Abstract
A hallmark of Parkinson’s disease (PD) is the progressive loss of the A9 midbrain dopaminergic (mDA) neurons in the substantia nigra pars compacta. Recently, multiple causative mutations have been identified in the leucine-rich repeat kinase 2 (LRRK2) gene for both familial and sporadic PD cases. Therefore, to investigate functional roles of LRRK2 in normal and/or diseased brain, it is critical to define LRRK2 expression in mDA neurons. To address whether LRRK2 mRNA and protein are expressed in mDA neurons, we purified DA neurons from the tyrosine hydroxylase (TH)-GFP transgenic mouse using FACS-sorting and analyzed the expression of LRRK2 and other mDA markers. We observed that all mDA markers tested in this study (TH, Pitx3, DAT, Nurr1 and Lmx1a) are robustly expressed only in GFP+ cells, but not in GFP− cells. Notably, LRRK2 was expressed in both GFP+ and GFP− cells. Consistent with this, our immunohistochemical analyses showed that LRRK2 is expressed in TH-positive mDA neurons as well as in surrounding TH-negative cells in the rat brain. Importantly, in the midbrain region, LRRK2 protein was preferentially expressed in A9 DA neurons of the substantia nigra, compared to A10 DA neurons of the ventral tegmental area. However, LRRK2 was also highly expressed in the cortical and hippocampal regions. Taken together, our results suggest that LRRK2 may have direct functional role(s) in the neurophysiology of A9 DA neurons and that dysfunction of these neurons by mutant LRRK2 may directly cause their selective degeneration.
Keywords: dopaminergic neurons, leucine-rich repeat kinase 2 (LRRK2), Parkinson’s disease, substantia nigra
Parkinson’s disease (PD) is the second most common neurodegenerative disease affecting more than 1% of the population over age 55. However, its etiology is largely unknown. Like other complex human disorders, PD is likely affected by both environmental and genetic factors. However, until recently the prevailing hypothesis was that PD-related neurodegeneration results from exposure to neurotoxin(s). While the sporadic form of PD afflicts the majority of sufferers, a small proportion of all PD cases (approximately 5%) is inherited representing the familial form [6]. During the last decade or so, intensive genetic linkage studies have mapped ten PD-related loci, PARK1-PARK13 [1]. Although these familial forms represent a minor population of PD, their identification and functional characterization are important because the underlying molecular genetic mechanisms may shed new insights into PD pathogenesis. Two groups recently reported that mutation of the leucine-rich repeat kinase 2 (LRRK2) gene, encoding dardarin (derived from the Basque word, dardara, for tremor), a 2527 amino acid protein, is responsible for PARK8-linked autosomal dominant PD [15, 22]. Strikingly, subsequent genetic studies indicated that LRRK2 mutations were found in approximately 3% to 7% of familial PD and 1% to 3% in sporadic PD in several ethnic populations [17], with the highest prevalence (up to 40%) in North Africans and Ashkanezi Jews [11, 14, 19]. Until now, studies showed that some mutant forms of LRRK2 (e.g., G2019S and R1441C) significantly increase kinase activity and cause cell death [5, 21]. Another study reported that overexpression of LRRK2 could induce cell death via apoptotic mechanisms [9].
To better understand the role of mutant LRRK2 in PD pathogenesis, it is critical to determine whether LRRK2 is expressed in mDA neurons, in particular in A9 DA neurons of the substantia nigra pars compacta, where major neuronal death is occurring in PD. At present, it is somewhat controversial whether LRRK2 mRNA is expressed in DA neurons of the brain. While two groups reported that LRRK2 mRNA is not expressed in the substantia nigra but rather in DA-innervated areas [4, 12], other groups concluded that it is expressed in DA neurons [5, 16, 20]. These conflicting data may be due to low expression of LRRK2 mRNA in DA neurons. In this report, we attempted to address this issue by analyzing LRRK2 mRNA expression in FACS-purified DA neurons and non-DA neurons. We also examined LRRK2 protein expression by immunohistochemistry using a specific antibody against LRRK2.
To address whether LRRK2 mRNA is expressed in mDA neurons, we attempted to isolate sufficient numbers of purified mDA neurons by FACS from the TH-GFP transgenic mouse [10].
GFP+ and GFP− cells have been isolated by fluorescence activated cell sorting (FACS) from E13-14 ventral mesencephalons (VM) of the TH-GFP transgenic mouse, as previously described [4]. Total RNAs from both GFP+ and GFP− cells were prepared using the TriReagent (Sigma) followed by treatment with DNase I (Ambion). For RT-PCR analysis, 5 μg RNA was transcribed into cDNA using the SuperScript™. Preamplification Kit (Life Technologies) and oligo (dT) or random primers. The cDNA was used as the template in the PCR assay using the following primers.
TH: 5′-TCCTGCACTCCCTGTCAGG-3′, 5′-CCAAGAGCAGCCCATCAAAGG-3′, 432bp;
Pitx3: 5′-CTCTCTGAAGAAGAAGCAGCG-3′, 5′-CCGAGGGCACCATGGAGGCAGC-3′, 491bp;
DAT: 5′-CAGAGAGGTGGAGCTCATC-3′, 5′-GGCAGATCTTCCAGACACC-3′, 328bp;
Nurr1: 5′-CGACATTTCTGCCTTCTCCT-3′, 5′-GAAAGGTAAGGTGTCCAGGA-3′, 300bp
Lmx1a: 5′-GGATCCCATATGGACGGCCTAAAGATGGAGGAGAA-3′, 5′-GGATCCCTCGAGTTAGAAGTAAGAATTCTGCATGGAGTA-3′, 1133bp, β-actin: 5′-GGTGATGACCTGGCCGTCAGGCAGCTCGTA-3′, 5′-AACCCCAAGGCCAACCGCGAGAAGATGACC-3′, 160bp
LRRK2: 5′-ACCAGAACAGTTTGCATGAGA-3′, 5′-AGCCCAGACACTGAATTTCTTG-3′, 674bp
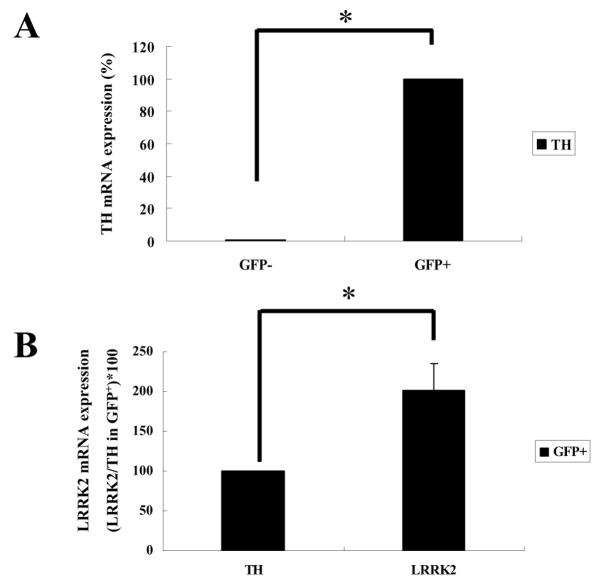
PCR reactions were carried out with 1x IN Reaction Buffer (Epicintre Technologies, Madison, WI), 1.4nM of each primer, and 2.5 units of Taq I DNA polymerase (Promega, Madison, WI). Samples were amplified in the Eppendorf Thermocycler (Brinkmann Instruments, Westbury, NY) under the following conditions: denaturing step at 95 °C, 40s; annealing step at 58°C, 30s; amplification step at 72°C, 1 min for 25–35 cycles and then the final amplification step at 72°C for 10min. For semi-quantitative RT-PCR, to avoid the saturation effect the number of PCR cycles was determined for each gene using the β-actin gene as the control (Fig. 1). In a previous study, these transgenic mice were successfully used to purify mDA neurons by FACS [3]. Consistent with this, the majority (>98%) of FACS-purified GFP+ cells from ventral mesencephalons (VM) of E13–14 transgenic mice were TH-positive (data not shown). Total RNAs were prepared from both GFP+ and GFP− cells and subjected to RT-PCR analysis for mRNA expression levels of LRRK2, midbrain DA marker (TH, Pitx3, DAT, Nurr1, Lmx1a), and control (β-actin) genes. As shown in Fig. 1, all DA marker genes tested were clearly detected in GFP+ cells. In GFP− cells, these markers were either non-detectable or only faintly detected, which could be due to contaminating cells during the FACS procedure. Notably, LRRK2 was prominently detected in both GFP+ and GFP− cells with higher expression in GFP+ DA neurons. To quantify LRRK2 expression levels, we performed real-time PCR analysis (Fig. 2). Real-time PCR was performed by SYBR green I using the Opticon DNA engine (MJ Research, Waltham, MA). Amplifications were performed in 25 μl containing 0.5 μM of each primer, 0.5X SYBR Green I (Molecular Probes, Inc., Eugene, OR) and 2 μl of five-fold diluted cDNAs that were generated using the same amount of total RNAs from GFP+ and GFP− cells. Fifty PCR cycles were performed with the temperature profile of 95°C for 30 seconds, 55°C for 30 seconds, 72°C for 30 seconds and 79°C for 5 seconds. The dissociation curve for each PCR product was determined to test the specificity of the fluorescent signals. After each PCR cycle, fluorescence was detected at 79°C to melt primer dimers (the Tm of all primer dimers used in this study was <76°C). Plasmid DNAs containing the glyceraldehydes-3-phosphate dehydrogenase (GAPDH) gene (from 104 to 109 molecules) were used to generate the standard curve. The fluorescent signals from specific TH and LRRK2 product were normalized against that of GAPDH gene, and then relative values were calculated by setting the value of TH in GFP+ cells as 100. Each experiment was performed in triplicate. As expected, the control GAPDH mRNA was comparably expressed in GFP+ and GFP− cells (data not shown), which was used for normalization of TH and LRRK2 mRNA levels. When the expression level of TH mRNA in GFP+ cells was set at 100, it was barely detectable (<0.5%) in GFP− cells (Fig. 2A). Surprisingly, in GFP+ cells the LRRK2 mRNA expression level was approximately twice that of the TH gene (Fig. 2B). In GFP− cells, the LRRK2 expression level was lower than in GFP+ cells (data not shown). Based on this result, we conclude that LRRK2 mRNA is abundantly expressed in mDA neurons while it is also expressed in non-DA cells in the midbrain area.
Fig. 1.
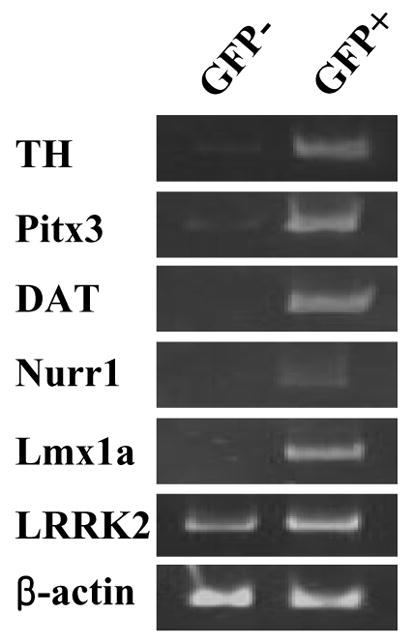
LRRK2 mRNAs are expressed in dopaminergic and non-dopaminergic neurons. (A) GFP+ cells (DA) and GFP− cells (non-DA) were sorted by FACS followed by RNAs extractions. RT-PCR analyses were performed using specific primers as described in Materials and Methods. The β-actin gene was used as a positive control.
Fig. 2.
Real-time PCR reactions were performed using SYBR green I. The standard curve was generated using plasmid DNA containing the GAPDH cDNA. The amount of the TH mRNA in GFP+ cells was set as 100 to calculate the relative amounts of each mRNA. Each reaction has been performed in triplicate using an identical amount of cDNAs as the template. The expression of TH and LRRK2 was measured as described in Materials and Methods. GAPDH expression levels were used to normalize mRNA levels of each gene. Asterisk indicates statistically significant difference from GFP− cells (*P< 0.01).
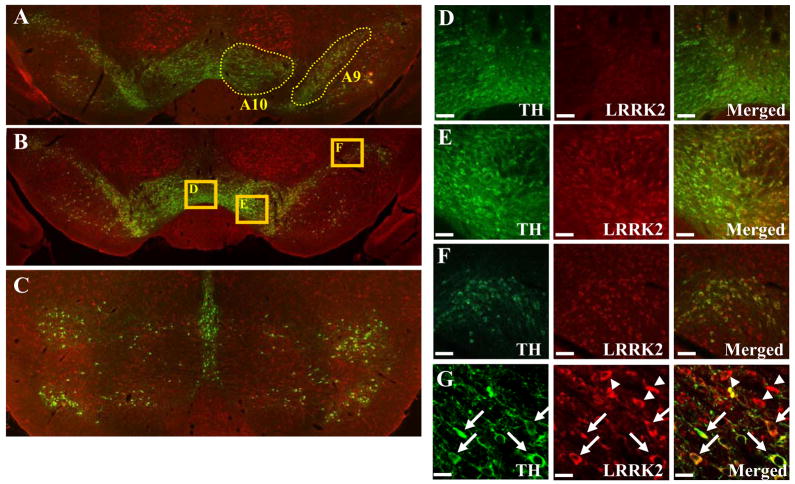
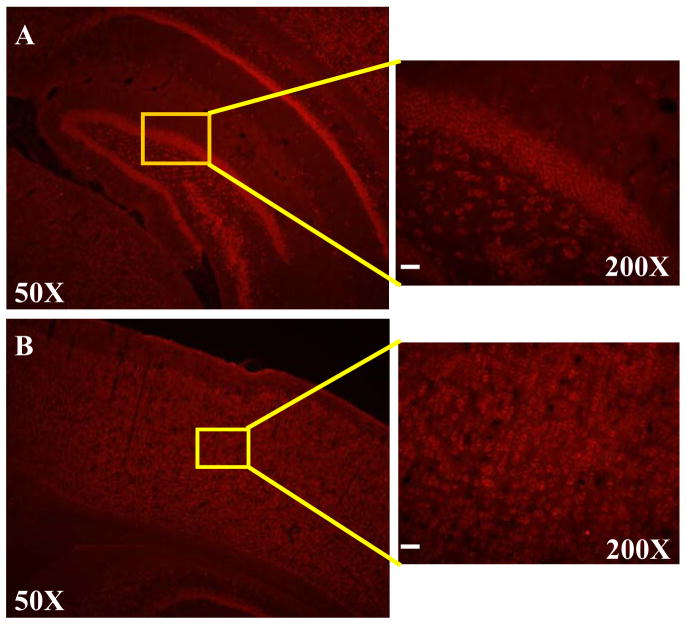
We next sought to examine the expression of LRRK2 protein in DA neurons by co-expression analysis using immunohistochemistry with anti-LRRK2 and anti-TH antibodies. Adult male Sprague-Dawley rats (3 months old with the weight of approximately 290g) were deeply anesthetized with an intraperitoneal injection of ketamine and xylazine, and perfused transcardially with saline, followed by 4% paraformaldehyde in 0.1M phosphate buffered saline (PBS). Brains were removed, postfixed overnight in the same fixative, and processed further as follows: brains were equilibrated in glycerol (20% in PBS), sectioned (30 μm) on a freezing microtome, and collected in PBS. Tissue sections were rinsed three times in PBS followed by pre-incubation in 10% normal goat serum (Jackson ImmunoResearch Labs., West Grove, PA, USA) for 1 h, and then incubated for overnight at 4°C with rabbit anti-LRRK2 (diluted 1:200, a gift from Dr. Hattori) and mouse anti-tyrosine hydroxylase (TH) (diluted 1:500; Pel-freez, Rogers, AR) antibody. For this purpose, we used a specific rabbit polyclonal antibody generated against synthetic peptides at the C-terminal end (2510–2527 a.a.) of human LRRK2. This antibody was characterized to be specific by immunohistochemistry and western blotting analyses. For instance, this antibody recognized a single band corresponding to LRRK2 (approximate molecular weight of 280 kDa) on Western blots from whole mouse brain extracts [7]. After a PBS rinse, sections were incubated with Alexa Fluor-conjugated secondary antibodies (Alexa Flour 488-conjugated anti-mouse IgG, Alexa Flour 594-conjugated anti-rabbit IgGs, diluted 1:200; Molecular Probes) for 1h at room temperature. Section were mounted and examined by fluorescent and by confocal microscopy (Zeiss, Zena, Germany). We sectioned adult rat brains study the expression pattern of LRRK2 in the broad midbrain area. As shown in Fig. 3, it appears that the majority of DA neurons co-express LRRK2 in the substantia nigra. In contrast, LRRK2 expression was either much weaker or undetectable in most DA neurons in the ventral tegmental area, suggesting that LRRK2 is preferentially expressed in A9 DA neurons of the substantia nigra, compared to A10 DA neurons of the ventral tegmental areas. In support this possibility, in the center of the ventral tegmental area (Fig. 3D), most of TH-positive neurons did not co-express LRRK2. At the borderline of the substantia nigra and the ventral tegmental area (Fig. 3E), the lateral part belonging to the substantia nigra showed more co-expression pattern compared to the medial part. At the far lateral part of the substantia nigra (Fig. 3F), the great majority of TH-positive neurons also expressed LRRK2. In a higher magnification, this co-expression pattern was clearly demonstrated (see arrows in Fig. 3G). In addition, there were many TH-negative cells that express LRRK2 (see arrowheads in Fig. 3G), which is consistent with RT-PCR results in Fig 1. In addition, consistent with previous studies, LRRK2 immunoreactivity was robustly detected in non-dopaminergic areas such as cortex and hippocampus (Fig. 4).
Fig. 3.
LRRK2 is continuously expressed in SN regions. Rat brain sections were incubated with LRRK2 antibody (red) and tyrosine hydroxylase (green) showing colocalization (yellow) in dopaminergic neurons in the substantia nigra. In (A), (B) and C, the expression of LRRK2 proteins in regions A9 and A10 is shown. High magnification of (B) is shown in (D), (E) and (F). Scale bar = 50μm. By using confocal microscopy, LRRK2 immunoreactivities are detected in both TH-positive and TH-negative neurons (G). Arrows indicate co-localization of LRRK2 and TH. Arrow heads indicate that some TH-negative cells also express LRRK2 proteins. Scale bar = 10μm.
Fig. 4.
LRRK2 is highly expressed in the cortical and the hippocampal regions. Rat brain sections were incubated with anti-LRRK2 antibody. Shown in the left column are representative fluorescence photographs of LRRK2 immunohistochemistry from rat brain at the hippocampus (A) and cortex (B) levels. In the right column, high magnification images corresponding to boxed areas from the overview image are shown for rat brain, hippocampal region (A) or cortical region (B). Scale bar=40μm.
Among the pathogenic genes whose mutations are linked to familial forms of PD, LRRK2 is the newest gene whose mutations account for substantial fractions of both familial and sporadic cases of PD [11, 14, 17]. Therefore, understanding the functional role(s) of LRRK2 and its mutant forms in normal and diseased brains will be critical for the etiology of PD. Initial northern blot analysis showed that LRRK2 is expressed in diverse brain areas such as the cerebellum, cerebral cortex, medulla, spinal cord, and putman [15]. Given that midbrain DA neurons and their nigrostriatal pathway are selectively degenerating in PD, it is important to determine whether LRRK2 is specifically expressed in these relevant neurons. Indeed, subsequent studies demonstrated that both LRRK2 mRNA and protein are abundantly expressed in the target area of the pathway, striatum, as well as in other brain areas such as cortex and cerebellum [4, 5, 12, 16]. However, whether LRRK2 is expressed in the midbrain DA neurons has been rather contentious. While some in situ hybridization analysis using oligonucleotide probes did not detect LRRK2 mRNA in the substantia nigra [4, 12], other studies using longer exposures of in situ hybridization with non-radioactive riboprobes detected low expression of LRRK2 mRNA [5, 16, 20]. While our study was in progress, more recent studies using specific LRRK2 antibodies showed that LRRK2 protein is abundantly and ubiquitously expressed in many brain regions, including the substantia nigra [8, 13]. In Biskup et al report, lrrk2 mRNA in brains from mouse embryos/neonates E6 to P28 was barely detectable at E15 then clearly visible at E17 [2] The difference between Biskup’s report and ours was releated to the brain retion used for our experiment. They used whole brain RNA but we used only GFP-TH positive neurons from the midbrain regions for RT-PCR.
The purpose of this study was to clarify the expression pattern of LRRK2 mRNA and protein in the midbrain DA neurons. In particular, we wished to address if LRRK2 is specifically expressed in A9 DA neurons of the substantia nigra, which are the major degenerating neurons in PD. Toward this goal, we attempted to isolate sufficient RNAs from FACS-purified DA neurons from embryonic ventral mesencephalon of the TH-GFP transgenic mice [3]. Indeed, the great majority of FACS-purified neurons were TH-positive while GFP-negative cells were TH-negative [3] (data not shown). In our RT-PCR analysis, we found that all midbrain DA marker genes tested were exclusively detected in GFP+ cells (Fig. 1). Thus, these GFP+ cells likely represent authentic midbrain DA neurons. LRRK2 mRNA was evidently detected in these GFP+ cells, leading us to conclude that midbrain DA neurons express LRRK2 mRNAs. Interestingly, our real-time PCR analysis indicates that the expression level of LRRK2 mRNA was approximately two-fold of TH mRNA. Therefore, our data suggest that midbrain DA neurons in fact express an abundant level of LRRK2 mRNAs, contrary to the notion of very low mRNA expression based on in situ hybridization studies [5, 8, 13, 16, 20]. While the absolute expression level of LRRK2 mRNA awaits further confirmation, we conclude that mDA neurons prominently and abundantly express LRRK2 mRNA. In addition, our RT-PCR and real-time PCR analyses show that LRRK2 mRNAs are also expressed in non-DA cells of the midbrain area, suggesting that LRRK2 expression is not restricted to DA neurons in the midbrain area. To define the LRRK2 protein expression pattern in the midbrain area, we next performed immunohistochemistry in a series of coronal rat brain sections containing A9-A10 regions. In this analysis, we used an antibody against LRRK2 which exhibited a high specificity to endogenous LRRK2 [7]. To examine the co-expression pattern, we also used a specific TH antibody as the DA marker. Interestingly, this co-immunohistochemistry revealed that most A9 DA neurons in the substantia nigra express LRRK2 while there are many LRRK2-expressing non-dopaminergic cells, which is consistent with our RT-PCR analysis (Fig. 2 and 3). In contrast, we found that LRRK2 expression was much weaker and/or undetectable in most A10 DA neurons of the ventral tegmental area. In addition, in agreement with previous studies, we also found that LRRK2 is robustly expressed in non-DA areas such as cortex and hippocampus (Fig. 4).
In summary, our results demonstrate that LRRK2 mRNA and protein are evidently expressed in midbrain DA neurons as well as in non-DA neurons in the rodent brain. Our findings furthermore indicate that LRRK2 is preferentially expressed in A9 DA neurons of the substantia nigra, compared to A10 DA neurons of the ventral tegmental areas. This finding may be important for our understanding of the functional role of LRRK2 in the pathogenesis of PD by its mutant forms. For instance, recent studies showed that some mutant forms of LRRK2 (e.g., G2019S and R1441C) showed significantly increased kinase activity and caused cell death [5, 21]. Other studies reported that overexpression of LRRK2 could induce cell death via apoptotic mechanisms [9]. If LRRK2 is only expressed in the dopaminoceptive areas, DA neuronal cell death by these LRRK2 mutant forms might be induced by alteration of synaptic communication between A9 DA neurons and target striatal neurons harboring mutant LRRK2. In contrast, since LRRK2 appears to be expressed in A9 DA neurons rather than in A10 DA neurons, mutant LRRK2 forms may directly affect DA neuronal death in these A9 neurons of the substantia nigra. Given that A10 neurons are largely spared in PD, our findings furthermore suggest an interesting possibility that selective A9 neuronal death is at least in part caused by preferential LRRK2 expression in the substantia nigra compared to the ventral tegmental area. Another important aspect is that LRRK2 is also expressed in several major non-DA brain regions. PD patients with dementia showed decrease of grey matter volume in several brain regions such as putamen, hippocampus or cingulated cortex [18]. Thus, it is also possible that mutant LRRK2 in these areas may confer cell death, leading to a decreased grey matter. Further investigation is warranted to address these important questions.
Acknowledgments
This work was supported by MH48866 and DC006501 (to KSK) and the Korea Foundation for International Cooperation of Science and Technology (M60602000011-06E0200-01010 to WS)
References
- 1.Belin AC, Westerlund M. Parkinson’s disease: A genetic perspective. FEBS J. 2008;275:1377–83. doi: 10.1111/j.1742-4658.2008.06301.x. [DOI] [PubMed] [Google Scholar]
- 2.Biskup S, Moore DJ, Rea A, Lorenz-Deperieux B, Coombes CE, Dawson VL, Dawson TM, West AB. Dynamic and redundant regulation of LRRK2 and LRRK1 expression. BMC Neurosci. 2007;8:102. doi: 10.1186/1471-2202-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donaldson AE, Marshall CE, Yang M, Suon S, Iacovitti L. Purified mouse dopamine neurons thrive and function after transplantation into brain but require novel glial factors for survival in culture. Mol Cell Neurosci. 2005;30:601–10. [PubMed] [Google Scholar]
- 4.Galter D, Westerlund M, Carmine A, Lindqvist E, Sydow O, Olson L. LRRK2 expression linked to dopamine-innervated areas. Ann Neurol. 2006;59:714–9. doi: 10.1002/ana.20808. [DOI] [PubMed] [Google Scholar]
- 5.Greggio E, Jain S, Kingsbury A, Bandopadhyay R, Lewis P, Kaganovich A, Van Der Brug MP, Beilina A, Blackinton J, Thomas KJ, Ahmad R, Miller DW, Kesavapany S, Singleton A, Lees A, Harvey RJ, Harvey K, Cookson MR. Kinase activity is required for the toxic effects of mutant LRRK2/dardarin. Neurobiol Dis. 2006;23:329–41. doi: 10.1016/j.nbd.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Gwinn-Hardy K. Genetics of parkinsonism. Mov Disord. 2002;17:645–56. doi: 10.1002/mds.10173. [DOI] [PubMed] [Google Scholar]
- 7.Hatano T, Kubo S, Imai S, Maeda M, Ishikawa K, Mizuno Y, Hattori N. Leucine-rich repeat kinase 2 associates with lipid rafts. Hum Mol Genet. 2007;16:678–90. doi: 10.1093/hmg/ddm013. [DOI] [PubMed] [Google Scholar]
- 8.Higashi S, Biskup S, West AB, Trinkaus D, Dawson VL, Faull RL, Waldvogel HJ, Arai H, Dawson TM, Moore DJ, Emson PC. Localization of Parkinson’s disease-associated LRRK2 in normal and pathological human brain. Brain Res. 2007;1155:208–19. doi: 10.1016/j.brainres.2007.04.034. [DOI] [PubMed] [Google Scholar]
- 9.Iaccarino C, Crosio C, Vitale C, Sanna G, Carri MT, Barone P. Apoptotic mechanisms in mutant LRRK2-mediated cell death. Hum Mol Genet. 2007;16:1319–26. doi: 10.1093/hmg/ddm080. [DOI] [PubMed] [Google Scholar]
- 10.Kessler MA, Yang M, Gollomp KL, Jin H, Iacovitti L. The human tyrosine hydroxylase gene promoter. Brain Res Mol Brain Res. 2003;112:8–23. doi: 10.1016/s0169-328x(02)00694-0. [DOI] [PubMed] [Google Scholar]
- 11.Lesage S, Durr A, Tazir M, Lohmann E, Leutenegger AL, Janin S, Pollak P, Brice A. LRRK2 G2019S as a cause of Parkinson’s disease in North African Arabs. N Engl J Med. 2006;354:422–3. doi: 10.1056/NEJMc055540. [DOI] [PubMed] [Google Scholar]
- 12.Melrose H, Lincoln S, Tyndall G, Dickson D, Farrer M. Anatomical localization of leucine-rich repeat kinase 2 in mouse brain. Neuroscience. 2006;139:791–4. doi: 10.1016/j.neuroscience.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 13.Melrose HL, Kent CB, Taylor JP, Dachsel JC, Hinkle KM, Lincoln SJ, Mok SS, Culvenor JG, Masters CL, Tyndall GM, Bass DI, Ahmed Z, Andorfer CA, Ross OA, Wszolek ZK, Delldonne A, Dickson DW, Farrer MJ. A comparative analysis of leucine-rich repeat kinase 2 (Lrrk2) expression in mouse brain and Lewy body disease. Neuroscience. 2007;147:1047–58. doi: 10.1016/j.neuroscience.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 14.Ozelius LJ, Senthil G, Saunders-Pullman R, Ohmann E, Deligtisch A, Tagliati M, Hunt AL, Klein C, Henick B, Hailpern SM, Lipton RB, Soto-Valencia J, Risch N, Bressman SB. LRRK2 G2019S as a cause of Parkinson’s disease in Ashkenazi Jews. N Engl J Med. 2006;354:424–5. doi: 10.1056/NEJMc055509. [DOI] [PubMed] [Google Scholar]
- 15.Paisan-Ruiz C, Jain S, Evans EW, Gilks WP, Simon J, Van Der Brug M, Lopez De Munain A, Aparicio S, Gil AM, Khan N, Johnson J, Martinez JR, Nicholl D, Carrera IM, Pena AS, De Silva R, Lees A, Marti-Masso JF, Perez-Tur J, Wood NW, Singleton AB. Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron. 2004;44:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 16.Simon-Sanchez J, Herranz-Perez V, Olucha-Bordonau F, Perez-Tur J. LRRK2 is expressed in areas affected by Parkinson’s disease in the adult mouse brain. Eur J Neurosci. 2006;23:659–66. doi: 10.1111/j.1460-9568.2006.04616.x. [DOI] [PubMed] [Google Scholar]
- 17.Singleton AB. Altered alpha-synuclein homeostasis causing Parkinson’s disease: the potential roles of dardarin. Trends Neurosci. 2005;28:416–21. doi: 10.1016/j.tins.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Summerfield C, Junque C, Tolosa E, Salgado-Pineda P, Gomez-Anson B, Marti MJ, Pastor P, Ramirez-Ruiz B, Mercader J. Structural brain changes in Parkinson disease with dementia: a voxel-based morphometry study. Arch Neurol. 2005;62:281–5. doi: 10.1001/archneur.62.2.281. [DOI] [PubMed] [Google Scholar]
- 19.Tan EK, Jankovic J. Genetic testing in Parkinson disease: promises and pitfalls. Arch Neurol. 2006;63:1232–7. doi: 10.1001/archneur.63.9.1232. [DOI] [PubMed] [Google Scholar]
- 20.Taymans JM, Van Den Haute C, Baekelandt V. Distribution of PINK1 and LRRK2 in rat and mouse brain. J Neurochem. 2006;98:951–61. doi: 10.1111/j.1471-4159.2006.03919.x. [DOI] [PubMed] [Google Scholar]
- 21.West AB, Moore DJ, Choi C, Andrabi SA, Li X, Dikeman D, Biskup S, Zhang Z, Lim KL, Dawson VL, Dawson TM. Parkinson’s disease-associated mutations in LRRK2 link enhanced GTP-binding and kinase activities to neuronal toxicity. Hum Mol Genet. 2007;16:223–32. doi: 10.1093/hmg/ddl471. [DOI] [PubMed] [Google Scholar]
- 22.Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, Stoessl AJ, Pfeiffer RF, Patenge N, Carbajal IC, Vieregge P, Asmus F, Muller-Myhsok B, Dickson DW, Meitinger T, Strom TM, Wszolek ZK, Gasser T. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–7. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]