Abstract
Tumor necrosis factor (TNF) is reputed to have very powerful antitumor effects, but it is also a strong proinflammatory cytokine. Injection of TNF in humans and mice leads to a systemic inflammatory response syndrome with major effects on liver and bowels. TNF is also a central mediator in several inflammatory diseases. We report that type I interferons (IFNs) are essential mediators of the lethal response to TNF. Mice deficient in the IFN-α receptor 1 (IFNAR-1) or in IFN-β are remarkably resistant to TNF-induced hypothermia and death. After TNF injection, IFNAR-1−/− mice produced less IL-6, had less bowel damage, and had less apoptosis of enterocytes and hepatocytes compared with wild-type (WT) mice. Extensive gene expression analysis in livers of WT and IFNAR-1−/− mice revealed a large deficiency in the response to TNF in the knockout mice, especially of IFN-stimulated response element–dependent genes, many of which encode chemokines. In livers of IFNAR-1−/− mice, fewer infiltrating white blood cells (WBCs) were detected by immunohistochemistry. Deficiency of type I IFN signaling provided sufficient protection for potentially safer therapeutic use of TNF in tumor-bearing mice. Our data illustrate that type I IFNs act as essential mediators in TNF-induced lethal inflammatory shock, possibly by enhancing cell death and inducing chemokines and WBC infiltration in tissues.
The cytokine TNF, especially in combination with IFN-γ, has a very strong antitumor effect. In mice, for example, established tumors completely regress during 10 d of treatment (Brouckaert et al., 1986). In patients, treatment of tumors with TNF leads to remarkable results, as in the treatment of melanoma metastasis in the limbs (Eggermont et al., 2004). However, TNF is a very strong inducer of inflammation (Aggarwal and Natarajan, 1996). After TNF binds to its major receptor TNFR1 (p55), it activates a broad range of transcription factors, leading to induction of cytokines, enzymes, and adhesion molecules (Kollias et al., 1999). Thus, injection of TNF leads to a systemic inflammatory response syndrome (SIRS) characterized by hypotension, hepatitis, and bowel necrosis (Tracey et al., 1986; Vassalli et al., 1992). Several molecules have been proven to mediate the acutely lethal effects of TNF: IL-1 (Everaerdt et al., 1994), NO (Cauwels et al., 2000), and, more recently, IL-17 (Takahashi et al., 2008). Because of these strong proinflammatory effects, treatment with TNF is restricted to loco-regional settings.
Three types of IFNs have been identified: type I, type II, and the recently discovered type III IFNs (Ank et al., 2006). Type I IFNs form a group of almost 20 members, of which IFN-β is one of the most important. They all signal through a heterodimeric receptor complex consisting of an IFN-α receptor 1 (IFNAR-1) and an IFNAR-2 chain (Uzé et al., 2007). Also, type I IFNs have antiviral, apoptotic, and antitumor activities, and they have been shown to mediate the acute inflammatory shock that occurs during endotoxemia (Karaghiosoff et al., 2003; Mahieu and Libert, 2007).
It was recently shown that TNF stimulates the expression of type I IFNs through IRF-1 in macrophages and, via IFNAR-1, the expression of IFN-stimulated response element (ISRE)–dependent genes (Yarilina et al., 2008). We therefore wondered whether type I IFNs play a role in the TNF-induced acutely lethal SIRS. Our data clearly support the idea that type I IFNs are strong mediators of the in vivo TNF effects and that their inhibition could lead to safer antitumor therapy based on TNF.
RESULTS AND DISCUSSION
IFNAR-1−/− mice and IFN-β−/− mice are protected against TNF-induced lethal SIRS
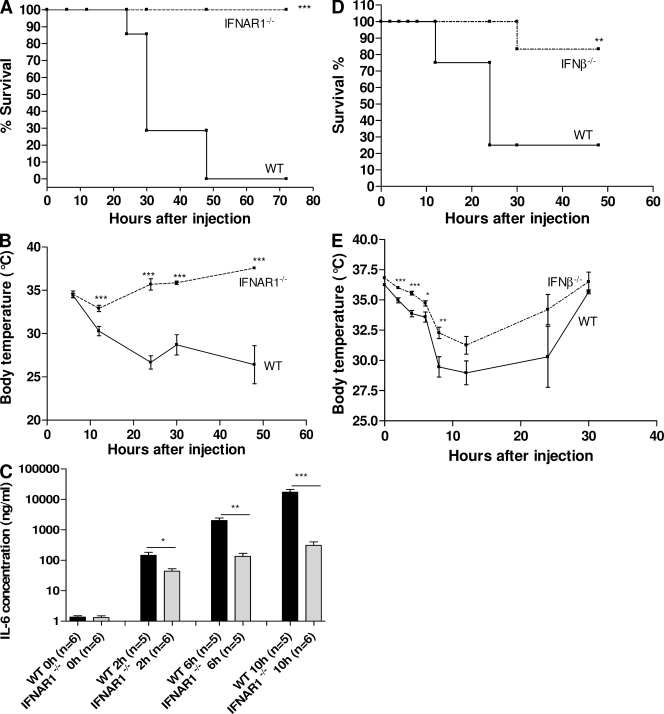
We studied the role of type I IFNs by using IFNAR-1−/− mice, IFN-β−/− mice, and WT mice. All mice had a matching genetic background of C57BL/6. IFNAR-1−/− and WT mice were injected i.p. with 30 µg of recombinant mouse TNF (2xLD100 for WT mice) and mortality was recorded during 72 h, after which no further deaths occurred. All WT mice died, whereas all IFNAR-1−/− mice survived (P < 0.0001; Fig. 1 A). Moreover, although IFNAR-1−/− mice displayed a mild and transient hypothermia, WT mice suffered pronounced hypothermia before dying (Fig. 1 B). In another experiment, 24 mice per genotype were injected i.p. with 30 µg TNF, and groups of six WT and six IFNAR-1−/− mice were bled at different times to study the induction profile of IL-6. Although this cytokine is functionally inert in this TNF model, its induction profile illustrates the extent of gene induction (Libert et al., 1994) and inflammation. As can be seen in Fig. 1 C, basal serum levels of IL-6 were comparable in WT and IFNAR-1−/− mice, but 2, 6, and 10 h after TNF challenge, the increase in IL-6 serum concentrations was significantly more pronounced in WT than in IFNAR-1−/− mice. It is notable that the difference from the WT developed relatively quickly. Furthermore, serum concentrations of IL-12p40 and IL-12p70 were also much lower in IFNAR-1−/− mice 6 h after challenge (unpublished data). Because we found that our IFNAR-1−/− mice displayed a mild reduction in the numbers of NK and NKT cells in liver but not in spleen (unpublished data), we also tested the response of IFN-β−/− mice to TNF. These mice were also significantly resistant to the lethal effects of TNF (P = 0.0027) and to hypothermia (Fig. 1, D and E).
Figure 1.
IFNAR-1−/− mice and IFN-β−/− mice are resistant to TNF-induced shock. 30 µg TNF was injected i.p. in WT (n = 14) and IFNAR-1−/− (n = 14) mice. (A) Mortality was monitored for 72 h (no further deaths occurred). (B) Body temperatures were measured regularly and plotted in function of time. (C) New groups of WT (black) and IFNAR-1−/− (gray) mice (n = 44 in total) were injected with 30 µg TNF i.p. and bled for IL-6 determination. IL-6 values are expressed on a log scale. 17.5 µg TNF was injected i.p. in WT (n = 12) and IFN-β−/− (n = 12) mice. (D) Mortality was monitored for 72 h (no further deaths occurred). (E) Body temperatures were measured regularly and plotted as a function of time. Experiments in A–C were performed three times; D and E were performed twice. Error bars represent SD of the means. *, 0.01 ≤ P ≤ 0.05; **, 0.001 ≤ P ≤ 0.01; ***, P ≤ 0.001.
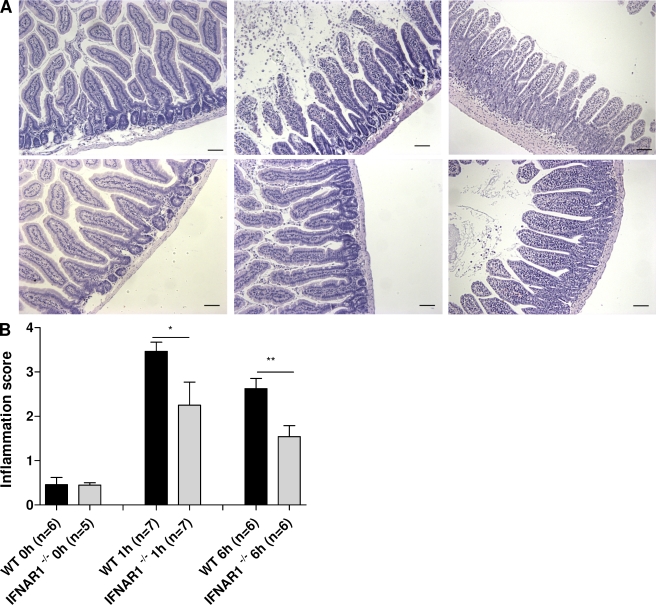
Sections of the small intestines (ileum) were made 1 and 6 h after challenge and stained with hematoxylin/eosin. As early as 1 h after challenge, the detrimental effect of TNF was much less pronounced in the IFNAR-1−/− mice than in WT mice. We observed less shrinkage and erosion of villi, less mucus formation, fewer apoptotic nuclei, and milder loss of Goblet cells (Fig. 2 A). The degree of tissue damage in these sections was quantified using the method described by Halpern et al. (2003). Significantly less damage was induced by TNF in IFNAR1−/− than in WT mice 1 and 6 h after challenge (P = 0.0255 and P = 0.0046, respectively; Fig. 2 B).
Figure 2.
Bowel damage after TNF injection in WT mice and IFNAR-1−/− mice. (A) IFNAR-1−/− mice are protected against TNF-induced bowel tissue destruction. 30 µg TNF was injected i.p. in WT mice (n = 18) and IFNAR-1−/− mice (n = 18), and jejunum was sampled 0 h (n = 6), 1 h (n = 6), and 6 h (n = 6) later. Standard hematoxylin/eosin staining of control samples (left), 1 h (middle), and 6 h (right) after TNF injection showed more extensive tissue damage in the bowel of WT mice (top) than in IFNAR-1−/− mice (bottom). Bars, 50 µm. The experiment was performed twice. (B) Neutral observers quantified the degree of bowel toxicity in sections from an independent experiment. The experiment was analogous to the one in A but used 19 WT mice and 18 IFNAR-1−/− mice. Error bars represent SD of the means. *, 0.01 ≤ P ≤ 0.05; **, 0.001 ≤ P ≤ 0.01.
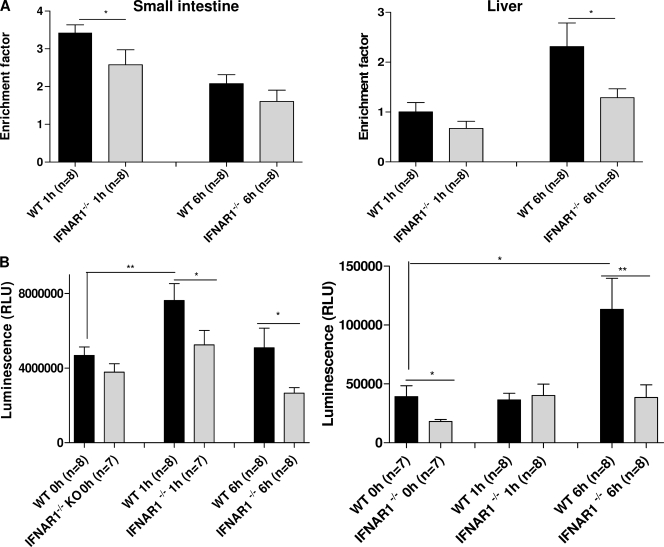
Acute cell death (apoptosis) of enterocytes after TNF injection has been reported (Piguet et al., 1998), and our data indicate that this TNF effect is enhanced by type I IFNs. The apoptosis-enhancing activity of type I IFN was recently shown for macrophages as well (Qiu et al., 2008). Furthermore, type I IFNs have been shown to exert apoptotic effects on renal epithelial cells (Lechner et al., 2008). TNF not only induces apoptosis of enterocytes, it also induces multiple mechanisms through the death receptor pathway to initiate hepatocyte apoptosis leading to liver injury (Ding and Yin, 2004). Liver injury has been identified as a major dose-limiting toxicity of TNF. We therefore compared the degree of acute enterocyte apoptosis and liver damage after TNF injection in WT and IFNAR-1−/− mice by several techniques. Mice were injected with 30 µg TNF, and the degree of apoptosis was estimated in bowels and livers 1 and 6 h after injection by measuring the extent of DNA fragmentation, caspase 3/caspase 7 activity, and TUNEL staining. As shown in Fig. 3 A, the extent of DNA fragmentation induced by TNF was very high in intestines 1 h after challenge and in liver 6 h after challenge, but it was significantly less in IFNAR-1−/− mice than in WT mice (P = 0.03986 and P = 0.0313, respectively). A similar pattern was observed when studying caspase 3/caspase 7 activity in TNF-injected mice (Fig. 3 B) or when staining sections for TUNEL activity (not depicted).
Figure 3.
TNF induces less apoptosis in IFNAR-1−/− mice than in WT mice. 30 µg TNF was injected i.p. in WT mice and IFNAR-1−/− mice, and jejunum and liver were sampled 0 h (n = 7–8), 1 h (n = 7–8), and 6 h (n = 8) later. (A) The degree of DNA fragmentation was measured and expressed as an enrichment factor compared with the 0-h values. (B) The sum of the activities of caspase 3 and caspase 7 was determined and expressed in relative luminescence units (RLU). The entire experiment was performed twice. Error bars represent SD of the means. *, 0.01 ≤ P ≤ 0.05; **, 0.001 ≤ P ≤ 0.01.
We conclude that abolition of type I IFN signaling protects mice against the acute inflammatory and toxic effects of TNF. The protective effect is initiated early and is reflected in the lower degree of hypothermia and lower level of circulating IL-6, as well as in reduced enterocyte and liver apoptosis, which are the major dose-limiting toxicities of TNF (Tracey et al., 1986).
Reduced expression of genes after TNF injection in IFNAR-1−/− mice
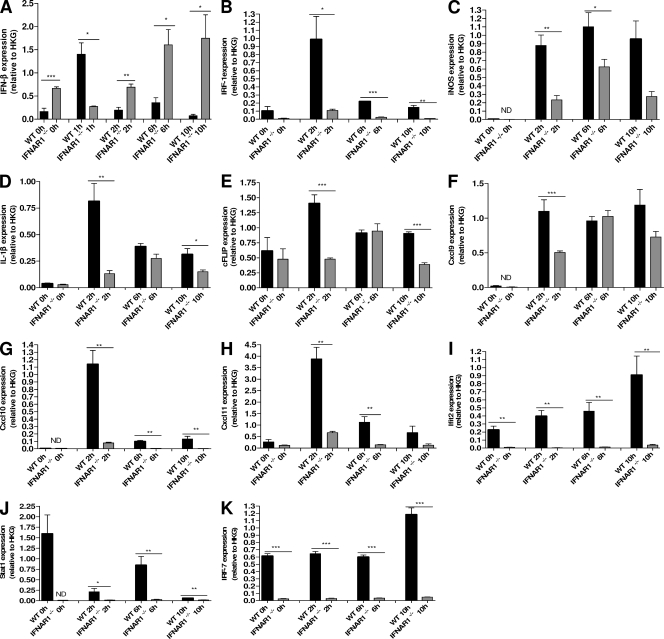
Very moderate induction of the major type I IFN (IFN-β) by TNF was recently shown in macrophages (Yaralina et al., 2008). Because the liver has been reported as a major target for TNF (Wielockx et al., 2001), we studied the levels of IFN-β messenger RNA in livers by quantitative PCR and found a sharp but transient induction in WT mice and a slow gradual increase in IFNAR-1−/− mice (Fig. 4 A). The late appearance of IFN-β at the RNA level after injection of TNF in IFNAR-1−/− mice might be, as has been proposed previously (Fenner et al., 2006), the result of weaker induction of inhibitors of the Jak–Stat pathway, such as SOCS molecules, by IFN-β. It should be noted that no IFN activity could be measured in serum or liver extract of TNF-injected mice by IFN-β–specific ELISA or by a sensitive bioassay based on the antiviral activity of IFN.
Figure 4.
TNF-induced gene expression in liver is lower in IFNAR-1−/− mice than in WT mice. WT (n = 4; black) and IFNAR-1−/− (n = 4; gray) mice were injected with 30 µg TNF i.p. and RNA was isolated 0, 1, 2, 6, and 10 h after injection. Quantitative PCR was used to measure gene-specific RNA levels of a set of early genes encoding IFN-β (A), IRF-1 (B), iNOS (C), IL-1β (D), and c-FLIP (E), as well as ISRE genes encoding CXCL9 (F), CXCL10 (G), CXCL11 (H), IFIT2 (I), STAT1 (J), and IRF-7 (K). The experiment was performed twice. Error bars represent SD of the means. *, 0.01 ≤ P ≤ 0.05; **, 0.001 ≤ P ≤ 0.01; ***, P ≤ 0.001.
To study the effects of IFNAR-1 deletion on TNF-induced gene expression in vivo, we measured the expression of several genes in the liver and spleen 0, 2, 6, and 10 h after injection of 30 µg TNF. In both the liver and spleen, the expression of all studied genes was compromised in IFNAR-1−/− mice. When we examined the induction of early genes by TNF, the large difference in induction of IRF-1 was remarkable (Fig. 4 B). The induction of other genes that depend on NF-κB, such as those coding for inducible nitric oxide synthase (iNOS), IL-1β, and c-Flip, was also substantially compromised in IFNAR-1−/− mice, especially at the early time point (Fig. 4, C–E). This can be explained, at least for the iNOS and IL-1β genes, by their coregulation by NF-κB and IRF factors and also in the promoter of the c-Flip-encoding gene because NF-κB, as well as IRF-responsive elements, was found by using the bioinformatics program Contra (not depicted; Hooghe et al., 2008). The lower level of serum IL-12 can also be explained by this reduced expression of IRF-1 (Liu et al., 2004).
When comparing the induction of later Jak–Stat-dependent genes, the expression in the IFNAR-1−/− mice was again found to be greatly reduced. The genes encoding CXCL9, CXCL10, CXCL11, IFIT2, STAT1, and IRF-7 were induced by TNF in the liver of WT mice but to a much smaller extent (or not at all) in the absence of the IFNAR-1 receptor (Fig. 4, F–K). These genes belong to the group of ISRE genes, the promoters of which are controlled by the transcription complex ISGF3. This complex consists of STAT-1–STAT-2–IRF-9 heterotrimers and is formed after stimulation of the IFNAR receptor (Maher et al., 2007). Most ISRE genes have immune modulatory, antiviral, or chemotactic functions (Der et al., 1998). Deficient induction of a whole set of chemokines, such as CXCL9, CXCL10, CXCL11, and IFIT2, should have profound effects on the chemotaxis of white blood cells (WBCs) in vivo.
Reduced infiltration of WBC in IFNAR-1−/− mice
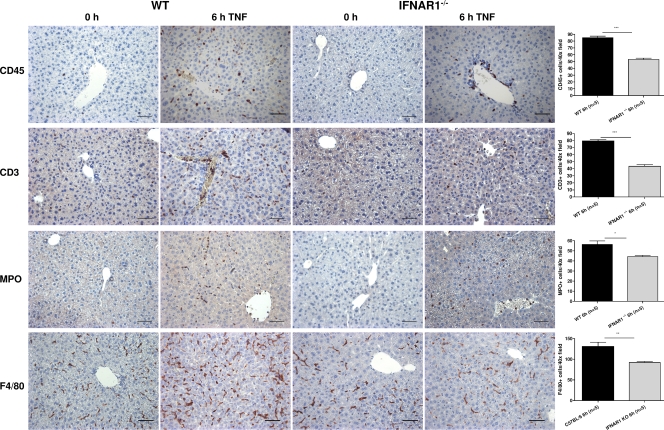
Because the induction of several chemokines upon TNF injection seemed much weaker (or altogether absent) in livers of IFNAR-1−/− mice compared with WT mice, we investigated whether infiltration of tissues by WBCs was reduced. Indeed, stimulation of the IFNAR-1 receptor in WT mice is expected to produce chemokines, which attract WBC from the blood stream into the tissue to cause further damage. Therefore, we injected mice i.p. with 30 µg TNF and sampled livers 6 h later. We also took samples from untreated mice. Tissue sections were stained for several WBC markers, namely the general WBC marker CD45, the T cell marker CD3, the marker for polymorphonuclear cells and monocytes MPO, and F4/80, a marker of macrophages (Fig. 5). The numbers of positive cells in WT and IFNAR-1−/− mice were compared. In the liver, we found that the numbers of WBC in the parenchyma were significantly lower in IFNAR1-1−/− mice whether they were CD3, CD45, MPO, or F4/80 positive. It is noteworthy that WBCs in IFNAR-1−/− mice seemed to accumulate in veins and arteries, whereas in WT mice they crossed the endothelial barrier into the parenchyma. Because CXCL9, CXCL10, and CXCL11 bind to the rather generally expressed receptor CxCR3 on WBCs, the reduced infiltration of CD3-, CD45-, MPO-, and F4/80-positive cells can be explained.
Figure 5.
Reduced tissue migration of WBC in IFNAR-1−/− mice after injection with TNF. WT (n = 6) and IFNAR-1−/− (n = 6) mice were injected i.p. with 30 µg TNF and livers were isolated 0 or 6 h later. Sections were cut and stained with CD45, CD3, MPO, and F4/80. Five representative fields were selected in all samples and the mean number of positive cells in five fields at 40× was counted by a neutral observer. Bars, 50 µm. IHC was performed three times. Error bars represent SD of the means. *, 0.01 ≤ P ≤ 0.05; **, 0.001 ≤ P ≤ 0.01; ***, P ≤ 0.001.
Safer antitumor therapy with TNF/IFN-γ in IFNAR-1−/− mice
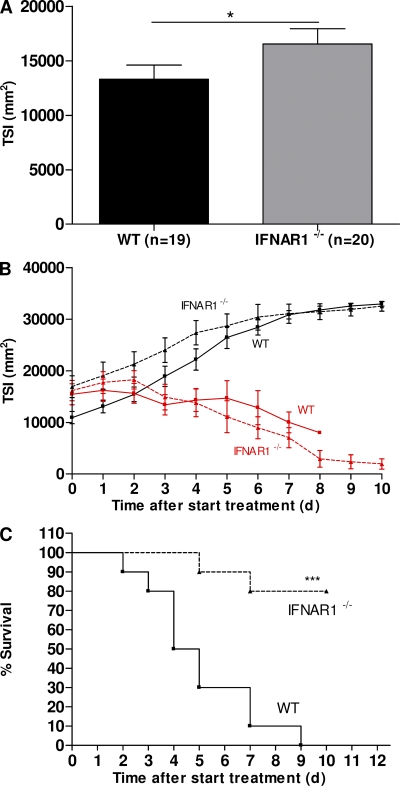
The protection provided by knockout of IFNAR-1 against acute lethal SIRS induced by a bolus injection of TNF raises hopes that it might be protective in an antitumor model as well. We studied this possibility in a standard tumor model. We inoculated 19 WT and 20 IFNAR-1−/− mice with 6 × 105 B16BL6 melanoma cells in the right thigh. 10 d later, tumors in IFNAR-1−/− mice had grown slightly larger than in WT mice (Fig. 6 A). This confirms earlier findings and illustrates that type I IFNs have some antiproliferative activity (Li et al., 2008). The mice were then divided into four groups and injected near the lesions with PBS or with 15 µg TNF + 5,000 IU IFN-γ per mouse per day. Daily measurement of tumor index showed that tumors regressed equally in WT (n = 10) and IFNAR-1−/− mice (n = 10; Fig. 6 B). All 10 WT mice and only 2 out of 10 IFNAR-1−/− mice died from the treatment (P = 0.0003; Fig. 6 C). This illustrates that type I IFNs also mediate much of the toxicity associated with the anticancer activity of TNF/IFN-γ.
Figure 6.
Protection of tumor-bearing IFNAR-1−/− mice against TNF/IFN-γ lethality. WT (n = 19; ▪) and IFNAR-1−/− (n = 20; ▴) mice were inoculated with B16BL6 melanoma cells. 10 d later, tumors were measured, TSI was calculated (A), and treatment was started. Treatment was daily for 10 d with PBS (black lines) in 9 WT and 10 IFNAR-1−/− mice or with TNF/IFN-γ (red) in 10 WT and 10 IFNAR-1−/− mice. Tumor regression (B) and mouse survival (C) were recorded until the end of the treatment (no further deaths occurred). The difference in survival between WT (n = 10) and IFNAR-1−/− (n = 10) mice was highly significant. The experiment was performed twice. Error bars represent SD of the means. *, 0.01 ≤ P ≤ 0.05; ***, P ≤ 0.001.
Many patients have been treated with type I IFNs for cancer, multiple sclerosis, and viral infections such as HBV and HCV (Maher et al., 2007). Although these patients suffer from severe side effects of the treatment, no serious accidents have been reported. However, although type I IFNs have been shown to possess some antiinflammatory activities, especially in the central nervous system, several authors have shown that they should be considered as important mediators in acute inflammatory conditions. Absence of IFN-β or IFNAR-1 in knockout mice leads to complete protection against LPS-induced lethal inflammatory shock (Karaghiosoff et al., 2003; Mahieu and Libert, 2007). In our study, we found that the presence or absence of type I IFN signaling also has a large impact on the outcome of TNF-induced inflammation. We expect that type I IFNs are also involved in other TNF-induced or TNF-mediated pathologies such as arthritis. Yarilina et al. (2008) found that TNF in macrophages induces a typical type I IFN program through IRF-1 and that IFN-β was elevated in synovial fluids of arthritic patients. Therefore, therapeutic inhibition of type I IFNs is conceivable. The endogenous type I IFNs, however, are crucial for antiviral defences, and IFNAR-1−/− mice are hypersensitive to many viral infections. Hence, although therapeutic inhibition of type I IFNs in inflammatory conditions seems logical, it is difficult to achieve in practice.
In conclusion, deficiency of type I IFNs in vivo leads to considerable protection against the deleterious effects of TNF. Two mechanisms might be involved in this protection. Type I IFNs seem to enhance TNF-induced apoptosis in enterocytes and hepatocytes. In contrast, deficient induction of chemokine genes is observed and is reflected in reduced infiltration of inflammatory cells in mice deficient in type I IFN receptor. Therapeutic inhibition of this family of IFNs should be evaluated for treatment of disorders in which TNF is implicated or for increasing the therapeutic index of TNF in cancer therapy. The safety of therapeutic inhibition with anti–type I IFN agents should be evaluated because these IFNs possess essential antiviral activities (Müller et al., 1994).
MATERIALS AND METHODS
Mice.
C57BL/6J WT mice were purchased from Charles River Laboratories. IFNAR-1 knockout mice (IFNAR-1−/−) and IFN-β knockout mice (IFN-β −/−), both with a C57BL/6J genetic background, were provided by D. Bonaparte (Gulbenkian Institute of Science, Oeiras, Portugal) and S. Weiss, respectively. The mice were kept in individually ventilated cages in a conventional animal house and were used at the age of 8–12 wk. Animal studies were approved by the ethics committee of Ghent University.
Agents.
Recombinant mouse TNF and IFN-γ were produced in Escherichia coli and purified to homogeneity in our laboratories. TNF and IFN-γ had specific activities of 1.2 × 108 IU/mg and 1.16 × 107 IU/mg, respectively, with no detectable endotoxin contamination.
Injections, monitoring, and sampling.
TNF was diluted in endotoxin-free PBS and injected i.p. in a volume of 0.2 ml. Mortality was scored for 72 h. Serum and tissue samples were collected 0, 1, 2, 6, and 10 h after injection. Blood was withdrawn by cardiac puncture and allowed to clot overnight at 4°C. The clot was removed, and serum was collected after centrifugation at 20,000 g for 10 min and stored at −20°C. Tissue samples were fixed briefly and embedded in paraffin by a standard protocol (Tissue-Tek VIP; Sakura). RNA samples were isolated by using RNeasy (QIAGEN).
Tumor cell culture and inoculation.
B16BL6 cells were cultured in DME supplemented with fetal calf serum, antibiotics, and l-glutamine. Cells were harvested, washed three times in LPS-free PBS, and brought to a density of 6 × 106/ml. From this cell suspension, 100 µl was injected s.c. into the right hind limbs of the mice.
Tumor size index (TSI) and body temperature.
The smaller and larger diameters of the tumors were measured with an electronic caliper, and TSI was calculated by multiplying these two values. Rectal body temperature was measured with an electronic thermometer (model 2001; Comark).
Determination of IL-6 and IFN-β.
IL-6 was determined in serum as previously described (Van Snick et al., 1986). Levels of IFN-β in serum and in liver extracts were determined by an ELISA system (PBL Biomedical Laboratories) according to the manufacturer’s instructions. The sensitivities of the IL-6 assay and the ELISA kit were 1 and 10 pg/ml, respectively.
Tissue section and histology.
Tissue sections of 4 µm were cut and stained with hematoxylin/eosin using standard techniques. After staining, the codes on the bowel sections were blinded and tissue damage was quantified with four neutral observers using the necrotizing enterocolitis scoring system published and validated by Halpern et al. (2003). The mean value of their estimations was used.
Immunohistochemistry (IHC).
4-µm liver sections were deparaffinized for antigen retrieval followed by rehydration. CD45 (Trowbridge and Thomas, 1994), CD3 (Haniffa et al., 2007), and MPO (Pinkus and Pinkus, 1991) IHC used target retrieval solution (Dako) or proteinase K treatment (10 µg/ml; Promega) in proteinase K buffer (100 mM Tris-HCl, pH 8.0, and 50 mM EDTA; for F4/80; van den Berg and Kraal, 2005), blocking in rabbit or swine serum (Dako), peroxidase blocking buffer (Dako), and incubation with anti-CD45 (BD), anti-CD3 (Abcam), anti-MPO (Dako), and anti-F4/80 (AbD Serotec) antibodies. Biotin-conjugated IgGs (Dako), the Vectastain ABC kit (Vector Laboratories), and AEC (Dako) were used for visualization. The slides were counterstained with hematoxylin solution (Sigma-Aldrich). CD45-, CD3-, MPO-, and F4/80-positive cells were counted in five random 40× fields in a double-blinded fashion. Negative controls were made by substituting buffer for primary antibody.
Real-time PCR.
RNA samples were reverse transcribed using oligo (dT) primers and MMLV reverse transcription. Primers were designed with Primer Express (Applied Biosystems) and purchased from Biolegio. Quantitative PCR was performed using the LightCycler 480 system (Applied Biosystems) for 10 ng of complementary DNA with SYBR Green Master mix. Genes were normalized to two housekeeping genes, GAPDH and actin. The relative messenger RNA level was expressed as 2−ΔCt (ΔCt, relative cycle threshold compared with GAPDH and actin).
Quantification of apoptosis in tissues.
Liver and small intestine homogenates were centrifuged for 30 min at 13,000 rpm and supernatant was stored at 4°C. Apoptosis was quantified by immunochemical determination of histone-complexed DNA fragments in a microtiter plate and by measuring caspase 3 and 7 activity. DNA fragmentation was measured by using the Cell Death Detection ELISAPLUS (Roche). In brief, plates coated with streptavidin were incubated with homogenates, anti–histone-biotin, and anti–DNA-POD. Detection was performed with anti–DNA-HRP and substrate. Livers and small intestines of untreated animals were used as negative control. To detect caspase 3 and 7 activity in livers and small intestines, we used the Caspase-Glo 3/7 Assay (Promega) with a slight modification. This modified procedure used a hypotonic extraction buffer (25 mM Hepes, pH 7.5, 5 mM MgCl2, 1 mM EGTA, and 1 µg/ml each of PMSF, leupeptin, and aprotinin) during dounce homogenization of livers and small intestines. After homogenization, the extracts were cleared by two centrifugations at 13,000 rpm for 30 min at 4°C. Protein concentrations in the cleared extracts were adjusted to 1 mg/ml before storage at −20°C. To perform the caspase assays, we mixed an equal volume (10 µg/ml) of diluted extract with the appropriate Caspase-Glo reagent in 96-well white-walled plates.
Statistical analysis.
Survival curves (Kaplan-Meyer plots) were compared using a log-rank test. Final mortality rates (deaths per total) were compared with a chi2 test. Means ± SD were compared with a Student’s t test. *, **, and *** represent 0.01 ≤ P ≤ 0.05, 0.001 ≤ P ≤ 0.01, and P ≤ 0.001, respectively.
Acknowledgments
We thank Dr. D. Bonaparte (Gulbenkian Institute of Science, Oeiras, Portugal) for generously providing the IFNAR-1−/− mice. We thank W. Burm for technical assistance and Dr. A. Bredan for editing the manuscript.
The work was supported by FWO Vlaanderen, Belgium, and the Interuniversity Attraction Poles, Belgium. L. Huys and L. Dejager are research assistants of the FWO-Vlaanderen, Belgium. F. Van Hauwermeiren was supported by a grant from Ghent University.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used: IFNAR-1, IFN-α receptor 1; IHC, immunohistochemistry; iNOS, inducible nitric oxide synthase; ISRE, IFN-stimulated response element; SIRS, systemic inflammatory response syndrome; TSI, tumor size index; WBC, white blood cell.
References
- Aggarwal B.B., Natarajan K. 1996. Tumor necrosis factors: developments during the last decade.Eur. Cytokine Netw. 7:93–124 [PubMed] [Google Scholar]
- Ank N., West H., Paludan S.R. 2006. IFN-lambda: novel antiviral cytokines.J. Interferon Cytokine Res. 26:373–379 [DOI] [PubMed] [Google Scholar]
- Brouckaert P.G., Leroux-Roels G.G., Guisez Y., Tavernier J., Fiers W. 1986. In vivo anti-tumour activity of recombinant human and murine TNF, alone and in combination with murine IFN-gamma, on a syngeneic murine melanoma.Int. J. Cancer. 38:763–769 [DOI] [PubMed] [Google Scholar]
- Cauwels A., Van Molle W., Janssen B., Everaerdt B., Huang P., Fiers W., Brouckaert P. 2000. Protection against TNF-induced lethal shock by soluble guanylate cyclase inhibition requires functional inducible nitric oxide synthase.Immunity. 13:223–231 [DOI] [PubMed] [Google Scholar]
- Der S.D., Zhou A., Williams B.R., Silverman R.H. 1998. Identification of genes differentially regulated by interferon alpha, beta, or gamma using oligonucleotide arrays.Proc. Natl. Acad. Sci. USA. 95:15623–15628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding W.X., Yin X.M. 2004. Dissection of the multiple mechanisms of TNF-alpha-induced apoptosis in liver injury.J. Cell. Mol. Med. 8:445–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermont A.M.M., Brunstein F., Grünhagen D., ten Hagen T.L. 2004. Regional treatment of metastasis: role of regional perfusion. State of the art isolated limb perfusion for limb salvage.Ann. Oncol. 15:iv107–iv112 [DOI] [PubMed] [Google Scholar]
- Everaerdt B., Brouckaert P., Fiers W. 1994. Recombinant IL-1 receptor antagonist protects against TNF-induced lethality in mice.J. Immunol. 152:5041–5049 [PubMed] [Google Scholar]
- Fenner J.E., Starr R., Cornish A.L., Zhang J.G., Metcalf D., Schreiber R.D., Sheehan K., Hilton D.J., Alexander W.S., Hertzog P.J. 2006. Suppressor of cytokine signaling 1 regulates the immune response to infection by a unique inhibition of type I interferon activity.Nat. Immunol. 7:33–39 [DOI] [PubMed] [Google Scholar]
- Halpern M.D., Holubec H., Dominguez J.A., Meza Y.G., Williams C.S., Ruth M.C., McCuskey R.S., Dvorak B. 2003. Hepatic inflammatory mediators contribute to intestinal damage in necrotizing enterocolitis.Am. J. Physiol. Gastrointest. Liver Physiol. 284:G695–G702 [DOI] [PubMed] [Google Scholar]
- Haniffa M.A., Wang X.N., Holtick U., Rae M., Isaacs J.D., Dickinson A.M., Hilkens C.M., Collin M.P. 2007. Adult human fibroblasts are potent immunoregulatory cells and functionally equivalent to mesenchymal stem cells.J. Immunol. 179:1595–1604 [DOI] [PubMed] [Google Scholar]
- Hooghe B., Hulpiau P., van Roy F., De Bleser P. 2008. ConTra: a promoter alignment analysis tool for identification of transcription factor binding sites across species.Nucleic Acids Res. 36:W128–W132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaghiosoff M., Steinborn R., Kovarik P., Kriegshäuser G., Baccarini M., Donabauer B., Reichart U., Kolbe T., Bogdan C., Leanderson T., et al. 2003. Central role for type I interferons and Tyk2 in lipopolysaccharide-induced endotoxin shock.Nat. Immunol. 4:471–477 [DOI] [PubMed] [Google Scholar]
- Kollias G., Douni E., Kassiotis G., Kontoyiannis D. 1999. On the role of tumor necrosis factor and receptors in models of multiorgan failure, rheumatoid arthritis, multiple sclerosis and inflammatory bowel disease.Immunol. Rev. 169:175–194 [DOI] [PubMed] [Google Scholar]
- Lechner J., Malloth N., Seppi T., Beer B., Jennings P., Pfaller W. 2008. IFN-alpha induces barrier destabilization and apoptosis in renal proximal tubular epithelium.Am. J. Physiol. Cell Physiol. 294:C153–C160 [DOI] [PubMed] [Google Scholar]
- Li W., Lewis-Antes A., Huang J., Balan M., Kotenko S.V. 2008. Regulation of apoptosis by type III interferons.Cell Prolif. 41:960–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert C., Takahashi N., Cauwels A., Brouckaert P., Bluethmann H., Fiers W. 1994. Response of interleukin-6-deficient mice to tumor necrosis factor-induced metabolic changes and lethality.Eur. J. Immunol. 24:2237–2242 [DOI] [PubMed] [Google Scholar]
- Liu J., Guan X., Tamura T., Ozato K., Ma X. 2004. Synergistic activation of interleukin-12 p35 gene transcription by interferon regulatory factor-1 and interferon consensus sequence-binding protein.J. Biol. Chem. 279:55609–55617 [DOI] [PubMed] [Google Scholar]
- Maher S.G., Romero-Weaver A.L., Scarzello A.J., Gamero A.M. 2007. Interferon: cellular executioner or white knight? Curr. Med. Chem. 14:1279–1289 [DOI] [PubMed] [Google Scholar]
- Mahieu T., Libert C. 2007. Should we inhibit type I interferons in sepsis? Infect. Immun. 75:22–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller U., Steinhoff U., Reis L.F., Hemmi S., Pavlovic J., Zinkernagel R.M., Aguet M. 1994. Functional role of type I and type II interferons in antiviral defense.Science. 264:1918–1921 [DOI] [PubMed] [Google Scholar]
- Piguet P.F., Vesin C., Guo J., Donati Y., Barazzone C. 1998. TNF-induced enterocyte apoptosis in mice is mediated by the TNF receptor 1 and does not require p53.Eur. J. Immunol. 28:3499–3505 [DOI] [PubMed] [Google Scholar]
- Pinkus G.S., Pinkus J.L. 1991. Myeloperoxidase: a specific marker for myeloid cells in paraffin sections.Mod. Pathol. 4:733–741 [PubMed] [Google Scholar]
- Qiu H., Fan Y., Joyee A.G., Wang S., Han X., Bai H., Jiao L., Van Rooijen N., Yang X. 2008. Type I IFNs enhance susceptibility to Chlamydia muridarum lung infection by enhancing apoptosis of local macrophages.J. Immunol. 181:2092–2102 [DOI] [PubMed] [Google Scholar]
- Takahashi N., Vanlaere I., de Rycke R., Cauwels A., Joosten L.A., Lubberts E., van den Berg W.B., Libert C. 2008. IL-17 produced by Paneth cells drives TNF-induced shock.J. Exp. Med. 205:1755–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey K.J., Beutler B., Lowry S.F., Merryweather J., Wolpe S., Milsark I.W., Hariri R.J., Fahey T.J., III, Zentella A., Albert J.D., et al. 1986. Shock and tissue injury induced by recombinant human cachectin.Science. 234:470–474 [DOI] [PubMed] [Google Scholar]
- Trowbridge I.S., Thomas M.L. 1994. CD45: an emerging role as a protein tyrosine phosphatase required for lymphocyte activation and development.Annu. Rev. Immunol. 12:85–116 [DOI] [PubMed] [Google Scholar]
- Uzé G., Schreiber G., Piehler J., Pellegrini S. 2007. The receptor of the type I interferon family.Curr. Top. Microbiol. Immunol. 316:71–95 [DOI] [PubMed] [Google Scholar]
- van den Berg T.K., Kraal G. 2005. A function for the macrophage F4/80 molecule in tolerance induction.Trends Immunol. 26:506–509 [DOI] [PubMed] [Google Scholar]
- Van Snick J., Cayphas S., Vink A., Uyttenhove C., Coulie P.G., Rubira M.R., Simpson R.J. 1986. Purification and NH2-terminal amino acid sequence of a T-cell-derived lymphokine with growth factor activity for B-cell hybridomas.Proc. Natl. Acad. Sci. USA. 83:9679–9683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalli P., Grau G.E., Piguet P.F. 1992. TNF in autoimmune diseases, graft-versus-host reactions, and pulmonary fibrosis.Immunol. Ser. 56:409–430 [PubMed] [Google Scholar]
- Wielockx B., Lannoy K., Shapiro S.D., Itoh T., Itohara S., Vandekerckhove J., Libert C. 2001. Inhibition of matrix metalloproteinases blocks lethal hepatitis and apoptosis induced by tumor necrosis factor and allows safe antitumor therapy.Nat. Med. 7:1202–1208 [DOI] [PubMed] [Google Scholar]
- Yarilina A., Park-Min K.H., Antoniv T., Hu X., Ivashkiv L.B. 2008. TNF activates an IRF1-dependent autocrine loop leading to sustained expression of chemokines and STAT1-dependent type I interferon-response genes.Nat. Immunol. 9:378–387 [DOI] [PubMed] [Google Scholar]