOI] [PubMed] [Google Scholar]
- 298.Ravaux L. Denoyelle C. Monne C. Limon I. Raymondjean M. El Hadri K. Inhibition of interleukin-1beta-induced group IIA secretory phospholipase A2 expression by peroxisome proliferator-activated receptors (PPARs) in rat vascular smooth muscle cells: cooperation between PPARbeta and the proto-oncogene BCL-6. Mol Cell Biol. 2007;27:8374–8387. doi: 10.1128/MCB.00623-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Razeghi P. Young ME. Abbasi S. Taegtmeyer H. Hypoxia in vivo decreases peroxisome proliferator-activated receptor alpha-regulated gene expression in rat heart. Biochem Biophys Res Commun. 2001;287:5–10. doi: 10.1006/bbrc.2001.5541. [DOI] [PubMed] [Google Scholar]
- 300.Reddy J. Svoboda D. Azarnoff D. Microbody proliferation in liver induced by nafenopin, a new hypolipidemic drug: comparison with CPIB. Biochem Biophys Res Commun. 1973;52:537–543. doi: 10.1016/0006-291x(73)90745-6. [DOI] [PubMed] [Google Scholar]
- 301.Reddy JK. Hepatic microbody proliferation and catalase synthesis induced by methyl clofenapate, a hypolipidemic analog of CPIB. Am J Pathol. 1974;75:103–118. [PMC free article] [PubMed] [Google Scholar]
- 302.Reddy JK. Krishnakantha TP. Hepatic peroxisome proliferation: induction by two novel compounds structurally unrelated to clofibrate. Science (New York) 1975;190:787–789. doi: 10.1126/science.1198095. [DOI] [PubMed] [Google Scholar]
- 303.Redondo S. Ruiz E. Santos-Gallego CG. Padilla E. Tejerina T. Pioglitazone induces vascular smooth muscle cell apoptosis through a peroxisome proliferator-activated receptor-gamma, transforming growth factor-beta1, and a Smad2-dependent mechanism. Diabetes. 2005;54:811–817. doi: 10.2337/diabetes.54.3.811. [DOI] [PubMed] [Google Scholar]
- 304.Richter EA. Kiens B. Wojtaszewski JF. Can exercise mimetics substitute for exercise? Cell Metab. 2008;8:96–98. doi: 10.1016/j.cmet.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 305.Ricote M. Glass CK. PPARs and molecular mechanisms of transrepression. Biochim Biophys Acta. 2007;1771:926–935. doi: 10.1016/j.bbalip.2007.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Ricote M. Huang J. Fajas L. Li A. Welch J. Najib J. Witztum JL. Auwerx J. Palinski W. Glass CK. Expression of the peroxisome proliferator-activated receptor gamma (PPARgamma) in human atherosclerosis and regulation in macrophages by colony stimulating factors and oxidized low density lipoprotein. Proc Natl Acad Sci U S A. 1998;95:7614–7619. doi: 10.1073/pnas.95.13.7614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Ricote M. Li AC. Willson TM. Kelly CJ. Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 308.Rieusset J. Touri F. Michalik L. Escher P. Desvergne B. Niesor E. Wahli W. A new selective peroxisome proliferator-activated receptor gamma antagonist with antiobesity and antidiabetic activity. Mol Endocr (Baltimore) 2002;16:2628–2644. doi: 10.1210/me.2002-0036. [DOI] [PubMed] [Google Scholar]
- 309.Rigamonti E. Fontaine C. Lefebvre B. Duhem C. Lefebvre P. Marx N. Staels B. Chinetti-Gbaguidi G. Induction of CXCR2 receptor by peroxisome proliferator-activated receptor gamma in human macrophages. Arterioscler Thromb Vasc Biol. 2008;28:932–939. doi: 10.1161/ATVBAHA.107.161679. [DOI] [PubMed] [Google Scholar]
- 310.Riserus U. Sprecher D. Johnson T. Olson E. Hirschberg S. Liu A. Fang Z. Hegde P. Richards D. Sarov-Blat L. Strum JC. Basu S. Cheeseman J. Fielding BA. Humphreys SM. Danoff T. Moore NR. Murgatroyd P. O'Rahilly S. Sutton P. Willson T. Hassall D. Frayn KN. Karpe F. Activation of peroxisome proliferator-activated receptor (PPAR)delta promotes reversal of multiple metabolic abnormalities, reduces oxidative stress, and increases fatty acid oxidation in moderately obese men. Diabetes. 2008;57:332–339. doi: 10.2337/db07-1318. [DOI] [PubMed] [Google Scholar]
- 311.Rival Y. Beneteau N. Taillandier T. Pezet M. Dupont-Passelaigue E. Patoiseau JF. Junquero D. Colpaert FC. Delhon A. PPARalpha and PPARdelta activators inhibit cytokine-induced nuclear translocation of NF-kappaB and expression of VCAM-1 in EAhy926 endothelial cells. Eur J Pharmacol. 2002;435:143–151. doi: 10.1016/s0014-2999(01)01589-8. [DOI] [PubMed] [Google Scholar]
- 312.Robyr D. Wolffe AP. Wahli W. Nuclear hormone receptor coregulators in action: diversity for shared tasks. Mol Endocr (Baltimore) 2000;14:329–347. doi: 10.1210/mend.14.3.0411. [DOI] [PubMed] [Google Scholar]
- 313.Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 314.Rossi A. Kapahi P. Natoli G. Takahashi T. Chen Y. Karin M. Santoro MG. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IkappaB kinase. Nature. 2000;403:103–108. doi: 10.1038/47520. [DOI] [PubMed] [Google Scholar]
- 315.Ruiz E. Redondo S. Gordillo-Moscoso A. Tejerina T. Pioglitazone induces apoptosis in human vascular smooth muscle cells from diabetic patients involving the transforming growth factor-beta/activin receptor-like kinase-4/5/7/Smad2 signaling pathway. J Pharmacol Exp Ther. 2007;321:431–438. doi: 10.1124/jpet.106.114934. [DOI] [PubMed] [Google Scholar]
- 316.Sambandam N. Morabito D. Wagg C. Finck BN. Kelly DP. Lopaschuk GD. Chronic activation of PPARalpha is detrimental to cardiac recovery after ischemia. Am J Physiol Heart Circ Physiol. 2006;290:H87–H95. doi: 10.1152/ajpheart.00285.2005. [DOI] [PubMed] [Google Scholar]
- 317.Santini E. Fallahi P. Ferrari SM. Masoni A. Antonelli A. Ferrannini E. Effect of PPAR-gamma activation and inhibition on glucose-stimulated insulin release in INS-1e cells. Diabetes. 2004;53(suppl 3):S79–S83. doi: 10.2337/diabetes.53.suppl_3.s79. [DOI] [PubMed] [Google Scholar]
- 318.Satoh H. Tsukamoto K. Hashimoto Y. Hashimoto N. Togo M. Hara M. Maekawa H. Isoo N. Kimura S. Watanabe T. Thiazolidinediones suppress endothelin-1 secretion from bovine vascular endothelial cells: a new possible role of PPARgamma on vascular endothelial function. Biochem Biophys Res Commun. 1999;254:757–763. doi: 10.1006/bbrc.1998.0126. [DOI] [PubMed] [Google Scholar]
- 319.Schopfer FJ. Lin Y. Baker PR. Cui T. Garcia-Barrio M. Zhang J. Chen K. Chen YE. Freeman BA. Nitrolinoleic acid: an endogenous peroxisome proliferator-activated receptor gamma ligand. Proc Natl Acad Sci U S A. 2005;102:2340–2345. doi: 10.1073/pnas.0408384102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Semple RK. Meirhaeghe A. Vidal-Puig AJ. Schwabe JW. Wiggins D. Gibbons GF. Gurnell M. Chatterjee VK. O'Rahilly S. A dominant negative human peroxisome proliferator-activated receptor (PPAR){alpha} is a constitutive transcriptional corepressor and inhibits signaling through all PPAR isoforms. Endocrinology. 2005;146:1871–1882. doi: 10.1210/en.2004-1405. [DOI] [PubMed] [Google Scholar]
- 321.Sethi S. Ziouzenkova O. Ni H. Wagner DD. Plutzky J. Mayadas TN. Oxidized omega-3 fatty acids in fish oil inhibit leukocyte-endothelial interactions through activation of PPAR alpha. Blood. 2002;100:1340–1346. doi: 10.1182/blood-2002-01-0316. [DOI] [PubMed] [Google Scholar]
- 322.Shalev A. Siegrist-Kaiser CA. Yen PM. Wahli W. Burger AG. Chin WW. Meier CA. The peroxisome proliferator-activated receptor alpha is a phosphoprotein: regulation by insulin. Endocrinology. 1996;137:4499–4502. doi: 10.1210/endo.137.10.8828512. [DOI] [PubMed] [Google Scholar]
- 323.Sharma S. Taegtmeyer H. Adrogue J. Razeghi P. Sen S. Ngumbela K. Essop MF. Dynamic changes of gene expression in hypoxia-induced right ventricular hypertrophy. Am J Physiol Heart Circ Physiol. 2004;286:H1185–H1192. doi: 10.1152/ajpheart.00916.2003. [DOI] [PubMed] [Google Scholar]
- 324.Shaw N. Elholm M. Noy N. Retinoic acid is a high affinity selective ligand for the peroxisome proliferator-activated receptor beta/delta. J Biol Chem. 2003;278:41589–41592. doi: 10.1074/jbc.C300368200. [DOI] [PubMed] [Google Scholar]
- 325.Shearer BG. Billin AN. The next generation of PPAR drugs: do we have the tools to find them? Biochim Biophys Acta. 2007;1771:1082–1093. doi: 10.1016/j.bbalip.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 326.Shearer BG. Steger DJ. Way JM. Stanley TB. Lobe DC. Grillot DA. Iannone MA. Lazar MA. Willson TM. Billin AN. Identification and characterization of a selective peroxisome proliferator-activated receptor beta/delta (NR1C2) antagonist. Mol Endocr (Baltimore) 2008;22:523–529. doi: 10.1210/me.2007-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Sherr CJ. G1 phase progression: cycling on cue. Cell. 1994;79:551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- 328.Sherr CJ. Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 329.Shi Y. Hon M. Evans RM. The peroxisome proliferator-activated receptor delta, an integrator of transcriptional repression and nuclear receptor signaling. Proc Natl Acad Sci U S A. 2002;99:2613–2618. doi: 10.1073/pnas.052707099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Shu H. Wong B. Zhou G. Li Y. Berger J. Woods JW. Wright SD. Cai TQ. Activation of PPARalpha or gamma reduces secretion of matrix metalloproteinase 9 but not interleukin 8 from human monocytic THP-1 cells. Biochem Biophys Res Commun. 2000;267:345–349. doi: 10.1006/bbrc.1999.1968. [DOI] [PubMed] [Google Scholar]
- 331.Sierra-Honigmann MR. Nath AK. Murakami C. Garcia-Cardena G. Papapetropoulos A. Sessa WC. Madge LA. Schechner JS. Schwabb MB. Polverini PJ. Flores-Riveros JR. Biological action of leptin as an angiogenic factor. Science (New York) 1998;281:1683–1686. doi: 10.1126/science.281.5383.1683. [DOI] [PubMed] [Google Scholar]
- 332.Siess W. Zangl KJ. Essler M. Bauer M. Brandl R. Corrinth C. Bittman R. Tigyi G. Aepfelbacher M. Lysophosphatidic acid mediates the rapid activation of platelets and endothelial cells by mildly oxidized low density lipoprotein and accumulates in human atherosclerotic lesions. Proc Natl Acad Sci U S A. 1999;96:6931–6936. doi: 10.1073/pnas.96.12.6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Skogsberg J. Kannisto K. Cassel TN. Hamsten A. Eriksson P. Ehrenborg E. Evidence that peroxisome proliferator-activated receptor delta influences cholesterol metabolism in men. Arterioscler Thromb Vasc Biol. 2003;23:637–643. doi: 10.1161/01.ATV.0000064383.88696.24. [DOI] [PubMed] [Google Scholar]
- 334.Smeets PJ. Teunissen BE. Planavila A. de Vogel-van den Bosch H. Willemsen PH. van der Vusse GJ. van Bilsen M. Inflammatory pathways are activated during cardiomyocyte hypertrophy and attenuated by peroxisome proliferator-activated receptors PPAR{alpha} and PPAR{delta} J Biol Chem. 2008;283:29109–29118. doi: 10.1074/jbc.M802143200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Smeets PJ. Teunissen BE. Willemsen PH. van Nieuwenhoven FA. Brouns AE. Janssen BJ. Cleutjens JP. Staels B. van der Vusse GJ. van Bilsen M. Cardiac hypertrophy is enhanced in PPAR alpha−/− mice in response to chronic pressure overload. Cardiovasc Res. 2008;78:79–89. doi: 10.1093/cvr/cvn001. [DOI] [PubMed] [Google Scholar]
- 336.Son NH. Park TS. Yamashita H. Yokoyama M. Huggins LA. Okajima K. Homma S. Szabolcs MJ. Huang LS. Goldberg IJ. Cardiomyocyte expression of PPARgamma leads to cardiac dysfunction in mice. J Clin Invest. 2007;117:2791–2801. doi: 10.1172/JCI30335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Song J. Walsh MF. Igwe R. Ram JL. Barazi M. Dominguez LJ. Sowers JR. Troglitazone reduces contraction by inhibition of vascular smooth muscle cell Ca2+ currents and not endothelial nitric oxide production. Diabetes. 1997;46:659–664. doi: 10.2337/diab.46.4.659. [DOI] [PubMed] [Google Scholar]
- 338.Sorrentino SA. Bahlmann FH. Besler C. Muller M. Schulz S. Kirchhoff N. Doerries C. Horvath T. Limbourg A. Limbourg F. Fliser D. Haller H. Drexler H. Landmesser U. Oxidant stress impairs in vivo reendothelialization capacity of endothelial progenitor cells from patients with type 2 diabetes mellitus: restoration by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. Circulation. 2007;116:163–173. doi: 10.1161/CIRCULATIONAHA.106.684381. [DOI] [PubMed] [Google Scholar]
- 339.Sprecher DL. Massien C. Pearce G. Billin AN. Perlstein I. Willson TM. Hassall DG. Ancellin N. Patterson SD. Lobe DC. Johnson TG. Triglyceride:high-density lipoprotein cholesterol effects in healthy subjects administered a peroxisome proliferator activated receptor delta agonist. Arterioscler Thromb Vasc Biol. 2007;27:359–365. doi: 10.1161/01.ATV.0000252790.70572.0c. [DOI] [PubMed] [Google Scholar]
- 340.Staels B. Koenig W. Habib A. Merval R. Lebret M. Torra IP. Delerive P. Fadel A. Chinetti G. Fruchart JC. Najib J. Maclouf J. Tedgui A. Activation of human aortic smooth-muscle cells is inhibited by PPARalpha but not by PPARgamma activators. Nature. 1998;393:790–793. doi: 10.1038/31701. [DOI] [PubMed] [Google Scholar]
- 341.Stout RD. Suttles J. Functional plasticity of macrophages: reversible adaptation to changing microenvironments. J Leukoc Biol. 2004;76:509–513. doi: 10.1189/jlb.0504272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Straus DS. Pascual G. Li M. Welch JS. Ricote M. Hsiang CH. Sengchanthalangsy LL. Ghosh G. Glass CK. 15-Deoxy-delta 12,14-prostaglandin J2 inhibits multiple steps in the NF-kappa B signaling pathway. Proc Natl Acad Sci U S A. 2000;97:4844–4849. doi: 10.1073/pnas.97.9.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Sugawara A. Takeuchi K. Uruno A. Ikeda Y. Arima S. Kudo M. Sato K. Taniyama Y. Ito S. Transcriptional suppression of type 1 angiotensin II receptor gene expression by peroxisome proliferator-activated receptor-gamma in vascular smooth muscle cells. Endocrinology. 2001;142:3125–3134. doi: 10.1210/endo.142.7.8272. [DOI] [PubMed] [Google Scholar]
- 344.Suh N. Wang Y. Williams CR. Risingsong R. Gilmer T. Willson TM. Sporn MB. A new ligand for the peroxisome proliferator-activated receptor-gamma (PPAR-gamma), GW7845, inhibits rat mammary carcinogenesis. Cancer Res. 1999;59:5671–5673. [PubMed] [Google Scholar]
- 345.Sznaidman ML. Haffner CD. Maloney PR. Fivush A. Chao E. Goreham D. Sierra ML. LeGrumelec C. Xu HE. Montana VG. Lambert MH. Willson TM. Oliver WR Jr. Sternbach DD. Novel selective small molecule agonists for peroxisome proliferator-activated receptor delta (PPARdelta): synthesis and biological activity. Bioorg Med Chem Lett. 2003;13:1517–1521. doi: 10.1016/s0960-894x(03)00207-5. [DOI] [PubMed] [Google Scholar]
- 346.Takata Y. Kitami Y. Okura T. Hiwada K. Peroxisome proliferator-activated receptor-gamma activation inhibits interleukin-1beta-mediated platelet-derived growth factor-alpha receptor gene expression via CCAAT/enhancer-binding protein-delta in vascular smooth muscle cells. J Biol Chem. 2001;276:12893–12897. doi: 10.1074/jbc.M011655200. [DOI] [PubMed] [Google Scholar]
- 347.Takata Y. Kitami Y. Yang ZH. Nakamura M. Okura T. Hiwada K. Vascular inflammation is negatively autoregulated by interaction between CCAAT/enhancer-binding protein-delta and peroxisome proliferator-activated receptor-gamma. Circ Res. 2002;91:427–433. doi: 10.1161/01.res.0000031271.20771.4f. [DOI] [PubMed] [Google Scholar]
- 348.Takata Y. Liu J. Yin F. Collins AR. Lyon CJ. Lee CH. Atkins AR. Downes M. Barish GD. Evans RM. Hsueh WA. Tangirala RK. PPARdelta-mediated antiinflammatory mechanisms inhibit angiotensin II-accelerated atherosclerosis. Proc Natl Acad Sci U S A. 2008;105:4277–4282. doi: 10.1073/pnas.0708647105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Takeda K. Ichiki T. Tokunou T. Funakoshi Y. Iino N. Hirano K. Kanaide H. Takeshita A. Peroxisome proliferator-activated receptor gamma activators downregulate angiotensin II type 1 receptor in vascular smooth muscle cells. Circulation. 2000;102:1834–1839. doi: 10.1161/01.cir.102.15.1834. [DOI] [PubMed] [Google Scholar]
- 350.Tan NS. Michalik L. Noy N. Yasmin R. Pacot C. Heim M. Fluhmann B. Desvergne B. Wahli W. Critical roles of PPAR beta/delta in keratinocyte response to inflammation. Genes Dev. 2001;15:3263–3277. doi: 10.1101/gad.207501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Tao L. Liu HR. Gao E. Teng ZP. Lopez BL. Christopher TA. Ma XL. Batinic-Haberle I. Willette RN. Ohlstein EH. Yue TL. Antioxidative, antinitrative, and vasculoprotective effects of a peroxisome proliferator-activated receptor-gamma agonist in hypercholesterolemia. Circulation. 2003;108:2805–2811. doi: 10.1161/01.CIR.0000097003.49585.5E. [DOI] [PubMed] [Google Scholar]
- 352.Teissier E. Nohara A. Chinetti G. Paumelle R. Cariou B. Fruchart JC. Brandes RP. Shah A. Staels B. Peroxisome proliferator-activated receptor alpha induces NADPH oxidase activity in macrophages, leading to the generation of LDL with PPAR-alpha activation properties. Circ Res. 2004;95:1174–1182. doi: 10.1161/01.RES.0000150594.95988.45. [DOI] [PubMed] [Google Scholar]
- 353.Tenenbaum A. Motro M. Fisman EZ. Tanne D. Boyko V. Behar S. Bezafibrate for the secondary prevention of myocardial infarction in patients with metabolic syndrome. Arch Intern Med. 2005;165:1154–1160. doi: 10.1001/archinte.165.10.1154. [DOI] [PubMed] [Google Scholar]
- 354.Teunissen BE. Smeets PJ. Willemsen PH. De Windt LJ. Van der Vusse GJ. Van Bilsen M. Activation of PPARdelta inhibits cardiac fibroblast proliferation and the transdifferentiation into myofibroblasts. Cardiovasc Res. 2007;75:519–529. doi: 10.1016/j.cardiores.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 355.Tham DM. Martin-McNulty B. Wang YX. Wilson DW. Vergona R. Sullivan ME. Dole W. Rutledge JC. Angiotensin II is associated with activation of NF-kappaB-mediated genes and downregulation of PPARs. Physiol Genom. 2002;11:21–30. doi: 10.1152/physiolgenomics.00062.2002. [DOI] [PubMed] [Google Scholar]
- 356.Thom T. Haase N. Rosamond W. Howard VJ. Rumsfeld J. Manolio T. Zheng ZJ. Flegal K. O'Donnell C. Kittner S. Lloyd-Jones D. Goff DC Jr. Hong Y. Adams R. Friday G. Furie K. Gorelick P. Kissela B. Marler J. Meigs J. Roger V. Sidney S. Sorlie P. Steinberger J. Wasserthiel-Smoller S. Wilson M. Wolf P. Heart disease and stroke statistics: 2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–e151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 357.Tolman KG. Chandramouli J. Hepatotoxicity of the thiazolidinediones. Clin Liver Dis. 2003;7:369–379. doi: 10.1016/s1089-3261(03)00020-5. vi. [DOI] [PubMed] [Google Scholar]
- 358.Tontonoz P. Nagy L. Alvarez JG. Thomazy VA. Evans RM. PPARgamma promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell. 1998;93:241–252. doi: 10.1016/s0092-8674(00)81575-5. [DOI] [PubMed] [Google Scholar]
- 359.Tordjman K. Bernal-Mizrachi C. Zemany L. Weng S. Feng C. Zhang F. Leone TC. Coleman T. Kelly DP. Semenkovich CF. PPARalpha deficiency reduces insulin resistance and atherosclerosis in apoE-null mice. J Clin Invest. 2001;107:1025–1034. doi: 10.1172/JCI11497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Tran H. Brunet A. Griffith EC. Greenberg ME. The many forks in FOXO's road. Sci STKE. 2003;2003:RE5. doi: 10.1126/stke.2003.172.re5. [DOI] [PubMed] [Google Scholar]
- 361.Tsukahara T. Tsukahara R. Yasuda S. Makarova N. Valentine WJ. Allison P. Yuan H. Baker DL. Li Z. Bittman R. Parrill A. Tigyi G. Different residues mediate recognition of 1-O-oleyllysophosphatidic acid and rosiglitazone in the ligand binding domain of peroxisome proliferator-activated receptor gamma. J Biol Chem. 2006;281:3398–3407. doi: 10.1074/jbc.M510843200. [DOI] [PubMed] [Google Scholar]
- 362.Tugwood JD. Issemann I. Anderson RG. Bundell KR. McPheat WL. Green S. The mouse peroxisome proliferator activated receptor recognizes a response element in the 5' flanking sequence of the rat acyl CoA oxidase gene. EMBO J. 1992;11:433–439. doi: 10.1002/j.1460-2075.1992.tb05072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.van der Lee KA. Vork MM. De Vries JE. Willemsen PH. Glatz JF. Reneman RS. Van der Vusse GJ. Van Bilsen M. Long-chain fatty acid-induced changes in gene expression in neonatal cardiac myocytes. J lipid Res. 2000;41:41–47. [PubMed] [Google Scholar]
- 364.Van Ginderachter JA. Meerschaut S. Liu Y. Brys L. De Groeve K. Hassanzadeh Ghassabeh G. Raes G. De Baetselier P. Peroxisome proliferator-activated receptor gamma (PPARgamma) ligands reverse CTL suppression by alternatively activated (M2) macrophages in cancer. Blood. 2006;108:525–535. doi: 10.1182/blood-2005-09-3777. [DOI] [PubMed] [Google Scholar]
- 365.Van Ginderachter JA. Movahedi K. Hassanzadeh Ghassabeh G. Meerschaut S. Beschin A. Raes G. De Baetselier P. Classical and alternative activation of mononuclear phagocytes: picking the best of both worlds for tumor promotion. Immunobiology. 2006;211:487–501. doi: 10.1016/j.imbio.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 366.Vecchione C. Maffei A. Colella S. Aretini A. Poulet R. Frati G. Gentile MT. Fratta L. Trimarco V. Trimarco B. Lembo G. Leptin effect on endothelial nitric oxide is mediated through Akt-endothelial nitric oxide synthase phosphorylation pathway. Diabetes. 2002;51:168–173. doi: 10.2337/diabetes.51.1.168. [DOI] [PubMed] [Google Scholar]
- 367.Verma S. Kuliszewski MA. Li SH. Szmitko PE. Zucco L. Wang CH. Badiwala MV. Mickle DA. Weisel RD. Fedak PW. Stewart DJ. Kutryk MJ. C-reactive protein attenuates endothelial progenitor cell survival, differentiation, and function: further evidence of a mechanistic link between C-reactive protein and cardiovascular disease. Circulation. 2004;109:2058–2067. doi: 10.1161/01.CIR.0000127577.63323.24. [DOI] [PubMed] [Google Scholar]
- 368.Verrier E. Wang L. Wadham C. Albanese N. Hahn C. Gamble JR. Chatterjee VK. Vadas MA. Xia P. PPARgamma agonists ameliorate endothelial cell activation via inhibition of diacylglycerol-protein kinase C signaling pathway: role of diacylglycerol kinase. Circ Res. 2004;94:1515–1522. doi: 10.1161/01.RES.0000130527.92537.06. [DOI] [PubMed] [Google Scholar]
- 369.Villacorta L. Schopfer FJ. Zhang J. Freeman BA. Chen YE. PPARgamma and its ligands: therapeutic implications in cardiovascular disease. Clin Sci (Lond) 2009;116:205–218. doi: 10.1042/CS20080195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Vosper H. Khoudoli GA. Palmer CN. The peroxisome proliferator activated receptor delta is required for the differentiation of THP-1 monocytic cells by phorbol ester. Nucl Recept. 2003;1:9. doi: 10.1186/1478-1336-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Vosper H. Patel L. Graham TL. Khoudoli GA. Hill A. Macphee CH. Pinto I. Smith SA. Suckling KE. Wolf CR. Palmer CN. The peroxisome proliferator-activated receptor delta promotes lipid accumulation in human macrophages. J Biol Chem. 2001;276:44258–44265. doi: 10.1074/jbc.M108482200. [DOI] [PubMed] [Google Scholar]
- 372.Wakino S. Hayashi K. Kanda T. Tatematsu S. Homma K. Yoshioka K. Takamatsu I. Saruta T. Peroxisome proliferator-activated receptor gamma ligands inhibit Rho/Rho kinase pathway by inducing protein tyrosine phosphatase SHP-2. Circ Res. 2004;95:e45–e55. doi: 10.1161/01.RES.0000142313.68389.92. [DOI] [PubMed] [Google Scholar]
- 373.Wakino S. Kintscher U. Kim S. Yin F. Hsueh WA. Law RE. Peroxisome proliferator-activated receptor gamma ligands inhibit retinoblastoma phosphorylation and G1- ->S transition in vascular smooth muscle cells. J Biol Chem. 2000;275:22435–22441. doi: 10.1074/jbc.M910452199. [DOI] [PubMed] [Google Scholar]
- 374.Wakino S. Kintscher U. Liu Z. Kim S. Yin F. Ohba M. Kuroki T. Schonthal AH. Hsueh WA. Law RE. Peroxisome proliferator-activated receptor gamma ligands inhibit mitogenic induction of p21(Cip1) by modulating the protein kinase Cdelta pathway in vascular smooth muscle cells. J Biol Chem. 2001;276:47650–47657. doi: 10.1074/jbc.M108719200. [DOI] [PubMed] [Google Scholar]
- 375.Wallace JM. Schwarz M. Coward P. Houze J. Sawyer JK. Kelley KL. Chai A. Rudel LL. Effects of peroxisome proliferator-activated receptor alpha/delta agonists on HDL-cholesterol in vervet monkeys. J lipid Res. 2005;46:1009–1016. doi: 10.1194/jlr.M500002-JLR200. [DOI] [PubMed] [Google Scholar]
- 376.Walter DH. Rittig K. Bahlmann FH. Kirchmair R. Silver M. Murayama T. Nishimura H. Losordo DW. Asahara T. Isner JM. Statin therapy accelerates reendothelialization: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation. 2002;105:3017–3024. doi: 10.1161/01.cir.0000018166.84319.55. [DOI] [PubMed] [Google Scholar]
- 377.Wang CH. Ciliberti N. Li SH. Szmitko PE. Weisel RD. Fedak PW. Al-Omran M. Cherng WJ. Li RK. Stanford WL. Verma S. Rosiglitazone facilitates angiogenic progenitor cell differentiation toward endothelial lineage: a new paradigm in glitazone pleiotropy. Circulation. 2004;109:1392–1400. doi: 10.1161/01.CIR.0000123231.49594.21. [DOI] [PubMed] [Google Scholar]
- 378.Wang N. Verna L. Chen NG. Chen J. Li H. Forman BM. Stemerman MB. Constitutive activation of peroxisome proliferator-activated receptor-gamma suppresses pro-inflammatory adhesion molecules in human vascular endothelial cells. J Biol Chem. 2002;277:34176–34181. doi: 10.1074/jbc.M203436200. [DOI] [PubMed] [Google Scholar]
- 379.Wang YX. Lee CH. Tiep S. Yu RT. Ham J. Kang H. Evans RM. Peroxisome-proliferator-activated receptor delta activates fat metabolism to prevent obesity. Cell. 2003;113:159–170. doi: 10.1016/s0092-8674(03)00269-1. [DOI] [PubMed] [Google Scholar]
- 380.Wang YX. Zhang CL. Yu RT. Cho HK. Nelson MC. Bayuga-Ocampo CR. Ham J. Kang H. Evans RM. Regulation of muscle fiber type and running endurance by PPARdelta. PLoS Biol. 2004;2:e294. doi: 10.1371/journal.pbio.0020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Watanabe K. Fujii H. Takahashi T. Kodama M. Aizawa Y. Ohta Y. Ono T. Hasegawa G. Naito M. Nakajima T. Kamijo Y. Gonzalez FJ. Aoyama T. Constitutive regulation of cardiac fatty acid metabolism through peroxisome proliferator-activated receptor alpha associated with age-dependent cardiac toxicity. J Biol Chem. 2000;275:22293–22299. doi: 10.1074/jbc.M000248200. [DOI] [PubMed] [Google Scholar]
- 382.Welch JS. Ricote M. Akiyama TE. Gonzalez FJ. Glass CK. PPARgamma and PPARdelta negatively regulate specific subsets of lipopolysaccharide and IFN-gamma target genes in macrophages. Proc Natl Acad Sci U S A. 2003;100:6712–6717. doi: 10.1073/pnas.1031789100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Werner C. Kamani CH. Gensch C. Bohm M. Laufs U. The peroxisome proliferator-activated receptor-gamma agonist pioglitazone increases number and function of endothelial progenitor cells in patients with coronary artery disease and normal glucose tolerance. Diabetes. 2007;56:2609–2615. doi: 10.2337/db07-0069. [DOI] [PubMed] [Google Scholar]
- 384.Wiley SE. Murphy AN. Ross SA. van der Geer P. Dixon JE. MitoNEET is an iron-containing outer mitochondrial membrane protein that regulates oxidative capacity. Proc Natl Acad Sci U S A. 2007;104:5318–5323. doi: 10.1073/pnas.0701078104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Wiley SE. Paddock ML. Abresch EC. Gross L. van der Geer P. Nechushtai R. Murphy AN. Jennings PA. Dixon JE. The outer mitochondrial membrane protein mitoNEET contains a novel redox-active 2Fe-2S cluster. J Biol Chem. 2007;282:23745–23749. doi: 10.1074/jbc.C700107200. [DOI] [PubMed] [Google Scholar]
- 386.Wright HM. Clish CB. Mikami T. Hauser S. Yanagi K. Hiramatsu R. Serhan CN. Spiegelman BM. A synthetic antagonist for the peroxisome proliferator-activated receptor gamma inhibits adipocyte differentiation. J Biol Chem. 2000;275:1873–1877. doi: 10.1074/jbc.275.3.1873. [DOI] [PubMed] [Google Scholar]
- 387.Xin X. Yang S. Kowalski J. Gerritsen ME. Peroxisome proliferator-activated receptor gamma ligands are potent inhibitors of angiogenesis in vitro and in vivo. J Biol Chem. 1999;274:9116–9121. doi: 10.1074/jbc.274.13.9116. [DOI] [PubMed] [Google Scholar]
- 388.Xiong C. Mou Y. Zhang J. Fu M. Chen YE. Akinbami MA. Cui T. Impaired expression of PPAR gamma protein contributes to the exaggerated growth of vascular smooth muscle cells in spontaneously hypertensive rats. Life Sci. 2005;77:3037–3048. doi: 10.1016/j.lfs.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 389.Xu HE. Lambert MH. Montana VG. Parks DJ. Blanchard SG. Brown PJ. Sternbach DD. Lehmann JM. Wisely GB. Willson TM. Kliewer SA. Milburn MV. Molecular recognition of fatty acids by peroxisome proliferator-activated receptors. Mol Cell. 1999;3:397–403. doi: 10.1016/s1097-2765(00)80467-0. [DOI] [PubMed] [Google Scholar]
- 390.Xu HE. Lambert MH. Montana VG. Plunket KD. Moore LB. Collins JL. Oplinger JA. Kliewer SA. Gampe RT Jr. McKee DD. Moore JT. Willson TM. Structural determinants of ligand binding selectivity between the peroxisome proliferator-activated receptors. Proc Natl Acad Sci U S A. 2001;98:13919–13924. doi: 10.1073/pnas.241410198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Xu HE. Stanley TB. Montana VG. Lambert MH. Shearer BG. Cobb JE. McKee DD. Galardi CM. Plunket KD. Nolte RT. Parks DJ. Moore JT. Kliewer SA. Willson TM. Stimmel JB. Structural basis for antagonist-mediated recruitment of nuclear co-repressors by PPARalpha. Nature. 2002;415:813–817. doi: 10.1038/415813a. [DOI] [PubMed] [Google Scholar]
- 392.Xu Y. Gen M. Lu L. Fox J. Weiss SO. Brown RD. Perlov D. Ahmad H. Zhu P. Greyson C. Long CS. Schwartz GG. PPAR-gamma activation fails to provide myocardial protection in ischemia and reperfusion in pigs. Am J Physiol Heart Circ Physiol. 2005;288:H1314–H1323. doi: 10.1152/ajpheart.00618.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Xu Y. Mayhugh D. Saeed A. Wang X. Thompson RC. Dominianni SJ. Kauffman RF. Singh J. Bean JS. Bensch WR. Barr RJ. Osborne J. Montrose-Rafizadeh C. Zink RW. Yumibe NP. Huang N. Luffer-Atlas D. Rungta D. Maise DE. Mantlo NB. Design and synthesis of a potent and selective triazolone-based peroxisome proliferator-activated receptor alpha agonist. J Med Chem. 2003;46:5121–5124. doi: 10.1021/jm034173l. [DOI] [PubMed] [Google Scholar]
- 394.Yamamoto K. Ohki R. Lee RT. Ikeda U. Shimada K. Peroxisome proliferator-activated receptor gamma activators inhibit cardiac hypertrophy in cardiac myocytes. Circulation. 2001;104:1670–1675. doi: 10.1161/hc4001.097186. [DOI] [PubMed] [Google Scholar]
- 395.Yang XY. Wang LH. Chen T. Hodge DR. Resau JH. DaSilva L. Farrar WL. Activation of human T lymphocytes is inhibited by peroxisome proliferator-activated receptor gamma (PPARgamma) agonists: PPARgamma co-association with transcription factor NFAT. J Biol Chem. 2000;275:4541–4544. doi: 10.1074/jbc.275.7.4541. [DOI] [PubMed] [Google Scholar]
- 396.Yang XY. Wang LH. Mihalic K. Xiao W. Chen T. Li P. Wahl LM. Farrar WL. Interleukin (IL)-4 indirectly suppresses IL-2 production by human T lymphocytes via peroxisome proliferator-activated receptor gamma activated by macrophage-derived 12/15-lipoxygenase ligands. J Biol Chem. 2002;277:3973–3978. doi: 10.1074/jbc.M105619200. [DOI] [PubMed] [Google Scholar]
- 397.Yoo J. Ghiassi M. Jirmanova L. Balliet AG. Hoffman B. Fornace AJ Jr. Liebermann DA. Bottinger EP. Roberts AB. Transforming growth factor-beta-induced apoptosis is mediated by Smad-dependent expression of GADD45b through p38 activation. J Biol Chem. 2003;278:43001–43007. doi: 10.1074/jbc.M307869200. [DOI] [PubMed] [Google Scholar]
- 398.Yoon CH. Hur J. Park KW. Kim JH. Lee CS. Oh IY. Kim TY. Cho HJ. Kang HJ. Chae IH. Yang HK. Oh BH. Park YB. Kim HS. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation. 2005;112:1618–1627. doi: 10.1161/CIRCULATIONAHA.104.503433. [DOI] [PubMed] [Google Scholar]
- 399.Young ME. McNulty P. Taegtmeyer H. Adaptation and maladaptation of the heart in diabetes, Part II: potential mechanisms. Circulation. 2002;105:1861–1870. doi: 10.1161/01.cir.0000012467.61045.87. [DOI] [PubMed] [Google Scholar]
- 400.Young PW. Buckle DR. Cantello BC. Chapman H. Clapham JC. Coyle PJ. Haigh D. Hindley RM. Holder JC. Kallender H. Latter AJ. Lawrie KW. Mossakowska D. Murphy GJ. Roxbee Cox L. Smith SA. Identification of high-affinity binding sites for the insulin sensitizer rosiglitazone (BRL-49653) in rodent and human adipocytes using a radioiodinated ligand for peroxisomal proliferator-activated receptor gamma. J Pharmacol Exp Ther. 1998;284:751–759. [PubMed] [Google Scholar]
- 401.Yu C. Chen L. Luo H. Chen J. Cheng F. Gui C. Zhang R. Shen J. Chen K. Jiang H. Shen X. Binding analyses between human PPARgamma-LBD and ligands. Eur J Biochem FEBS. 2004;271:386–397. doi: 10.1046/j.1432-1033.2003.03937.x. [DOI] [PubMed] [Google Scholar]
- 402.Yue L. Ye F. Gui C. Luo H. Cai J. Shen J. Chen K. Shen X. Jiang H. Ligand-binding regulation of LXR/RXR and LXR/PPAR heterodimerizations: SPR technology-based kinetic analysis correlated with molecular dynamics simulation. Protein Sci. 2005;14:812–822. doi: 10.1110/ps.04951405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Yue TL. Nerurkar SS. Bao W. Jucker BM. Sarov-Blat L. Steplewski K. Ohlstein EH. Willette RN. In vivo activation of peroxisome proliferator-activated receptor-delta protects the heart from ischemia/reperfusion injury in Zucker fatty rats. J Pharmacol Exp Thera. 2008;325:466–474. doi: 10.1124/jpet.107.135327. [DOI] [PubMed] [Google Scholar]
- 404.Zahradka P. Yurkova N. Litchie B. Moon MC. Del Rizzo DF. Taylor CG. Activation of peroxisome proliferator-activated receptors alpha and gamma1 inhibits human smooth muscle cell proliferation. Mol Cell Biochem. 2003;246:105–110. [PubMed] [Google Scholar]
- 405.Zhan Q. Lord KA. Alamo I., Jr. Hollander MC. Carrier F. Ron D. Kohn KW. Hoffman B. Liebermann DA. Fornace AJ., Jr The gadd and MyD genes define a novel set of mammalian genes encoding acidic proteins that synergistically suppress cell growth. Mol Cell B. 1994;14:2361–2371. doi: 10.1128/mcb.14.4.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Zhang C. Baker DL. Yasuda S. Makarova N. Balazs L. Johnson LR. Marathe GK. McIntyre TM. Xu Y. Prestwich GD. Byun HS. Bittman R. Tigyi G. Lysophosphatidic acid induces neointima formation through PPARgamma activation. J Exp Med. 2004;199:763–774. doi: 10.1084/jem.20031619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Zhang F. Sowers JR. Ram JL. Standley PR. Peuler JD. Effects of pioglitazone on calcium channels in vascular smooth muscle. Hypertension. 1994;24:170–175. doi: 10.1161/01.hyp.24.2.170. [DOI] [PubMed] [Google Scholar]
- 408.Zhang H. Pi R. Li R. Wang P. Tang F. Zhou S. Gao J. Jiang J. Chen S. Liu P. PPARbeta/delta activation inhibits angiotensin II-induced collagen type I expression in rat cardiac fibroblasts. Arch Biochem Biophys. 2007;460:25–32. doi: 10.1016/j.abb.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 409.Zhang J. Fu M. Zhao L. Chen YE. 15-Deoxy-prostaglandin J(2) inhibits PDGF-A and -B chain expression in human vascular endothelial cells independent of PPAR gamma. Biochem Biophys Res Commun. 2002;298:128–132. doi: 10.1016/s0006-291x(02)02401-4. [DOI] [PubMed] [Google Scholar]
- 410.Zhang J. Fu M. Zhu X. Xiao Y. Mou Y. Zheng H. Akinbami MA. Wang Q. Chen YE. Peroxisome proliferator-activated receptor delta is up-regulated during vascular lesion formation and promotes post-confluent cell proliferation in vascular smooth muscle cells. J B Chem. 2002;277:11505–11512. doi: 10.1074/jbc.M110580200. [DOI] [PubMed] [Google Scholar]
- 411.Zhou G. Cummings R. Li Y. Mitra S. Wilkinson HA. Elbrecht A. Hermes JD. Schaeffer JM. Smith RG. Moller DE. Nuclear receptors have distinct affinities for coactivators: characterization by fluorescence resonance energy transfer. Mol Endocr (Baltimore) 1998;12:1594–1604. doi: 10.1210/mend.12.10.0176. [DOI] [PubMed] [Google Scholar]
- 412.Ziouzenkova O. Perrey S. Asatryan L. Hwang J. MacNaul KL. Moller DE. Rader DJ. Sevanian A. Zechner R. Hoefler G. Plutzky J. Lipolysis of triglyceride-rich lipoproteins generates PPAR ligands: evidence for an antiinflammatory role for lipoprotein lipase. Proc Natl Acad Sci U S A. 2003;100:2730–2735. doi: 10.1073/pnas.0538015100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Zungu M. Felix R. Essop MF. Wy-14,643 and fenofibrate inhibit mitochondrial respiration in isolated rat cardiac mitochondria. Mitochondrion. 2006;6:315–322. doi: 10.1016/j.mito.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 414.Zuo X. Wu Y. Morris JS. Stimmel JB. Leesnitzer LM. Fischer SM. Lippman SM. Shureiqi I. Oxidative metabolism of linoleic acid modulates PPAR-beta/delta suppression of PPAR-gamma activity. Oncogene. 2006;25:1225–1241. doi: 10.1038/sj.onc.1209160. [DOI] [PMC free article] [PubMed] [Google Scholar]