Abstract
Immunity declines during aging, however the mechanisms involved in this decline are not known. In this study, we show that cutaneous delayed type hypersensitivity (DTH) responses to recall antigens are significantly decreased in older individuals. However, this is not related to CC chemokine receptor 4, cutaneous lymphocyte-associated antigen, or CD11a expression by CD4+ T cells or their physical capacity for migration. Instead, there is defective activation of dermal blood vessels in older subject that results from decreased TNF-α secretion by macrophages. This prevents memory T cell entry into the skin after antigen challenge. However, isolated cutaneous macrophages from these subjects can be induced to secrete TNF-α after stimulation with Toll-like receptor (TLR) 1/2 or TLR 4 ligands in vitro, indicating that the defect is reversible. The decreased conditioning of tissue microenvironments by macrophage-derived cytokines may therefore lead to defective immunosurveillance by memory T cells. This may be a predisposing factor for the development of malignancy and infection in the skin during aging.
Immunity declines with age (Yoshikawa, 2000). This may explain the age-associated increase in the frequency of cutaneous tumors and infections (Laube, 2004; Diffey and Langtry, 2005). Most studies directed at identifying defects in the immune system during aging have focused on circulating leukocyte populations, but it is not clear if the results obtained can be extrapolated to the behavior of leukocytes within tissues. Clearly, a more informative experimental system is required to define the nature of the age-related attenuation of cutaneous immunity.
The intradermal injection of recall antigens such as tuberculin purified protein derivative (PPD) or Candida albicans (Orteu et al., 1998; Reed et al., 2004; Vukmanovic-Stejic et al., 2008) induces a delayed type hypersensitivity reaction (DTH) in the skin, which has been used as a measure of systemic immunity in vivo (Turk, 1980). There are distinct phases of the DTH response. First, the presence of antigen and trauma to the skin induce nonspecific “danger signals,” that recruit and activate cells of the innate immune system (Matzinger, 2002). This is crucial for conditioning of the inflammatory environment to enable the recruitment of antigen-specific T cells from the blood (Kupper and Fuhlbrigge, 2004). Coincident with this, antigen is sequestered and processed by Langerhans and dendritic cells that migrate via the lymphatics to the lymph node, where they present antigen to and activate memory T cells populations (Jakob et al., 1998; Saeki et al., 1999). These memory T cells then migrate via the blood stream to the site of the antigenic insult, where they traverse activated endothelium in tissue that had been conditioned by “innate signals” and amplify the response (Campbell and Butcher, 2002; Greening et al., 2003; Kupper and Fuhlbrigge, 2004). Nonimmune individuals do not mount a DTH response to antigen, despite the fact that equal levels of antigen and trauma to the skin are present (Orteu et al., 1998; Reed et al., 2004; Vukmanovic-Stejic et al., 2008). This highlights the necessity of memory T cell recruitment for the amplification step of this response to take place (Vukmanovic-Stejic et al., 2008). Antigen-specific and -nonspecific events therefore have to be induced and coordinated to enable a DTH response to occur (Turk, 1980).
We previously characterized the kinetics of memory and regulatory T cell infiltration into the skin of healthy humans during DTH responses (Orteu et al., 1998; Reed et al., 2004; Vukmanovic-Stejic et al., 2008). To do this, we performed histological analysis of infiltrating cells in biopsies taken from the site of the response at different times after antigen injection (Orteu et al., 1998; Reed et al., 2004; Vukmanovic-Stejic et al., 2008). We also induced skin suction blisters at the site of antigen challenge that allowed us to harvest live infiltrating leukocytes for phenotypic and functional analyses (Reed et al., 2004; Vukmanovic-Stejic et al., 2008). Using these techniques, we now show that old humans have decreased DTH responses to bacterial (PPD), fungal (C. albicans), or viral (varicella-zoster virus [VZV]) antigens. This was caused by decreased endothelial activation by macrophage-secreted TNF-α that prevents the recruitment of antigen-specific CD4+ T cells from the blood. Furthermore, this defect is caused by a lack of macrophage activation in the skin and not a defect in the macrophages per se, as these cells could be induced to synthesize TNF-α after stimulation with TLR ligands in vitro. We suggest that decreased TNF-α secretion occurs in part through inhibition by CD4+Foxp3+ regulatory T cells, which can directly inhibit TNF-α secretion by macrophages (Taams et al., 2005; Tiemessen et al., 2007), and these cells were significantly increased in the skin of old subjects. Collectively, our results suggest that strategies that boost the function of macrophages or other components of the cutaneous innate immune system may promote the infiltration of and immunosurveillance by T cells from the blood. This may be a way to reduce the risk of infections and malignancy during aging.
RESULTS
Decreased cutaneous DTH responses in healthy old individuals
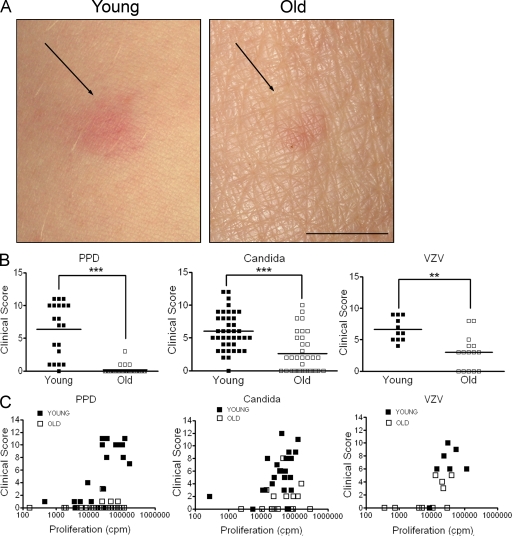
We first examined whether the clinical (erythema and induration) or cellular response to antigen challenge was reduced in the skin of old subjects after injection of recall antigens. At 72 h after injection, young subjects had obvious clinical responses to tuberculin PPD, C. albicans (Candin), and VZV skin test antigen, whereas the clinical score for old subjects was significantly lower (Fig. 1, A and B; P = <0.0001). We next investigated whether the older subjects had a generalized defect in the ability to respond to antigen. To do this, we obtained PBMCs from young and old volunteers at the same time we injected the recall antigens, and then examined the proliferative responses of their T cells to stimulation with these antigens in vitro. In young individuals, good proliferative responses (>10,000 cpm) were associated with a robust clinical response in the skin. However, a large number of old individuals who failed to develop a clinical response in the skin had robust PBMC responses to antigen in vitro (Fig. 1 C). This indicates that they have defective local immune responses to secondary antigen challenge in the skin that does not reflect a global loss of systemic immunity.
Figure 1.
Cutaneous DTH responses are reduced in old individuals. Young and old volunteers were injected intradermally with 0.1 ml of 10 U/ml tuberculin PPD (n = 20 young, 32 old), 0.02 ml Candin skin antigen test (n = 40 young, 34 old), or 0.02 ml VZV skin test antigen (n = 11 young, 15 old). (A) A photograph of the injection site 3 d after PPD injection. Bar, 1 cm. (B) Induration diameter, erythema index, and palpability of the lesion were assessed and graded at day 3, and a clinical score was assigned based on these parameters (***, P < 0.0001; **, P < 0.001; Mann-Whitney test). (C) Freshly isolated PBMCs were stimulated with PPD (n = 18 young, 28 old), C. albicans (n = 23 young, 19 old), or VZV (n = 7 young, 10 old) in vitro for 5–6 d. Proliferation was assessed by [3H]thymidine incorporation and plotted against corresponding clinical score. Each symbol represents the result from one individual.
Reduced CD3+ and CD4+ T cell infiltration during secondary immune responses in old subjects
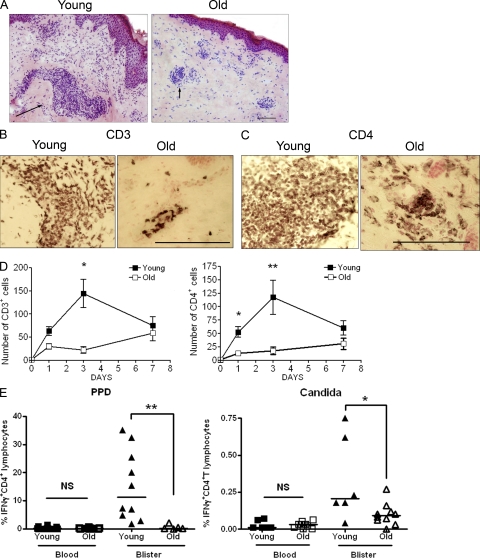
Optimal secondary immune responses in the skin are dependent on antigen-specific CD4+ T cells (Poulter et al., 1982). We next determined whether the defective clinical response in old subjects was associated with decreased CD4+ T cell infiltration after antigen challenge. Immunocytochemical staining of skin sections 3 d after challenge with C. albicans antigens showed that lymphocytic perivascular infiltrates in the papillary and reticular dermis of young skin were larger than those in corresponding sections from old subjects (Fig. 2 A). Furthermore, there were reduced numbers of CD3+ (Fig. 2 B) and CD4+ T lymphocytes (Fig. 2 C) in perivascular infiltrates in old skin sections at all time points studied (Fig. 2 D), indicating that the results obtained were not caused by a shift in the kinetics of the response in old individuals. In addition, old and young subjects have similar proportions of PPD and C. albicans-specific CD4+T lymphocytes in the blood; however, the former had significantly lower proportions of Ag-specific CD4+ T cells in the skin (Fig. 2 E). This indicates that the inability to mount a clinical response to recall antigen challenge in older individuals is associated with decreased cutaneous antigen-specific CD4+ T cell infiltration.
Figure 2.
Cellular infiltrate at the site of cutaneous DTH response is reduced in the old. Young and old volunteers were injected with 0.02 ml Candin skin antigen test and 5-mm punch biopsies were performed on days 1, 3, or 7 after injection. (A) Hematoxylin and eosin staining of skin sections from one young and one old individual on day 3 after Candin injection showing perivascular infiltrates (black arrow). The results are representative stains of three young and three old subjects that were tested. Day 3 biopsies were stained for CD3 (B) and CD4 (C) using an indirect immunoperoxidase immunohistochemical technique. The results shown are representative of five young and four old subjects and the quantification of this data are included in D. (D) The number of CD3+ and CD4+ T cells within perivascular infiltrates was determined by counting the number of positive cells within the five largest perivascular infiltrates per skin section. Mean ± SEM of 4–7 individuals per time point is shown (*, P = 0.01; **, P = 0.007; Mann-Whitney test; n = 20 old, 22 young with 4–7 volunteers per time point). (E) The percentage IFN-γ–producing antigen specific CD4+ T lymphocytes in old blisters is significantly reduced compared with young (n = 10 young, 5 old; **, P = 0.001, for PPD specific cells and n = 6 young, 10 old; *, P = 0.04 for C. albicans). Each symbol represents the result of a single subject. Horizontal bars represent the median. Bars, 100 µm.
Expression of skin homing receptors and capacity of CD4+ T cells to migrate is not reduced during aging
As circulating Ag-specific cells are not decreased in the old subjects, we hypothesized that these cells might be impaired in their capacity to migrate into the skin after antigen injection. We therefore investigated the expression of cutaneous lymphocyte-associated antigen (CLA; Fuhlbrigge et al., 1997), CC chemokine receptor 4 (CCR4), a chemokine receptor found on skin homing T cells (Imai et al., 1997), and CD11a, which is essential for firm adhesion to endothelium via interaction with the β2-integrin intercellular adhesion molecule-1 (ICAM-1; von Andrian and Mackay, 2000), on CD4+ T cells from old and young individuals. We found that the decreased migration of T cells into the skin after antigen challenge in old subjects was not caused by decreased expression of key cell surface molecules on circulating CD4+ T cells (Fig. S1).
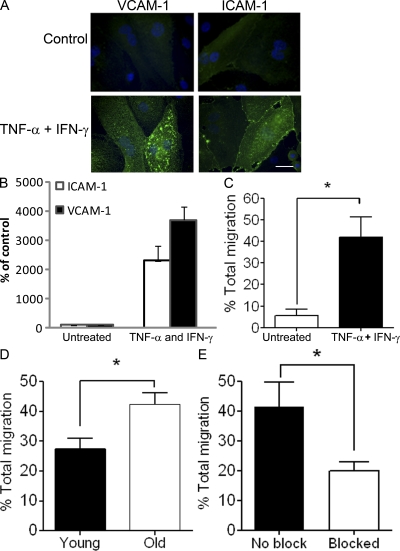
We next used time-lapse phase-contrast microscopy to investigate the physical capacity of T cells from old subjects to migrate across primary human dermal microvascular endothelial cells (HDMECs) in vitro. The HDMECs were preactivated with TNF-α and IFN-γ to stimulate E-selectin, ICAM-1, and vascular cell adhesion molecule-1 (VCAM-1) expression (Swerlick et al., 1992). Adhesion molecule expression and CD4 cell migration was minimal when using resting endothelium (Fig. 3, A–C). However, CD4+ T cells from the blood of old subjects migrated more efficiently than those from the young group across activated endothelium (Fig. 3 D). Furthermore, this migration was blocked by the addition of anti–ICAM-1 and anti–VCAM-1 antibodies (Fig. 3 E), supporting the direct requirement for these molecules in the migration process (McHale et al., 1999). Hence, the defect in accumulation of antigen-specific CD4+ T cells in the skin of old donors after antigen challenge was not caused by alterations in the physical migratory capacity of the T cells themselves.
Figure 3.
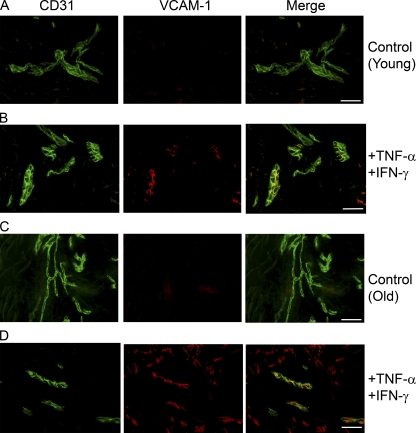
CD4+ T cells from old volunteers show adequate ability to migrate across the dermal endothelial monolayer in vitro. (A) Photomicrographs immunostaining of HDMEC monolayer for VCAM-1 and ICAM-1 (green) on unactivated (top) and activated (bottom) endothelium. Endothelial monolayers were activated with TNF-α (110,000 U/ml) and IFN-γ (100 U/ml) for 24 h before addition of T cells. Bar,10 µm. (B) Graph showing ICAM-1 and VCAM-1 up-regulation upon HDMEC activation. Results were expressed as mean ± SEM of triplicate wells. (C) Bar graph showing increased total CD4+ T cell migration across stimulated HDMEC compared with unstimulated endothelium. To evaluate the level of migration, CD4+ T cells isolated from young and old volunteers were plated onto confluent dermal endothelial monolayers for 4 h at 37°C and 5% CO2 (n = 3 young, 3 old). (D) The percentage total migration for old versus young CD4+ T cells across stimulated endothelium was significantly different (Mann Whitney *, P = 0.02; n = 4 young, 4 old). (E) Graph showing effect of adding blocking antibodies to ICAM-1, VCAM-1 and E-selectin on total CD4+ lymphocyte migration (Paired t test *P = 0.04). Results were expressed as mean ± SEM of three separate experiments.
Reduced expression of adhesion molecules by dermal endothelium during a DTH response in old subjects
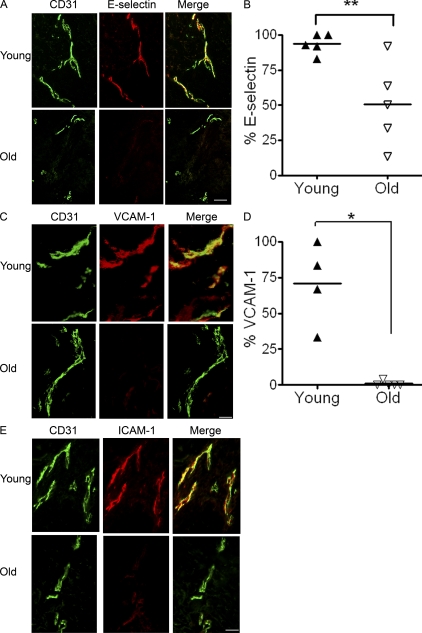
The activation of dermal blood vessel endothelial cells induces expression of E-selectin, ICAM-1, and VCAM-1, the ligands to CLA, CD11a, and VLA-4 on T cells (Berg et al., 1991; Alon et al., 1995; Berlin et al., 1995). We therefore investigated whether the lack of T cell infiltration into the skin in these individuals was caused by defective endothelial cell activation. We found that the number of capillary loops, identified by their expression of CD31 (Newman, 1997), was not decreased in old compared with young subjects (unpublished data). Skin biopsies from day 3 post–C. albicans skin test injections from young and old skin were stained for these adhesion molecules in conjunction with CD31 to highlight the dermal endothelial vessels. There was a significant reduction in the proportion of capillary loops that expressed E-selectin in old skin at 24 h (not depicted) and at day 3 (Mann-Whitney test; P = 0.008; Fig. 4, A and B). Furthermore, dermal endothelial cells from the site of injection in old subjects also showed significantly reduced VCAM-1 expression compared with young controls (P = 0.016; Fig. 4, C and D). The expression of ICAM-1 was found on all dermal capillary loops throughout skin sections from old subjects, but the intensity of staining was reduced in skin sections from all six old donors compared with five young donors as assessed independently by two investigators. The defective accumulation of T cells during the response to recall antigen in the skin in old donors is therefore linked to reduced expression of adhesion molecules on the endothelium of dermal vessels.
Figure 4.
Dermal endothelium in old individuals shows reduced expression of adhesion molecules. Histological sections (6 µm) from skin injected with C. albicans (n = 5 young, 6 old) at day 3 after injection were stained with antibody to CD31 to highlight dermal capillary loops and endothelial adhesion molecules E-selectin VCAM-1 and ICAM-1. (A) Double immunofluorescence staining of representative biopsies for one young and one old individual showing CD31 (green) and E-selectin (red). Capillary loops expressing adhesion molecules appear yellow (merge). This staining was repeated on biopsies of skin 3 d after C. albicans injection in five different young and five different old subjects. The collective results are shown in B. The graph shows percentage of capillary loops expressing E-selectin and the horizontal line indicates the mean (**, P = 0.008, young vs. old, Mann-Whitney test). (C) VCAM-1 (red) and CD31 (green) expression by skin biopsies taken 3 d after C. albicans injection. Representative staining of one young and one old subject is shown. These experiments were performed on four young and five old subjects, and the collective results are shown in D. Graph shows the percentage of capillary loops expressing VCAM-1; each symbol represents an individual, and lines indicate the mean (*, P = 0.015, young vs. old). (E) ICAM-1 (red) and CD31 (green) immunofluorescence staining of skin biopsies from one representative young and one representative old individual 3 d after C. albicans injection. These experiments are representative results of staining that was performed in five young and five old subjects. Bars, 100 µm.
To determine if the reduction in endothelial adhesion molecule expression in the skin of old donors was caused by an intrinsic defect in the endothelium, we obtained skin biopsies from normal young and old volunteers (n = 3 young, 3 old) and incubated the tissue with medium containing TNF-α and IFN-γ or medium alone for 16 h (Fig. 5). We found that there was an increase in the intensity of E-selectin, ICAM-1, and VCAM-1 on the endothelium in the presence of TNF-α and IFN-γ in all the old and young subjects (as assessed independently by two investigators). A representative VCAM-1 staining profile is shown in Fig. 5. Similar results were obtained for E-selectin and ICAM-1 (not depicted). This suggests that old endothelium is able to express adhesion molecules in the presence of appropriate cytokines and is therefore not inherently defective.
Figure 5.
Old endothelium is not defective and can up-regulate adhesion molecules in vitro. Young and old normal skin explants were cultured in RPMI + 10% human serum or in RPMI + 10% human serum supplemented with TNF-α (110,000 U/ml) and IFN-γ (100 U/ml) for 16 h at 37°C and 5% CO2. Histological sections from skin explants were stained with antibodies to CD31 and E-selectin, ICAM-1, and VCAM-1. The results shown represent one young (A and B) and one old (C and D) subject showing VCAM-1 (red) and CD31 (green) staining. These experiments were performed in a total of three different young and three different old subjects that showed similar results. Bars, 100 µm.
Dermal CD163+ macrophages do not secrete TNF-α in old subjects
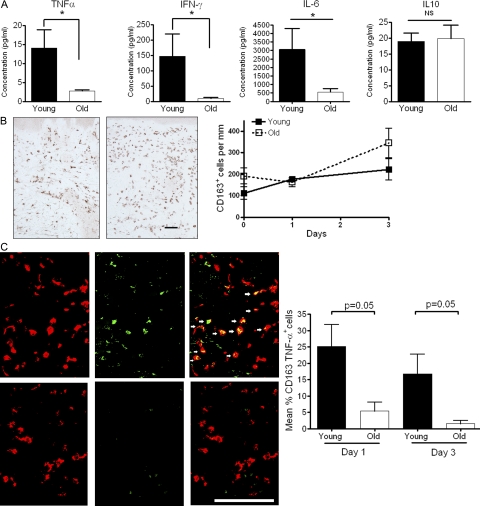
To investigate whether there was a reduction in cytokines that activate endothelium, including TNF-α (McHale et al., 1999), IFN-γ, and IL-6 (Swerlick et al., 1992; Kluger et al., 1997; Wung et al., 2005) in the skin of old individuals, we measured the levels of these mediators in the fluid from skin blisters that were induced after 48 h at the site of C. albicans injection (n = 10 old, 6 young). The concentrations of TNF-α, IFN-γ, and IL-6 were significantly lower in old compared with young subjects (P = 0.014 for TNF-α; P = 0.013 for IFN-γ; and P = 0.031 for IL-6; Fig. 6 A). In contrast, the concentration of IL-10 was not different between both groups (Fig. 6 A). IL-2, IL-7, and IL-15 were also assessed, but were found to be undetectable in blister fluid from young and old subjects (unpublished data). The reduction in endothelial adhesion molecule expression in old subjects was therefore related to decreased induction of proinflammatory cytokines in the skin after antigen challenge.
Figure 6.
Levels of TNF-α are reduced in blister fluid and in CD163+ macrophages in old skin. (A) Skin suction blisters were raised over the site of C. albicans antigen injection at day 3. Levels of TNF-α, IFN-γ, IL-10 and IL-6 were measured in the blister fluid using cytometric bead arrays. The results shown are the mean and SEM of 6 young and 10 old subjects for each cytokine tested. A significant reduction in the level of TNF-α, IFN-γ, and IL-6 was noted in the old group compared with the young group (Mann-Whitney test; *, P = 0.01 for TNF-α and IFN-γ; *, P = 0.03 for IL-6). The number of CD163+ cells within the dermis was determined by indirect immunoperoxidase staining of 6-µm tissue sections. (B, left) Representative staining of one young and one old subject 3 d after C. albicans injection. We investigated a total of five young and six old volunteers, and the collective results are shown (B, right). We also investigated the presence of CD163+ macrophages at different times after C. albicans injection (B, right). Graph shows mean number of CD163+ macrophages before injection (n = 5 young, 5 old), day 1 (n = 5 young, 7 old), and day 3 after injection (n = 5 young, 6 old). The data represent the mean ± SEM. Bar, 100 µm. Double immunofluorescence staining of TNF–α (green) and CD163 (red) in skin biopsies 3 d after C. albicans injection. Results shown are representative of one young and one old subject (C, left). These experiments were performed in three young and three old subjects and the collective results showing the mean and SEM of the percentage of CD163+ macrophages producing TNF-α per perivascular infiltrate are shown in (C, right). Bar, 100 µm.
Previous studies have shown that skin macrophages are mainly responsible for TNF-α and IL-6 secretion after cutaneous challenge with recall antigens, whereas IFN-γ is secreted mainly by T lymphocytes (Chu et al., 1992). Using CD163 as a marker for macrophages and CD11c as a marker for dendritic cells (Zaba et al., 2007), we found similar numbers of both populations in skin of young and old subjects after injection with C. albicans antigens (Fig. 6 B and not depicted). This was confirmed when macrophages were identified by CD68 and CD14 (unpublished data). There was extensive TNF-α expression in CD163+ macrophages from young donors after 24 h and at 3 d after antigen challenge (Fig. 6 C). In contrast, TNF-α was undetectable in these cells from old subjects at both time points (Fig. 6 C). Furthermore, TNF-α was not detectable in CD11c+ cells and keratinocytes (Fig. S2 and not depicted). These results were confirmed by immunohistochemical staining for TNF-α (Fig. S2). We therefore extend previous observations that macrophages are the main source of TNF-α after cutaneous antigen challenge and show that this synthesis is defective in old individuals (Chu et al., 1992).
Dermal macrophages from old individuals can be induced to secrete TNF-α in vitro
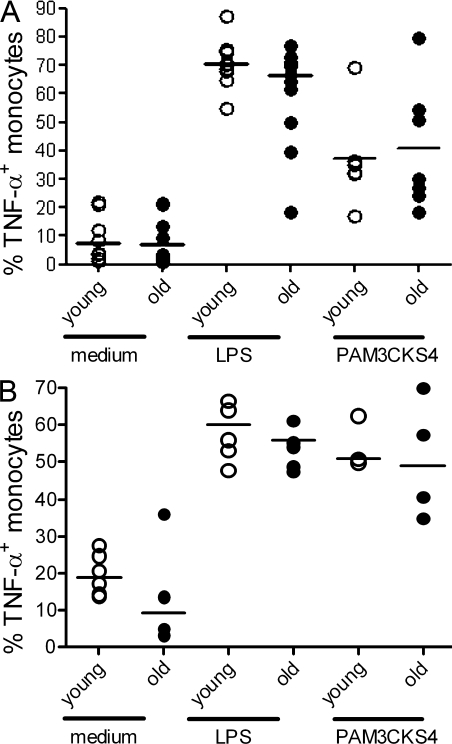
The lack of TNF-α secretion by macrophages in old subjects could have been caused by lack of activating signals for these cells in the aged skin microenvironment after antigen injection, or a generalized defect in macrophage activation in old individuals. To clarify this, we investigated the capacity of macrophages that were isolated from the skin of old and young subjects 48 h after injection with C. albicans, to secrete TNF-α upon challenge with the TLR-1/2 ligand PAM3CKS4 or a TLR-4 ligand LPS in vitro. Peripheral blood monocytes were examined in parallel. Peripheral blood CD14+ monocytes and cutaneous macrophages from both young and old donors secreted significantly more TNF-α in response to LPS and PAM3CKS4 compared with unstimulated controls in vitro (Fig. 7, A and B). However, in contrast to the low TNF-α production of old macrophages in vivo, cutaneous macrophages from old and young subjects secreted similar levels of TNF-α in response to LPS and PAM3CKS4 stimulation in vitro (Fig. 7, A and B). To determine whether the activation threshold of monocytes from old volunteers was higher than that in the young, we stimulated peripheral blood CD14+ monocytes with both high and low doses of LPS. No difference was observed in the TNF-α production between these cells from both groups (Fig. S3). Thus, once removed from the skin environment macrophages can synthesize TNF-α, suggesting that the cutaneous immune defect in old individuals may be reversible.
Figure 7.
Macrophages isolated from the skin of old donors secrete TNF-α in response to TLR signals in vitro. Cells removed from skin suction blisters 48 h after C. albicans injection were stimulated with LPS (100 ng/ml; Sigma-Aldrich), Pam3CSK4 (1 µg/ml), or medium as a control for 4 h in the presence of GolgiStop, and then stained for CD14, CD163, and TNF–α. PBMCs were stimulated in parallel. (A) Graph shows percentage of TNF-α–secreting CD14+ cells after in vitro stimulation of PBMCs with medium alone or LPS (n = 11 young, 11 old) and Pam3CSK4 (n = 6 young, 7 old). Each symbol represents the results from one individual. The percentage of CD14+ cells producing TNF-α after in vitro stimulation of blister cells with LPS (n = 6 young, 7 old) and Pam3CSK4 (n = 3 young, 4 for old). (B) Each symbol represents the results from one individual.
Increased representation of CD4+Foxp3+ regulatory T (T reg) cells in the skin of old humans
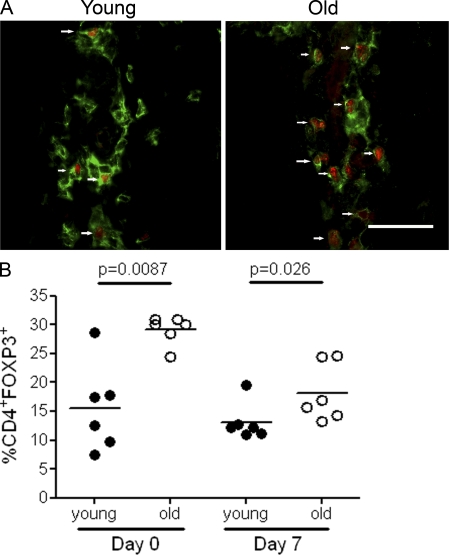
Previous studies have shown that CD4+Foxp3+ T reg cells can inhibit TNF-α secretion by macrophages (Taams et al., 2005; Tiemessen et al., 2007). We therefore investigated whether the decreased macrophage production of TNF-α in old subjects was associated with increased proportions of these cells in the skin. In a previous study, we showed that cutaneous CD4+Foxp3+ T cells expressed typical phenotypic and functional characteristics of regulatory cells (Vukmanovic-Stejic et al., 2008). There was a significant increase in the proportion of these cells in the skin of old subjects compared with the young group either before or after injection of antigen (Fig. 8, A and B). In addition, T reg cells from young and old subjects suppress responses to C. albicans antigens equally (Fig. S4), which is in line with previous studies using other antigens (Vukmanovic-Stejic et al., 2006). Collectively, this data suggests that the overrepresentation of T reg cells in the skin may explain, in part, the decreased TNF-α secretion by cutaneous macrophages in old humans.
Figure 8.
Numbers of CD4+Foxp3+ T reg cells are increased in the skin of old subjects. (A) Double immunofluorescence staining of representative biopsies from young and old normal skin; CD4+ (green) and Foxp3+ (red) cells in a perivascular lymphocytic infiltrate. CD4+Foxp3+ cells are indicated by a white arrow. Bar, 100 µm. (B) Percentage of CD4+ cells expressing Foxp3 per perivascular infiltrate counted. The five largest perivascular infiltrates present in the upper and mid-dermis were selected for analysis for each subject. Each symbol represents an average of five perivascular infiltrates counted for each individual (n = 5–7 subjects per time point, line indicates the mean).
DISCUSSION
The cutaneous DTH response to antigen is widely considered to be a manifestation of memory T cell responsiveness in vivo. The central observation of this study is that this response to challenge by a panel of recall antigens was defective in older humans. In addition, the incidence of positive contact hypersensitivity reactions is also decreased in the elderly (Piaserico et al., 2004; Balato et al., 2008). This suggests that there may be a generalized defect in skin reactivity during aging. This may be the biological basis for the increased susceptibility of old subjects to both cutaneous malignancy and infections (Laube, 2004; Diffey and Langtry, 2005). An unexpected observation was that the defect in the response to cutaneous antigenic challenge was not in the memory T cell compartment, but in the innate conditioning of the skin after antigen stimulation.
Although T cell numbers are decreased after antigen challenge, macrophages identified by CD163 (this study) or CD68 and CD14 (unpublished data) are present in equal numbers in young and old skin. This is likely because of the different trafficking requirements of different leukocytes. It has been shown that DC-SIGN–ICAM-2 interactions mediate dendritic cell trafficking (Geijtenbeek et al., 2000), and DC-SIGN is also strongly expressed on CD163+ macrophages in both normal and inflamed skin (Zaba et al., 2007). We have also found high levels of DC-SIGN expression by activated peripheral blood monocytes (unpublished data). ICAM-2 is constitutively expressed on endothelial cells and, in contrast to E-selectin, VCAM-1, and ICAM-1, its expression is not dependent on the presence of TNF-α (Silverman et al., 2001). Activated monocytes may therefore enter the skin by alternative pathways of transmigration through the endothelium compared with memory T cells. Furthermore, significant numbers of CD163+ macrophages are present in the skin of both young and old subjects, even without injection of antigen; therefore, the recruitment of circulating myeloid cells at the very early stages of the response may not be a limiting step in DTH responses of old humans.
The dominant role of TNF-α in regulating leukocyte migration via the activation of endothelial cells is supported by studies using models of contact hypersensitivity (McHale et al., 1999). Furthermore, it was found that anti–TNF-α therapy in humans decreases the expression of the adhesion molecules E-selectin, ICAM-1, and VCAM-1 in various assays, leading to reduced trafficking of leukocytes (Paleolog et al., 1998). This decreased endothelial activation is associated with an increase in the frequency of skin and soft tissue infections in anti–TNF-α–treated rheumatoid arthritis patients compared with those receiving traditional disease-modifying antirheumatic drugs (Dixon et al., 2006).
The need for appropriate leukocyte migration for immunosurveillance and the prevention of disease is further underscored by the clinical use of anti-α4β1 integrin mAb that binds to VLA-4, the main homing molecule involved in lymphocyte migration to inflamed brain in rodent models of multiple sclerosis (Engelhardt et al., 1995). A mAb to this molecule reduced the inflammatory infiltrate in brain tissue and blocked clinical paralysis in various animal models of multiple sclerosis (Yednock et al., 1992; Theien et al., 2003; Deloire et al., 2004). However, the use of Natalizumab, which is a humanized mAb to α4β1 integrin, for treatment of multiple sclerosis led to the development of severe opportunistic brain infections (Steinman, 2005). Thus, inhibiting leukocyte migration in humans can lead to severe pathological consequences. The defect in macrophage triggering to secrete TNF-α in the skin raises the question of whether there is a defect in endothelial activation during immune responses in other organs during aging, as the potential decrease in T cell immunosurveillance may contribute to the reported increase in susceptibility of old individuals to a wide range of infections (Nicholson et al., 1997; Yoshikawa, 2000; Schmader, 2001).
An important unanswered question is why macrophages in the old skin are not triggered to secrete TNF-α. Previous studies have shown that there is an age associated defect in human TLR1 and 2 function that results in a significant defect in TNF-α secretion after ligation of these receptors (van Duin et al., 2007). Because C. albicans activates TLR1 and 2, this may explain the cutaneous defect in macrophage-derived TNF-α that we have observed. It has also been shown that there is a defect in TLR4 function and expression and function during aging (Renshaw et al., 2002; Krabbe et al., 2004; van den Biggelaar et al., 2004), although this has not been confirmed in other studies (van Duin and Shaw, 2007). However, we found that both peripheral blood monocytes and isolated cutaneous macrophages from donors of both age groups synthesized similar levels of TNF-α after TLR-1/2 and TLR-4 stimulation in vitro. We therefore conclude that cutaneous macrophages in old humans are not inherently defective in terms of TNF-α synthesis, but do not rule out the possibility that other functions in these cells may be altered during aging. This is currently under investigation.
In addition to the need for TLR signaling for macrophage activation, these cells must also interact with cytokines such as IFN-γ to become fully functional. Previous studies have shown that IFN-γ is mainly secreted by T cells during DTH responses (Chu et al., 1992). The lack of IFN-γ in the skin in our studies may reflect the decreased infiltration of T cells into the site of antigen challenge. Activation by IFN-γ leads to the expression of several genes that regulate macrophage biology, including expression of MHC class II genes (Celada and Maki, 1991; Goñalons et al., 1998; Cullell-Young et al., 2001), which are crucial for antigen presentation to T lymphocytes. It is possible that the lack of T cell signals such as IFN-γ early in the DTH response in old subjects may prevent the cascade of events required for amplification of the response.
An alternative possibility is that macrophages in the skin of old individuals may be inhibited functionally in situ. It has been shown that human CD4+Foxp3+ T reg cells are potent inhibitors of macrophage activation and TNF-α secretion by these cells (Taams et al., 2005; Tiemessen et al., 2007). In addition, in a previous study we showed that there is a significant increase in functional circulating T reg cells in old individuals (Vukmanovic-Stejic et al., 2006), which is in agreement with the observation that functional T reg cells accumulate in tissues of aged mice (Lages et al., 2008). We now show that there are significantly higher proportions of CD4+Foxp3+ T cells in the skin of old volunteers either before or 7 d after injection of C. albicans antigens. Although circumstantial, this suggests that the accumulation of T reg cells in the skin may be one possible mechanism for the observed decrease of TNF-α secretion by cutaneous macrophages in old individuals. This is currently under investigation.
With increasing age, cellular debris is not cleared optimally and accumulates within blood vessels and tissues, triggering nonspecific immune responses (for review see Richards et al., 2007). This may manifest as atherosclerosis, the accumulation of amyloid proteins leading to Alzheimer's disease, lipofuscin pigments leading to age-related macular degeneration, and crystal arthritis that results from the defective removal of uric acid (Richards et al., 2007). The reduced activation of macrophages in the skin and possibly other tissues may reduce the occurrence of chronic nonspecific inflammatory responses in old individuals. However, this reduced activation can also lead to decreased tissue-specific immunosurveillance by memory T cells. For successful aging to occur, it is therefore crucial to reduce nonspecific inflammation, yet retain sufficient protective immunity. The regulation of this homeostatic status quo clearly requires further investigation.
MATERIALS AND METHODS
Subjects.
This work was approved by the Ethics Committee of the Royal Free Hospital. Healthy young individuals under the age of 40 yr (n = 81; median age = 30 yr) and old individuals over 70 yr (n = 81, median age = 79.50 yr) were recruited for the study. Exclusion criteria based on a modified version of the SENIEUR protocol were used to recruit old people to reduce confounding factors caused by associated significant comorbidity (Ligthart et al., 1984). All volunteers provided written informed consent, and study procedures were performed in accordance with the principles of the declaration of Helsinki.
Skin tests.
DTH responses were induced by intradermal injection of antigen on non–sun-exposed skin of the medial proximal volar forearm. 0.02 ml candin skin test solution (n = 40 young, 34 old; Allermed Laboratories, Inc), 0.1 ml of 10 U/ml tuberculin PPD (Evans Vaccines Ltd; n = 37 young, 35 old), and VZV skin test antigen (gift from M. Takahashi, Osaka University, Osaka, Japan; n = 11 young, 15 old) were used as skin test antigens. Induration, palpability, and the change in erythema from baseline were measured and scored on day 3 as described previously (Orteu et al., 1998). A clinical score (range 0–15) based on these parameters was then assigned. The injection site was sampled by skin biopsy or skin suction blister at an allotted time point between 0 and 7 d after the skin test injection.
Skin biopsies.
Punch biopsies (5 mm diam) from the site of antigen injection were obtained from 20 young volunteers and 21 old volunteers at various time points (24 h, day 3 and day 7) after C. albicans skin test injection. Control skin-punch biopsies from normal noninjected forearm skin (n = 5 young, 5 old) were also obtained. Biopsies were frozen in optimal cutting temperature compound (Bright Instrument Company Ltd). 6-µm sections were cut and left to dry overnight, and then fixed in ethanol and acetone and stored at −80°C.
Suction blisters.
Skin suction blisters were induced by the application of a negative pressure of 25–40 kPa (200–300 mmHg) below atmospheric pressure via a suction chamber for 2–4 h using a clinical suction pump (VP25; Eschmann) until a unilocular blister measuring 10–15 mm in diameter was formed. Suction blisters were raised over the sites of PPD injection (mantoux reactions), C. albicans skin test injection, or normal skin 18–24 h before sampling to ensure maximum cell recovery. The blister fluid was microcentrifuged at 650 g for 4 min to pellet the cells present. The pellet was resuspended in complete medium (Invitrogen) containing 10% human AB serum, 100 U/ml penicillin, 100 g/ml streptomycin, and 2 mM l-glutamine (all obtained from Sigma-Aldrich).
Immunohistochemistry.
Skin sections from normal and C. albicans–injected skin (n = 25 young, 26 old) were stained with optimal dilutions of purified mouse anti–human CD3 antibody (Dako), purified mouse anti–human CD4 antibody (BD), purified mouse anti–human CD8 antibody (Dako), purified mouse anti–human CD163 antibody (Acris), and anti–TNF-α FITC (BD). Rabbit anti–mouse horseradish peroxidase–conjugated antibody (Dako) was added to detect anti-CD3, -CD4, and -CD8. The staining signal for these antibodies was developed using chromagen 3′-diaminobenzidine tetrahydrochloride. The five largest perivascular infiltrates in the upper and mid-dermis were photographed using a camera (model DXM1200F; Nikon) and Eclipse Net software version 1.16.3 for Nikon. The number of positive cells in these infiltrates was counted using Adobe Photoshop CS2 software. Biotin-labeled horse anti–mouse antibodies (Vector Laboratories) were used to detect CD163 and TNF-α antibodies, respectively. The staining signal was amplified with avidin–biotin complex (Vector Laboratories) and developed using chromogen 3-amino-9-ethylcarbazole (Sigma-Aldrich). The number of positive cells per millimeter was counted manually using computer-assisted image analysis (NIH Image 6.1; http://rsb.info.nih.gov/nih-image).
Immunofluorescence.
6-µm skin sections were blocked with Dako nonserum protein block for 20 min. Primary antibodies E-selectin, ICAM-1, VCAM-1 were incubated for 1 h at room temperature and amplified with the appropriate secondary antibody: goat anti–mouse IgG1 conjugated with Alexa Fluor 546. For colocalization with CD31, sections were then costained with anti–human CD31FITC. For CD4 and Foxp3 staining, primary antibodies (biotin anti–human Foxp3, mouse anti–human CD4) were incubated overnight at 4°C, followed by strepCy3 and anti–mouse IgG1 conjugated with Alexa Fluor 488. Slides were then washed twice in PBS and mounted with Vectashield containing DAPI (Vector laboratory). Images were acquired using appropriate filters of a Leica DMLB microscope with Leica N PLAN 20×/0.40 objective and a Cool SNAP-Pro cf Monochrome Media Cybernetics camera, controlled by Image-Pro PLUS 6.2 software. When counting the numbers of cells in perivascular infiltrates, at least five largest perivascular infiltrates present in the upper and mid-dermis were selected for analysis (Vukmanovic-Stejic et al., 2008). Cell numbers were expressed as the mean absolute cell number counted within the frame. For double staining, skin sections from day 3 C. albicans–stimulated skin were blocked in 10% normal goat serum (Vector Laboratories) for 30 min. Primary antibody CD163 was incubated overnight at 4°C and amplified with the following: goat anti–mouse IgG1 conjugated with Alexa Fluor 568, followed by overnight incubation with directly conjugated TNF-α FITC and amplified with goat anti-FITC Alexa Fluor 488. Images were acquired using appropriate filters of an Axioplan 2I microscope (Carl Zeiss, Inc.) with Plan Apochromat 20 × 0.7 numerical aperture lens and a Hamamatsu orca ER-cooled charge-coupled device camera, controlled by METAVUE software (Universal Imaging).
PBMC preparation and proliferation experiments.
Heparinized blood was collected from young and old volunteers at the time of blister aspiration or as specified. PBMCs were prepared by density centrifugation on Ficoll-Paque (GE Healthcare) and resuspended in complete medium. For migration experiments, CD4 T cells were isolated by positive selection using CD4 microbeads according to the manufacturer's instructions (Miltenyi Biotec). For measurement of cellular proliferation by [3H]thymidine incorporation, PBMCs were added to 96-well round-bottomed tissue culture plates (Falcon; BD) at a concentration of 105 cells/well. The cells were incubated at 37°C in a humidified 5% CO2 atmosphere for 5–6 d (5 d for PPD antigen, 6 d for C. albicans and VZV antigens) before adding [3H]thymidine (GE Healthcare). The mean of triplicate wells was calculated and used for the purpose of data analysis.
T reg cell suppression assays.
CD4+ T cell subsets (CD4+CD25− and CD4+CD25+) were isolated using the CD4 isolation kit and CD25 microbeads according to the manufacturer's instructions (Miltenyi Biotec; Vukmanovic-Stejic et al., 2006). For suppression assays, 5 × 104 CD4+CD25− T cells were stimulated with C. albicans antigens and irradiated (5,000 rad) autologous PBMC as APC (5 × 104 cells per well) in 96-well round-bottom plates (Nunc). CD4+CD25+ T cells were added at responder/suppressor ratios of 1:0, 1:0.1, 1:0.5, and 1:1. Cells were cultured in RPMI 1640 medium (Invitrogen), supplemented with 1% penicillin/streptomycin, 1% l-glutamine, and 10% human AB serum (all from Sigma-Aldrich), for 6 d with [3H]thymidine added at 1 µCi/well for the last 18 h of culture. Proliferative responses are expressed as percentage of proliferation in comparison to the proliferation of CD4+CD25− subset alone.
Flow cytometric analysis.
Four-parameter analysis of blister and blood T cell phenotype was performed on a FACSCalibur (BD). PBMCs were stained with antibodies to CD3 (Dako), CD4, CLA, CCR4, and CD11a (all from BD). Appropriate isotype controls were used. For the detection of antigen-specific cells, PBMCs (106) or blister cells were stimulated with PPD at a final concentration of 10 µg/ml or C. albicans antigen at a final concentration of 40 µg/ml for 15 h at 37°C in a humidified 5% CO2 atmosphere. Brefeldin A (Sigma-Aldrich) was added at a final concentration of 5 µg/ml after 2 h of incubation. Unstimulated controls were also included. The cells were fixed and permeabilized (Fix & Perm Cell Permeabilization kit; Invitrogen) before staining for CD4 and IFN-γ, as previously described (Reed et al., 2004). For the analysis of macrophage function, PBMCs and blister cells were washed and resuspended in RPMI 1460 supplemented with 1% penicillin/streptomycin, 1% glutamine, and 10% heat-inactivated fetal calf serum (Cambrex). Cells were stimulated with the TLR ligands N-palmitoyl-S-[2,3-bis(palmitoyloxy)-(2R,S)-propyl]-Cys-[S]-Serl-[S]-Lys(4) trihydrochloride (Pam3CSK4; 10 µg/ml) and LPS (100 ng/ml; Sigma-Aldrich) or medium as a control for 4 h in the presence of GolgiStop (BD). Cells were stained for cell surface markers for 30 min at 4°C, fixed in 2% paraformaldehyde, and permeabilized with 0.5% saponin, and then labeled with either anti-IgG1 APC or TNF-α APC (eBioscience). Cells were analyzed on FACSCanto II using FACSDiva software (both from BD) and further analyzed using FlowJo software (Tree Star, Inc.).
Cytokine analysis.
The simultaneous measurement of TNF-α, IFN-γ, IL-10, IL-7, IL-2, IL-15, and IL-6 in blister fluids was performed using multiplex bead-based immunoassays (Invitrogen). Samples were diluted 1:1 with PBS, 1% BSA, and 0.05% Tween 20, and incubated with mAb-coated capture beads for 2 h at 20°C. Washed beads were further incubated with biotin-labeled anti–human cytokine antibody for 2 h, followed by streptavidin-phycoerythrin for 30 min. Samples were analyzed using a Luminex 100 (Luminex) and STtarStation 2.0 software (Applied Cytometry Systems). Standard curves of known concentrations of recombinant human cytokines were used to convert fluorescence units to cytokine concentration.
T lymphocyte transendothelial migration.
Normal nontransformed HDMECs (HDMEC-c; PromoCell) derived from young human dermis were cultured at 37°C in a humidified 5% CO2, in endothelial cell basal medium supplemented with 5% FCS (supplement pack C39220; PromoCell). Migration assays were performed as previously described (Adamson et al., 1999). In brief, HDMEC monolayers were grown to confluency in 96-well plates and stimulated with TNF-α (110,000 U/ml) and IFN-γ (100 U/ml) for 24 h before migration assay. By FACS analysis, TNF-α– and IFN-γ–treated cells were >99% and 96% positive for ICAM-1 and VCAM-1, respectively, after 24 h of treatment. CD4+ T cells, isolated from young and old PBMCs by negative selection and kept overnight in X-vivo medium (to prevent down-regulation of CLA; Lonza Group Ltd.) were added (20,000 cells per well) with a minimum of 6 wells per condition per experiment. To evaluate the level of migration, cocultures were placed on the stage of a phase-contrast inverted microscope housed in a temperature controlled (37°C), 5% CO2 gassed chamber (Carl Zeiss, Inc.). For each well, a 200 × 200-mm field was randomly chosen and recorded for 5 min spanning the 4-h time point using a camera linked to a time-lapse video recorder. Recordings were replayed at 160× normal speed, and lymphocytes that had migrated through the monolayer were identified and counted. Lymphocytes on the surface of the monolayer were identified by their highly refractive morphology (phase-bright) and rounded or partially spread appearance. In contrast, cells that had migrated through the monolayer were phase-dark and highly attenuated. Data were expressed as the percentage of total lymphocytes within a field that had migrated through the monolayer.
Statistics.
Statistical analysis was performed using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, California, USA). Nonparametric tests were predominantly used, as the data could not be assumed to be normally distributed. The Kruskall-Wallis test was used to compare three or more unpaired groups, and a two-tailed Mann-Whitney test was used when comparing only two unpaired groups. The Wilcoxon matched pairs test was used when comparing two groups of matched data.
Online supplemental material.
Fig. S1 shows the expression of skin homing receptors on young and old lymphocytes. Peripheral blood mononuclear cells from old and young donors were stained with antibodies to CD3, CD4, CLA, CD11a, and CCR4 and analyzed by flow cytometry. Fig. S2 shows representative immunohistochemical staining of TNF-α–positive cells in biopsies taken on day 3 after C. albicans injection. Fig. S3 shows the production of TNF-α by young and old peripheral blood monocytes stimulated with different doses of LPS. Fig. S4 shows representative suppression assay comparing the capacity of young and old T reg cells to suppress proliferation in response to C. albicans antigens in vitro. Online supplemenatl material is available at http://www.jem.org/cgi/content/full/jem.20090896/DC1.
Acknowledgments
We are grateful to all the volunteers who took part in this study. We would like to thank Fari Tahami (Royal Free Hospital) for help/advice with skin tissue preparation, immunohistochemical staining, and cryosectioning. We would like to thank Prof. David Adams (Birmingham) for help/discussion of migration studies.
This work was funded by grants from the Biotechnology and Biological Sciences Research Council, The Henry Smith Charity, The British Skin Foundation, and Dermatrust.
The authors have no competing financial interests.
Footnotes
Abbreviations used: CCR, CC chemokine receptor; CLA, cutaneous lymphocyte-associated antigen; DTH, delayed type hypersensitivity; HDMEC, human dermal microvascular endothelial cell; ICAM-1, intercellular adhesion molecule-1; Pam3CSK4, N-palmitoyl-S-[2,3-bis(palmitoyloxy)-(2R,S)-propyl]-Cys-[S]-Serl-[S]-Lys(4) trihydrochloride; PPD, purified protein derivative; VCAM-1, vascular cell adhesion molecule-1; VZV, varicella-zoster virus.
References
- Adamson P., Etienne S., Couraud P.O., Calder V., Greenwood J. 1999. Lymphocyte migration through brain endothelial cell monolayers involves signaling through endothelial ICAM-1 via a rho-dependent pathway.J. Immunol. 162:2964–2973 [PubMed] [Google Scholar]
- Alon R., Kassner P.D., Carr M.W., Finger E.B., Hemler M.E., Springer T.A. 1995. The integrin VLA-4 supports tethering and rolling in flow on VCAM-1.J. Cell Biol. 128:1243–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balato A., Balato N., Di Costanzo L., Ayala F. 2008. Contact sensitization of older patients in an academic department in Naples, Italy.Dermatitis. 19:209–212 [PubMed] [Google Scholar]
- Berg E.L., Yoshino T., Rott L.S., Robinson M.K., Warnock R.A., Kishimoto T.K., Picker L.J., Butcher E.C. 1991. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1.J. Exp. Med. 174:1461–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin C., Bargatze R.F., Campbell J.J., von Andrian U.H., Szabo M.C., Hasslen S.R., Nelson R.D., Berg E.L., Erlandsen S.L., Butcher E.C. 1995. alpha 4 integrins mediate lymphocyte attachment and rolling under physiologic flow.Cell. 80:413–422 [DOI] [PubMed] [Google Scholar]
- Campbell D.J., Butcher E.C. 2002. Rapid acquisition of tissue-specific homing phenotypes by CD4+ T cells activated in cutaneous or mucosal lymphoid tissues.J. Exp. Med. 195:135–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada A., Maki R.A. 1991. IFN-gamma induces the expression of the genes for MHC class II I-A beta and tumor necrosis factor through a protein kinase C-independent pathway.J. Immunol. 146:114–120 [PubMed] [Google Scholar]
- Chu C.Q., Field M., Andrew E., Haskard D., Feldmann M., Maini R.N. 1992. Detection of cytokines at the site of tuberculin-induced delayed-type hypersensitivity in man.Clin. Exp. Immunol. 90:522–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullell-Young M., Barrachina M., López-López C., Goñalons E., Lloberas J., Soler C., Celada A. 2001. From transcription to cell surface expression, the induction of MHC class II I-A alpha by interferon-gamma in macrophages is regulated at different levels.Immunogenetics. 53:136–144 [DOI] [PubMed] [Google Scholar]
- Deloire M.S., Touil T., Brochet B., Dousset V., Caillé J.M., Petry K.G. 2004. Macrophage brain infiltration in experimental autoimmune encephalomyelitis is not completely compromised by suppressed T-cell invasion: in vivo magnetic resonance imaging illustration in effective anti-VLA-4 antibody treatment.Mult. Scler. 10:540–548 [DOI] [PubMed] [Google Scholar]
- Diffey B.L., Langtry J.A. 2005. Skin cancer incidence and the aging population.Br. J. Dermatol. 153:679–680 [DOI] [PubMed] [Google Scholar]
- Dixon W.G., Watson K., Lunt M., Hyrich K.L., Silman A.J., Symmons D.P.; British Society for Rheumatology Biologics Register 2006. Rates of serious infection, including site-specific and bacterial intracellular infection, in rheumatoid arthritis patients receiving anti-tumor necrosis factor therapy: results from the British Society for Rheumatology Biologics Register.Arthritis Rheum. 54:2368–2376 [DOI] [PubMed] [Google Scholar]
- Engelhardt B., Conley F.K., Kilshaw P.J., Butcher E.C. 1995. Lymphocytes infiltrating the CNS during inflammation display a distinctive phenotype and bind to VCAM-1 but not to MAdCAM-1.Int. Immunol. 7:481–491 [DOI] [PubMed] [Google Scholar]
- Fuhlbrigge R.C., Kieffer J.D., Armerding D., Kupper T.S. 1997. Cutaneous lymphocyte antigen is a specialized form of PSGL-1 expressed on skin-homing T cells.Nature. 389:978–981 [DOI] [PubMed] [Google Scholar]
- Geijtenbeek T.B., Krooshoop D.J., Bleijs D.A., van Vliet S.J., van Duijnhoven G.C., Grabovsky V., Alon R., Figdor C.G., van Kooyk Y. 2000. DC-SIGN-ICAM-2 interaction mediates dendritic cell trafficking.Nat. Immunol. 1:353–357 [DOI] [PubMed] [Google Scholar]
- Goñalons E., Barrachina M., García-Sanz J.A., Celada A. 1998. Translational control of MHC class II I-A molecules by IFN-gamma.J. Immunol. 161:1837–1843 [PubMed] [Google Scholar]
- Greening J.E., Tree T.I.M., Kotowicz K.T., van Halteren A.G., Roep B.O., Klein N.J., Peakman M. 2003. Processing and presentation of the islet autoantigen GAD by vascular endothelial cells promotes transmigration of autoreactive T-cells.Diabetes. 52:717–725 [DOI] [PubMed] [Google Scholar]
- Imai T., Baba M., Nishimura M., Kakizaki M., Takagi S., Yoshie O. 1997. The T cell-directed CC chemokine TARC is a highly specific biological ligand for CC chemokine receptor 4.J. Biol. Chem. 272:15036–15042 [DOI] [PubMed] [Google Scholar]
- Jakob T., Walker P.S., Krieg A.M., Udey M.C., Vogel J.C. 1998. Activation of cutaneous dendritic cells by CpG-containing oligodeoxynucleotides: a role for dendritic cells in the augmentation of Th1 responses by immunostimulatory DNA.J. Immunol. 161:3042–3049 [PubMed] [Google Scholar]
- Kluger M.S., Johnson D.R., Pober J.S. 1997. Mechanism of sustained E-selectin expression in cultured human dermal microvascular endothelial cells.J. Immunol. 158:887–896 [PubMed] [Google Scholar]
- Krabbe K.S., Pedersen M., Bruunsgaard H. 2004. Inflammatory mediators in the elderly.Exp. Gerontol. 39:687–699 [DOI] [PubMed] [Google Scholar]
- Kupper T.S., Fuhlbrigge R.C. 2004. Immune surveillance in the skin: mechanisms and clinical consequences.Nat. Rev. Immunol. 4:211–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lages C.S., Suffia I., Velilla P.A., Huang B., Warshaw G., Hildeman D.A., Belkaid Y., Chougnet C. 2008. Functional regulatory T cells accumulate in aged hosts and promote chronic infectious disease reactivation.J. Immunol. 181:1835–1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laube S. 2004. Skin infections and aging.Aging Res. Rev. 3:69–89 [DOI] [PubMed] [Google Scholar]
- Ligthart G.J., Corberand J.X., Fournier C., Galanaud P., Hijmans W., Kennes B., Müller-Hermelink H.K., Steinmann G.G. 1984. Admission criteria for immunogerontological studies in man: the SENIEUR protocol.Mech. Aging Dev. 28:47–55 [DOI] [PubMed] [Google Scholar]
- Matzinger P. 2002. An innate sense of danger.Ann. N. Y. Acad. Sci. 961:341–342 [DOI] [PubMed] [Google Scholar]
- McHale J.F., Harari O.A., Marshall D., Haskard D.O. 1999. Vascular endothelial cell expression of ICAM-1 and VCAM-1 at the onset of eliciting contact hypersensitivity in mice: evidence for a dominant role of TNF-alpha.J. Immunol. 162:1648–1655 [PubMed] [Google Scholar]
- Newman P.J. 1997. The biology of PECAM-1.J. Clin. Invest. 99:3–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson K.G., Kent J., Hammersley V., Cancio E. 1997. Acute viral infections of upper respiratory tract in elderly people living in the community: comparative, prospective, population based study of disease burden.BMJ. 315:1060–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orteu C.H., Poulter L.W., Rustin M.H., Sabin C.A., Salmon M., Akbar A.N. 1998. The role of apoptosis in the resolution of T cell-mediated cutaneous inflammation.J. Immunol. 161:1619–1629 [PubMed] [Google Scholar]
- Paleolog E.M., Young S., Stark A.C., McCloskey R.V., Feldmann M., Maini R.N. 1998. Modulation of angiogenic vascular endothelial growth factor by tumor necrosis factor alpha and interleukin-1 in rheumatoid arthritis.Arthritis Rheum. 41:1258–1265 [DOI] [PubMed] [Google Scholar]
- Piaserico S., Larese F., Recchia G.P., Corradin M.T., Scardigli F., Gennaro F., Carriere C., Semenzato A., Brandolisio L., Peserico A., Fortina A.B.; North-East Italy Contact Dermatitis Group 2004. Allergic contact sensitivity in elderly patients.Aging Clin. Exp. Res. 16:221–225 [DOI] [PubMed] [Google Scholar]
- Poulter L.W., Seymour G.J., Duke O., Janossy G., Panayi G. 1982. Immunohistological analysis of delayed-type hypersensitivity in man.Cell. Immunol. 74:358–369 [DOI] [PubMed] [Google Scholar]
- Reed J.R., Vukmanovic-Stejic M., Fletcher J.M., Soares M.V., Cook J.E., Orteu C.H., Jackson S.E., Birch K.E., Foster G.R., Salmon M., et al. 2004. Telomere erosion in memory T cells induced by telomerase inhibition at the site of antigenic challenge in vivo.J. Exp. Med. 199:1433–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renshaw M., Rockwell J., Engleman C., Gewirtz A., Katz J., Sambhara S. 2002. Cutting edge: impaired Toll-like receptor expression and function in aging.J. Immunol. 169:4697–4701 [DOI] [PubMed] [Google Scholar]
- Richards A., Kavanagh D., Atkinson J.P. 2007. Inherited complement regulatory protein deficiency predisposes to human disease in acute injury and chronic inflammatory statesthe examples of vascular damage in atypical hemolytic uremic syndrome and debris accumulation in age-related macular degeneration.Adv. Immunol. 96:141–177 [DOI] [PubMed] [Google Scholar]
- Saeki H., Moore A.M., Brown M.J., Hwang S.T. 1999. Cutting edge: secondary lymphoid-tissue chemokine (SLC) and CC chemokine receptor 7 (CCR7) participate in the emigration pathway of mature dendritic cells from the skin to regional lymph nodes.J. Immunol. 162:2472–2475 [PubMed] [Google Scholar]
- Schmader K. 2001. Herpes zoster in older adults.Clin. Infect. Dis. 32:1481–1486 [DOI] [PubMed] [Google Scholar]
- Silverman M.D., Zamora D.O., Pan Y., Texeira P.V., Planck S.R., Rosenbaum J.T. 2001. Cell adhesion molecule expression in cultured human iris endothelial cells.Invest. Ophthalmol. Vis. Sci. 42:2861–2866 [PubMed] [Google Scholar]
- Steinman L. 2005. Blocking adhesion molecules as therapy for multiple sclerosis: natalizumab.Nat. Rev. Drug Discov. 4:510–518 [DOI] [PubMed] [Google Scholar]
- Swerlick R.A., Lee K.H., Li L.J., Sepp N.T., Caughman S.W., Lawley T.J. 1992. Regulation of vascular cell adhesion molecule 1 on human dermal microvascular endothelial cells.J. Immunol. 149:698–705 [PubMed] [Google Scholar]
- Taams L.S., van Amelsfort J.M., Tiemessen M.M., Jacobs K.M., de Jong E.C., Akbar A.N., Bijlsma J.W., Lafeber F.P. 2005a. Modulation of monocyte/macrophage function by human CD4+CD25+ regulatory T cells.Hum. Immunol. 66:222–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theien B.E., Vanderlugt C.L., Nickerson-Nutter C., Cornebise M., Scott D.M., Perper S.J., Whalley E.T., Miller S.D. 2003. Differential effects of treatment with a small-molecule VLA-4 antagonist before and after onset of relapsing EAE.Blood. 102:4464–4471 [DOI] [PubMed] [Google Scholar]
- Tiemessen M.M., Jagger A.L., Evans H.G., van Herwijnen M.J., John S., Taams L.S. 2007. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages.Proc. Natl. Acad. Sci. USA. 104:19446–19451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk J.L. : Delayed Hypersensitivity. 3rd Edition 1980. Amsterdam: Elsevier [Google Scholar]
- van den Biggelaar A.H., Huizinga T.W., de Craen A.J., Gussekloo J., Heijmans B.T., Frölich M., Westendorp R.G. 2004. Impaired innate immunity predicts frailty in old age. The Leiden 85-plus study.Exp. Gerontol. 39:1407–1414 [DOI] [PubMed] [Google Scholar]
- van Duin D., Shaw A.C. 2007. Toll-like receptors in older adults.J. Am. Geriatr. Soc. 55:1438–1444 [DOI] [PubMed] [Google Scholar]
- van Duin D., Mohanty S., Thomas V., Ginter S., Montgomery R.R., Fikrig E., Allore H.G., Medzhitov R., Shaw A.C. 2007. Age-associated defect in human TLR-1/2 function.J. Immunol. 178:970–975 [DOI] [PubMed] [Google Scholar]
- von Andrian U.H., Mackay C.R. 2000. T-cell function and migration. Two sides of the same coin.N. Engl. J. Med. 343:1020–1034 [DOI] [PubMed] [Google Scholar]
- Vukmanovic-Stejic M., Zhang Y., Cook J.E., Fletcher J.M., McQuaid A., Masters J.E., Rustin M.H., Taams L.S., Beverley P.C., Macallan D.C., Akbar A.N. 2006. Human CD4+ CD25hi Foxp3+ regulatory T cells are derived by rapid turnover of memory populations in vivo.J. Clin. Invest. 116:2423–2433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukmanovic-Stejic M., Agius E., Booth N., Dunne P.J., Lacy K.E., Reed J.R., Sobande T.O., Kissane S., Salmon M., Rustin M.H., Akbar A.N. 2008. The kinetics of CD4+Foxp3+ T cell accumulation during a human cutaneous antigen-specific memory response in vivo.J. Clin. Invest. 118:3639–3650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wung B.S., Ni C.W., Wang D.L. 2005. ICAM-1 induction by TNFalpha and IL-6 is mediated by distinct pathways via Rac in endothelial cells.J. Biomed. Sci. 12:91–101 [DOI] [PubMed] [Google Scholar]
- Yednock T.A., Cannon C., Fritz L.C., Sanchez-Madrid F., Steinman L., Karin N. 1992. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin.Nature. 356:63–66 [DOI] [PubMed] [Google Scholar]
- Yoshikawa T.T. 2000. Epidemiology and unique aspects of aging and infectious diseases.Clin. Infect. Dis. 30:931–933 [DOI] [PubMed] [Google Scholar]
- Zaba L.C., Fuentes-Duculan J., Steinman R.M., Krueger J.G., Lowes M.A. 2007. Normal human dermis contains distinct populations of CD11c+BDCA-1+ dendritic cells and CD163+FXIIIA+ macrophages.J. Clin. Invest. 117:2517–2525 [DOI] [PMC free article] [PubMed] [Google Scholar]