Abstract
Innate immune cells detect pathogens via pattern recognition receptors (PRRs), which signal for initiation of immune responses to infection. Studies with Dectin-1, a PRR for fungi, have defined a novel innate signaling pathway involving Syk kinase and the adaptor CARD9, which is critical for inducing Th17 responses to fungal infection. We show that another C-type lectin, Dectin-2, also signals via Syk and CARD9, and contributes to dendritic cell (DC) activation by fungal particles. Unlike Dectin-1, Dectin-2 couples to Syk indirectly, through association with the FcRγ chain. In a model of Candida albicans infection, blockade of Dectin-2 did not affect innate immune resistance but abrogated Candida-specific T cell production of IL-17 and, in combination with the absence of Dectin-1, decreased Th1 responses to the organism. Thus, Dectin-2 constitutes a major fungal PRR that can couple to the Syk–CARD9 innate signaling pathway to activate DCs and regulate adaptive immune responses to fungal infection.
The sensing of pathogens by innate immune cells is mediated by germline-encoded pattern recognition receptors (PRRs) that recognize pathogen-associated molecular patterns (PAMPs; Janeway, 1989). Although some PRRs may be involved primarily in phagocytic clearance of invading organisms, others engage a plethora of signaling pathways that lead to the expression of genes that encode chemokines, cytokines, and other mediators of innate immune responses to infection. In addition, PRR signaling in DCs renders them competent to prime T cells, thereby initiating adaptive immunity (Reis e Sousa, 2004). Established families of PRRs regulating both innate and adaptive immunity include the Toll-like receptors (TLRs), NOD-like receptors (NLRs), and RIG-I–like receptors (RLRs; Akira et al., 2006; Lee and Kim, 2007; Pichlmair and Reis e Sousa, 2007). However, recent work by us and others has indicated that Dectin-1 and related members of the C-type lectin receptor (CLR) family may also function as PRRs that can signal to regulate immunity (Brown, 2006; Robinson et al., 2006).
Dectin-1 is expressed primarily by neutrophils, macrophages, and DCs, and recognizes β-glucans present in cell walls of fungi and some bacteria (Brown and Gordon, 2001; Dennehy and Brown, 2007). The intracellular tail of Dectin-1 contains a single YxxL motif, termed a hemITAM, that is able to recruit and activate Syk upon engagement by agonist ligands (Rogers et al., 2005; Underhill et al., 2005). Dectin-1 coupling to Syk leads to downstream activation of MAPKs, NFAT, and, via the adaptor CARD9, NF-κB, which coordinate the transcription of innate response genes (Gross et al., 2006; Goodridge et al., 2007; LeibundGut-Landmann et al., 2007). In DCs, the pattern of cytokines triggered by selective Dectin-1 engagement is somewhat different from that induced by agonists of TLR, RLR, or NLR pathways, and is typified by robust induction of proinflammatory cytokines such as TNF, IL-6, and IL-23 accompanied by high levels of IL-2 and IL-10 (LeibundGut-Landmann et al., 2007). Consistent with its stimulatory effects on DCs, a Dectin-1 agonist acts as an adjuvant in vivo to induce adaptive immune responses against a coadministered model antigen, resulting in induction of antigen-specific Th1 and Th17 cells, as well as CTL and antibody responses (LeibundGut-Landmann et al., 2007; LeibundGut-Landmann et al., 2008). In addition, Dectin-1–activated DCs are able to convert regulatory T cells into IL-17 producers (Osorio et al., 2008). In view of its immunomodulatory activities, Dectin-1 is therefore thought to modulate both the innate and adaptive responses to fungal infection. In line with this notion, Dectin-1–deficient mice display increased susceptibility to Candida albicans and Pneumocystis carinii infection in some models (Saijo et al., 2007; Taylor et al., 2007).
Innate immune cells express many receptors that can potentially recognize fungi, including TLR-2, TLR-4, Dectin-2, mannose receptor, DC-SIGN, SIGN-R1, and Mincle (Netea et al., 2008; Willment and Brown, 2008). Although many of these receptors signal independently of Syk, Syk-deficient DCs fail to produce IL-2 and IL-10 upon stimulation with zymosan, a heat-killed and trypsinized preparation of Saccharomyces cerevisiae (Rogers et al., 2005). This would be consistent with a dominant role for Dectin-1 in DC responses to fungi, as we have observed in macrophages (Taylor et al., 2007). However, surprisingly, Dectin-1–deficient DCs display only a mild impairment in their ability to produce the same two cytokines in response to zymosan (LeibundGut-Landmann et al., 2007; Saijo et al., 2007; Taylor et al., 2007). Along the same lines, Card9−/− mice appear to be much more susceptible to infection by C. albicans (Gross et al., 2008) than Dectin-1–deficient mice (Saijo et al., 2007; Taylor et al., 2007). In addition, CARD9-deficient mice fail to mount a Candida-specific Th17 response, yet this response is preserved in mice lacking Dectin-1 (LeibundGut-Landmann et al., 2007). Finally, a recent paper suggests that the mannose receptor contributes to human Th17 responses to C. albicans (van de Veerdonk et al., 2009), although there is as yet no evidence linking that receptor to the Syk–CARD9 pathway. Collectively, these different observations imply that Dectin-1 is not the only Syk–CARD9–coupled fungal PRR in DCs and suggest that multiple receptors capable of activating this signaling pathway could regulate DC-dependent Th17 responses to fungal infection.
Dectin-2 is a type II transmembrane CLR that was originally cloned from a DC line (Ariizumi et al., 2000a) but is most abundantly expressed on tissue macrophages and inflammatory monocytes and has specificity for high mannose structures (Taylor et al., 2005; McGreal et al., 2006). Dectin-2 lacks any obvious signaling motif within its short cytoplasmic domain and, despite its name, is only 20–25% homologous to Dectin-1 (Ariizumi et al., 2000a). Nevertheless, like Dectin-1, Dectin-2 can bind to zymosan and to many fungi, including C. albicans, Cryptococcus neoformans, Histoplasma capsulatum, Microsporum audouinii, and Trichophyton rubrum, displaying a preference for the hyphal form over yeast (McGreal et al., 2006; Sato et al., 2006). Notably, Dectin-2 can associate with the immunoreceptor tyrosine-based activation motif (ITAM)–bearing FcRγ chain (Sato et al., 2006; Barrett et al., 2009), and Dectin-2–transfected macrophages respond to hyphal stimulation with protein tyrosine phosphorylation, NF-κB activation, and secretion of TNF and IL-1ra (Sato et al., 2006). Whether this reflects signaling by Dectin-2 or is a consequence of fungal binding allowing engagement of other signaling receptors has not been explored (Sato et al., 2006) but led us to hypothesize that Dectin-2 might substitute for Dectin-1 in activating Syk and CARD9 in DCs in response to fungal presence.
In this paper, we demonstrate that Dectin-2, together with Dectin-1, explains most of the Syk- and CARD9-dependence of DC responses to fungal stimuli. We show that triggering of endogenous Dectin-2 in DCs activates Syk and the MAPK cascades, and leads to CARD9- and Syk-dependent cytokine production. Furthermore, by blocking Dectin-2 in vivo, we identify a role for this CLR in the induction of adaptive immune responses, particularly Th17, in a model of systemic C. albicans infection. These results not only confirm Dectin-2 as a PRR for fungi but also describe it as another Syk-coupled myeloid CLR involved in regulating DC function and the nature of the adaptive immune response to fungal infection.
RESULTS
Induction of IL-2, IL-10, and TNF by fungal stimuli in DCs is dependent on Syk but not on Dectin-1
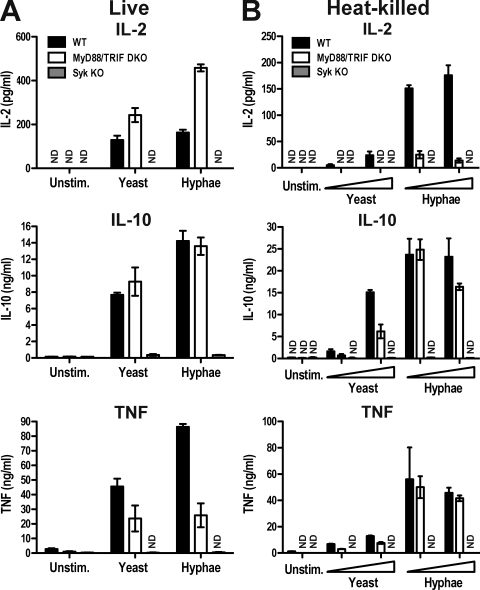
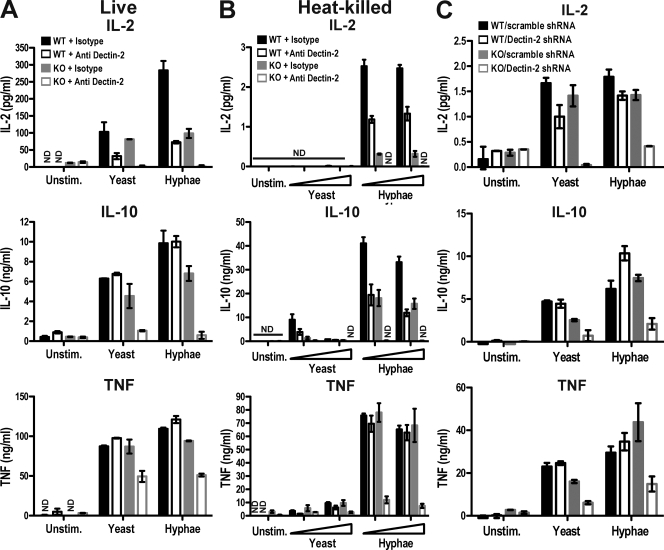
In previous studies using DCs stimulated with zymosan, we found that production of IL-10 and IL-2 but not production of IL-12/23 p40 depends on Syk (Rogers et al., 2005). In more recent experiments, we have found that zymosan-induced production of TNF is also Syk dependent and independent of MyD88 and TRIF, two adaptors essential for TLR signaling (Fig. S1 A). To extend those studies to a potential fungal pathogen, we compared the response of GM-CSF–cultured bone marrow–derived DCs (BMDCs) to different preparations of C. albicans (Fig. S2 A). As for zymosan, both live and heat-killed yeast and hyphal forms of C. albicans elicited production of IL-2, IL-10, and TNF by BMDCs. Production of these cytokines was not greatly affected by the antifungal agents used to prevent overgrowth in cultures with live C. albicans, although slight variations between samples treated with fungizone and with caspofungin were noted (Fig. 1 and Fig. S2 B). However, none of the antifungal agents stimulated cytokine production on their own or altered the response to curdlan, a selective Dectin-1 agonist (Fig. S2 B). Notably, IL-2, IL-10, and TNF were strictly dependent on Syk whether live or heat-killed organisms were used (Fig. 1). In contrast, the production of IL-12/23 p40 and IL-6 was dependent on MyD88/TRIF adaptors and largely independent of Syk (not depicted). Interestingly, the three Syk-dependent cytokines were more strongly induced by the hyphal than the yeast form of C. albicans, especially when the organisms were subjected to heat inactivation (Fig. 1). The latter also unmasked a MyD88/TRIF dependence for IL-2 induction, which was not seen with live organisms (Fig. 1, A and B). The dual dependence of IL-2 on Syk and MyD88/TRIF pathways has been previously noted for zymosan (Rogers et al., 2005), and may reflect denaturation of some PAMPs and exposure of others during the heat inactivation process (Gantner et al., 2005). Consistent with PAMPs being altered by boiling, the MyD88/TRIF adaptors contributed partially to TNF induction by live but not heat-inactivated C. albicans (Fig. 1). IL-10 remained largely MyD88/TRIF independent regardless of the fungal stimulus (Fig. 1), consistent with our data using zymosan (Rogers et al., 2005). We conclude that, independently of possible contributions from TLR pathways, IL-2, IL-10, and TNF production by BMDCs in response to fungal stimuli requires signaling via Syk and can be used to monitor the activity of Syk-coupled PRRs for fungi that are expressed on DCs.
Figure 1.
The contribution of Syk and TLR signaling to cytokine induction by C. albicans. (A) BMDCs from C57BL/6 wild-type (WT, black bars), Myd88−/−/Trif−/− (MyD88/TRIF DKO, white bars), or Syk−/− chimeric (Syk KO, gray bars) mice were stimulated with 105 live C. albicans yeast or hyphae. Fungizone was added 2 h later, and cytokine levels in the supernatants were measured after overnight incubation. (B) BMDCs as in A were stimulated overnight with 105 or 5 × 105 heat-killed C. albicans. Data are means ± SD of duplicate wells and are representative of at least three independent experiments. ND, not detectable.
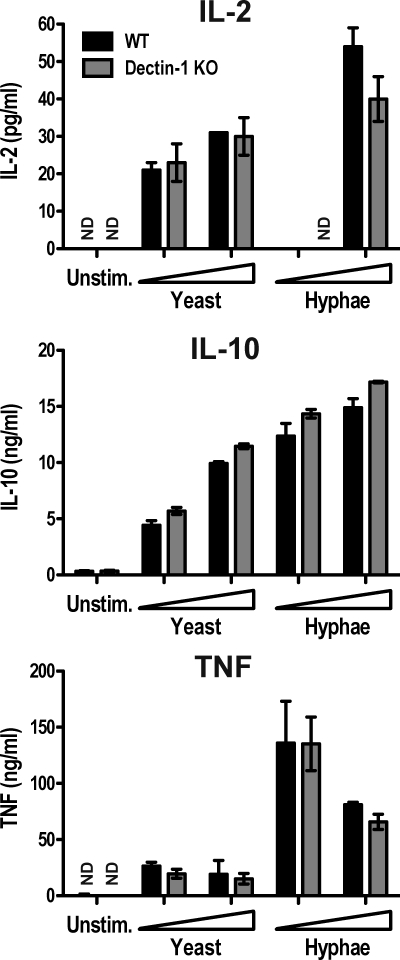
Dectin-1 can trigger the production of IL-2, IL-10, and TNF upon engagement by agonistic β-glucans (Yoshitomi et al., 2005; LeibundGut-Landmann et al., 2007), so we next analyzed whether the Syk-dependent responses were attributable to Dectin-1. Compared with wild-type cells, Dectin-1–deficient BMDCs produced similar levels of IL-2, IL-10, and TNF in response to heat-killed (Fig. 2) or live C. albicans (not depicted). Similarly, in response to zymosan, the induction of the Syk-dependent cytokines was only marginally reduced by Dectin-1 deficiency (Fig. S1 B). This is consistent with previous observations made in DCs (LeibundGut-Landmann et al., 2007; Saijo et al., 2007; Taylor et al., 2007) but, interestingly, contrasts with data indicating that in macrophages induction of TNF by zymosan and Candida is predominantly mediated by Dectin-1 (LeibundGut-Landmann et al., 2007; Saijo et al., 2007; Taylor et al., 2007). The near complete Syk dependence and the relative Dectin-1 independence of TNF, IL-2, and IL-10 responses to fungal stimuli suggests the existence of additional Syk-coupled fungal PRRs in DCs that remain active in triggering this signaling pathway in the absence of Dectin-1.
Figure 2.
The contribution of Dectin-1 to cytokine induction by C. albicans. BMDCs from C57BL/6 wild-type (WT, black bars) or Clec7a−/− chimeric (Dectin-1 KO, gray bars) mice were stimulated overnight with 105 or 5 × 105 heat-killed C. albicans yeast or hyphae. Data are means ± SD of duplicate wells and are representative of at least three independent experiments. ND, not detectable.
Dectin-2 is a Syk-coupled receptor in DCs
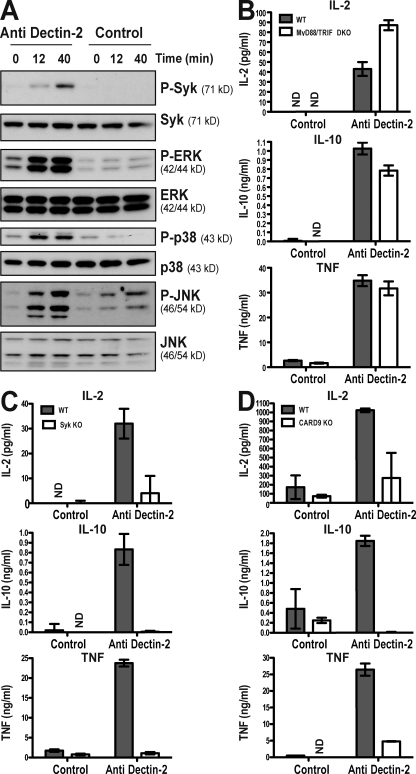
Dectin-2 is capable of binding both C. albicans and zymosan (McGreal et al., 2006) and could therefore potentially act as a PRR for fungi. Despite previous reports of limited expression of Dectin-2 on DCs (Taylor et al., 2005; Carter et al., 2006), using a more sensitive detection method we found surface expression by the CD4+, CD8+, and double-negative subsets of splenic DCs (Fig. S3 A). As reported recently (Barrett et al., 2009), BMDCs also expressed Dectin-2 (Fig. S3 B). This broad expression of Dectin-2 on conventional DCs is similar to that of Dectin-1 (Fig. S5 C; LeibundGut-Landmann et al., 2007; Saijo et al., 2007; Taylor et al., 2007). Next, we cultured BMDCs with anti–Dectin-2 immobilized on plastic to assess whether selective stimulation through Dectin-2 alone was sufficient to promote DC activation. F(ab′)2 preparations of secondary antibody and Fab preparations of anti–Dectin-2 were used to avoid Fc receptor engagement, but in all cases, similar data were obtained with intact antibodies (unpublished data). Contact of DCs with the anti–Dectin-2 surfaces induced phosphorylation of Syk and of ERK, p38, and JNK MAPKs, whereas no increase above basal phosphorylation was observed with control antibodies (Fig. 3 A). In addition, overnight stimulation with plated anti–Dectin-2 induced accumulation of high levels of IL-2, IL-10, and TNF in culture supernatants (Fig. 3, B–D). This was not caused by contamination of the Fab/F(ab′)2 preparations with TLR agonists, because a similar response was observed with BMDCs doubly deficient in MyD88 and TRIF (Fig. 3 B). We therefore explored the notion that Dectin-2 might signal via the Syk–CARD9 pathway. Consistent with that hypothesis, Dectin-2 cross-linking completely failed to induce IL-2, IL-10, and TNF from Syk- or CARD9-deficient BMDCs (Fig. 3, C and D) despite the fact that those cells expressed normal levels of Dectin-2 and responded normally to stimulation with TLR agonists (not depicted). We conclude that triggering of endogenous Dectin-2 in DCs is sufficient to activate Syk and downstream signaling pathways in DCs, leading to TLR-independent production of cytokines.
Figure 3.
Syk- and CARD9-dependent activation of DCs by Dectin-2. (A) Activation of Syk and MAPK cascades by plated anti–Dectin-2. C57BL/6 BMDCs were centrifuged onto wells coated with anti–Dectin-2 Fab or control Fab. After the indicated times, the cells were lysed and immunoblotted for active (phosphorylated) and total Syk, p38, ERK, or JNK. (B) Wild-type C57BL/6 (WT, gray bars) or Myd88−/−/Trif−/− (MyD88/TRIF DKO, white bars) BMDCs were stimulated overnight with anti–Dectin-2 or control Fab as in A, and cytokines levels in the supernatants were measured. (C and D) BMDCs from Syk−/− chimeric (Syk KO, white bars; C) and Card9−/− (Card9 KO, white bars; D) mice were stimulated as in B, and cytokine levels were compared with those made by WT cells (gray bars). Representatives of at least three independent experiments are shown. Cytokine data are means ± SD of duplicate wells. ND, not detectable.
Dectin-2 signals through association with FcRγ chain
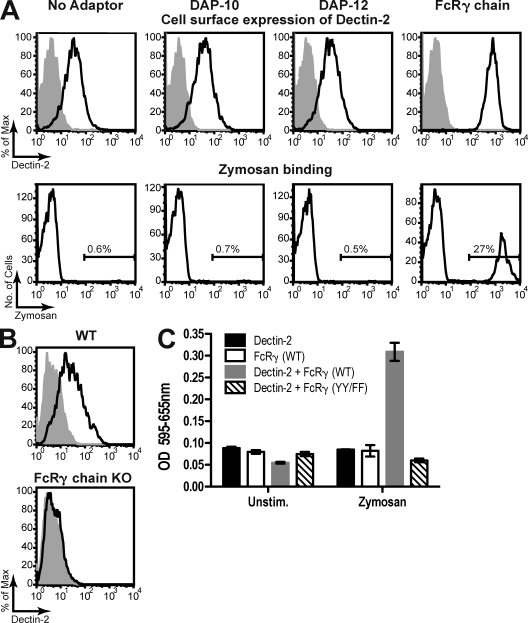
Unlike Dectin-1, Dectin-2 does not have an intracellular hemITAM motif that would allow it to couple directly to Syk. However, exogenously expressed Dectin-2 in myeloid cells coimunoprecipitates with the ITAM-bearing γ chain of the Fc receptor (Sato et al., 2006), and its surface expression is increased by FcRγ cotransfection (Barrett et al., 2009). To analyze the interaction between Dectin-2 and possible signaling adaptors, including FcRγ chain, DAP-10, or DAP-12, we used B3Z T hybridoma cells, which express a β-galactosidase NFAT reporter of ITAM signaling (Karttunen et al., 1992). Dectin-2–transduced B3Z cells expressed modest amounts of receptor at the cell surface that were not significantly increased by previous transduction with DAP-10 or DAP-12 (Fig. 4 A). However, in B3Z cells coexpressing FcRγ chain, cell-surface staining of Dectin-2 was greatly enhanced (Fig. 4 A). This increase in surface expression conferred the ability to bind fluorescent zymosan, which was not detectable in the other B3Z cell lines (Fig. 4 A). To analyze whether endogenous Dectin-2 expression similarly required FcRγ chain, BMDCs from wild-type and FcRγ chain–deficient mice were analyzed. Surface expression of Dectin-2 was not detectable on FcRγ chain–deficient BMDCs (Fig. 4 B), which also failed to respond to plated anti–Dectin-2 Fab stimulation (Fig. S4). Collectively, these results demonstrate that association with FcRγ chain is necessary for functional surface expression of Dectin-2.
Figure 4.
Dectin-2 requires FcRγ chain for surface expression and signaling. (A) B3Z cells (No Adaptor) or sublines derived by transduction with DAP-10, DAP-12, or FcRγ chain were subsequently transduced with Dectin-2–IRES–EGFP. (top) Anti–Dectin-2 staining of GFP-positive parental, DAP-10, DAP-12, or FcRγ chain (open histogram) cell lines compared with isotype control (shaded histogram). (bottom) Cy5-zymosan binding to the same cell lines. (B) BMDCs from C57BL/6 (WT) or Fcer1g−/− (FcRγ chain KO) mice stained with anti–Dectin-2 (open histogram) or an isotype-matched control mAb (shaded histogram). (C) B3Z cells expressing Dectin-2, wild-type FcRγ chain (FcRγ (WT)), Dectin-2 and FcRγ chain (Dectin-2 + FcRγ (WT)), or Dectin-2 and a signaling-deficient mutant of FcRγ chain (Dectin-2 + FcRγ (YY/FF)) were stimulated with or without 100 µg/ml zymosan. NFAT activity was measured using a β-galactosidase reporter. Data are means ± SD of duplicate wells (C), and are representative of two (C) or at least three (A and B) independent experiments.
To analyze the ability of FcRγ chain to mediate Dectin-2 signaling, we stimulated B3Z cells with zymosan and measured activation of the NFAT reporter. Reporter activity was seen in B3Z cells cotransduced with Dectin-2 and FcRγ chain but not in B3Z cells expressing either protein alone (Fig. 4 C). To dissect the role of the ITAM motif of FcRγ chain on Dectin-2 signaling, we mutated the two key tyrosines to phenylalanines. The double tyrosine mutation did not impair the ability of FcRγ chain to promote Dectin-2 cell-surface expression or zymosan binding (Fig. S5). However, B3Z cells expressing the mutant FcRγ chain and Dectin-2 were unable to signal in response to zymosan, as determined by induction of the NFAT reporter (Fig. 4 C). We conclude that the ITAM motif of the FcRγ chain mediates signaling by Dectin-2.
Induction of Syk-dependent cytokines by fungal stimuli requires Dectin-1 and -2
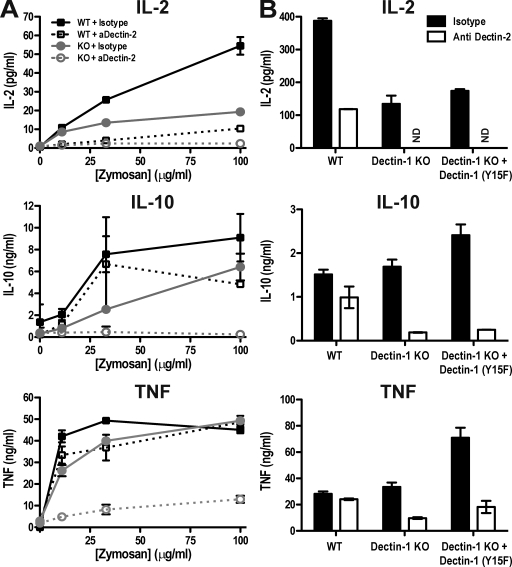
These results suggested that, through its association with FcRγ chain, Dectin-2 might potentially function as a Syk-coupled PRR and contribute, in conjunction with Dectin-1, to Syk–CARD9–dependent DC activation by fungal stimuli. To test this hypothesis, we compared fungal stimulation of Dectin-1–sufficient and –deficient BMDCs with or without Dectin-2 function. As has been observed for many antibodies against other ITAM-coupled receptors, anti–Dectin-2 did not trigger activation unless immobilized on plastic or beads (unpublished data). Instead, in solution, the mAb caused down-regulation of Dectin-2 and could consequently be used as a blocking reagent (unpublished data). Therefore, we treated BMDCs with anti–Dectin-2 or an isotype-matched irrelevant antibody control for 2 h before stimulation with zymosan. As with loss of Dectin-1, down-regulation of Dectin-2 in wild-type DCs only modestly reduced the levels of IL-2, IL-10, or TNF produced in response to zymosan (Fig. 5, A and B). However, blockade of Dectin-2 in Dectin-1–deficient BMDCs led to a profound reduction in the same cytokines (Fig. 5, A and B), approaching the decrease observed in Syk-deficient cells (Fig. S1 A; Rogers et al., 2005). Similar results were obtained using live C. albicans: blockade of Dectin-2 in wild-type BMDCs or genetic loss of Dectin-1 led to modest and variable reductions in the levels of IL-2 and IL-10, but blockade of Dectin-2 in Dectin-1–deficient BMDCs caused an almost complete loss in production of all three Syk-dependent cytokines (Fig. 6 A). The exception was TNF, which was only partially dependent on the two CLRs (Fig. 6 A), possibly reflecting the TLR contribution noted in Fig. 1 A. Induction of IL-2, IL-10, and TNF by heat-killed C. albicans was mediated by Dectin-1 and -2, as seen with zymosan. We also analyzed the induction of IL-12 and IL-23 and found that IL-12 p35 mRNA was at the limit of detection (unpublished data), whereas IL-23p19 mRNA was elicited by Candida stimulation in a manner dually dependent on Dectin-1 and -2 (Fig. S6). To verify the contribution of Dectin-2 by an independent means, we used short hairpin RNA (shRNA) to knock down Dectin-2 expression (Barrett et al., 2009). Infection of BMDCs with a lentivirus encoding Dectin-2 shRNA but not with a lentivirus encoding a scrambled control insert reduced the surface expression of Dectin-2 by >50% as determined by flow cytometry (not depicted). Notably, knockdown of Dectin-2 in Dectin-1–deficient cells almost completely prevented the induction of IL-2, IL-10, and TNF by heat-killed C. albicans (Fig. 6 C). These results suggest that signaling by Dectin-1 and -2 together could account for the Syk–CARD9 dependence of DC activation by fungi.
Figure 5.
Dectin-1 and -2 mediate Syk-dependent responses to zymosan. (A) BMDCs from wild-type C57BL/6 (WT) or Clec7a−/− chimeric (Dectin-1 KO) mice were treated with 10 µg/ml anti–Dectin-2 or isotype-matched control mAb for 2 h before stimulation with increasing doses of zymosan overnight. (B) BMDCs from Clec7a−/− chimeric mice were transduced with signaling-deficient Dectin-1 (Dectin-1 KO + Dectin-1 (Y15F)) or mock transduced (Dectin-1 KO). These cells, as well as mock-transduced C57BL/6 BMDCs (WT) were then stimulated overnight with 33 µg/ml zymosan. Cytokines in the supernatants were quantified by ELISA. Data are means ± SD of duplicate wells and are representative of at least three independent experiments. ND, not detectable.
Figure 6.
Dectin-1 and -2 mediate Syk-dependent responses to C. albicans. (A) BMDCs from wild-type C57BL/6 (WT) or Clec7a−/− chimeric (Dectin-1 KO) mice were treated with 10 µg/ml anti–Dectin-2 or isotype control for 2 h before stimulation with 105 live C. albicans yeast or hyphae. Fungizone was added 2 h after stimulation, and the cytokines levels in the supernatants were measured after overnight incubation. (B) BMDCs pretreated with mAbs as in A were stimulated overnight with 105 or 5 × 105 heat-killed C. albicans. (C) BMDCs from wild-type 129/Sv (WT) or Clec7a−/− (Dectin-1 KO) mice were infected with lentivirus encoding Dectin-2 shRNA or a scrambled control. After puromycin selection, cells were stimulated overnight with 105 heat-killed C. albicans. Data are means ± SD of duplicate wells and are representative of at least three (A and B) or two (C) independent experiments. ND, not detectable.
In addition to the loss of Syk-dependent cytokines, we noted that binding of fluorescent zymosan was markedly decreased when Dectin-2 function was blocked in Dectin-1–deficient BMDCs (Fig. S7). These results indicate that the primary binding receptors for zymosan on DCs are Dectin-1 and -2. At the same time, they raise a caveat with the interpretation that Syk-dependent DC activation is caused by signals propagated by Dectin-1 and -2 because the loss of cytokines seen with Dectin-2 blockade in Dectin-1–deficient cells could, at least in principle, be caused by lack of binding of fungal particles, which would then prevent triggering of any other Syk-coupled receptors. To address this possibility, we transduced Dectin-1–deficient BMDCs with a Y15F mutant form of Dectin-1 that allows restoration of zymosan binding without Syk signaling (Rogers et al., 2005). The transduced cells bound zymosan even more avidly than wild-type controls, and in this setting, the binding was only marginally decreased by Dectin-2 blockade (Fig. S7). Yet, production of IL-2, IL-10, and TNF was profoundly compromised by the same Dectin-2 blockade (Fig. 5 B). Indeed, anti–Dectin-2 blocked cytokine production by Y15F-transduced cells to the same extent as in Dectin-1–deficient mock-transduced cells (Fig. 5 B). Thus, anti–Dectin-2 nearly completely blocks Syk-dependent cytokine responses in a condition in which Dectin-1 is unable to signal but promotes a high degree of binding of fungal particles. This, in turn, argues against a major contribution from other signaling receptors and indicates that the requirement for Dectin-1 and -2 in Syk-dependent DC activation by fungi is caused by signals propagated by these receptors and not just by their ability to mediate binding of fungal particles.
Dectin-2 and -1 direct the Th cell response to fungal infection
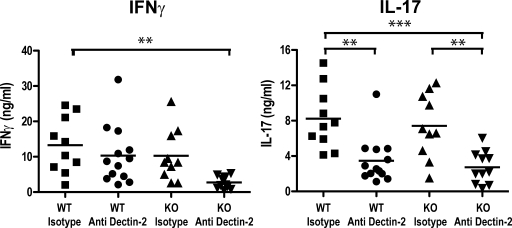
In light of our in vitro results with DCs, we assessed the contribution of Dectin-2 to adaptive immunity to Candida infection in vivo. As seen in vitro, anti–Dectin-2 antibody was able to promote clearance of Dectin-2 from the cell surface of DCs in vivo (unpublished data). Wild-type and Dectin-1–deficient mice were therefore injected with the mAb 6 h before and 2 and 4 d after systemic infection with a sublethal dose of C. albicans and were compared with mice injected with an isotype-matched irrelevant control mAb. Th1 and Th17 responses were monitored by measuring IFN-γ and IL-17, respectively, after restimulation of splenocytes with heat-killed C. albicans for 2 d (LeibundGut-Landmann et al., 2007). Remarkably, the Th17 response to C. albicans was markedly reduced in mice treated with anti–Dectin-2, independent of Dectin-1 deficiency (Fig. 7). This was seen even at a higher dose of C. albicans used for restimulation (Fig. S8 A), which indicates that, in this model, Dectin-2 is required for driving the Th17 response to the organism. In contrast, little effect of Dectin-2 blockade was apparent on the Th1 response to the same pathogen, although when anti–Dectin-2 was used in combination with Dectin-1 deficiency, a statistically significant decrease in IFN-γ production was observed in cells restimulated with the lower but not the higher dose of heat-killed C. albicans (Fig. 7 and Fig. S8 A). Splenocytes from Dectin-1–deficient mice given isotype-matched control mAb produced similar levels of IFN-γ and IL-17 upon restimulation as similarly treated wild-type animals (Fig. 7 and Fig. S8 A), consistent with previous data (LeibundGut-Landmann et al., 2007). Therefore, Dectin-2 is required for Th17 responses to Candida and, with Dectin-1, contributes to Th1 cell differentiation in response to the same organism.
Figure 7.
Dectin-1 and -2 are required during systemic C. albicans infection for subsequent splenocyte recall responses. Wild-type 129/Sv (WT) or Clec7a−/− (Dectin-1 KO) mice were given anti–Dectin-2 or isotype-matched control mAb intraperitoneally before and after i.v. C. albicans infection. After 7 d, splenocytes were restimulated with heat-killed C. albicans for 2 d, and IFN-γ and IL-17 accumulation in the supernatants was measured by ELISA. The data are pooled from two independent experiments, and each symbol represents the mean of triplicate stimulations from an individual mouse. Statistically different groups are indicated (**, 0.001 < P < 0.01; and ***, P < 0.001).
Dectin-2 blockade does not impair innate resistance to C. albicans infection
Despite the marked impact of Dectin-2 blockade on Th17 responses to C. albicans, mice receiving anti–Dectin-2 showed similar kidney fungal burdens at day 7 after infection as mice receiving isotype-matched control antibody (Fig. S8 B). This was true even in mice deficient for Dectin-1 (Fig. S8 B). In addition, weight loss during the course of infection was also not affected by Dectin-2 blockade independently of Dectin-1 sufficiency or deficiency (Fig. S8 C). Therefore, in this model of systemic disease, Dectin-2 blockade does not impair resistance, and Dectin-2 may therefore be redundant for innate immunity to C. albicans infection.
DISCUSSION
In the broadest sense of the term, PRRs include all receptors that detect PAMPs (Janeway, 1989). However, a subset of PRRs possesses the ability to trigger intracellular signaling pathways that lead to expression of innate response genes, including those encoding chemokines and cytokines. These types of PRRs play a key role in orchestrating the innate and adaptive immune responses to infection, and have previously been described as belonging to the TLR, RLR, and NLR families. The C-type lectin Dectin-1 constituted a notable exception, as it is not a member of any of these families yet it is able to induce immunity via a Syk and CARD9-dependent pathway. In this study, we identify Dectin-2 as an additional Syk–CARD9–coupled C-type lectin that mediates DC activation by fungi. Notably, we show that Dectin-2 accounts for the seemingly paradoxical Syk- and CARD9-dependent yet Dectin-1–independent aspects of DC activation by fungal stimuli. This is in contrast to most macrophages that respond to C. albicans primarily through Dectin-1 (Taylor et al., 2007) and to some types of DCs that may rely primarily on TLR signaling via TRIF to respond to the same organism (De Luca et al., 2007). The importance of Dectin-2 in DC function is underscored by the fact that in vivo blockade of Dectin-2 severely reduces Th17 responses to C. albicans infection and, in the context of Dectin-1 deficiency, affects Th1 immunity to the same organism. Thus, Dectin-2 constitutes a major receptor regulating T cell responses to fungi by engaging the Syk–CARD9 pathway in DCs.
The fungal PAMP recognized by Dectin-2 has not been fully identified. The C-type lectin domain of Dectin-2, unlike that of Dectin-1, possesses a classical carbohydrate recognition site that coordinates Ca2+ and specifically recognizes high mannose structures (McGreal et al., 2006). Binding of this domain to zymosan is blocked by mannose, fucose, or mannan, suggesting that the yeast ligand is probably a high-mannose–like structure such as mannan (McGreal et al., 2006). Both O- and N-linked yeast mannans possess immunostimulatory properties, and the activity of the former has been ascribed to its ability to engage TLR4 (Netea et al., 2006). In contrast, many innate receptors, including SIGNR-1, DC-SIGN, mannose receptor, and TLR2, have been proposed to bind structures within N-mannan (Jouault et al., 2003; Taylor et al., 2004; Cambi et al., 2008). Dectin-2 may fall in the same group, although competition studies suggest that the molecular pattern recognized by Dectin-2 is distinct from that bound by SIGNR-1 and mannose receptor (McGreal et al., 2006). Thus, different receptors may sense different forms of mannan, which could be differentially expressed during the fungal life cycle. Indeed, Sato et al. (2006) have shown that Dectin-2 preferentially binds the hyphal form of C. albicans and suggested that the ligand may not be expressed or could be concealed in the yeast form. Our results, however, show that, although hyphae induce the Dectin-1/2–dependent cytokines more strongly than yeasts, Dectin-2 is also involved in the sensing of the latter. Consistent with the notion that Dectin-2 can act as a yeast receptor, we have previously reported that the recombinant C-type lectin domain of Dectin-2 binds to zymosan and to the yeast form of C. albicans (McGreal et al., 2006). Finally, the PAMPs recognized by Dectin-2 may not be restricted to fungi, as this receptor appears to play a role in the response to house dust mite allergens (Barrett et al., 2009) and the extracellular domain of Dectin-2 is capable of binding bacterial polysaccharides (McGreal et al., 2006).
Stimulation through Dectin-2 defines a new mechanism for DC activation by a PRR. We show that Dectin-2 requires association with the FcRγ chain to be expressed on the surface of cells but, more critically, we provide evidence that the ITAM of this adaptor is required for signaling. Cross-linking of Dectin-2 triggered the activation of Syk and the MAPK cascades, leading to cytokine production. The latter process was dependent on CARD9, the adaptor necessary for induction of NF-κB downstream of Dectin-1 and other ITAM-signaling myeloid receptors (Gross et al., 2006; Hara et al., 2008). However, in contrast to Dectin-1 that binds Syk directly, Dectin-2 couples to Syk in trans, through association with FcRγ chain. In this regard, Dectin-2 behaves like lymphocyte antigen receptors and activatory Fc receptors, all of which engage Syk or ZAP-70 indirectly, through association with ITAM-bearing signaling chains (Isakov, 1997). Likewise, other myeloid receptors, including Mincle, MAIR-II, TREM-1, TREM-2, OSCAR, and DCAR, engage ITAM pathways through association with FcRγ chain or DAP12 (Bouchon et al., 2001; Kanazawa et al., 2003; Hara et al., 2007; Yamasaki et al., 2008). All of these receptors can be easily triggered by means of antibody cross-linking, as shown in this study for Dectin-2. In contrast, antibody cross-linking does not induce measurable Dectin-1–mediated cytokine responses. Thus, although our study highlights the equivalence of Dectin-1 and -2 in activating the Syk–CARD9 pathway, hemITAM and ITAM receptors may have distinct signaling requirements, which could lead to subtle differences in downstream responses not apparent in our study.
As shown in this paper, IL-2, IL-10, and TNF, the hallmark cytokines triggered by either Dectin-1 or -2 engagement in DCs, are the same cytokines that are critically dependent on Syk and CARD9 for induction in response to fungal stimuli. Why TLR signaling in response to agonists present in fungal particles (Hohl et al., 2006; Netea et al., 2008) is unable to mimic Syk-dependent signals in eliciting IL-2, IL-10, and TNF but can induce IL-6 and IL-12 p40 is at present unclear. This phenomenon may be DC subtype dependent, as TLR signaling via TRIF can also lead to IL-10 production by mesenteric lymph node DCs in response to Candida (De Luca et al., 2007). However, it is consistent with our previous study showing a marked dependence of zymosan-induced IL-2 and IL-10 on ERK, which was predominantly activated via Syk but not MyD88 (Slack et al., 2007). Also, C. albicans and zymosan activate NFAT via Dectin-1 and not via TLRs in macrophages, and this NFAT activation is required for IL-2 and IL-10 production (Goodridge et al., 2007). Therefore, a robust engagement of the ERK and NFAT pathways by Dectin-1 and -2 may underlie the induction of the Syk-signature cytokines.
It is important to note that IL-2, IL-10, and TNF may be only part of the Syk–CARD9 “signature” of DC activation by fungi, which will need to be investigated fully using genome-wide screens. Interestingly, a recent report indicates that, in DCs, Dectin-2/Syk signaling can additionally couple to the generation of cysteinyl leukotrienes (Barrett et al., 2009). The contribution of Dectin-2 to DC activation by fungi is not fully elucidated, but in vivo, it maps out at the level of the Th17 response to the organism. This follows from previous work in which we found that systemic infection with C. albicans induces potent Th1 and Th17 responses and that the latter but not the former are completely abrogated in CARD9-deficient mice (LeibundGut-Landmann et al., 2007). In this paper, we show that Dectin-2 is the major regulator of the Th17 response to systemic Candida infection and confirm earlier evidence that Dectin-1–deficient mice show little or no impairment in this response in the absence of concomitant Dectin-2 blockade (LeibundGut-Landmann et al., 2007). However, combined Dectin-2 blockade and Dectin-1 deficiency impaired the Th1 response to the organism, indicating that Dectin-1 is not fully dispensable for adaptive immunity to Candida. This is an identical phenotype to that seen previously in the CARD9-deficient mice (LeibundGut-Landmann et al., 2007), implying that Dectin-1 and -2 account for most of the CARD9-dependent adaptive immune response to Candida infection.
In contrast to Th1 and Th17 induction, Dectin-1 and -2 do not fully account for the CARD9-dependent innate immune response to C. albicans. Indeed, we observed no significant increase in renal fungal burden, even when Dectin-2 was blocked in Dectin-1–deficient mice, which indicates that Dectin-2 function may be redundant for antifungal resistance during the first week of systemic infection. This is consistent with previous data showing that fungal burden in the kidneys of Dectin-1–deficient mice infected i.v. with similar doses was significantly increased only in those mice that succumbed to the infection and not at these early time points (Taylor et al., 2007). In contrast, Card9−/− mice are highly susceptible to a similar dose of the fungus and have large increases in kidney mass and fungal burden as early as 4 d after infection (Gross et al., 2006). As it is believed that resistance to disseminated candidiasis in acute infection primarily reflects the activity of innate immunity (Romani, 2008), it seems likely that additional Syk–CARD9–coupled receptors are expressed by other immune cells, such as granulocytes, which are involved in the killing of Candida organisms during the acute phase of the antifungal response. A distinct question is whether the adaptive Th17 response to Candida infection is involved in protection from infection. In favor of that notion, a recent paper has shown that mice deficient in IL-23p19, a critical cytokine for Th17 responses, or in IL-17RA are very susceptible to oropharyngeal candidiasis (Conti et al., 2009). This is in agreement with earlier observations that IL-17RA–deficient mice are very susceptible to the organism (Huang et al., 2004). Notably, human memory CD4+ T cells specific for C. albicans are skewed toward Th17 (Acosta-Rodriguez et al., 2007; Zhou et al., 2008), and STAT3-mediated Th17 deficiency in humans is associated with increased susceptibility to C. albicans infection (Ma et al., 2008). However, Th17 responses have also been argued to contribute not to protection but to Candida-associated immunopathology (Zelante et al., 2007). In addition, we have recently found that IL-17 responses initiated by Dectin-1–activated DCs can involve FoxP3+ cells that, by other criteria, would be classified as regulatory and thought to have a downmodulatory effect on immunity (Osorio et al., 2008). Therefore, the protective role of type 17 immunity in fungal infection remains controversial (Zelante et al., 2009). It should be noted that any protective effect of Th17 immunity might only be apparent in a longer term follow up of animals infected with sublethal doses of C. albicans. Unfortunately, in the absence of a Dectin-2–deficient mouse, we are restricted to short-term experiment infection with mAb blockade such as described in this paper, in which any protective (or detrimental) effect of Th17 cells may be masked by innate resistance mechanisms.
Dectin-1 was previously considered an exception as it does not belong to the three most prominent families of PRRs, namely the TLRs, NLRs, and RLRs. Given our present and past findings, we propose that Dectin-1 and -2 may represent a novel class of myeloid innate receptors characterized by Syk–CARD9 coupling that can function to initiate immunity. It is important to note that Dectin-1 and -2 possess structurally different C-type lectin domains, fall into different subgroups of the CLR family, and use markedly different strategies for engaging the Syk pathway. Therefore, members of this class of CLRs will need to be defined by reference to function rather than structure. A possible candidate to be included in this family is Clec5a, which has recently been shown to be a receptor for Dengue virus involved in the induction of proinflammatory cytokines, including TNF (Chen et al., 2008). Clec5a associates with DAP-12 (Bakker et al., 1999), an ITAM-bearing adaptor similar to the FcRγ chain, and therefore may link to Syk. In addition, Mincle, an inducible myeloid CLR, was recently implicated in macrophage sensing of C. albicans and Malassezia species and the subsequent induction of proinflammatory mediators (Wells et al., 2008; Yamasaki et al., 2009). Interestingly, Mincle has also been shown to associate with the FcRγ chain and signal via CARD9 in response to self-ligands such as the ribonucleoprotein SAP130 released by necrotic cells (Yamasaki et al., 2008). Notably, both Dectin-1 and -2 have also been reported to possess an endogenous ligand (Ariizumi et al., 2000b; Aragane et al., 2003), and DNGR-1, an additional hemITAM-bearing CLR, mediates Syk-dependent crosspriming responses to dead cells (Sancho et al., 2009). Thus, Syk-coupled CLRs may have additional functions not only as PRRs but also as innate receptors for initiating proinflammatory responses to sterile injury.
MATERIALS AND METHODS
Mice.
Wild-type C57BL/6 and Myd88−/−/Trif−/− mice were bred at Cancer Research UK in specific pathogen-free conditions. The bone marrow from Clec7a−/− mice (Taylor et al., 2007) and fetal liver cells from Syk−/− embryos were used to generate radiation chimeras in C57BL/6 hosts, as described previously (Rogers et al., 2005). Card9−/− mice (Gross et al., 2006) were bred at the Technische Universität München, and Fcer1g−/− (FcRγ chain knockout) mice (Park et al., 1998) were bred at the Leiden University Medical Center. All mice were on a C57BL/6 background with the exception of those used for knockdown and infection studies. For these, both Clec7a−/− and wild-type mice were on a 129/Sv genetic background with breeding, and the in vivo experiments were performed at Cardiff University. All animal protocols were approved by the London Research Institute Animal Ethics Committee or the Cardiff University Local Ethical Review Process, and were performed under the authority of the Animal Scientific Procedures Act 1986 (UK).
Cells, fungal stimuli, constructs, and antibodies.
Culture medium was RPMI 1640 supplemented with glutamine, penicillin, streptomycin, 2-mercaptoethanol (all from Invitrogen), and 10% heat-inactivated fetal calf serum (Autogen Bioclear). Mouse BMDCs were generated using GM-CSF (Inaba et al., 1992) and purified from bulk cultures by magnetic selection with anti-CD11c microbeads. This routinely gave purities of >98%. Retroviral transduction of BMDCs has previously been described (LeibundGut-Landmann et al., 2007). Spleen cells were prepared by digestion with liberase and DNase, followed by enriching for conventional DCs by positive selection with anti-CD11c microbeads.
A single colony of C. albicans strain SC5314 was grown overnight at 30°C in yeast peptone dextrose media. The cells were washed twice with PBS before use as live yeasts or were heat-killed by boiling for 30–45 min. For the hyphal forms, the washed yeast were resuspended at 107 cells/ml in RPMI 1640 with 10% FCS and grown overnight. After washing in PBS, the hyphae were used for live stimulations or were heat killed as described. The morphology was verified by differential interference contrast microscopy using a 40× objective (Plan Fluor; Nikon; Fig. S2). Zymosan was purchased from InvivoGen and labeled with Cy5 as previously described (Rogers et al., 2005). Curdlan was purchased from Wako Chemicals USA, Inc.
The cDNA for FcRγ chain, DAP-10, and DAP-12 was amplified from either the RAW264.7 macrophage cell line or C57BL/6 bone marrow cells and cloned into the retroviral vector pMX-IP (a gift from T. Kitamura, University of Tokyo, Tokyo, Japan; Kitamura et al., 2003). The signaling-defective FcRγ chain was made by mutating tyrosines 65 and 76 to phenylalanine by standard molecular biology techniques and verified by sequencing. The full-length α isoform of Dectin-2 was cloned from wild-type BMDC cDNA by PCR, sequenced, and subcloned into pFB-IRES-EGFP (donated by R. Germain, National Institute of Allergy and Infectious Diseases, Bethesda, MD).
B3Z cells containing a reporter plasmid for NFAT coupled to β-galactosidase activity have been previously described (Karttunen et al., 1992) and were a gift from N. Shastri (University of California, Berkeley, Berkeley, CA). B3Z cells were retrovirally transduced with the pMX-IP vectors separately and selected under 10 µg/ml puromycin (Sigma-Aldrich) for at least 7 d. These lines were subsequently retrovirally transduced with pFB–Dectin-2–IRES–EGFP or the empty vector. For functional assays, the cells were FACS sorted based on EGFP expression.
The anti–Dectin-2 mAb, clone D2.11E4 (Taylor et al., 2005), and a rat IgG2a isotype-matched control (BD) were used in vitro. For the in vivo model of C. albicans infection, the rat IgG2a clone OX11 was used as the isotype-matched control. F(ab′) preparations of D2.11E4 and control antibody (Y13-259, a rat IgG1 raised against p21 V-ras; CRUK Monoclonal Antibody Service) were made using immobilized papain digestion followed by protein G depletion of Fc and undigested antibody (Thermo Fisher Scientific). All immunoblotting antibodies were rabbit polyclonal antibodies purchased from Cell Signaling Technology, with the exception of those against phospho-JNK (Promega) and total Syk (Turner et al., 1995).
DC stimulations.
For analysis of cytokine production, 105 BMDCs were cultured overnight in a 96-well U-bottomed plate with 105 live C. albicans, 105 or 5 × 105 heat-killed C. albicans, or increasing doses of zymosan. In blocking experiments, the BMDCs were preincubated for 2 h with 10 µg/ml D2.11E4 or isotype-matched control mAb before addition of the stimuli. 2.5 µg/ml fungizone (Invitrogen) or 50 ng/ml caspofungin (Merck) was added to the cultures 2 h after live C. albicans stimulation. Cytokine levels in the supernatant were measured by sandwich ELISA.
For stimulation with plated antibodies, UV-irradiated MaxiSorp plates (Thermo Fisher Scientific) were coated overnight with 40 µg/ml goat F(ab′)2 anti–rat F(ab′)2 (Jackson ImmunoResearch Laboratories) in PBS. After washing, the plates were incubated with 10 µg/ml of control or anti–Dectin-2 F(ab′) for 1–3 h at 37°C before washing with PBS. 2 × 105 BMDCs were incubated on the plate overnight and cytokines were measured as described.
Immunoblotting.
BMDCs were stimulated by plated F(ab′) as described, except that 2.5 × 105 cells were spun at 233 g onto the coated plate. At the times after centrifugation indicated in the figures, the medium was removed and the cells were lysed for 30 min on ice in RIPA buffer (50 mM Tris [pH 7.5], 1% Nonidet-P40, 0.5% deoxycholic acid, 0.1% SDS, 150 mM NaCl, 10 mM NaF, 1 mM Na3VO4, 2 mM Na4P2O7, and a mixture of protease inhibitors; Roche). Insoluble material was discarded and a fixed amount of lysate was mixed with sample buffer (LeibundGut-Landmann et al., 2007) before separation by SDS-PAGE. After transfer to a polyvinyldifluoride membrane (Millipore), proteins were analyzed by immunoblotting and visualized by enhanced chemiluminescence (Thermo Fisher Scientific).
FACS staining, zymosan binding, and B3Z cell stimulation.
Cell suspensions were stained in ice-cold FACS wash (PBS containing 5mM EDTA and 1% FCS), which was further supplemented with 1% normal mouse serum when staining for Dectin-2. BMDCs, splenic DCs, and B3Z cells were stained with 10 µg/ml D2.11E4, 2A11 (anti–Dectin-1), or isotype-matched control mAb for 30 min, followed by anti–rat IgG-PE (Jackson ImmunoResearch Laboratories) and, in the case of DCs, anti–CD8-FITC, anti–CD4-PerCP, and/or anti–CD11c-allophycocyanin (BD).
For zymosan binding experiments, 2 × 105 B3Z cells or 105 BMDCs were pretreated or not for 2 h at 37°C with 10 µg/ml anti–Dectin-2 or an isotype-matched antibody control. After cooling on ice, the cells were incubated with 10–100 µg/ml Cy5-zymosan for 10–90 min and washed three times with ice-cold media before flow cytometric analysis.
105 B3Z cells/well in a 96-well plate were incubated overnight with 100 µg/ml zymosan. LacZ activity was measured by lysis in CPRG-containing buffer (Roche). OD 595 was measured using OD 655 as a reference.
Lentiviral knockdown.
The shRNA construct for mouse Dectin-2 in a pLKO.1 lentiviral vector (TRCN0000066785; Open Biosystems) was used for this study based on a previous publication (Barrett et al., 2009). A scrambled shRNA sequence was used as the control. Infectious viral particles were generated after cotransfection of 293 T cells with the PLKO.1 construct, the packaging vector Δ8.9, and the envelope vector VSV-G.
105 bone marrow precursors/well in complete media containing GM-CSF were seeded into a 96-well plate. On day 2, the media was removed and replaced by the viral stocks in the presence of 8 µg/ml hexadimethrine bromide. The cells were centrifuged at 1,130 g for 90 min, after which the virus was replaced by fresh media. 5 µg/ml puromycin was added on day 4. Cells were harvested on day 6 and stimulated as described. In each experiment, Dectin-2 expression was analyzed by flow cytometry and the median fluorescence was reduced by at least 50% as compared with the scrambled control.
Systemic C. albicans infection model.
Female mice aged 12–16 wk were injected with 200 µg D2.11E4 or control mAb, OX11, intraperitoneally. After 6 h, the mice were infected i.v. with 3 × 104 live C. albicans yeast. The mice were further injected with mAbs 2 and 4 d after infection. After a total of 7 d, the mice were sacrificed. Total splenocytes were harvested and restimulated at 2 × 106 cells/well in a 96-well U-bottomed plate with 105 heat-killed C. albicans for 2 d. ELISA was used to measure the IL-17 and IFN-γ levels in the supernatant. The kidneys were removed and disrupted in 0.5 ml PBS using a Dounce homogenizer. Serial dilutions were plated on agar plates, and the total number of colonies was counted and calculated back to colony forming units.
Statistical analysis.
Statistical significance of the infection study was determined by one-way analysis of variance with Tukey multiple comparison of all pairs for posttest analysis.
RNA isolation and quantitative RT-PCR.
Total RNA from BMDCs was extracted 3 h after stimulation with live yeast or hyphae and prepared with an RNeasy Mini Kit (QIAGEN). cDNA was synthesized from total RNA with random hexamer primers and Superscript II RT (Invitrogen). Quantitative real-time PCR was performed using SYBR green incorporation. Measurements were performed in duplicate wells using a sequence detection system (ABI PRISM 7700; Applied Biosystems). Normalization was performed against HPRT and results are shown as relative mRNA quantities.
Online supplemental material.
Fig. S1 demonstrates that zymosan induction of cytokines from DCs is dependent on Syk but not on MyD88, TRIF, or Dectin-1. Fig. S2 shows the morphology of the C. albicans preparations and demonstrates that the use of fungizone or caspofungin, two distinct antifungals, does not substantially alter the Syk-dependent cytokine response to C. albicans or to curdlan. Fig. S3 depicts Dectin-2 expression on conventional DC subpopulations. Fig. S4 demonstrates the requirement for FcRγ chain in anti–Dectin-2 triggering of cytokine production. In Fig. S5, cell-surface expression of Dectin-2 and the subsequent binding of fluorescent zymosan is similarly enhanced by expression of either wild-type or an ITAM-mutated FcRγ chain. Fig. S6 demonstrates that combined loss of Dectin-1 and blockade of Dectin-2 abolishes the up-regulation of IL-23p19 mRNA by heat-killed C. albicans. Fig. S7 shows that both Dectin-1 and -2 contribute to the binding of fluorescent zymosan by BMDCs and that this is increased by transduction of the signaling-defective Dectin-1. Fig. S8 shows that anti–Dectin-2 mAb treatment of C. albicans–infected mice, but not Dectin-1 deficiency, compromises IL-17 but not IFN-γ production in an assay in which splenocytes are restimulated with a high dose of C. albicans. It further shows that the kidney fungal burden and mouse weight loss are not significantly different in wild-type and Dectin-1–deficient mice with and without anti–Dectin-2 treatment early after C. albicans infection. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20082818/DC1.
Acknowledgments
We thank the FACS service and Equipment Park of the London Research Institute for technical support, and all of our animal facilities for the care of the animals used in these studies. We are grateful to C. Swanton and U. Banerji for assistance in procuring caspofungin. We thank members of the Immunobiology Laboratory, Cancer Research UK for advice and discussions.
This work was funded by Cancer Research UK and the Medical Research Council (MRC). P.R. Taylor is an MRC Senior Fellow (grant G0601617). L.F. Moita is a Young Investigator from the Human Frontier Science Program and receives support from Fundação Luso-Americana para o Desenvolvimento and Fundação para a Ciência e a Tecnologia (grant PTDC/SAU-MII/69280/2006 and PTDC/SAU-MII/78333/2006). F. Osorio is supported by Graduate School Research Scholarship and Overseas Research Scholarship studentships from University College London and by a Cancer Research UK PhD studentship.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used: BMDC, bone marrow–derived DC; CLR, C-type lectin receptor; ITAM, immunoreceptor tyrosine-based activation motif; NLR, NOD-like receptor; PAMP, pathogen-associated molecular pattern; PRR, pattern recognition receptor; RLR, RIG-I–like receptor; shRNA, short hairpin RNA; TLR, Toll-like receptor.
References
- Acosta-Rodriguez E.V., Rivino L., Geginat J., Jarrossay D., Gattorno M., Lanzavecchia A., Sallusto F., Napolitani G. 2007. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells.Nat. Immunol. 8:639–646 [DOI] [PubMed] [Google Scholar]
- Akira S., Uematsu S., Takeuchi O. 2006. Pathogen recognition and innate immunity.Cell. 124:783–801 [DOI] [PubMed] [Google Scholar]
- Aragane Y., Maeda A., Schwarz A., Tezuka T., Ariizumi K., Schwarz T. 2003. Involvement of dectin-2 in ultraviolet radiation-induced tolerance.J. Immunol. 171:3801–3807 [DOI] [PubMed] [Google Scholar]
- Ariizumi K., Shen G.L., Shikano S., Ritter R., III, Zukas P., Edelbaum D., Morita A., Takashima A. 2000a. Cloning of a second dendritic cell-associated C-type lectin (dectin-2) and its alternatively spliced isoforms.J. Biol. Chem. 275:11957–11963 [DOI] [PubMed] [Google Scholar]
- Ariizumi K., Shen G.L., Shikano S., Xu S., Ritter R., III, Kumamoto T., Edelbaum D., Morita A., Bergstresser P.R., Takashima A. 2000b. Identification of a novel, dendritic cell-associated molecule, dectin-1, by subtractive cDNA cloning.J. Biol. Chem. 275:20157–20167 [DOI] [PubMed] [Google Scholar]
- Bakker A.B., Baker E., Sutherland G.R., Phillips J.H., Lanier L.L. 1999. Myeloid DAP12-associating lectin (MDL)-1 is a cell surface receptor involved in the activation of myeloid cells.Proc. Natl. Acad. Sci. USA. 96:9792–9796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett N.A., Maekawa A., Rahman O.M., Austen K.F., Kanaoka Y. 2009. Dectin-2 recognition of house dust mite triggers cysteinyl leukotriene generation by dendritic cells.J. Immunol. 182:1119–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchon A., Hernández-Munain C., Cella M., Colonna M. 2001. A DAP12-mediated pathway regulates expression of CC chemokine receptor 7 and maturation of human dendritic cells.J. Exp. Med. 194:1111–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G.D. 2006. Dectin-1: a signalling non-TLR pattern-recognition receptor.Nat. Rev. Immunol. 6:33–43 [DOI] [PubMed] [Google Scholar]
- Brown G.D., Gordon S. 2001. Immune recognition. A new receptor for beta-glucans.Nature. 413:36–37 [DOI] [PubMed] [Google Scholar]
- Cambi A., Netea M.G., Mora-Montes H.M., Gow N.A., Hato S.V., Lowman D.W., Kullberg B.J., Torensma R., Williams D.L., Figdor C.G. 2008. Dendritic cell interaction with Candida albicans critically depends on N-linked mannan.J. Biol. Chem. 283:20590–20599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter R.W., Thompson C., Reid D.M., Wong S.Y., Tough D.F. 2006. Induction of CD8+ T cell responses through targeting of antigen to Dectin-2.Cell. Immunol. 239:87–91 [DOI] [PubMed] [Google Scholar]
- Chen S.T., Lin Y.L., Huang M.T., Wu M.F., Cheng S.C., Lei H.Y., Lee C.K., Chiou T.W., Wong C.H., Hsieh S.L. 2008. CLEC5A is critical for dengue-virus-induced lethal disease.Nature. 453:672–676 [DOI] [PubMed] [Google Scholar]
- Conti H.R., Shen F., Nayyar N., Stocum E., Sun J.N., Lindemann M.J., Ho A.W., Hai J.H., Yu J.J., Jung J.W., et al. 2009. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis.J. Exp. Med. 206:299–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca A., Montagnoli C., Zelante T., Bonifazi P., Bozza S., Moretti S., D'Angelo C., Vacca C., Boon L., Bistoni F., et al. 2007. Functional yet balanced reactivity to Candida albicans requires TRIF, MyD88, and IDO-dependent inhibition of Rorc.J. Immunol. 179:5999–6008 [DOI] [PubMed] [Google Scholar]
- Dennehy K.M., Brown G.D. 2007. The role of the beta-glucan receptor Dectin-1 in control of fungal infection.J. Leukoc. Biol. 82:253–258 [DOI] [PubMed] [Google Scholar]
- Gantner B.N., Simmons R.M., Underhill D.M. 2005. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments.EMBO J. 24:1277–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodridge H.S., Simmons R.M., Underhill D.M. 2007. Dectin-1 stimulation by Candida albicans yeast or zymosan triggers NFAT activation in macrophages and dendritic cells.J. Immunol. 178:3107–3115 [DOI] [PubMed] [Google Scholar]
- Gross O., Gewies A., Finger K., Schäfer M., Sparwasser T., Peschel C., Förster I., Ruland J. 2006. Card9 controls a non-TLR signalling pathway for innate anti-fungal immunity.Nature. 442:651–656 [DOI] [PubMed] [Google Scholar]
- Gross O., Grupp C., Steinberg C., Zimmermann S., Strasser D., Hannesschläger N., Reindl W., Jonsson H., Huo H., Littman D.R., et al. 2008. Multiple ITAM-coupled NK-cell receptors engage the Bcl10/Malt1 complex via Carma1 for NF-kappaB and MAPK activation to selectively control cytokine production.Blood. 112:2421–2428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara H., Ishihara C., Takeuchi A., Imanishi T., Xue L., Morris S.W., Inui M., Takai T., Shibuya A., Saijo S., et al. 2007. The adaptor protein CARD9 is essential for the activation of myeloid cells through ITAM-associated and Toll-like receptors.Nat. Immunol. 8:619–629 [DOI] [PubMed] [Google Scholar]
- Hara H., Ishihara C., Takeuchi A., Xue L., Morris S.W., Penninger J.M., Yoshida H., Saito T. 2008. Cell type-specific regulation of ITAM-mediated NF-kappaB activation by the adaptors, CARMA1 and CARD9.J. Immunol. 181:918–930 [DOI] [PubMed] [Google Scholar]
- Hohl T.M., Rivera A., Pamer E.G. 2006. Immunity to fungi.Curr. Opin. Immunol. 18:465–472 [DOI] [PubMed] [Google Scholar]
- Huang W., Na L., Fidel P.L., Schwarzenberger P. 2004. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice.J. Infect. Dis. 190:624–631 [DOI] [PubMed] [Google Scholar]
- Inaba K., Inaba M., Romani N., Aya H., Deguchi M., Ikehara S., Muramatsu S., Steinman R.M. 1992. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor.J. Exp. Med. 176:1693–1702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isakov N. 1997. Immunoreceptor tyrosine-based activation motif (ITAM), a unique module linking antigen and Fc receptors to their signaling cascades.J. Leukoc. Biol. 61:6–16 [DOI] [PubMed] [Google Scholar]
- Janeway C.A., Jr. 1989. Approaching the asymptote? Evolution and revolution in immunology.Cold Spring Harb. Symp. Quant. Biol. 54:1–13 [DOI] [PubMed] [Google Scholar]
- Jouault T., Ibata-Ombetta S., Takeuchi O., Trinel P.A., Sacchetti P., Lefebvre P., Akira S., Poulain D. 2003. Candida albicans phospholipomannan is sensed through toll-like receptors.J. Infect. Dis. 188:165–172 [DOI] [PubMed] [Google Scholar]
- Kanazawa N., Tashiro K., Inaba K., Miyachi Y. 2003. Dendritic cell immunoactivating receptor, a novel C-type lectin immunoreceptor, acts as an activating receptor through association with Fc receptor gamma chain.J. Biol. Chem. 278:32645–32652 [DOI] [PubMed] [Google Scholar]
- Karttunen J., Sanderson S., Shastri N. 1992. Detection of rare antigen-presenting cells by the lacZ T-cell activation assay suggests an expression cloning strategy for T-cell antigens.Proc. Natl. Acad. Sci. USA. 89:6020–6024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T., Koshino Y., Shibata F., Oki T., Nakajima H., Nosaka T., Kumagai H. 2003. Retrovirus-mediated gene transfer and expression cloning: powerful tools in functional genomics.Exp. Hematol. 31:1007–1014 [PubMed] [Google Scholar]
- Lee M.S., Kim Y.J. 2007. Signaling pathways downstream of pattern-recognition receptors and their cross talk.Annu. Rev. Biochem. 76:447–480 [DOI] [PubMed] [Google Scholar]
- LeibundGut-Landmann S., Gross O., Robinson M.J., Osorio F., Slack E.C., Tsoni S.V., Schweighoffer E., Tybulewicz V., Brown G.D., Ruland J., Reis e Sousa C. 2007. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17.Nat. Immunol. 8:630–638 [DOI] [PubMed] [Google Scholar]
- LeibundGut-Landmann S., Osorio F., Brown G.D., Reis e Sousa C. 2008. Stimulation of dendritic cells via the dectin-1/Syk pathway allows priming of cytotoxic T-cell responses.Blood. 112:4971–4980 [DOI] [PubMed] [Google Scholar]
- Ma C.S., Chew G.Y., Simpson N., Priyadarshi A., Wong M., Grimbacher B., Fulcher D.A., Tangye S.G., Cook M.C. 2008. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3.J. Exp. Med. 205:1551–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGreal E.P., Rosas M., Brown G.D., Zamze S., Wong S.Y., Gordon S., Martinez-Pomares L., Taylor P.R. 2006. The carbohydrate-recognition domain of Dectin-2 is a C-type lectin with specificity for high mannose.Glycobiology. 16:422–430 [DOI] [PubMed] [Google Scholar]
- Netea M.G., Gow N.A., Munro C.A., Bates S., Collins C., Ferwerda G., Hobson R.P., Bertram G., Hughes H.B., Jansen T., et al. 2006. Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors.J. Clin. Invest. 116:1642–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea M.G., Brown G.D., Kullberg B.J., Gow N.A. 2008. An integrated model of the recognition of Candida albicans by the innate immune system.Nat. Rev. Microbiol. 6:67–78 [DOI] [PubMed] [Google Scholar]
- Osorio F., LeibundGut-Landmann S., Lochner M., Lahl K., Sparwasser T., Eberl G., Reis e Sousa C. 2008. DC activated via dectin-1 convert Treg into IL-17 producers.Eur. J. Immunol. 38:3274–3281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.Y., Ueda S., Ohno H., Hamano Y., Tanaka M., Shiratori T., Yamazaki T., Arase H., Arase N., Karasawa A., et al. 1998. Resistance of Fc receptor- deficient mice to fatal glomerulonephritis.J. Clin. Invest. 102:1229–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichlmair A., Reis e Sousa C. 2007. Innate recognition of viruses.Immunity. 27:370–383 [DOI] [PubMed] [Google Scholar]
- Reis e Sousa C. 2004. Activation of dendritic cells: translating innate into adaptive immunity.Curr. Opin. Immunol. 16:21–25 [DOI] [PubMed] [Google Scholar]
- Robinson M.J., Sancho D., Slack E.C., LeibundGut-Landmann S., Reis e Sousa C. 2006. Myeloid C-type lectins in innate immunity.Nat. Immunol. 7:1258–1265 [DOI] [PubMed] [Google Scholar]
- Rogers N.C., Slack E.C., Edwards A.D., Nolte M.A., Schulz O., Schweighoffer E., Williams D.L., Gordon S., Tybulewicz V.L., Brown G.D., Reis e Sousa C. 2005. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins.Immunity. 22:507–517 [DOI] [PubMed] [Google Scholar]
- Romani L. 2008. Cell mediated immunity to fungi: a reassessment.Med. Mycol. 46:515–529 [DOI] [PubMed] [Google Scholar]
- Saijo S., Fujikado N., Furuta T., Chung S.H., Kotaki H., Seki K., Sudo K., Akira S., Adachi Y., Ohno N., et al. 2007. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans.Nat. Immunol. 8:39–46 [DOI] [PubMed] [Google Scholar]
- Sancho D., Joffre O.P., Keller A.M., Rogers N.C., Martínez D., Hernanz-Falcón P., Rosewell I., Reis e Sousa C. 2009. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity.Nature. 458:899–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Yang X.L., Yudate T., Chung J.S., Wu J., Luby-Phelps K., Kimberly R.P., Underhill D., Cruz P.D., Jr., Ariizumi K. 2006. Dectin-2 is a pattern recognition receptor for fungi that couples with the Fc receptor gamma chain to induce innate immune responses.J. Biol. Chem. 281:38854–38866 [DOI] [PubMed] [Google Scholar]
- Slack E.C., Robinson M.J., Hernanz-Falcón P., Brown G.D., Williams D.L., Schweighoffer E., Tybulewicz V.L., Reis e Sousa C. 2007. Syk-dependent ERK activation regulates IL-2 and IL-10 production by DC stimulated with zymosan.Eur. J. Immunol. 37:1600–1612 [DOI] [PubMed] [Google Scholar]
- Taylor P.R., Brown G.D., Herre J., Williams D.L., Willment J.A., Gordon S. 2004. The role of SIGNR1 and the beta-glucan receptor (dectin-1) in the nonopsonic recognition of yeast by specific macrophages.J. Immunol. 172:1157–1162 [DOI] [PubMed] [Google Scholar]
- Taylor P.R., Reid D.M., Heinsbroek S.E., Brown G.D., Gordon S., Wong S.Y. 2005. Dectin-2 is predominantly myeloid restricted and exhibits unique activation-dependent expression on maturing inflammatory monocytes elicited in vivo.Eur. J. Immunol. 35:2163–2174 [DOI] [PubMed] [Google Scholar]
- Taylor P.R., Tsoni S.V., Willment J.A., Dennehy K.M., Rosas M., Findon H., Haynes K., Steele C., Botto M., Gordon S., Brown G.D. 2007. Dectin-1 is required for beta-glucan recognition and control of fungal infection.Nat. Immunol. 8:31–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M., Mee P.J., Costello P.S., Williams O., Price A.A., Duddy L.P., Furlong M.T., Geahlen R.L., Tybulewicz V.L. 1995. Perinatal lethality and blocked B-cell development in mice lacking the tyrosine kinase Syk.Nature. 378:298–302 [DOI] [PubMed] [Google Scholar]
- Underhill D.M., Rossnagle E., Lowell C.A., Simmons R.M. 2005. Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production.Blood. 106:2543–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Veerdonk F.L., Marijnissen R.J., Kullberg B.J., Koenen H.J., Cheng S.C., Joosten I., van den Berg W.B., Williams D.L., van der Meer J.W., Joosten L.A., Netea M.G. 2009. The macrophage mannose receptor induces IL-17 in response to Candida albicans.Cell Host Microbe. 5:329–340 [DOI] [PubMed] [Google Scholar]
- Wells C.A., Salvage-Jones J.A., Li X., Hitchens K., Butcher S., Murray R.Z., Beckhouse A.G., Lo Y.L., Manzanero S., Cobbold C., et al. 2008. The macrophage-inducible C-type lectin, mincle, is an essential component of the innate immune response to Candida albicans.J. Immunol. 180:7404–7413 [DOI] [PubMed] [Google Scholar]
- Willment J.A., Brown G.D. 2008. C-type lectin receptors in antifungal immunity.Trends Microbiol. 16:27–32 [DOI] [PubMed] [Google Scholar]
- Yamasaki S., Ishikawa E., Sakuma M., Hara H., Ogata K., Saito T. 2008. Mincle is an ITAM-coupled activating receptor that senses damaged cells.Nat. Immunol. 9:1179–1188 [DOI] [PubMed] [Google Scholar]
- Yamasaki S., Matsumoto M., Takeuchi O., Matsuzawa T., Ishikawa E., Sakuma M., Tateno H., Uno J., Hirabayashi J., Mikami Y., et al. 2009. C-type lectin Mincle is an activating receptor for pathogenic fungus, Malassezia.Proc. Natl. Acad. Sci. USA. 106:1897–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshitomi H., Sakaguchi N., Kobayashi K., Brown G.D., Tagami T., Sakihama T., Hirota K., Tanaka S., Nomura T., Miki I., et al. 2005. A role for fungal β-glucans and their receptor Dectin-1 in the induction of autoimmune arthritis in genetically susceptible mice.J. Exp. Med. 201:949–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelante T., De Luca A., Bonifazi P., Montagnoli C., Bozza S., Moretti S., Belladonna M.L., Vacca C., Conte C., Mosci P., et al. 2007. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance.Eur. J. Immunol. 37:2695–2706 [DOI] [PubMed] [Google Scholar]
- Zelante T., De Luca A., D'Angelo C., Moretti S., Romani L. 2009. IL-17/Th17 in anti-fungal immunity: what's new? Eur. J. Immunol. 39:645–648 [DOI] [PubMed] [Google Scholar]
- Zhou M., Yang B., Ma R., Wu C. 2008. Memory Th-17 cells specific for C. albicans are persistent in human peripheral blood.Immunol. Lett. 118:72–81 [DOI] [PubMed] [Google Scholar]