Abstract
Allergen-specific desensitization is the only disease-modifying therapy currently available for the treatment of allergies. These therapies require application of allergen over several years and some may induce life-threatening anaphylactic reactions. An ideal vaccine for desensitization should be highly immunogenic and should alleviate allergic symptoms upon few injections while being nonreactogenic. We describe such a vaccine for the treatment of cat allergy, consisting of the major cat allergen Fel d1 coupled to bacteriophage Qβ-derived virus-like particles (Qβ–Fel d1). Qβ–Fel d1 was highly immunogenic, and a single vaccination was sufficient to induce protection against type I allergic reactions. Allergen-specific immunoglobulin G antibodies were shown to be the critical effector molecules and alleviated symptoms by two distinct mechanisms. Although allergen-induced systemic basophil degranulation was inhibited in an FcγRIIb-dependent manner, inhibition of local mast cell degranulation in tissues occurred independently of FcγRIIb. In addition, treatment with Qβ–Fel d1 abolished IgE memory responses upon antigen recall. Despite high immunogenicity, the vaccine was essentially nonreactogenic and vaccination induced neither local nor systemic anaphylactic reactions in sensitized mice. Moreover, Qβ–Fel d1 did not induce degranulation of basophils derived from human volunteers with cat allergies. These data suggest that vaccination with Qβ–Fel d1 may be a safe and effective treatment for cat allergy.
Allergic reactions are associated with several hypersensitivity diseases including asthma, rhinoconjunctivitis, contact dermatitis, urticaria, anaphylaxis, and insect, drug, and food allergy. These diseases can affect all age groups and have reached epidemic proportions worldwide with increasing incidence over the last decades (Holgate, 1999). The most common forms of allergies, such as pollen, house dust, or animal dander allergies, are dependent on type 2 T cell responses (Georas et al., 2005), leading to the generation of IL-4 and IgE. IgE antibodies have a short half-life in serum but are stable if bound to Fcε receptors on circulating basophils and, in particular, tissue mast cells (Vieira and Rajewsky, 1988). Cross-linking of the IgE–FcεI receptor complex on these cells by allergen leads to degranulation within seconds, liberating a variety of preformed inflammatory mediators. The clinical effects attended by such allergic reactions vary according to the site of basophil and mast cell activation. Although inhalation or ingestion of allergens activates mucosal mast cells, i.v. or s.c. antigen entry activates circulating basophils and connective tissue mast cells.
Most current therapies for the treatment of allergies block mast cell effector molecules (e.g., histamines) or nonspecifically suppress immune responses (e.g., steroids). Although effective, these treatments fail to affect the immunological conditions causing the allergies. In addition, different vaccination or desensitization strategies have been investigated, including the usage of allergen-derived peptides (Francis and Larché, 2005), recombinant hypoallergenic derivates (Niederberger et al., 2004; Saarne et al., 2005), oligonucleotides with CpG motives (Hessel et al., 2005), or allergen conjugated to carbohydrate-based particles (Andersson et al., 2004). However, only a few disease-modifying therapies for allergy have been approved so far. These immunotherapies consist of either multiple s.c. injections of increasing doses of allergen or multiple sublingual or oral administration of the allergen, resulting in long-term desensitization (Till et al., 2004). Although these allergen-specific immunotherapies have shown successes (Durham and Till, 1998), the procedures are time consuming and require 1–3 yr of regular treatments (Hedlin et al., 1986, 1991). Moreover, this therapy bears a high risk for anaphylactic reactions, especially after administration of higher allergen doses (Cox and Coulter, 1997; Bousquet et al., 1998).
The mechanism by which this specific immunotherapy affects allergies is poorly understood. The reduction in allergic symptoms by this treatment has been hypothesized to be at least partly mediated by a shift from Th2 toward a Th1 response or the induction of regulatory T (T reg) cells (Akdis et al., 2005). Alternatively, and not mutually exclusive, the balance between allergen-specific IgE and IgG antibodies may regulate mast cell and basophil activity. In fact, Hulett et al. (1993) identified an FcR, with a binding site for IgG, which regulates high affinity IgE receptor–mediated mast cell activation (Daëron et al., 1995b). It is well established that the FcγRIIb (FcγIIb receptor), expressed on mouse and human basophils and mast cells (Bischoff, 2007), can down-regulate FcεRI signaling by cross-linking of the FcεRI–IgE and FcγRIIb–IgG-Ag complex (Daëron et al., 1995a; Katz, 2002; Bruhns et al., 2005; Kraft and Kinet, 2007; Nimmerjahn and Ravetch, 2008). Indeed, a fusion molecule between the cat allergen Fel d1 and the constant part of IgG1 has recently been shown to inhibit allergic symptoms by cross-linking of FcγRIIb with FcεRI in a mouse model of asthma (Zhu et al., 2005; Terada et al., 2006). In addition to signaling through FcγRIIb, allergen-specific IgG antibodies may sequester allergens and hence prevent their binding to IgE–FcεRI complexes. Moreover, it has been shown that the ratio of allergen-specific IgE and IgG antibodies may affect presentation of allergen-derived epitopes to T cells (Wachholz and Durham, 2004). Thus, allergen-specific antibodies may have multiple ways to modulate allergic responses.
We have previously shown that antigens displayed in a repetitive fashion on virus-like particles (VLPs) derived from the coat protein of the bacteriophage Qβ are highly immunogenic in mice (Jegerlehner et al., 2002a,b; Lechner et al., 2002; Spohn et al., 2005) and humans (Maurer et al., 2005; Kündig et al., 2006; Ambühl et al., 2007; Tissot et al., 2008). Because bacterial host RNA is incorporated into the VLPs during self-assembly inside bacteria, Qβ-VLPs provide Toll-like receptor ligands, which induce strong IgG2a/c-dominated antibody responses (Forsbach et al., 2007, 2008; Jegerlehner et al., 2007). In the present study, the major cat allergen Fel d1 was coupled in an oriented fashion to Qβ-VLPs, resulting in a highly repetitive form of the allergen. This vaccine was strongly immunogenic in mice, yielding high and long-lasting antigen-specific serum IgG titers after only a single immunization. Vaccination with Qβ–Fel d1 resulted in strongly reduced immediate type I allergic responses in a mouse model of mast cell degranulation, vascular leakage, and anaphylaxis and completely abolished IgE B cell memory responses upon antigen recall. Strikingly, coupling of Fel d1 to Qβ-VLPs essentially abrogated its ability to induce allergic responses in mice or the degranulation of human basophils derived from allergic individuals. Thus, displaying Fel d1 on VLPs enhanced its immunogenicity and therapeutic efficacy while strongly reducing its reactogenicity.
RESULTS
Generation and structure of recombinant Fel d1 protein and vaccine design
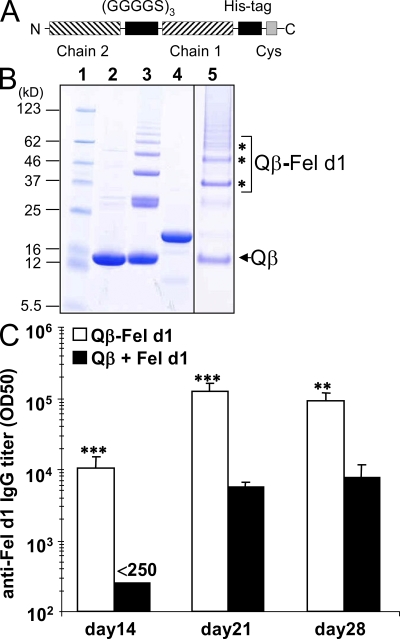
To generate a vaccine for the treatment of cat allergies, we displayed the major cat allergen Fel d1 in a highly ordered fashion on Qβ VLPs, which have been shown to be a potent carrier for various antigens in mice and humans (Bachmann et al., 1993; Jegerlehner et al., 2002a,b; Maurer et al., 2005; Spohn et al., 2005; Kündig et al., 2006; Ambühl et al., 2007; Tissot et al., 2008). To this end, a covalent dimer of Fel d1 was generated and expressed in Escherichia coli (rFel d1). The details of the constructs are schematically shown in Fig. 1 A. rFel d1 was coupled to Qβ VLPs (Qβ–Fel d1) and analyzed by SDS-PAGE. Densitometric analysis of the coupling products indicated 40% coupling efficiency, corresponding to 70 rFel d1 molecules per Qβ VLP (Fig. 1 B). Thus, there is roughly 20 µg rFel d1 per 50 µg of vaccine. Identities of the different bands were confirmed by Western blotting using anti-His tag and anti-Qβ antibodies (unpublished data).
Figure 1.
Production and coupling of recombinant Fel d1 protein. Fel d1 was cloned, expressed, purified, and coupled to VLPs as described in Materials and methods. (A) Schematic representation of rFel d1. Chain 1 and 2, His tag with (G4S)x3 linker, and the Cystein used for coupling are shown. (B) Analysis of coupling reactions. Fel d1 was coupled to Qβ-VLPs and analyzed by SDS PAGE (12%). 1, prestained protein marker (broad range 5–123); 2, untreated Qβ; 3, SMPH-derivatized Qβ; 4, Fel d1-15aa-HC; 5, purified Qβ–Fel d1-15aa-HC. Coupling products are marked with asterisks. All lanes (1–5) originate from one gel. Lanes presenting products of unimportant purification steps were cropped out between Fel d1-15aa-HC and the purified end product Qβ–Fel d1-15aa-HC. (C) Naive BALB/c mice were immunized s.c. with either 50 µg Qβ–Fel d1 or 20 µg Fel d1 mixed with 30 µg Qβ on days 0 and 14. Mice were bled on day 14, 21, and 28 and anti–Fel d1 IgG serum antibody titers determined by ELISA. Subclass titers are provided in Fig. S1. Mean Fel d1–specific IgG titers ± SD (n = 3) are shown. Data are representative of three independent experiments with three mice per group. **, P < 0.005; ***, P < 0.0005.
To determine the immunogenicity of the vaccine, BALB/c mice were immunized on days 0 and 14 by s.c. injections with Qβ–Fel d1 or, as a control, with equivalent amounts of free rFel d1 mixed with Qβ. Fel d1–specific IgG titers were determined at the indicated time points by ELISA. A single vaccination of mice with Qβ–Fel d1 induced a Fel d1–specific IgG response (Fig. 1 C), which consisted of similar amounts of antigen-specific IgG1 and IgG2a isotypes (Fig. S1). This response could be boosted by the second injection of the vaccine on day 14 (Fig. 1 C). rFel d1 mixed with Qβ also induced similar amounts of allergen-specific IgG1 and IgG2a isotypes (Fig. S1); however, titers were much lower overall than those achieved with the coupled product. (Fig. 1 C). These data demonstrate that coupling of Fel d1 to Qβ strongly enhances its immunogenicity. Of note, antigen-specific IgE titer could not be detected upon Qβ–Fel d1 immunization (Fig. S1), indicating that the construct is nonallergenic.
Coupling of Fel d1 to Qβ strongly reduces its reactogenicity in vivo
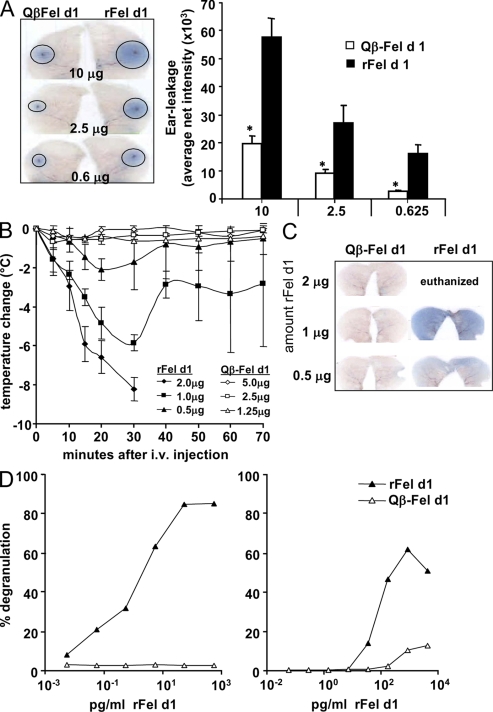
Considering that anaphylactic reactions are the major drawback of current desensitization therapies, we set out to investigate the ability of Qβ–Fel d1 to trigger mast cell–mediated type I hypersensitivity reactions. To this end, BALB/c mice were sensitized with Fel d1 and, 3 wk later, the right ears were pricked with Qβ–Fel d1 and the left ears with rFel d1. As expected, ear pricks performed with rFel d1 induced strong mast cell degranulation, as visualized by dye extravasation in a dose dependent manner (Fig. 2 A, left). In contrast, vascular leakage was strongly reduced if the pricks were performed with equivalent amounts of rFel d1 coupled to Qβ. Densitometric quantification of dye extravasation revealed that Qβ–Fel d1 induced three to five times less vascular leakage than equivalent amounts of rFel d1 (Fig. 2 A, right).
Figure 2.
Reduced in vitro and in vivo reactogenicity of Fel d1 coupled to Qβ. BALB/c mice were sensitized as described in Materials and methods and challenged on day 21 with rFel d1 or Qβ–Fel d1. (A) Intradermal ear prick test with different amounts of free rFel d1 into the right ears or coupled rFel d1 (Qβ–Fel d1) into the left ears. One representative ear pair out of three is shown (left). Mean net intensity of pricked ears ± SD (n = 3) from the densitometric quantification of dye extravasation is also shown (right). Data are representative of two independent experiments with three mice per group for both experiments. (B) Mice were injected with 2, 1, or 0.5 µg of free or coupled rFel d1 i.v. Mean temperature changes (°C) in Fel d1– (filled symbols) or Qβ–Fel d1-injected mice (open symbols) ± SD are shown (n = 3). (C) 70 min after i.v. injection of either free or coupled Fel d1, active systemic anaphylaxis documented by diffuse dye leakage in the skin was examined. One representative mouse out of three is shown. Data are representative of two independent experiments with three mice per group. (D) Heparinized human whole blood of two cat allergic donors was incubated with rFel d1 or Qβ–Fel d1. Basophil degranulation (in percentage) was determined by CD63 surface expression on CD123+/HLA-DR− cells by flow cytometry (Sanz et al., 2002). Data are representative of three independent experiments with these volunteers. *, P < 0.05.
To assess systemic anaphylactic reactions, sensitized mice were challenged i.v. with Qβ–Fel d1 or rFel d1. Injection of rFel d1 induced anaphylactic syndromes in a dose-dependent manner as documented by a rapid drop in temperature (Fig. 2 B). It is important to note that animals challenged with the highest dose of allergen had to be euthanized after 30 min because of the strong anaphylactic reaction induced. In marked contrast, injection of Qβ–Fel d1 did not induce systemic anaphylaxis at any concentration tested. Likewise, vascular leakage in ears upon systemic mast cell degranulation was only observed in animals that had been challenged with rFel d1 (Fig. 2C). Because endotoxin contaminations in either Qβ–Fel d1 or rFel d1 preparations could interfere with the anaphylactic response, the endotoxin content of the Qβ–Fel d1 and r Fel d1 batches used in our experiments were determined. Qβ–Fel d1 and rFel d1 exhibited similar low levels of endotoxin (Fig. S2), excluding an influence of endotoxin on the allergic response observed.
Having demonstrated that covalent coupling of rFel d1 to Qβ VLPs strongly reduces its potential to trigger allergic responses in mice, we set out to confirm this with human cells. Therefore, heparinized whole blood of cat allergic and nonallergic donors was collected and incubated with increasing concentrations of either rFel d1 or Qβ–Fel d1. rFel d1 induced strong degranulation of basophils in cat-allergic individuals. In accordance with the observations in the previous paragraph, Qβ–Fel d1 hardly induced human basophil degranulation in three out of three allergic individuals tested (Fig. 2 D and not depicted). Neither rFel d1 nor Qβ–Fel d1 induced basophil degranulation in nonallergic individuals (unpublished data). Collectively, these results demonstrate that coupling of Fel d1 to Qβ VLPs dramatically reduces its reactogenicity in mice in vivo and human basophils in vitro.
Vaccination with Qβ–Fel d1 protects from systemic anaphylaxis and inhibits local mast cell degranulation in vivo in an allergen-specific manner
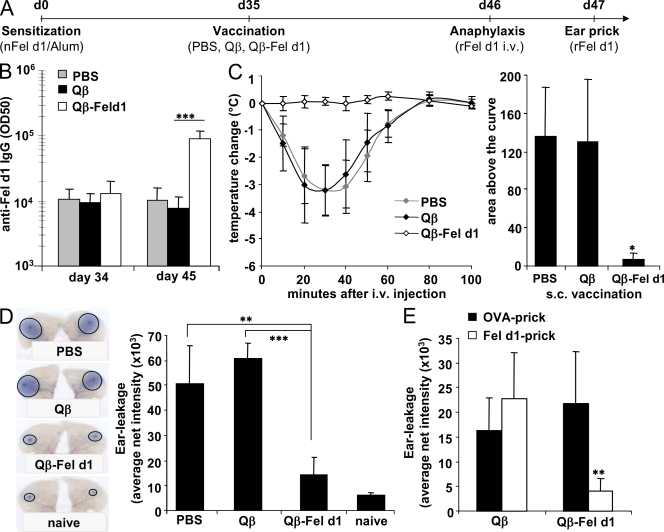
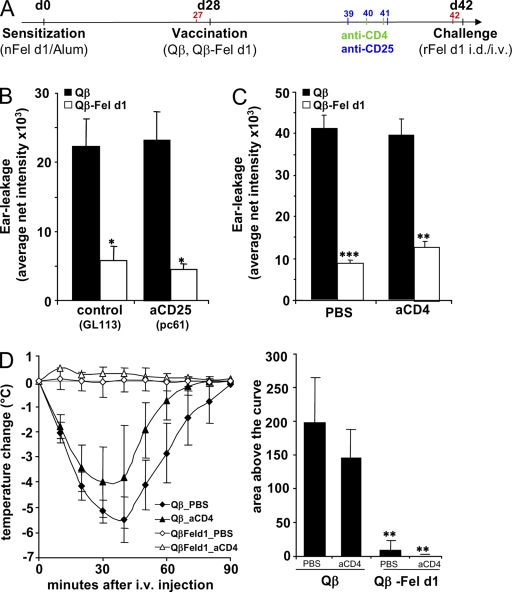
Next, we addressed whether Qβ–Fel d1 was able to desensitize allergic mice. To this end Fel d1–sensitized BALB/c mice were vaccinated once with Qβ–Fel d1 or, as a control, injected with Qβ, PBS, or Fel d1 alone (Fig. 3 A). s.c. administration of PBS, Qβ, or Qβ–Fel d1 did not induce anaphylactic reactions in allergic mice, as documented by constant body temperature after injection (not depicted). In marked contrast, s.c. vaccination with free Fel d1 induced such strong anaphylactic reactions that mice had to be euthanized 30–40 min after injection and could not be used for further experiments (unpublished data). Because of sensitization, all experimental groups had mounted a Fel d1–specific IgG response by day 34 and vaccination with Qβ–Fel d1 led to a 10-fold increase in Fel d1–specific IgG titers at day 45 (Fig. 3 B). The predominant subclass was IgG1, presumably because Fel d1–specific memory B cells expressing IgG1 were preferentially boosted (Fig. S3). To assess whether vaccinated animals were protected from allergic reactions, all groups were challenged i.v. with Fel d1 and body temperature was monitored at regular intervals. A severe drop in body temperature was observed in mice that had been treated with PBS or Qβ (Fig. 3 C). In contrast, Qβ–Fel d1-treated animals were completely protected from anaphylactic reactions.
Figure 3.
Inhibition of mast cell degranulation and protection from active systemic anaphylaxis in Qβ–Fel d1-vaccinated mice. (A) Schematic outline of the experiment. (B) Serum of individual mice was collected before (d35) and after (d45) vaccination. Mean Fel d1–specific IgG titers ± SD are shown (n = 7). (C) 11 d after vaccination, all groups were challenged i.v. with rFel d1 and body temperature was measured at regular intervals. Changes in body temperature in degrees celsius ± SD (n = 3) are shown on the left and mean area above the curves ± SD (n = 3) on the right. Individual areas above the curves were determined and compared by one-way analysis of variance (ANOVA) using Bonferroni's post test (PBS/Qβ, NS; PBS/Qβ–Fel d1, P < 0.01; Qβ/Qβ–Fel d1, P < 0.01. (D) Ear prick tests with rFel d1 in naive, PBS-, Qβ-, or Qβ–Fel d1-treated mice. One representative mouse of each group is shown on the left. Mean net intensity of pricked ears ± SD (n = 4) from the densitometric quantification of dye extravasation is shown on the right. (E) OVA/rFel d1–sensitized mice were vaccinated with Qβ or Qβ–Fel d1 and pricked with OVA in the left ear and rFel d1 in the right ear. Mean net intensities ± SD (n = 5) are shown. Qβ versus Qβ–Fel d1/OVA challenge was NS. Net intensity of the Qβ–Fel d1/Fel d1-challenged group is significantly reduced as compared with all other groups. All data shown are representative of four independent experiments with at least the same number of mice per group as shown in A–D. *, P < 0.01; **, P < 0.005; ***, P < 0.0005.
To investigate the effect of vaccination on local allergic reactions, ear skin prick tests with rFel d1 were performed in a different set of mice as outlined in Fig. 3 A. Allergen challenge induced mast cell degranulation and subsequent vascular leakage in Qβ and PBS control groups (Fig. 3 D). In contrast, Qβ–Fel d1-treated animals were protected from mast cell degranulation. In fact, dye extravasation was almost reduced to the levels observed in nonsensitized naive mice (Fig. 3 D). These experiments show that treatment with Qβ–Fel d1 is safe, prevents local and systemic allergic reactions in vivo, and, thus, efficiently desensitizes mice against Fel d1.
To address whether Qβ–Fel d1 vaccination inhibits mast cell degranulation in an antigen-specific manner, mice were sensitized against OVA and rFel d1. Sensitized mice were vaccinated subsequently with either Qβ or Qβ–Fel d1, 2 wk before ear prick tests with either OVA (left ear) or rFel d1 (right ear) were performed. Strong mast cell–mediated vascular leakage was observed in the control group, which was pricked either with rFel d1 or OVA, indicating that the animals had been equally sensitized to both allergens (Fig. 3 E). As observed in the previous paragraph, mice treated with Qβ–Fel d1 hardly reacted to rFel d1 challenge. However, Qβ–Fel d1 treatment did not influence vascular leakage induced by OVA, demonstrating allergen specificity (Fig. 3 E).
Qβ–Fel d1 vaccination prevents the immediate type I allergic response upon i.p. antigen challenge
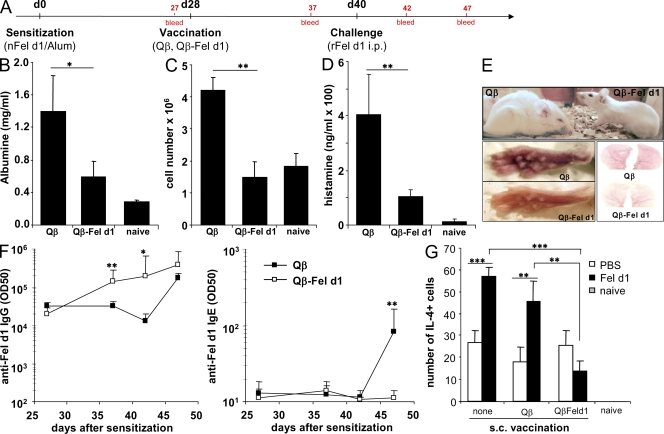
To further characterize an influence of vaccination on the immediate type I allergic response, sensitized and vaccinated mice were challenged i.p. with rFel d1 (Fig. 4 A). In the Qβ-immunized control group, strong i.p. vascular leakage was observed as documented by increased levels of albumin and number of infiltrating cells in the peritoneum and increased histamine levels in serum (Fig. 4, B–D). In fact, i.p. rFel d1 administration resulted in strong anaphylactic reactions 40 min after challenge in Qβ-vaccinated control mice, as documented by a lethargic state, shaggy hair, strong hunched back, closed eyes, and red skin (Fig. 4 E). In contrast, mice treated with Qβ–Fel d1 displayed significantly lower i.p. albumin levels and cell counts as well as reduced serum histamine levels than control mice (Fig.4, B–D). Moreover, these mice showed no physical signs of anaphylaxis (Fig. 4 E).
Figure 4.
Qβ–Fel d1 vaccination blocks type I allergic reactions after i.p. allergen challenge. (A) Schematic outline of the experiment. BALB/c mice were sensitized and, 4 wk later, vaccinated s.c. with Qβ or Qβ–Fel d1. 12 d later, mice were challenged i.p. with rFel d1. (B–D) Peritoneal vascular leakage was determined by measurement of peritoneal albumin concentrations (B), peritoneal cell influx (C), and serum histamine levels (D) upon antigen challenge. Mean values ± SD are shown (n = 5). (E) Pictures of representative animals of each experimental group, 40 min after i.p. rFel d1 challenge. Qβ control animals displayed typical signs of an anaphylactic reaction including reduced activity, shaggy hair, closed eyes, and swollen and red feet and ears. (F) Fel d1–specific IgG (left) and Fel d1–specific IgE (right) titers. Subclass titers are provided in Fig. S4. Mean half-maximal antibody titers ± SD (n = 5) are shown. (G) ELISPOT assays were performed 1 wk after Fel d1 challenge as described in Materials and methods. Numbers of IL-4 producing cells per 2 × 105 spleen cells ± SD (n = 4) are shown (Qβ–Fel d1–PBS vs. Qβ–Fel d1–Fel d1 challenge, NS). In addition, similar results were obtained with purified CD4+ T cells (not depicted). The data shown are representative of two independent experiments with the same number of mice per group. *, P < 0.05; **, P < 0.005; ***, P < 0.0005.
We next monitored Fel d1–specific IgG and IgE titers in these mice at the indicated time points (Fig. 4 A). After sensitization, similar Fel d1–specific IgG titers were measured in all animals. Vaccination with Qβ–Fel d1 boosted the anti–Fel d1 IgG response and allergen challenge further increased anti–Fel d1 IgG titers in serum (Fig. 4 F, left). The subclass produced upon Qβ–Fel d1 vaccination was predominated by boosted preexisting IgG1 isotypes but also antigen-specific IgG2a and IgG2b antibodies were induced upon Qβ–Fel d1 vaccination (Fig. S3). As expected, upon Qβ vaccination no Fel d1–specific IgGs were induced or boosted (Fig. 4 F, left) and antigen-specific IgG1 and IgG2b isotypes were only induced upon allergen challenge on day 40 (Fig. S3). Interestingly, although Qβ-injected control animals showed a strong boosting of the preexisting IgE response upon allergen challenge, in Qβ–Fel d1-treated animals no boosting of the IgE response was observed upon challenge with allergen (Fig. 4 F, right). This result indicates that vaccination with Qβ–Fel d1 not only boosts antigen-specific IgG responses but also prevents IgE B cell memory responses upon allergen recall. In an attempt to elucidate how Qβ–Fel d1 vaccination prevents boosting of IgE responses upon antigen challenge, we examined whether the Fel d1–specific T helper cell response was affected upon Qβ–Fel d1 vaccination. To this end, we vaccinated sensitized mice with Qβ or Qβ–Fel d1 and challenged mice 2 wk later either with Fel d1 or PBS. 7 d later, IL-4 ELISPOT assays were performed with spleen cells from the different groups of mice. Sensitized and nonvaccinated mice challenged with PBS or Fel d1 were used as controls. Confirming previous experiments, Qβ–Fel d1 vaccination boosted antigen-specific IgG titers and prevented IgE memory responses upon later antigen challenge (unpublished data). Furthermore, Fel d1 challenge induced an increase in IL-4–producing Fel d1–specific T helper cell numbers in sensitized control and Qβ-vaccinated animals (Fig. 4 G). In contrast, Fel d1 challenge in sensitized Qβ–Fel d1-vaccinated mice did not lead to a significant increase of IL-4–producing T helper cells numbers, indicating that vaccination with Qβ–Fel d1 prevents expansion of these cells and, perhaps, thereby also IgE isotype switching.
Qβ–Fel d1 desensitization is mediated by Fel d1-specific serum IgG
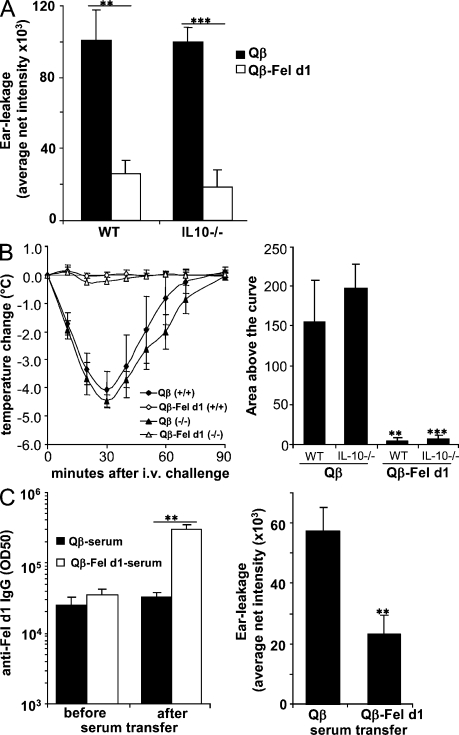
In light of the recent studies implicating T cells (Lu et al., 2006; Kashyap et al., 2008) and IL-10 (Grimbaldeston et al., 2007; Kennedy Norton et al., 2008) in mast cell function, we set out to test whether these components of the immune system were involved in Qβ–Fel d1-mediated desensitization. Therefore, we first depleted either T reg cells or CD4+ T cells in sensitized and vaccinated mice by the injection of anti-CD25 (pc61) or anti-CD4 antibodies, respectively. Depletion of these cell populations was confirmed by FACS (Figs. S4 and S5). As shown in Fig. 5 (B and C), T reg cells and CD4+ T cells are not required for Qβ–Fel d1-mediated desensitization against local allergen challenge. Likewise, Qβ–Fel d1 treatment prevented systemic anaphylactic reactions independent of CD4+ T cells (Fig. 5 D). In addition, experiments with IL-10−/− mice showed no dependence of Qβ–Fel d1-mediated local (Fig. 6 A) and systemic (Fig. 6 B) desensitization on the regulatory cytokine IL-10.
Figure 5.
T reg cells and CD4+ effector T cells are dispensable for Qβ–Fel d1 desensitization. (A) Schematic outline of the experiment. After sensitization and vaccination, T reg cells or CD4+ T cells were depleted by administration of anti-CD25 or anti-CD4, respectively. Control mice received either GL113 isotype Abs or PBS. (B and C) Mean net intensity of dye extravasation after skin pricking in T reg cell–depleted ± SD (n = 4; B) and CD4+-depleted mice ± SD (n = 5; C). (D) Anaphylaxis in sensitized mice treated with Qβ or Qβ–Fel d1, 2 d after CD4+ T cell depletion. Mean changes in body temperature ± SD (n = 4) are shown on the left and mean areas above the curves ± SD (n = 4) are shown on the right. Individual areas above the curves were determined and compared by one-way ANOVA using Bonferroni's post test (PBS: Qβ/Qβ–Fel d1, P < 0.001; anti-CD4: Qβ/Qβ–Fel d1, P < 0.001; Qβ–Fel d1: PBS vs. anti-CD4, NS). Data shown are representative of three independent experiments with four mice per group. B and C: *, P < 0.05; **, P < 0.005; ***, P < 0.0005. D: **, P< 0.001.
Figure 6.
Qβ–Fel d1-mediated desensitization is mediated by antigen-specific IgG. Mice were sensitized and vaccinated as described in Fig. 5 A. (A) Mean net intensity of dye extravasation after skin pricking of Qβ or Qβ–Fel d1-treated WT and IL-10−/− mice ± SD (n = 3). (B) Anaphylaxis in sensitized WT (+/+) and IL-10−/− mice (−/−) treated with Qβ or Qβ–Fel d1. Mean changes in body temperature ± SD (n = 4) are shown on the left and mean areas above the curves ± SD (n = 4) are shown on the right. Individual areas above the curves were determined and compared by one-way ANOVA using Bonferroni's post test (WT: Qβ/Qβ–Fel d1, P < 0.001; IL-10−/−: Qβ/Qβ–Fel d1, P < 0.0001; Qβ–Fel d1: WT vs. IL-10−/−, NS). (C) Serum from sensitized and Qβ- or Qβ–Fel d1-vaccinated mice was transferred into sensitized mice. Mean half-maximal antibody titers before (day 34) and after (day 37) transfer ± SD (n = 3) are shown on the left. Mean net intensity of pricked skin areas ± SD (n = 3) are shown on the right. Data shown are representative of two independent experiments with the same number of mice per group. A and C: **, P < 0.005; ***, P < 0.0005. B: **, P < 0.001; ***, P < 0.0001.
To investigate the role of Fel d1–specific antibodies in the desensitization process, serum from sensitized mice that had been vaccinated with Qβ or Qβ–Fel d1 was transferred into recipient mice, 4 wk after their sensitization. The Fel d1–specific IgG titers in all experimental groups were similar before the transfer and increased in the experimental groups that had received serum from Qβ–Fel d1-vaccinated mice (Fig. 6 C, left). As shown in Fig. 6 C (right), transfer of serum from sensitized and Qβ–Fel d1-vaccinated mice strongly reduced vascular leakage in allergic recipient mice challenged with allergen, 3 d after serum transfer. Similar results were obtained with purified total IgG (unpublished data). Thus, Fel d1–specific IgGs are key effector molecules in the desensitization process and are able to block IgE-mediated mast cell degranulation.
The inhibitory FcγRIIb-receptor is essential for Qβ–Fel d1-mediated protection from systemic anaphylaxis
To investigate the mechanism of protection by Fel d1–specific IgG, we investigated whether an inhibitory signaling event through FcγRIIb was required. Hence, WT and FcγRIIb−/− mice were sensitized and vaccinated 4 wk later with Qβ or Qβ–Fel d1 (Fig. 7 A). In line with previous studies (Takai et al., 1996), FcγRIIb−/− mice exhibited slightly higher antigen-specific antibody titers upon sensitization compared with WT mice (unpublished data). Nevertheless, vaccination with Qβ–Fel d1 strongly increased Fel d1–specific IgGs in WT and FcγRIIb−/− mice reaching similar levels (Fig. 7 B). i.v. antigen challenge 2 wk after control Qβ treatment led to a strong anaphylactic reaction in both WT and FcγRIIb−/− mice. As reported previously (Takai et al., 1996; Ujike et al., 1999), FcγRIIb−/− mice displayed a stronger anaphylactic response than WT control mice (Fig. 7 C). Strikingly, although Qβ–Fel d1-vaccinated WT mice were completely protected from acute systemic anaphylaxis, Qβ–Fel d1-vaccinated FcγRIIb−/− mice were not protected at all (Fig. 7 C). To exclude that the increased sensitivity for anaphylaxis in FcγRIIb−/− mice was responsible for the absence of protection upon Qβ–Fel d1 vaccination, we repeated this anaphylaxis experiment using a lower challenge dose of Fel d1 for FcγRIIb−/− than for WT mice. As shown in Fig. S6, control Qβ-vaccinated WT and FcγRIIb−/− mice experienced an almost identical anaphylactic response under these experimental conditions. Although Qβ–Fel d1-vaccinated WT mice were protected from anaphylaxis, Qβ–Fel d1-vaccinated FcγRIIB−/− mice experienced a strong anaphylactic response, which was not significantly different from the response of control mice, demonstrating that FcγRIIb signaling was indeed essential for Qβ–Fel d1-mediated prevention of anaphylaxis.
Figure 7.
Anti–Fel d1 IgG-mediated inhibition of systemic anaphylaxis is mediated by FcγRIIb. (A) Schematic outline of the experiment. WT and FcγRIIb−/− mice were sensitized and vaccinated as described in Materials and methods. (B) Anti-Fel d1 IgG serum antibody titers after vaccination. Mean half-maximal antibody titers ± SD are shown (WT, n = 4; FcγRIIb−/−, n = 3). (C) 14 d after vaccination, mice were challenged i.v. with rFel d1 and body temperature was monitored at regular intervals. Mean changes in body temperature ± SD are shown (n = 3) on the left and mean areas above the curves ± SD (n = 3) are shown on the right. Individual areas above the curves were determined and compared by one-way ANOVA using Bonferroni's post test (WT: Qβ/Qβ–Fel d1, P < 0.01; FcγRIIb−/−: Qβ/Qβ–Fel d1, NS; Qβ: WT/FcγRIIb−/−, P < 0.01; Qβ–Fel d1: WT/FcγRIIb−/−, P < 0.0001). (C) Ear prick tests 2 wk after vaccination with Qβ or Qβ–Fel d1. Dye extravasation was quantified by densitometry and mean net intensities ± SD (WT, n = 4; FcγRIIb−/−, n = 3) are shown (WT: Qβ/Qβ–Fel d1, P < 0.05; FcγRIIB−/−: Qβ/Qβ–Fel d1, P < 0.005; Qβ–Fel d1: WT/FcγRIIb−/−, NS). All data shown are representative of four independent experiments with three mice per group. B and D: *, P < 0.05; **, P < 0.005. C: *, P < 0.01; ***, P < 0.0001.
To address whether local mast cell degranulation in the skin was also affected by FcγRIIB expression, we challenged sensitized and vaccinated FcγRIIb−/− mice intradermally with Fel d1. In contrast to the systemic reaction to allergen, FcγRIIb−/− mice treated with Qβ–Fel d1 were equally well protected from intradermal allergen challenge as WT mice (Fig. 7 D).
Collectively, these results strongly suggest that acute systemic anaphylactic reactions can be prevented by inhibitory signaling mediated by IgG-Fel d1 immune complexes bound to FcγIIb receptor on circulating basophils. Interestingly, inhibitory signaling through FcγIIb receptor is not needed to prevent local mast cell degranulation upon intradermal allergen challenge. Under those circumstances, the slowly diffusing allergen might be sequestered within the tissue by the induced IgGs at the injection site and hence prevented from reaching IgEs bound to FcεRI on mast cells.
DISCUSSION
In the present study, we describe a therapy for cat allergy based on immunization with the recombinant cat allergen Fel d1 displayed on VLPs derived from the bacteriophage Qβ (Qβ–Fel d1). The therapy is characterized by three key features: (a) Qβ–Fel d1 is highly immunogenic and a single vaccination is sufficient for therapy; (b) Qβ–Fel d1 is essentially nonreactogenic; and (c) the effector mechanism of the therapy is based upon the induction of allergen-specific IgGs.
Qβ-VLPs consist of 180 subunits of a 14-kD coat protein. These VLPs elicit strong B cell responses as a result of their highly organized and repetitive structures (Jegerlehner et al., 2002a,b). This feature can be exploited to enhance the immunogenicity of self- and foreign antigens. Chemical coupling of antigens via a cysteine to surface lysine residues on the VLP renders these antigens equally repetitive and consequently immunogenic. Moreover, host cell RNA, a ligand for TLR3 and TLR7 (Kanzler et al., 2007), is incorporated into the VLPs during self assembly of the coat proteins in E. coli serving as a build-in adjuvant. Together, these features explain the strong IgG responses induced by a single injection of Qβ–Fel d1.
Interestingly, vaccination of sensitized mice with Qβ–Fel d1 resulted in a shutdown of memory IgE responses. Specifically, although a strong boosting of the IgE response was observed in sensitized control animals, no increase in sensitized and vaccinated mice was observed upon subsequent allergen challenge. There are different possibilities for how IgE responses might be down-regulated upon Qβ–Fel d1 vaccination. First, it is possible that binding of IgG–Fel d1 complexes to the FcγIIb receptor, which is also expressed on B cells, would mediate inhibition of B cell function (Muta et al., 1994; Daëron et al., 1995a). In this case, inhibition would specifically affect IgE and not IgG B cell responses because antigen-specific IgG is strongly boosted upon vaccination. However, using sensitized FcγRIIb−/− mice for Qβ–Fel d1 vaccination, we found that despite the absence of the inhibitory FcγIIb receptor on B cells, vaccination with Qβ–Fel d1 still blocked IgE B cell memory responses (unpublished data). We therefore believe that an inhibitory FcγRIIb signaling on B cells is not the cause for the abrogated IgE B cell response upon antigen recall. A second possibility for how Qβ–Fel d1 vaccination could affect long-term IgE responses may be via a direct or indirect inhibitory effect of Qβ–Fel d1 on T helper cell responses, lowering IL-4 production and, consequently, IgE isotype switching. Indeed, ELISPOT assays performed with splenocytes from sensitized, vaccinated, and antigen-challenged mice showed that Qβ–Fel d1-vaccinated mice did not develop an increased IL-4 response upon allergen challenge, which is different from control mice. Thus, Qβ–Fel d1 seems to be able to block expansion of Th2 cells upon allergen challenge. We also performed IFN-γ ELISPOT assays but have not observed an increased Th1 response in Qβ–Fel d1-vaccinated mice (unpublished data). This suggests that the reduced Th2 response is not caused by a shift to Th1 cells. The mechanism of this inhibition will need to be further investigated. One further plausible explanation for the Qβ–Fel d1-mediated effect could be found in the composition of the Qβ-VLP itself. It is possible that only moderate, and not strong, cross-linking of surface Ig is able to drive IgE responses. Indeed, viruses rather than parasites exhibit highly organized surface structures in a manner similar to Fel d1 displayed on VLPs. IgE responses, however, protect against parasitic rather than viral infections. Furthermore, Qβ-VLPs contain RNA, a ligand for TLR 3/7, which also drives Th1 and IgG2a responses. Thus, the combination of high cross-linking activity with TLR7 ligands may be able to block IgE memory responses long term. Indeed, some of our preliminary results indicated that the shutdown of the allergen-specific IgE response was the result of the single-stranded RNA within the VLPs. Finally, available antigen may be limited in Qβ–Fel d1-vaccinated mice as a result of the presence of high anti–Fel d1 IgG titers abolishing the IgE response. The abrogated IgE response upon vaccination with Qβ–Fel d1 may have important clinical implications because exposure to allergen results in increased IgE levels in allergic individuals, which may worsen the allergy. Shutting down this allergen-induced IgE response may therefore facilitate long-term curing of the patients. Indeed, seasonal allergen exposure usually boosts IgE responses in allergic individuals, maintaining or even worsening the disease.
The observation that repetitive display of Fel d1 on the VLPs resulted in reduced ability to trigger mast cell degranulation may seem somewhat unexpected. Allergens, which are typically not multivalent, are not able to strongly cross-link IgE-FcεRI on mast cells. Hence, allergens displayed on VLPs are nonphysiological ligands for mast cells and may, therefore, not be able to properly activate the degranulation cascade. In fact, it is well known that supraoptimal levels of IgE cross-linking on mast cells lead to reduced rather than enhanced degranulation (Daëron and Lesourne, 2006). Furthermore, because of the linkage of the allergen to the relatively large VLPs (30 nm), the in vivo distribution will be fundamentally different from free Fel d1. It is important to note that the failure of Qβ–Fel d1 to trigger mast cell degranulation is not the result of a failure of IgE antibodies to recognize the vaccine because both polyclonal and a panel of monoclonal Fel d1–specific IgE antibodies recognized Fel d1 on Qβ (unpublished data). Hence, the nonreactogenicity of Qβ–Fel d1 is not caused by an absence of recognition by IgE antibodies but by a failure to stimulate mast cells in a productive way.
In this context, T reg cells have been recently described as a regulator of mast cell activity by altering IgE receptor expression and signaling (Kashyap et al., 2008). T reg cells may also activate mast cells to mediate local immune suppression (Lu et al., 2006). In addition, the regulatory cytokine IL-10, which is produced by T reg cells, has been shown to silence mast cell function in vivo and in vitro (Grimbaldeston et al., 2007; Kennedy Norton et al., 2008). In contrast to these studies, we have found that our VLP-based vaccination primarily works through the induction of allergen-specific IgGs rather than T reg cells. This is based on our findings that transferred IgG antibodies could block mast cell degranulation in the mouse skin and that depletion of T reg cells with pc61 did not affect Qβ–Fel d1-mediated desensitization. It is known that administration of pc61 leads to incomplete T reg cell depletion because CD4+CD25−Foxp3+ cells cannot be depleted and may remain functionally present in vivo (Zelenay et al., 2005; Couper et al., 2007). We therefore performed experiments in mice in which all CD4+ T cells were depleted and, again, saw no effect on desensitization. Furthermore, IL-10 was also not required for the Qβ–Fel d1-induced desensitization. Although successful immunotherapy in humans may involve both T and B cells (Till et al., 2004), as well as induction of T reg cells producing IL-10 (Francis et al., 2003; Robinson et al., 2004), our data demonstrate that these mediators are not important in the immediate type of allergic response upon Qβ–Fel d1 treatment in our mouse model.
Antigen-specific IgG1 was shown to induce anaphylaxis in several in vitro and in vivo studies (Ovary et al., 1970; Oettgen et al., 1994; Oshiba et al., 1996; Dombrowicz et al., 1997; Miyajima et al., 1997; Ujike et al., 1999). Yet we find in this paper that polyclonal antibodies specific for Fel d1 dominated by the IgG1 isotype not only fail to sensitize mice for anaphylaxis but even prevent IgE-mediated anaphylaxis upon allergen challenge. This difference may have several explanations. In the studies where IgG1-mediated anaphylaxis was observed, the reaction was induced by passive transfer of either antigen-specific IgE or IgG. These elegant studies clearly demonstrate that either activation of FcεRI–IgE or FcγRIII–IgG1 upon passive transfer and antigen stimulation was able to induce anaphylaxis in mice. Interestingly, IgG1-mediated active systemic anaphylaxis has so far only been demonstrated in FcεRI−/− mice, which show increased anaphylactic responses caused by up-regulation of FcγRIII receptor expression (Dombrowicz et al., 1997; Miyajima et al., 1997). Under these nonphysiological conditions, IgG1 antibodies may preferentially bind to the FcγRIII, activating mast cell degranulation. In contrast to these studies, we used active sensitization with Fel d1 in WT mice. Under these conditions, mast cells/basophils express all relevant Fc receptors on their surface and IgG–Ag immune complexes will also bind to the inhibitory FcγRIIb, inhibiting IgE-mediated anaphylaxis. In addition, vaccination of sensitized mice results in specific IgG1 as well as IgG2a antibodies. The presence of Fel d1–specific IgG2a antibodies may have masked a potential ability of IgG1 antibodies to trigger anaphylactic reactions.
The results with FcγRIIB-deficient mice offer an explanation for how allergen-specific IgGs may inhibit allergic reactions. Interestingly, we found that IgG antibodies may inhibit the allergic reaction dependent on the allergen localization. The inhibitory FcγRIIb was essential for IgG-mediated inhibition of anaphylaxis upon systemic administration of the allergen. This suggests that because of the quick diffusion upon systemic exposure complete neutralization of the allergen may not be efficient by the circulating antibodies and some Fel d1 molecules will reach basophils. Nevertheless, Fel d1 will be complexed with antigen-specific IgG, engaging cell-bound FcγRIIb, capable of blunting FcεRI-mediated signals in WT mice. In contrast, local stimulation of mast cells in the skin was inhibited by polyclonal IgG antibodies even in the absence of negative FcγRIIb signaling. This suggests that Fel d1, which slowly diffuses within tissues, is recognized by specific IgG antibodies, covering up all available epitopes on the allergen, and may even result in local immune complexes, sequestering the allergen. Hence, Fel d1–specific IgG antibodies may simply neutralize the allergen in the periphery. Indeed, earlier studies with blood from allergic patients have suggested that IgG4 antibodies might exhibit blocking activities in tissues as they compete with IgE for binding to mast cells (García et al., 1993). It has also been shown that serum obtained from subjects after antigen-specific immunotherapy could inhibit IgE-facilitated presentation of allergen to grass pollen–specific (van Neerven et al., 1999; van Neerven et al., 2004) or birch pollen–specific T cells (Wachholz et al., 2003; Nouri-Aria et al., 2004). Thus, anti–Fel d1-specific IgGs could also compete with IgE for antigen binding, inhibiting activation of mast cells in tissues in the absence of an inhibitory FcγIIb receptor. In summary, our data demonstrate that allergens displayed on VLPs have the potential to rapidly treat established allergies and delineate two different mechanisms how IgG antibodies are able to block type I allergic reactions.
MATERIALS AND METHODS
Mice
BALB/c mice were purchased (Harlan) at the age of 6 wk and BALB/c FcγRIIb−/− mice (N12; Taconic) were purchased at the age of 5–7 wk. BALB/c IL-10−/− mice were provided by M. Kopf (Swiss Federal Institute, Zürich, switzerland) and by U. Eriksson (University Hospital, Zürich, Switzerland). All animals were kept under specific pathogen-free conditions at the Biosupport AG Wagistrasse, Zürich-Schlieren. All animals were used for experimentation according to protocols approved by the Swiss Federal Veterinary Office.
Cloning, expression, purification, and coupling of rFel d1
A covalent fusion of rFel d1 was generated as previously described (Grönlund et al., 2003) with modifications. In brief, a complementary DNA encoding a covalent dimer of chain 2 and chain 1 of Fel d1 spaced by a 15aa-linker (GGGGS)x3 was obtained by PCR amplification using sets of overlapping DNA-primers. This complementary DNA was cloned in frame into a modified version of pET-42a(+) (EMD), leading to the addition of the coding sequence for LEHHHHHHGGC at the C terminus of the covalent dimer. This sequence contains a His tag for purification, followed by a GGC linker used for the coupling of the protein to Qβ (pET-42T × Fel d1-15aa-HC). The plasmid pET-42T × Fel d1-15aa-HC was transformed into the E. coli strain BL21(DE3). Expression of rFel d1 was induced at 20°C with 1 mM IPTG. After 20 h, cells were harvested, resuspended in native lysis buffer (50 mM NaH2PO4, 300 mM NaCl, and 10 mM imidazol, pH 8.0) and disrupted by sonification. rFel d1 was Ni2+ affinity purified, and native folding was achieved by intramolecular reshuffling of the disulfide bonds with oxidized glutathione (GSSG; Applichem) and reduced glutathione (GSH; Applichem) at a molar ratio of 1:1. The reaction was performed for 24 h immediately after elution of rFel d1 in the elution buffer by adding 2.5 mM GSSG and 2.5 mM GSH at room temperature. Refolded rFel d1 was further purified to homogeneity by size-exclusion chromatography (Superdex 75 pg; GE Healthcare) equilibrated in PBS.
VLP derived from the bacteriophage Qβ were expressed in E. coli strain JM109 harboring the expression plasmid pQ10 and purified as previously described (Cielens et al., 2000). For coupling, Qβ-VLPs were first reacted at room temperature for 30 min with a fivefold molar excess of the heterobifunctional chemical cross-linker succinimidyl-6-(b-maleimidopropionamide) hexanoate. Nonreacted cross-linker was removed by dialysis against 150 mM PBS, pH 7.4. A 1:1 ratio of rFel d1 and SMPH-derivatized Qβ-VLP was incubated for 4 h at room temperature while shaking. After covalent coupling, noncoupled rFel d1 was removed by gel filtration. The vaccine was analyzed by SDS-PAGE, and immunoblotting with anti-Qβ and anti-penta His antibodies indicated free Qβ-VLP and Qβ–Fel d1 coupling bands. The intensities of Coomassie blue–stained Qβ–Fel d1 coupling bands were used to calculate coupling efficiency by densitometry.
Human basophil degranulation assay
100 µl of heparinized human whole blood of cat allergic and nonallergic donors was incubated with 20 µl of basophil stimulation buffer, with or without the indicated rFel d1 or Qβ–Fel d1 for 20 min at 37°C. 2 mM fMLP was used as a positive control for maximal basophil degranulation. The reaction was stopped at 4°C, and cells were stained with anti–CD63-FITC, anti–CD123-PE, and anti–HLA-DR-PerCP antibodies for 20 min. 2 ml FACS lysis solution (BD) were added to lyse erythrocytes and to fix blood cells. After a 15-min incubation at room temperature, cells were washed twice with PBS and analyzed by flow cytometry. Degranulation was detected via CD63 surface expression on CD123+ HLA-DR− cells (Sanz et al., 2002; FastImmune Assay kit; BD).
Determination of antigen-specific IgG and IgE antibodies
For determination of Fel d1–specific IgG, 96-well ELISA plates (MaxiSorp; Nunc) were coated either with 5 µg rFel d1 or with 2 µg Qβ-VLPs in Carbonate buffer at 4°C overnight. After blocking with 2% PBS/BSA solution for 2 h, plates were washed five times with PBS/0.05% Tween. Serial dilutions of sera were added to the plates and incubated for 2 h at room temperature. Plates were than washed five times with PBS/0.05% Tween (PBST). Thereafter, HRP0-labeled goat anti–mouse IgG (Jackson ImmunoResearch Laboratories) antibodies were incubated at room temperature for 2 h, followed by addition of the substrate OPD (O-Phenylenediamine dihydrochloride; Sigma-Aldrich). Optical densities were measured at 450 nm. For determination of IgE antibodies, a special ELISA was established. 96-well plates were coated with 2 µg/ml of rat anti–mouse IgE mAb (BD) diluted in carbonate buffer at 4°C overnight. After washing with PBST and blocking with PBS/5% BSA solution for 2 h at room temperature, sera of individual mice was prediluted 1:20 and then serially diluted 1:3 and incubated for 2 h at room temperature. Thereafter, each well was incubated with 500 ng/ml rFel d1, diluted in 0.1% PBS/BSA for 30 min at room temperature. After washing with PBST, plates were incubated with an anti–Fel d1 IgG-biotin antibody (Indoors Biotechnologies) for 1 h at room temperature, followed by incubation with HRPO-labeled Streptavidin (BD) for a further 30 min at RT. After a final wash with PBST, OPD substrate was added and incubated for at least 30 min at room temperature before optical densities were measured at 450 nm. Half-maximal antibody titers are defined as the reciprocal of the dilution leading to half of the OD measured at saturation.
Limulus amebocyte lysate (LAL) test
The LAL endochrome-K assay (Endosafe; Charles River Laboratories) was performed according to the manufacturer's protocol. For standard curve preparation, lyophilized control endotoxin standard was dissolved and diluted 10× in LAL reagent endotoxin-free water starting with 50 EU in the first step. All standard dilutions were prepared in pyrogen-free glass tubes. In parallel, samples, including rFel d1 and Qβ–Fel d1 batches, were also diluted 10× starting with a 1:100 dilution in the first step. After standard and sample preparation, each 100 µl of the standard and sample dilutions was transferred to a 96-well microplate in duplicates. As further control, a standard-spiked sample was used in which 10 µl of standard was added to each 100-µl sample dilution. After transfer of standards and samples, the microtiter plate was incubated for 10 min at 37°C. In the meantime, substrate solutions were prepared and 100 µl of substrate was added to each well starting with the lowest concentration. The kinetic measurement with 30-s intervals was performed at OD405 nm.
Immunization, sensitization, and vaccination
To test the immunogenicity of the vaccine, 6-wk-old naive BALB/c mice were immunized s.c. either with rFel d1 coupled to Qβ-VLP (50 µg Qβ–Fel d1) or with a mixture of rFel d1 and Qβ-VLP (30 µg Qβ + 20 µg rFel d1) on days 0 and 14. For Fel d1 sensitization, 6-wk-old naive mice were injected i.p. with 1 µg of natural Fel d1 (Indoors Biotechnologies) mixed in 200 µl Alum (10 mg/ml Al(OH)3; Alhydrogel; Brenntag Biosector). Alternatively, mice were sensitized with a mixture of 1 µg Fel d1 and 1 µg OVA (Grade V; Sigma-Aldrich) diluted in 200 µl Alum. For efficacy experiments, sensitized mice were vaccinated once s.c. either with PBS, 50 µg Qβ, or Qβ–Fel d1 in 200 µl PBS, 4–5 wk after sensitization.
rFel d1 antigen challenge in vivo
Ear prick tests.
Mice were injected i.v. with 200 µl of Evans blue solution (0.5%; Sigma-Aldrich) and anesthetized 30 min later with isoflurane. Afterward a drop of rFel d1 solution (10 µg/20 µl PBS) was placed onto the outer ear skin. Pricks through the ear skin were performed with 23G (0.6 mm × 25 mm) needles (Microlance; BD). For intradermal ear prick tests, an rFel d1 solution (0.6, 2.5, and 10 µg/10 µl PBS) was injected intradermally into the ears. Dye extravasations started immediately after antigen challenge. 1 hr later, mice were sacrificed and ears were prepared from individual mice for evaluation of dye leakage by densitometry. To this end, the area of dye leakage was defined within the densitometer by gating around the leaked dye. A combination of size and color intensity of the pricked area defines the strength of dye leakage per ear, which is expressed as net intensity by the densitometer. Mean net intensity of different mice per group is shown.
Acute systemic anaphylaxis.
For the induction of anaphylaxis, sensitized mice were challenged i.v. with 1 µg rFel d1/200 µl PBS. Temperature was measured with a rectal probe digital thermometer (Thermalert TH-5, RET-3; Physitemp Instruments INC) immediately after i.v. antigen challenge and monitored for a maximum of 100 min after challenge. For statistical analysis, the area above the curve was determined using Prism (GraphPad Software, Inc.).
Analysis of immediate type allergic responses.
Sensitized mice were challenged i.p. with 10 µg rFel d1 in 300 µl PBS 12 d after vaccination with Qβ or Qβ–Fel d1. Immediately after i.p. challenge, typical signs of anaphylactic reactions, including activity, ear, feet, and skin redness, or swelling, were monitored. Pictures were taken 40 min after rFel d1 administration. 1 h after i.p. antigen challenge, mice were sacrificed and albumin levels and cellular infiltrates in the peritoneum were determined as described elsewhere (Sun et al., 2007). In brief, peritoneal lavages were centrifuged and cell counts determined from the cell pellet with a Coulter Counter (Instrumenten Gesellschaft AG). The content of albumin was quantified from peritoneal lavage supernatants with a commercial Bicinchoninic Acid kit (Sigma-Aldrich) using a BSA standard curve. Albumin levels of naive mice, challenged with rFel d1 antigen, were used as background levels. Serum histamine was determined from plasma of individual mice with an enzyme immunoassay kit (DRG Instruments GmbH).
ELISPOT assays
Sensitized BALB/c mice were challenged either i.p. or i.v. with 10 µg rFel d1 diluted in PBS, 2 wk after Qβ- and Qβ–Fel d1 vaccination. Sensitized and nonvaccinated mice, challenged either with PBS or rFel d1, were used as control mice. 7 d after antigen challenge, spleens of individual mice were isolated and single cell suspensions were prepared in IMDM medium, supplemented with 10% FCS and antibiotics. For IFN-γ ELISPOT assays, spleen cells of individual mice were threefold titrated in 96-well round bottom plates starting with 3 × 106 cells in the first well. Cells were stimulated for 2 d with 200 µl Fel d1 (10 µg/ml), 200 µl PMA/ionomycin (1mM/1mg/ml), or medium alone. After 2 d of incubation at 37°C and 7% CO2, cells were transferred on IFN-γ–coated and FCS-blocked ELISPOT plates (MultiScreen 96-well plates; Millipore), followed by incubation for a further 24 h at 37°C. For IL-4 ELISPOT assays, spleen cells of individual mice were plated directly on IL-4–coated and FCS-blocked ELISPOT plates, followed by incubation of 6 d at 37°C. Alternatively to the usage of whole splenocytes, CD4+ T cells isolated by magnetic cell sorting (MACS microbeads; Miltenyi Biotec) were also used for IFN-γ and IL-4 ELISPOT assays. After incubation of cells, ELISPOT assays were performed according to standard protocol procedures. In brief, cells were flicked off the plates and washed five times with PBS/1% Tween 20, followed by incubation with biotinylated anti–IFN-γ or anti–IL-4 antibodies for 2 h at room temperature. Afterward, plates were washed five times with PBS/1% Tween 20 and incubated with Streptavidin-AP for a further 30 min before the substrate (alkaline phosphatase) was added. The substrate reaction was stopped after 8 min by rinsing plates with tap water. Plates were dried overnight and ELISPOTs were counted in each well.
Depletion of CD4+ and T reg cells and serum transfer
T reg cells were depleted with an anti-CD25 mAb (clone pc61; provided by A. Gallimore, Cardiff University, Cardiff, Wales) by two i.p. administrations of 250 µg of antibody, 3 and 1 d before antigen challenge. Control mice received isotype control Abs (clone GL113; A. Gallimore). CD4+ T lymphocytes were depleted with an anti-CD4 mAb (clone YTS 191) by two i.p. administration of 200 µg of antibody, 2 and 1 d before antigen challenge. Control mice received PBS. Depletion of the respective cell populations (CD4+foxp3+CD25+ or CD4+ cells) was determined by flow cytometry 1 d after the last injection of the antibodies. With these treatments a reduction of the respective cell types between 95 and 99% was achieved.
For transfer experiments, serum of sensitized and either Qβ- or Qβ–Fel d1-vaccinated mice was collected on several days after vaccination for 5 wk. Collected serum was pooled and anti-Qβ and Fel d1 antibody titer were determined by ELISA as described in an earlier Materials and methods section. Before transfer, complement activity in serum needs to be inactivated. To this end, serum was inactivated at 56°C for 30 min just before i.p. Qβ or Fel d1 serum transfer was performed, 4 wk after their sensitization. The presence of antigen-specific IgG antibodies in recipient mice was confirmed by ELISA. 3 d after serum transfer, prick tests with rFel d1 were performed as described above.
Statistical analysis
All statistical analysis comparing two different groups were performed with two tailed Student's t tests. The resulting p-values are either indicated in figure legends or with asterisks in figures (*, P < 0.05; **, P < 0.005; ***, P < 0.0005). For statistical analysis of anaphylaxis experiments, individual areas above the curves were determined and compared by one-way ANOVA using Bonferroni's multiple comparison post tests (*, P < 0.01; **, P < 0.001; ***, P < 0.0001).
Online supplemental material
Fig. S1 shows Fel d1–specific IgG1 and IgG2a titers in mice immunized with Fel d1 mixed or coupled to Qβ and Fel d1–specific IgE levels in mice immunized with Fel d1 coupled to Qβ. These parameters were measure in the samples from mice shown in Fig. 1 C. Fig. S2 shows the quantification of LPS in Fel d1 and Qβ–Fel d1 samples used for the in vivo experiments. Fig. S3 shows a time course of the IgG subclasses from the experiment shown in Fig. 4 F. Figs. S4 and S5 refer to the experiment shown in Fig. 5 and illustrate the efficiency of the depletion of CD25- and CD4-positive cells, respectively, after injection of monoclonal antibodies. Fig. S6 shows a similar anaphylaxis experiment as the one shown in Fig. 7 with WT and FcγRIIB−/− mice but under challenge conditions which lead to similar anaphylaxis in both mouse strains. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20090199/DC1.
Acknowledgments
We thank Prof. Dr. Manfred Kopf and Prof. Dr. U. Eriksson for providing BALB/c IL-10−/− mice and we thank Prof. Dr. A. Gallimore for providing anti-CD25 pc61 mAbs.
All authors are employees of Cytos Biotechnology AG and hold stocks or stock options in the company. The authors have no additional financial interests.
Footnotes
Abbreviations used: ANOVA, analysis of variance; LAL, limulus amebocyte lysate; T reg cell, regulatory T cell; VLP, virus-like particle.
References
- Akdis M., Blaser K., Akdis C.A. 2005. T regulatory cells in allergy: novel concepts in the pathogenesis, prevention, and treatment of allergic diseases.J. Allergy Clin. Immunol. 116:961–968 [DOI] [PubMed] [Google Scholar]
- Ambühl P.M., Tissot A.C., Fulurija A., Maurer P., Nussberger J., Sabat R., Nief V., Schellekens C., Sladko K., Roubicek K., et al. 2007. A vaccine for hypertension based on virus-like particles: preclinical efficacy and phase I safety and immunogenicity.J. Hypertens. 25:63–72 [DOI] [PubMed] [Google Scholar]
- Andersson T.N., Ekman G.J., Grönlund H., Buentke E., Eriksson T.L., Scheynius A., Van Hage-Hamsten M., Gafvelin G. 2004. A novel adjuvant-allergen complex, CBP-rFel d 1, induces up-regulation of CD86 expression and enhances cytokine release by human dendritic cells in vitro.Immunology. 113:253–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann M.F., Rohrer U.H., Kündig T.M., Bürki K., Hengartner H., Zinkernagel R.M. 1993. The influence of antigen organization on B cell responsiveness.Science. 262:1448–1451 [DOI] [PubMed] [Google Scholar]
- Bischoff S.C. 2007. Role of mast cells in allergic and non-allergic immune responses: comparison of human and murine data.Nat. Rev. Immunol. 7:93–104 [DOI] [PubMed] [Google Scholar]
- Bousquet J., Lockey R., Malling H.J. 1998. Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paper.J. Allergy Clin. Immunol. 102:558–562 [DOI] [PubMed] [Google Scholar]
- Bruhns P., Frémont S., Daëron M. 2005. Regulation of allergy by Fc receptors.Curr. Opin. Immunol. 17:662–669 [DOI] [PubMed] [Google Scholar]
- Cielens I., Ose V., Petrovskis I., Strelnikova A., Renhofa R., Kozlovska T., Pumpens P. 2000. Mutilation of RNA phage Qbeta virus-like particles: from icosahedrons to rods.FEBS Lett. 482:261–264 [DOI] [PubMed] [Google Scholar]
- Couper K.N., Blount D.G., de Souza J.B., Suffia I., Belkaid Y., Riley E.M. 2007. Incomplete depletion and rapid regeneration of Foxp3+ regulatory T cells following anti-CD25 treatment in malaria-infected mice.J. Immunol. 178:4136–4146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J.C., Coulter A.R. 1997. Adjuvants—a classification and review of their modes of action.Vaccine. 15:248–256 [DOI] [PubMed] [Google Scholar]
- Daëron M., Lesourne R. 2006. Negative signaling in Fc receptor complexes.Adv. Immunol. 89:39–86 [DOI] [PubMed] [Google Scholar]
- Daëron M., Latour S., Malbec O., Espinosa E., Pina P., Pasmans S., Fridman W.H. 1995a. The same tyrosine-based inhibition motif, in the intracytoplasmic domain of Fc gamma RIIB, regulates negatively BCR-, TCR-, and FcR-dependent cell activation.Immunity. 3:635–646 [DOI] [PubMed] [Google Scholar]
- Daëron M., Malbec O., Latour S., Arock M., Fridman W.H. 1995b. Regulation of high-affinity IgE receptor-mediated mast cell activation by murine low-affinity IgG receptors.J. Clin. Invest. 95:577–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrowicz D., Flamand V., Miyajima I., Ravetch J.V., Galli S.J., Kinet J.P. 1997. Absence of Fc epsilonRI alpha chain results in upregulation of Fc gammaRIII-dependent mast cell degranulation and anaphylaxis. Evidence of competition between Fc epsilonRI and Fc gammaRIII for limiting amounts of FcR beta and gamma chains.J. Clin. Invest. 99:915–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham S.R., Till S.J. 1998. Immunologic changes associated with allergen immunotherapy.J. Allergy Clin. Immunol. 102:157–164 [DOI] [PubMed] [Google Scholar]
- Forsbach A., Nemorin J.G., Völp K., Samulowitz U., Montino C., Müller C., Tluk S., Hamm S., Bauer S., Lipford G.B., Vollmer J. 2007. Characterization of conserved viral leader RNA sequences that stimulate innate immunity through TLRs.Oligonucleotides. 17:405–417 [DOI] [PubMed] [Google Scholar]
- Forsbach A., Nemorin J.G., Montino C., Müller C., Samulowitz U., Vicari A.P., Jurk M., Mutwiri G.K., Krieg A.M., Lipford G.B., Vollmer J. 2008. Identification of RNA sequence motifs stimulating sequence-specific TLR8-dependent immune responses.J. Immunol. 180:3729–3738 [DOI] [PubMed] [Google Scholar]
- Francis J.N., Larché M. 2005. Peptide-based vaccination: where do we stand? Curr. Opin. Allergy Clin. Immunol. 5:537–543 [DOI] [PubMed] [Google Scholar]
- Francis J.N., Till S.J., Durham S.R. 2003. Induction of IL-10+CD4+CD25+ T cells by grass pollen immunotherapy.J. Allergy Clin. Immunol. 111:1255–1261 [DOI] [PubMed] [Google Scholar]
- García B.E., Sanz M.L., Gato J.J., Fernández J., Oehling A. 1993. IgG4 blocking effect on the release of antigen-specific histamine.J. Investig. Allergol. Clin. Immunol. 3:26–33 [PubMed] [Google Scholar]
- Georas S.N., Guo J., De Fanis U., Casolaro V. 2005. T-helper cell type-2 regulation in allergic disease.Eur. Respir. J. 26:1119–1137 [DOI] [PubMed] [Google Scholar]
- Grimbaldeston M.A., Nakae S., Kalesnikoff J., Tsai M., Galli S.J. 2007. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B.Nat. Immunol. 8:1095–1104 [DOI] [PubMed] [Google Scholar]
- Grönlund H., Bergman T., Sandström K., Alvelius G., Reininger R., Verdino P., Hauswirth A., Liderot K., Valent P., Spitzauer S., et al. 2003. Formation of disulfide bonds and homodimers of the major cat allergen Fel d 1 equivalent to the natural allergen by expression in Escherichia coli.J. Biol. Chem. 278:40144–40151 [DOI] [PubMed] [Google Scholar]
- Hedlin G., Graff-Lonnevig V., Heilborn H., Lilja G., Norrlind K., Pegelow K.O., Sundin B., Løwenstein H. 1986. Immunotherapy with cat- and dog-dander extracts. II. In vivo and in vitro immunologic effects observed in a 1-year double-blind placebo study.J. Allergy Clin. Immunol. 77:488–496 [DOI] [PubMed] [Google Scholar]
- Hedlin G., Graff-Lonnevig V., Heilborn H., Lilja G., Norrlind K., Pegelow K., Sundin B., Lowenstein H. 1991. Immunotherapy with cat- and dog-dander extracts. V. Effects of 3 years of treatment.J. Allergy Clin. Immunol. 87:955–964 [DOI] [PubMed] [Google Scholar]
- Hessel E.M., Chu M., Lizcano J.O., Chang B., Herman N., Kell S.A., Wills-Karp M., Coffman R.L. 2005. Immunostimulatory oligonucleotides block allergic airway inflammation by inhibiting Th2 cell activation and IgE-mediated cytokine induction.J. Exp. Med. 202:1563–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holgate S.T. 1999. The epidemic of allergy and asthma.Nature. 402:B2–B4 [DOI] [PubMed] [Google Scholar]
- Hulett M.D., McKenzie I.F., Hogarth P.M. 1993. Chimeric Fc receptors identify immunoglobulin-binding regions in human Fc gamma RII and Fc epsilon RI.Eur. J. Immunol. 23:640–645 [DOI] [PubMed] [Google Scholar]
- Jegerlehner A., Storni T., Lipowsky G., Schmid M., Pumpens P., Bachmann M.F. 2002a. Regulation of IgG antibody responses by epitope density and CD21-mediated costimulation.Eur. J. Immunol. 32:3305–3314 [DOI] [PubMed] [Google Scholar]
- Jegerlehner A., Tissot A., Lechner F., Sebbel P., Erdmann I., Kündig T., Bächi T., Storni T., Jennings G., Pumpens P., et al. 2002b. A molecular assembly system that renders antigens of choice highly repetitive for induction of protective B cell responses.Vaccine. 20:3104–3112 [DOI] [PubMed] [Google Scholar]
- Jegerlehner A., Maurer P., Bessa J., Hinton H.J., Kopf M., Bachmann M.F. 2007. TLR9 signaling in B cells determines class switch recombination to IgG2a.J. Immunol. 178:2415–2420 [DOI] [PubMed] [Google Scholar]
- Kanzler H., Barrat F.J., Hessel E.M., Coffman R.L. 2007. Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists.Nat. Med. 13:552–559 [DOI] [PubMed] [Google Scholar]
- Kashyap M., Thornton A.M., Norton S.K., Barnstein B., Macey M., Brenzovich J., Shevach E., Leonard W.J., Ryan J.J. 2008. Cutting edge: CD4 T cell-mast cell interactions alter IgE receptor expression and signaling.J. Immunol. 180:2039–2043 [DOI] [PubMed] [Google Scholar]
- Katz H.R. 2002. Inhibitory receptors and allergy.Curr. Opin. Immunol. 14:698–704 [DOI] [PubMed] [Google Scholar]
- Kennedy Norton S., Barnstein B., Brenzovich J., Bailey D.P., Kashyap M., Speiran K., Ford J., Conrad D., Watowich S., Moralle M.R., et al. 2008. IL-10 suppresses mast cell IgE receptor expression and signaling in vitro and in vivo.J. Immunol. 180:2848–2854 [DOI] [PubMed] [Google Scholar]
- Kraft S., Kinet J.P. 2007. New developments in FcepsilonRI regulation, function and inhibition.Nat. Rev. Immunol. 7:365–378 [DOI] [PubMed] [Google Scholar]
- Kündig T.M., Senti G., Schnetzler G., Wolf C., Prinz Vavricka B.M., Fulurija A., Hennecke F., Sladko K., Jennings G.T., Bachmann M.F. 2006. Der p 1 peptide on virus-like particles is safe and highly immunogenic in healthy adults.J. Allergy Clin. Immunol. 117:1470–1476 [DOI] [PubMed] [Google Scholar]
- Lechner F., Jegerlehner A., Tissot A.C., Maurer P., Sebbel P., Renner W.A., Jennings G.T., Bachmann M.F. 2002. Virus-like particles as a modular system for novel vaccines.Intervirology. 45:212–217 [DOI] [PubMed] [Google Scholar]
- Lu L.F., Lind E.F., Gondek D.C., Bennett K.A., Gleeson M.W., Pino-Lagos K., Scott Z.A., Coyle A.J., Reed J.L., Van Snick J., et al. 2006. Mast cells are essential intermediaries in regulatory T-cell tolerance.Nature. 442:997–1002 [DOI] [PubMed] [Google Scholar]
- Maurer P., Jennings G.T., Willers J., Rohner F., Lindman Y., Roubicek K., Renner W.A., Müller P., Bachmann M.F. 2005. A therapeutic vaccine for nicotine dependence: preclinical efficacy, and Phase I safety and immunogenicity.Eur. J. Immunol. 35:2031–2040 [DOI] [PubMed] [Google Scholar]
- Miyajima I., Dombrowicz D., Martin T.R., Ravetch J.V., Kinet J.P., Galli S.J. 1997. Systemic anaphylaxis in the mouse can be mediated largely through IgG1 and Fc gammaRIII. Assessment of the cardiopulmonary changes, mast cell degranulation, and death associated with active or IgE- or IgG1-dependent passive anaphylaxis.J. Clin. Invest. 99:901–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muta T., Kurosaki T., Misulovin Z., Sanchez M., Nussenzweig M.C., Ravetch J.V. 1994. A 13-amino-acid motif in the cytoplasmic domain of Fc gamma RIIB modulates B-cell receptor signalling.Nature. 369:340. [DOI] [PubMed] [Google Scholar]
- Niederberger V., Horak F., Vrtala S., Spitzauer S., Krauth M.T., Valent P., Reisinger J., Pelzmann M., Hayek B., Kronqvist M., et al. 2004. Vaccination with genetically engineered allergens prevents progression of allergic disease.Proc. Natl. Acad. Sci. USA. 101:14677–14682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn F., Ravetch J.V. 2008. Fcgamma receptors as regulators of immune responses.Nat. Rev. Immunol. 8:34–47 [DOI] [PubMed] [Google Scholar]
- Nouri-Aria K.T., Wachholz P.A., Francis J.N., Jacobson M.R., Walker S.M., Wilcock L.K., Staple S.Q., Aalberse R.C., Till S.J., Durham S.R. 2004. Grass pollen immunotherapy induces mucosal and peripheral IL-10 responses and blocking IgG activity.J. Immunol. 172:3252–3259 [DOI] [PubMed] [Google Scholar]
- Oettgen H.C., Martin T.R., Wynshaw-Boris A., Deng C., Drazen J.M., Leder P. 1994. Active anaphylaxis in IgE-deficient mice.Nature. 370:367–370 [DOI] [PubMed] [Google Scholar]
- Oshiba A., Hamelmann E., Takeda K., Bradley K.L., Loader J.E., Larsen G.L., Gelfand E.W. 1996. Passive transfer of immediate hypersensitivity and airway hyperresponsiveness by allergen-specific immunoglobulin (Ig) E and IgG1 in mice.J. Clin. Invest. 97:1398–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovary Z., Vaz N.M., Warner N.L. 1970. Passive anaphylaxis in mice with gamma-G antibodies. V. Competitive effects of different immunoglobulins and inhibition of reactions with antiglobulin sera.Immunology. 19:715–727 [PMC free article] [PubMed] [Google Scholar]
- Robinson D.S., Larché M., Durham S.R. 2004. Tregs and allergic disease.J. Clin. Invest. 114:1389–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarne T., Kaiser L., Grönlund H., Rasool O., Gafvelin G., van Hage-Hamsten M. 2005. Rational design of hypoallergens applied to the major cat allergen Fel d 1.Clin. Exp. Allergy. 35:657–663 [DOI] [PubMed] [Google Scholar]
- Sanz M.L., Maselli J.P., Gamboa P.M., Oehling A., Diéguez I., de Weck A.L. 2002. Flow cytometric basophil activation test: a review.J. Investig. Allergol. Clin. Immunol. 12:143–154 [PubMed] [Google Scholar]
- Spohn G., Schwarz K., Maurer P., Illges H., Rajasekaran N., Choi Y., Jennings G.T., Bachmann M.F. 2005. Protection against osteoporosis by active immunization with TRANCE/RANKL displayed on virus-like particles.J. Immunol. 175:6211–6218 [DOI] [PubMed] [Google Scholar]
- Sun J., Arias K., Alvarez D., Fattouh R., Walker T., Goncharova S., Kim B., Waserman S., Reed J., Coyle A.J., Jordana M. 2007. Impact of CD40 ligand, B cells, and mast cells in peanut-induced anaphylactic responses.J. Immunol. 179:6696–6703 [DOI] [PubMed] [Google Scholar]
- Takai T., Ono M., Hikida M., Ohmori H., Ravetch J.V. 1996. Augmented humoral and anaphylactic responses in Fc gamma RII-deficient mice.Nature. 379:346–349 [DOI] [PubMed] [Google Scholar]
- Terada T., Zhang K., Belperio J., Londhe V., Saxon A. 2006. A chimeric human-cat Fcgamma-Fel d1 fusion protein inhibits systemic, pulmonary, and cutaneous allergic reactivity to intratracheal challenge in mice sensitized to Fel d1, the major cat allergen.Clin. Immunol. 120:45–56 [DOI] [PubMed] [Google Scholar]
- Till S.J., Francis J.N., Nouri-Aria K., Durham S.R. 2004. Mechanisms of immunotherapy.J. Allergy Clin. Immunol. 113:1025–1034 [DOI] [PubMed] [Google Scholar]
- Tissot A.C., Maurer P., Nussberger J., Sabat R., Pfister T., Ignatenko S., Volk H.D., Stocker H., Müller P., Jennings G.T., et al. 2008. Effect of immunisation against angiotensin II with CYT006-AngQb on ambulatory blood pressure: a double-blind, randomised, placebo-controlled phase IIa study.Lancet. 371:821–827 [DOI] [PubMed] [Google Scholar]
- Ujike A., Ishikawa Y., Ono M., Yuasa T., Yoshino T., Fukumoto M., Ravetch J.V., Takai T. 1999. Modulation of immunoglobulin (Ig)E-mediated systemic anaphylaxis by low-affinity Fc receptors for IgG.J. Exp. Med. 189:1573–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Neerven R.J., Wikborg T., Lund G., Jacobsen B., Brinch-Nielsen A., Arnved J., Ipsen H. 1999. Blocking antibodies induced by specific allergy vaccination prevent the activation of CD4+ T cells by inhibiting serum-IgE-facilitated allergen presentation.J. Immunol. 163:2944–2952 [PubMed] [Google Scholar]
- van Neerven R.J., Arvidsson M., Ipsen H., Sparholt S.H., Rak S., Würtzen P.A. 2004. A double-blind, placebo-controlled birch allergy vaccination study: inhibition of CD23-mediated serum-immunoglobulin E-facilitated allergen presentation.Clin. Exp. Allergy. 34:420–428 [DOI] [PubMed] [Google Scholar]
- Vieira P., Rajewsky K. 1988. The half-lives of serum immunoglobulins in adult mice.Eur. J. Immunol. 18:313–316 [DOI] [PubMed] [Google Scholar]
- Wachholz P.A., Durham S.R. 2004. Mechanisms of immunotherapy: IgG revisited.Curr. Opin. Allergy Clin. Immunol. 4:313–318 [DOI] [PubMed] [Google Scholar]
- Wachholz P.A., Soni N.K., Till S.J., Durham S.R. 2003. Inhibition of allergen-IgE binding to B cells by IgG antibodies after grass pollen immunotherapy.J. Allergy Clin. Immunol. 112:915–922 [DOI] [PubMed] [Google Scholar]
- Zelenay S., Lopes-Carvalho T., Caramalho I., Moraes-Fontes M.F., Rebelo M., Demengeot J. 2005. Foxp3+ CD25- CD4 T cells constitute a reservoir of committed regulatory cells that regain CD25 expression upon homeostatic expansion.Proc. Natl. Acad. Sci. USA. 102:4091–4096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D., Kepley C.L., Zhang K., Terada T., Yamada T., Saxon A. 2005. A chimeric human-cat fusion protein blocks cat-induced allergy.Nat. Med. 11:446–449 [DOI] [PubMed] [Google Scholar]