Abstract
BACKGROUND
Fetal exposure of animals to antiepileptic drugs at doses lower than those required to produce congenital malformations can produce cognitive and behavioral abnormalities, but cognitive effects of fetal exposure of humans to antiepileptic drugs are uncertain.
METHODS
Between 1999 and 2004, we enrolled pregnant women with epilepsy who were taking a single antiepileptic agent (carbamazepine, lamotrigine, phenytoin, or valproate) in a prospective, observational, multicenter study in the United States and the United Kingdom. The primary analysis is a comparison of neurodevelopmental outcomes at the age of 6 years after exposure to different antiepileptic drugs in utero. This report focuses on a planned interim analysis of cognitive outcomes in 309 children at 3 years of age.
RESULTS
At 3 years of age, children who had been exposed to valproate in utero had significantly lower IQ scores than those who had been exposed to other antiepileptic drugs. After adjustment for maternal IQ, maternal age, antiepileptic-drug dose, gestational age at birth, and maternal preconception use of folate, the mean IQ was 101 for children exposed to lamotrigine, 99 for those exposed to phenytoin, 98 for those exposed to carbamazepine, and 92 for those exposed to valproate. On average, children exposed to valproate had an IQ score 9 points lower than the score of those exposed to lamotrigine (95% confidence interval [CI], 3.1 to 14.6; P = 0.009), 7 points lower than the score of those exposed to phenytoin (95% CI, 0.2 to 14.0; P = 0.04), and 6 points lower than the score of those exposed to carbamazepine (95% CI, 0.6 to 12.0; P = 0.04). The association between valproate use and IQ was dose dependent. Children’s IQs were significantly related to maternal IQs among children exposed to carbamazepine, lamotrigine, or phenytoin but not among those exposed to valproate.
CONCLUSIONS
In utero exposure to valproate, as compared with other commonly used antiepileptic drugs, is associated with an increased risk of impaired cognitive function at 3 years of age. This finding supports a recommendation that valproate not be used as a first-choice drug in women of childbearing potential.
Women with epilepsy are at increased risk for poor pregnancy outcomes, although most of their children are normal.1 In animals, fetal exposure to antiepileptic drugs at doses lower than those that result in structural malformations can produce behavioral and cognitive deficits, alter neurochemistry, and reduce brain weight.2,3 The effects of in utero exposure to antiepileptic drugs in humans may contribute to poor neurodevelopmental outcomes,4 but this risk must be balanced against potentially grave risks to both mother and fetus posed by seizures.5 Antiepileptic drugs may have differential risks in pregnancy, but studies have been lacking to guide the choice of antiepileptic drugs in women who are pregnant or may become pregnant. The most recent consensus guidelines by the American Academy of Neurology,6 the American College of Obstetricians and Gynecologists,7 and the International League against Epilepsy8 do not distinguish among antiepileptic drugs in terms of teratogenetic risks. We performed a cohort study to assess the neurodevelopmental outcomes of children who were exposed in utero to one of several antiepileptic drugs.
METHODS
STUDY DESIGN
The Neurodevelopmental Effects of Antiepileptic Drugs (NEAD) study is a prospective observational study that enrolled pregnant women who were using any of several antiepileptic-drug monotherapies from October 1999 through February 2004 in 25 epilepsy centers in the United States and the United Kingdom. Separate investigations in the United States and the United Kingdom, which collaborated during design, were later combined. The primary outcome is cognitive performance of the children at 6 years of age. Here we report a planned interim analysis conducted when the children were 3 years of age.
PARTICIPANTS
The institutional review boards at each center approved the study, and written informed consent was obtained from the women before enrollment. Pregnant women with epilepsy who were receiving monotherapy with carbamazepine, lamotrigine, phenytoin, or valproate were enrolled. Women taking other antiepileptic drugs were not included because of insufficient numbers of patients. Women receiving polytherapy were not included because of the association of polytherapy with poorer outcomes1 and the inability of the study to assess multiple possible combinations of drugs. A control group of nonexposed children was not included, at the direction of a National Institutes of Health review panel. Mothers with IQ scores below 70 were excluded to avoid floor effects and because maternal IQ is the major predictor of child IQ.9 Other exclusion criteria included positive serologic tests for syphilis or human immunodeficiency virus, progressive cerebral disease, other major disease (e.g., diabetes), exposure to teratogenic agents other than antiepileptic drugs, poor adherence to antiepileptic-drug use, drug abuse in the previous year, and drug-abuse sequelae.
PROCEDURES
Information was collected on potentially confounding variables, including maternal IQ, age at delivery, education, employment, race or ethnic group, type of epilepsy, type and frequency of seizures, antiepileptic-drug dose, compliance with antiepileptic therapy, socioeconomic status,10 preconception use of folate, and use of alcohol, tobacco, or other drugs during pregnancy; whether the site was located in the United Kingdom or the United States; whether the current pregnancy was unwanted; abnormalities or complications in the current pregnancy or previous pregnancies; gestational age at enrollment and at birth; weight of the infant at birth; whether the child was breast-fed; and the child’s history of disease. Assessors who were unaware of drug exposure evaluated cognitive outcomes with the use of the Mental Developmental Index of the Bayley Scales of Infant Development, second edition11 (conducted in children from 21 to 34 months of age) and Differential Ability Scales12 (conducted in children from 33 to 45 months of age); standardized scores were calculated. Several measures were used to determine maternal IQ because of the later merger of data from the U.S. and United Kingdom sites; these measures included the Test of Nonverbal Intelligence13 in 267 mothers, the Wechsler Abbreviated Scale of Intelligence14 in 18, and the National Adult Reading Test15 in 18. The neuropsychological evaluations were monitored to ensure quality and consistency. Each assessor was given face-to-face training on all neuropsychological test batteries annually. The assessor was required to identify errors in administration and scoring from videotaped test sessions and provide appropriate corrections. In addition, for each test instrument, the assessors submitted videotapes of themselves administering the test and record forms from the test to the neuropsychology core director for review, feedback, and approval. Assessors whose performance was not approved were required to submit additional videotapes and records for approval before they were allowed to test children in the study.
STATISTICAL ANALYSIS
The primary analysis was conducted on the intention-to-treat principle. There were 309 live births, including 6 twins. Secondary analyses were conducted of children who completed testing at either 2 or 3 years of age or at both ages (258 children), at 3 years only (232 children), and at 2 years only (187 children). Analyses were performed at the NEAD data and statistical center with the use of SAS software, version 9.1, and R software, version 2.7.1.
Linear regression models were used to examine group differences in IQ after adjustment for maternal IQ, standardized maternal antiepileptic-drug dose, maternal age at delivery, gestational age of the infant at delivery, and maternal preconception use of folate. Additional covariates were type of epilepsy; type and frequency of seizures during pregnancy; maternal education, employment, race or ethnic group, socioeconomic status, alcohol and tobacco use, and adherence to antiepileptic-drug use; whether the site was located in the United States or the United Kingdom; weight of the infant at birth; whether the pregnancy was unwanted; whether the infant was breast-fed; birth defects or complications in previous pregnancies; and complications of the current pregnancy. The dose of antiepileptic drug was standardized relative to the ranges observed within each group according to the formula 100 × (observed dose – minimum dose) ÷ range of doses. The a priori hypothesis was that the specific antiepileptic drug used, the dose, and maternal IQ were important covariates, and thus these were included as predictors in a linear model with child IQ as the outcome. Other covariates were added individually to the model and were included if they were found to be significant (P<0.05) or if they did not exhibit collinearity with existing predictors according to inspection of condition indexes16; that is, maternal age, gestational age of the infant at delivery, and maternal preconception use of folate were included in the model along with the antiepileptic drug (four categories), drug dose, and maternal IQ. When they were added to the model, none of the baseline variables substantively changed inferences regarding differences related to the type of antiepileptic drug.
Data were available on seizure frequency for 275 mothers (90.1%), breast-feeding for 217 mothers (71.6%), antiepileptic-drug adherence for 237 mothers (78.2%), and birth weight for 308 infants (99.7%). The data for the remaining covariates were complete. Outcomes at 3 years of age were missing for 77 children (24.9%). IQ scores measured at 2 and 3 years of age were highly correlated (r = 0.70, P<0.001) in the 161 children who were tested at both ages. Markov-chain Monte Carlo methods for monotone missing data17–19 were used in primary and secondary analyses to impute missing outcomes for 3-year-old children. In the primary analysis, missing outcomes for children at 3 years of age were imputed from the outcomes at 2 years for those tested at 2 years of age (26 children) or from baseline variables related to IQ outcome or to the probability of having missing IQ-outcome data (51 children). Baseline variables in the imputation model included type and dose of antiepileptic drug, maternal IQ and age, infant’s gestational age at delivery, maternal preconception use of folate, maternal socioeconomic status, and whether the site was in the United States or the United Kingdom. In the secondary analysis of the 258 children who completed testing at 2 or 3 years or at both ages, 26 missing outcomes were imputed from the outcomes at 2 years. Standard errors and confidence intervals of parameter estimates incorporated uncertainty of imputation. Least-squares mean IQ scores were estimated for each group after adjustment for maternal IQ and age, dose of antiepileptic drug, infant’s gestational age at delivery, and maternal preconception use of folate.
To investigate whether baseline differences explain the association of valproate with poorer cognitive outcomes, post hoc subgroup analyses were conducted and forest plots were created. Subgroups were defined by individual covariates related to valproate exposure and propensity scores.20 Covariates related to valproate exposure were identified with the use of logistic-regression models with valproate group as outcome and baseline variables as individual predictors. Forest plots were created for each individual baseline variable when the valproate group showed differences from the other antiepileptic-drug groups.
Propensity scores are predicted probabilities of receiving a treatment given baseline covariates. Covariates are approximately equally distributed within subgroups defined by propensity scores.20,21 Propensity scores were estimated with the use of predicted probabilities from a logistic-regression model, with exposure to valproate as the outcome. Variables related to valproate exposure (Table 1) were predictors in the propensity-score model, along with variables significantly related to IQ at 3 years of age.22 Given the resulting distributions of estimated propensity scores in the two groups (subjects exposed and those not exposed to valproate), the subjects were initially partitioned into two subgroups depending on whether their estimated propensity score was above or below the median estimated propensity score for subjects exposed to valproate. The below-median propensity group was further classified according to type of epilepsy (localization-related, idiopathic generalized, or generalized tonic–clonic) to remove residual imbalances in this covariate. Within each of the four resulting subgroups, covariates were balanced between the groups exposed and those not exposed to valproate (P>0.05 by the t-test for continuous variables or chi-square test for categorical variables), which permitted us to compare mean IQ outcomes in the valproate group with those in the other three groups with the use of forest plots.
Table 1.
Baseline Characteristics of the 303 Mothers According to Antiepileptic Drug Used during Pregnancy.*
| Characteristic | Carbamazepine (N = 92) | Lamotrigine (N = 99) | Phenytoin (N = 52) | Valproate (N = 60)† |
|---|---|---|---|---|
| mean (95% CI) | ||||
| Maternal IQ | 99 (95–102) | 101 (98–104) | 92 (88–97) | 96 (92–100) |
| Maternal age at delivery (yr) | 30 (29–31) | 30 (29–31) | 31 (29–32) | 28 (27–30) |
| Dose (mg/day)‡ | 786 (698–873) | 457 (406–507) | 400 (364–435) | 1040 (882–1197) |
| Standardized dose§ | 32 (29–36) | 35 (31–39) | 49 (44–54) | 26 (22–31) |
| Gestational age of infant at delivery (wk) | 39 (38–39) | 39 (39–40) | 39 (38–39) | 39 (39–40) |
| no./total no. (%) | ||||
| Preconception folate use | 54/92 (59) | 59/99 (60) | 21/52 (40) | 38/60 (63) |
| United Kingdom site | 36/92 (39) | 29/99 (29) | 7/52 (13) | 31/60 (52) |
| Breast-feeding | 27/62 (44) | 34/73 (47) | 21/46 (46) | 11/36 (31) |
| Epilepsy type¶ | ||||
| Localization-related | 80/92 (87) | 51/99 (52) | 40/52 (77) | 12/60 (20) |
| Idiopathic generalized | 7/92 (8) | 39/99 (39) | 8/52 (15) | 42/60 (70) |
| Generalized tonic–clonic seizures | 5/92 (5) | 9/99 (9) | 4/52 (8) | 6/60 (10) |
| No. of convulsions during pregnancy | ||||
| 0 | 71/81 (88) | 68/86 (79) | 40/49 (82) | 45/59 (76) |
| >5 | 3/81 (4) | 1/86 (1) | 3/49 (6) | 1/59 (2) |
The maternal racial or ethnic distribution was 80.2% white, 10.2% Hispanic, 4.6% black, and 5.0% other. Race or ethnic group was self- reported.
The valproate group differed significantly from the other groups combined in maternal age, standardized dose of antiepileptic drug, presence or absence of breast-feeding, U.S. versus United Kingdom site, and type of epilepsy (P<0.05, odds ratio from logistic-regression model with membership in the valproate group as the response variable).
The average dose during the pregnancy is given.
The dose was standardized relative to the ranges observed within each group according to the formula 100 × (observed dose - minimum dose) ÷ range of doses.
There were three types of epilepsy: localization-related, idiopathic generalized, and generalized tonic–clonic (uncertain whether partial or generalized). Of the seizures that occurred, 60% were partial (simple, complex, or secondary generalized tonic–clonic), 32% were generalized (absence, myoclonic, tonic–clonic, or tonic seizures with initial bilateral cerebral involvement as indicated by electroencephalogram or clinical syndrome), and 8% were generalized tonic–clonic (uncertain whether partial or generalized). All partial seizures were localization- related epilepsies (40% symptomatic and 60% cryptogenic). All generalized seizures were idiopathic epilepsies (17% genetic, 6% juvenile myoclonic, 5% absence, and 72% idiopathic and not otherwise classified).
RESULTS
Cognitive assessments were conducted in 258 children (of 252 mothers) at either 2 or 3 years of age or at both ages. Of these children, 73 had been exposed to carbamazepine, 84 to lamotrigine, 48 to phenytoin, and 53 to valproate. This sample constituted 83.5% of the initial (i.e., intention-to-treat) group of 309 live births (93 exposed to carbamazepine, 100 to lamotrigine, 55 to phenytoin, and 61 to valproate) from 303 mothers. The different sample sizes reflect the different frequencies of antiepileptic-drug use in NEAD centers. The original United Kingdom study design did not include testing at 2 years of age, and 63 United Kingdom children passed this age before the study was merged with the U.S. study. Assessments included 187 children at 2 years of age and 232 children at 3 years of age. There were no significant differences among the four antiepileptic-drug groups in the frequency of missing data or the method of assessment of maternal IQ.
Table 1 summarizes maternal demographic information. Logistic-regression analyses showed significant associations between the use of valproate and maternal age, dose of antiepileptic drug, type of seizure, type of epilepsy, whether the site was in the United States or the United Kingdom, and presence or absence of breast-feeding.
Table 2 presents the IQ scores and group differences for the children at 3 years of age according to the antiepileptic drug to which they were exposed in utero.23 Table 1 in the Supplementary Appendix (available with the full text of this article at NEJM.org) summarizes results of the regression models for the primary analysis. Significant independent predictors of child IQ included antiepileptic drug, maternal IQ, maternal age, standardized dose of antiepileptic drug, gestational age at birth, and maternal preconception use of folate. No other variables were significant when they were added to the model. During pregnancy, 2 women changed their antiepileptic drug and 12 added a second antiepileptic drug; the results were materially unchanged after these women were excluded from the study. The results of analyses of children assessed at either 2 or 3 years of age or at both ages (258 children) were similar to those assessed at 3 years of age (232 children) (Tables 2 and 3 in the Supplementary Appendix). The results of analyses of the group of children who had cognitive assessments at 2 years of age (187 children) are shown in Table 4 in the Supplementary Appendix (P = 0.08 for the association between antiepileptic drug and IQ).
Table 2.
IQ Scores of Children at 3 Years of Age According to In Utero Exposure to Antiepileptic Drugs.*
| Variable | Carbamazepine (N = 73) | Lamotrigine (N = 84) | Phenytoin (N = 48) | Valproate (N = 53) |
|---|---|---|---|---|
| Mean IQ (95% CI)† | 98 (95–102) | 101 (98–104) | 99 (94–104) | 92 (88–97) |
| Mean difference in IQ from valproate group (95% CI)‡ | 6 (0.6–12.0) | 9 (3.1–14.6) | 7 (0.2–14.0) | |
| P value§ | 0.04 | 0.009 | 0.04 |
The results are based on regression models for the intention-to-treat population (309 children). See Table 1 in the Supplementary Appendix for full results of the regression models. IQ at 3 years of age was imputed for 77 of the original 309 children born alive who were not assessed at that age (1 of these children died from severe heart malformation, 6 were enrolled in the NEAD study from the United Kingdom study after they had reached 3 years of age, 31 withdrew before 3 years of age, and 39 did not present for testing).
Least-squares means from the primary analysis are given after adjustment for maternal IQ and age, antiepileptic-drug dose, infant’s gestational age at birth, and maternal preconception use of folate.
Although the confidence intervals for carbamazepine and phenytoin overlap with the confidence interval for valproate, the confidence intervals for the differences between carbamazepine and valproate and between phenytoin and valproate do not include zero.
P values are for the comparison with the valproate group. P values from tests of the null hypothesis of no difference from the valproate-group mean were adjusted for multiple comparisons.23
Children exposed to valproate in utero had lower IQs than did children exposed to any of the other antiepileptic drugs (Table 2). On average, children exposed to valproate had an IQ score 9 points lower than those exposed to lamotrigine (95% confidence interval [CI], 3.1 to 14.6; P = 0.009), 7 points lower than those exposed to phenytoin (95% CI, 0.2 to 14.0; P = 0.04), and 6 points lower than those exposed to carbamazepine (95% CI, 0.6 to 12.0; P = 0.04). IQ scores did not differ significantly among children exposed to any of the other three antiepileptic drugs (P = 0.68). The association of valproate with poor cognitive outcomes persisted after adjustment for confounders with the use of two approaches: linear regression analysis and subgroup analysis with propensity scores and individual confounding variables. In each propensity-score subgroup, the mean IQ for children exposed to valproate is lower than the mean IQ for children exposed to any of the other antiepileptic drugs (Fig. 1 and 2, and Fig. 1 through 4 in the Supplementary Appendix). The pattern in the forest plots implies that baseline differences do not explain the association of valproate with poor IQ outcome.
Figure 1. IQ Scores of Children Who Were Exposed to Antiepileptic Drugs In Utero, According to Drug and Dose.
The means (black squares) and 95% confidence intervals (horizontal lines) are given for the children’s IQ as a function of antiepileptic drug and dose. The children’s IQs were adjusted for factors in the primary model, except dose. Low doses are below the median for each antiepileptic drug, and high doses are equal to or above the median. The median doses were 750 mg per day for carbamazepine, 433 mg per day for lamotrigine, 398 mg per day for phenytoin, and 1000 mg per day for valproate. The vertical line is the mean IQ for all children, and the size of the black squares is proportional to the sample size.
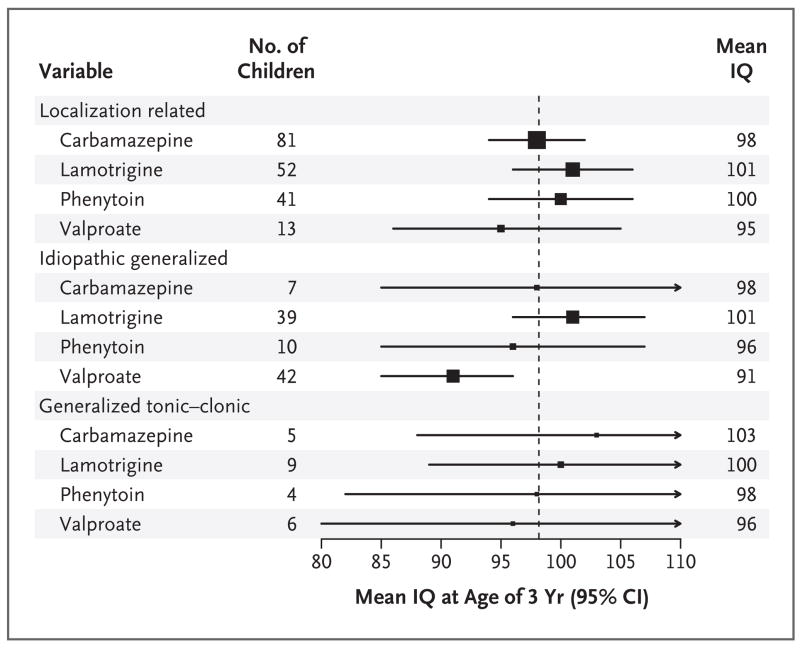
Figure 2. IQ Scores of Children Who Were Exposed to Antiepileptic Drugs In Utero, According to Drug and Type of Maternal Epilepsy.
The means (black squares) and 95% confidence intervals (horizontal lines) are given for the children’s IQ as a function of antiepileptic drug and type of maternal epilepsy. The children’s IQs were adjusted for factors in the primary model. The vertical line is the mean IQ for all children, and the size of the black squares is proportional to the sample size.
In analyses assessing associations between the average dose of an antiepileptic drug in pregnancy and a child’s IQ at the age of 3 years, only the dose of valproate was significantly correlated (inversely) with IQ (r = −0.38, P = 0.005) (see Fig. 5 in the Supplementary Appendix for the relation between the drug dose and the difference in IQ between child and mother). The results were similar when average doses in the third trimester, rather than those throughout pregnancy, were included in the model. Among the subgroup of 38 women in whom blood levels of valproate were measured, the dose was correlated with the blood level (r = 0.42, P = 0.008), but the level was not significantly associated with the child’s IQ in this small sample. IQs did not differ significantly among children exposed to different antiepileptic drugs when the analysis was restricted to children exposed to low doses (below the median) (Fig. 1).
Higher maternal IQs were significantly associated with higher IQs of children overall and within each antiepileptic-drug group except valproate (Fig. 3). Children with major malformations (previously described24) had lower IQs, but this effect did not explain the differences in cognitive outcomes associated with exposure to different antiepileptic drugs.
Figure 3. Relationship between Child and Maternal IQ According to Antiepileptic Drug.
Child IQ at 3 years of age and maternal IQ are significantly correlated for all antiepileptic drugs except valproate. The data are from an intention-to-treat sample of 309 children. IQ was imputed for 77 children.
DISCUSSION
In utero exposure to valproate was associated with poorer cognitive outcomes than was exposure to other commonly used antiepileptic drugs. The effect of valproate was dose dependent. As in previous population studies,9 maternal IQ was strongly related to child IQ except for mothers who took valproate during pregnancy, a result suggesting that valproate disrupted this normally robust relationship.
The strengths of our study include its prospective design, blinded cognitive assessments with the use of standardized measures, and detailed monitoring of multiple potential confounding factors. Its limitations include its relatively small sample, lack of randomization, and lack of a control group of unexposed children. The relatively young age of the children at this planned interim analysis is an additional limitation, and we plan to obtain more detailed assessments of the children at the age of 6 years.
Because the NEAD study is not a randomized trial, a potential concern is that the results may be due to confounding factors related to baseline characteristics that might affect the child’s IQ. For example, antiepileptic-drug assignment was not randomized, and therefore the largest proportion of mothers taking valproate had idiopathic generalized epilepsy. However, the association between maternal valproate use and poor cognitive outcomes in the children persisted in analyses adjusted for many baseline characteristics, including the propensity analyses.
Our results are consistent with those of previous investigations. Retrospective studies in the United Kingdom found poorer outcomes among children exposed to valproate in utero as compared with children who were unexposed and with those exposed to other antiepileptic-drug monotherapies.5,25 These poor outcomes for valproate included increased developmental delays in a cohort younger than 6 years of age, increased special-education needs in a cohort 5 to 18 years of age, and reduced verbal IQ in a cohort 6 to 16 years of age, as compared with unexposed children (7 points), children exposed to carbamazepine (10 points), and children exposed to phenytoin (15 points). A prospective Finnish study reported a reduction in verbal IQ among children exposed to valproate as compared with unexposed controls (13 points) and with children exposed to carbamazepine (14 points).26 This prospective study was limited by a small sample of subjects exposed to valproate monotherapy (13 children) and lack of information on maternal IQ.26
Several studies have also shown that valproate is associated with an increased risk of congenital malformations24,27–32; this association is dose-dependent.24–26,28,30 A recent meta-analysis indicated that of various antiepileptic drug monotherapies, valproate was associated with the highest incidence of malformations (10.7%; 95% CI, 8.2 to 13.3).33 The results of several studies showing an increased incidence of anatomical or behavioral teratogenesis in children exposed to valproate as compared with other antiepileptic drugs raise serious concern that valproate poses a particular risk to the unborn child.
Additional studies are needed to better define the risks associated with antiepileptic drugs and to understand the underlying mechanisms. Data currently being collected in registries of pregnant women taking antiepileptic drugs (e.g., the North American Antiepileptic Drug Pregnancy Registry and the International Registry of Antiepileptic Drugs and Pregnancy [EURAP]) should provide further information on the risks associated with antiepileptic drugs and modifiers of these risks.
The present results, together with other data, suggest that valproate should not be used as a first-line antiepileptic drug in pregnant women or — since data indicate that half of pregnancies are unplanned34 — in women of childbearing potential. Our finding that associations between the use of valproate in pregnancy and lower IQ in the offspring are dose-dependent suggests that lower doses may be safe. However, it should be recognized that there is considerable individual variability among children exposed to similar doses.
For some patients, valproate is the only medication that adequately controls seizures. Such women should be informed of the potential risks associated with the use of this medication in pregnancy. If a woman taking valproate is already pregnant, it is critical that she not stop valproate without consultation with her physician, since stopping an antiepileptic drug could lead to seizures and serious consequences for both the woman and her fetus.
Fewer than half of antiepileptic-drug prescriptions are written for epilepsy or seizures; the majority are intended for pain management and psychiatric indications. Although our study did not include women who were prescribed antiepileptic drugs for indications other than seizure, a previous report suggested that the risk of malformations in the offspring of these women is similar to that of children of women taking antiepileptic drugs for epilepsy.35
In summary, our interim analysis of the NEAD study indicates that the maternal use of valproate during pregnancy is associated with an increased risk of cognitive impairment in children at 3 years of age. This information is relevant to counseling women of reproductive age regarding this drug class.
Supplementary Material
Acknowledgments
Supported by grants from the National Institute of Neurological Disorders and Stroke (R01NS038455 and R01NS050659) and the United Kingdom Epilepsy Research Foundation (RB219738).
We thank the children and families who have given their time to participate in the NEAD study.
APPENDIX
The members of the NEAD Study Group are as follows: Arizona Health Sciences Center, Tucson: D. Labiner, J. Moon, S. Sherman; Baylor Medical Center, Irving, TX: D.T. Combs-Cantrell; University of Texas Southwestern Medical Center, Dallas: C. Silver; Case Western Reserve University, Cleveland: M. Goyal, M.R. Schoenberg; Columbia University, New York: A. Pack, C. Palmese, J. Echo; Emory University, Atlanta: K.J. Meador, D. Loring, P. Pennell, D. Drane, E. Moore, M. Denham, C. Epstein, J. Gess, S. Helmers, T. Henry; Georgetown University, Washington, DC: G. Motamedi, E. Flax; Harvard Medical School and Brigham and Women’s Hospital, Boston: E. Bromfield, K. Boyer, B. Dworetzky; Harvard Medical School and Massachusetts General Hospital, Boston: A. Cole, L. Halperin, S. Shavel-Jessop; Henry Ford Hospital, Detroit: G. Barkley, B. Moir; Medical College of Cornell University, New York: C. Harden, T. Tamny-Young; Medical College of Georgia, Augusta: G. Lee, M. Cohen; Minnesota Epilepsy Group, St. Paul: P. Penovich, D. Minter; Ohio State University, Columbus: L. Moore, K. Murdock; Riddle Health Care, Media, PA: J. Liporace, K. Wilcox; Rush University Medical Center, Chicago: A. Kanner, M.N. Nelson; Comprehensive Epilepsy Care Center for Children and Adults, St. Louis: W. Rosenfeld, M. Meyer; St. Mary’s Hospital, Manchester, United Kingdom: J. Clayton-Smith, G. Mawer, U. Kini; University of Alabama, Birmingham: R. Martin; University of Cincinnati, Cincinnati: M. Privitera, J. Bellman, D. Ficker; University of Kansas School of Medicine, Wichita: L. Baade, K. Liow; University of Liverpool, Merseyside, United Kingdom: D. Chadwick, G. Baker, A. Booth, R. Bromley, S. Dutton, J. Kelly, J. Mallows, L. McEwan, L. Purdy; University of Miami, Miami: E. Ramsay, P. Arena; University of Southern California, Los Angeles: L. Kalayjian, C. Heck, S. Padilla; University of Washington, Seattle: J. Miller, G. Rosenbaum, A. Wilensky; University of Utah, Salt Lake City: T. Constantino, J. Smith; Walton Centre for Neurology and Neurosurgery, Liverpool, United Kingdom: N. Adab, Gisela Veling-Warnke; Wake Forest University, Winston-Salem, NC: M. Sam, C. O’Donovan, C. Naylor, S. Nobles, C. Santos, W. Bell. Executive Committee: Dartmouth Medical School, Hanover, NH: G. Holmes; Stanford University, Stanford, CA: M. Druzin, M. Morrell, L. Nelson; Texas A&M University Health Science Center, Houston: R. Finnell; University of Oregon, Portland: M. Yerby; University of Toronto, Toronto: K. Adeli, P. Wells. Data and Statistical Center: EMMES Corporation, Rockville, MD: T. Blalock, N. Browning, T. Crawford, L. Hendrickson, B. Jolles, M.K. Kunchai, M. LaMotteo, C. Murray-Krezan, S. Russell, C. Servis, J. Winestone, M. Wolff, P. Zaia, T. Zajdowicz.
Footnotes
Dr. Meador reports receiving research support from the McKnight Brain Institute, the MCG Foundation, GlaxoSmithKline, Eisai Medical Research, Myriad Pharmaceuticals, Marinus Pharmaceuticals, NeuroPace, SAM Technology, and UCB Pharma and serving on the professional advisory board of the Epilepsy Foundation. Dr. Baker reports serving on paid advisory boards for UCB Pharma; receiving lecture fees from Pfizer, UCB Pharma, and Janssen; receiving grant support from Sanofi-Aventis and Pfizer; and serving as an expert witness in litigation related to neurodevelopmental effects of antiepileptic drugs. Dr. Clayton-Smith reports serving as an expert witness in litigation related to neurodevelopmental effects of antiepileptic drugs. Dr. Combs-Cantrell reports receiving lecture fees from GlaxoWellcome. Dr. Kalayjian reports receiving lecture fees from GlaxoSmithKline and Ortho-McNeil and grant support from Marinus Pharmaceuticals. Dr. Kanner reports receiving lecture fees from GlaxoSmithKline, Ortho-McNeil, Pfizer, and UCB Pharma; advisory-board fees from GlaxoSmithKline, Ortho-McNeil, Valeant Pharmaceuticals, and UCB Pharma; and grant support from GlaxoSmithKline and Novartis. Dr. Liporace reports serving on the professional board of the Epilepsy Foundation of Eastern Pennsylvania. Dr. Pennell reports serving on the Keppra Pregnancy Registry Expert Panel (UCB Pharma) and receiving grant support from UCB Pharma, Marinus Pharmaceuticals, and GlaxoSmithKline. Dr. Privitera reports receiving consulting and advisory-board fees from Ortho-McNeil, UCB Pharma, Johnson & Johnson, and the National EpiFellows Foundation; lecture fees from Ortho-McNeil, Pfizer, GlaxoSmithKline, Janssen, and UCB Pharma; and grant support from UCB Pharma, Ortho-McNeil, and the American Epilepsy Society. Dr. Loring reports receiving consulting fees from UCB Pharma, NeuroPace, and Sanofi-Aventis and grant support from Myriad Pharmaceuticals, SAM Technology, and Novartis. No other potential conflict of interest relevant to this article was reported.
References
- 1.Pennell PB. Pregnancy in women who have epilepsy. Neurol Clin. 2004;22:799–820. doi: 10.1016/j.ncl.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Fisher JE, Vorhees C. Developmental toxicity of antiepileptic drugs: relationship to postnatal dysfunction. Pharmacol Res. 1992;26:207–21. doi: 10.1016/1043-6618(92)90210-3. [DOI] [PubMed] [Google Scholar]
- 3.Gaily E, Meador KJ. Neurodevelopmental effects. In: Engel J Jr, Pedley TA, editors. Epilepsy: a comprehensive textbook. 2. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 1225–33. [Google Scholar]
- 4.Finnell RH, Nau H, Yerby MS. General principles: teratogenicity of antiepileptic drugs. In: Levy RH, Mattson RH, Meldrum BS, editors. Antiepileptic drugs. 4. New York: Raven Press; 1995. pp. 209–30. [Google Scholar]
- 5.Adab N, Kini U, Vinten J, et al. The longer term outcome of children born to mothers with epilepsy. J Neurol Neurosurg Psychiatry. 2004;75:1575–83. doi: 10.1136/jnnp.2003.029132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Practice parameter: management issues for women with epilepsy (summary statement) — report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 1998;51:944–8. doi: 10.1212/wnl.51.4.944. [DOI] [PubMed] [Google Scholar]
- 7.Committee on Educational Bulletins of the American College of Obstetricians and Gynecologists. ACOG educational bulletin: seizure disorders in pregnancy. Int J Gynaecol Obstet. 1997;56:279–86. [PubMed] [Google Scholar]
- 8.Commission on Genetics, Pregnancy and the Child, International League against Epilepsy. Guidelines for the care of women of childbearing age with epilepsy. Epilepsia. 1993;34:588–9. doi: 10.1111/j.1528-1157.1993.tb00431.x. [DOI] [PubMed] [Google Scholar]
- 9.Sattler JM. Assessment of children. 3. San Diego, CA: Jerome M. Sattler; 1992. [Google Scholar]
- 10.Hollingshead AB. Four factor index of social status. New Haven, CT: Yale University; 1975. [Google Scholar]
- 11.Bayley N. Bayley scales of infant development. 2. San Antonio, TX: Psychological Corporation; 1993. [Google Scholar]
- 12.Elliott CD. Differential ability scales. San Antonio, TX: Psychological Corporation; 1990. [Google Scholar]
- 13.Brown L, Sherbenou RJ, Johnsen SK. Test of nonverbal intelligence. 3. Austin, TX: Pro-Ed; 1996. [Google Scholar]
- 14.Wechsler D. Wechsler abbreviated scale of intelligence (WASI) San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- 15.Nelson H, Willison J. National adult reading test (NART) Oxford, England: NFER–Nelson Publishing; 1991. [Google Scholar]
- 16.Belsley DA. Conditioning diagnostics, collinearity and weak data in regression. New York: Wiley; 1991. [Google Scholar]
- 17.Little RJA, Rubin DB. Statistical analysis with missing data. 2. New York: John Wiley; 2002. [Google Scholar]
- 18.Li KH. Imputation using Markov chains. J Stat Comput Simul. 1988;30:57–79. [Google Scholar]
- 19.Schafer JL. Analysis of incomplete multivariate data. New York: Chapman & Hall; 1997. [Google Scholar]
- 20.Rosenbaum P, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 21.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–81. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 22.Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Stürmer T. Variable selection for propensity score models. Am J Epidemiol. 2006;163:1149–56. doi: 10.1093/aje/kwj149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hochberg Y. A sharper Bonferroni procedure for multiple significance testing. Biometrika. 1988;75:800–3. [Google Scholar]
- 24.Meador KJ, Baker GA, Finnell RH, et al. In utero antiepileptic drug exposure: fetal death and malformations. Neurology. 2006;67:407–12. doi: 10.1212/01.wnl.0000227919.81208.b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adab N, Jacoby A, Smith D, Chadwick D. Additional educational needs in children born to mothers with epilepsy. J Neurol Neurosurg Psychiatry. 2001;70:15–21. doi: 10.1136/jnnp.70.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaily E, Kantola-Sorsa E, Hiilesmaa V, et al. Normal intelligence in children with prenatal exposure to carbamazepine. Neurology. 2004;62:28–32. doi: 10.1212/wnl.62.1.28. [DOI] [PubMed] [Google Scholar]
- 27.Wyszynski DF, Nambisan M, Surve T, Alsdorf RM, Smith CR, Holmes LB. Increased rate of major malformations in offspring exposed to valproate during pregnancy. Neurology. 2005;64:961–5. doi: 10.1212/01.WNL.0000154516.43630.C5. [DOI] [PubMed] [Google Scholar]
- 28.Vajda FJ, Eadie MJ. Maternal valproate dosage and foetal malformations. Acta Neurol Scand. 2005;112:137–43. doi: 10.1111/j.1600-0404.2005.00458.x. [DOI] [PubMed] [Google Scholar]
- 29.Wide K, Winbladh B, Källén B. Major malformations in infants exposed to antiepileptic drugs in utero, with emphasis on carbamazepine and valproic acid: a nationwide population-based register study. Acta Paediatr. 2004;93:174–6. doi: 10.1080/08035250310021118. [DOI] [PubMed] [Google Scholar]
- 30.Artama M, Auvinen A, Raudaskoski T, Isojärvi I, Isojärvi J. Antiepileptic drug use of women with epilepsy and congenital malformations in offspring. Neurology. 2005;64:1874–8. doi: 10.1212/01.WNL.0000163771.96962.1F. [DOI] [PubMed] [Google Scholar]
- 31.Morrow J, Russell A, Gutherie E, et al. Malformation risks of antiepileptic drugs in pregnancy: a prospective study from the UK Epilepsy and Pregnancy Register. J Neurol Neurosurg Psychiatry. 2006;77:193–8. doi: 10.1136/jnnp.2005.074203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cunnington M, Tennis P. Lamotrigine and the risk of malformations in pregnancy. Neurology. 2005;64:955–60. doi: 10.1212/01.WNL.0000154515.94346.89. [DOI] [PubMed] [Google Scholar]
- 33.Meador K, Reynolds MW, Crean S, Fahrbach K, Probst C. Pregnancy outcomes in women with epilepsy: a systematic review and meta-analysis of published pregnancy registries and cohorts. Epilepsy Res. 2008;81:1–13. doi: 10.1016/j.eplepsyres.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finer LB, Henshaw SK. Disparities in rates of unintended pregnancy in the United States, 1994 and 2001. Perspect Sex Reprod Health. 2006;38:90–6. doi: 10.1363/psrh.38.090.06. [DOI] [PubMed] [Google Scholar]
- 35.Holmes LB, Harvey EA, Coull BA, et al. The teratogenicity of anticonvulsant drugs. N Engl J Med. 2001;344:1132–8. doi: 10.1056/NEJM200104123441504. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.