Abstract
Toll-like receptor (TLR) signals perform a crucial role in innate immune responses to pathogens. In this study, we found that the aryl hydrocarbon receptor (Ahr) negatively regulates inflammatory responses mediated by lipopolysaccharide (LPS) in macrophages. Ahr was induced in macrophages stimulated by LPS, but not by transforming growth factor (TGF)-β plus interleukin (IL)-6, which can induce Ahr in naive T cells. The production of IL-6 and tumor necrosis factor (TNF)-α by LPS was significantly elevated in Ahr-deficient macrophages compared with that in wild-type (WT) cells. Ahr-deficient mice were more highly sensitive to LPS-induced lethal shock than WT mice. Signal transducer and activator of transcription 1 (Stat1) deficiency, as well as Ahr deficiency, augmented LPS-induced IL-6 production. We found that Ahr forms a complex with Stat1 and nuclear factor-kappa B (NF-κB) in macrophages stimulated by LPS, which leads to inhibition of the promoter activity of IL-6. Ahr thus plays an essential role in the negative regulation of the LPS signaling pathway through interaction with Stat1.
APCs such as macrophages are important for innate immune defense and for the generation and regulation of adaptive immunity against various pathogens. Activated macrophages produce proinflammatory cytokines, including IL-6, IL-12, and TNF-α, which activate T cells and induce their differentiation. It has been demonstrated that IL-6 combined with TGF-β participates in the differentiation of naive T cells into IL-17–producing T helper (Th17) cells (Bettelli et al., 2006). More recently, our group and others demonstrated that Aryl hydrocarbon receptor (Ahr), also known as dioxin receptor, is induced by TGF-β plus IL-6 in naive T cells and participates in the differentiation of Th17 cells (Kimura et al., 2008; Quintana et al., 2008; Veldhoen et al., 2008). We proved that Ahr participates in Th17 cell development through regulating activation of signal-transducer-and-activator-of-transcription 1 (Stat1), which suppresses Th17 cell differentiation (Stumhofer et al. 2006; Kimura et al., 2008).
Ahr is a ligand-activated transcription factor that belongs to the basic-helix-loop-helix-PER-ARNT-SIM family (Burbach et al., 1992; Ema et al., 1992; Fujii-Kuriyama et al., 1994). Upon binding with a ligand, Ahr undergoes a conformation change, translocates to the nucleus, and dimerizes with the Ahr nuclear translocator (Arnt). Within the nucleus, the Ahr/Arnt heterodimer binds to a specific sequence, designated a xenobiotic responsive element, which causes a variety of toxicological effects (Dragan and Schrenk, 2000; Ohtake et al., 2003; Puga et al., 2005). In immune responses, Ahr activated by ligands such as 2,3,7,8-tetrachlorodibenzo-p-dioxin regulates the generation of regulatory T cells and modulates Th1/Th2 balance (Funatake et al., 2005; Negishi et al., 2005). Although it has been established that Ahr performs an important role in immune regulation as well as in toxic responses, it remains unclear how Ahr modulates immune responses in individual immune cell populations. Ahr-deficient (KO) mice all die within 5 wk of birth under conventional conditions where environmental pathogens are common, in contrast to their survival in a specific pathogen–free state, which led us to hypothesize that Ahr also may play an essential role in innate immune signaling in macrophages.
The Toll-like receptor (TLR) family is a diverse group of transmembrane receptors that recognize microbial components. TLRs are expressed mainly on APCs such as macrophages and DCs and recognition of microbial products by TLRs leads to generation of a variety of signal transduction pathways that elicit rapid inflammatory reactions (Akira and Takeda, 2004). LPS is the principal active agent in the pathogenesis of endotoxin shock, which is triggered by the interaction of LPS with TLR4 and leads to the production of cytokines and other inflammatory mediators, including IL-1, IL-6, TNF-α, IL-12 and IFNs (Beutler and Rietschel, 2003). TLR4 signaling can occur via two independent pathways. One depends on myeloid differentiation factor 88 (MyD88), which results in the activation of NF-κB. This MyD88-dependent pathway is critical for the production of IL-6 and TNF-α. The other pathway is a TIR domain–containing adaptor that induces an IFN-β (TRIF)–dependent pathway, which in turn induces IFN-β via IFN regulatory factor-3 (Fitzgerald et al., 2003). A splice variant of MyD88 (MyD88s) inhibits TLR pathways by its failure to recruit IRAK4, while TGF-β also inhibits the MyD88-dependent pathway for LPS–TLR4 signaling (Burns et al., 2003; Naiki et al., 2005). We also reported that suppressor of cytokine signaling 1 (SOCS-1) negatively regulates the LPS signal pathway (Nakagawa et al., 2002; Kimura et al., 2005). Thus, while it has been demonstrated that various regulatory systems are involved in TLR signaling, the mechanisms underlying the negative regulation in TLRs signaling have not been fully elucidated.
This study deals with a novel regulatory system for TLR signaling in which Ahr negatively regulates the inflammatory responses by LPS. We demonstrate that LPS-induced proinflammatory cytokines are augmented in Ahr-deficient macrophages compared with those in WT cells, and that Ahr-deficient mice are more susceptible to endotoxin shock induced by LPS. We also provide evidence that Ahr interacts with Stat1 and NF-κB and that the Ahr–Stat1 complex controls NF-κB-dependent proinflammatory responses by LPS.
RESULTS
Increased LPS-induced production of proinflammatory cytokines in Ahr deficient macrophages
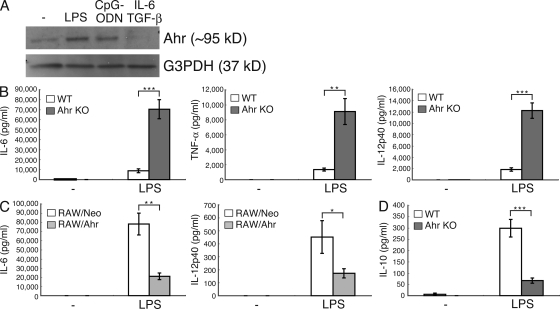
Our group and others previously reported that Ahr is induced in naive T cells stimulated by TGF-β plus IL-6, which participates in the induction of Th17 cell differentiation (Kimura et al., 2008; Quintana et al., 2008; Veldhoen et al., 2008). In this study, we used Western blot analysis to investigate Ahr expression in peritoneal macrophages stimulated by LPS, CpG-oligodeoxynucleotides (ODNs), and TGF-β plus IL-6. Ahr was expressed in peritoneal macrophages stimulated by LPS and CpG-ODN, but not by TGF-β plus IL-6 (Fig. 1 A), indicating that Ahr is induced by TLR signaling in those cells and that its expression pattern in macrophages and T cells is different. We next used Ahr KO peritoneal macrophages to examine whether Ahr affects LPS-induced proinflammatory cytokine production. As shown in Fig. 1 B, the levels of IL-6, TNF-α, and IL-12p40 were significantly elevated by LPS in Ahr KO peritoneal macrophages compared with those in WT cells. Next, we used a retroviral system to investigate whether Ahr reconstitution could reverse the phenotype in Ahr KO peritoneal macrophages and found that infection with Ahr in Ahr-deficient cells restored the overproduction of IL-6 (Fig. S1 A). We also examined TLR4 expression in WT and Ahr KO peritoneal macrophages, which showed the same pattern (unpublished data), indicating that the LPS signal is normally transmitted from the plasma membrane to the cytoplasm between WT and Ahr KO cells. To examine the effect of Ahr on LPS signaling, we established a mouse macrophage-like cell line (RAW cells) that constitutively expressed Ahr (RAW/Ahr). With RAW/Neo cells functioning as control, RAW/Ahr cells were treated with LPS, and LPS-induced production of proinflammatory cytokines was examined by means of ELISA. It was found that IL-6 and IL-12p40 production by LPS was inhibited in Ahr-overexpressing RAW cells compared with that in RAW/Neo cells (Fig. 1 C).
Figure 1.
Ahr deficiency augments LPS-induced proinflammatory responses in macrophages. (A) Peritoneal macrophages were stimulated with LPS, CpG-ODN, and TGF-β plus IL-6 for 24 h. The cells were lysed and subjected to immunoblotting (IB) analysis for the expression of Ahr and G3PDH. Data are from one representative of three independent experiments. (B–D) WT and Ahr KO peritoneal macrophages or RAW/Neo and RAW/Ahr cells were stimulated with LPS. Supernatants were collected 24 h after stimulation, and the production of IL-6, TNF-α, IL-12p40, and IL-10 were measured by means of ELISA. Data show means ± SEM of three independent experiments (*, P < 0.05; **, P < 0.005; ***, P < 0.001).
It has been recently reported that Ahr agonists in culture medium are important for Th17 cell differentiation, in which Iscove’s modified Dulbecco’s medium (IMDM), a medium that is richer in amino acids that can give rise to Ahr agonists, enhances Th17 cell development more than RPMI medium (Veldhoen et al., 2009). We therefore tested whether IMDM affects increased LPS-induced production of proinflammatory cytokines in WT- and Ahr-deficient macrophages. Although IMDM suppressed LPS-induced IL-6 production in WT peritoneal macrophages when compared with RPMI medium, its production was inhibited at the same rate as in Ahr KO cells (Fig. S1 B). These results indicate that natural ligands for Ahr in this culture medium do not affect the regulation of LPS signaling by Ahr.
Because it is known that macrophages produce an antiinflammatory cytokine, IL-10, to control the overproduction of inflammatory cytokines (Moore et al., 2001), we compared LPS-induced IL-10 production in WT and Ahr KO peritoneal macrophages. In contrast to proinflammatory cytokine production, LPS-induced IL-10 production was inhibited in Ahr KO peritoneal macrophages compared with that in WT cells (Fig. 1 D). These results demonstrate that Ahr has an antiinflammatory function in macrophages under the LPS–TLR4 signaling pathway. Because hypoproduction of IL-10 may cause hyperproduction of proinflammatory cytokines in Ahr KO peritoneal macrophages under LPS stimulation, we tested whether the addition of IL-10 to Ahr KO cells stimulated by LPS normalizes the overproduction of proinflammatory cytokine. Although IL-10 inhibited LPS-induced IL-6 production in Ahr KO cells by ∼40% compared with that by LPS stimulation only, its production was higher than that in WT cells stimulated by LPS (Fig. S2). Additionally, we found that RAW cells were not able to produce IL-10 under LPS stimulation (unpublished data), which suggests that the inhibition of LPS-induced proinflammatory cytokines in RAW/Ahr cells is unrelated to IL-10. These results indicate that Ahr regulates the production of LPS-induced proinflammatory cytokines independently of IL-10.
Ahr-deficient mice are hyperresponsive to LPS
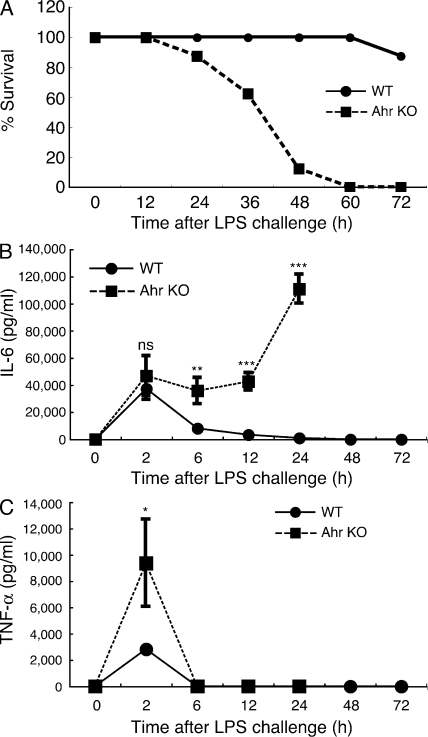
Because Ahr KO peritoneal macrophages showed a higher level of LPS-induced proinflammatory cytokine production than WT cells, we asked whether Ahr KO mice were more susceptible to LPS-induced toxicity. 6-wk-old WT and Ahr KO mice were injected intraperitoneally with 7.5 mg/kg of LPS. As shown in Fig. 2 A, all Ahr KO mice died within 60 h of being injected, but their WT littermates did not. We next measured serum levels of IL-6 and TNF-α in WT and Ahr KO mice after the LPS challenge. The serum IL-6 level in WT mice peaked 2 h after LPS administration, and then returned to the baseline level by 24 h, which is consistent with previously reported findings (Basu et al., 1997). In contrast, although serum IL-6 levels in Ahr KO mice increased similarly to those in WT mice until 2 h after LPS challenge, serum IL-6 in Ahr KO mice maintained the same level for 2–12 h and then increased again (Fig. 2 B). On the other hand, serum TNF-α levels in Ahr KO mice were significantly higher than in WT mice 2 h after LPS administration, but the kinetics were similar in the two groups of mice (Fig. 2 C). These results demonstrate that Ahr is involved in the negative regulation of LPS responses in vivo as well.
Figure 2.
Hypersensitivity of Ahr KO mice to LPS in vivo. 6-wk-old Ahr KO mice and littermate WT mice (n = 10 for each) were i.p. injected with 7.5 mg/kg of LPS. (A) Lethality was observed over 60 h after LPS challenge. Data are representative of two independent experiments. (B and C) Serum levels of IL-6 and TNF-α between WT and Ahr KO mice were measured by ELISA at indicated time points after LPS challenge. Data show means ± SEM of three independent experiments (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
Stat1 interacts with Ahr and regulates LPS-induced inflammatory responses in macrophages
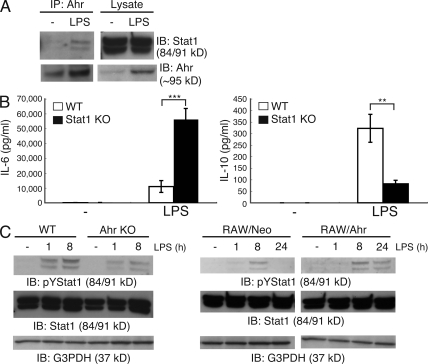
We previously reported that Ahr interacts with Stat1 and inhibits its activation in the process of Th17 cell differentiation (Kimura et al., 2008). To examine whether Ahr can bind with Stat1 in macrophages as it does in T cells, peritoneal macrophages were stimulated with LP, followed by verification (via immunoprecipitation and Western blotting) of the interaction between Ahr and Stat1. The results demonstrated that Ahr interacted with Stat1 in macrophages after activation with LPS (Fig. 3 A). To verify the involvement of Stat1 in LPS-stimulated cytokine production, WT and Stat1 KO peritoneal macrophages were stimulated with LPS, and the protein levels of IL-6 and IL-10 were measured by means of ELISA. Similar to that in Ahr KO peritoneal macrophages, LPS-induced IL-6 production was significantly augmented in Stat1 KO cells, whereas IL-10 production was inhibited compared with that in WT cells (Fig. 3 B). We confirmed that Ahr was normally induced by LPS in the absence of Stat1 (Fig. S3 A), indicating that hyperproduction of IL-6 in Stat1 KO peritoneal macrophages stimulated by LPS is not caused by the absence of Ahr.
Figure 3.
Association between Ahr and Stat1 in macrophages. (A) Peritoneal macrophages were isolated from BALB/c mice and stimulated by LPS for 24 h. Interaction between Ahr and Stat1 was examined by means of immunoprecipitation (IP) and Western blotting. Data are from one representative of three independent experiments. IB, immunoblot. (B) WT and Stat1 KO peritoneal macrophages were stimulated with LPS. Supernatants were collected 24 h after stimulation, and the production of IL-6 and IL-10 were measured by means of ELISA. Data show means ± SEM of three independent experiments (**, P < 0.005; ***, P < 0.001). (C) WT and Ahr KO peritoneal macrophages or RAW/Neo and RAW/Ahr cells were incubated with LPS at the indicated time points. Whole-cell lysates were used for immunoblotting analysis with anti–phospho-tyrosine Stat1, Stat1, and G3PDH antibodies (Ab). Data are from one representative of three independent experiments.
We previously demonstrated that Ahr inhibits Stat1 activation in naive T cells under Th17-polarizing conditions (TGF-β plus IL-6; Kimura et al., 2008). In macrophages, however, Ahr prolonged Stat1 activation by LPS. LPS-induced Stat1 activation was diminished in Ahr KO macrophages compared with that in WT cells (Fig. 3 C). On the other hand, LPS-induced Stat1 activation was prolonged in RAW/Ahr cells compared with that in RAW/Neo cells (Fig. 3 C). Because it has been reported that LPS-dependent Stat1 phosphorylation is mainly dependent on IFN-β (Toshchakov et al., 2002) and that SOCS proteins are important for regulating Stat1 activation (Yoshimura et al., 2007), we examined both LPS-induced IFN-β production and the expression of SOCS-1 and SOCS-3 in WT and Ahr KO peritoneal macrophages stimulated with LPS. We found no changes in IFN-β production or SOCSs expression in WT and Ahr KO cells after LPS stimulation (Fig. S3, B and C), indicating that the suppression of Stat1 activation by LPS in Ahr KO macrophages occurs independently of IFN-β and SOCSs. Collectively, these findings suggest that Ahr may directly protect the inactivation of Stat1 in macrophages through interacting with it, followed by regulation of LPS signaling.
Ahr–Stat1 complex binds to NF-κB and suppresses its transcriptional activity, but not its DNA-binding capacity
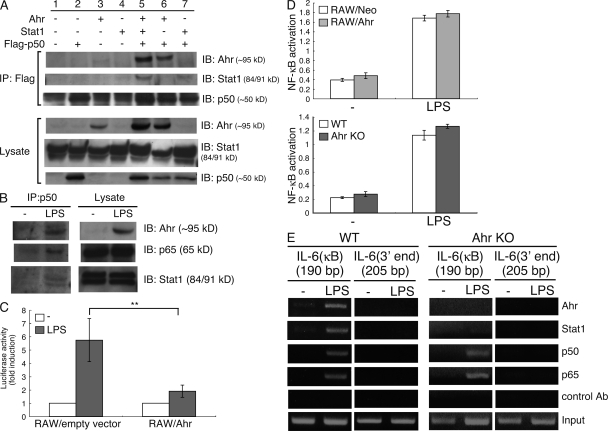
The production of proinflammatory cytokines such as IL-6 and TNF-α by LPS is induced via the MyD88-dependent NF-κB pathway (Kawai et al., 1999; Beutler and Rietschel, 2003). It has also been reported that Ahr combines with NF-κB, and that this complex regulates several signal pathways (Tian et al., 1999, 2002; Vogel et al., 2007). We speculated that the Ahr–Stat1 complex might interact with NF-κB, followed by regulation of the NF-κB pathway by the resultant complex. To test this hypothesis, we first examined whether Ahr interacts with NF-κB together with Stat1. COS7 cells were transiently transfected with Ahr, NF-κB p50, and Stat1 and subjected to coimmunoprecipitation analysis. As shown in Fig. 4 A, Ahr interacted with NF-κB p50 (lane 6) and formed a complex together with NF-κB p50 and Stat1 (lane 5). Furthermore, to determine whether endogenous Ahr forms a complex together with endogenous Stat1 and NF-κB p50, peritoneal macrophages were stimulated with LPS, followed by verification by means of immunoprecipitation and Western blotting of the association of their endogenous proteins. We also found that Ahr interacts with Stat1 and NF-κB p50 endogenously in peritoneal macrophages activated by LPS (Fig. 4 B).
Figure 4.
Ahr inhibits LPS-induced NF-κB transcriptional activity together with Stat1. (A) COS7 cells were cotransfected with Ahr, Stat1, and p50-Flag. After 24 h, the cells were lysed and immunoprecipitated with anti-Flag Ab, followed by detection of Ahr, Stat1, and p50 by means of Western blotting. Data are from one representative of three independent experiments. IP, immunoprecipitation; IB, immunoblot. (B) Peritoneal macrophages were stimulated with LPS for 24 h. Whole-cell lysates were immunoprecipitated with anti-p50 antibody, after which Ahr, p65, and Stat1 were detected with Western blotting. Data are from one representative of three independent experiments. (C) RAW cells were transiently cotransfected with luciferase reporter gene construct of the murine IL-6 promoter and an expression vector for Ahr (RAW/Ahr) or empty control expression vector (RAW/empty vector). 6 h after transfection, cells were stimulated with LPS for 12 h. Luciferase assay and quantitation were performed as described in Materials and methods. Data show means ± SEM of three independent experiments (**, P < 0.02). (D) RAW/Neo and RAW/Ahr or WT and Ahr KO peritoneal macrophages were stimulated with LPS for 24 h NF-κB binding activity was examined using TransAM assay. Data show means ± SEM of three independent experiments. (E) Peritoneal macrophages from WT and Ahr KO mice were stimulated with LPS for 4 h, and the ChIP assay was performed using anti-p50, anti-p65, anti-Stat1, and anti-Ahr antibodies. Purified DNA fragments were amplified using primers specific for the IL-6 promoter. Data are from one representative of three independent experiments.
We next examined the effect of Ahr on LPS-induced activation of the IL-6 promoter. RAW cells were transiently transfected with a reporter plasmid containing the promoter of IL-6 combined with either Ahr or a control vector. After treatment with LPS, luciferase activities were measured with the dual luciferase reporter assay system. LPS-induced activation of the IL-6 promoter was significantly suppressed in RAW cells overexpressing Ahr (Fig. 4 C), which suggests that Ahr inhibits the NF-κB transcriptional activity on LPS-induced IL-6 production. For further investigation of how Ahr regulates LPS-induced NF-κB activation, we used the TransAM assay to assess NF-κB DNA binding activity between RAW/Neo and RAW/Ahr cells stimulated by LPS. Ahr showed no significant influence on LPS-induced NF-κB DNA binding activity between those cells (Fig. 4 D). Similarly, NF-κB bound to its target DNA upon LPS stimulation of both WT- and Ahr-KO peritoneal macrophages (Fig. 4 D). Cytosolic IκB-α is reportedly degraded upon activation of NF-κB (Brown et al., 1993), and we also found no difference in IκB-α degradation in macrophages stimulated by LPS with or without Ahr (Fig. S4). These findings demonstrate that Ahr suppresses the NF-κB transcriptional activity of the IL-6 promoter, but not its DNA-binding capacity. It has further been reported that IL-6 production is required to induce IκBζ via the Myd88-dependent NF-κB pathway in LPS signaling, followed by the association of IκBζ with p50 and recruitment of the resultant complex to the IL-6 promoter (Yamamoto et al., 2004). We therefore examined whether Ahr affects IκBζ induction by LPS and found no difference in its induction by LPS in RAW/Ahr and RAW/Neo cells (Fig. S5). This result is consistent with that illustrated in Fig. 4 D, which shows that Ahr does not affect the NF-κB DNA-binding activity. These findings indicate that Ahr selectively inhibits NF-κB transcriptional activity in the LPS signaling pathway.
We further examined whether upon LPS stimulation the Ahr–Stat1 complex can interact with NF-κB on the promoter region of proinflammatory cytokines and then suppress LPS-induced NF-κB transcriptional activation and inflammatory cytokine production. We performed the chromatin immunoprecipitation (ChIP) assay to determine whether Ahr and Stat1 are recruited to the IL-6 promoter in response to LPS in combination with NF-κB. Peritoneal macrophages from WT and Ahr KO mice were stimulated with LPS for 4 h, and the ChIP assay was performed using antibodies for detection of Ahr, Stat1, p50, and p65, and it was found that although p50 and p65 were recruited to the IL-6 promoter in response to LPS in both cells, Ahr and Stat1 bound to the IL-6 promoter region in WT, but not in Ahr KO cells (Fig. 4 E). These results indicate that Ahr, in combination with Stat1, regulates LPS-induced proinflammatory cytokine production in macrophages through inhibition of NF-κB transcriptional activity in their promoter region.
Ahr does not participate in CpG-ODN signaling
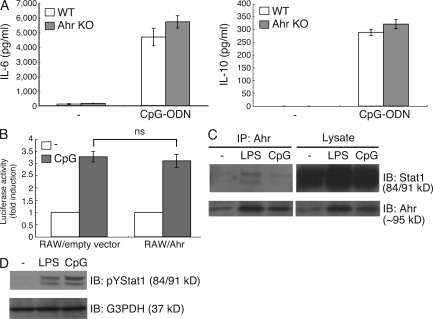
As shown in Fig. 1 A, Ahr was induced in peritoneal macrophages stimulated by CpG-ODN and LPS. We therefore asked whether Ahr regulates the CpG-ODN–TLR9 pathway. WT and Ahr KO peritoneal macrophages were stimulated with CpG-ODN, and the protein levels of IL-6 and IL-10 were measured by means of ELISA. Surprisingly, we found that Ahr deficiency had no effect on their production by CpG-DNA (Fig. 5 A) and that CpG-ODN–induced activation of the IL-6 promoter was similar in RAW cells with or without Ahr (Fig. 5 B). Thus, Ahr is not capable of regulating the CpG-ODN signaling pathway despite its expression in peritoneal macrophages stimulated with CpG-ODN.
Figure 5.
Ahr has no influence in CpG-ODN signaling pathway. (A) WT and Ahr KO peritoneal macrophages were stimulated with CpG-ODN for 24 h. The production of IL-6 and IL-10 were measured by means of ELISA. Data show means ± SE of three independent experiments. (B) RAW cells were transiently cotransfected with luciferase reporter gene construct of the murine IL-6 promoter and an expression vector for Ahr (RAW/Ahr) or empty control expression vector (RAW/empty vector). 6 h after transfection, cells were stimulated with CpG-ODN for 12 h. Luciferase assay and quantitation were performed as described in Materials and methods. Data show means ± SEM of three independent experiments. Peritoneal macrophages were stimulated with LPS and CpG-ODN. (C) Cells were lysed, immunoprecipitated by Ahr, and analyzed by Western blotting with anti-Stat1 Ab. IP, immunoprecipitation; IB, immunoblot. (D) Whole-cell lysates subjected to Western analysis with anti-pYStat1 antibodies. Data are from one representative of three independent experiments.
To understand why Ahr has no effect on CpG-ODN-induced pro- and antiinflammatory cytokine production, we assessed the interaction between Ahr and Stat1 in LPS- or CpG-ODN–treated peritoneal macrophages. As shown in Fig. 3 A, although Ahr interacted with Stat1 under LPS stimulation, hardly any binding of Ahr with Stat1 could be detected in CpG-ODN–treated cells (Fig. 5 C). However, CpG-ODN activated Stat1 to the same degree as did LPS stimulation (Fig. 5 D), indicating that the complex formation of Ahr with Stat1 is independent of Stat1 activation. These results suggest that it may be required for some natural ligand for Ahr to form the complex with Stat1 and that LPS may be able to induce some natural ligand for Ahr, but not CpG-ODN.
DISCUSSION
Ahr is a ligand-inducible transcription factor, which has been shown to regulate the expression of a variety of genes, including those encoding for cytochrome P450 enzymes. In addition, Ahr activation by ligands such as dioxin has been linked to alterations in cell proliferation, apoptosis, tumor promotion, development, and reproductive functions (Puga et al., 2000; Shimizu et al., 2000; Bonnesen et al., 2001). A growing number of studies have recently detailed the various effects of Ahr on the immune system, especially the development of Th17 cells (Kimura et al., 2008; Quintana et al., 2008; Veldhoen et al., 2008). Because we found that Ahr-KO mice all die under conventional conditions, it was expected that Ahr might also participate in the innate immune system, which is capable of recognizing a wide variety of pathogens and rapidly inducing various antimicrobial and inflammatory responses. In this study, we identified an important role of Ahr in TLR signaling, that is, Ahr combined with Stat1 controls LPS–TLR4–mediated pro- and antiinflammatory cytokine production.
Initially, we demonstrated that TLR ligands such as LPS, but not IL-6 in combination with TGF-β, induced Ahr expression in macrophages and that, whereas the production of proinflammatory cytokines such as IL-6, TNF-α, and IL-12p40 was drastically increased upon LPS stimulation, production of the antiinflammatory cytokine IL-10 was inhibited in the absence of Ahr. In addition, we found that Ahr-deficient mice were highly susceptible to LPS-induced toxicity. The levels of serum IL-6 and TNF-α in Ahr KO mice were higher than those in WT mice after LPS challenge. These findings indicate that Ahr contributes to the negative regulation of the LPS signal pathway both in vivo and in vitro.
We also found that Ahr forms a complex with Stat1 and NF-κB, which is consistent with previous findings that Ahr interacts with several transcriptional factors, such as Stat1 and NF-κB (Tian et al., 1999, 2002; Vogel et al., 2007; Kimura et al., 2008). An important finding of our current study is that Stat1 deficiency, like Ahr deficiency, led to an increase in LPS-induced IL-6 production, but suppressed production of the LPS-induced antiinflammatory cytokine IL-10. However, it was previously reported that Stat1-deficient mice are resistant to LPS-induced shock (Karaghiosoff et al., 2003), which seems to conflict with our finding that Stat1-deficient macrophages produce more IL-6 and less IL-10 compared with those produced in WT cells. Stat1 contributes to the development of endotoxin shock through its central role in IFN responses, which are secondarily induced by LPS (Karaghiosoff et al., 2003). Stat1 deficiency therefore shows resistance to LPS-induced shock in vivo through blocking LPS-induced secondary cytokine (IFN) signaling. We speculate that Stat1 takes part in not only LPS-induced secondary responses (IFN responses) in vivo but also in direct signaling of LPS in vitro through interacting with NF-κB and Ahr; in the latter function Stat1 has the property to suppress LPS-NF-κB signaling.
The findings that Ahr inhibits LPS-induced activation of the IL-6 promoter and interacts in combination with both NF-κB and Stat1 on the same region of the IL-6 promoter suggest that the Ahr–Stat1 complex may control LPS-induced proinflammatory responses by inhibiting NF-κB transcriptional activity. In fact, NF-κB DNA-binding activity was not inhibited by Ahr, which is consistent with the finding that Ahr did not affect the expression of IκBζ via the LPS-MyD88-dependent pathway. At present, however, the detailed mechanism of Ahr in suppressing NF-κB transcriptional activity remains poorly understood. Nuclear receptors in combination with coactivators and corepressors can switch the transcriptional activity of several transcriptional factors on and off, respectively. It was recently reported that Ahr repressor (Ahrr), known as an Ahr negative regulator, represses estrogen receptor α–mediated transcriptional activation through interacting directly with ERα on the promoter sequences of estrogen receptor–target genes (Kanno et al., 2008). This seems to imply that the Ahr–Stat1 complex may inhibit LPS-induced NF-κB transcriptional activity via a corepressor such as Ahrr.
Two groups in addition to ours recently reported that Ahr participates in Th17 cell differentiation (Kimura et al., 2008; Quintana et al., 2008; Veldhoen et al., 2008). In our study, we provided evidence that Ahr is involved in the differentiation of Th17 cells by inhibiting Stat1 activation, which suppresses Th17 cell differentiation, under Th17-polarizing conditions (TGF-β plus IL-6). Stat1 activation was eliminated 24 h after stimulation with TGF-β plus IL-6 in WT naive T cells, whereas its activation was maintained in Ahr-deficient naive T cells (Kimura et al., 2008). In contrast, Stat1 activation by LPS was inhibited in Ahr-deficient macrophages, compared with that in normal macrophages. These findings indicate that Stat1 activation is differentially regulated by Ahr in T cells and macrophages. Ahr is known to perform a dual function in controlling intracellular protein levels, serving both as a transcriptional factor and as a ligand-dependent E3 ubiquitin ligase (Ohtake et al., 2007). It is also possible that, although Ahr regulates the activation of Stat1 through the degradation of activated Stat1 by functioning as a ligand-dependent E3 ubiquitin ligase in the generation of Th17 cells, it acts like a transcriptional factor and cooperates with Stat1 to regulate NF-κB transcriptional activation in LPS-activated macrophages. Thus, the Ahr–Stat1 combination controls immune responses in different ways depending on the immune cell population.
The CpG-ODN–TLR9 signaling pathway and the LPS–TLR4 signaling pathway induce proinflammatory cytokines such as IL-6 via MyD88-NF-κB (Akira and Takeda, 2004). However, our findings demonstrate that Ahr is incapable of regulating the production of pro- and antiinflammatory cytokines by CpG-ODN, although it is induced in peritoneal macrophages stimulated with CpG-ODN. Interestingly, Ahr interacted with Stat1 in the peritoneal macrophages under stimulation with LPS, but not with CpG-ODN, even though the level of Stat1 activation was the same for these two stimulations, which may account for the difference between the LPS and CpG-ODN signaling pathways in the regulation by Ahr of pro- and antiinflammatory cytokine production. Given that Ahr forms the complex together with Stat1 and p50 on the IL-6 promoter region and regulates NF-κB transcriptional activity, Stat1 may be required for the inhibition of NF-κB transcriptional activity by Ahr. Some natural ligand may be required when Ahr forms the complex with Stat1, followed by the regulation of NF-κB.
Our preliminary data show that IL-6 suppresses LPS-induced Ahr expression in macrophages (unpublished data). As seen in Fig. 1 A, the expression of Ahr in macrophages was inhibited by IL-6 in combination with TGF-β. In this study, we demonstrated that Ahr performs an antiinflammatory function in macrophages. It can thus be speculated that IL-6 may amplify proinflammatory responses in macrophages through inhibiting the expression of Ahr, which suppresses LPS-induced proinflammatory responses. In T cells, on the other hand, IL-6 combined with TGF-β induces the expression of Ahr, which participates in Th17 cell differentiation. IL-6 thus promotes proinflammatory responses through the differential regulation of Ahr expression in macrophages and T cells.
To summarize, we have identified and characterized a novel regulatory mechanism of the TLR signaling pathway in which Ahr in combination with Stat1 concurrently controls LPS-induced pro- and antiinflammatory cytokine production. We have also provided evidence that Ahr differentially regulates Stat1 activation and NF-κB transcriptional activity in T cells and macrophages, respectively. This suggests that Ahr may control several immune responses through the regulation of transcriptional factors, such as the Stat and NF-κB families, and be involved in several autoimmune diseases. It is necessary to gain an understanding of how Ahr regulates the immune system in various immune cells such as T cells, B cells, macrophages and dendritic cells. Further studies using each immune cell–specific Ahr conditional KO mice will define the functions of Ahr in immunity and several autoimmune diseases.
MATERIALS AND METHODS
Mice.
C57BL/6 WT mice were obtained from CLEA Japan, Inc. Ahr KO mice and Stat1 KO mice (C57BL/6 background) were provided by Y. Fujii-Kuriyama (University of Tsukuba, Tsukuba, Japan) and T. Naka (National Institute of Biomedical Innovation, Osaka, Japan), respectively. All mice were maintained under specific pathogen-free conditions. All animal experiments were performed in accordance with protocols approved by the Institutional Animal Care and Use Committees of the Graduate School of Frontier Bioscience, Osaka University. WT and Ahr KO mice were injected i.p. with the indicated amounts of LPS (Escherichia coli; Sigma-Aldrich) for the indicated periods of time.
Cell culture and reagents.
Peritoneal macrophages were prepared as previously described (Kimura et al., 2005). The thioglycolate-elicited peritoneal macrophages and a mouse macrophage cell line (RAW cells) were cultured in RPMI 1640 with 10% FCS, 100 µg/ml streptomycin, and 100 U/ml penicillin G. RAW cells were stably transfected with Ahr cDNAs (donated by Y. Fujii-Kuriyama). Stably transfected RAW mutant lines (RAW/Neo, RAW/Ahr) were maintained in the presence of 500 µg/ml G418. COS7 cells were cultured in DME with 10% FCS, 100 µg/ml streptomycin, and 100 U/ml penicillin G. We stimulated the cells with 1 µg/ml LPS (E. coli; Sigma-Aldrich), 1 µM phosphorothioate-modified CpG-ODN, 20 ng/ml mouse IL-6 and 2 ng/ml human TGF-β1 (both from R&D Systems) for the indicated periods of time.
Cytokine ELISA.
The cells were stimulated with 1 µg/ml LPS (E. coli; Sigma-Aldrich) and 1 µM phosphorothioate-modified CpG-ODN for 24 h. Mouse IL-6, TNF-α, IL-12p40, and IL-10 from either the supernatants or the serum were measured by means of ELISA according to the manufacturer’s instructions (R&D Systems).
Immunoprecipitation and Western blotting.
Peritoneal macrophages were cultured with 1 µg/ml LPS or 1 µM phosphorothioate-modified CpG-ODN for 24 h and then lysed with a lysis buffer (1% NP-40, 20 mM Tris-HCl, ph 7.5, 150 mM NaCl, 10 mM Na2VO4, 0.5 mM DTT, and 1/100 protease inhibitor cocktail). Ahr and p50 were immunoprecipitated with anti-Ahr (BIOMOL International) and anti-p50 (Santa Cruz Biotechnology, Inc.), respectively, and then subjected to SDS-PAGE. Whole-cell lysates and the immunocomplex were analyzed with Western blotting using anti-Stat1 (BD), anti-p65 (Santa Cruz Biotechnology, Inc.), or anti-Ahr (BIOMOL International L.P.). COS7 cells were cotransfected with 1 µg of pEF-BOS-Ahr, pEF-BOS-Stat1, and pEF-BOS-p50-Flag with the aid of FuGENE 6 (Roche). Cells were lysed with lysis buffer and lysates were immunoprecipitated with anti-Flag M2 (Sigma-Aldrich). Immunoprecipitated samples were analyzed by means of Western blotting by using anti-Ahr (BIOMOL), anti-Stat1 (BD) and anti-p50 (Santa Cruz Biotechnology, Inc.).
Activation of Stat1.
WT and Ahr KO peritoneal macrophages or RAW/Neo and RAW/Ahr cells were incubated with 1 µg/ml LPS for the time indicated, and cells were lysed with a lysis buffer. Whole-cell lysates were then analyzed by means of Western blotting using anti–phospho-Stat1 (Tyr701; Cell Signaling Technology).
Luciferase assay.
RAW cells were transfected with 1 µg of the reporter plasmid and, in cotransfection experiments, with 0.1 µg of pRL-TK for use as an internal control reporter and 1 µg of pEF-BOS-Ahr or an empty vector (pEF-BOS). Cells were stimulated with 1 µg/ml LPS or 1 µM phosphorothioate-modified CpG-ODN for 12 h and lysed with luciferase lysis reagent (Promega). Luciferase activity was determined with a commercial dual-luciferase reporter assay system (Promega) according to the manufacturer’s instructions. Relative light units of Firefly luciferase activity were normalized with Renilla luciferase activity.
TransAM assay.
RAW/Neo and RAW/Ahr cells or WT and Ahr KO peritoneal macrophages were stimulated with 1 µg/ml LPS for 24 h. Nuclear extraction was performed with a nuclear extraction kit (Active Motif). 10 µg of nuclear extraction protein was used for assessing the NF-κB binding activity with the NF-κB (p50) TransAM Assay (Active Motif) according to the manufacturer’s instructions.
ChIP assay.
The ChIP assay was performed essentially according to Upstate Biotechnology’s protocol. In brief, WT and Ahr KO peritoneal macrophages were stimulated with 1 µg/ml LPS for 12 h, and then fixed with formaldehyde for 10 min. The cells were lysed, sheared by sonication, and incubated overnight with specific antibodies, followed by incubation with protein A-agarose saturated with salmon sperm DNA (Vector Laboratories). Precipitated DNAs were analyzed by quantitative PCR (35 cycles) using primers 5′-CGATGCTAAACGACGTCACATTGTGCA-3′ and 5′-CTCCAGAGCAGAATGAGCTACAGACAT-3′ for the κB site in the IL-6 promoter.
Statistical analysis.
Student’s t test was used to analyze data for significant differences. Values of P < 0.05 were regarded as significant.
Online supplemental material.
Fig. S1 shows that Ahr regulates LPS-induced production of IL-6 with or without natural ligands for Ahr in culture medium. Fig. S2 shows that that hypoproduction of IL-10 does not cause hyperproduction of proinflammatory cytokines in Ahr KO peritoneal macrophages under LPS stimulation. Fig. S3 shows the expression of Ahr in Stat1 KO peritoneal macrophages and the induction of IFN-β and SOCS family members in Ahr KO cells. Fig. S4 shows the IκB-α degradation in WT and Ahr KO macrophages stimulated by LPS. Fig. S5 shows the IκBζ expression in RAW/Neo and RAW/Ahr cells stimulated by LPS. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20090560/DC1.
Acknowledgments
This work was supported by the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation and Chugai-Roche Pharmaceutical Co. Ltd, Tokyo, Japan.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used: Ahr, aryl hydrocarbon receptor; ChIP, chromatin immunoprecipitation; IMDM, Iscove's modified Dulbecco's medium; MyD88, myeloid differentiation factor 88; ODN, oligodeoxynucleotide; Stat1, signal transducer and activator of transcription 1; SOCS, suppressor of cytokine signaling 1; TLR, Toll-like receptor.
References
- Akira S., Takeda K. 2004. Toll-like receptor signalling.Nat. Rev. Immunol. 4:499–511 [DOI] [PubMed] [Google Scholar]
- Basu S., Dunn A.R., Marino M.W., Savoia H., Hodgson G., Lieschke G.J., Cebon J. 1997. Increased tolerance to endotoxin by granulocyte-macrophage colony-stimulating factor-deficient mice.J. Immunol. 159:1412–1417 [PubMed] [Google Scholar]
- Bettelli E., Carrier Y., Gao W., Korn T., Strom T.B., Oukka M., Weiner H.L., Kuchroo V.K. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells.Nature. 441:235–238 [DOI] [PubMed] [Google Scholar]
- Beutler B., Rietschel E.T. 2003. Innate immune sensing and its roots: the story of endotoxin.Nat. Rev. Immunol. 3:169–176 [DOI] [PubMed] [Google Scholar]
- Bonnesen C., Eggleston I.M., Hayes J.D. 2001. Dietary indoles and isothiocyanates that are generated from cruciferous vegetables can both stimulate apoptosis and confer protection against DNA damage in human colon cell lines.Cancer Res. 61:6120–6130 [PubMed] [Google Scholar]
- Brown K., Park S., Kanno T., Franzoso G., Siebenlist U. 1993. Mutual regulation of the transcriptional activator NF-kappa B and its inhibitor, I kappa B-alpha.Proc. Natl. Acad. Sci. USA. 90:2532–2536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbach K.M., Poland A., Bradfield C.A. 1992. Cloning of the Ah-receptor cDNA reveals a distinctive ligand-activated transcription factor.Proc. Natl. Acad. Sci. USA. 89:8185–8189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns K., Janssens S., Brissoni B., Olivos N., Beyaert R., Tschopp J. 2003. Inhibition of interleukin 1 receptor/Toll-like receptor signaling through the alternatively spliced, short form of MyD88 is due to its failure to recruit IRAK-4.J. Exp. Med. 197:263–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragan Y.P., Schrenk D. 2000. Animal studies addressing the carcinogenicity of TCDD (or related compounds) with an emphasis on tumour promotion.Food Addit. Contam. 17:289–302 [DOI] [PubMed] [Google Scholar]
- Ema M., Sogawa K., Watanabe N., Chujoh Y., Matsushita N., Gotoh O., Funae Y., Fujii-Kuriyama Y. 1992. cDNA cloning and structure of mouse putative Ah receptor.Biochem. Biophys. Res. Commun. 184:246–253 [DOI] [PubMed] [Google Scholar]
- Fitzgerald K.A., Rowe D.C., Barnes B.J., Caffrey D.R., Visintin A., Latz E., Monks B., Pitha P.M., Golenbock D.T. 2003. LPS-TLR4 signaling to IRF-3/7 and NF-kappaB involves the toll adapters TRAM and TRIF.J. Exp. Med. 198:1043–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii-Kuriyama Y., Ema M., Miura J., Sogawa K. 1994. Ah receptor: a novel ligand-activated transcription factor.Exp. Clin. Immunogenet. 1:65–74 [DOI] [PubMed] [Google Scholar]
- Funatake C.J., Marshall N.B., Steppan L.B., Mourich D.V., Kerkvliet N.I. 2005. Cutting edge: activation of the aryl hydrocarbon receptor by 2,3,7,8-tetrachlorodibenzo-p-dioxin generates a population of CD4+ CD25+ cells with characteristics of regulatory T cells.J. Immunol. 175:4184–4188 [DOI] [PubMed] [Google Scholar]
- Kanno Y., Takane Y., Takizawa Y., Inouye Y. 2008. Suppressive effect of aryl hydrocarbon receptor repressor on transcriptional activity of estrogen receptor alpha by protein-protein interaction in stably and transiently expressing cell lines.Mol. Cell. Endocrinol. 291:87–94 [DOI] [PubMed] [Google Scholar]
- Karaghiosoff M., Steinborn R., Kovarik P., Kriegshäuser G., Baccarini M., Donabauer B., Reichart U., Kolbe T., Bogdan C., Leanderson T., et al. 2003. Central role for type I interferons and Tyk2 in lipopolysaccharide-induced endotoxin shock.Nat. Immunol. 4:471–477 [DOI] [PubMed] [Google Scholar]
- Kawai T., Adachi O., Ogawa T., Takeda K., Akira S. 1999. Unresponsiveness of MyD88-deficient mice to endotoxin.Immunity. 11:115–122 [DOI] [PubMed] [Google Scholar]
- Kimura A., Naka T., Muta T., Takeuchi O., Akira S., Kawase I., Kishimoto T. 2005. Suppressor of cytokine signaling-1 selectively inhibits LPS-induced IL-6 production by regulating JAK-STAT.Proc. Natl. Acad. Sci. USA. 102:17089–17094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A., Naka T., Nohara K., Fujii-Kuriyama Y., Kishimoto T. 2008. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells.Proc. Natl. Acad. Sci. USA. 105:9721–9726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K.W., de Waal Malefyt R., Coffman R.L., O’Garra A. 2001. Interleukin-10 and the interleukin-10 receptor.Annu. Rev. Immunol. 19:683–765 [DOI] [PubMed] [Google Scholar]
- Naiki Y., Michelsen K.S., Zhang W., Chen S., Doherty T.M., Arditi M. 2005. Transforming growth factor-beta differentially inhibits MyD88-dependent, but not TRAM- and TRIF-dependent, lipopolysaccharide-induced TLR4 signaling.J. Biol. Chem. 280:5491–5495 [DOI] [PubMed] [Google Scholar]
- Nakagawa R., Naka T., Tsutsui H., Fujimoto M., Kimura A., Abe T., Seki E., Sato S., Takeuchi O., Takeda K., et al. 2002. SOCS-1 participates in negative regulation of LPS responses.Immunity. 17:677–687 [DOI] [PubMed] [Google Scholar]
- Negishi T., Kato Y., Ooneda O., Mimura J., Takada T., Mochizuki H., Yamamoto M., Fujii-Kuriyama Y., Furusako S. 2005. Effects of aryl hydrocarbon receptor signaling on the modulation of TH1/TH2 balance.J. Immunol. 175:7348–7356 [DOI] [PubMed] [Google Scholar]
- Ohtake F., Takeyama K., Matsumoto T., Kitagawa H., Yamamoto Y., Nohara K., Tohyama C., Krust A., Mimura J., Chambon P., et al. 2003. Modulation of oestrogen receptor signalling by association with the activated dioxin receptor.Nature. 423:545–550 [DOI] [PubMed] [Google Scholar]
- Ohtake F., Baba A., Takada I., Okada M., Iwasaki K., Miki H., Takahashi S., Kouzmenko A., Nohara K., Chiba T., et al. 2007. Dioxin receptor is a ligand-dependent E3 ubiquitin ligase.Nature. 446:562–566 [DOI] [PubMed] [Google Scholar]
- Puga A., Barnes S.J., Dalton T.P., Chang C., Knudsen E.S., Maier M.A. 2000. Aromatic hydrocarbon receptor interaction with the retinoblastoma protein potentiates repression of E2F-dependent transcription and cell cycle arrest.J. Biol. Chem. 275:2943–2950 [DOI] [PubMed] [Google Scholar]
- Puga A., Tomlinson C.R., Xia Y. 2005. Ah receptor signals cross-talk with multiple developmental pathways.Biochem. Pharmacol. 69:199–207 [DOI] [PubMed] [Google Scholar]
- Quintana F.J., Basso A.S., Iglesias A.H., Korn T., Farez M.F., Bettelli E., Caccamo M., Oukka M., Weiner H.L. 2008. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor.Nature. 453:65–71 [DOI] [PubMed] [Google Scholar]
- Shimizu Y., Nakatsuru Y., Ichinose M., Takahashi Y., Kume H., Mimura J., Fujii-Kuriyama Y., Ishikawa T. 2000. Benzo[a]pyrene carcinogenicity is lost in mice lacking the aryl hydrocarbon receptor.Proc. Natl. Acad. Sci. USA. 97:779–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumhofer J.S., Laurence A., Wilson E.H., Huang E., Tato C.M., Johnson L.M., Villarino A.V., Huang Q., Yoshimura A., Sehy D., et al. 2006. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system.Nat. Immunol. 7:937–945 [DOI] [PubMed] [Google Scholar]
- Tian Y., Ke S., Denison M.S., Rabson A.B., Gallo M.A. 1999. Ah receptor and NF-kappaB interactions, a potential mechanism for dioxin toxicity.J. Biol. Chem. 274:510–515 [DOI] [PubMed] [Google Scholar]
- Tian Y., Rabson A.B., Gallo M.A. 2002. Ah receptor and NF-kappaB interactions: mechanisms and physiological implications.Chem. Biol. Interact. 141:97–115 [DOI] [PubMed] [Google Scholar]
- Toshchakov V., Jones B.W., Perera P.Y., Thomas K., Cody M.J., Zhang S., Williams B.R., Major J., Hamilton T.A., Fenton M.J., Vogel S.N. 2002. TLR4, but not TLR2, mediates IFN-beta-induced STAT1alpha/beta-dependent gene expression in macrophages.Nat. Immunol. 3:392–398 [DOI] [PubMed] [Google Scholar]
- Veldhoen M., Hirota K., Westendorf A.M., Buer J., Dumoutier L., Renauld J.C., Stockinger B. 2008. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins.Nature. 453:106–109 [DOI] [PubMed] [Google Scholar]
- Veldhoen M., Hirota K., Christensen J., O’Garra A., Stockinger B. 2009. Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 T cells.J. Exp. Med. 206:43–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel C.F., Sciullo E., Li W., Wong P., Lazennec G., Matsumura F. 2007. RelB, a new partner of aryl hydrocarbon receptor-mediated transcription.Mol. Endocrinol. 21:2941–2955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Yamazaki S., Uematsu S., Sato S., Hemmi H., Hoshino K., Kaisho T., Kuwata H., Takeuchi O., Takeshige K., et al. 2004. Regulation of Toll/IL-1-receptor-mediated gene expression by the inducible nuclear protein IkappaBzeta.Nature. 430:218–222 [DOI] [PubMed] [Google Scholar]
- Yoshimura A., Naka T., Kubo M. 2007. SOCS proteins, cytokine signalling and immune regulation.Nat. Rev. Immunol. 7:454–465 [DOI] [PubMed] [Google Scholar]