Abstract
Post-traumatic immune suppression renders individuals with spinal cord injury (SCI) susceptible to infection. Normally, proper immune function is regulated by collaboration between the sympathetic nervous system (SNS) and hypothalamic-pituitary-adrenal (HPA) axis and involves the controlled release of glucocorticoids (GCs) and norepinephrine (NE). Recently, we showed that after high thoracic (T3) SCI, aberrant levels of GCs and NE accumulate in the blood and spleen, respectively. These changes are associated with splenic atrophy, splenic leucopenia, increased intrasplenic caspase-3 levels and suppressed B lymphocyte function. Since GCs boost SNS function, in part by increasing the expression and affinity of beta-2 adrenergic receptors (β2ARs) while simultaneously preventing β2AR down-regulation, we predicted that surges in stress hormones (i.e., GCs and NE) in the blood and spleen of mice with high-level SCI would act concurrently to adversely affect lymphocyte function and survival. Here, we show that post-SCI concentrations of GCs enhance the sensitivity of lymphocytes to β2AR stimulation causing an increase in intracellular Bim (Bcl2-Interacting Mediator of Cell Death) and subsequent apoptosis. In vivo, the combined antagonism of GC receptors and β2ARs significantly diminished lymphocyte Bim levels and SCI-induced splenic lymphopenia. Together, these data suggest that pharmacological antagonists of the HPA/SNS axes should be considered as adjunct therapies for ameliorating post-traumatic immune suppression in quadriplegics and high paraplegics.
Keywords: CNS injury, sympathetic nervous system, hypothalamic-pituitary-adrenal axis, Bim
Introduction
Individuals with spinal cord injury (SCI) have deficits in immune function that increase susceptibility to infections of the lung (e.g., pneumonia) and gastrointestinal or urogenital tract (Cruse et al. 1993, DeVivo et al. 1989, Nash 2000). Those with SCIs at the high thoracic and cervical level are most susceptible with infections being the leading cause of death in these individuals (DeVivo et al. 1989). A similar type of secondary immunodeficiency – a concept recently designated as CNS injury-induced immunodepression (CIDS) – also increases morbidity and mortality after traumatic brain injury and stroke (Meisel et al. 2005, Offner et al. 2006, Prass et al. 2003). In each case, CIDS can be explained by dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic nervous system (SNS).
In response to any stress (physical or psychological), the HPA axis and SNS collaborate to promote lipid metabolism and gluconeogenesis, i.e., processes that fuel mechanisms of tissue repair and evasion (“flight” from a threatening stimulus). During these reactions, a dynamic interaction exists at the molecular level between mediators and signaling intermediates of the HPA and SNS. Catecholamines (e.g., norepinephrine; NE) enhance glucocorticoid (GC) receptor (GR) stability (Dong et al. 1989) and binding to DNA (Rangarajan et al. 1992). Conversely, GCs boost SNS function in part by increasing the expression and affinity of beta-2 adrenergic receptors (β2ARs) while simultaneously preventing β2AR down-regulation (Davies & Lefkowitz 1981, Mak et al. 1995a, Mak et al. 1995b). Therefore, during stress (e.g., SCI), cells expressing β2ARs could become hyper-responsive to catecholamines.
Recently, we showed that in mice with a high thoracic (T3) SCI, in which spinal control over SNS innervation of peripheral lymphoid tissues is disrupted, there is marked splenic atrophy and leucopenia; with a concomitant increase in intrasplenic caspase-3 levels that suggests high-level SCI induces splenocyte apoptosis (Lucin et al. 2007). These changes were associated with increased levels of circulating GCs (corticosterone or CORT) and splenic norepinephrine (NE). Since the spleen filters the blood and is also densely innervated by the SNS, we predicted that surges in stress hormones (i.e., GCs and NE) in the blood and spleen of mice with high-level SCI would act in tandem to adversely affect lymphocyte function and survival.
Here, using in vitro models, we show that CORT and NE signaling pathways synergize within lymphocytes to increase expression of the pro-apoptotic protein Bim (Bcl-2 interacting mediator of cell death) (Willis et al. 2007). Importantly, Bim levels and the marked loss of lymphocytes after T3 SCI can be blocked in vivo by delivering a combination of RU486 (a GR antagonist) and butoxamine (a selective β2AR antagonist). Together, these data suggest that the stress response to high level SCI, mediated in part through enhanced GR and β2AR signaling in lymphocytes, could cause post-SCI immune suppression thereby increasing the susceptibility to infection in affected individuals. Consequently, commonly used drugs (e.g, beta-blockers or Mifepristone) may be useful for improving host defense and/or bolstering the effects of post-traumatic immunizations in quadriplegic or high paraplegic individuals. Similar benefits may be attained in immune compromised individuals suffering from traumatic brain injury or stroke.
Materials and methods
Mice
Adult pathogen-free C57BL/6 female mice (6–8 weeks old; 16–21g, n=87) were purchased from Taconic Laboratories (Germantown, NY). All experimental procedures were approved by the Animal Review Committee at The Ohio State University and are in accord with the US Department of Health, Education and Welfare.
Spinal cord injury
A total of 29 mice received spinal cord injuries as described previously (Lucin et al. 2007). Briefly, mice were anesthetized (i.p.) with a cocktail of ketamine (80 mg/kg)/xylazine (40 mg/kg) then were given prophylactic antibiotics (Gentocin; 1 mg/kg; s.q.). Using aseptic technique, a partial laminectomy was performed at vertebral level T3 to expose the dorsal spinal cord. Sham injured animals received a laminectomy (LAM) but no SCI. Complete spinal transection injuries were performed after which the muscles and skin were sutured separately. All mice receiving surgery received injections of sterile saline (2 mL, s.q.) to prevent dehydration then were placed individually into warmed HEPA-filtered cages. Post-operative care included manual bladder expression 2–3x/day and daily antibiotics (Gentocin, 50 mg/kg; s.q.).
Beta-2 adrenergic and CORT receptor antagonists
β2AR antagonists (butoxamine; 30mg/kg in PBS) and/or the GR antagonist Mifepristone (a.k.a. RU486; 30mg/kg in ethanol/sesame oil (1:10)) were injected (i.p.) immediately before SCI and at 1 and 2 days post-injury. All drugs were obtained from Sigma. Control injections consisted of sterile PBS only (i.p.).
Blood cell counts
Mice were anesthetized and blood was collected via retro-orbital puncture. 100µL of blood was diluted in 5mL of PBS (Ca2+ and Mg2+ free) + EDTA (2.7mM EDTA; pH 7.3) to prevent clotting. Red blood cells were lysed with 5mL of 0.8% ammonia chloride and washed with 10mL RPMI + 5% FBS. Red blood cell lysis was performed twice to ensure complete lysis. Washed cells were resuspended in 1mL of RPMI + 5% FBS then counted using a hemacytometer.
Splenocyte isolation
We focused our analyses on mixed and purified splenocytes since the ability to mount an immune response to a pathogen or a vaccine, involves effective interactions between splenic leukocytes. Moreover, sympathetic nervous system (SNS) innervation of the spleen is critical for controlling the survival and function of lymphocytes and as we have shown previously, this “hard-wiring” is adversely affected by a high-level SCI (Lucin et al. 2007).
After blood was collected, a laparatomy was performed under aseptic conditions to expose then excise the spleen. Single cell suspensions were prepared from individual spleens by processing spleens through 40μm nylon mesh strainers. Strainers were washed 3x with 5mL RPMI + 5%FBS. Red blood cells were lysed with 0.8% ammonia chloride. Splenocytes then were washed with RPMI + 5% FBS, resuspended in complete RPMI (cRPMI; RPMI 1640, 10% FBS (Atlas Biologicals, Ft. Collins, CO), 0.01M HEPES, 0.1nM non-essential amino acids, 1mM sodium pyruvate, 5×105M β-mercaptoethanol (Sigma), 0.01% penicillin-streptomycin, and 2nM L-glutamine), then counted on a hemacytometer. Unless specified, all chemical were obtained from Invitrogen, Inc. (Carlsbad, CA).
B and T cell isolation
Magnetic microbeads (Miltenyi Biotec, Auburn, CA) were used to purify B and T cells from splenocyte suspensions. Briefly, splenocytes were washed with PBS then were resuspended in MACS buffer (consisting of PBS, 0.5% BSA and 2 mM EDTA; pH 7.2) with the appropriate dilution of magnetic beads specific for B cell (CD45R or B220) or T cell antigens (CD4). Bead-labeled cells were passed though a magnetic column then were washed 3x with MACS buffer. After removing the magnet, the column containing labeled cells was flushed with MACS buffer and total numbers of B or T cells were counted on a hemacytometer. Flow cytometry confirmed that our isolated B and T cell preparations were ~95% pure (see Fig. 3A&B).
Figure 3. Stimulation of glucocorticoid and β2AR receptors causes apoptosis in purified T and B lymphocytes.
Naive B- and T-cells were isolated from whole splenocytes then were cultured in vitro for 24 hrs with the appropriate drugs. Percent apoptosis was determined using AO/EB staining. Suspensions of B- and T-cells were confirmed to be ~95% pure via flow cytometry (A and B, respectively). Isotype-matched control antibodies were used to delineate non-specific staining (white peaks, A&B). Unlike mixed splenocyte cultures (see Fig. 2), CORT or terbutaline (Terb) were able to induce apoptosis in purified B and T cells. However, the synergistic induction of apoptosis by CORT+Terb was prominent in B cells (C) (***p<0.001,**p<0.01, *p<0.05 vs. media control and vs. CORT or Terb alone; n=4/group). This can be explained by a higher baseline of apoptosis in T cells with CORT or Terb alone (D) (***p<0.001 vs. media control). All data analyzed via ANOVA with Tukey’s Post-test.
Cell culture
To mimic the corticosterone (CORT) and NE surges that occur after a high-level SCI, we used hydrocortisone and terbutaline, respectively. Hydrocortisone was used in our studies because it is a water-soluble derivative of CORT making it ideal for in vitro studies. Terbutaline is a widely used NE analogue and selective β2AR agonist. Order-of-potency studies, using immune cell function as an endpoint readout, have shown that NE and terbutaline activate lymphocyte β2ARs with similar potency (Sanders & Munson 1984). All cells were cultured in cRPMI at 37ºC (5% CO2) and supplemented with 10µg/mL LPS and 4µg/mL concanavalin A. When used in vitro, RU486, CORT (i.e., hydrocortisone), and Rolipram were first dissolved in ethanol alone and then diluted in PBS. Dibutyryl cAMP and terbutaline were prepared in PBS only. All drugs were obtained from Sigma.
Cytofluorometric analysis
Flow cytometry (FACScalibur; Becton-Dickinson, San Jose, CA) was used to confirm the purity of splenocyte preparations and to assess apoptosis. Isolated B or T cell suspensions (5×105 cells/sample) were analyzed separately. All samples were incubated for 15 minutes with 0.25 μg Fc-block (anti-mouse CD16/CD32) then labeled for B cells (CD19-PE; 1µg) or T cells (CD3-APC; 0.25µg). Isotype-matched control antibodies at equal concentrations were used to delineate non-specific staining. Apoptosis was quantified using Annexin-V-FITC according to the manufacture’s protocol (BD Pharmingen; Franklin Lakes, NJ). At least 10,000 events were collected per sample. Fc-block was obtained from BD Pharmingen while all other antibodies were obtained from eBioscience (San Diego, CA).
Analysis of Apoptosis
Acridine Orange and Ethidium Bromide (AO/EB)
For AO/EB counting of apoptotic cells, 5×105 cells were plated in cRPMI. Cells were exposed to the appropriate treatment for 24hrs then were pelleted at 500×g for 5 mins. Supernatants were dumped and cells were washed with 1mL of cold PBS. Cells were pelleted again, supernatants dumped and cells were resuspended in 25µL PBS and 1µL AO/EB (100µg/mL each). After incubating cells in the dark for 10 mins at room temperature, a 10µL aliquot was placed on a glass slide, coverslipped and analyzed on an Axioplan 2 imaging system equipped with a UV filter (Carl Zeiss Inc., Thornwood, NY). 100 cells per slide were counted in random fields using a 40x objective. Apoptotic cells were defined as having a condensed or fragmented nucleus and/or exhibiting membrane blebbing (see Fig. 2A).
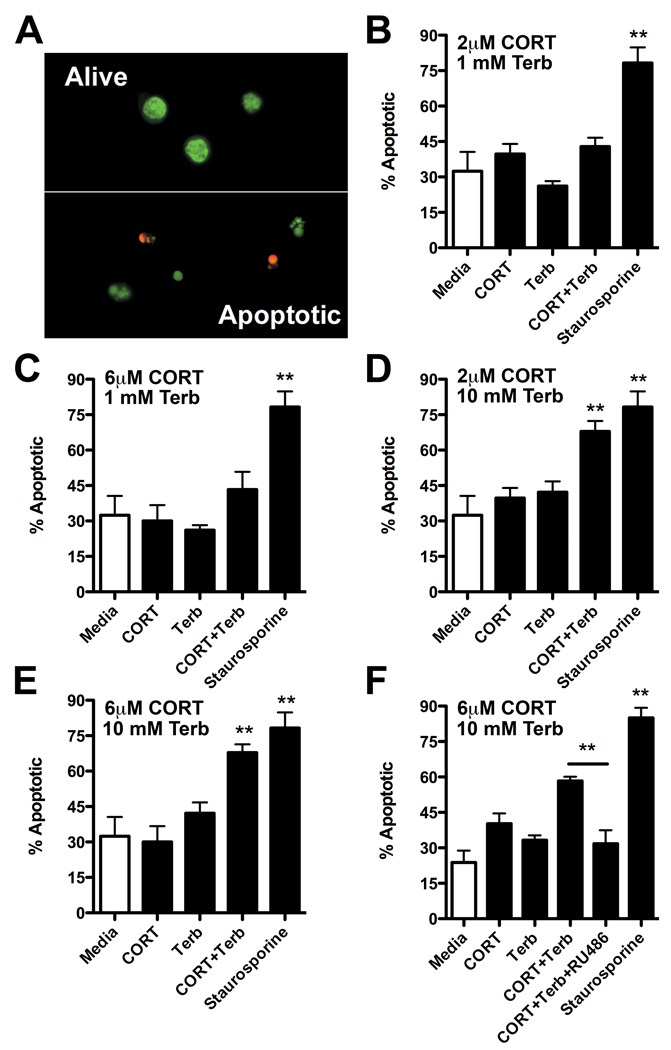
Figure 2. Acridine orange and ethidium bromide (AO/EB) staining reveal enhanced splenocyte apoptosis following activation of glucocorticoid and β2AR receptors.
(A) A representative image of AO/EB-stained lymphocytes. Apoptotic cells are easily distinguished from live cells based on the appearance of condensed and/or fragmented nuclei. (B–E) Apoptosis was quantified in naive splenocytes cultured in vitro for 24hrs with the varying combinations and concentrations of CORT and terbutaline (Terb); **p<0.01 vs. media control or CORT or Terb alone; n=4/group. (F) Induction of apoptosis by CORT+NE synergy was reversed by co-incubating with the glucocorticoid receptor antagonist, RU486 (**p<0.01 vs. CORT+Terb). Staurosporine (1µM) was used as a positive apoptotic control stimulus (B–F). All were analyzed using one-way ANOVA with Tukey’s Post-test.
DNA laddering
To confirm our AO/EB data, DNA fragmentation, a hallmark of apoptosis was analyzed in splenocytes treated with NE, CORT or NE+CORT. 5×105 splenocytes were plated with the appropriate treatment for 24hrs and were then centrifuged at 200×g at 4ºC for 10 mins. The cell pellets were resuspended and permeabilized with TTE buffer (10mM Tris, 0.2% Triton X-100, and 1mM EDTA). To separate fragmented DNA from intact chromatin, cells were centrifuged at 20,000×g for 10 mins at 4ºC. Cell supernatants containing fragmented DNA were collected and treated with 5M NaCl to dissociate histones from DNA. DNA was precipitated overnight at −20ºC with isopropanol and recovered by centrifuging (20,000×g, 10mins @ 4ºC). Supernatants were discarded and the DNA pellet was washed with 70% ethanol. DNA pellets were air dried for ~4hrs. DNA was dissolved in 20µL TE buffer and incubated at 37ºC overnight. DNA was mixed with gel loading solution (Sigma), heated at 65ºC for 10 mins then was added to a 1% agarose gel containing 0.5µg/mL ethidium bromide. Samples were run at 70 volts for 60 mins. Fragmented DNA segments were visualized on a UV trans-illuminator (Fotodyne Inc., Hartland, WI).
Quantitative real-time PCR
For mRNA analysis, 3×106 cells were plated with the appropriate drug treatment for ~6hrs. RNA was then purified from cells using Trizol (Invitrogen) and quantified by spectrophotometry. Resultant RNA was DNase-treated (Invitrogen) and reverse transcribed into cDNA using MULV DNA polymerase and DNA nucleotides (Applied Biosystems, Foster City, CA). Gene-specific primer pairs were used to detect mRNA expression via quantitative real-time PCR (Q-RT-PCR). Primer sequence specificity was confirmed via BLAST analysis for highly similar sequences against known sequence databases. Briefly, 20µL PCR reactions were performed using 10ng of cDNA, 500nmol/L of each primer and SYBR Green master mix (Applied Biosystems). Standard curves were generated for each gene using a control cDNA dilution series. Melting point analyses for each reaction confirmed a single amplified product. β-actin served as an internal control to ensure the efficiency of reverse transcription and to normalize for the concentration of cDNA used in each real-time PCR reaction. The following primers were used for β-actin: forward primer, 5'-TACAGCTTCACCACCACAGC-3' and reverse primer, 5'-AAGGAAGGCTGGAAAAGAGC-3'; β2AR: forward primer, 5'-ACTTCCTTAGGGATGAGGTTGTCC-3' and reverse primer, 5'-TTGCCTATCCAGATGCACTGGTAC -3' (Sanders et al. 2003); Bim: forward primer, 5'- CGACAGTCTCAGGAGGAACC -3' and reverse primer, 5'-CCTTCTCCATACCAGACGGA-3' (Zhang & Insel 2004). All primers were synthesized and purchased from Integrated DNA Technologies (Coralville, IA).
cAMP assay
cAMP was analyzed using a fluorescence polarization Biotrak Immunoassay system (Amersham Biosciences, Piscataway, NJ) per the manufacturer’s specifications. Briefly, 5×105 splenocytes were cultured for ~24hrs as described above (±CORT). After 24hrs, terbutaline was added and splenocytes were incubated for an additional 5hrs. Cells were washed with PBS, lysed with a proprietary lysis buffer and were incubated overnight in a humidity chamber (at room temperature protected from light) with rabbit anti-cAMP serum and a Cy3B cAMP fluorescent conjugate. Fluorescent cAMP conjugates that bound rabbit antibodies could be displaced by endogenous cAMP in the splenocyte lysates. Thus, the amount of unbound fluorescent conjugate acted as a surrogate marker for the levels of endogenous cAMP. Bound vs. unbound fluorescent cAMP conjugates were quantified by fluorescence polarization (525nm/580nm) using a SpectraMax M5 fluorescence plate reader (Molecular Devices, Sunnyvale, CA) based on a standard curve of known amounts of cAMP.
Western blot
3×106 splenocytes were homogenized in 200µL Mammalian cell Protein Extraction Reagent (M-PER™; Pierce, Rockford, IL) and 5µL Halt™ Protease Inhibitor cocktail (Pierce). The resulting homogenate was centrifuged for 5 mins at 4500×g and supernatants were transferred into fresh tubes for protein quantification. Protein concentrations were determined using Coomassie Plus™ Protein Assay Kit (Pierce). 15µg of protein was added to 6.25µL NuPAGE™ LDS sample buffer with 5% beta-mercaptoethanol and brought up to a volume of 25µL with H2O. Samples were heated at 95°C for 5 minutes. Samples were loaded and run on NuPAGE™ 4–12% Bis-Tris gels. Gels were run at 200V for ~40 min after which proteins were transferred to immobilon-P membranes (Millipore, Medford, MA) at 30V for 90 mins. Membranes were blocked with 5% milk and 0.5% Tween for 1 hr at RT then hybridized with antibodies against Bim (1:1000 @ 4°C overnight; BD Pharmingen, San Jose, CA). Antibodies against α-tubulin (1:2000; Sigma) were used to ensure consistent protein loading. Goat anti-mouse HRP and goat anti-rabbit HRP (Sigma; 1:5000) were used as secondary antibodies for α-tubulin and Bim, respectively. Blots were developed using Pierce WestPico chemiluminescent substrate kit and visualized on Kodak Biomax film (Rochester, NY).
Statistical analyses
All data are expressed as mean ± SEM. Group means were compared using ANOVA with Tukey’s post-test or, when applicable, an unpaired t-test. Significance for all analyses was set at p<0.05. All tests were performed using GraphPad Prism version 4.03 (GraphPad Software, San Diego, CA, USA).
Results
CORT and β2AR stimulation induce lymphocyte apoptosis in vitro
Previously, we showed that T3 SCI causes CORT and NE to increase in the blood and spleen, respectively, and that these changes in stress hormones are associated with splenic atrophy and increased levels of intrasplenic caspase-3, an effector of cellular apoptosis (Lucin et al. 2007). Also, humoral (antibody-mediated) immunity was significantly impaired in T3 SCI mice but could be restored using butoxamine, a selective β2AR inhibitor. Collectively, these data implicate stress hormones as a critical regulator of immune cell function and survival after a high level SCI. Indeed, although physiological levels of CORT and NE are important for regulating glucose availability and other cellular functions, when leukocytes are exposed to stress hormones for prolonged periods or at supraphysiological concentrations, like those found after SCI, cell death may ensue (Del Rey et al. 2003). This prompted us to ask whether the acute reduction in splenocytes after SCI was a result of NE and/or CORT initiating cell death cascades.
To test this hypothesis, mixed splenocyte preparations were exposed to hydrocortisone and terbutaline, i.e., analogues of CORT and NE, respectively (see Methods for rationale). Importantly, lymphocytes were exposed to 2–6µM of hydrocortisone or 1–10mM of terbutaline, concentrations that approximate the levels of CORT and NE found in vivo after T3 SCI. After SCI, circulating CORT levels rise reaching concentrations up to ~ 700ng/mL (~2µM; see Lucin et al. 2007). To investigate dose-response effects of hydrocortisone, higher concentrations (6µM) also were used as described in independent reports (Eisen et al. 1973).
To mimic the concentrations of NE that splenic lymphocytes are exposed to in vivo, we used 1–10mM terbutaline. Indeed, changes in circulating NE are likely to be irrelevant since NE is rapidly degraded in the blood with a half-life of ~1s (Capella et al. 1993). In contrast, splenic lymphocytes are closely apposed to sympathetic nerve terminals and as NE is released, it acts locally before it is recycled back into the nerve terminals (Felten et al. 1987). Neurotransmitter concentrations in nerve terminals are predicted to be ~60–210mM (Riveros et al. 1986, Shupliakov et al. 1992) with synaptic concentrations reaching ~10mM (Clements et al. 1992, Vizi & Kiss 1998). Therefore, in SCI mice, splenic lymphocytes could easily be exposed to concentrations of NE reaching or exceeding ~10mM.
We first tested whether terbutaline and/or hydrocortisone were capable of inducing apoptosis in a mixed splenocyte population. Flow cytometry for Annexin V suggested that when combined, hydrocortisone and terbutaline synergized to cause lymphocyte apoptosis (Fig. 1A). This finding was confirmed with DNA laddering (Fig. 1B). Next, we stained cells with acridine orange and ethidium bromide (AO/EB) and confirmed the ability of a range of concentrations of hydrocortisone and terbutaline to induce apoptosis. In contrast to flow cytometry or DNA laddering analysis, microscopic analysis of AO/EB-stained cells is fast and inexpensive and can be used to quickly and reliability distinguish between live and apoptotic cells. Indeed, condensed or fragmented nuclei, membrane blebbing or increased membrane permeability to EB were clearly visible in apoptotic cells (Fig. 2A). Thus, AO/EB is an ideal tool for rapidly screening different concentrations of terbutaline and hydrocortisone (Fig. 2B–E). As seen in Fig. 2, when given alone, neither hydrocortisone nor terbutaline adversely affected splenocyte survival. However, when combined, they induced significant apoptosis (Fig. 2D&E; p<0.01). When RU486, a GR antagonist, was combined with hydrocortisone and terbutaline, their ability to induce apoptosis was abolished (Fig. 2F).
Figure 1. Stimulation via glucocorticoid and β2AR receptors synergizes to induce splenocyte apoptosis in vitro.
Naive splenocytes were exposed to increasing concentrations of terbutaline (β2AR agonist) for 24hrs in the presence (open circles; 2µM) or absence (closed circles; 0µM) hydrocortisone (referred to throughout this and subsequent figures as CORT. (A) Apoptosis was analyzed using flow cytometry (Annexin V staining) or (B) DNA laddering. Numbers displayed on the DNA laddering image correspond with the following stimulation conditions: 1:Media; 2:2µM CORT; 3:1mM Terb; 4:10mM Terb; 5:2µM CORT+1mM Terb; 6:2µM CORT+10mM Terb; 7:Staurosporine.
CORT and NE synergize to cause B- and T-cell apoptosis in vitro
Data in Figure 1&Figure 2 reveal the cytotoxic effect of simultaneously stimulating GRs and β2ARs in mixed splenocyte preparations consisting of T and B cells, dendritic cells and macrophages. We previously showed that each of these leukocyte subsets is adversely affected by SCI with B cells being most affected (Lucin et al. 2007). To determine if post-SCI elevations of CORT and NE could explain the massive loss of B cells and the corresponding loss of antibody synthesis that we observed previously after T3 SCI, we repeated the above experiments using purified B and T cells (97% and 93% pure; Fig. 3A,B, respectively).
Unlike whole splenocytes, apoptosis was induced in purified B and T cells following exposure to either hydrocortisone or terbutaline (Fig. 3C&D). However, when hydrocortisone and terbutaline were combined, apoptosis was significantly increased in B cells (Fig. 3C; p<0.001 vs. CORT or Terb alone). The lack of synergistic apoptotic signaling in T cells could be explained by the higher level of apoptosis induced in these cells by hydrocortisone or terbutaline alone (Fig. 3D).
CORT increases β2AR expression and cAMP accumulation in lymphocytes
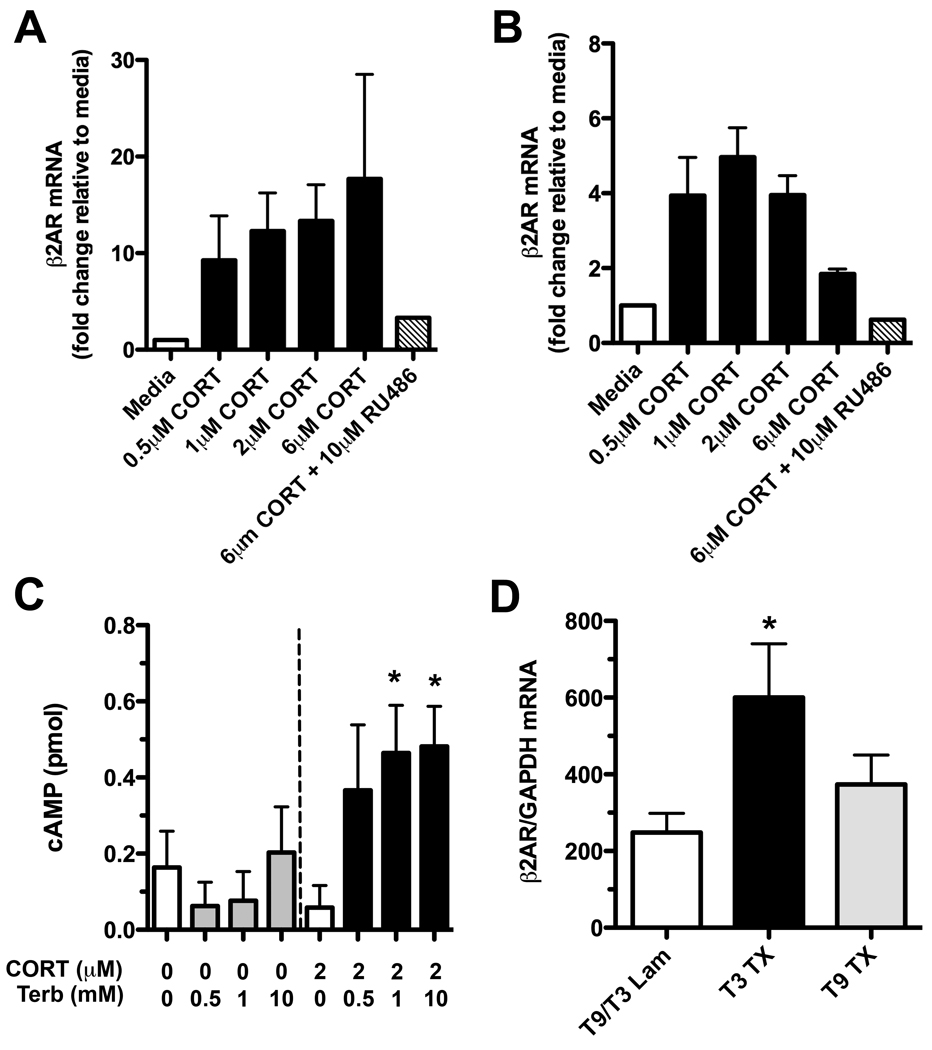
Clearly, the parallel activation of GRs and β2ARs causes apoptosis in mature lymphocytes. A number of possible mechanisms could explain this synergy. For example, the β2AR gene has several GC response elements (Nakada et al. 1989) at which CORT could act to enhance β2AR expression and affinity while simultaneously preventing downregulation (Davies & Lefkowitz 1981, Hadcock & Malbon 1988, Mak et al. 1995a, Mak et al. 1995b). Glucocorticoids also enhance β2AR-mediated induction of cAMP signaling (Abraham et al. 2003, Parker et al. 1973). These interactions have been primarily described in neutrophils and lung parenchymal cells but not in lymphocytes. To determine if similar interactions exist in B cells, we exposed purified naïve B220+ B cells to increasing concentrations of hydrocortisone. In the presence of hydrocortisone, β2AR mRNA expression was increased ~20-fold (Fig. 4A). In contrast, the non-B cell fraction of purified cells (~82% CD3+ T cells) were less responsive to hydrocortisone with β2AR mRNA expression increasing ~5-fold but only at low hydrocortisone concentrations (Fig. 4B). In both cell populations, changes in β2AR mRNA expression were dependent on GC signaling since co-administering RU486 blocked the effect (Fig. 4A&B).
Figure 4. CORT increases β2AR expression in lymphocytes after T3 SCI.
(A) Naïve B220+ B cells or (B) B220− cells were incubated in vitro with varying concentrations of CORT for 6hrs. CORT increased β2AR mRNA expression ~20-fold and ~5-fold, respectively. This could be reversed by antagonizing GRs (representative of n=2 independent experiments; RNA pooled from triplicate wells). (C) Using naive splenocytes treated with or without CORT (24 hr pre-treatment) and subsequently stimulated with terbutaline (5hrs before cell harvest), it was confirmed that CORT increases functional β2ARs on lymphocytes. Note the increased intracellular concentrations of cAMP in Terb-stimulated cells that were pre-treated with 2µM CORT; *p<0.05 vs. CORT alone, n=4/group. (D) β2AR mRNA expression is increased in vivo on B cells after T3 but not a T9 SCI (3 days post injury); *p<0.05 vs. laminectomy (Lam) control and T9 SCI. Data were analyzed using a one-way ANOVA with Tukey’s Post-test.
To verify the functional significance of these mRNA changes, we measured intracellular cAMP in splenocytes that were first cultured with/without hydrocortisone then were subsequently stimulated via β2ARs. In cells pre-treated with hydrocortisone, β2AR signaling was markedly enhanced as indicated by elevated intracellular cAMP (Fig. 4C; p<0.05 vs. CORT only).
Based on the above data and our past data showing that circulating CORT and splenic NE are increased only after a T3 SCI (but not mid-thoracic SCI; Lucin et al. 2007), we predicted that only T3 SCI would induce β2AR expression in lymphocytes. Real-time PCR analysis of purified B cell RNA confirmed this hypothesis and showed that β2AR mRNA expression was only increased in cells isolated from T3 SCI mice (Fig. 4D; p<0.05 vs. T3 and T9 lam).
Elevated levels of cAMP do not induce lymphocyte apoptosis
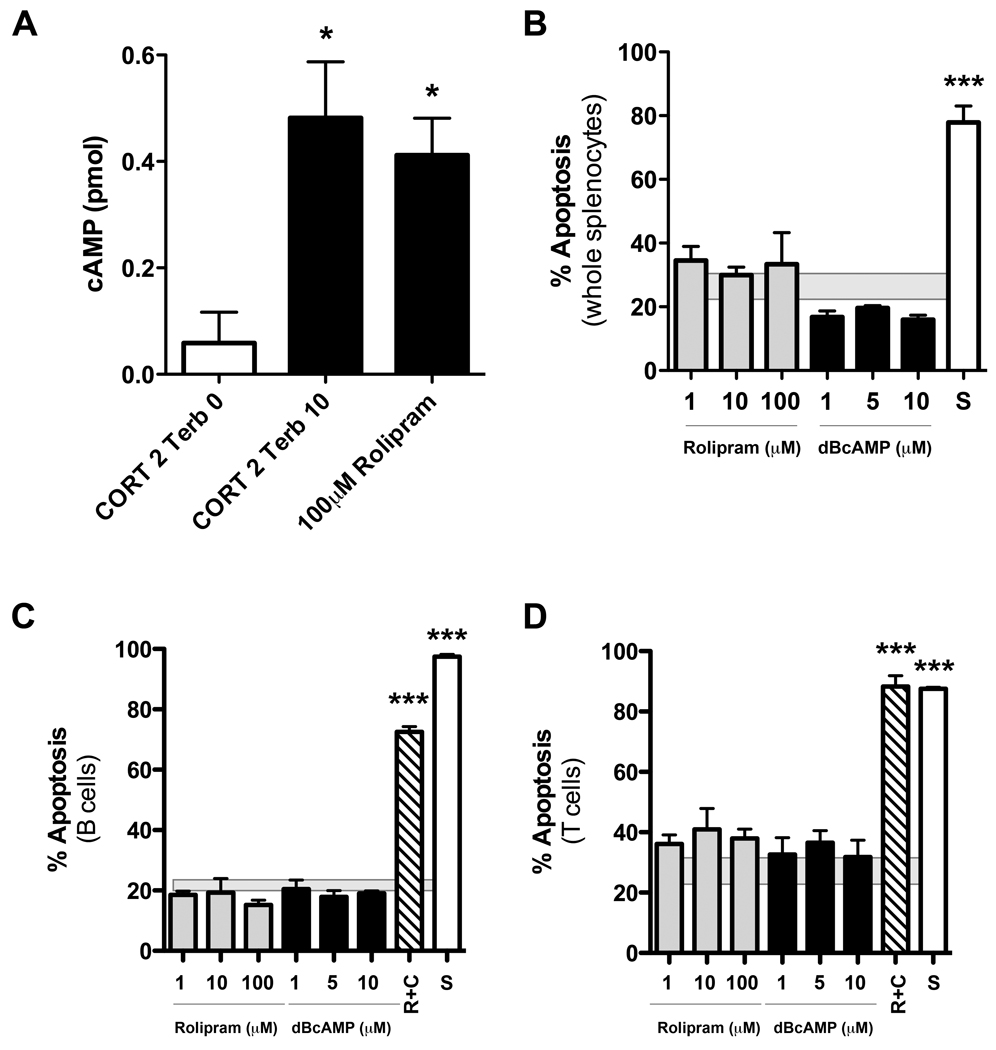
After a T3 SCI, increased expression of β2ARs on B cells could render them “hypersensitive” to stimulation by the supraphysiological levels of NE that exist in the lymphoid tissue (del Rey et al. 2006, Marra & Hoffman-Goetz 2004). Subsequent increases in intracellular cAMP signaling could then trigger growth arrest and/or apoptosis (Zhang & Insel 2004). To determine if high levels of intracellular cAMP were responsible for apoptosis of lymphocytes by hydrocortisone and terbutaline synergy (see Fig. 2&Fig. 3), we cultured naïve splenocytes in the presence of different concentrations of Rolipram, an inhibitor of the cAMP-degrading enzyme, phosphodiesterase-4. Rolipram markedly elevates intracellular cAMP to levels similar to those found in cells stimulated with hydrocortisone+terbutaline (Fig. 5A) but did not cause apoptosis (Fig. 5B). In separate cultures, addition of dibutyryl cAMP (dBcAMP), a soluble membrane permeable cAMP analogue, also failed to cause splenocyte apoptosis (Fig. 5B).
Figure 5. Elevation of intracellular cAMP is not sufficient to induce lymphocyte apoptosis.
(A) Rolipram elevates intracellular cAMP to the same extent as CORT+terbutaline but does not trigger lymphocyte apoptosis (see B–D). (B) Mixed splenocytes stimulated in vitro with increasing concentrations of Rolipram or dibutyryl cAMP (dBcAMP) for 24hrs fail to induce apoptosis. (C&D) Similarly, neither Rolipram nor dBcAMP was able to trigger apoptosis in purified B or T lymphocytes. Apoptosis was induced only when lymphocytes were simultaneously stimulated with 2µM CORT and Rolipram. Apoptosis was quantified in AO/EB-stained cells (see Fig. 2). 1µM staurosporine (S) acted as a positive control. For each graph, n=4/group from replicate experiments. All data were analyzed using one-way ANOVA with Tukey’s Post-test; *p<0.05 or ***p<0.001 vs. control stimulus; mean±SEM of apoptosis caused by media alone is represented by shaded box behind individual column data in B–D.
When we repeated these studies using purified B and T cells, neither Rolipram nor dBcAMP induced apoptosis (Fig. 5C&D). In contrast, stimulation of GRs in the presence of Rolipram caused apoptosis in ~80–90% of lymphocytes (Fig. 5C,D). Collectively, these data suggest that potent activation of β2ARs is not sufficient to induce apoptosis of lymphocytes. Instead, the simultaneous activation of β2ARs and GRs causes lymphocyte apoptosis.
CORT and NE synergize to induce the apoptotic protein Bim
Bcl-2 interacting mediator of cell death (Bim) is a recently described mediator of apoptosis that can be activated by GC and β2AR signaling (Wang et al. 2003, Zhang & Insel 2004). We tested whether induction of Bim in lymphocytes was dependent on synergistic signaling via β2ARs and GRs. Using naïve splenocytes, we found that when used alone, hydrocortisone or terbutaline had little effect on Bim mRNA expression. However, expression of Bim mRNA was significantly increased in lymphocytes exposed to both hydrocortisone and terbutaline (Fig. 6A; p<0.05 vs. media-stimulated cells). Co-incubating cells with the GR antagonist RU486 reduced this synergistic effect (Fig. 6A). Each of these effects was confirmed at the protein level using Western blot (Fig. 6B&C; p<0.01).
Figure 6. CORT and NE synergize to induce Bim expression in lymphocytes in vitro and in vivo after T3 SCI.
(A–C) The combination of CORT and terbutaline signaling induces mRNA (A) and protein (B) expression of the pro-apoptotic molecule, Bim in splenocytes. This effect was blocked by co-treatment with the glucocorticoid receptor antagonist, RU486 (A&C). (D) In vivo following a T3 SCI (3 days post injury), Bim was induced in splenocytes. This induction was inhibited by the combined administration of β2AR and glucocorticoid receptor antagonists (i.e., butoxamine and RU486, respectively). Western blots in B&C show Bim EL isoform and are representative of two independent experiments. n=4/group; *p<0.05 or **p<0.01 vs. media-stimulated (A–C) or sham-injured control (D); One-way ANOVA with Tukey’s Post-test.
GR and β2AR antagonists block Bim induction and lymphocyte apoptosis after T3 SCI
We next tested whether lymphocyte apoptosis that occurs after T3 SCI was associated with an increase in Bim mRNA. Real-time PCR analysis of splenocyte mRNA isolated from T3 SCI mice revealed marked induction of Bim (Fig. 6D; p<0.05 vs. T3 laminectomy control). This could be inhibited by pre-treating mice with RU486 and butoxamine prior to SCI suggesting that apoptotic signaling in lymphocytes is mediated via GR and β2AR signaling (Fig. 6D).
Since these drugs limit signaling via GRs and β2ARs with a concomitant reduction in Bim (see Fig. 4A&Fig. 6), we predicted they would reverse the splenic atrophy and splenic leucopenia caused by T3 SCI. Confirming our hypothesis we found that pre-injury injections of RU486 and butoxamine significantly attenuated splenic atrophy and partially blocked the loss of leukocytes in spleen (Fig. 7A–B). However, when used alone, β2AR or GR antagonists failed to significantly restore splenocyte numbers. Importantly, splenic lymphopenia after T3 SCI is not associated with enhanced lymphocyte migration from spleen into the blood (as determined by circulating leukocyte numbers), suggesting the loss of splenic leukocytes after T3 SCI cannot be explained by cellular efflux (Fig. 7C). In parallel with the spleen, pre-injury injections of RU486 and butoxamine partially block the loss of circulating leukocytes after T3 SCI (Fig. 7C).
Figure 7. The induction of splenic leukocyte apoptosis after T3 SCI is attenuated using antagonists of GRs and β2ARs.
(A–C) Spleen weight (A), total numbers of splenocytes and circulating white blood cell counts (B&C, respectively) are reduced after T3 SCI (3 days post injury). These SCI-specific effects were partially reversed by co-administration of butoxamine (Butox; a selective β2AR antagonist) and RU486 (a GR antagonist). n=5/group; ***p<0.001, **p<0.01, *p<0.05 as indicated in A&B or vs. laminectomy control in C; One-way ANOVA with Tukey’s Post-test.
Discussion
Previously, we showed that high thoracic (T3) SCI causes marked splenic atrophy with a parallel increase in intrasplenic caspase-3. These changes were accompanied by an increase in circulating corticosterone (CORT) and splenic norepinephrine (NE) and suppressed immune function (Lucin et al. 2007). In the present study we provide an explanation for these changes. Namely, post-SCI elevations of CORT and NE synergize to increase apoptosis of leukocytes in general and B cells in particular. Enhanced apoptosis is associated with the induction of Bim, a BH-3-only protein that is important for inducing apoptosis. These effects can be inhibited using antagonists of GRs and β2ARs, a strategy that we previously showed was effective in restoring humoral immune function after T3 SCI (Lucin et al. 2007).
These data provide a unique perspective on how to reverse immune deficits caused by severe CNS injury. Indeed, the HPA axis and SNS become over-activated or are inappropriately regulated after traumatic brain injury and stroke (Offner et al. 2006, Prass et al. 2003), mimicking the consequences of major systemic stress. For example, burn injury (Fukuzuka et al. 2000, Maekawa et al. 2002), hemorrhagic shock (Oberbeck et al. 2002) and intense endurance training (Mars et al. 1998, Mooren et al. 2002) trigger aberrant activation of the HPA/SNS causing severe immune suppression and lymphocyte apoptosis. Based on our present data, we predict that in each case, immune suppression is caused by the convergence of GC and catecholamine signaling in leukocytes with subsequent induction of Bim and apoptosis.
Increased expression of Bim mRNA has been described in several models of GC-induced apoptosis (Wang et al. 2003). More recently, Bim was found to be elevated in a synergistic manner by GCs and NE in T-lymphoma cells (Zhang & Insel 2004). Here we extend those observations and show that synergistic signaling via GRs and β2ARs elevates Bim and promotes apoptosis in mature primary lymphocytes. Since all immune cells express GRs and nearly all express β2ARs, the convergence of these signaling pathways may also explain why innate immune function is impaired in people with high-level SCI (Campagnolo et al. 2000).
While our data provide a mechanism to explain how/why lymphocytes undergo apoptosis after severe CNS injury, the advantage of this apparently conserved physiological response is not immediately apparent. In fact, intuitively one would predict that suppression of immune function after any injury would be an unfavorable response for the host. It is possible that this has evolved as a mechanism to prevent hyperactivation of the immune system. In response to trauma or infection, the HPA axis and SNS are activated in parallel with the release of pro-inflammatory cytokines and acute phase proteins (Maier et al. 1998). This systemic inflammatory response syndrome (SIRS), if left unchecked, can exacerbate pathology or cause death. The role of stress hormones in limiting immune hyperactivation is evident in adrenalectomized animals where sub-lethal doses of endotoxin become lethal (Kapcala et al. 1995). Similarly, adrenalectomized mice remain efficient at clearing virus but experience exaggerated TNF levels and increased lethality without endogenous glucocorticoids (Ruzek et al. 1999). This is why diseases associated with GC deficiency (e.g., Addison’s disease or pituitary ACTH deficiency) require GC replacement therapy during episodes of fever, infection or inflammatory stress (Kapcala et al. 1995).
It is also possible that trauma-induced activation of the HPA/SNS axis helps prevent pathological autoimmune reactions. In humans and rodents, spinal trauma, TBI and stroke can activate autoreactive T and B cells (Ankeny et al. 2006, Becker et al. 2005, Jones et al. 2002, Kil et al. 1999). However, in most cases, these responses fail to cause clinically evident autoimmune pathology. Signaling via GRs and β2ARs may limit lymphocyte proliferation and expansion. Indeed, GCs cause apoptosis of myelin-reactive T cells and limit neuropathology in experimental autoimmune encephalomyelitis (EAE), a model of multiple sclerosis (McCombe et al. 1996, Nguyen et al. 1997). Moreover, minimal T cell apoptosis occurs in adrenalectomized rats with EAE. These rats experience a more rapid disease onset with increased mortality when compared with animals with an intact HPA axis (Smith et al. 1996). GR/β2AR signaling may also be important for regulating lymphocyte apoptosis in the context of other autoimmune diseases (Del Rey et al. 2003). Individuals with rheumatoid arthritis express fewer β2ARs on their B cells and exhibit corresponding impairments in β2AR signaling (Wahle et al. 2001). As a result, their B cells are less responsive to catecholamine-induced apoptosis (Wahle et al. 2002). Also, β2AR polymorphisms are associated with an increased incidence of rheumatoid arthritis, a cell-mediated autoimmune disease (Xu et al. 2005). Interestingly, in individuals with systemic lupus erythematosus (SLE) and systemic sclerosis, β2AR expression and signaling are impaired in B cells (Wahle et al. 2001). Independent studies suggest that this may be a result of decreased function in the HPA axis of individuals with SLE (Harle et al. 2006). Indeed, despite evidence of enhanced sympathetic outflow in SLE patients (as measured by neuropeptide Y), the same individuals had low levels of circulating ACTH and cortisol (Harle et al. 2006).
Whether GC/β2AR signaling induces apoptosis in lymphocytes may depend on the maturation state or the prior state of activation of a given cell. Indeed, sustained elevations of CORT can deplete developing cells of the B lymphocyte lineage in secondary lymphoid tissues (Garvy et al. 1993). However, once these cells mature and become activated, pro-survival proteins, including Bcl-xL, are increased and limit the effects of apoptotic stimuli (Fang et al. 1997). This suggests that newly formed or naive lymphocytes may be more sensitive to the stress response elicited by SCI than activated or memory lymphocytes. Still, in all of our in vitro studies, lymphocytes were activated with LPS or conA, potent B- or T-cell mitogens, respectively; yet, GCs and terbutaline still caused apoptosis. Thus, combined signaling through GC and β2ARs can apparently overcome pro-survival signaling in mature activated lymphocytes.
Our current data provide a mechanism to explain why immune suppression occurs after a high level SCI. Specifically, post-traumatic SCI increases circulating GCs and catecholamine (e.g., NE) concentrations in spleen (and presumably other lymphoid tissues) (Lucin et al. 2007). As lymphocytes are exposed to these stress hormones, β2AR expression is increased. This effectively reduces their threshold for activation via NE and provokes massive increases in intracellular cAMP with subsequent induction of Bim. Bim was previously found to be a convergence point for inducing apoptosis of cells by GCs and agents that elevate cAMP (Zhang & Insel 2004). Although our present data confirm this observation, we find that unlike transformed cell lines (e.g., S49 lymphoma cells) in which Bim expression and subsequent apoptosis can be induced by cAMP analogues or β-adrendergic agonists, simply elevating intracellular cAMP levels does not induce apoptosis in primary mouse lymphocytes. Instead, GRs and β2ARs must be activated in parallel to induce apoptosis. Also, our studies highlight the difference in apoptotic sensitivity between cells cultured in isolation or as a heterogeneous population directly isolated from lymphoid organs (compare Fig. 2 & Fig. 3). This disparity is likely due to the complex network of intercellular communication that exists in vivo, which acts to provide pro-survival signals to complementary cells (e.g., via CD40L or CD80/86).
Presently, we do not know how long the effects of lymphocyte apoptosis linger after high-level SCI. Based on rat and human SCI data, a similar decline in lymphocyte numbers is observed within 24 hrs and persists up to one week post-injury (Riegger et al. 2007, Riegger et al. 2008, Furlan et al. 2006). Even if immune function is restored as the lymphoid organs spontaneously repopulate, it is likely that functionally significant levels of lymphocyte apoptosis will be continuously induced throughout the lifetime of a quadriplegic or high-level paraplegic. Indeed, episodic catecholamine “storms” and spikes in circulating GCs will be triggered repeatedly as a consequence of recurrent bouts of autonomic dysreflexia (AD). AD is a life-long problem for most people with a SCI above the T5–6 spinal level. We predict that the episodic and recurrent onset of AD with subsequent suppression of immune function (due to lymphocyte apoptosis) may explain why these individuals are at high risk for recurrent infections throughout their lifetime.
Annual flu vaccines are recommended by the US Centers for Disease Control and Prevention for SCI individuals (Goldstein et al. 2005). Also, therapeutic myelin vaccines have been shown to promote axon regeneration in rodents with mid-thoracic SCI (Huang et al. 1999). Since the efficacy of any vaccine is dependent on an intact and functional immune system, it is unlikely that these vaccines will consistently elicit the intended therapeutic benefits in individuals with high-level SCI, stroke or traumatic brain injury (TBI) where immune function is suppressed. Based on our present data, it is practical to consider using β2AR or GR antagonists (e.g., beta-blockers and Mifepristone) as adjunct therapies for enhancing immune function in a subpopulation of SCI, stroke or TBI patients.
Acknowledgments
The authors thank Zhen Guan, Ming Wang, Pat Walters, Daniel Ankeny, and Kristina Kigerl for their technical assistance. The authors also thank Jessica Alexander for her critical review. Funding was provided by NIH T32 AI55411 (KML), NIH AI37326 (VMS), and NIH NS047175 (PGP). The authors of this manuscript have no conflicts of interest.
Abbreviations Used
- ACTH
adrenocorticotropic hormone
- AD
autonomic dysreflexia
- AO/EB
acridine orange/ethidium bromide
- β2AR
β2-adrenergic receptors
- Bim
Bcl-2 interacting mediator of cell death
- CIDS
CNS injury-induced immunodepression
- CORT
corticosterone/hydrocortisone
- cRPMI
complete RPMI media
- GC
glucocorticoid
- GR
glucocorticoid receptor
- HPA
hypothalamic-pituitary-adrenal
- LAM
laminectomy
- MAG
myelin-associated glycoprotein
- NE
norepinephrine
- SCI
spinal cord injury
- SIRS
systemic inflammatory response syndrome
- SNS
sympathetic nervous system
- T3
spinal cord injury at thoracic level 3
- TNF
tumor necrosis factor
References
- Abraham G, Schusser GF, Ungemach FR. Dexamethasone-induced increase in lymphocyte beta-adrenergic receptor density and cAMP formation in vivo. Pharmacology. 2003;67:1–5. doi: 10.1159/000066787. [DOI] [PubMed] [Google Scholar]
- Ankeny DP, Lucin KM, Sanders VM, McGaughy VM, Popovich PG. Spinal cord injury triggers systemic autoimmunity: evidence for chronic B lymphocyte activation and lupus-like autoantibody synthesis. J Neurochem. 2006;99:1073–1087. doi: 10.1111/j.1471-4159.2006.04147.x. [DOI] [PubMed] [Google Scholar]
- Becker KJ, Kindrick DL, Lester MP, Shea C, Ye ZC. Sensitization to brain antigens after stroke is augmented by lipopolysaccharide. J Cereb Blood Flow Metab. 2005;25:1634–1644. doi: 10.1038/sj.jcbfm.9600160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagnolo DI, Bartlett JA, Keller SE. Influence of neurological level on immune function following spinal cord injury: a review. J Spinal Cord Med. 2000;23:121–128. doi: 10.1080/10790268.2000.11753519. [DOI] [PubMed] [Google Scholar]
- Capella P, Ghasemzadeh MB, Adams RN, Wiedemann DJ, Wightman RM. Real-time monitoring of electrically stimulated norepinephrine release in rat thalamus: II. Modeling of release and reuptake characteristics of stimulated norepinephrine overflow. J Neurochem. 1993;60:449–453. doi: 10.1111/j.1471-4159.1993.tb03171.x. [DOI] [PubMed] [Google Scholar]
- Clements JD, Lester RA, Tong G, Jahr CE, Westbrook GL. The time course of glutamate in the synaptic cleft. Science. 1992;258:1498–1501. doi: 10.1126/science.1359647. [DOI] [PubMed] [Google Scholar]
- Cruse JM, Lewis RE, Jr, Bishop GR, Kliesch WF, Gaitan E, Britt R. Decreased immune reactivity and neuroendocrine alterations related to chronic stress in spinal cord injury and stroke patients. Pathobiology. 1993;61:183–192. doi: 10.1159/000163790. [DOI] [PubMed] [Google Scholar]
- Davies AO, Lefkowitz RJ. Agonist-promoted high affinity state of the beta-adrenergic receptor in human neutrophils: modulation by corticosteroids. J Clin Endocrinol Metab. 1981;53:703–708. doi: 10.1210/jcem-53-4-703. [DOI] [PubMed] [Google Scholar]
- Del Rey A, Kabiersch A, Petzoldt S, Besedovsky HO. Sympathetic abnormalities during autoimmune processes: potential relevance of noradrenaline-induced apoptosis. Ann N Y Acad Sci. 2003;992:158–167. doi: 10.1111/j.1749-6632.2003.tb03146.x. [DOI] [PubMed] [Google Scholar]
- del Rey A, Roggero E, Kabiersch A, Schafer M, Besedovsky HO. The role of noradrenergic nerves in the development of the lymphoproliferative disease in Fas-deficient, lpr/lpr mice. J Immunol. 2006;176:7079–7086. doi: 10.4049/jimmunol.176.11.7079. [DOI] [PubMed] [Google Scholar]
- DeVivo MJ, Kartus PL, Stover SL, Rutt RD, Fine PR. Cause of death for patients with spinal cord injuries. Arch Intern Med. 1989;149:1761–1766. [PubMed] [Google Scholar]
- Dong Y, Aronsson M, Gustafsson JA, Okret S. The mechanism of cAMP-induced glucocorticoid receptor expression. Correlation to cellular glucocorticoid response. J Biol Chem. 1989;264:13679–13683. [PubMed] [Google Scholar]
- Eisen HJ, Goldfine ID, Glinsmann WH. Regulation of hepatic glycogen synthesis during fetal development: roles of hydrocortisone, insulin, and insulin receptors. Proc Natl Acad Sci U S A. 1973;70:3454–3457. doi: 10.1073/pnas.70.12.3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang W, Nath KA, Mackey MF, Noelle RJ, Mueller DL, Behrens TW. CD40 inhibits B cell apoptosis by upregulating bcl-xL expression and blocking oxidant accumulation. Am J Physiol. 1997;272:C950–C956. doi: 10.1152/ajpcell.1997.272.3.C950. [DOI] [PubMed] [Google Scholar]
- Felten DL, Ackerman KD, Wiegand SJ, Felten SY. Noradrenergic sympathetic innervation of the spleen: I. Nerve fibers associate with lymphocytes and macrophages in specific compartments of the splenic white pulp. J Neurosci Res. 1987;18:28–36. 118–121. doi: 10.1002/jnr.490180107. [DOI] [PubMed] [Google Scholar]
- Fukuzuka K, Edwards CK, 3rd, Clare-Salzler M, Copeland EM, 3rd, Moldawer LL, Mozingo DW. Glucocorticoid-induced, caspase-dependent organ apoptosis early after burn injury. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1005–R1018. doi: 10.1152/ajpregu.2000.278.4.R1005. [DOI] [PubMed] [Google Scholar]
- Furlan JC, Krassioukov AV, Fehlings MG. Hematologic abnormalities within the first week after acute isolated traumatic cervical spinal cord injury: a case-control cohort study. Spine. 2006;31:2674–2683. doi: 10.1097/01.brs.0000244569.91204.01. [DOI] [PubMed] [Google Scholar]
- Garvy BA, King LE, Telford WG, Morford LA, Fraker PJ. Chronic elevation of plasma corticosterone causes reductions in the number of cycling cells of the B lineage in murine bone marrow and induces apoptosis. Immunology. 1993;80:587–592. [PMC free article] [PubMed] [Google Scholar]
- Goldstein B, Weaver FM, Hammond MC. New CDC recommendations: annual influenza vaccination recommended for individuals with spinal cord injuries. J Spinal Cord Med. 2005;28:383–384. doi: 10.1080/10790268.2005.11753837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadcock JR, Malbon CC. Regulation of beta-adrenergic receptors by "permissive" hormones: glucocorticoids increase steady-state levels of receptor mRNA. Proc Natl Acad Sci U S A. 1988;85:8415–8419. doi: 10.1073/pnas.85.22.8415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harle P, Straub RH, Wiest R, Mayer A, Scholmerich J, Atzeni F, Carrabba M, Cutolo M, Sarzi-Puttini P. Increase of sympathetic outflow measured by neuropeptide Y and decrease of the hypothalamic-pituitary-adrenal axis tone in patients with systemic lupus erythematosus and rheumatoid arthritis: another example of uncoupling of response systems. Ann Rheum Dis. 2006;65:51–56. doi: 10.1136/ard.2005.038059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DW, McKerracher L, Braun PE, David S. A therapeutic vaccine approach to stimulate axon regeneration in the adult mammalian spinal cord. Neuron. 1999;24:639–647. doi: 10.1016/s0896-6273(00)81118-6. [DOI] [PubMed] [Google Scholar]
- Jones TB, Basso DM, Sodhi A, Pan JZ, Hart RP, MacCallum RC, Lee S, Whitacre CC, Popovich PG. Pathological CNS autoimmune disease triggered by traumatic spinal cord injury: implications for autoimmune vaccine therapy. J Neurosci. 2002;22:2690–2700. doi: 10.1523/JNEUROSCI.22-07-02690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapcala LP, Chautard T, Eskay RL. The protective role of the hypothalamic-pituitary-adrenal axis against lethality produced by immune, infectious, and inflammatory stress. Ann N Y Acad Sci. 1995;771:419–437. doi: 10.1111/j.1749-6632.1995.tb44699.x. [DOI] [PubMed] [Google Scholar]
- Kil K, Zang YC, Yang D, Markowski J, Fuoco GS, Vendetti GC, Rivera VM, Zhang JZ. T cell responses to myelin basic protein in patients with spinal cord injury and multiple sclerosis. J Neuroimmunol. 1999;98:201–207. doi: 10.1016/s0165-5728(99)00057-0. [DOI] [PubMed] [Google Scholar]
- Lucin KM, Sanders VM, Jones TB, Malarkey WB, Popovich PG. Impaired antibody synthesis after spinal cord injury is level dependent and is due to sympathetic nervous system dysregulation. Exp Neurol. 2007;207:75–84. doi: 10.1016/j.expneurol.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa T, Kajihara H, Okabayashi K, Otani M, Yuge O. Impairment of splenic B and T lymphocytes in the early period after severe thermal injury: immunohistochemical and electron microscopic analysis. Burns. 2002;28:329–339. doi: 10.1016/s0305-4179(01)00104-8. [DOI] [PubMed] [Google Scholar]
- Maier SF, Goehler LE, Fleshner M, Watkins LR. The role of the vagus nerve in cytokine-to-brain communication. Ann N Y Acad Sci. 1998;840:289–300. doi: 10.1111/j.1749-6632.1998.tb09569.x. [DOI] [PubMed] [Google Scholar]
- Mak JC, Nishikawa M, Barnes PJ. Glucocorticosteroids increase beta 2-adrenergic receptor transcription in human lung. Am J Physiol. 1995a;268:L41–L46. doi: 10.1152/ajplung.1995.268.1.L41. [DOI] [PubMed] [Google Scholar]
- Mak JC, Nishikawa M, Shirasaki H, Miyayasu K, Barnes PJ. Protective effects of a glucocorticoid on downregulation of pulmonary beta 2-adrenergic receptors in vivo. J Clin Invest. 1995b;96:99–106. doi: 10.1172/JCI118084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra S, Hoffman-Goetz L. Beta-adrenergic receptor blockade during exercise decreases intestinal lymphocyte apoptosis but not cell loss in mice. Can J Physiol Pharmacol. 2004;82:465–473. doi: 10.1139/y04-072. [DOI] [PubMed] [Google Scholar]
- Mars M, Govender S, Weston A, Naicker V, Chuturgoon A. High intensity exercise: a cause of lymphocyte apoptosis? Biochem Biophys Res Commun. 1998;249:366–370. doi: 10.1006/bbrc.1998.9156. [DOI] [PubMed] [Google Scholar]
- McCombe PA, Nickson I, Tabi Z, Pender MP. Corticosteroid treatment of experimental autoimmune encephalomyelitis in the Lewis rat results in loss of V beta 8.2+ and myelin basic protein-reactive cells from the spinal cord, with increased total T-cell apoptosis but reduced apoptosis of V beta 8.2+ cells. J Neuroimmunol. 1996;70:93–101. doi: 10.1016/s0165-5728(96)00043-4. [DOI] [PubMed] [Google Scholar]
- Meisel C, Schwab JM, Prass K, Meisel A, Dirnagl U. Central nervous system injury-induced immune deficiency syndrome. Nat Rev Neurosci. 2005;6:775–786. doi: 10.1038/nrn1765. [DOI] [PubMed] [Google Scholar]
- Mooren FC, Bloming D, Lechtermann A, Lerch MM, Volker K. Lymphocyte apoptosis after exhaustive and moderate exercise. J Appl Physiol. 2002;93:147–153. doi: 10.1152/japplphysiol.01262.2001. [DOI] [PubMed] [Google Scholar]
- Nakada MT, Haskell KM, Ecker DJ, Stadel JM, Crooke ST. Genetic regulation of beta 2-adrenergic receptors in 3T3-L1 fibroblasts. Biochem J. 1989;260:53–59. doi: 10.1042/bj2600053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash MS. Known and plausible modulators of depressed immune functions following spinal cord injuries. J Spinal Cord Med. 2000;23:111–120. doi: 10.1080/10790268.2000.11753518. [DOI] [PubMed] [Google Scholar]
- Nguyen KB, McCombe PA, Pender MP. Increased apoptosis of T lymphocytes and macrophages in the central and peripheral nervous systems of Lewis rats with experimental autoimmune encephalomyelitis treated with dexamethasone. J Neuropathol Exp Neurol. 1997;56:58–69. doi: 10.1097/00005072-199701000-00006. [DOI] [PubMed] [Google Scholar]
- Oberbeck R, van Griensven M, Nickel E, Tschernig T, Wittwer T, Pape HC. Influence of beta-adrenoceptor antagonists on hemorrhage-induced cellular immune suppression. Shock. 2002;18:331–335. doi: 10.1097/00024382-200210000-00007. [DOI] [PubMed] [Google Scholar]
- Offner H, Subramanian S, Parker SM, Wang C, Afentoulis ME, Lewis A, Vandenbark AA, Hurn PD. Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. J Immunol. 2006;176:6523–6531. doi: 10.4049/jimmunol.176.11.6523. [DOI] [PubMed] [Google Scholar]
- Parker CW, Huber MG, Baumann ML. Alterations in cyclic AMP metabolism in human bronchial asthma. 3. Leukocyte and lymphocyte responses to steroids. J Clin Invest. 1973;52:1342–1348. doi: 10.1172/JCI107306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prass K, Meisel C, Hoflich C, et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med. 2003;198:725–736. doi: 10.1084/jem.20021098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangarajan PN, Umesono K, Evans RM. Modulation of glucocorticoid receptor function by protein kinase A. Mol Endocrinol. 1992;6:1451–1457. doi: 10.1210/mend.6.9.1435789. [DOI] [PubMed] [Google Scholar]
- Riegger T, Conrad S, Liu K, Schluesener HJ, Adibzahdeh M, Schwab JM. Spinal cord injury-induced immune depression syndrome (SCI-IDS) The European journal of neuroscience. 2007;25:1743–1747. doi: 10.1111/j.1460-9568.2007.05447.x. [DOI] [PubMed] [Google Scholar]
- Riegger T, Conrad S, Schluesener HJ, et al. Immune depression syndrome following human spinal cord injury (SCI): A pilot study. Neuroscience. 2008 doi: 10.1016/j.neuroscience.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Riveros N, Fiedler J, Lagos N, Munoz C, Orrego F. Glutamate in rat brain cortex synaptic vesicles: influence of the vesicle isolation procedure. Brain Res. 1986;386:405–408. doi: 10.1016/0006-8993(86)90181-2. [DOI] [PubMed] [Google Scholar]
- Ruzek MC, Pearce BD, Miller AH, Biron CA. Endogenous glucocorticoids protect against cytokine-mediated lethality during viral infection. J Immunol. 1999;162:3527–3533. [PubMed] [Google Scholar]
- Sanders VM, Kasprowicz DJ, Swanson-Mungerson MA, Podojil JR, Kohm AP. Adaptive immunity in mice lacking the beta(2)-adrenergic receptor. Brain Behav Immun. 2003;17:55–67. doi: 10.1016/s0889-1591(02)00056-9. [DOI] [PubMed] [Google Scholar]
- Sanders VM, Munson AE. Beta adrenoceptor mediation of the enhancing effect of norepinephrine on the murine primary antibody response in vitro. J Pharmacol Exp Ther. 1984;230:183–192. [PubMed] [Google Scholar]
- Shupliakov O, Brodin L, Cullheim S, Ottersen OP, Storm-Mathisen J. Immunogold quantification of glutamate in two types of excitatory synapse with different firing patterns. J Neurosci. 1992;12:3789–3803. doi: 10.1523/JNEUROSCI.12-10-03789.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T, Schmied M, Hewson AK, Lassmann H, Cuzner ML. Apoptosis of T cells and macrophages in the central nervous system of intact and adrenalectomized Lewis rats during experimental allergic encephalomyelitis. J Autoimmun. 1996;9:167–174. doi: 10.1006/jaut.1996.0020. [DOI] [PubMed] [Google Scholar]
- Vizi ES, Kiss JP. Neurochemistry and pharmacology of the major hippocampal transmitter systems: synaptic and nonsynaptic interactions. Hippocampus. 1998;8:566–607. doi: 10.1002/(SICI)1098-1063(1998)8:6<566::AID-HIPO2>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Wahle M, Kolker S, Krause A, Burmester GR, Baerwald CG. Impaired catecholaminergic signalling of B lymphocytes in patients with chronic rheumatic diseases. Ann Rheum Dis. 2001;60:505–510. doi: 10.1136/ard.60.5.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahle M, Pierer M, Krause A, Kolker S, Baerwald CG. Decreased catecholamine-induced cell death in B lymphocytes from patients with rheumatoid arthritis. Ann N Y Acad Sci. 2002;966:425–428. doi: 10.1111/j.1749-6632.2002.tb04243.x. [DOI] [PubMed] [Google Scholar]
- Wang Z, Malone MH, He H, McColl KS, Distelhorst CW. Microarray analysis uncovers the induction of the proapoptotic BH3-only protein Bim in multiple models of glucocorticoid-induced apoptosis. J Biol Chem. 2003;278:23861–23867. doi: 10.1074/jbc.M301843200. [DOI] [PubMed] [Google Scholar]
- Willis SN, Fletcher JI, Kaufmann T, et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–859. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- Xu BY, Arlehag L, Rantapaa-Dahlquist SB, Lefvert AK. beta2 Adrenoceptor gene single nucleotide polymorphisms are associated with rheumatoid arthritis in northern Sweden. Ann Rheum Dis. 2005;64:773–776. doi: 10.1136/ard.2004.027532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Insel PA. The pro-apoptotic protein Bim is a convergence point for cAMP/protein kinase A- and glucocorticoid-promoted apoptosis of lymphoid cells. J Biol Chem. 2004;279:20858–20865. doi: 10.1074/jbc.M310643200. [DOI] [PubMed] [Google Scholar]