Abstract
Mesenteric lymph node (mLN) CD103 (αE integrin)+ dendritic cells (DCs) induce regulatory T cells and gut tolerance. However, the function of intestinal CD103− DCs remains to be clarified. CD47 is the ligand of signal regulatory protein α (SIRPα) and promotes SIRPα+ myeloid cell migration. We first show that mucosal CD103− DCs selectively express SIRPα and that their frequency was augmented in the lamina propria and mLNs of mice that developed Th17-biased colitis in response to trinitrobenzene sulfonic acid. In contrast, the percentage of SIRPα+CD103− DCs and Th17 responses were decreased in CD47-deficient (CD47 knockout [KO]) mice, which remained protected from colitis. We next demonstrate that transferring wild-type (WT), but not CD47 KO, SIRPα+CD103− DCs in CD47 KO mice elicited severe Th17-associated wasting disease. CD47 expression was required on the SIRPα+CD103− DCs for efficient trafficking to mLNs in vivo, whereas it was dispensable on both DCs and T cells for Th17 polarization in vitro. Finally, administration of a CD47-Fc molecule resulted in reduced SIRPα+CD103− DC–mediated Th17 responses and the protection of WT mice from colitis. We thus propose SIRPα+CD103− DCs as a pathogenic DC subset that drives Th17-biased responses and colitis, and the CD47–SIRPα axis as a potential therapeutic target for inflammatory bowel disease.
DCs are located throughout mucosal surfaces and orchestrate the delicate balance between tolerance to innocuous antigens and the generation of protective immune responses upon exposure to pathogens. Different DC subsets populate the gut mucosa and mesenteric lymph nodes (mLNs), and their functions vary according to their anatomical location and conditioning by epithelial cells (Iwasaki, 2007). For instance, CD103+ and CD103− DC subsets isolated from mLNs display distinct phenotypes and cytokine profiles. A substantial proportion of CD103+ DCs continuously emigrate from the intestine to the mLNs in a CCR7-dependent manner (Johansson-Lindbom et al., 2005; Jang et al., 2006), where they represent from 40 to 70% of the DC population (Coombes et al., 2007; Jaensson et al., 2008). Under steady-state conditions, mLN CD103+ DCs are prone, in the presence of retinoic acid, to convert naive CD4+CD25− T cells into CD4+CD25+Foxp3+ T reg cells (Coombes et al., 2007; Sun et al., 2007), whereas CD103− DCs isolated from the mLNs and lamina propria (LP) of the small intestine express CX3CR1 and drive Th17 polarization in vitro (Denning et al., 2007; Atarashi et al., 2008). The mLN CD103− DCs appear to be directly derived from blood-borne precursors; express Toll-like receptor (TLR) 2, TLR4, and TBX21 to a greater extent than CD103+ DCs; and secrete high levels of TNF and IL-6 in response to stimulation (Coombes and Powrie, 2008). However, little is known about the precise contribution of each of these DC subsets during the development of intestinal inflammation.
Crohn's disease (CD) is a chronic, relapsing, T cell–driven inflammatory disease of the gastrointestinal tract thought to result from inappropriate mucosal immune responses to commensal bacteria in genetically susceptible individuals (Cho, 2008). DCs have been implicated in CD pathogenesis, because dysregulation in DC–epithelial cell interactions, defective migration and function of tolerogenic DCs, and/or aberrant immunogenic DC responses to pathogens have been proposed to participate in the induction of intestinal inflammation (Kelsall, 2008). Once initiated, the inflamed tissue is characterized by the expression of innate (IL-6, IL-12, TNF, and IL-23) and adaptive-derived (IL-6, IFN-γ, and IL-17) proinflammatory cytokines (Sanchez-Munoz et al., 2008). Antigen-presenting cell–derived TGF-β, IL-1β, IL-6, and IL-23 drive Th17 responses, whereas IL-12 and IFN-γ promote Th1 and suppress Th17 responses (Trinchieri, 1993; Bettelli et al., 2007).
Trinitrobenzene sulfonic acid (TNBS)–induced colitis is a mouse model of intestinal inflammation sharing many key features with human CD. TNBS administration first induces acute inflammation, characterized by the infiltration of neutrophils and macrophages, followed by antigen-specific priming of T cells. Challenge with a second dose of TNBS provokes a T cell–predominant reaction associated with tissue damage and mimics the chronic inflammation seen in CD (te Velde et al., 2006). Although classically considered a Th1 disease, the recently identified Th17 cell lineage appears to play an important role in the development of both TNBS colitis and CD (Annunziato et al., 2007; Sheibanie et al., 2007).
CD47, a marker of self on immune and nonimmune cells, is implicated at several levels in the generation of immune responses (van den Berg and van der Schoot, 2008). On the one hand, ligation of CD47 by the extracellular matrix protein thrombospondin-1, which is abundantly and rapidly expressed in tissues in response to inflammation (Reed et al., 1993), down-regulates IL-12p70 production by antigen-presenting cells (Armant et al., 1999) and impairs human naive T cell differentiation to Th1 cells in vitro without any immune deviation to Th2 cells (Avice et al., 2000). Recent data have also shown that CD47 expression on T cells is a self-control negative regulator of Th1 immune responses in vivo (Bouguermouh et al., 2008). On the other hand, CD47 interacts with signal regulatory protein α (SIRPα), its counterreceptor, which is selectively expressed on myeloid, endothelial, and neuronal cells (Adams et al., 1998; Ticchioni et al., 2001). The expression of CD47 on epidermal and dermal DCs promotes their migration to draining lymph nodes, where T cell priming and immune responses are initiated (Van et al., 2006). We first show that SIRPα is selectively expressed on one of the two major mucosal DC subsets, i.e., the CD103− DCs. We next demonstrate that SIRPα+CD103− DCs are the primary immunogenic DC subset involved in the development and perpetuation of Th17-associated TNBS-induced colitis, and that their migration is controlled by CD47.
RESULTS
CD47 regulates SIRPα+CD103− DC homeostasis in the LP and mLNs of naive and TNBS-treated mice
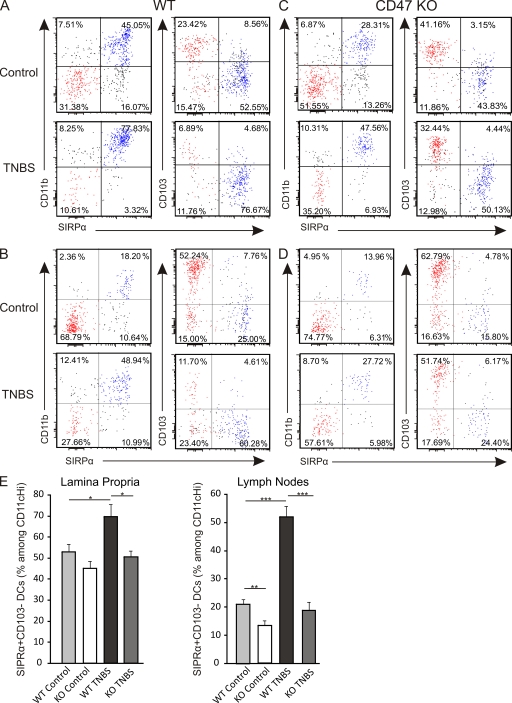
Multiple DC subpopulations have been identified and characterized by their expression of CD11c, CD11b, and CD103 in the intestinal LP and mLNs. Because CD47 has previously been shown to regulate cell migration of innate SIRPα+ cells (Liu et al., 2002; Van et al., 2006), we first thought to examine the expression of SIRPα on the two major mucosal DC subsets, i.e., CD103+ and CD103− DCs. We found that SIRPα was preferentially expressed with high intensity on CD11b+CD103− DCs in the LP and mLNs (Fig. 1, A and B). To investigate the dynamic regulation of these DC subsets during inflammation, their distribution profiles were assessed at steady state and in response to TNBS challenge. We observed an increased percentage of SIRPα+CD103− DCs in the LP as well as in the mLNs after the intrarectal administration of TNBS (Fig. 1, A and B), suggesting a role for this DC subset in the response to hapten-induced colonic inflammation. We next used CD47 KO mice to examine the potential role of CD47 in modulating the frequency of SIRPα+CD103− DCs in the LP and mLNs. Although no significant differences in SIRPα+CD103− DC proportions were observed in the LP of naive WT and CD47 KO mice (Fig. 1, A and C), a reduction in SIRPα+CD103− DCs was observed in the mLNs of naive CD47 KO mice when compared with their WT littermates (Fig. 1, B and D). Upon TNBS challenge, the increase in SIRPα+CD103− DCs normally observed in WT mice did not occur in either the LP or the mLNs of CD47 KO mice (Fig. 1, C and D), suggesting that CD47 is required for the mobilization of SIRPα+CD103− DCs in response to TNBS challenge. Although we show results from mLNs on day 2 after TNBS instillation, similar data were observed on day 4 and in caudal lymph nodes (unpublished data). Notably, the proportion of SIRPα−CD103+ DCs, which have been shown to promote tolerance, were not increased in the LP of either strain of TNBS-treated mice. However, their frequency was strongly reduced in the mLNs of WT but not CD47 KO TNBS-treated mice (Fig. S1). Of note, the intensity of CD47 expression was identical between SIRPα+CD103− and SIRPα−CD103+ DCs in the LP and mLNs of WT mice (unpublished data). Collectively, our results suggest that CD47 regulates the mobilization of mucosal SIRPα+CD103−DCs in response to inflammation and, thus, may play a role in gut DC homeostasis.
Figure 1.
CD47 regulates SIRPα+CD103− DC homeostasis in the LP and mLNs. (A–D) CD11b, CD103, and SIRPα expression among CD45.2+CD11cHi DCs at steady state (control; top) and 2 d after intrarectal administration of 3 mg TNBS (TNBS; bottom) in the LP (A and C) and mLNs (B and D) of WT (A and B) and CD47 KO (C and D) mice. Data shown are one representative mouse per group. (E) Percentage of SIRPα+CD103− DCs among CD45.2+CD11cHi DCs in the LP (left) and mLNs (right). Data represent the means ± SEM of more than eight mice per group. Each experiment was independently performed a minimum of four times. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
CD47 ablation ameliorates the development of chronic TNBS colitis
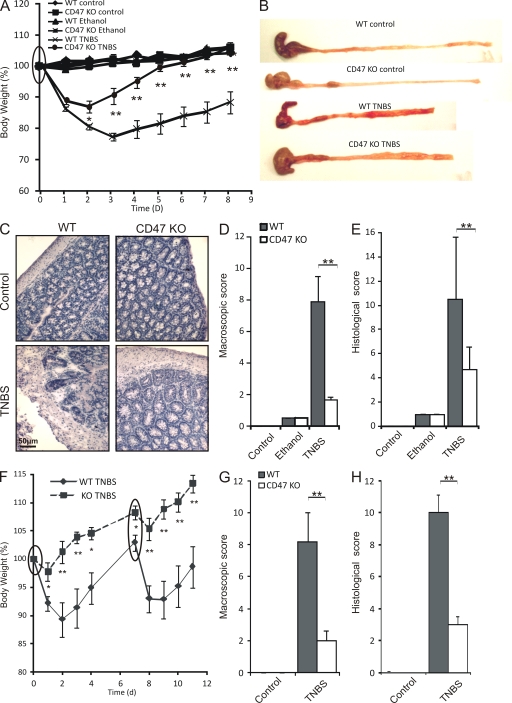
We therefore hypothesized that the increased proportion of SIRPα+CD103− DCs in the LP and mLNs of WT mice would be correlated with colitis development, in which case CD47 KO mice would be protected from disease. Analysis of body weight loss as early as day 2 after primary TNBS instillation revealed a significant reduction of wasting disease in CD47 KO compared with WT mice (Fig. 2 A). This protection was enhanced daily until day 8, by which time CD47 KO, as opposed to WT mice, had fully regained their original body weight (Fig. 2 A). To evaluate the degree of colonic inflammation, both the macroscopic and histological scores of tissue damage were assessed. Colons from WT mice with TNBS colitis displayed shortening and thickening, with patchy areas of severe ulceration and prominent adhesions typical of this form of colitis. On the contrary, CD47 KO mice developed fewer of these characteristics and displayed significantly milder inflammation than WT mice (Fig. 2, B and D). Upon histological assessment, we confirmed that colons from WT mice with colitis exhibited a substantial infiltration with mononuclear cells, edema, and loss of goblet cells, whereas CD47 KO mice were resistant to these changes (Fig. 2, C and E).
Figure 2.
CD47 ablation protects from the development of TNBS colitis. TNBS colitis was induced on day 0 alone or on days 0 and 7 (circled). (A) Weight loss curves of WT and CD47 KO mice injected intrarectally with saline (control), ethanol alone, or TNBS dissolved in 50% ethanol on day 0. (B–E) Macroscopic (B and D) and histological (C and E) analyses of inflammation in WT and CD47 KO mice. Data represent means ± SEM of more than eight mice per group, and one representative experiment out of three is shown. (F) Weight loss curves of WT and CD47 KO mice injected intrarectally with 2 mg TNBS dissolved in 50% ethanol on days 0 and 7. (G and H) Macroscopic (G) and histological (H) analyses of inflammation in WT and CD47 KO mice after reinduction of TNBS colitis. Data represent means ± SEM of six mice per group. One representative experiment out of three is shown. *, P < 0.05; **, P < 0.01.
We next confirmed the disease resistance of CD47 KO mice in a reactivated form of colitis, where a second instillation of TNBS was administered 7 d after the primary instillation. This model is said to mimic the relapsing behavior of human CD. Here, upon reinduction of TNBS colitis, CD47 KO mice remained resistant to body weight loss, whereas WT mice displayed severe wasting once again (Fig. 2 F). Macroscopic (Fig. 2 G) and microscopic (Fig. 2 H) scores confirmed that CD47 KO mice were largely devoid of signs of chronic colonic inflammation. Collectively, our data demonstrate that the CD47-dependent increase in SIRPα+CD103− DCs in the LP and mLNs is correlated with the development of chronic intestinal inflammation.
CD47 ablation impairs Th17 responses in vivo
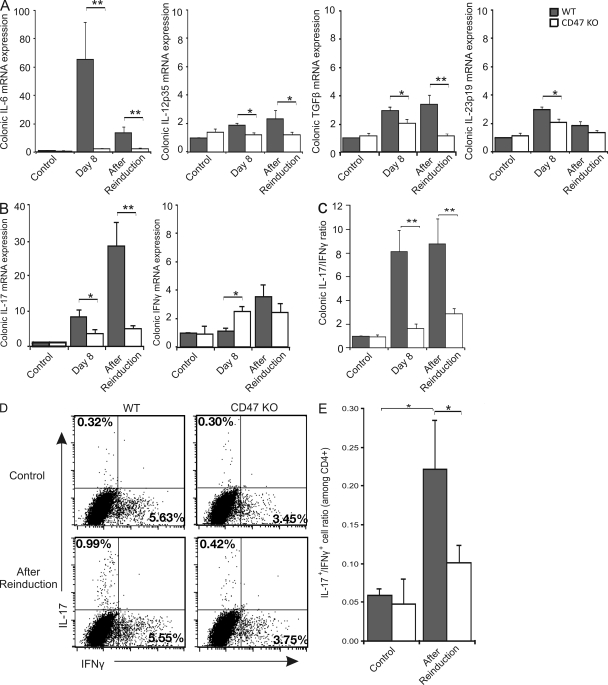
The chronic stage of TNBS colitis is driven by an important interplay between the innate and adaptive immune systems. We sought to further characterize this inflammation in WT and CD47 KO mice. Both on day 8 and after reinduction of TNBS colitis, a strong up-regulation of IL-6 mRNA expression was observed in WT but not in protected CD47 KO mice (Fig. 3 A). The same trend, but of a lesser magnitude, was observed for TGF-β, IL-12p35, and IL-23p19, where mRNA expression was increased in TNBS-treated WT but not CD47 KO mice (Fig. 3 A). Corroborating the increase in IL-6 and TGF-β expression, IL-17 mRNA was highly up-regulated at both time points in WT but not in CD47 KO mice (Fig. 3 B). IFN-γ mRNA expression was greater in CD47 KO than WT mice, but only on day 8, with no significant difference observed after reinduction (Fig. 3 B). Interestingly, although both forms of colitis displayed qualitatively and quantitatively distinct IL-17 and IFN-γ mRNA expression profiles, the IL-17/IFN-γ mRNA expression ratios were nearly identical, with both being higher in WT than CD47 KO mice (Fig. 3 C). We therefore chose to focus on the reinduction model of colitis for the rest of this study because it more accurately reflects the chronic relapsing behavior of CD.
Figure 3.
Decreased proinflammatory cytokine and Th17-biased responses in CD47 KO mice. (A–C) Colonic mRNA expression of cytokines. (A) Colonic IL-6, IL-12p35, TGF-β, and IL-23p19 mRNA expression. (B) Colonic IL-17 and IFN-γ mRNA expression. (C) Ratio of colonic IL-17/IFN-γ mRNA expression. Data represent mean fold changes relative to WT control ± SEM of more than eight mice per group (two independent experiments). (D) mLNs were isolated after reinduction of TNBS colitis and stimulated for 4 d in the presence of IL-23. Intracytoplasmic staining was performed after restimulation. The percentage IL-17+ or IFN-γ+ cells among total CD4+ T cells is shown. (E) Ratio of IL-17+/IFN-γ+ cells among CD4+ T cells. Data represent the mean percentages ± SEM of more than seven mice per group (two independent experiments). *, P < 0.05; **, P < 0.01.
We next postulated that the reduction in IL-17–associated cytokines in colon tissues was partly attributable to an impaired capacity to generate IL-17–producing T cells (Th17 cells) in the mLNs of CD47 KO mice. Therefore, we examined IFN-γ and IL-17 production in 4-d cultures of mLNs isolated from mice with chronic TNBS colitis. Analysis by flow cytometry revealed that the percentage of Th17 cells was significantly higher in WT compared with CD47 KO mice with colitis (Fig. 3 D). Similar data were observed by measuring IL-17 production in the culture supernatants by ELISA or by examining IL-17 expression in mLN cells after 6 h of PMA/ionomycin restimulation (unpublished data). Under these pro-Th17 culture conditions, no significant difference was observed with respect to CD4+ T cells producing IFN-γ (Fig. 3 D). When expressed as a ratio of IL-17+/IFN-γ+ cells, CD47 KO mice exposed to TNBS displayed a significantly reduced propensity toward IL-17 production (Fig. 3 E), corroborating with tissue mRNA expression and the recent shift in the Th1/Th17 paradigm reported in TNBS colitis (Sheibanie et al., 2007). Thus, CD47 ablation impairs the induction and perpetuation of colitis associated with a Th17-biased response in mLNs.
SIRPα+CD103− DCs promote Th17 responses in vitro
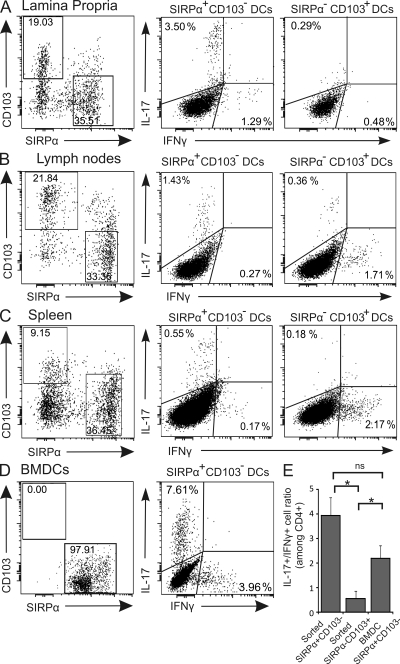
To establish causative links between the increased frequency of mucosal SIRPα+CD103− DCs and Th17 responses observed in vivo, we first sought to assess the in vitro Th1- versus Th17-promoting capacities of SIRPα+CD103− and SIRPα−CD103+ DCs. These two DC subpopulations were isolated from the LP and mLNs (Fig. S2) and co-cultured with CD4+ T cells from the same anatomical origin. We found that SIRPα+CD103− DCs were more efficient than SIRPα−CD103+ DCs at promoting IL-17 production by CD4+ T cells (Fig. 4, A and B). Interestingly, DCs purified from spleens behaved in a very similar manner as those isolated from LP and mLNs, with SIRPα+CD103− DCs favoring Th17 (Fig. 4 C).
Figure 4.
SIRPα+CD103− DCs promote Th17 responses in vitro. (A–C) Percentage of SIRPα+CD103− and SIRPα−CD103+ DCs (gated on CD11cHi; left) and their IFN-γ– versus IL-17–promoting capacities (middle and right) when isolated from the LP (A), mLNs (B), or spleen (C) and co-cultured with purified anti-CD3–stimulated CD4+ T cells of the same anatomical origin. Dot plots represent one independent experiment out of two or three. (D) Percentage of SIRPα+CD103− and SIRPα−CD103+ BMDCs (gated on CD11c+; left) and co-culture with OVA-stimulated CD4+ transgenic T cells under Th17-polarizing conditions (right). Data represent one independent experiment out of three. (E) IL-17+/IFN-γ+ cell ratio (among CD4+ T cells) after co-culture with either sorted SIRPα+CD103− or SIRPα−CD103+ DCs or SIRPα+CD103− BMDCs. The data of sorted cells represents mean of all sources of DCs (LP, mLNs, and spleen) ± SEM of a minimum of three independent experiments. *, P < 0.05.
When bone marrow–derived DCs (BMDCs) that express SIRPα (>99%) but not CD103 (<1%) were co-cultured with naive transgenic T cells under Th17-promoting conditions, they elicited a similar Th17-biased profile as SIRPα+CD103− mLN DCs, suggesting that these properties are not limited to gut-derived DCs (Fig. 4 D). Moreover, SIRPα+CD103− mucosal or splenic DCs and SIRPα+CD103− BMDCs induced statistically equivalent IL-17+/IFN-γ+ ratios, both of which were significantly higher compared with sorted SIRPα−CD103+ DCs (Fig. 4 E). Therefore, we propose that SIRPα+CD103− but not SIRPα−CD103+ DCs possess Th17-promoting properties in vitro.
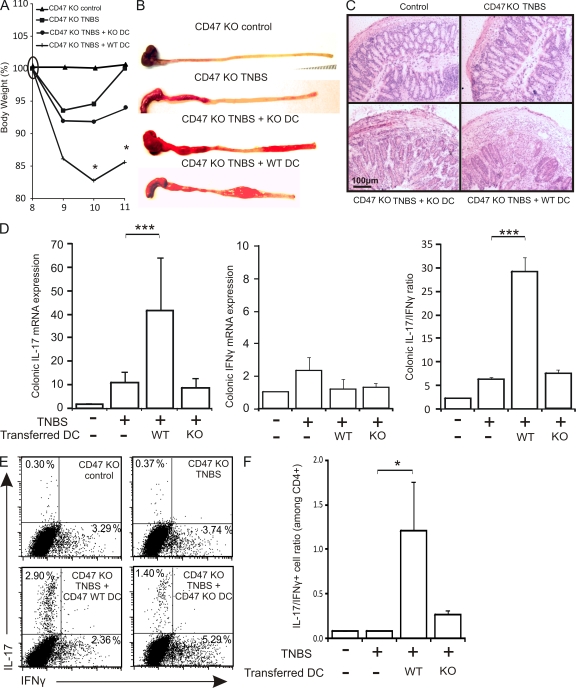
Transfer of SIRPα+CD103− BMDCs induces colitis and promotes a Th17 response in CD47 KO mice
To directly examine the proinflammatory capacity of SIRPα+CD103− DCs in vivo and the role of CD47 on the DCs for disease induction, CD47 KO mice were administered mature WT or CD47 KO SIRPα+CD103− BMDCs on the day of colitis induction. BMDCs were injected i.p. because previous reports have shown that the mLNs drain the peritoneal cavity, in addition to the gut tissues (Johansson-Lindbom et al., 2003; Kool et al., 2008). Moreover, the i.v. route was deemed unsuitable because i.v.-injected BMDCs migrate very poorly to the LNs, even when administered at high concentrations (3–8 × 106 BMDCs/mouse; Robert et al., 2003; Cavanagh et al., 2005). Upon reinduction of TNBS colitis on day 8, CD47 KO mice injected with WT BMDCs developed severe wasting disease (Fig. 5 A), indicating that CD47 expression was not required in the host for colitis development. Injection of WT BMDCs had no effect on disease outcome when injected in WT mice (Fig. S3). In contrast to WT BMDCs, injection of CD47 KO BMDCs in CD47 KO hosts appeared to induce only a mild form of inflammation that resulted in a slight, nonsignificant reduction of body weight (Fig. 5 A). The changes in body weight were reflected both in the macroscopic (Fig. 5 B) and microscopic (Fig. 5 C) extent of inflammation, with WT BMDC injection resulting in an aggressive form of disease. Notably, injection of WT BMDCs also increased the mortality rate of CD47 KO mice more than injection of CD47 KO BMDCs (uninjected, <5%; CD47 KO BMDCs, 25%; and WT BMDCs, 45%).
Figure 5.
CD47 expression on SIRPα+CD103− DCs promotes the development of intestinal inflammation and Th17 responses. CD47 KO mice were injected i.p. with saline or WT or CD47 KO BMDCs 30 min before the induction of TNBS colitis on day 0. TNBS was reinduced on day 8. (A) Weight-loss curves after transfer of WT or CD47 KO BMDCs in CD47 KO hosts and reinduction of TNBS colitis, normalized to body weight on day 8. (B and C) Macroscopic (B) and histological (C) appearance of colonic inflammation. Images are of one representative mouse per experimental group. (D) Colonic mRNA expression of IL-17 and IFN-γ and IL-17/IFN-γ mRNA expression ratio. Data represent the mean fold changes relative to control. (E) mLNs were isolated after reinduction of TNBS colitis and stimulated for 4 d in the presence of IL-23. The percentages of IL-17+ or IFN-γ+ CD4+ T cells and the ratio of IL-17+/IFN-γ+ cells among CD4+ T cells are shown. Data represent means ± SEM of more than five mice per group (two pooled independent experiments). *, P < 0.05; ***, P < 0.001.
We next examined whether disease development after WT BMDC injection was associated with the induction of Th17-biased responses in CD47 KO hosts. Injection of WT BMDCs significantly enhanced the colonic mRNA expression of IL-17 but not IFN-γ, whereas injection of CD47 KO BMDCs had no effect on the colonic mRNA expression of either cytokine (Fig. 5 D). Therefore, only WT BMDCs resulted in a significant increase in the IL-17/IFN-γ mRNA ratio (Fig. 5 D). To verify whether the induction of colonic IL-17 mRNA expression was linked to an increase in Th17 cells, we examined the mLNs of reconstituted mice. Injection of WT BMDCs increased the proportion of IL-17+ CD4+ T cells, leading to a significant enhancement in the IL-17+/IFN-γ+ CD4+ T cell ratio (Fig. 5, E and F). On the other hand, injection of CD47 KO BMDCs elicited IL-17 expression that did not translate into a significant increase in the IL-17/IFN-γ ratio (Fig. 5, E and F). Finally, SIRPα+CD103− DCs purified from WT mLNs and injected i.p. were also capable of inducing severe wasting disease, resulting in 25% mortality after a single dose of TNBS in CD47 KO mice (Fig. S4). Severe weight loss in these mice was correlated with pronounced colonic inflammation and Th17-polarized responses, corroborating the similar in vitro pro-Th17 responses of SIRPα+CD103− BMDCs and mucosal DCs. These data demonstrate that CD47 expression is dispensable on the endothelium, epithelium, and T cells of the host but appears to be critical on SIRPα+CD103− DCs for Th17-associated colonic disease induction in vivo.
SIRPα+CD103− DC trafficking but not phenotype or function is governed by CD47
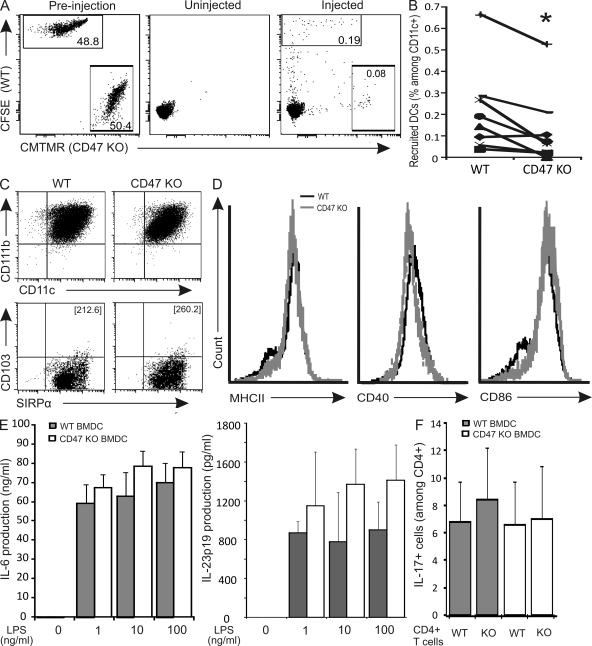
We next sought to delineate the mechanisms by which CD47 expression on SIRPα+CD103− BMDCs controls the development of Th17 responses and colonic inflammation. To this end, we evaluated the in vivo migratory properties and the in vitro function of WT and CD47 KO BMDCs. First, WT and CD47 KO BMDCs were fluorescently labeled and injected i.p. at a 1:1 ratio in CD47 KO mice and retraced in the mLNs after 2 d. By using this competitive migration assay, we provide evidence that CD47 expression on BMDCs significantly favors their accumulation in the mLNs (Fig. 6, A and B), corroborating the increased mobilization of SIRPα+CD103− mucosal DCs observed in WT but not CD47 KO mice upon induction of TNBS colitis (Fig. 1).
Figure 6.
CD47 regulates SIRPα+CD103− DC trafficking but not phenotype or function. (A, left) 1:1 ratio of labeled BMDCs before injection on day 0. (middle and right) TNBS colitis was induced in CD47 KO mice, and mLNs were harvested after 2 d. (middle) CD11c+ DCs in the mLNs of uninjected CD47 KO mice. (right) Labeled WT and CD47 KO BMDCs (gated on CD11c+) in the mLNs of BMDC-injected CD47 KO mice. One representative mouse is shown for each condition. (B) Percentages of labeled WT and CD47 KO BMDCs (gated on CD11c+) in the mLNs of CD47 KO mice. Data were generated in two independent experiments (n = 8). (C and D) Analysis of phenotype (C) and activation status (D) of LPS-stimulated BMDCs. The numbers in brackets in C represent the mean fluorescence intensity of SIRPα. Data are representative of three independent experiments. (E) Cytokine production by BMDCs stimulated overnight with LPS (0–100 ng/ml) and IFN-γ (10 ng/ml) in vitro. Data represent the means ± SEM (n = 4 independent experiments). (F) Percentage of IL-17+ cells (among CD4+ T cells) after criss-cross co-cultures with anti-CD3–stimulated WT versus CD47 KO splenic CD4+ T cells and LPS-stimulated WT versus CD47 KO BMDCs under Th17-polarizing conditions. Data represent means ± SEM (n = 3 independent experiments with two to three mice per experimental group).
Next, we assessed the phenotype of BMDCs in terms of their expression of CD11c, CD11b, SIRPα, and CD103 and observed no difference between WT and CD47 KO BMDCs (Fig. 6 C). Importantly, the level of expression of SIRPα was not altered on a per-cell basis by the expression of CD47, as determined by its mean fluorescence intensity (Fig. 6 C). The surface expression of MHC II and the co-stimulatory molecules CD40 and CD86 were also independent of CD47 expression (Fig. 6 D). We next examined the function of WT and CD47 KO BMDCs by measuring the production of cytokines such as IL-6 and IL-23p19, as well as their ability to drive Th17 polarization. Both cytokine production and Th17-promoting capacity were found to be unrelated to CD47 expression on the DCs (Fig. 6, E and F). Also, lack of CD47 expression on T cells did not alter the percentage of IL-17–producing CD4+ T cells, thereby eliminating the possibility of a T cell–intrinsic defect in Th17 polarization in CD47 KO T cells as a mechanism for decreased Th17 responses (Fig. 6 F). Because CD47 does not alter the phenotype, activation status, cytokine production, or Th17-polarizing capacity of SIRPα+CD103− BMDCs, we propose that CD47 expression on SIRPα+CD103− DCs is critical for their trafficking to mLNs, a property mechanistically related to the ensuing Th17 responses and colitis development.
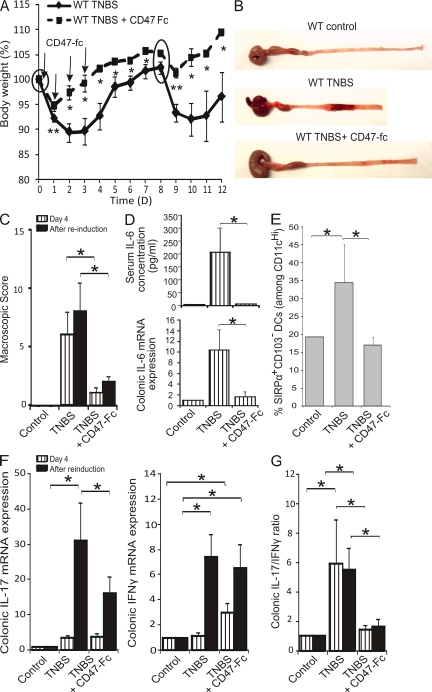
Administration of CD47-Fc ameliorates disease development in BALB/c mice
Finally, we circumvented the multiple defects of CD47 KO hosts and directly examined the impact of targeting the SIRPα–CD47 axis on the recruitment of SIRPα+CD103− DCs in mLNs, Th17 polarization, and the development of chronic colitis in WT mice. To this end, BALB/c mice were administered a CD47-Fc molecule i.p. 30 min before TNBS injection and on days 1–3 (Fig. 7 A, arrows). Mice administered CD47-Fc were largely protected from colonic inflammation as assessed by body weight, starting as early as 1 d after the induction of colitis (Fig. 7 A). Furthermore, CD47-Fc–treated mice remained resistant to colitis even after a second induction of TNBS colitis, as demonstrated by a reduction in body weight loss and the extent of tissue damage (Fig. 7, A–C). Injection of a control human CD47-Fc with no cross-reactivity in mice did not ameliorate disease (not depicted). Serum levels and colonic mRNA expression of IL-6 were determined early in disease onset (day 4) and were dramatically reduced in CD47-Fc–treated mice (Fig. 7 D). Importantly, the percentage of SIRPα+CD103− DCs was specifically and significantly reduced by CD47-Fc treatment (Fig. 7 E). On day 4, IL-17 and IFN-γ mRNA expression were unaffected. After reinduction, IL-17 mRNA expression was effectively suppressed by CD47-Fc, whereas IFN-γ mRNA expression was unaltered (Fig. 7 F). Regardless of the time of sacrifice (day 4 or after reinduction), the IL-17/IFN-γ mRNA ratio was nearly identical and equally suppressed by administration of CD47-Fc (Fig. 7 G). Similarly, the Th17/Th1 ratio in mLNs was also reduced by CD47-Fc (unpublished data). We therefore conclude that CD47-Fc treatment protects mice from the development of chronic colitis and is correlated with an altered development of SIRPα+CD103− DC–mediated Th17 responses.
Figure 7.
Administration of CD47-Fc protects BALB/c mice from the development of TNBS colitis. WT BALB/c mice were injected i.p. with saline or CD47-Fc, TNBS colitis was induced on days 0 and 8 (circles), and mice were sacrificed on day 4 or 12 (after reinduction). (A) Weight-loss curves normalized to body weight on day 0. (B) Macroscopic appearance and (C) mean macroscopic score inflammation. (D) Serum concentration (top) and colonic mRNA expression (bottom) of IL-6. (E) mLN SIRPα+CD103− DCs as a percentage of total CD11cHi. (F) Colonic mRNA expression of IL-17 and IFN-γ. (G) IL-17/IFN-γ mRNA expression ratio. Data represent means ± SEM of more than six mice per group of two independent experiments. *, P < 0.05; **, P < 0.01.
DISCUSSION
DCs lie at the interface between the innate and the adaptive immune system, and although they are poised to maintain gut homeostasis and tolerance, they also appear to play a critical role in CD pathogenesis. In this paper, we propose SIRPα-expressing CD11b+CD103− DCs as one primary candidate for the induction and perpetuation of chronic TNBS colitis and implicate CD47 as a key player in controlling their homeostasis and trafficking to the LP and mLNs. We first observed a correlation between the frequency of SIRPα+CD103− DCs in the LP and mLNs, Th17 responses, and the development of intestinal inflammation, parameters that were all impaired in CD47 KO mice. Next, we demonstrated that CD47 expression is required on SIRPα+CD103− DCs for efficient trafficking to mLNs and induction of severe wasting Th17-associated disease in vivo, but is dispensable on both SIRPα+ DCs and T cells to promote Th17 responses in vitro. Finally, early administration of a CD47-Fc molecule treated and prevented the recurrence of experimental colitis, which was correlated with defective homing of SIRPα+CD103− DCs and Th17 responses. We thus provide evidence that CD47 expression on SIRPα+CD103− DCs governs their trafficking and DC-driven Th17 responses, which are likely implicated in the development of intestinal inflammation.
Although the importance of DCs in the initiation of intestinal inflammation is now well accepted (Coombes and Powrie, 2008), the specific contributions of individual DC subsets and the key molecules involved in their function/migration remain to be clarified. This is the first study to identify SIRPα expression on mucosal CD103− DCs and to support a role for this DC subset in the in vivo promotion of intestinal inflammation. Nevertheless, although the proportion of total SIRPα+CD103− DCs increased in the LP and mLNs of WT mice upon induction of TNBS colitis, the percentage of the tolerogenic CD103+ DC subset, which we describe as being primarily SIRPα−, decreased in the mLNs. Therefore, it may have been postulated that the reduction in the proportion of CD103+ DCs migrating to the mLNs of WT mice was the key event in triggering intestinal inflammation, as opposed to the increased recruitment of the pathogenic SIRPα+CD103− DC subset in both the tissues and mLNs. Yet, CD47 KO mice do not display any change in the proportion of tolerogenic CD103+ DCs in mLNs upon exposure to TNBS, and a single administration of WT SIRPα+CD103− DCs in CD47 KO mice was sufficient to induce severe wasting disease. Consequently, our data support a proinflammatory role for the SIRPα+CD103− DC subset, the effect of which may be amplified by, but is not exclusively dependent on, the reduction in the proportion of tolerogenic CD103+ DCs in the mLNs. In support of this concept, CD103+ DCs are found in equal proportions in the mLNs draining the small intestines of healthy individuals and those of CD patients, implying that intestinal inflammation is not dependent on a reduction in CD103+ DCs (Jaensson et al., 2008).
In the gut, CCR7 largely controls CD103+ DC trafficking at steady state (Jang et al., 2006; Worbs et al., 2006). We have previously reported that the absence of CD47 does not alter CCR7 expression or the in vitro migration of skin SIRPα+ DCs toward CCL19 or sphingosine-1-phosphate (Van et al., 2006). The reduced proportion of the SIRPα+CD103− DC subset in the mLNs of CD47 KO and CD47-Fc–treated mice upon exposure to TNBS strongly suggests that CD47 promotes this process. These findings corroborate previous in vivo studies in which a reduction of SIRPα+ DCs was observed in skin-draining LNs of CD47 KO and CD47-Fc–treated mice, which was found to be independent of CD47 expression on lymphatic vessels (Van et al., 2006). In support of these findings, we demonstrate in this paper that injected WT SIRPα+CD103− DCs migrate more efficiently to mLNs and elicit a more aggressive form of disease in CD47 KO hosts when compared with CD47 KO DCs. However, the underlying mechanisms by which CD47 governs SIRPα+CD103− DC migration remains to be elucidated. One hypothesis is that CD47–SIRPα interactions that may occur in cis on the DC indirectly control integrin-mediated transendothelial trafficking.
The expression of CD62L on CD103− DCs (Johansson-Lindbom et al., 2005) and a more recent report examining the turnover of intestinal DC subsets (Jaensson et al., 2008) favor the hypothesis of a direct recruitment of CD103− DCs or their precursors from the bloodstream to the mLNs via high endothelial venules at steady state. Our study supports this hypothesis, because there is no defect of SIRPα+CD103− DCs in the LP, whereas there is a significant reduction in the proportion of these DCs in the mLNs of CD47 KO mice at steady state. Therefore, SIRPα+CD103− DCs or their precursors may bypass the LP to seed the mLNs in a CD47-dependent manner. However, during the induction of colonic inflammation, SIRPα+CD103− DCs or their precursors may be recruited de novo to inflamed tissues before migrating to the mLNs. Interestingly, under inflammatory conditions, CX3CR1intGR1highCD62L+CCR2+ monocytes (GR1high monocytes) have been shown to differentiate into LP DCs (Varol et al., 2007), but i.v.-injected bone marrow monocytes are also capable of directly entering LNs through high endothelial venules (Nakano et al., 2009). Thus, under inflammatory conditions, CD47 may be implicated in the recruitment of SIRPα+CD103− DC precursors to the LP and/or the mLNs, resulting in a reduction in the frequency of SIRPα+CD103− DCs at those sites in CD47 KO or CD47-Fc–treated mice. Nevertheless, we cannot exclude the possibility that CD47 is required for the migration of SIRPα+CD103− DCs directly from the LP to the mLNs (Fig. S5). Thus, further examination of the physiological routes of SIRPα+CD103− DC trafficking under inflammatory conditions is required to delineate the precise role of CD47 in this process.
The early phases of TNBS colitis have been successfully induced in the absence of T cells. Therefore, innate-mediated mechanisms are also likely to be implicated in disease pathogenesis (Fiorucci et al., 2002; Katakura et al., 2005). SIRPα+ neutrophils, which are rapidly recruited to sites of inflammation, have been shown to exhibit CD47-mediated transmigration (Cooper et al., 1995). A significant reduction in colonic myeloperoxidase activity, a marker of neutrophil infiltration, occurred early in disease development in CD47 KO mice (unpublished data). We thus hypothesized that a defect in CD47-mediated neutrophil transmigration may be protective in the acute phase of disease. However, a previously published report has suggested a protective role for neutrophils in disease initiation (Kühl et al., 2007). Therefore, although a reduction of neutrophil infiltration was observed in CD47 KO mice, we largely excluded this phenomenon as playing a protective role in the development of TNBS colitis.
In the present study, we observed causative links between an increased percentage of SIRPα+CD103− DCs, Th17-skewed responses, and disease manifestation, suggesting a role for Th17 cells in the development of colitis. The in vitro Th17-promoting capacity of CD11cHiSIRPα+CD103− DCs seems to be shared by the previously described CD11cHiCX3CR1+CD103− LP DC subset, supporting their contribution in the initiation of Th17 responses (Denning et al., 2007). Until recently, both CD and TNBS colitis have been considered Th1 diseases characterized by IL-12, TNF-α, and IFN-γ production (Neurath et al., 1995; Parronchi et al., 1997). However, several recent observations have strongly implicated a role for IL-23, a Th17 survival factor, and Th17 cells in disease pathogenesis. In humans, elevated serum levels of IL-17 and colonic mucosal levels of both IL-17 and IL-23 have been detected in CD patients (Fujino et al., 2003; Schmidt et al., 2005), whereas a mutation in the IL-23R gene (rs11209026, c.1142G>A, p.Arg381Gln) is protective from disease development (Duerr et al., 2006). It is established that the intestinal mucosal environment, via TGF-β, thymic stromal lymphopoietin, and IL-10, acts to condition resident antigen-presenting cells to exert immunosuppressive activities (Jarry et al., 2008; Zeuthen et al., 2008). Epithelial cells from CD patients express less thymic stromal lymphopoietin, leading to an enhanced release of the pro-Th1 and -Th17 inflammatory cytokines IL-12, IL-6, and IL-23, and expression of TLR2 and TLR4 by DCs (Hart et al., 2005; Rimoldi et al., 2005). Moreover, the combination of intestinal epithelial-derived TGF-β and retinoid acid has also recently been shown to convert CD103− DCs into CD103+ DCs capable of generating T reg cells (Iliev et al., 2009). IL-23 is abundantly produced by ileal but not colonic mouse DCs (Becker et al., 2003). Therefore, TNBS colitis, a model in which the inflammation is localized to the colon, might be less suitable than others for examining IL-23 regulation, as underscored by the low colonic mRNA expression of IL-23p19 in this study, even under conditions of severe inflammation. Nonetheless, the expression of IL-17 was strongly up-regulated by TNBS in WT mice as compared with CD47 KO mice. IL-17 is considered to be pathogenic in TNBS colitis because IL-17R–deficient mice are protected from disease development, and administration of an anti–IL-17 mAb ameliorates intestinal inflammation despite high levels of IFN-γ (Zhang et al., 2006). In contrast, IFN-γ receptor– and IFN-γ–deficient mice, or mice administered a neutralizing anti–IFN-γ mAb developed colitis as severe as WT mice (Camoglio et al., 2000; Tozawa et al., 2003), suggesting that IFN-γ and IL-12 play a less essential role in disease development than once believed. On the other hand, both IL-17 and IFN-γ synergize to create intestinal inflammation in Helicobacter hepaticus–induced T cell–dependent colitis (Kullberg et al., 2006). In Th17-associated autoimmune diseases, such as experimental autoimmune uveitis (Luger et al., 2008) and experimental autoimmune encephalomyelitis, either Th1 or Th17 cells alone can drive tissue damage (Bettelli et al., 2004; Kroenke et al., 2008; Stromnes et al., 2008). T cell clones isolated from CD patients have been identified as being either single IL-17+ or double IL-17+IFN-γ+ cells, underscoring the potentially pathogenic role of both cytokines (Annunziato et al., 2007). Some studies have even suggested a differential role for Th1 versus Th17 cells at various stages of disease (Kobayashi et al., 2008). Moreover, the developmental plasticity of Th17 cells has been reported, whereby IL-23 and IL-12 convert terminally differentiated mouse Th17 cells into IFN-γ–producing cells (Lee et al., 2009). Thus, it is likely an oversimplification to implicate Th17 cells alone in the development of TNBS colitis, and as such, we monitored the Th17/Th1 ratio rather than an exclusive decrease in the frequency of Th17 cells as a common thread of disease protection throughout this study. Finally, although Th17 cells are often categorized as pathogenic in autoimmune disease, it should be emphasized that they critically participate in protecting the host from microbial invasion and maintaining the epithelial barrier, as was demonstrated for the Th17-associated cytokine IL-22 (Mudter et al., 2008).
We also report that the mRNA expression of IL-6, a key Th17 cell differentiation factor, was impaired in the colon tissues of protected mice. IL-6 plays an important role in both T cell–dependent and –independent models of colitis (Atreya et al., 2000; te Velde et al., 2007), and IL-6–deficient mice were reported to show resistance to TNBS-induced colitis (Atreya et al., 2000; Gay et al., 2006). Although the precise cellular source of IL-6 was not examined in this study, epithelial cells and T cells themselves, in addition to antigen-presenting cells, contribute to IL-6 production during colitis (Dann et al., 2008; Mudter et al., 2008). We demonstrate in this paper that CD47 expression does not regulate IL-6 production by SIRPα+CD103− DCs. Therefore, the reduction of IL-6 expression observed in this study likely reflects an indirect consequence of reduced tissue infiltration.
To eliminate the developmental alterations in CD47 KO mice, such as the expansion of the CD103+ T reg cell population (Van et al., 2008) and Th1-biased phenotype (Bouguermouh et al., 2008), as a potential explanation for disease resistance, we demonstrate that the short-term interruption of CD47 ligation by the administration of a CD47-Fc molecule protected WT BALB/c mice from disease development. At an early time point after disease induction, we readily observed a reduced proportion of SIRPα+CD103− DCs in the mLNs and an inverted IL-17/IFN-γ mRNA ratio in treated compared with untreated mice. The therapeutic efficacy of CD47-Fc could not be attributed to increased T reg cell activity, because CD4+CD25+FoxP3+ T reg cell numbers were positively correlated with inflammation and were lower in CD47-Fc–treated mice (unpublished data). Finally, early administration of CD47-Fc was shown to be highly effective in preventing both disease initiation and the recurrence of colitis after a second TNBS challenge. In that respect, the therapeutic efficacy of most published compounds has only been assessed in the prevention of acute disease (te Velde et al., 2006).
In conclusion, we have identified SIRPα+CD103− DCs as an immunogenic Th17-inducing DC subset and propose their role in the pathogenesis of TNBS-induced colitis. We further demonstrate that CD47 expression on SIRPα+CD103− DCs promotes their trafficking and the development of severe intestinal inflammation. We therefore propose that targeting the CD47–SIRPα axis may serve as the basis for the development of novel therapeutic strategies in CD.
MATERIALS AND METHODS
Animals
6–8-wk-old WT BALB/c and BALB/c CD47 KO mice were bred and maintained in our animal care facility in standard animal cages and under specific pathogen-free conditions. CD47 KO mice are viable and do not exhibit any overt phenotype. All mice were handled according to institutionally recommended animal care guidelines, and all experiments were approved by the Animal Studies Ethics Committee of McGill University and the Centre de Recherche du Centre Hospitalier de Montreal.
Culture medium, antibodies, and reagents
Ex vivo and in vitro experiments were performed using complete RPMI 1640 medium (Wisent Inc.) supplemented with 10% fetal calf serum (Wisent Inc), 500 U/ml penicillin, 500 µg/ml streptomycin, 10 mM Hepes buffer, and 1 mM 2-mercaptoethanol (Invitrogen). IMDM (Invitrogen) supplemented with 5% fetal calf serum, 500 U/ml penicillin, 500 µg/ml streptomycin, and 1 mM 2-mercaptoethanol was used for in vitro CD4+ T cell co-cultures with sorted DCs. Escherichia coli LPS was obtained from Sigma-Aldrich, and GM-CSF was purchased from PeproTech. The anti-CD3 antibody (145-2C11) used for in vitro polyclonal stimulation was obtained from BD. Allophycocyanin (APC)-labeled anti-CD4, FITC-labeled anti–IFN-γ, and PE-labeled anti–IL-17 were used for intracytoplasmic cytokine staining of CD4+ T cells and were obtained from BD. For DC phenotype staining and DC/CD4+ T cell sorting, FITC-labeled anti-CD11b, anti–MHC II, anti-CD40, and anti-CD86, PE-labeled anti-SIRPα, biotinylated anti-CD103 plus streptavidin-PerCP, APC-labeled anti-CD11c, anti-CD45.2–APC–Cy7, and anti-CD4–PeCy7 were obtained from BD.
TNBS-induced colitis
Two different models of chronic TNBS colitis were induced. In the first model, 3 mg TNBS (Sigma-Aldrich) was dissolved in 50% ethanol and a total volume of 50 µl was injected intrarectally in isopropanol-anesthetized mice (Scheiffele and Fuss, 2002). Controls included those administered intrarectal saline or ethanol alone. Mice were sacrificed 8 d after TNBS injection to assess colonic inflammation. The second form of chronic TNBS colitis was induced by two injections of 2 mg TNBS dissolved in 50% ethanol. The second injection was administered 7 or 8 d after the primary injection. Mice were sacrificed either 2 d after primary TNBS injection to assess early events in disease development or 4 d after the secondary injection to assess colonic inflammation.
For treatment of TNBS colitis, 100 µg of mouse CD47-Fc (Van et al., 2006), control human CD47-Fc (Latour et al., 2001), or saline was injected i.p. on days 0–3, inclusively. 2 mg TNBS colitis was induced on days 0 and 8, and mice were sacrificed on day 4 or 12. In all cases, the macroscopic score of inflammation was assessed based on the degree of ulceration (0–10), the presence of diarrhea (0–1) and adhesions (0–2), and on the thickness of the colon wall (0–1). For histological assessment, colon samples were embedded in optimum cutting temperature compound (Sakura Finetek) and stained with hematoxylin and eosin. Histological changes were semiquantitatively graded based on a set of previously established criteria (Ameho et al., 1997). The grading scale ranged from 0–16 and was calculated as the sum of scores for expansion of submucosa (0–4), expansion of LP (0–4), loss of goblet cells (0–4), and neutrophil infiltration (0–4). All macroscopic and microscopic scoring was performed in a blinded fashion.
Cytokine quantification by ELISA
Whole blood was withdrawn from mice immediately postmortem and sera were frozen at −20°C until use. Serum IL-6 was quantified by the Quantikine ELISA kit (R&D Systems). IL-6 and IL-23p19 production was assessed in BMDC culture supernatants after overnight culture using the mouse IL-6 DuoSet (R & D Systems) and IL-23p19 (eBioscience) ELISA kits.
Real-time PCR
Colons were immediately immersed in RNAlater (QIAGEN) upon dissection and frozen at −20°C until use. mRNA was extracted according to the TRIzol protocol and was reverse transcribed using the cDNA RT kit (Applied Biosystems). Quantitative real-time PCR was performed using a sequence detection system (1 PCR cycle at 95°C for 10 min; 40 PCR cycles at 60°C for 1 min and at 95°C for 15 s; ABI Prism 7900HT; Applied Biosystems). cDNA was amplified in a 10-µl final reaction mix containing TaqMan Universal PCR Master Mix (Applied Biosystems) and the corresponding TaqMan gene expression assays (IL-6, Mm00446190_m1; Eukaryotic 18s rRNA, Hs99999901_s1; IL-17a, Mm00439619_m1; IL-12p35, Mm00434165_m1; thrombospondin-1, Mm00449022_m1; IL-23p19, Mm00518984_m1; IFN-γ, Mm00801778_m1; TGF-β, Mm00441724_m1; Applied Biosystems). Signals were analyzed by the ABI Prism sequence detection system software (version 2.2; Applied Biosystems). The comparative Ct method for relative quantification was used, whereby all Ct were first normalized to the expression of 18s rRNA. Cytokine expression is represented as a fold change relative to control mice.
Ex vivo cultures of mLNs
For ex vivo cultures, mLNs were harvested 3–4 d after secondary TNBS injection and 106 cells/ml were cultured in 48-well plates. Cells were stimulated with 2 µg/ml anti-CD3 antibody for 4 d in the presence of 20 ng/ml IL-23, followed by restimulation with 5 ng/ml PMA and 0.5 µg/ml ionomycin in the presence of 1 µg/ml Brefeldin A for the last 6 h of culture. In some experiments, cells were immediately stimulated after harvest with PMA/ionomycin in the presence of Brefeldin A. Cells were collected, stained for CD4, fixed and permeabilized with the Fix/Perm kit (BD), and stained for intracellular IL-17 and IFN-γ for analysis by flow cytometry. Data were acquired on a FACSCalibur (BD) and were analyzed with CellQuest software (BD).
DC phenotype in LP and mLNs
LP cells were isolated as previously described (Drakes et al., 2004). In brief, colons were extracted, thoroughly cleaned, and digested in a solution of collagenase IV (Roche) and DNase I (Roche). mLNs were harvested 2 d after primary TNBS instillation and treated with 0.7 mg/ml Liberase (Roche) for 15 min at 37°C. 4 × 106 cells were stained with anti-CD45.2, anti-CD11c, anti-CD11b, anti-SIRPα, and anti-CD103 antibodies and analyzed by flow cytometry. Data were acquired on a FACSAria II (BD) and analyzed with CellQuest software.
Generation of BMDCs
BMDCs were generated from bone marrow as previously described (Van et al., 2006). In brief, bone marrow was harvested from WT and CD47 KO mice and cultured in RPMI 1640 supplemented with 5% FBS, 500 U/ml penicillin, 500 µg/ml streptomycin, 1 mM 2-mercaptoethanol, and 40 ng/ml GM-CSF, which was replenished on days 3, 7, and 12. BMDCs were harvested on day 13.
In vitro and in vivo assessment of DC function
In vitro.
WT BALB/c mice were injected with 10 µg of human Flt3-L daily for 13 d to increase DC numbers. LP and mLN cells were purified as described, and cells were isolated from spleens using the same Liberase digestion protocol as mLNs. All cells were stained with anti-CD11b, anti-SIRPα, anti-CD103, anti-CD11c, anti-CD45.2, and anti-CD4, and sorted using a FACSAria II (Fig. S2 shows the gating strategy). The purity of CD4+ T cells and DC subsets was >99% and >96%, respectively. CD4+ T cells were co-cultured for 5 d with sorted SIRPα+CD103− or SIRPα−CD103+ DCs at a 25:1 ratio in the presence of soluble 2 µg/ml anti-CD3, 2 ng/ml TGF-β, and 10 µg/ml anti–IFN-γ. Transgenic (D011.10) CD4+ T cells were co-cultured with BMDCs (1:2 ratio) in the presence of 2 µg/ml OVA peptide, 2 ng/ml TGF-β, and 10 µg/ml anti–IFN-γ. On day 5, 5 ng/ml PMA, 0.5 µg/ml ionomycin, and 1 µg/ml Brefeldin A were added to the cell cultures, and intracytoplasmic staining for IL-17 and IFN-γ was performed as described above.
WT and CD47 KO BMDCs were stimulated with 100 ng/ml LPS and the expression of CD11c, CD11b, CD103, and SIRPα, and the activation markers CD86, CD40, and MHC II were assessed after overnight culture by flow cytometry. To assess cytokine production (IL-6 and IL-23p19), BMDCs were stimulated overnight with 100 ng/ml LPS in the presence of 10 ng/ml IFN-γ. For WT/CD47 KO BMDC/T cell criss-cross co-cultures, splenic CD4+ T cells were purified using the EasySep Biotin Selection Kit (StemCell Technologies Inc.). Anti-CD3–stimulated (2 µg/ml) CD4+ T cells were co-cultured with LPS-stimulated (500 ng/ml) BMDCs at a 10:1 (T cell/DC) ratio in the presence of 1 ng/ml TGF-β. After 5 d, cells were restimulated with PMA/ionomycin in the presence of Brefeldin A and stained for intracytoplasmic IL-17.
In vivo.
LPS-stimulated (500 ng/ml) WT and CD47 KO BMDCs were harvested and transferred to CD47 KO hosts by i.p. injection of 2 × 106 cells per mouse 30 min before induction of TNBS colitis (Gonzalez-Rey and Delgado, 2006).
Competitive DC migration assay
WT and CD47 KO BMDCs were stimulated with 500 ng/ml LPS overnight and labeled with CFSE (WT) or CMTMR (CD47 KO) and injected i.p. in CD47 KO hosts. TNBS colitis was induced 30 min after DC injection, and mLNs were harvested on day 2 from host mice to assess DC migration. mLNs were digested with 0.7 mg/ml Liberase for 15 min at 37°C, and cells were analyzed by gating on CD11c+ cells by flow cytometry.
Statistical analyses
Statistical analyses were performed using the Instat program (GraphPad Software, Inc.). All values are expressed as means ± SEM. The Student's t test, paired t test, and Mann-Whitney U test were used to assess statistical significance.
Online supplemental material
Fig. S1 indicates the frequency of SIRPα−CD103+ DCs in the LP and mLNs of WT and CD47 KO control mice or mice administered TNBS. Fig. S2 indicates the purity of DCs and CD4+ T cells isolated by cell sorting from mLNs of 10 pooled Flt3-L–treated mice. Fig. S3 demonstrates that WT BMDCs administered i.p. in WT mice has no effect on TNBS-induced colitis outcome (body weight loss and cytokine mRNA expression in colons). Fig. S4 indicates that SIRPα+CD103− DCs isolated from mLNs and injected i.p. into CD47 KO mice promote TNBS-induced colitis (body weight loss and macroscopic score) and Th17 responses in mLNs. Fig. S5 represents, in a schematic model, the potential routes of CD47-dependent SIRPα+CD103− DC or DC precursor migration to gut tissues and mLNs in response to TNBS administration, and the role of recruited SIRPα+CD103− DCs in the induction of Th17/Th1 polarization and perpetuation of colonic inflammation. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20082805/DC1.
Acknowledgments
This work was supported by the Crohn's and Colitis Foundation of Canada (D. Franchimont, M. Sarfati, and G. Fortin), the Canada Research Chair (D. Franchimont), the Canadian Foundation for Innovation (D. Franchimont and M. Sarfati), and the Research Institute of the McGill University Health Centers (D. Franchimont). D. Franchimont is senior clinical scientist at the Belgium National Foundation for Research.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used: BMDC, bone marrow–derived DC; CD, Crohn's disease; LP, lamina propria; mLN, mesenteric lymph node; SIRPα, signal regulatory protein α; TLR, Toll-like receptor; TNBS, trinitrobenzene sulfonic acid.
References
- Adams S., van der Laan L.J., Vernon-Wilson E., Renardel de Lavalette C., Döpp E.A., Dijkstra C.D., Simmons D.L., van den Berg T.K. 1998. Signal-regulatory protein is selectively expressed by myeloid and neuronal cells.J. Immunol. 161:1853–1859 [PubMed] [Google Scholar]
- Ameho C.K., Adjei A.A., Harrison E.K., Takeshita K., Morioka T., Arakaki Y., Ito E., Suzuki I., Kulkarni A.D., Kawajiri A., Yamamoto S. 1997. Prophylactic effect of dietary glutamine supplementation on interleukin 8 and tumour necrosis factor alpha production in trinitrobenzene sulphonic acid induced colitis.Gut. 41:487–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annunziato F., Cosmi L., Santarlasci V., Maggi L., Liotta F., Mazzinghi B., Parente E., Filì L., Ferri S., Frosali F., et al. 2007. Phenotypic and functional features of human Th17 cells.J. Exp. Med. 204:1849–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armant M., Avice M.N., Hermann P., Rubio M., Kiniwa M., Delespesse G., Sarfati M. 1999. CD47 ligation selectively downregulates human interleukin 12 production.J. Exp. Med. 190:1175–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atarashi K., Nishimura J., Shima T., Umesaki Y., Yamamoto M., Onoue M., Yagita H., Ishii N., Evans R., Honda K., Takeda K. 2008. ATP drives lamina propria T(H)17 cell differentiation.Nature. 455:808–812 [DOI] [PubMed] [Google Scholar]
- Atreya R., Mudter J., Finotto S., Müllberg J., Jostock T., Wirtz S., Schütz M., Bartsch B., Holtmann M., Becker C., et al. 2000. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo.Nat. Med. 6:583–588 [DOI] [PubMed] [Google Scholar]
- Avice M.N., Rubio M., Sergerie M., Delespesse G., Sarfati M. 2000. CD47 ligation selectively inhibits the development of human naive T cells into Th1 effectors.J. Immunol. 165:4624–4631 [DOI] [PubMed] [Google Scholar]
- Becker C., Wirtz S., Blessing M., Pirhonen J., Strand D., Bechthold O., Frick J., Galle P.R., Autenrieth I., Neurath M.F. 2003. Constitutive p40 promoter activation and IL-23 production in the terminal ileum mediated by dendritic cells.J. Clin. Invest. 112:693–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E., Sullivan B., Szabo S.J., Sobel R.A., Glimcher L.H., Kuchroo V.K. 2004. Loss of T-bet, but not STAT1, prevents the development of experimental autoimmune encephalomyelitis.J. Exp. Med. 200:79–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E., Korn T., Kuchroo V.K. 2007. Th17: the third member of the effector T cell trilogy.Curr. Opin. Immunol. 19:652–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouguermouh S., Van V.Q., Martel J., Gautier P., Rubio M., Sarfati M. 2008. CD47 expression on T cell is a self-control negative regulator of type 1 immune response.J. Immunol. 180:8073–8082 [DOI] [PubMed] [Google Scholar]
- Camoglio L., te Velde A.A., de Boer A., ten Kate F.J., Kopf M., van Deventer S.J. 2000. Hapten-induced colitis associated with maintained Th1 and inflammatory responses in IFN-gamma receptor-deficient mice.Eur. J. Immunol. 30:1486–1495 [DOI] [PubMed] [Google Scholar]
- Cavanagh L.L., Bonasio R., Mazo I.B., Halin C., Cheng G., van der Velden A.W., Cariappa A., Chase C., Russell P., Starnbach M.N., et al. 2005. Activation of bone marrow-resident memory T cells by circulating, antigen-bearing dendritic cells.Nat. Immunol. 6:1029–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J.H. 2008. The genetics and immunopathogenesis of inflammatory bowel disease.Nat. Rev. Immunol. 8:458–466 [DOI] [PubMed] [Google Scholar]
- Coombes J.L., Powrie F. 2008. Dendritic cells in intestinal immune regulation.Nat. Rev. Immunol. 8:435–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes J.L., Siddiqui K.R., Arancibia-Cárcamo C.V., Hall J., Sun C.M., Belkaid Y., Powrie F. 2007. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid–dependent mechanism.J. Exp. Med. 204:1757–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D., Lindberg F.P., Gamble J.R., Brown E.J., Vadas M.A. 1995. Transendothelial migration of neutrophils involves integrin-associated protein (CD47).Proc. Natl. Acad. Sci. USA. 92:3978–3982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dann S.M., Spehlmann M.E., Hammond D.C., Iimura M., Hase K., Choi L.J., Hanson E., Eckmann L. 2008. IL-6-dependent mucosal protection prevents establishment of a microbial niche for attaching/effacing lesion-forming enteric bacterial pathogens.J. Immunol. 180:6816–6826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning T.L., Wang Y.C., Patel S.R., Williams I.R., Pulendran B. 2007. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses.Nat. Immunol. 8:1086–1094 [DOI] [PubMed] [Google Scholar]
- Drakes M.L., Czinn S.J., Blanchard T.G. 2004. Isolation and purification of colon lamina propria dendritic cells from mice with colitis.Cytotechnology. 46:151–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerr R.H., Taylor K.D., Brant S.R., Rioux J.D., Silverberg M.S., Daly M.J., Steinhart A.H., Abraham C., Regueiro M., Griffiths A., et al. 2006. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene.Science. 314:1461–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorucci S., Mencarelli A., Palazzetti B., Sprague A.G., Distrutti E., Morelli A., Novobrantseva T.I., Cirino G., Koteliansky V.E., de Fougerolles A.R. 2002. Importance of innate immunity and collagen binding integrin alpha1beta1 in TNBS-induced colitis.Immunity. 17:769–780 [DOI] [PubMed] [Google Scholar]
- Fujino S., Andoh A., Bamba S., Ogawa A., Hata K., Araki Y., Bamba T., Fujiyama Y. 2003. Increased expression of interleukin 17 in inflammatory bowel disease.Gut. 52:65–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay J., Kokkotou E., O'Brien M., Pothoulakis C., Karalis K.P. 2006. Interleukin-6 genetic ablation protects from trinitrobenzene sulfonic acid-induced colitis in mice. Putative effect of antiinflammatory cytokines.Neuroimmunomodulation. 13:114–121 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rey E., Delgado M. 2006. Therapeutic treatment of experimental colitis with regulatory dendritic cells generated with vasoactive intestinal peptide.Gastroenterology. 131:1799–1811 [DOI] [PubMed] [Google Scholar]
- Hart A.L., Al-Hassi H.O., Rigby R.J., Bell S.J., Emmanuel A.V., Knight S.C., Kamm M.A., Stagg A.J. 2005. Characteristics of intestinal dendritic cells in inflammatory bowel diseases.Gastroenterology. 129:50–65 [DOI] [PubMed] [Google Scholar]
- Iliev I.D., Mileti E., Matteoli G., Chieppa M., Rescigno M. 2009. Intestinal epithelial cells promote colitis-protective regulatory T-cell differentiation through dendritic cell conditioning.Mucosal Immunol. 2:340–350 [DOI] [PubMed] [Google Scholar]
- Iwasaki A. 2007. Mucosal dendritic cells.Annu. Rev. Immunol. 25:381–418 [DOI] [PubMed] [Google Scholar]
- Jaensson E., Uronen-Hansson H., Pabst O., Eksteen B., Tian J., Coombes J.L., Berg P.-L., Davidsson T., Powrie F., Johansson-Lindbom B., Agace W.W. 2008. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans.J. Exp. Med. 205:2139–2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang M.H., Sougawa N., Tanaka T., Hirata T., Hiroi T., Tohya K., Guo Z., Umemoto E., Ebisuno Y., Yang B.G., et al. 2006. CCR7 is critically important for migration of dendritic cells in intestinal lamina propria to mesenteric lymph nodes.J. Immunol. 176:803–810 [DOI] [PubMed] [Google Scholar]
- Jarry A., Bossard C., Bou-Hanna C., Masson D., Espaze E., Denis M.G., Laboisse C.L. 2008. Mucosal IL-10 and TGF-beta play crucial roles in preventing LPS-driven, IFN-gamma-mediated epithelial damage in human colon explants.J. Clin. Invest. 118:1132–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson-Lindbom B., Svensson M., Wurbel M.A., Malissen B., Márquez G., Agace W. 2003. Selective generation of gut tropic T cells in gut-associated lymphoid tissue (GALT): requirement for GALT dendritic cells and adjuvant.J. Exp. Med. 198:963–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson-Lindbom B., Svensson M., Pabst O., Palmqvist C., Marquez G., Förster R., Agace W.W. 2005. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing.J. Exp. Med. 202:1063–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katakura K., Lee J., Rachmilewitz D., Li G., Eckmann L., Raz E. 2005. Toll-like receptor 9-induced type I IFN protects mice from experimental colitis.J. Clin. Invest. 115:695–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsall B.L. 2008. Innate and adaptive mechanisms to control pathological intestinal inflammation.J. Pathol. 214:242–259 [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Okamoto S., Hisamatsu T., Kamada N., Chinen H., Saito R., Kitazume M.T., Nakazawa A., Sugita A., Koganei K., et al. 2008. IL23 differentially regulates the Th1/Th17 balance in ulcerative colitis and Crohn's disease.Gut. 57:1682–1689 [DOI] [PubMed] [Google Scholar]
- Kool M., Soullié T., van Nimwegen M., Willart M.A., Muskens F., Jung S., Hoogsteden H.C., Hammad H., Lambrecht B.N. 2008. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells.J. Exp. Med. 205:869–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke M.A., Carlson T.J., Andjelkovic A.V., Segal B.M. 2008. IL-12– and IL-23–modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition.J. Exp. Med. 205:1535–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühl A.A., Kakirman H., Janotta M., Dreher S., Cremer P., Pawlowski N.N., Loddenkemper C., Heimesaat M.M., Grollich K., Zeitz M., et al. 2007. Aggravation of different types of experimental colitis by depletion or adhesion blockade of neutrophils.Gastroenterology. 133:1882–1892 [DOI] [PubMed] [Google Scholar]
- Kullberg M.C., Jankovic D., Feng C.G., Hue S., Gorelick P.L., McKenzie B.S., Cua D.J., Powrie F., Cheever A.W., Maloy K.J., Sher A. 2006. IL-23 plays a key role in Helicobacter hepaticus–induced T cell–dependent colitis.J. Exp. Med. 203:2485–2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latour S., Tanaka H., Demeure C., Mateo V., Rubio M., Brown E.J., Maliszewski C., Lindberg F.P., Oldenborg A., Ullrich A., et al. 2001. Bidirectional negative regulation of human T and dendritic cells by CD47 and its cognate receptor signal-regulator protein-alpha: down-regulation of IL-12 responsiveness and inhibition of dendritic cell activation.J. Immunol. 167:2547–2554 [DOI] [PubMed] [Google Scholar]
- Lee Y.K., Turner H., Maynard C.L., Oliver J.R., Chen D., Elson C.O., Weaver C.T. 2009. Late developmental plasticity in the T helper 17 lineage.Immunity. 30:92–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Bühring H.J., Zen K., Burst S.L., Schnell F.J., Williams I.R., Parkos C.A. 2002. Signal regulatory protein (SIRPalpha), a cellular ligand for CD47, regulates neutrophil transmigration.J. Biol. Chem. 277:10028–10036 [DOI] [PubMed] [Google Scholar]
- Luger D., Silver P.B., Tang J., Cua D., Chen Z., Iwakura Y., Bowman E.P., Sgambellone N.M., Chan C.C., Caspi R.R. 2008. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category.J. Exp. Med. 205:799–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudter J., Amoussina L., Schenk M., Yu J., Brüstle A., Weigmann B., Atreya R., Wirtz S., Becker C., Hoffman A., et al. 2008. The transcription factor IFN regulatory factor-4 controls experimental colitis in mice via T cell-derived IL-6.J. Clin. Invest. 118:2415–2426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano H., Lin K.L., Yanagita M., Charbonneau C., Cook D.N., Kakiuchi T., Gunn M.D. 2009. Blood-derived inflammatory dendritic cells in lymph nodes stimulate acute T helper type 1 immune responses.Nat. Immunol. 10:394–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath M.F., Fuss I., Kelsall B.L., Stüber E., Strober W. 1995. Antibodies to interleukin 12 abrogate established experimental colitis in mice.J. Exp. Med. 182:1281–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parronchi P., Romagnani P., Annunziato F., Sampognaro S., Becchio A., Giannarini L., Maggi E., Pupilli C., Tonelli F., Romagnani S. 1997. Type 1 T-helper cell predominance and interleukin-12 expression in the gut of patients with Crohn's disease.Am. J. Pathol. 150:823–832 [PMC free article] [PubMed] [Google Scholar]
- Reed M.J., Puolakkainen P., Lane T.F., Dickerson D., Bornstein P., Sage E.H. 1993. Differential expression of SPARC and thrombospondin 1 in wound repair: immunolocalization and in situ hybridization.J. Histochem. Cytochem. 41:1467–1477 [DOI] [PubMed] [Google Scholar]
- Rimoldi M., Chieppa M., Salucci V., Avogadri F., Sonzogni A., Sampietro G.M., Nespoli A., Viale G., Allavena P., Rescigno M. 2005. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells.Nat. Immunol. 6:507–514 [DOI] [PubMed] [Google Scholar]
- Robert C., Klein C., Cheng G., Kogan A., Mulligan R.C., von Andrian U.H., Kupper T.S. 2003. Gene therapy to target dendritic cells from blood to lymph nodes.Gene Ther. 10:1479–1486 [DOI] [PubMed] [Google Scholar]
- Sanchez-Munoz F., Dominguez-Lopez A., Yamamoto-Furusho J.K. 2008. Role of cytokines in inflammatory bowel disease.World J. Gastroenterol. 14:4280–4288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiffele F., Fuss I.J. 2002. Induction of TNBS colitis in mice.Curr. Protoc Immunol. Chapter 15:Unit 15 19 [DOI] [PubMed] [Google Scholar]
- Schmidt C., Giese T., Ludwig B., Mueller-Molaian I., Marth T., Zeuzem S., Meuer S.C., Stallmach A. 2005. Expression of interleukin-12-related cytokine transcripts in inflammatory bowel disease: elevated interleukin-23p19 and interleukin-27p28 in Crohn's disease but not in ulcerative colitis.Inflamm. Bowel Dis. 11:16–23 [DOI] [PubMed] [Google Scholar]
- Sheibanie A.F., Yen J.H., Khayrullina T., Emig F., Zhang M., Tuma R., Ganea D. 2007. The proinflammatory effect of prostaglandin E2 in experimental inflammatory bowel disease is mediated through the IL-23→IL-17 axis.J. Immunol. 178:8138–8147 [DOI] [PubMed] [Google Scholar]
- Stromnes I.M., Cerretti L.M., Liggitt D., Harris R.A., Goverman J.M. 2008. Differential regulation of central nervous system autoimmunity by T(H)1 and T(H)17 cells.Nat. Med. 14:337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C.M., Hall J.A., Blank R.B., Bouladoux N., Oukka M., Mora J.R., Belkaid Y. 2007. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid.J. Exp. Med. 204:1775–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Velde A.A., Verstege M.I., Hommes D.W. 2006. Critical appraisal of the current practice in murine TNBS-induced colitis.Inflamm. Bowel Dis. 12:995–999 [DOI] [PubMed] [Google Scholar]
- te Velde A.A., de Kort F., Sterrenburg E., Pronk I., ten Kate F.J., Hommes D.W., van Deventer S.J. 2007. Comparative analysis of colonic gene expression of three experimental colitis models mimicking inflammatory bowel disease.Inflamm. Bowel Dis. 13:325–330 [DOI] [PubMed] [Google Scholar]
- Ticchioni M., Raimondi V., Lamy L., Wijdenes J., Lindberg F.P., Brown E.J., Bernard A. 2001. Integrin-associated protein (CD47/IAP) contributes to T cell arrest on inflammatory vascular endothelium under flow.FASEB J. 15:341–350 [DOI] [PubMed] [Google Scholar]
- Tozawa K., Hanai H., Sugimoto K., Baba S., Sugimura H., Aoshi T., Uchijima M., Nagata T., Koide Y. 2003. Evidence for the critical role of interleukin-12 but not interferon-gamma in the pathogenesis of experimental colitis in mice.J. Gastroenterol. Hepatol. 18:578–587 [DOI] [PubMed] [Google Scholar]
- Trinchieri G. 1993. Interleukin-12 and its role in the generation of TH1 cells.Immunol. Today. 14:335–338 [DOI] [PubMed] [Google Scholar]
- Van V.Q., Lesage S., Bouguermouh S., Gautier P., Rubio M., Levesque M., Nguyen S., Galibert L., Sarfati M. 2006. Expression of the self-marker CD47 on dendritic cells governs their trafficking to secondary lymphoid organs.EMBO J. 25:5560–5568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van V.Q., Darwiche J., Raymond M., Lesage S., Bouguermouh S., Rubio M., Sarfati M. 2008. Cutting edge: CD47 controls the in vivo proliferation and homeostasis of peripheral CD4+ CD25+ Foxp3+ regulatory T cells that express CD103.J. Immunol. 181:5204–5208 [DOI] [PubMed] [Google Scholar]
- van den Berg T.K., van der Schoot C.E. 2008. Innate immune ‘self’ recognition: a role for CD47-SIRPalpha interactions in hematopoietic stem cell transplantation.Trends Immunol. 29:203–206 [DOI] [PubMed] [Google Scholar]
- Varol C., Landsman L., Fogg D.K., Greenshtein L., Gildor B., Margalit R., Kalchenko V., Geissmann F., Jung S. 2007. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells.J. Exp. Med. 204:171–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worbs T., Bode U., Yan S., Hoffmann M.W., Hintzen G., Bernhardt G., Förster R., Pabst O. 2006. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells.J. Exp. Med. 203:519–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuthen L.H., Fink L.N., Frokiaer H. 2008. Epithelial cells prime the immune response to an array of gut-derived commensals towards a tolerogenic phenotype through distinct actions of thymic stromal lymphopoietin and transforming growth factor-beta.Immunology. 123:197–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Zheng M., Bindas J., Schwarzenberger P., Kolls J.K. 2006. Critical role of IL-17 receptor signaling in acute TNBS-induced colitis.Inflamm. Bowel Dis. 12:382–388 [DOI] [PubMed] [Google Scholar]