Abstract
We previously reported that cell wall polysaccharide (CWPS) given to mice intranasally with adjuvant induces serotype-independent immunity to pneumococci. Some strains make CWPS with one phosphocholine group (CWPS/1), but most express two per tetrasaccharide repeat unit (CWPS/2). Here, CWPS/1 and CWPS/2 were equally protective against colonization by CWPS/2-type pneumococci, but the related Streptococcus mitis polymer lacking phosphocholine was non-protective. Previously the protection was shown to be CD4+ T cell-dependent, abrogated by antiserum to interleukin (IL)-17A, and demonstrable in antibody-defective mice. Here, CWPS failed to protect IL-17A receptor-knockout mice, further indicating IL-17A-dependence. When commercial CWPS/1 was size-fractionated preparatively, the larger exceeded the smaller molecules in their capacity to prime for IL-17A responses, and only the larger protected against pneumococcal colonization. However, a CWPS-tetanus toxoid conjugate -- despite raising high titers of phosphocholine antibody -- was non-protective, confirming the irrelevance of humoral immunity in this model. The results strengthen the concept that IL-17A-mediated T cell immunity is inducible by zwitterionic polysaccharides with sufficient chain-length to provide coiled secondary structure. Coupling CWPS to protein, which paradoxically prevents protection, may occlude this regular linear conformation. We suggest that mucosal immunization with CWPS primes TH17 cells, which - upon contact with the phosphocholine of colonizing pneumococci - elaborate IL-17A, enhancing phagocytosis.
Keywords: Streptococcus pneumoniae, cell-wall polysaccharide, vaccine, colonization
INTRODUCTION
The cell wall polysaccharide (CWPS) of Streptococcus pneumoniae (pneumococcus) is a peptidoglycan-attached teichoic acid common to all capsular serotypes examined, and the same polymer with a lipid anchor - called lipoteichoic acid - is associated with the cell membrane 1. In the strain examined (R6), these two morphologic forms of the teichoic acid are structurally identical 2. A major antigenic determinant of the polymer is the phosphocholine (PCho) sidechain 1. Beginning with the work of Briles and colleagues, PCho has been viewed as a possible focus of serotype-independent immunity: passive protection with antibody to PCho 3, 4 and active immunization with PCho-protein conjugates 5, 6 have been demonstrated in murine models. Other studies, however, reported non-protection 7–9, attributed to inaccessibility of the teichoic acids to antibody in fully encapsulated pneumococci 9, 10. Apart from this controversy, we reported that intranasal immunization of mice with purified CWPS (without protein conjugation), using cholera toxin as adjuvant, induced immunity measurable as increased clearance of serotype 6B pneumococci from the nasopharynx or as survival in an aspiration pneumonia model by a heavily encapsulated serotype 3 strain 11. Here, the mechanism of the protection is further examined.
Fischer and colleagues defined the repeating unit in strain R6 as -6)-β-D-Glcp-(1–3)-α-D-AATGalp-(1–4)-α-D-[6-PCho]GalNAc-(1–3)-β-D- [6-PCho] *GalNAc-(1-1)-D-ribitol-5-P(O- 2. However, the commercial CWPS reagent, made from strain CSR SCS2, lacks the PCho sidechain designated by the asterix 12. These forms are here designated CWPS/2 and CWPS/1, respectively. CWPS/2, found in most strains, expresses an antigenic specificity distinct from CWPS/1, which loses one PCho by mutation in the Licd 2 genetic region 13. An analogous cell wall polysaccharide in Streptococcus mitis has the identical tetrasaccharide-ribitolphosphate backbone as pneumococcal CWPS but contains no PCho 14; this is designated here as CWPS/0. Our previous intranasal immunization study 11 used the CWPS/1 commercial reagent, and the role of PCho was not specifically examined. Here the CWPS type of the challenge strain has been defined, the strain’s surface expression of PCho measured, and the protective activity of CWPS/0, CWPS/1, and CWPS/2 compared.
Previously the intranasal protection was shown to be CD4+ T cell-dependent and could be abrogated by administration of antiserum to interleukin (IL)-17A at the time of challenge 11. Here, to further examine the dependence upon the IL-17A pathway, receptor-knockout mice were tested. Although polysaccharides in general behave as T cell-independent antigens 15, CWPS is an example of a “zwitterionic” polysaccharide (in which the repeating unit contains both positively and negatively-charged ionic groups). Kasper and colleagues showed that such polysaccharides when injected into rats induce abscess formation through a CD4+ T cell- and IL-17A-dependent process 16, and we are exploring whether the intranasal pneumococcal immunity in mice is induced through the same mechanism. This T-cell activity of zwitterionic polysaccharides requires longer chains, which permit a coiled secondary structure displaying the charged groups laterally with regular spacing 17. The chain length variable had not been examined in our pneumococcal system, which used the somewhat size-disperse commercial (CWPS/1) reagent 11. Here we have used additional preparative molecular sieving to test the effect of size upon protection and the capacity to prime mice for IL-17A expression as determined in cell culture.
In the context of humoral immunity, polysaccharides are rendered more “T cell-dependent” by coupling to protein carriers 15. To test for a protein carrier effect in this particular form of T cell immunity and to further examine the effect of antibody in the system, a CWPS-tetanus toxoid conjugate potent in antibody induction was evaluated in the colonization model.
RESULTS
The Licd1-Licd2 genomic regions of the serotype 6B strain 0603, used herein and previously for colonization challenges 18, and the serotype 3 strain (WU2) used in our aspiration pneumonia model 11 were sequenced. Compared to the known CWPS/2 strain R6 13, strains 0603 and WU2 contain five wobble mutations — at position 166 C→T, 168 G→A, 498 C→T, 504 A→G, 585 A→G, and 1226 T→C, plus mutation 885 C→T that causes an amino acid change from histidine to tyrosine in the LicD2 gene. Since this histidine is not conserved among sequenced pneumococci, it is likely that 0603 and WU2 are of the common CWPS/2 phenotype.
The expression of the PCho determinant in the serotype 6B challenge strain 0603, as defined by accessibility to the IgA Mab TEPC-15, was assayed and compared to cultures of “opaque” and “transparent” variants of a strain of serotype 6A (6Ao and 6At, respectively). In this assay the 0603 challenge strain expressed PCho similarly to the transparent 6At culture, which exceeded the opaque variant 6Ao by about 1 log-fold (Table). Colonies of 0603, viewed in oblique light, resembled the transparent 6At variant (not shown).
TABLE.
PNEUMOCOCCAL SURFACE EXPRESSION OF THE PHOSPHORYL CHOLINE DETERMINANT AS DEFINED BY INHIBITION OF BINDING OF TEPC-15 TO CWPS/1 IN ELISA
| Strain | CWPS/1-equivalence, μg/109 cells |
|---|---|
| 0603 - challenge strain of present study | 6.5 |
| 6At - “transparent” phase selected from 6A strain | 9.1 |
| 6Ao - “opaque” phase selected from 6A strain | 0.7 |
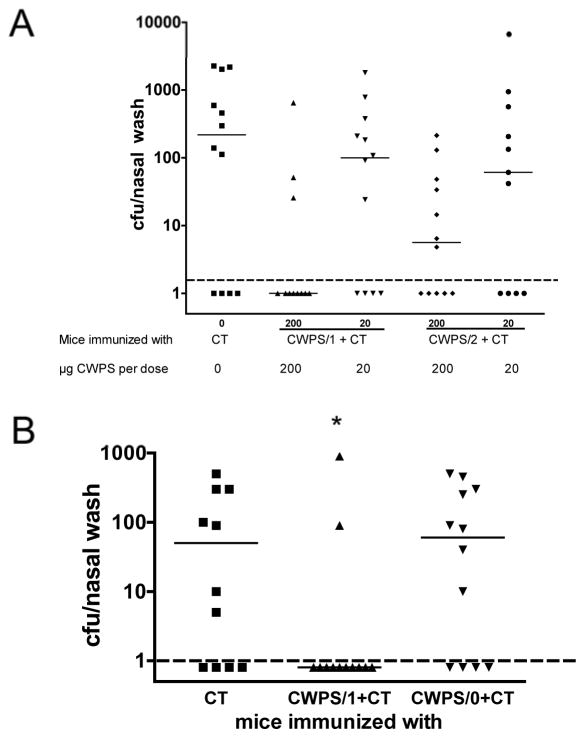
Immunization with various CWPS preparations was done intranasally twice with a 1-week interval using 1 μg of cholera toxin (CT) as adjuvant, and protection against colonization by intranasal challenge with strain 0603 was assayed 1 month post-immunization; all experiments included a control group receiving CT alone. Figure 1A compares CWPS/1 and CWPS/2 at doses of 20 μg and 200 μg, showing similar protection by both polymers. Figure 1B compares CWPS/0 with CWPS/1, showing protection only by the latter. Thus protection appeared to require the polymer to express PCho, but there was cross-protection by CWPS/1 and/2 and no evidence that the additional PCho group conferred enhanced protection.
Figure 1. Role of phosphorylcholine (PCho) in protection by CWPS.
(A) Mice (n=11–12 per group) were immunized with CT alone or CT with CWPS with either one (CWPS/1) or two (CWPS/2) PCho moieties per repeat unit, at doses of 20 or 200 μg. Four weeks after the second immunization, mice were challenged with strain 0603, and the density of colonization determined one week later. Shown are the medians with interquartile range for the density of colonization as determined by the cfu of nasal washes. There are no differences in protection afforded by immunization with CWPS/1 or CWPS/2 at either dose tested. (B). In contrast, CWPS from S. mitis (which lacks PCho, and is designated CWPS/0) is not protective as compared to CWPS/1 (n=12 per group, *P=0.045 by Mann-Whitney U for comparison of CWPS/1 vs. CT). Dashed line represents the lower limit of detection.
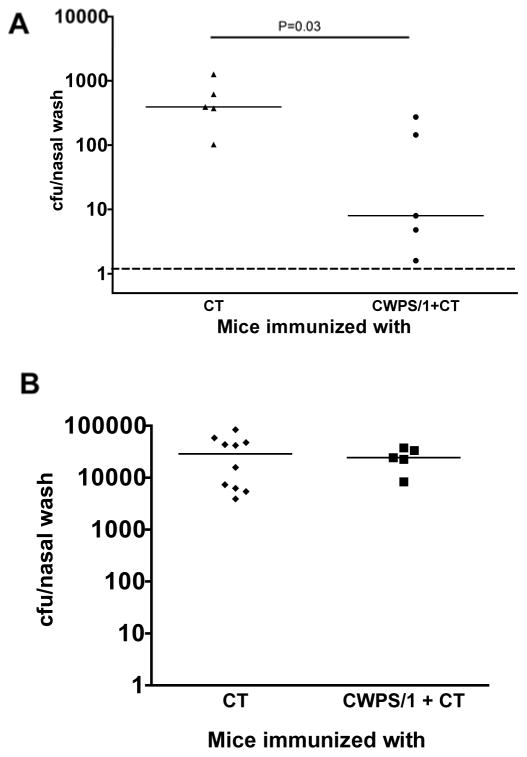
IL-17A-receptor knockout mice immunized with CWPS/1 were not protected (Figure 2B, P=NS) in contrast to wild-type animals (Figure 2A, P=0.035)
Figure 2. Role of IL-17A in protection from nasopharyngeal colonization induced by intranasal immunization with CWPS/1.
(A) Wild-type and (B) IL-17A-receptor knockout C57BL/6 mice (n=5–10 per group) were immunized, then challenged with type 6B pneumococcal strain 0603. IL-17A receptor-deficient mice were not protected (P>0.5 vs. CT by Mann-Whitney U) whereas wild type mice were significantly protected against NP colonization (P=0.03 by Mann-Whitney U). Depicted are the median and the interquartile range of density of colonization as determined by cfu of the nasal wash. Dashed line represents the lower limit of detection.
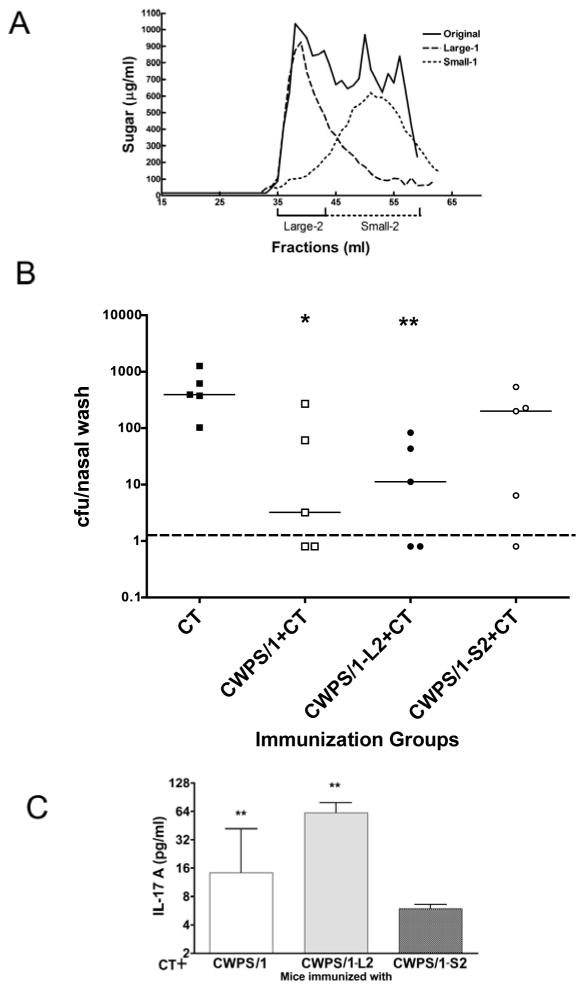
Figure 3A shows the size distribution on Sephacryl S300 of the standard CWPS/1 reagent and also of our separately rechromatographed larger (L1) and smaller (S1) fractions thereof. For immunization, the larger fractions of the L1 and smaller fractions of S1 were pooled as indicated as L2 and S2 respectively. As a relative estimate of chain length, the ratios of total to reducing terminal sugars in the preparations were determined to be 17 and 5.6 respectively, compared to 11 for the unfractionated CWPS/1. Figure 3B compares pools L2 and S2 with unfractionated CWPS/1 for protection against colonization: the unfractionated and L2 fractions gave about 20-fold reduction in cfu [P=0.03 and P=0.008, respectively, by Mann-Whitney U, compared to the controls receiving CT alone] while the S2 fraction was not protective (<3 fold reduction, P=0.16). Blood samples had been obtained from these mice 3 weeks post-immunization (one week before pneumococcal challenge) and assayed for IL-17A expression in vitro in response to killed pneumococcal cells (Figure 3C): mice immunized with the L2 fraction made significantly more IL-17A in response to in vitro pneumococcal stimulation than mice immunized with the S2 fraction (P=0.03 by Mann-Whitney U), an assay shown to correlate with protection against pneumococcal colonization 19.
Figure 3. Molecular sieve fractionation of CWPS/1 and the effect of size on protection against NP colonization and priming for IL17A elicitation in vitro.
(A) CWPS/1 was size-fractionated on a 1×115 cm column of Sephacryl S300 with PBS as eluant. A 10-mg sample was applied, and the elution profile of 1-ml fractions determined by assay of sugar content. Fractions 35–43 were pooled as L1 and fractions 44–59 as S1. The pools were dialyzed vs water, concentrated by lyophilization, then separately refractionated. Fractions 35–43 of L1 were pooled as L2 and fractions 44–59 of S1 pooled as S2; these pools were dialyzed and lyophilized. (B) C57BL/6 mice (n=5 per group) were immunized with CT alone, or CT with CWPS/1, the large fraction of CWPS/1 (CWPS/1-L2) or the small fraction (CWPS/1-S2). Mice immunized with CWPS or CWPS/1-L2 were significantly protected against colonization compared to mice immunized with CT alone (P<0.05 and <0.01 respectively by Mann-Whitney U), whereas mice immunized with CWPS/1-S2 were not protected. (C) Whole blood from mice immunized with CWPS/1 or CWPS/1-L2 produce significantly more IL-17A in response to stimulation with killed whole-cell pneumococcal antigen (WCA) than from mice immunized with CWPS/1-S2 (*P=0.03 by Mann-Whitney U). Dashed line represents the lower limit of detection.
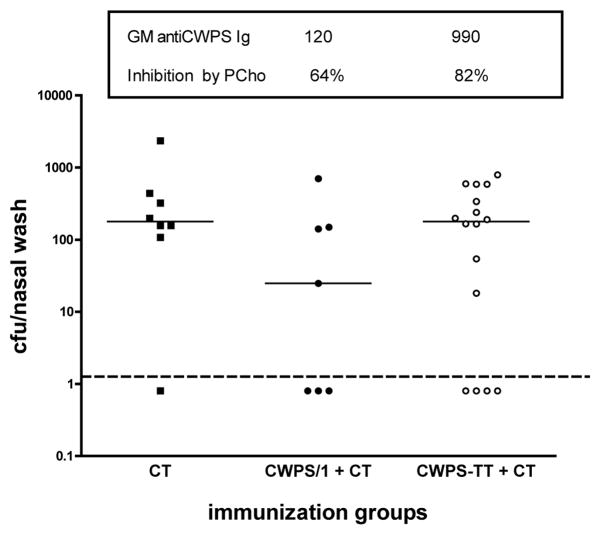
Mice were vaccinated with 20 μg of CWPS/1 (intended as a marginally protective dosage) or with 20 μg of CWPS/1 coupled to tetanus toxoid (CWPS-TT). Geometric mean serum antibody titers to CWPS/1 at 3 weeks post-immunization in the CWPS/1 and CWPS-TT groups were 120 and 990 respectively (P<0.001 by Mann-Whitney U test) with responses mainly against the PCho determinant (64% and 82% inhibition by excess PCho, respectively). Figure 4 shows, however, that while the marginal dose of unmodified polysaccharide reduced cfu by a log-fold (P=0.09 vs. group that received CT alone, by Mann-Whitney U test), the CWPS-TT induced no reduction of nasopharyngeal colonization (P>0.5 by Mann-Whitney vs. group that received CT).
Figure 4.
Effect of covalent coupling of CWPS/1 with tetanus toxoid upon activity as an intranasal immunogen: differential between antibody response and protection against colonization. Mice (n=7–16 per group) were vaccinated twice at a 1-week interval with 20 μg of CWPS/1 coupled to tetanus toxoid or the uncoupled CWPS/1; 1 μg of cholera toxin (CT) was used as adjuvant. At 3 weeks post-immunization, blood samples were taken for determination of serum antibody to CWPS/1 without and with inhibition with phosphocholine. Shown are geometric mean titers +/− 50 μg PCho/ml. At four weeks post immunization, mice were challenged intranasally with pneumococcus type 6B strain 0603, and nasopharyngeal carriage was determined one week later. Dashed line represents the lower limit of detection.
DISCUSSION
Previously, investigators found that injection of zwitterionic bacterial polysaccharides in rats would induce immunity through abscess formation by a mechanism dependent upon CD4+ T cells and IL-17A 16. Both positive and negative charged groups within the repeat unit of the polymer backbone and longer-chain polymers are required for activity 17.
In our previous study of pneumococcal immunity induced intranasally by CWPS in mice, activity was eliminated by N-acetylation, indicating that the positively-charged free amino group on the backbone AATgal was critical. Protection required CD4+ T cells. Splenocytes from CWPS-immunized mice produced IL-17A in vitro upon exposure to pneumococci, and administration of polyclonal antibody to IL-17A prior to challenge reduced protection 11. Thus there were indications that mucosal vaccination with CWPS operated by the mechanism described for zwitterionic polysaccharides. Here, in further examination, IL-17A-receptor knockout mice were not protected, confirming the importance of this pathway. The polymer length requirement had not been examined previously, but the present study has shown that protection requires the longer chains of CWPS/1 and that the longer were more active than the shorter chains in priming the animals for IL-17A expression in vitro. The size difference data per se would be ambiguous since antibody induction also increases with polysaccharide chain length 15 This reservation is irrelevant, however, since protection can be induced in antibody-negative mice 11, and increasing the serum antibody responses by coupling CWPS to TT did not increase protection in the present study. Interestingly, the coupling appears to have reduced protection. This result is consistent with the hypothesis that the polymer must be long enough to assume a coiled secondary structure displaying the charged groups laterally with regular spacing--for effective presentation to T cells 16, 17, 20. Possibly, random attachment of the TT protein along the CWPS chain would interfere with this conformation. Thus the induction by CWPS of pneumococcal immunity by the intranasal route further seems a particular example of the capacity of zwitterionic polysaccharides to generate memory TH17 cells. The mechanism is potentially antibody-independent: IL-17A was recently shown to stimulate killing of pneumococci by human polymorphonuclear leukocytes in vitro in the absence of opsonins in a system mimicking “surface phagocytosis” 19.
Coincidentally the commercial CWPS reagent is made from a strain expressing a single PCho group per repeat unit rather than the CWPS/2 made by most pneumococcal strains. It seemed worthwhile here to show that the serotype 6B strain routinely used in our nasopharyngeal colonization model is probably of the majority (CWPS/2) type. Interestingly CWPS/1 was as protective as CWPS/2 against this strain, thus there is no indication of importance of the extra antigenic specificity of the latter in induction of the immunity. Also, the serotype 3 strain WU2 used in our fatal aspiration pneumonia challenge model 11 is probably of the majority CWPS type; thus the cross-protection by CWPS/1 applies in this model as well. Both models begin by intranasal application of the pathogen. One might predict that i.n. vaccination with CWPS, like active and passive systemic immunization, would be ineffective in model infections wherein highly capsulated pneumococci are injected systemically. However, such models bypass the need to establish mucosal colonization--considered the prerequisite for natural pneumococcal infection 21.
CWPS has antigenic determinants other than PCho within the polymer “backbone”, and these appear to induce antibodies when CWPS/1 is presented intranasally (Fig. 4). The same backbone structure, lacking PCho, comprises the analogous cell-wall polymer of S. mitis 14. This polymer, here designated CWPS/0, was prepared for the present study using the same Sephacryl S300 size fractions as for CWPS/2 and the commercial CWPS/1. However, CWPS/0 did not induce protection, thus the backbone determinants appear unable to induce immunity effective in recognizing and clearing strain 0603 pneumococci from the nasopharynx. Kim and Weiser found that pneumococci can alternatively express a “transparent” phase that predominate in the nasopharynx and an “opaque” phase in the bloodstream. The former contain between 2.1 to 3.8-fold more total teichoic acid than the latter, which contain more capsular polysaccharide -- tending to occlude surface exposure of PCho. Exposure of PCho by transparent-phase pneumococci is thought to facilitate colonization by binding to the receptor for platelet-activating factor on mucosal cell surfaces 22. Here we examined surface expression of the PCho determinant, found it to be a log-fold greater in the characterized transparent 6A than in the opaque strain, and found our type 6B challenge strain to be similar to the transparent variant. The interaction of pneumococci with the mucosa depends upon a number of components23–25 but it appears that PCho contributes significantly to immune recognition. Thus we hypothesize that the zwitterionic property of intranasally applied CWPS permits presentation to and activation of CD4+ T cells, including those specific for PCho. When the mucosa is challenged with pneumococci, TH17 cells specific for PCho are activated and release IL-17A, which in turn recruits and activates polymorphonuclear leukocytes for phagocytic clearance of the pathogen from the mucosa. It has recently become appreciated that T cells are involved in clearance of pneumococci, independently of antibody 26–28. The specificity is by no means limited to CWPS--various surface antigens capable of T cell recognition may work 29, 30.
There is increasing interest in the possibility of prevention of pneumococcal infection by reducing colonization, for which a number of non-capsular surface antigens other than CWPS have been considered; e.g., a mixture of the proteins PspA and PsaA 31 or killed capsule-negative pneumococcal cells 18 protected against colonization. The potential of CWPS as a practical vaccine by itself is uncertain due to the large dosage required. It may be more promising as part of a three-component construct with pneumococcal proteins, as we have recently shown 32. In this study, a fusion protein of PsaA and the nonhemolytic pneumolysoid PdT conjugated to CWPS was protective against colonization, while a mixture of the fusion protein with CWPS was not. The dosage of CWPS in both instances (10 μg) would be non-protective per se, and the coupling affects the immunogenicity of the protein components as well as the polysaccharide. Thus the the role of CWPS in the fusion conjugate may be more complex than simply the target of immunity. The major significance of the protection induced by CWPS following mucosal administration is to shed light upon the natural acquisition of immunity to pneumococcal colonization, which increasingly appears to include mechanisms other than antibody to the capsule 27, 28, 33, 34.
MATERIALS AND METHODS
Biologics
CWPS/1 was the reagent “Pneumococcal Cell Wall Polysaccharide, Purified” of the Pneumococcal Reference Laboratory, Statens Seruminstitut (SSI), Copenhagen, Denmark. The purification of CWPS/1 from strain CSR SCS2 and its properties have been described 12. CWPS/2 was isolated from pneumococcal strain 22F-R-LSA/ICS and CWPS/0 from Streptococcus mitis strain SK598 14. The purification from these cultures was as described 12; in brief: lysis (CWPS/1 and 2 with deoxycholate, CWPS/0 with mutanolysin), deproteinization by chloroform-butanol, ethanol fractionation, treatment with DNase, RNase, and trypsin, and molecular sieving on Sephacryl S300. For all three CWPS preparations, the same Sephacryl S300 fractions were used, so the size distributions were similar. CWPS/1 coupled to tetanus toxoid was kindly furnished by Maya Koster: the CWPS was activated with 1-cyano-4-dimethylaminopuridinium tetrafluoroborate and coupled as described 35; the preparation contained 20 μg of protein per 20-μg immunizing dose of CWPS. Cholera toxin (CT) was from List Biological Laboratories (Campbell, CA). Cultures of a serotype 6A pneumococcus in the transparent (6At) or the opaque (6Ao) phases were kindly furnished by Jeffrey Weiser (University of Pennsylvania).
Sequencing of the Licd1-Licd2 genomic region
To infer the CWPS type expressed, sequence analysis of serotype 6B strain 0603 and serotype 3 strain WU2 was done just as described 13.
Biochemical techniques
Total sugar content of preparations was assayed by a microtiter adaptation of the anthrone method 36: to 20 μl of sample or standards was added 100 μl of reagent containing anthrone, 3 mg/ml in sulfuric acid; after heating 20 min at 100°C the A620 was determined. The assay was standardized with CWPS\1. Reducing terminal sugars were estimated by the Park-Johnson assay 37 standardized with D-glucose. The ratios of total to reducing sugars were calculated for an estimate of relative chain length of CWPS/1 fractions.
CWPS/1 was size-fractionated on a 1×115 cm column of Sephacryl S300 with PBS as eluant. A 10-mg sample was applied, and the elution profile of 1-ml fractions determined by carbohydrate assay. Fractions 35–43 were pooled as L1 and fractions 44–59 as S1. The pools were dialyzed vs. water, concentrated by lyophilization, and separately refractionated. Fractions 35–43 of L1 were pooled as L2 and fractions 44–59 of S1 pooled as S2; these pools were dialyzed and lyophilized.
Mouse immunization and colonization model
C57Bl/6 mice were obtained from Jackson Laboratories (Bar Harbor, Maine) and IL-17A receptor knockout mice (B6.129 IL17Ra−/−) were a kind gift of Jay Kolls (Louisiana State University, New Orleans, LA). Mice were randomized to receive between 10–20 μl of antigen (containing, except where indicated, 200 μg per dose of CWPS/1, CWPS/2, CWPS/0 or large and small fractions used in these experiments) with cholera intranasally twice at one-week interval. Three weeks following the last inoculation, mice were anesthetized for retro-orbital blood sampling. To determine susceptibility to nasopharyngeal (NP) colonization, i.n. challenge with live encapsulated pneumococci was done as described 18 4 weeks after the last immunization. Briefly, serotype 6B strain 0603 was grown to mid-log phase in Todd-Hewitt broth with 0.5% yeast extract, harvested by centrifugation, resuspended in saline at a concentration of 108 colony-forming units (cfu)/ml and stored at −80°C, then thawed just prior to challenge. In unimmunized animals this challenge results in robust colonization. All the studies here determined density of colonization at day 7 post-challenge: the mice were euthanized by CO2 inhalation, and a nasal wash was done by instilling sterile saline retrograde through the transected trachea, collecting the first 6 drops (about 0.1 ml) from the nostrils, and plating neat or diluted samples on blood agar plates containing 2.5 μg gentamicin/ml. For calculations of geometric means, a sterile sample was assigned half the lower limit of detection, or 0.8 cfu/nasal wash.
Enzyme-linked immunosorbent assay (ELISA)
Assays for murine serum antibodies to CWPS/1 were done in NUNC-immuno 96 microwell plates (Nalge Nunc International, Rochester NY) coated overnight with CWPS/1 (5 μg/ml). After antigen adsorption, the plates were washed with phosphate-buffered saline-0.05% Tween (PBS-T) and blocked with 5% fetal calf serum in PBS-T. To distinguish PCho-specific from CWPS “backbone”-specific antibodies, the diluted antibody samples were first co-incubated with PBS-T alone or PBS-T with phosphorylcholine (100 μg/ml, Sigma) for 30 minutes at room temperature, after which samples were added to the ELISA plates and incubated at room temperature for 2 hours. Plates were washed with PBS-T, and secondary antibody to mouse immunoglobulin (Sigma) was added and incubated at room temperature for one hour. The plates were washed and developed with SureBlue TMB microwell peroxidase substrate (KPL, Gaithersburg, MD). Titers were determined against a standard serum sample from a mouse immunized systemically with CWPS/1, whose value was arbitrarily set at 1000 units/ml. Percent reduction by phosphorylcholine was calculated to ascertain to what extent the measured antibodies were directed against PCho. The surface expression of the PCho determinant by pneumococci was assayed by inhibition of the ELISA in which 50 μl of mouse monoclonal antibody to PCho (TEPC-15, Sigma) diluted 1/5000 was premixed with 50 ul of serial dilutions of the bacteria or of CWPS/1 (as the standard) in PBS-5%BSA before addition to the CWPS-coated plates; Tween was omitted in the initial incubation so as not to disrupt the bacterial surface; the results were expressed as concentration-equivalence to CWPS/1.
Assay of IL-17A production in whole blood samples
Fifty μl of heparinized blood was added to 450 μl DMEM (BioWhittaker, Walkersville, MD) containing 10% low-endotoxin defined FBS (Hyclone, Logan, UT) and Ciprofloxacin (10 μg/ml, Cellgro, Manassas, VA). The cultures were incubated at 37 °C for 6 days with 107 killed pneumococcal cells. Supernatants were collected following centrifugation and stored at −80°C until analyzed by ELISA for IL-17A concentration (R&D Systems, Minneapolis, MN).
Acknowledgments
The authors wish to thank Dr. Maya Koster for providing the CWPS-TT conjugate, Dr. Jay Kolls for providing the IL-17A receptor knockout mice, and Dr. Jeffrey Weiser for providing the opaque and transparent variants of the serotype 6A pneumococcal strain and for helpful discussions. This work was supported by a grant from the National Institutes of Health (AI067737 to RM).
Footnotes
The authors declare that they have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tomasz A. Surface components of Streptococcus pneumoniae. Rev Infect Dis. 1981 Mar-Apr;3(2):190–211. doi: 10.1093/clinids/3.2.190. [DOI] [PubMed] [Google Scholar]
- 2.Fischer W, Behr T, Hartmann R, Peter-Katalinic J, Egge H. Teichoic acid and lipoteichoic acid of Streptococcus pneumoniae possess identical chain structures. A reinvestigation of teichoid acid (C polysaccharide) Eur J Biochem. 1993 Aug 1;215(3):851–857. doi: 10.1111/j.1432-1033.1993.tb18102.x. [DOI] [PubMed] [Google Scholar]
- 3.Briles DE, Nahm M, Schroer K, et al. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 streptococcus pneumoniae. J Exp Med. 1981;153(3):694–705. doi: 10.1084/jem.153.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldenberg HB, McCool TL, Weiser JN. Cross-reactivity of human immunoglobulin G2 recognizing phosphorylcholine and evidence for protection against major bacterial pathogens of the human respiratory tract. J Infect Dis. 2004 Oct 1;190(7):1254–1263. doi: 10.1086/424517. [DOI] [PubMed] [Google Scholar]
- 5.Wallick S, Claflin JL, Briles DE. Resistance to Streptococcus pneumoniae is induced by a phosphocholine-protein conjugate. J Immunol. 1983 Jun;130(6):2871–2875. [PubMed] [Google Scholar]
- 6.Trolle S, Chachaty E, Kassis-Chikhani N, et al. Intranasal immunization with protein-linked phosphorylcholine protects mice against a lethal intranasal challenge with streptococcus pneumoniae. Vaccine. 2000 Jul 1;18(26):2991–2998. doi: 10.1016/s0264-410x(00)00089-x. [DOI] [PubMed] [Google Scholar]
- 7.Szu SC, Schneerson R, Robbins JB. Rabbit antibodies to the cell wall polysaccharide of Streptococcus pneumoniae fail to protect mice from lethal challenge with encapsulated pneumococci. Infect Immun. 1986 Nov;54(2):448–455. doi: 10.1128/iai.54.2.448-455.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Musher DM, Watson DA, Baughn RE. Does naturally acquired IgG antibody to cell wall polysaccharide protect human subjects against pneumococcal infection? Journal of Infectious Diseases. 1990;161(4):736–740. doi: 10.1093/infdis/161.4.736. [DOI] [PubMed] [Google Scholar]
- 9.Nielsen SV, Sorensen UB, Henrichsen J. Antibodies against pneumococcal C-polysaccharide are not protective. Microb Pathog. 1993 Apr;14(4):299–305. doi: 10.1006/mpat.1993.1029. [DOI] [PubMed] [Google Scholar]
- 10.Skov Sorensen UB, Blom J, Birch-Andersen A, Henrichsen J. Ultrastructural localization of capsules, cell wall polysaccharide, cell wall proteins, and F antigen in pneumococci. Infect Immun. 1988 Aug;56(8):1890–1896. doi: 10.1128/iai.56.8.1890-1896.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malley R, Srivastava A, Lipsitch M, et al. Antibody-independent, interleukin-17A-mediated, cross-serotype immunity to pneumococci in mice immunized intranasally with the cell wall polysaccharide. Infect Immun. 2006 Apr;74(4):2187–2195. doi: 10.1128/IAI.74.4.2187-2195.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karlsson C, Jansson PE, Skov Sorensen UB. The pneumococcal common antigen C-polysaccharide occurs in different forms. Mono-substituted or di-substituted with phosphocholine. Eur J Biochem. 1999 Nov;265(3):1091–1097. doi: 10.1046/j.1432-1327.1999.00835.x. [DOI] [PubMed] [Google Scholar]
- 13.Skovsted IC, Kerrn MB, Sonne-Hansen J, et al. Purification and structure characterization of the active component in the pneumococcal 22F polysaccharide capsule used for adsorption in pneumococcal enzyme-linked immunosorbent assays. Vaccine. 2007 Aug 29;25(35):6490–6500. doi: 10.1016/j.vaccine.2007.06.034. [DOI] [PubMed] [Google Scholar]
- 14.Bergstrom N, Jansson PE, Kilian M, Skov Sorensen UB. A unique variant of streptococcal group O-antigen (C-polysaccharide) that lacks phosphocholine. Eur J Biochem. 2003 May;270(10):2157–2162. doi: 10.1046/j.1432-1033.2003.03569.x. [DOI] [PubMed] [Google Scholar]
- 15.Janeway CA, Travers P, Walport M, Shlomchik MJ. Immunobiology. New York: Garland Publishing; 2001. [Google Scholar]
- 16.Chung DR, Kasper DL, Panzo RJ, et al. CD4+ T cells mediate abscess formation in intra-abdominal sepsis by an IL-17-dependent mechanism. J Immunol. 2003 Feb 15;170(4):1958–1963. doi: 10.4049/jimmunol.170.4.1958. [DOI] [PubMed] [Google Scholar]
- 17.Kalka-Moll WM, Tzianabos AO, Wang Y, et al. Effect of molecular size on the ability of zwitterionic polysaccharides to stimulate cellular immunity. J Immunol. 2000 Jan 15;164(2):719–724. doi: 10.4049/jimmunol.164.2.719. [DOI] [PubMed] [Google Scholar]
- 18.Malley R, Lipsitch M, Stack A, et al. Intranasal immunization with killed unencapsulated whole cells prevents colonization and invasive disease by capsulated pneumococci. Infect Immun. 2001 Aug;69(8):4870–4873. doi: 10.1128/IAI.69.8.4870-4873.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu YJ, Gross J, Bogaert D, et al. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog. 2008 Sep;4(9):e1000159. doi: 10.1371/journal.ppat.1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cobb BA, Wang Q, Tzianabos AO, Kasper DL. Polysaccharide processing and presentation by the MHCII pathway. Cell. 2004 May 28;117(5):677–687. doi: 10.016/j.cell.2004.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Austrian R. Some aspects of the pneumococcal carrier state. J Antimicrob Chemother. 1986;18 (Suppl A):35–45. doi: 10.1093/jac/18.supplement_a.35. [DOI] [PubMed] [Google Scholar]
- 22.Kim JO, Weiser JN. Association of intrastrain phase variation in quantity of capsular polysaccharide and teichoic acid with the virulence of Streptococcus pneumoniae. Journal of Infectious Diseases. 1998;177(2):368–377. doi: 10.1086/514205. [DOI] [PubMed] [Google Scholar]
- 23.van Rossum AM, Lysenko ES, Weiser JN. Host and bacterial factors contributing to the clearance of colonization by Streptococcus pneumoniae in a murine model. Infect Immun. 2005 Nov;73(11):7718–7726. doi: 10.1128/IAI.73.11.7718-7726.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiser JN. Phase variation in colony opacity by Streptococcus pneumoniae. Microbial Drug Resistance. 1998;4(2):129–135. doi: 10.1089/mdr.1998.4.129. [DOI] [PubMed] [Google Scholar]
- 25.Cundell DR, Gerard NP, Gerard C, Idanpaan-Heikkila I, Tuomanen EI. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature. 1995 Oct 5;377(6548):435–438. doi: 10.1038/377435a0. [DOI] [PubMed] [Google Scholar]
- 26.Kadioglu A, Coward W, Colston MJ, Hewitt CR, Andrew PW. CD4-T-lymphocyte interactions with pneumolysin and pneumococci suggest a crucial protective role in the host response to pneumococcal infection. Infect Immun. 2004 May;72(5):2689–2697. doi: 10.1128/IAI.72.5.2689-2697.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCool TL, Weiser JN. Limited Role of Antibody in Clearance of Streptococcus pneumoniae in a Murine Model of Colonization. Infect Immun. 2004 Oct;72(10):5807–5813. doi: 10.1128/IAI.72.10.5807-5813.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malley R, Trzcinski K, Srivastava A, Thompson CM, Anderson PW, Lipsitch M. CD4+ T cells mediate antibody-independent acquired immunity to pneumococcal colonization. Proc Natl Acad Sci U S A. 2005 Mar 29;102(13):4848–4853. doi: 10.1073/pnas.0501254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trzcinski K, Thompson C, Malley R, Lipsitch M. Antibodies to conserved pneumococcal antigens correlate with, but are not required for, protection against pneumococcal colonization induced by prior exposure in a mouse model. Infect Immun. 2005 Oct;73(10):7043–7046. doi: 10.1128/IAI.73.10.7043-7046.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Basset A, Thompson CM, Hollingshead SK, et al. Antibody-independent, CD4+ T-cell-dependent protection against pneumococcal colonization elicited by intranasal immunization with purified pneumococcal proteins. Infect Immun. 2007 Nov;75(11):5460–5464. doi: 10.1128/IAI.00773-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Briles DE, Ades E, Paton JC, et al. Intranasal immunization of mice with a mixture of the pneumococcal proteins PsaA and PspA is highly protective against nasopharyngeal carriage of Streptococcus pneumoniae. Infect Immun. 2000 Feb;68(2):796–800. doi: 10.1128/iai.68.2.796-800.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu YJ, Forte S, Thompson CM, Anderson PW, Malley R. Protection against Pneumococcal colonization and fatal pneumonia by a trivalent conjugate of a fusion protein with the cell wall polysaccharide. Infect Immun. 2009 May;77(5):2076–2083. doi: 10.1128/IAI.01554-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Granat SM, Ollgren J, Herva E, Mia Z, Auranen K, Makela PH. Epidemiological evidence for serotype-independent acquired immunity to pneumococcal carriage. J Infect Dis. 2009 Jul 1;200(1):99–106. doi: 10.1086/599364. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Z, Clarke TB, Weiser JN. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J Clin Invest. 2009 Jun 8; doi: 10.1172/JCI36731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lees A, Nelson BL, Mond JJ. Activation of soluble polysaccharides with 1-cyano-4- dimethylaminopyridinium tetrafluoroborate for use in protein- polysaccharide conjugate vaccines and immunological reagents. Vaccine. 1996;14(3):190–198. doi: 10.1016/0264-410x(95)00195-7. [DOI] [PubMed] [Google Scholar]
- 36.Roe JH. The determination of sugar in blood and spinal fluid with anthrone reagent. J Biol Chem. 1955 Jan;212(1):335–343. [PubMed] [Google Scholar]
- 37.Park JT, Johnson MJ. A submicrodetermination of glucose. J Biol Chem. 1949 Nov;181(1):149–151. [PubMed] [Google Scholar]