Abstract
This functional magnetic resonance imaging (fMRI) study investigated the role of Broca’s region for selecting semantic, syntactic, and phonological information during picture naming. According to psycholinguistic theory, selection is reflected in speech latency differences, e.g. during priming. Here, homogenous (priming) blocks in which German picture names had the same semantic category, syntactic gender, or initial phoneme alternated with heterogeneous (non-priming) blocks. Speech latencies revealed a negative priming effect. Speech latencies were used as regressors for the fMRI data in order to tap selection processes. In Broca’s region (BA 44), among others, fMRI data showed repetition priming, which was positive for semantic and syntactic but negative for phonological selection. The different effects in area 44 are discussed in terms of psycholinguistic theory. Overall, the activation pattern is in line with the hypothesis that area 44 generally supports selection processes during noun production at several levels of the mental lexicon.
Keywords: Broca’s region, Area 44, BA 44, Semantic, Phonological, Syntactic gender, fMRI
Introduction
The present study investigates the neural basis underlying the selection of semantic, phonological and syntactic information from the mental lexicon during speaking. Based upon a psycholinguistic definition of the term “selection”, we will demonstrate the involvement of cytoarchitectonically defined area 44 (Amunts et al. 2004) in Broca’s speech region during selection of information of all three types. Moreover, we will pinpoint the differential role of area 44 for selection processes at the different levels of the mental lexicon. Finally, we will discuss that the data on syntactic selection have some repercussion on psycholinguistic theories of language production.
Several psycholinguistic models of language production (e.g. Caramazza 1997; Dell 1986; Levelt et al. 1999) assume that, prior to uttering a word, activation spreads between nodes in the mental lexicon, each node representing some type of information. Such information may be, e.g. conceptual-semantic, syntactic, or phonological. According to the language production model by Levelt (e.g. Levelt et al. 1999), a node that receives the highest amount of activation is then selected, i.e. used, for the production process. This selection is based on a statistical procedure, with the selection probability of a node equalling the ratio of its activation to that of all other nodes (the “Luce ratio”). A similar mechanism is implemented in Dell’s model (e.g. Dell 1986). Thus, on the psycholinguistic level, there is a distinction between “activation” and “selection” of information in the mental lexicon. A node can receive a certain level of activation, but nonetheless not become selected. In other words, selection always implies prior activation, whereas activation not necessarily also includes selection. As a consequence, “selection” but not necessarily “activation” relates to speaking itself insofar as it is correlated with speech latencies. In other words, only selected information will be part of the utterance, and the time it takes to select this information (based e.g. on competition between alternatives and, thus, on the Luce ratio) is reflected in the time necessary to start producing the word. Transferring this logic to a functional imaging study implies that “selection” is reflected in the neuroimaging signal that correlates with speech latencies. A priming effect in this part of the neuroimaging signal thus represents a modulation of selection in the mental lexicon. Other priming effects which do not relate to speech latencies can also be viewed as indication of changes in the mental lexicon, but only as far as the activation level in a lexical node is concerned (which may be changed by priming) which does not lead to selection of this node. We will return to this issue when discussing the logic of the data analysis.
With respect to selection processes in the brain, Broca’s region in the left inferior frontal gyrus (IFG) has since long been regarded as a relevant structure. Several neuroimaging studies related activation in Broca’s region to the selection of semantic (e.g. Amunts et al. 2004; Badre et al. 2005; Thompson-Schill et al. 1997, Thompson-Schill et al. 1999), syntactic (e.g. Longoni et al. 2005), or phonological information (for reviews see e.g. Costafreda et al. 2006; Indefrey and Levelt 2004; Vigneau et al. 2006). However, the term “selection” in neuroimaging studies was not necessarily identical to that from psycholinguistics. For instance, in a study on semantic memory processes, Badre et al. (2005) contrasted “selection” (as a top-down process) with “top-down activation” (controlled retrieval) and “bottom-up activation”. Obviously, the latter process but not the one termed “selection” reflects the definition from psycholinguistics given above. Other studies defined selection demands based on lexical frequency values (Thompson-Schill et al. 1997) or compared the retrieval of lexical entries from the mental lexicon with that of overlearned sequences of words (e.g. months of the year; Amunts et al. 2004), which have lower selection demands. These examples illustrate that, at present, it is difficult to link neuroimaging data on selection processes with psycholinguistic theory because of the partly different definitions of the term “selection”. Moreover, most studies investigated only one aspect, e.g. the conceptual-semantic level, but did not provide the direct comparison of selection processes for semantic, syntactic and phonological information in the mental lexicon. Hence, in order to test the role of Broca’s region for selection at different levels of the mental lexicon, it is necessary to demonstrate that the haemodynamic response in Broca’s region covaries with, e.g. speech latencies as an indicator of selection processes in psycholinguistic terms. Furthermore, in order to generalise the findings, one would have to tap different levels in the mental lexicon in the same study.
Consequently, the present functional magnetic resonance imaging (fMRI) study addressed this issue, investigating several related questions. (1) First, we tested whether activation in Broca’s region was correlated with the speech latencies during overt picture naming, thus reflecting selection. (2) We further sought to elucidate whether such effects were comparable for semantic and phonological selection. (3) Finally, we investigated whether there was a similar effect also for the selection of syntactic gender information.
This last issue is of relevance since it has been debated in psycholinguistic research over the past decade. Syntactic gender is a lexical information used in many languages (e.g. Spanish, French, Dutch, or German) in order to indicate the correspondence of, e.g. a noun and a determiner or adjective (Spanish: “la casa blanca”—thefeminine house whitefeminine; German: “das Haus”—theneuter house; “weißes Haus”—whiteneuter house). But what if the speaker simply says “casa” or “Haus”, i.e. produces a bare noun with no gender-marking? Psycholinguistic models of language production featuring syntactic gender information (e.g. Caramazza 1997; Levelt et al. 1999) agree that the syntactic gender node in the mental lexicon might become activated even then. However, based on earlier behavioural data (La Heij et al. 1998), Levelt et al. (1999) assumed that syntactic gender only becomes selected when it is actually needed, e.g. for the correct retrieval of a determiner or an adjective, but not during bare noun production. This view was challenged by a more recent study (Cubelli et al. 2005) that did observe syntactic gender effects even in bare noun production. The authors demonstrated an influence of gender-related distractor words on picture naming latencies in the picture-word interference paradigm.
One research question in the present study, therefore, was whether, on the neural level, we would obtain indication for the selection of syntactic gender information during bare noun production after having established these selection effects for semantics and phonology. From a theoretical viewpoint, one might well assume that syntactic gender should become activated in noun production even if it is not needed, since syntactic word category information, which is not needed either in bare noun production, is nonetheless assumed to be activated in bare noun production (Levelt et al. 1999). One argument for the selection of syntactic word category is that substitution errors in speaking usually occur in the same syntactic category. However, a similar argument can also be made for syntactic gender. Badecker et al. (1995) reported the case of an anomic patient who was very impaired in naming but always could tell the target noun’s gender even though it was not needed for any form encoding.
The present fMRI study addressed these questions as follows. First, we adopted the psycholinguistic definition of “selection” (as opposed to “activation”). As outlined above, the selection process is reflected in the speech latencies. Consequently, we investigated haemodynamic effects which were correlated to speech latencies. Furthermore, in order to distinguish semantic, phonological and syntactic selection, we applied a priming paradigm in which semantic, syntactic or phonological information in the mental lexicon was repeatedly activated (as opposed to the unprimed conditions), which in turn altered the Luce ratio for the selection of this information in the primed, but not in the unprimed conditions. Participants overtly named pictures with simple noun phrases (e.g. “Haus”). Blocks in which all German picture names had the same gender (gender-homogeneous, GEN_HOM) alternated with blocks with mixed genders (gender-heterogeneous, GEN_HET; created from the same stimuli as the gender homogeneous blocks). Similarly, blocks in which all picture names started with the same phoneme (phonologically homogeneous, PHO_HOM) alternated with blocks of different phonemes (phonologically heterogeneous, PHO_HET; containing the same stimuli in a different order). Finally, in the semantic condition (SEM_HOM and SEM_HET), blocks with objects from the same category (e.g. animals) alternated with mixed blocks. Significant differences in the speech-latency-related haemodynamic effect for homogeneous (i.e. primed) versus heterogeneous (i.e. unprimed) trials thus reveal the neural correlates of selection in the psycholinguistic sense. In order to distinguish the empirical data from their neurofunctional interpretation, we will refer to the technical term “priming” when reporting differences between homogeneous and heterogeneous conditions, while we use the term “selection” for the interpretation of these priming effects with respect to the mental lexicon and its neural correlates.
Materials and methods
Participants
Twenty-three healthy participants (mean age 27.7 years; 12 women) participated in the experiment. They were all native German speakers and had normal or corrected-to-normal vision. Informed consent was obtained from all participants. The experimental standards were approved by the local ethics committee of the University of Aachen.
Materials
Eighteen sets of pictures were used, each containing 10 stimuli. Six of the sets were phonologically homogeneous, i.e. all German picture names in a given set started with the same phoneme (/k/, /m/, /sh/, /t/, /b/, or /f/). In contrast, the stimuli within each block all had different genders and belonged to different semantic categories. Six other sets were gender-homogeneous, i.e. all picture names in a particular set had the same syntactic gender (masculine, feminine or neuter) but started with different phonemes and were not in the same semantic category. Finally, the last six sets were semantically homogeneous, i.e. all pictures depicted objects from the same category (birds, mammals, food, weapons, tools or toys.). These items, however, differed with respect to their initial phonemes and syntactic genders. For each of the tree types of homogeneous sets (semantic, syntactic phonological) an equal number of heterogeneous sets was constructed by recombination of stimuli across the individual sets. For example, for the ensuing six semantic heterogeneous sets, pictures from the six semantic homogeneous sets were rearranged in such a way that different categories were distributed across all sets. These heterogeneous sets thus contained the same items as the homogeneous sets but did not feature the (semantic/syntactic/phonological) commonalities and hence should not evoke any priming effects. All German picture names and their translations are listed in Appendix 1. Some phonological commonalities between picture names which did not have the same initial phoneme (but, e.g. the same final letter) could not be excluded due to the large amount of pictures required for the study. However, such commonalities were present in all sets and are thus a constant, unspecific for any condition or set. The only exceptions from this rule were the feminine nouns in the syntactic sets, which frequently end with a schwa sound in German. However, as will be discussed below, the relevant syntactic effects in the fMRI data were comparable to the semantic but not the phonological effects, ruling out a systematic influence of this subset of all nouns from the syntactic set on the results.
Procedure
The pictures were presented to the participants via goggles (VisuaStim™, Resonance Technology, CA, USA). Stimulus presentation was controlled by a computer placed in the control room using Presentation software (Neurobehavioral Systems, Albany, CA, USA). The participants had to name each picture as quickly as possible after its onset.
The study employed an implicit block design. The blocks were not obvious to the participants since they were not separated by pauses. This was done in order not to draw the participants’ attention to the differences between blocks and hence the presence of semantic, syntactic, phonological sets. Each block comprised ten pictures. There were six blocks for the gender homogeneous, phonologically homogeneous, gender heterogeneous, phonologically heterogeneous, semantically homogeneous and semantically heterogeneous condition, respectively. As heterogeneous blocks were constructed from reordering the items of the corresponding homogenous blocks, each picture was consequently presented twice, once in a homogeneous block and once in a heterogeneous block. The order of appearance of an item in a homogeneous or heterogeneous block was pseudorandomised over subjects and thus did not induce a systematic habituation in the haemodynamic response for the one or other condition.
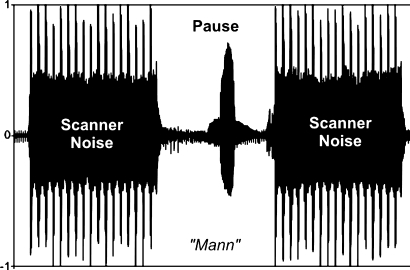
Each trial started with the presentation of a fixation cross in the middle of the screen for 1,000 ms. During this time the functional data were acquired (see Fig. 1 and the section “Data acquisition and analysis”). Then scanning paused for 1,000 ms. During the period of silence a picture appeared on the screen for 900 ms, which was named by the participant. Such procedure combines several advantages. First, it prevents motion-induced susceptibility artefacts, since subjects only speak when no fMRI data are recorded. Second, the scanner noise is not superimposed on the verbal response. Consequently, the subjects’ speech latencies are easier to assess as a behavioural variable in the experiment. The resulting stimulus onset asynchrony (SOA) between two subsequent pictures (with the first always being the prime for the second) was thus 2,000 ms, which is in the range usually applied in priming experiments (e.g. de Zubicaray et al. 2008: 1,750 ms; Raposo et al. 2006: 2,500 ms).
Fig. 1.
Speaking during scanning: A bunched-early EPI sequence was used for the acquisition of the fMRI data. All slices were recorded in the first 1.04 s of the TR, resulting in a silent period of 0.96 s in the second half of the TR. During this silent period, the participants generated the words. The speech signal is clearly discernable, since it is not obscured by the scanner noise
Speech recordings
The participants’ speech production was recorded using the microphone of the goggle system. The cable from the microphone to the patient intercom in the MR control room was plugged there into a splitter, from which one cable led to the intercom and one to the line-in port of an external sound card attached to a Toshiba notebook used for digital recording. From these recordings the speech latencies were obtained manually using the WavePad software (NCH Software Pty Ltd, Canberra, Australia).
Data acquisition and analysis
fMRI data
The fMRI experiment was carried out on a 3T Siemens Trio scanner. A standard birdcage head coil was used with foam paddings reducing head motion (cf. Heim et al. 2006). The functional data were recorded from 17 sagittal slices in the left hemisphere using a gradient-echo EPI sequence with echo time (TE) = 30 ms, flip angle = 90°, and repetition time (TR) = 2 s. The sagittal orientation of the slices was chosen in order to correct head motion in-plane (which is highest in the y-z plane; Heim et al. 2006), thus preventing “slice shuffling”. Acquisition of the slices within the TR was arranged so that all slices were acquired in the first 1,040 ms, followed by a 960-ms period of no acquisition to complete the TR during which the subjects spoke. The field of view (FOV) was 200 mm, with an in-plane resolution of 3.1 mm × 3.1 mm. The slice thickness was 3 mm with an inter-slice gap of 1 mm.
The data processing was performed using MATLAB 6.5 (The Mathworks Inc., Natick, USA), and SPM5 (Wellcome Department of Cognitive Neurology, UK). Two dummy scans before the beginning of the experiment were discarded to allow for magnetic saturation. Data pre-processing included the standard procedures of realignment, normalisation to the MNI single subject template and spatial smoothing (FWHM = 8 mm).
We performed an event-related statistical analysis at the first (single subject) level. To this end, homogeneous and heterogeneous trials in each set were identified from the individual PRESENTATION log-files. If a subject failed to name a picture correctly or the speech onset was after the presentation of the subsequent trial, the trial was excluded, and the subsequent trial was coded as a non-repetition trial. For each condition, a stick function (i.e. duration = 0, onset time = trial onset) was convolved with a canonical haemodynamic response function (HRF) and its first derivative. For each participant, the contrasts of each condition versus the implicit resting baseline were calculated. Each of the regressors was then contrasted against the implicit (resting) baseline. For the group analysis, these individual contrast images were entered into a repeated-measures ANCOVA (with appropriate non-sphericity modelling) allowing random effects analysis of our fMRI activation data. Moreover, the speech latencies for each condition were entered as covariates into the model, capturing the variance in the fMRI data that was due to the variance in the speech latencies and thus reflected selection processes. The regressors for the six conditions are subsequently called “condition regressors”, and the six speech latency regressors are subsequently called “RT regressors”. Following the logic outlined in the Introduction, differences in the RT regressors between primed and unprimed conditions reflect selection in the mental lexicon plus prior activation, whereas differences in the condition regressors (i.e. technically speaking, the residuals of the ANCOVA) reflect differences in the activation level in the mental lexicon without selection.
From the ANOVA the following contrasts were calculated. First, the overall main effect, i.e. the F-test over all 12 regressors, was performed, yielding the entire brain network involved in the picture naming task. Within this network, those regions that reflected the variability in the speech latencies, were identified by computing the main effect only for the six RT regressors. In order to constrain this analysis to effectively task-driven regions, the main effect was masked with the aforementioned overall main effect for picture naming. In a next step, brain regions were identified that revealed priming effects by calculating F-tests for the contrasts of the RT regressors for heterogeneous minus homogeneous trials (i.e. SEM_HET > SEM_HOM, SYN_HET > SYN_HOM, PHO_HET > PHO_HOM). In order to test for differences between the priming effects in the RT regressors, the F-test for the interaction term of the factors CONDITION (SEM, SYN, PHO) × PRIMING (HOM, HET) was also calculated. Moreover, priming effects for the RT regressors were contrasted pair-wise, i.e. priming for SEM versus SYN, SEM versus PHO, and SYN versus PHO. This analysis represents potentially different selection mechanisms for conceptual-semantic, syntactic and phonological information in the mental lexicon. Finally, for the condition regressors, F-tests for the contrasts of primed and unprimed conditions (i.e. SEM_HET vs. SEM_HOM, SYN_HET vs. SYN_HOM, PHO_HET vs. PHO_HOM) were calculated, which reflect the psycholinguistic activation levels between the conditions in the absence of selection processes.
Localisation of effects with cytoarchitectonic probability maps
For the anatomical localisation of the activations we used cytoarchitectonic probability maps (Amunts et al. 2004). These maps are based on an observer-independent analysis of the cytoarchitecture in a sample of ten post-mortem brains (Schleicher et al. 2005; Zilles et al. 2002). They provide information about the location and variability of cortical regions in standard MNI reference space. For the assignment of MNI coordinates to the cytoarchitectonically defined regions we used the SPM Anatomy Toolbox (Eickhoff et al. 2005) available with all published cytoarchitectonic probability maps and references from www.fz-juelich.de/inm/spm_anatomy_toolbox). In order to assess the role of Broca’s region for selection processes in the mental lexicon, the analysis of differential priming effects in the RT regressors was confined to cytoarchitectonically defined area 44 as provided in the SPM Anatomy Toolbox (Eickhoff et al. 2005).
Results
Behavioural data
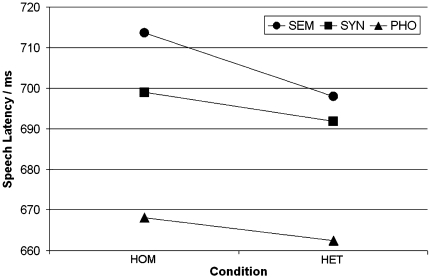
The average picture naming latencies per condition are presented in Fig. 2. The 3 × 2 repeated-measures ANOVA with factors CONDITION (SEM, SYN, PHO) and PRIMING (HOM, HET) yielded significant main effects of CONDITION (F2,21 = 22.26; P < 0.001) and PRIMING (F2,21 = 8.06; P = 0.010). Overall, the speech latencies were about 10 ms longer in the homogeneous than in the heterogeneous conditions. No significant effect was obtained for the interaction CONDITION × PRIMING (F2,21 < 1). Planned contrasts between homogeneous and heterogeneous trials for each condition yielded no significant differences (all P > 0.05).
Fig. 2.
Picture naming latencies in the six experimental conditions. SEM semantic, SYN syntactic, PHO phonological, HOM homogeneous, HET heterogeneous
fMRI data
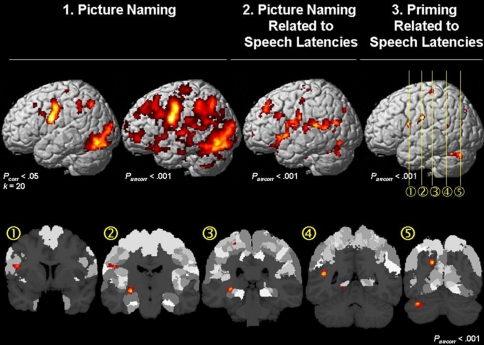
The effect of interest for picture naming yielded widespread activation in the left hemisphere (Pcorr < 0.001). Within this network, some brain regions revealed a significant correlation with the speech latencies (Fig. 3 top middle; for cytoarchitectonic details cf. Table 1). These regions included the IFG (area 44/45; Amunts et al. 2004), insula, parietal operculum (areas OP1/OP2/OP4; Eickhoff et al. 2007a), superior temporal gyrus (area TE1.0; Morosan et al. 2001), middle temporal gyrus, precentral gyrus (areas 4a/4p/6; Geyer et al. 1996; Geyer 2003), postcental gyrus (areas 1/2/3a/3b; Geyer et al. 1999, 2000; Grefkes et al. 2001), superior and inferior occipital gyrus (areas 17/18; Amunts et al. 2000), hippocampus (subiculum, SUB; cornu ammonis, CA; entorhinal cortex, EC; Amunts et al. 2005) and amygdala (superficial group, SF; Amunts et al. 2005).
Fig. 3.
Top Left: Surface rendering of the main effect for picture naming in the fMRI data at an FWE-corrected (Pcorr < 0.05) and uncorrected (Puncorr < 0.001) threshold. Top Middle fMRI signal that is correlated with the speech latencies. Top Rightand Bottom Within the brain regions where the fMRI signal correlated with the speech latencies (Top Middle), the main effect for priming (i.e. homogeneous vs. heterogeneous trials in each condition) was significant (Puncorr < 0.001) in (1) area 44 in the left inferior frontal gyrus; (2) area 6 in the precentral gyrus; (3) the hippocampus and the postcentral gyrus (area 3b); (4) the posterior middle temporal gyrus; and (5) precuneus and the cerebellum
Table 1.
Brain activation during overt picture naming correlated with the naming latencies (Puncorr < 0.001, k > 50 voxels)
| Local maximum in macroanatomical structure | x | y | z | F | Percent overlap of cluster with cytoarchitectonic areas | |
|---|---|---|---|---|---|---|
| Left middle temporal gyrus | −44 | −50 | 15 | 8.22 | 11.3 | Left OP 1 |
| 2.4 | Left TE 1.0 | |||||
| Left hippocampus | −16 | −16 | −13 | 8.94 | 17.3 | Left Hipp. (CA) |
| 6.1 | Left Hipp. (SUB) | |||||
| 5.7 | Left Amyg. (SF) | |||||
| 3.4 | Left Hipp. (EC) | |||||
| Left cerebellum | −36 | −70 | −29 | 8.07 | 11.3 | Left area 18 |
| 4.3 | Left area 17 | |||||
| Left postcentral gyrus | −18 | −22 | 61 | 7.92 | 23.5 | Left area 6 |
| 19.8 | Left area 3a | |||||
| 19.2 | Left area 4p | |||||
| 12.4 | Left area 4a | |||||
| 2.2 | Left area 3b | |||||
| Left superior occipital gyrus | −14 | −72 | 29 | 6.27 | 7.7 | Left area 17 |
| 1.3 | Left area 18 | |||||
| Left postcentral gyrus | −50 | −14 | 27 | 6.85 | 32.3 | Left OP 4 |
| 17.4 | Left area 3b | |||||
| 10.3 | Left area 3a | |||||
| 9.9 | Left area 44 | |||||
| 5 | Left OP 3 | |||||
| 1.7 | Left area 1 | |||||
| 1.1 | Left area 2 | |||||
| 1 | Left OP 1 | |||||
| Left superior frontal gyrus | −20 | 34 | 47 | 7.00 | ||
| Left insula | −46 | 8 | −3 | 8.75 | 1.2 | Left area 44 |
| Left inferior frontal gyrus | −54 | 20 | 25 | 5.72 | 50.1 | Left area 44 |
| 48.7 | Left area 45 | |||||
| Left middle orbital gyrus | −22 | 32 | −15 | 6.15 | ||
| Left inferior occipital gyrus | −42 | −74 | −13 | 7.03 | ||
| Left hippocampus | −30 | −32 | −3 | 7.12 | 26.1 | Left Hipp. (CA) |
| 1.8 | Left Hipp. (FD) | |||||
| Left superior medial gyrus | −16 | 54 | 3 | 7.93 | ||
The main effect for priming in the RT regressors, i.e. differences in the RT regressors for homogeneous versus heterogeneous trials, yielded significant results (Puncorr < 0.001) in the left IFG (area 44), precentral gyrus (areas 4a/4p/6), postcentral gyrus (areas 1/2/3a/3b), parietal operculum (areas OP1/OP2/OP4), lingual gyrus, precuneus, visual cortex (areas 17/18), middle temporal gyrus, hippocampus (CA and fascia dentata, FD), amygdala (centro-medial group, CM) and cerebellum (Fig. 3 top right and bottom; Table 2).
Table 2.
Effect of interest for priming in the speech latency regressors (Puncorr < 0.001; k > 30 voxels)
| Local maximum in macroanatomical structure | x | y | z | F | Percent overlap of cluster with cytoarchitectonic areas | |
|---|---|---|---|---|---|---|
| Left precuneus | −14 | −72 | 31 | 10.14 | ||
| Left cerebellum | −36 | −70 | −29 | 12.34 | ||
| Left postcentral gyrus | −50 | −14 | 27 | 9.20 | 24.8 | Left area 2 |
| 19.2 | Left OP 4 | |||||
| 15.6 | Left area 3b | |||||
| 10 | Left area 3a | |||||
| 2.9 | Left OP 3 | |||||
| 2.9 | Left OP 1 | |||||
| Left amygdala | −28 | −16 | −7 | 10.51 | 4.9 | Left Amyg. (CM) |
| 3.5 | Left Hipp. (CA) | |||||
| Left precentral gyrus | −26 | −28 | 63 | 9.33 | 77.4 | Left area 6 |
| 12.8 | Left area 4p | |||||
| 8 | Left area 4a | |||||
| Left middle temporal gyrus | −44 | −50 | 13 | 10.12 | ||
| Left lingual gyrus | −16 | −58 | −3 | 7.58 | 43.6 | Left area 18 |
| 16.1 | Left area 17 | |||||
| Left inferior frontal gyrus | −46 | 6 | 17 | 8.45 | 30.2 | Left area 44 |
| Left hippocampus | −30 | −32 | −3 | 9.22 | 14.9 | Left Hipp. (CA) |
| 5.2 | Left Hipp. (FD) | |||||
In order to assess which regions involved in selection (i.e. that showed a priming effect in the RT regressors) were also involved in picture naming in general, a conjunction analysis of the main effect of picture naming and the main effect for priming in the RT regressors was computed. This conjuction analysis revealed shared effects only in the left cuneus (MNI coordinates −14, −70, 29), but not in Broca’s region.
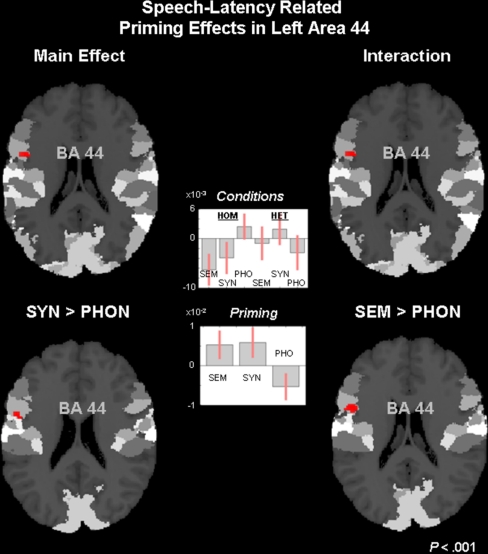
Since area 44 showed the hypothesised main effect for priming, we further analysed the actual pattern in the fMRI data in area 44. Testing for the interaction of CONDITION × PRIMING also yielded a significant effect in area 44 (Puncorr < 0.001; F = 8.09) at MNI coordinates (x,y,z) −54,6,19. The subsequent tests for pair-wise differences in priming between the three conditions were significant in area 44 for SYN > PHO (Puncorr < 0.001; F = 14.06) at MNI coordinates −52,2,21. Similarly, the difference in priming for SEM > PHO was significant (Puncorr < 0.001; F = 15.88) at MNI coordinates −50,8,19. No differential priming effect was observed for SEM versus SYN (Puncorr < 0.001) in area 44. All differential priming effects are displayed in Fig. 4, which also shows the beta estimates for the fMRI signal in the six RT regressors as well as their differences, i.e. the priming effects. These data demonstrate positive priming for both SEM and SYN, but negative priming for PHO. The implication of this pattern is discussed below.
Fig. 4.
In left area 44, there was a main effect for priming in the RT regressors (top left) as well as a significant interaction (top right). Pair-wise comparisons of priming in the semantic (SEM), syntactic (SYN), and phonological (PHO) conditions revealed differences between SYN and PHO (bottom left) and between SEM and PHO (bottom right). The beta values for the fMRI activation and those for priming in SEM, SYN and PHO (with 90% confidence intervals) are also shown (middle). All effects are displayed at Puncorr < 0.001, masked with the maximum probability map of area 44
Finally, analysing priming effects in the condition regressors, which, on the psycholinguistic level, reflect activation without subsequent selection, yielded the following results (Puncorr < 0.001; k = 30). Overall, there was a main effect for priming in the left angular gyrus (areas PGa, PF and PFm; Caspers et al. 2006), left inferior parietal lobule (areas hIP1, hIP2 and hIP3; Choi et al. 2006; Scheperjans et al. 2008), left superior parietal lobule (areas 7A and 7PC; Scheperjans et al. 2008), left middle frontal gyrus, left motor and premotor cortex (areas 4a, 4p, and 6), left Broca’s region (areas 44 and 45), left middle temporal gyrus and left precuneus (Table 3). Among these regions, phonological priming yielded massive priming effects in left superior and inferior parietal cortex (areas PGa, PF, PFm, hIP1, hIP2, hIP3, 7A and 7PC), Broca’s region (areas 44 and 45), left precuneus, left middle temporal gyurs and left middle frontal gyrus (Table 4). In the semantic conditions, there was one single effect in the left superior parietal lobule (areas 7A and hIP3), whereas syntactic priming was observed in one region close to the insula/parietal operculum.
Table 3.
Effect of interest for priming in the condition regressors (Puncorr < 0.001; k > 30 voxels)
| Local maximum in macroanatomical structure | x | y | z | F | Percent overlap of cluster with cytoarchitectonic areas | |
|---|---|---|---|---|---|---|
| Left angular gyrus (PFm) | −38 | −62 | 47 | 14.71 | 17.2 | Left area PGa |
| 15.8 | Left area 7A | |||||
| 11 | Left area hIP3 | |||||
| 7.5 | Left area hIP1 | |||||
| 2.4 | Left area PFm | |||||
| Left inferior parietal lobule (hIP1) | −46 | −48 | 41 | 11.17 | 25.8 | Left area hIP2 |
| 25.4 | Left area hIP1 | |||||
| 22.3 | Left area PFm | |||||
| 15.0 | Left area PF | |||||
| 9.4 | Left area PGa | |||||
| 1.1 | Left area 2 | |||||
| Left middle frontal gyrus | −38 | 54 | 1 | 9.15 | ||
| −36 | 20 | 45 | 9.41 | |||
| Left precentral gyrus (Area 4a) | −18 | −26 | 57 | 10.99 | 37.8 | Left area 4a |
| 35.9 | Left area 6 | |||||
| 23.6 | Left area 4p | |||||
| 2.3 | Left area 3a | |||||
| Left inferior and middle frontal gyrus | −48 | 16 | 39 | 7.37 | 66.7 | Left area 44 |
| 5.1 | Left area 45 | |||||
| Left middle temporal gyrus | −66 | −30 | −13 | 9.84 | ||
Table 4.
F-tests for priming effects in the condition regressors for phonological processing (Puncorr < 0.001; k > 30 voxels)
| Local maximum in macroanatomical structure | x | y | z | F | Percent overlap of cluster with cytoarchitectonic areas | |
|---|---|---|---|---|---|---|
| Left angular gyrus (PFm) | −38 | −62 | 47 | 36.96 | 31.2 | Left area PGa |
| 24.7 | Left area 7A | |||||
| 16.5 | Left area hIP3 | |||||
| 15.5 | Left area hIP1 | |||||
| 3.9 | Left area PFm | |||||
| Left inferior parietal lobule (hIP1) | −46 | −48 | 41 | 30.72 | 22.1 | Left area hIP2 |
| 23.0 | Left area hIP1 | |||||
| 21.4 | Left area PFm | |||||
| 17.7 | Left area PF | |||||
| 9.3 | Left area PGa | |||||
| 2.3 | Left area 2 | |||||
| Left middle frontal gyrus | −38 | 54 | 1 | 22.75 | ||
| Left middle frontal gyrus | −36 | 6 | 57 | 17.58 | ||
| Left inferior and middle frontal gyrus | −36 | 20 | 45 | 26.22 | 24.7 | Left area 44 |
| Left middle temporal gyrus | −64 | −30 | −11 | 24.70 | ||
| Left precuneus | −8 | −56 | 29 | 22.80 | ||
Discussion
The present fMRI study investigated the selection of semantic, syntactic and phonological information from the mental lexicon during language production using a priming paradigm. Priming was realised by the block-wise repetition of the same semantic category, syntactic gender, or initial phoneme. The speech latencies revealed a main effect for priming. Related to this behavioural effect, priming in the fMRI data recruited a brain network including left area 44 in Broca’s region. In area 44, positive priming was observed for semantic and syntactic selection, whereas negative priming was observed for phonological selection. A further aim of the present study was to assess whether syntactic priming, i.e. indication for syntactic gender selection, would occur at all, and if so, in area 44. The data demonstrated the existence of such effect, which was comparable to that for semantic priming but different from that for phonological priming. The implication of these findings is now discussed in detail.
fMRI evidence for selection in the mental lexicon
The logic behind the fMRI data analysis was to investigate brain activation effects that related to effects in the speech latency data, thus representing selection processes on the conceptual-semantic, syntactic and phonological level. The speech latencies revealed a significant effect of priming, i.e. an overall difference between homogeneous and heterogeneous trials across conditions. Accordingly, such effect was also observed for the RT regressors in the fMRI data analysis. Priming effects occurred in those regions whose activation co-varied with the speech latencies, including area 44, posterior middle temporal gyrus, the cerebellum and the hippocampus. This finding replicates a number of earlier neuroimaging results from studies investigating repetition priming (e.g. Meister et al. 2007; Raposo et al. 2006), semantic priming (e.g. Copland et al. 2003; Giesbrecht et al. 2004; Rissman et al. 2003; Tivarus et al. 2006) and phonological priming (e.g. Kouider et al. 2007), all of which reported effects in the posterior portion of the left inferior frontal gyrus. However, the findings of the present study go beyond these preliminary data in several respects. First, the present study related priming to selection mechanisms in the mental lexicon on the basis of a psycholinguistic definition. Second, it directly related the fMRI effects to the speech latencies by including them as regressors in the analysis. Third, it investigated three different stages of the production process (conceptual-semantic, syntactic and phonological) in the same experiment. Fourth, the neuroanatomical localisation was based on a cytoarchitectonically defined atlas of the brain.
Semantic versus phonological selection in area 44
Despite a main effect of priming in area 44, this priming effect differed for semantic (and syntactic) versus phonological priming. One explanation for this difference could be that selection in the mental lexicon does not universally rely on the same neural mechanisms. Rather, different processing stages in the mental lexicon could require different neural underpinnings. In the present study, the effects in left area 44 for both semantic and syntactic priming differed from those for phonological priming, while semantic and syntactic priming in area 44 did not differ significantly from each other. This pattern of results could also be explained on the basis of the psycholinguistic theory holding that semantic and syntactic processing both occur during an earlier stage of processing in the mental lexicon (in a former version of the Levelt model subsumed as the lemma level; Levelt 1989) which contains the abstract features of an entry such as meaning, word category, gender, etc. In contrast, phonological processing is located at the form/lexeme level on which the segmental structure of the word, its syllables and phonemes are specified (see e.g. Levelt 2001). Both processing stages are separated by a “rift” (Levelt et al. 1999) which becomes evident in non-impaired speakers in the tip-of-the-tongue state, when meaning and syntax of a word but not its sound form are available. The pattern of effects in area 44 might reflect processing on the lemma level which is tapped by syntactic and semantic priming. Neuroimaging evidence for this notion comes from a series of studies (Hauk et al. 2008; Heim et al. 2007; Longoni et al. 2005) that demonstrated the involvement of left area 44 in both lexical and syntactic processing.
The nature of the processes on the two stages in the mental lexicon might be better understood with a closer look at the direction of the effects in area 44. In particular, one needs to examine whether selection before and after the “rift” could still rely on the same neural mechanism despite the different effects in area 44. Whereas there was a positive priming effect for semantic (and syntactic) selection, it was negative for phonological selection. The positive priming effect for semantic selection indicates that in the primed/homogeneous condition, brain activation was lower than in the unprimed/heterogeneous condition. In other words, when selection demands were higher (in the unprimed condition), activation in area 44 was higher. In terms of the psycholinguistic definition of selection as a statistical mechanism evaluating Luce ratio, this effect implies that activation in area 44 is the stronger, the lower Luce ratio is, i.e. the smaller the difference between the activation of the target and its competitors. This consideration reveals that the haemodynamic effect in area 44 possibly reflects the effort to distinguish for which node in the mental lexicon Luce ratio is highest, a procedure becoming more difficult the lesser the differences in the activation of competing nodes in the mental lexicon are. This pattern of results and the conclusions drawn from it are well in line with earlier neuroimaging studies investigating semantic selection demands in a number of different paradigms (e.g. Thompson-Schill et al. 1997; or Badre et al. 2005). For instance, in the study by Thompson-Schill et al. (1997), subjects generated a verb for each noun presented as a cue. A ratio of the lexical frequency of the most common completion to the frequency of the second-most common completion was calculated as a measure of response strength. This response strength ratio is somewhat similar to Luce ratio, and accordingly, the haemodynamic effect in the posterior IFG in that study was higher for stimuli with a low versus high response strength. These findings corroborate our interpretation that the semantic selection effect in area 44 represents the effort it takes to actually perform the selection from the mental lexicon.
As noted above, the effect for phonological selection in area 44 was reversed in comparison to that for semantic selection. If area 44 supports a selection process which is identical for all levels of processing (independent of the “rift” in the mental lexicon) one would assume that the direction of the haemodynamic effect in area 44 was also identical for all levels. The following argumentation might explain that, despite these seemingly different effects, area 44 has the same role for selecting semantic and phonological information in the mental lexicon. In fact, the explanation for the reversed effect for phonological selection may be the relationship of matching versus mismatching phonemes in two subsequent words, i.e. the prime word and the target word. Schiller (2008) showed in a behavioural picture naming study that primes containing one or more phonemes of the target picture name reduced the speech latencies, i.e. the selection demands (e.g. %b%%%%%%—BANAAN; %ba%%%%%—BANAAN). However, when entire words were used as primes, which again shared one or more phonemes with the target picture name (e.g. beroep—BANAAN), speech latencies were longer, i.e. selection demands were higher. Schiller argued that this effect could be due to the activation of non-target segments in the phonological output lexicon, which possibly competed with the target segments for selection. Consequently, in the present study, the phonologically homogeneous sets actually required more rather than less selection than the heterogeneous sets, resulting in the reversed haemodynamic effect in area 44.
The present results differ from that of a recent fMRI study (Abel et al. 2009) which also tapped different processing stages of the language production process. These authors used distractor words rather than priming in order to interfere with the production process, which were phonologically, categorically or associatively related to the picture name. In contrast to the findings of the present study, Abel et al. (2009) only observed phonological but no semantic effects in the posterior part of the left IFG. There are possibly two reasons explaining this divergence of findings. One is the differential method, since the processing of a distractor word while retrieving a picture name from the mental lexicon has other task requirements than simply naming pictures (with residual activation levels in the mental lexicon from previous trials). The other is the differential scope of the two studies. Although both studies aimed at tapping different levels in the mental lexicon, the present paper explicitly investigated selection effects based on a neuroanatomically defined hypothesis. Therefore, the present study considered brain activation effects related to speech latencies, whereas Abel et al. (2009) reported brain activation effects related to conditions (rather than speech latencies). Therefore, the two studies may be regarded as complementary, providing insights into different aspects of the language production process.
Syntactic gender selection in area 44
Of particular importance for the research question in the present study was the fact that syntactic priming occurred and differed from phonological priming. In terms of “selection”, this means that there was a syntactic selection process going on which differed from phonological selection but resembled semantic selection.
Preliminary neuroimaging studies have already demonstrated that the left posterior IFG (and sometimes, more precisely, cytoarchitectonically defined area 44) supports syntactic gender processing in different tasks and modalities (Hernandez et al. 2004; Longoni et al. 2005; Miceli et al. 2002; Padovani et al. 2005). On the psycholinguistic level the presence of a gender priming effect in left area 44 implies that syntactic gender information must have been selected in the mental lexicon while the participants named pictures with bare nouns—otherwise no priming effect could have occurred. Thus, the present fMRI study demonstrated the selection of syntactic gender information in bare noun production even though such selection was not required for the actual form of the utterance (unlike in gender-marked determiner-noun phrases or adjective-noun phrases). This finding is in accordance with one behavioural language production study (Cubelli et al. 2005), but in opposition to several other studies (e.g. La Heij et al. 1998) and the model assumptions based thereupon (Levelt et al. 1999). However, if in the present study a non-significant effect in the fMRI data had occurred, one would still have to face the criticism of insufficient power. In contrast, the actual positive findings for syntactic selection in this study, which moreover occurred in a plausible brain region, make a point in favour of the selection of syntactic gender in bare noun production. This reasoning would make sense considering that word category information is also taken to be selected in bare noun production.
Priming effects in the RT regressors outside Broca’s region
Besides the selection effects in Broca’s region, the main effect for priming in the RT regressors yielded further results in a number of other regions including the precentral gyrus and the cerebellum involved in motor control, the hippocampus, amygdala and middle temporal gyrus (involved in lexical processing and memory), as well as the postcentral gyrus, parietal operculum, occipital lobe (somatosensory and visual processing). Among these regions, only the left middle temporal gyrus is commonly associated with linguistic processing (e.g. Friederici 2002; Indefrey and Levelt 2004) on several levels of the mental lexicon. Following the initial hypothesis that Broca’s region is particularly involved in the bottom-up selection processes in the mental lexicon, the involvement of the middle temporal cortex could possibly reflect the consequence of this selection process, i.e. that a lexical entry is now available for further processing. This entry could then activate its sensory features from semantic memory via the sensory and memory regions. This is the more plausible since all lexical entries refer to concrete, depictable objects with well-defined sensory features in the present picture naming study. Finally, the modulated production speed of the word, which is reflected in the speech latencies correlated with this effect, is also reflected in modulated involvement of the speech motor system. So far, only few is known about the effective connectivity of brain regions during priming (e.g. Nakamura et al. 2007) or language production (e.g. Eickhoff et al. 2009). For this reason, the here-proposed-interplay of the regions during priming in language production must remain speculative and be subjected to further research in this field.
Activation without subsequent selection in the mental lexicon
Priming effects that were not related to the RT regressors were interpreted to reflect activation level changes in the mental lexicon, however; without subsequent selection of an entry. The analysis of such priming effects in the fMRI data revealed a very particular pattern. Among all regions that were observed in the main effect, most regions were involved in phonological priming and only very few in semantic or syntactic priming. Thus, the distinction between phonological processing (lexeme level) on the one hand and semantic/syntactic processing (conceptual/lemma level) on the other hand, which was already discussed with respect to the function of Broca’s region, is apparently also relevant here.
Priming in the condition regressors for phonological processing, i.e. phonological activation in the mental lexicon, involved a left hemispheric network that has (as a whole or in parts) been reported in numerous other studies on phonological processing (e.g. Abel et al. 2009; Burton et al. 2000; Démonet et al. 1992; Indefrey and Levelt 2004; Vigneau et al. 2006). By distinguishing activation from selection in the mental lexicon, as we did in the present study, the assignment of parts of this network to the one or the other function becomes more obvious than on the basis of meta-analysis data (e.g. Vigneau et al. 2006).
With respect to the left IFG (probably area 44) Burton et al. (2000) argued that this region was involved in the segmentation of phonological information during language comprehension, since activation in this region was only observed when whole phonemes (but not single features of phonemes) had to be analysed for phonological same-different judgements. “Segmentation” in their sense requires the identification or retrieval of an entire phoneme and therefore can be regarded as synonymous to the term “selection” used in the present study. Thus, the study by Burton et al. (2000) and the present study provide concurrent evidence, stressing the importance of left area 44 (rather than temporo-parietal regions) for phonological selection; however, the present study goes beyond these earlier findings by demonstrating a role of left area 44 for phonological activation in the mental lexicon prior to selection.
The present findings also contribute to testing the claim by Indefrey and Levelt (2004) that left posterior IFG (including area 44) is involved in syllabification rather than phonological code retrieval, while phonological code retrieval is regarded as a function of posterior temporo-parietal regions. The data from the present study indicate that activation of phonemes in the mental lexicon and their subsequent retrieval, rely on left area 44. Consequently, area 44 is involved in the language production process prior to syllabification, i.e. at an earlier stage than hypothesised on the basis of the data available for the meta-analysis of Indefrey and Levelt (2004).
To conclude, the residual activation not correlated with the speech latencies provides some complimentary information about brain regions involved primarily in the activation and selection of phonemes in the mental lexicon.
Speech latencies
The ANOVA of the speech latencies yielded a significant priming effect. This priming effect consisted of increased speech latencies for the homogeneous as compared to the heterogeneous trials. Commonly, longer speech latencies are taken as indicators for inhibitory processes in the psycholinguistic literature (see e.g. Jescheniak et al. 2003 and references therein). However, this notion seems counter-intuitive in a priming paradigm as employed in the present study. In particular, priming is usually regarded as a form of implicit learning, resulting in facilitated rather than inhibited processing (e.g. Seger 1994). However, such notion only directly applies to repetition priming, i.e. the repeated presentation of a particular stimulus. In psycholinguistics, however, the situation is more complex. Depending on the experimental context, priming effects may occur in both directions, resulting in either increased or decreased response times, as will now be outlined in some more detail.
Phonological priming
For phonological priming, positive and negative priming effects have been reported in the literature. Whereas some studies reported facilitation of naming latencies by phonologically or segmentally homogeneous primes (e.g. Collins and Ellis 1992; Schiller 1999), other authors found the reversed effect (e.g. O’Seaghdha and Marin 2000). A possible solution to this dilemma was offered by Jescheniak et al. (2003) who argued that the direction of the phonological priming effect crucially depends on the distance between prime and target. By using a mathematical calculus they demonstrated that, whereas short prime-target SOAs usually result in facilitation, longer SOAs tend to produce inhibition. These seemingly differential effects result from the relation of gains and costs of activation of the corresponding phonological nodes in the mental lexicon. This model could well account for the longer speech latencies in the phonologically homogeneous versus heterogeneous trials in the present study which were separated by an SOA of 2,000 ms. Such interval is relatively long when compared to intervals of 0 or 150 ms which were reported to facilitate naming responses (e.g. in Jescheniak et al. 2003).
Syntactic gender priming
With respect to syntactic gender priming, the only psycholinguistic study that ever reported speech latency differences between gender-homogeneous and gender-heterogeneous trials in bare noun production (Cubelli et al. 2005) found that homogeneous trials had longer speech latencies than heterogeneous trials. While this result has not been replicated in other experiments, it is well in line with the data presented here, which consequently corroborate these previous findings.
The discrepancy of the present study with earlier behavioural studies (e.g. Van Berkum 1997; La Heij et al. 1998) possibly has several reasons. For instance, increasing the prime-target interval from one trial (in the present study) to 3–7 trials (Van Berkum 1997) led to the complete disappearance of gender priming even when gender-marked adjective-noun phrases served as primes and targets, indicating the subtlety of gender priming in general. Using the picture-word interference paradigm instead of gender priming, La Heij et al. (1998) did obtain a gender congruency effect (i.e. a significant influence of the gender of the distractor word) on the speech latencies for bare nouns (his Experiment 2) as well as for determiner phrases (his Experiment 3b). However, these effects were only present in the by-subject analysis but not in the by-item analysis. Interestingly, again, this was the case for both bare noun naming and determiner-phrase naming. The weakness of the congruency effect in the determiner-noun condition raises doubts about the statistical power of the experiment in general, which may also account for the seeming absence of a gender-congruency effect in bare noun production.
To summarise, the pattern of picture naming latencies obtained in the present study, which is characterised by longer latencies for semantically, phonologically or syntactically homogeneous as compared to heterogeneous trials, is in accordance with previous psycholinguistic studies of language production and thus a sound basis for using the speech latency regressors in the fMRI data analysis. Future research (such as the approach by Jescheniak et al. 2003) may elucidate under which conditions access to syntactic gender information on the lemma level is present, be it facilitating or inhibitory.
Conclusion
The present fMRI study demonstrated the involvement of left cytoarchitectonically defined area 44 in the selection of semantic, syntactic and phonological information from the mental lexicon during picture naming. The term selection was based on a psycholinguistic definition and implies bottom-up processing. The present data complement earlier findings from neuroimaging studies reporting more anterior activation (in or close to area 45; e.g. Amunts et al. 2004; Badre et al. 2005; Heim et al. 2008) related to selection processes that were top-down modulated by task demands. The view of area 44 supporting bottom-up processing and area 45 supporting top-down processing was corroborated by previous neuroimaging studies (e.g. Noesselt et al. 2003).
A second important aspect of this study was the presence of a syntactic selection effect in left area 44. This finding implies that syntactic gender information becomes selected in bare noun production. The absence of a correspondence to this haemodynamic effect in the speech latencies indicates the subtlety of the effect and may thus explain why most of the previous behavioural studies failed to obtain evidence for the selection of syntactic gender during language production. This finding might inspire future behavioural research to obtain more corroborating evidence while considering the subtlety of the effect and, therefore, the weak experimental power which must be expected. Such research, in turn, would have repercussion on psycholinguistic models of language production.
Acknowledgments
This Human Brain Project/Neuroinformatics research is funded by the National Institute of Biomedical Imaging and Bioengineering, the National Institute of Neurological Disorders and Stroke, and the National Institute of Mental Health (KA). Further support by Helmholtz-Gemeinschaft (VH-N6-012 to KA), the Brain Imaging Center West (BMBF 01GO0204) and the BMBF (01GW0771) is gratefully acknowledged. We thank Barbara Elghahwagi for her assistance with fMRI data recording. Moreover, we appreciate the discussions with Ralph Weidner and Simone Vossel with respect to the fMRI data analysis and the peripheral stimulation devices. We also wish to thank Helen Schreiber for her assistance with the analysis of the behavioural data. Finally, we appreciate the helpful comments by Max Coltheart, Jörg Jescheniak, and four anonymous reviewers on earlier versions of this manuscript.
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Appendix 1: German picture names
| Semantic sets | |||||
| Weapons | Mammals | Toys | |||
| Degen | Rapier | Giraffe | Giraffe | Ball | Ball |
| Dolch | Dagger | Ratte | Rat | Bogen | Bows |
| Bogen | Bows | Schaf | Sheep | Fahrrad | Bike |
| Messer | Knife | Schwein | Pig | Fußball | Football |
| Revolver | Revolver | Hase | Hare | Kreisel | Top |
| Säbel | Cutlass | Hund | Dog | Roller | Scooter |
| Gewehr | Rifle | Löwe | Lion | Schaukel | Swing |
| Bombe | Bomb | Pferd | Horse | Seil | Rope |
| Schwert | Sword | Zebra | Zebra | Wippe | See-saw |
| Kanone | Cannon | Ziege | Goat | Würfel | Dice |
| Tools | Food | Birds | |||
| Beil | Axe | Apfel | Appel | Adler | Eagle |
| Bohrer | Drill | Birne | Pear | Ente | Duck |
| Feile | Rasp | Brot | Bread | Eule | Owl |
| Hammer | Hammer | Käse | Cheese | Geier | Vulture |
| Kelle | Trowel | Kürbis | Pumpkin | Kranich | Crane |
| Hobel | Smoothing plane | Möhre | Carrot | Möwe | Gull |
| Säge | Saw | Paprika | Pepper | Papagei | Parrot |
| Schaufel | Shovel | Spargel | Asparagus | Schwan | Swan |
| Zange | Pliers | Traube | Grape | Storch | Stork |
| Spaten | Spade | Zwiebel | Onion | Taube | Pigeon |
| Syntactic sets | |||||
| Masculine | Feminine | Neuter | |||
| Ambos | Anvil | Angel | Fishing rod | Auge | Eye |
| Anker | Anchor | Ampel | Traffic light | Auto | Car |
| Ballon | Balloon | Blume | Flower | Bein | Leg |
| Eimer | Bucket | Erde | Earth | Eis | Ice cream |
| Gürtel | Belt | Gabel | Fork | Geschenk | Present |
| Hai | Shark | Hand | Hand | Haus | House |
| Haken | Hook | Geige | Violin | Gift | Poison |
| Igel | Hedgehog | Insel | Island | Iglu | Igloo |
| Löffel | Spoon | Lampe | Lamp | Lineal | Ruler |
| Nagel | Nail | Nuss | Nut | Nest | Nest |
| Ofen | Oven | Orgel | Organ | Ohr | Ear |
| Panzer | Tank | Palme | Palm tree | Paket | Packet |
| Pfeil | Arrow | Pfanne | Pan | Puzzle | Puzzle |
| Pilz | Mushroom | Pfeife | Pipe | Radio | Radio |
| Ring | Ring | Rakete | Rocket | Rad | Wheel |
| Roboter | Robot | Rose | Rose | Regal | Shelf |
| Sack | Sack | Schleife | Bow | Saxophon | Saxophone |
| Sattel | Saddle | Sonne | Sun | Sofa | Sofa |
| Sessel | Easy chair | Spinne | Spider | Stativ | Tripod |
| Wecker | Alarm clock | Waage | Balance | Wappen | Coat-of-arms |
| Phonological sets | |||||
| /k/ | /m/ | /sch/ | |||
| Käfer | Bug | Magnet | Magnet | Schach | Chess |
| Kaktus | Cactus | Moped | Moped | Schlange | Snake |
| Kanu | Canoo | Maske | Mask | Schere | Scissors |
| Kasse | Till | Mauer | Wall | Schiff | Ship |
| Katze | Cat | Maus | Mouse | Schal | Scarf |
| Kerze | Candle | Mixer | Mixer | Schirm | Umbrella |
| Kissen | Pillow | Mond | Moon | Schloss | Castle |
| Koffer | Suitcase | Muschel | Shell | Schraube | Screw |
| Krone | Crown | Mühle | Mill | Schrank | Cupboard |
| Krug | Jar | Mund | Mouth | Schuh | Shoe |
| /t/ | /b/ | /f/ | |||
| Tanne | Fir tree | Bank | Bank | Fahne | Flag |
| Tasse | Cup | Bus | Bus | Fisch | Fish |
| Telefon | Telephone | Buch | Book | Feder | Feather |
| Tisch | Table | Baum | Tree | Fuß | Foot |
| Toaster | Toaster | Besen | Broom | Flasche | Bottle |
| Topf | Pot | Bett | Bed | Floß | Float |
| Treppe | Stairs | Blatt | Leaf | Flugzeug | Plane |
| Trommel | Drum | Bombe | Bomb | Frosch | Frog |
| Tuba | Tuba | Boot | Boat | Füller | Fountain pen |
| Tube | Tube | Brille | Glasses | Fenster | Window |
References
- Abel S, Dressel K, Bitzer R, Kümmerer D, Mader I, Weiller C, Huber W (2009) The separation of processing stages in a lexical interference fMRI-paradigm. Neuroimage 44:1113–1124 [DOI] [PubMed]
- Amunts K, Malikovic A, Mohlberg H, Schormann T, Zilles K (2000) Brodmann’s areas 17 and 18 brought into stereotaxic space—where and how variable? Neuroimage 11:66–84 [DOI] [PubMed]
- Amunts K, Weiss PH, Mohlberg H, Pieperhoff P, Eickhoff S, Gurd JM et al (2004) Analysis of neural mechanisms underlying verbal fluency in cytoarchitectonically defined stereotaxic space—the roles of Brodmann areas 44 and 45. Neuroimage 22:42–56 [DOI] [PubMed]
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ et al (2005) Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol 210:343–352 [DOI] [PubMed]
- Badecker W, Miozzo M, Zanuttini R (1995) The two-stage model of lexical retrieval: evidence from a case of anomia with selective preservation of grammatical gender. Cognition 57:193–216 [DOI] [PubMed]
- Badre D, Poldrack RA, Paré-Blagoev EJ, Insler RZ, Wagner AD (2005) Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron 47:907–918 [DOI] [PubMed]
- Burton MW, Small SL, Blumstein SE (2000) The role of segmentation in phonological processing: an fMRI investigation. J Cogn Neurosci 12(4):679–690 [DOI] [PubMed]
- Caramazza A (1997) How many levels of processing are there in lexical access? Cogn Neuropsychol 14:177–208 [DOI]
- Caspers S, Geyer S, Schleicher A, Mohlberg H, Amunts K, Zilles K (2006) The human inferior parietal cortex: cytoarchitectonic parcellation and interindividual variability. Neuroimage 33:430–448 [DOI] [PubMed]
- Choi HJ, Zilles K, Mohlberg H, Schleicher A, Fink GR, Armstrong E, Amunts K (2006) Cytoarchitectonic identification and probabilistic mapping of two distinct areas within the anterior ventral bank of the human intraparietal cortex. J Comp Neurol 495:53–69 [DOI] [PMC free article] [PubMed]
- Collins AF, Ellis AW (1992) Phonological priming of lexical retrieval in speech production. Br J Psychol 83:375–388 [DOI] [PubMed]
- Copland DA, de Zubicaray GI, McMahon K, Wilson SJ, Eastburn M, Chenery HJ (2003) Brain activity during automatic semantic priming revealed by event-related functional magnetic resonance imaging. Neuroimage 20:302–310 [DOI] [PubMed]
- Costafreda SG, Fu CH, Lee L, Everitt B, Brammer MJ, David AS (2006) A systematic review and quantitative appraisal of fMRI studies of verbal fluency: role of the left inferior frontal gyrus. Hum Brain Mapp 27:799–810 [DOI] [PMC free article] [PubMed]
- Cubelli R, Lotto L, Paolieri D, Girelli M, Job R (2005) Grammatical gender is selected in bare noun production: evidence from the picture-word interference paradigm. J Mem Lang 53:42–59 [DOI]
- de Zubicaray GI, McMahon KL, Eastburn MM, Pringle AJ (2008) Negative priming in naming of categorically related objects: an fMRI study. Cortex 44:881–889 [DOI] [PubMed]
- Dell GS (1986) A spreading-activation theory of retrieval in sentence production. Psychol Rev 93:283–321 [DOI] [PubMed]
- Démonet J-F, Chollet F, Ramsay S, Cardebat D, Nespoulous J-L, Wise R, Rascol A, Frackowiak R (1992) The anatomy of phonological and semantic processing in normal subjects. Brain 115:1753–1768 [DOI] [PubMed]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K et al (2005) A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25:1325–1335 [DOI] [PubMed]
- Eickhoff SB, Grefkes C, Zilles K, Fink GR (2007a) The somatotopic organization of cytoarchitectonic areas on the human parietal operculum. Cereb Cortex 17:1800–1811 [DOI] [PubMed]
- Eickhoff SB, Paus T, Caspers S, Grosbras MH, Evans AC, Zilles K et al (2007b) Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage 36:511–521 [DOI] [PubMed]
- Eickhoff SB, Heim S, Zilles K, Amunts K (2009) A systems perspective on the effective connectivity of overt speech production. Phil Trans Royal Soc A 367:2399–2421 [DOI] [PMC free article] [PubMed]
- Friederici AD (2002) Towards a neural basis of auditory sentence processing. Trends Cogn Sci 6:78–84 [DOI] [PubMed]
- Geyer S (2003) The microstructural border between the motor and the cognitive domain in the human cerebral cortex. Springer, Wien [DOI] [PubMed]
- Geyer S, Ledberg A, Schleicher A, Kinomura S, Schormann T, Bürgel U et al (1996) Two different areas within the primary motor cortex of man. Nature 382:805–807 [DOI] [PubMed]
- Geyer S, Schleicher A, Zilles K (1999) Areas 3a, 3b, and 1 of human primary somatosensory cortex. Neuroimage 10:63–83 [DOI] [PubMed]
- Geyer S, Schormann T, Mohlberg H, Zilles K (2000) Areas 3a, 3b, and 1 of human primary somatosensory cortex. Part 2. Spatial normalization to standard anatomical space. Neuroimage 11:684–696 [DOI] [PubMed]
- Giesbrecht B, Camblin CC, Swaab TY (2004) Separable effects of semantic priming and imageability on word processing in human cortex. Cereb Cortex 14:521–529 [DOI] [PubMed]
- Grefkes C, Geyer S, Schormann T, Roland P, Zilles K (2001) Human somatosensory area 2: observer-independent cytoarchitectonic mapping, interindividual variability, and population map. Neuroimage 14:617–631 [DOI] [PubMed]
- Hauk O, Davis MH, Pulvermüller F (2008) Modulation of brain activity by multiple lexical and word form variables in visual word recognition: a parametric fMRI study. Neuroimage 42(3):1185–1195 [DOI] [PubMed]
- Heim S, Amunts K, Mohlberg H, Wilms M, Friederici AD (2006) Head motion during overt language production in fMRI. Neuroreport 17:579–582 [DOI] [PubMed]
- Heim S, Eickhoff SB, Ischebeck AK, Supp G, Amunts K (2007) Modality-independent involvement of the left BA 44 during lexical decision making. Brain Struct Funct 212(1):95–106 [DOI] [PubMed]
- Heim S, Eickhoff SB, Amunts K (2008) Specialisation in Broca’s region for semantic, phonological, and syntactic fluency? Neuroimage 40:1362–1368 [DOI] [PubMed]
- Hernandez AE, Kotz SA, Hofmann J, Valentin VV, Dapretto M, Bookheimer SY (2004) The neural correlates of grammatical gender decisions in Spanish. Neuroreport 15:863–866 [DOI] [PubMed]
- Indefrey P, Levelt WJM (2004) The spatial and temporal signatures of word production components. Cognition 92:101–144 [DOI] [PubMed]
- Jescheniak JD, Schriefers H, Hantsch A (2003) Utterance format affects phonological priming in the picture-word task: implications for models of phonological encoding in speech production. J Exp Psychol Hum Percept Perform 29:441–454 [DOI] [PubMed]
- Kouider S, Dehaene S, Jobert A, Le Bihan D (2007) Cerebral bases of subliminal and supraliminal priming during reading. Cereb Cortex 17:2019–2029 [DOI] [PubMed]
- La Heij W, Mak P, Sander J, Willeboordse E (1998) The gender-congruency effect in picture-word tasks. Psychol Res 61:209–219 [DOI]
- Levelt WJM (1989) Speaking. From intention to articulation. MIT Press, Cambridge
- Levelt WJM (2001) Spoken word production: a theory of lexical access. Proc Natl Acad Sci USA 98:13464–13471 [DOI] [PMC free article] [PubMed]
- Levelt WJM, Roelofs A, Meyer AS (1999) A theory of lexical access in speech production. Behav Brain Sci 22:1–75 [DOI] [PubMed]
- Longoni F, Grande M, Hendrich V, Kastrau F, Huber W (2005) An fMRI study on conceptual, grammatical, and morpho-phonological processing. Brain Cogn 57:131–134 [DOI] [PubMed]
- Meister IG, Buelte D, Sparing R, Boroojerdi B (2007) A repetition suppression effect lasting several days within the semantic network. Exp Brain Res 183:371–376 [DOI] [PubMed]
- Miceli G, Turriziani P, Caltagirone C, Capasso R, Tomaiuolo F, Caramazza A (2002) The neural correlates of grammatical gender: an fMRI investigation. J Cogn Neurosci 14:618–628 [DOI] [PubMed]
- Morosan P, Rademacher J, Schleicher A, Amunts K, Schormann T, Zilles K (2001) Human primary auditory cortex: cytoarchitectonic subdivisions and mapping into a spatial reference system. Neuroimage 13:684–701 [DOI] [PubMed]
- Nakamura K, Dehaene S, Jobert A, Le Bihan D, Kouider S (2007) Task-specific change of unconscious neural priming in the cerebral language network. Proc Natl Acad Sci USA 104:19643–19648 [DOI] [PMC free article] [PubMed]
- Noesselt T, Shah NJ, Jäncke L (2003) Top-down and bottom-up modulation of language related areas—an fMRI study. BMC Neurosci 4:13 [DOI] [PMC free article] [PubMed]
- O’Seaghdha PG, Marin JW (2000) Phonological competition and cooperation in form-related priming: Sequential and nonsequential processes in word production. J Exp Psychol Hum Percept Perform 26:57–73 [DOI] [PubMed]
- Padovani R, Calandra-Buonaura G, Cacciari C, Benuzzi F, Nichelli P (2005) Grammatical gender in the brain: evidence from an fMRI study on Italian. Brain Res Bull 65:301–308 [DOI] [PubMed]
- Raposo A, Moss HE, Stamatakis EA, Tyler LK (2006) Repetition suppression and semantic enhancement: an investigation of the neural correlates of priming. Neuropsychologia 44:2284–2295 [DOI] [PubMed]
- Rissman J, Eliassen JC, Blumstein SE (2003) An event-related FMRI investigation of implicit semantic priming. J Cogn Neurosci 15:1160–1175 [DOI] [PubMed]
- Scheperjans F, Hermann K, Eickhoff SB, Amunts K, Schleicher A, Zilles K (2008) Observer-independent cytoarchitectonic mapping of the human superior parietal cortex. Cereb Cortex 18:846–867 [DOI] [PubMed]
- Schiller NO (1999) Masked syllable priming of English nouns. Brain Lang 68:300–305 [DOI] [PubMed]
- Schiller NO (2008) The masked onset priming effect in picture naming. Cognition 106:952–962 [DOI] [PubMed]
- Schleicher A, Palomero-Gallagher N, Morosan P, Eickhoff SB, Kowalski T, de Vos K et al (2005) Quantitative architectural analysis: a new approach to cortical mapping. Anat Embryol 210:373–386 [DOI] [PubMed]
- Seger CA (1994) Implicit learning. Psychol Bull 115:163–196 [DOI] [PubMed]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, Farah MJ (1997) Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proc Natl Acad Sci USA 94:14792–14797 [DOI] [PMC free article] [PubMed]
- Thompson-Schill SL, D’Esposito M, Kan IP (1999) Effects of repetition and competition on activity in left prefrontal cortex during word generation. Neuron 23:513–522 [DOI] [PubMed]
- Tivarus ME, Ibinson JW, Hillier A, Schmalbrock P, Beversdorf DQ (2006) An fMRI study of semantic priming: modulation of brain activity by varying semantic distances. Cogn Behav Neurol 19:194–201 [DOI] [PubMed]
- van Berkum JJ (1997) Syntactic processes in speech production: the retrieval of grammatical gender. Cognition 64:115–152 [DOI] [PubMed]
- Vigneau M, Beaucousin V, Herve PY, Duffau H, Crivello F, Houde O, Mazoyer B, Tzourio-Mazoyer N (2006) Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. Neuroimage 30:1414–1432 [DOI] [PubMed]
- Zilles K, Schleicher A, Palomero-Gallagher N, Amunts K (2002) Quantitative analysis of cyto- and receptor architecture of the human brain. In: Mazziotta J, Toga A (eds) Brain mapping, the methods. Academic Press, San Diego, pp 573–602