Abstract
Eye specification in Drosophila is thought be controlled by a set of seven nuclear factors that includes the Pax6 homolog, Eyeless. This group of genes is conserved throughout evolution and has been repeatedly recruited for eye specification. Several of these genes are expressed within the developing eyes of vertebrates and mutations in several mouse and human orthologs are the underlying causes of retinal disease syndromes. Ectopic expression in Drosophila of any one of these genes is capable of inducing retinal development, while loss-of-function mutations delete the developing eye. These nuclear factors comprise a complex regulatory network and it is thought that their combined activities are required for the formation of the eye. We examined the expression patterns of four eye specification genes, eyeless (ey), sine oculis (so), eyes absent (eya), and dachshund (dac) throughout all time points of embryogenesis and show that only eyeless is expressed within the embryonic eye anlagen. This is consistent with a recently proposed model in which the eye primordium acquires its competence to become retinal tissue over several time points of development. We also compare the expression of Ey with that of a putative antennal specifying gene Distal-less (Dll). The expression patterns described here are quite intriguing and raise the possibility that these genes have even earlier and wide ranging roles in establishing the head and visual field.
Keywords: Eye, Evolution, Embryogenesis, Drosophila
Introduction
The specification of the compound eye of the fruit fly, Drosophila melanogaster, has been shown to be under the concerted action of seven nuclear proteins. This group of proteins is comprised of the Pax6 homologs Eyeless (Ey) and Twin of Eyeless (Toy), the Pax2 homolog Eye Gone (Eyg), the Six homologs Sine Oculis (So) and Optix, and two novel genes Dachshund (Dac) and Eyes Absent (Eya, Bonini et al. 1993; Cheyette et al. 1994; Mardon et al. 1994; Quiring et al. 1994; Serikaku and O'Tousa 1994; Jun et al. 1998; Czerny et al. 1999; Seimiya and Gehring 2000). Evidence from genetic, molecular and biochemical studies show that these proteins do not lie in a linear biochemical pathway, but rather form a complicated and interwoven regulatory network. For instance, the transcription of so and eya is activated by the direct binding of Ey protein to upstream promoter sequences within these genes and ectopic expression of eya requires ey expression to induce ectopic eyes (Bonini et al. 1997; Halder et al. 1998; Niimi et al. 1999; Bui et al. 2000; Zimmerman et al. 2000). Additional protein-protein interactions between So, Dac, and Eya have also been shown to occur in in vitro assays (Chen et al. 1997; Pignoni et al. 1997).
Homologs of the Drosophila eye specification genes have been found throughout the animal kingdom (Gehring and Ikeo 1999; Wawersik et al. 2000). Indeed several orthologs of toy, ey, so, eya, and dac are expressed in mouse and human retinas (Ton et al. 1991; Grindley et al. 1995; Oliver et al. 1995; Xu et al. 1997; Zimmerman et al. 1997; Hammond et al. 1998; Caubit et al. 1999; Davis et al. 1999; Leppert et al. 1999; Nishina et al. 1999; Terzic and Saraga-Babic 1999; Winchester et al. 1999; Davis et al. 2001). The expression patterns of these genes suggest that they have been used several times during evolution to specify eye development. Indeed, mutations in the mouse and human homologs of toy, ey, so, and eya, and possibly dac, underlie several severe retinal disease conditions (Hogan et al. 1986; Hill et al. 1991; Abdelhak et al. 1997a,b; Wallis et al. 1999; Azuma et al. 2000; Klesert et al. 2000; Pasquier et al. 2000; Sarkar et al. 2000; Davis et al. 2001).
The widespread use Eyeless/Pax6 in retinal development throughout the animal kingdom has led to the title of “master control gene” for eye specification (Halder et al. 1995b; Gehring 1996; Callaerts et al. 1997). In Drosophila this title has been justified by (1) the deletion of the adult compound eye in loss of function ey mutants, (2) the induction of ectopic eyes by the misexpression of ey, and (3) the ability of the mouse and human homologs of ey to induce retinal development in the fly despite the tremendous evolutionary distance between vertebrates and flies (Quiring et al. 1994; Halder et al. 1995a). Recently, the downstream targets and interacting components of ey have been identified and have been given the same title based on the above criteria (Bonini et al. 1997; Shen and Mardon 1997; Pan and Rubin 1998; Czerny et al. 1999; Seimiya and Gehring 2000).
The evidence from genetic, molecular, and biochemical experiments suggests that these nuclear factors should act together as a transcriptional unit within the retinal primordium to specify the eye. This is also expected if this set of genes does in fact represent master control genes for eye specification. However, it is known that each of the eye specification genes is also expressed in other tissues that do not give rise to retinal tissue (Fasano et al. 1991; Cheyette et al. 1994; Mardon et al. 1994; Quiring et al. 1994; Serikaku and O'Tousa 1994; Bonini et al. 1998; Jones et al. 1998; Leiserson et al. 1998; Czerny et al. 1999; Seimaya and Gehring 2000; Kumar and Moses 2001). Furthermore, since these are without exception nuclear factors, these genes should be expected to receive instructions from upstream signal transduction cascades. We have recently shown that both the epidermal growth factor receptor (Egfr) and Notch signaling cascades lie upstream of the above-mentioned eye specification genes. Hyperactivation of Egfr or downregulation of Notch signaling results in the homeotic transformation of the eye into an antenna (Kumar and Moses 2001). In such cases, the transcription of the eye specification genes is downregulated and is accompanied by the upregulation of antennal specification genes (Kumar and Moses 2001). These two pathways join the Hedgehog and transforming growth factor-beta (TGFb) pathways in regulating the eye specification genes (Chen et al. 1999; Curtiss and Mlodzik 2000; Kumar and Moses 2001).
This dramatic effect on eye and antennal specification surprisingly does not take place during embryogenesis, as was expected based on previous interpretations, but rather it occurs during the latter half of the second larval instar (Younossi-Hartenstein et al. 1993; Kumar and Moses 2001). Concomitant with eye specification at this time point is the co-expression of the Notch receptor and of all eye specification genes within the eye imaginal disc (Kumar and Moses 2001). We therefore argued that the co-expression of all seven master control genes during the second larval instar is crucial for eye specification. This view of eye development is in conflict with established models in which eye specification is thought to take place during embryogenesis. For this to be correct the eye specification genes should be co-expressed within the retinal primordium during its early development. However, at the latest phases of embryogenesis (time point 16) only toy, ey, and eyg have been localized to the embryonic eye imaginal disc primordium (Quiring et al. 1994; Jones et al. 1998; Czerny et al. 1999; Kumar and Moses 2001). They are joined later by the remaining eye specification genes in a spatially and temporally sequential manner (Bui et al. 2000; Kumar and Moses 2001).
The expression patterns of the eye specification genes during early embryonic development is less well described. We set out to describe in detail the expression pattern of ey, so, eya, and dac in relation to each other throughout embryogenesis. We find that all four of these genes are expressed in dynamic patterns during embryogenesis, but that only ey is expressed within the eye imaginal disc. However, the early expression patterns of so, eya, and dac suggest that they may be involved in the specification of the head and visual field. Such a role for these genes in evolution is supported by the head and visual system defects that are seen in mouse models and human patients (Abdelhak et al. 1997a,b; Wallis et al. 1999; Azuma et al. 2000; Klesert et al. 2000; Pasquier et al. 2000; Sarkar et al. 2000; Davis et al. 2001). We also find that contrary to the suggestion made by in vitro biochemical interactions, these three proteins have very distinct and in most cases non-overlapping expression patterns. In addition, a comparison of Ey expression with that of the antennal specifying gene Distal-less (Dll) indicates that, although Dll is expressed in several domains within the embryonic head, these do not appear to correspond to the future antennal segment. These results are consistent with a model in which eye and antennal specification occurs (1) during the second larval instar, (2) requires the Egfr, Notch, and TGFb signal transduction cascades, and (3) requires the co-expression of the eye and antennal specification genes in the appropriate spatial compartment.
Materials and methods
Drosophila
stocks and antibodies
The following fly strains were used in this study: ey-lacZ (Halder et al. 1998) and so-lacZ (Cheyette et al. 1994). Both lacZ lines are known to be faithful to the normal expression patterns of the eyeless and sine oculis genes and are used here to facilitate comparisons with other gene products in double labeling experiments within individual embryos. The following antibodies were used in this study: mouse anti-Dac 1:100 (Mardon et al. 1994), mouse anti-Eya 1:100 (Bonini et al. 1993), and rabbit anti Dll 1:100 (Dong et al. 2000).
Staging and staining of embryos
Embryos harboring an ey-lacZ or a so-lacZ insertion were collected for 1 h at 18°C. Embryos were then aged at 25°C to form a 1-h series starting at 1 h and continuing until 16 h (i.e., 1 h, 2 h, 3 h, 4 h, 5 h, 6 h, 7 h, 8 h, 9 h, 10 h, 11 h, 12 h, 13 h, 14 h, 15 h, 16 h). Embryos at each time point were then stained to look for the following combinations: ey-lacZ/Dll, ey-lacZ/Dac, ey-lacZ/Eya, so-lacZ/Dac, and so-lacZ/Eya. Embryos were stained as described in Kumar and Moses (2001). We did not stain embryos for both Dac and Eya, since all available antibodies to these two proteins are made in mouse and cross-reactivity between antibodies will compromise the analysis of the staining patterns.
Results
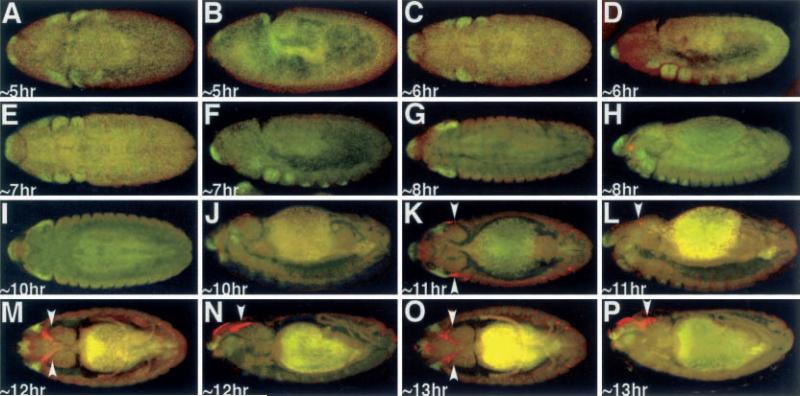
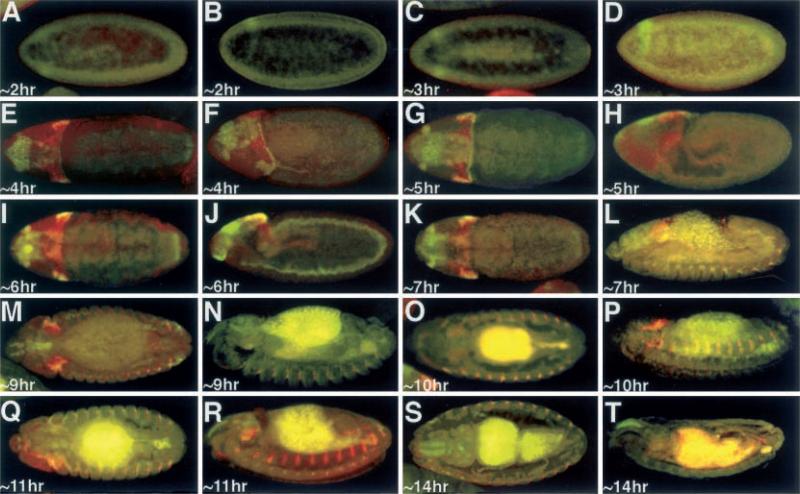
Clonal analysis and fate mapping data have suggested that the antenna and compound eyes are specified during embryogenesis (Postlethwait and Schneiderman 1971). Morphological analysis has further indicated that the antennal and eye disc primordia fuse to form a single complex near the end of embryogenesis (Younossi-Hartenstein et al. 1993). We analyzed the expression of an ey-lacZ transgene, which is known to faithfully reflect ey expression within the developing imaginal disc, in batches of embryos that were separated by 1 h and compared this with the expression of Dll, an antennal specifying gene. We found that Dll precedes ey-lacZ expression and is first seen in anterior regions of the head at approximately 5 h AED (Fig. 1A, BM). Dll expression can be found in the maxillary and mandibular segments along with the leg precursors at approximately 6 h AED (Fig. 1C–H). ey-lacZ expression is first detectable at approximately 11 h AED in two symmetrical regions on the dorso-lateral surface of the embryo (Fig. 1K, L). At this time point the ey-lacZ domains are adjacent to two domains of Dll expression. Interestingly, Dll expression is not seen in the presumptive antennal discs. However, as development proceeds and the eye imaginal disc adopts a more dorso-medial position and the domains of Dll expression become further separated from that of ey-lacZ (Fig. 1M–P). This separation continues through to the end of embryonic development. While ey expression is seen throughout the eye-antennal disc complex at this time point, Dll expression is not detected in the eye-antennal imaginal disc complex until the second instar larval time point, and is not restricted to just the antennal portion of the disc until the beginning of the third larval instar (Kumar and Moses 2001).
Fig. 1A–P.
Expression of Dll and ey-lacZ. A, C, E, G, I, K, M, O Dorsal view. B, D, F, H, J, L, N, P Lateral view. Arrowheads denote the eye imaginal discs. Age of embryos listed at bottom left of each panel. Red ey-lacZ, green Dll. Note the red spot in 1H is a microscope artifact and does not reflect ey-lacZ signal. Note: each panel has a low level of background autofluoresence, while older embryos have high levels of background staining in the presumptive gut region,.
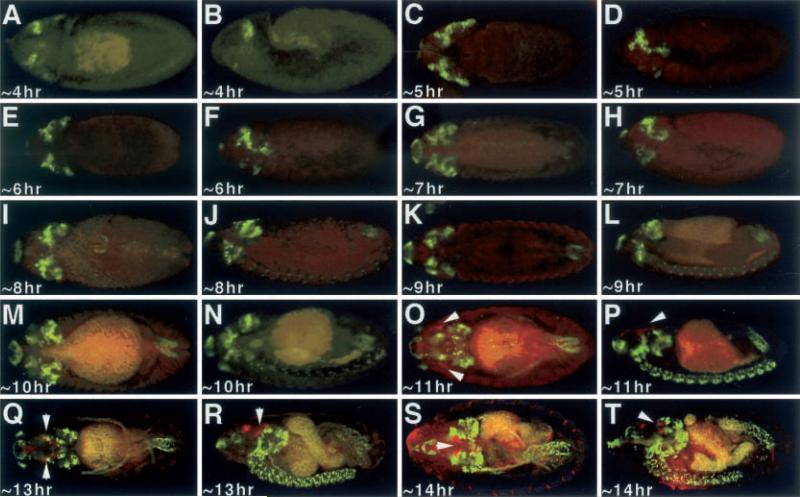
Genetic experiments have established ey as residing near the top of the eye specification hierarchy and dac as the most-downstream member of this signaling cascade. Nevertheless, genetic and molecular epistasy experiments have shown a complex reciprocal interaction between the two genes. Both are able to induce the transcription of the other in ectopic expression experiments, and both require the function of the other for ectopic eye development (Shen and Mardon 1997). Although it is still unclear if these connections are though direct binding of these proteins to each other's promoters, it suggests that both genes should be expressed within the same cells during eye imaginal disc development. We expected dac expression to be induced in all places where ey-lacZ was visualized and visa versa. We compared the expression of ey-lacZ with that of dac and were surprised to find that dac is expressed in an ey-independent manner during embryogenesis. dac expression is first detected in two anterior-dorsal domains at approximately 4 h AED and increases in complexity throughout the embryonic head by approximately 5 h and 6 h AED (Fig. 2A–F). Subsets of cells within the brain hemispheres express dac beginning at approximately 7 h AED (Fig. 2G, H), while cells within each segment of the central nervous system (CNS) express dac at approximately 8 h AED (Fig. 2I, J). At approximately 11 h AED ey-lacZ expression, which demarcates the eye imaginal disc, is not co-localized with dac, which is predominant in the optic lobes, brain and CNS (Fig. 2O. P). As the imaginal disc continues to develop through embryogenesis, dac and ey-lacZ expression are never co-localized to the same groups of cells (Fig. 2Q–T). This is further surprising, since ectopic dac expression in imaginal discs is sufficient on its own to induce ey transcription, and thus we did expect to see regions of ey-lacZ and dac colocalization.
Fig. 2A–T.
Expression of Dac and ey-lacZ. A, C, E, G, I, K, M, O, Q, S Dorsal view. B, D, F, H, J, L, N, P, R, T Lateral view. Arrowheads denote the eye imaginal discs. Age of embryos listed at bottom left of each panel. Red ey-lacZ, green Dac. Note that Dac is not co-expressed with ey-lacZ at any time point in development. Note: each panel has a low level of background autofluoresence, while older embryos have high levels of background staining in the presumptive gut region
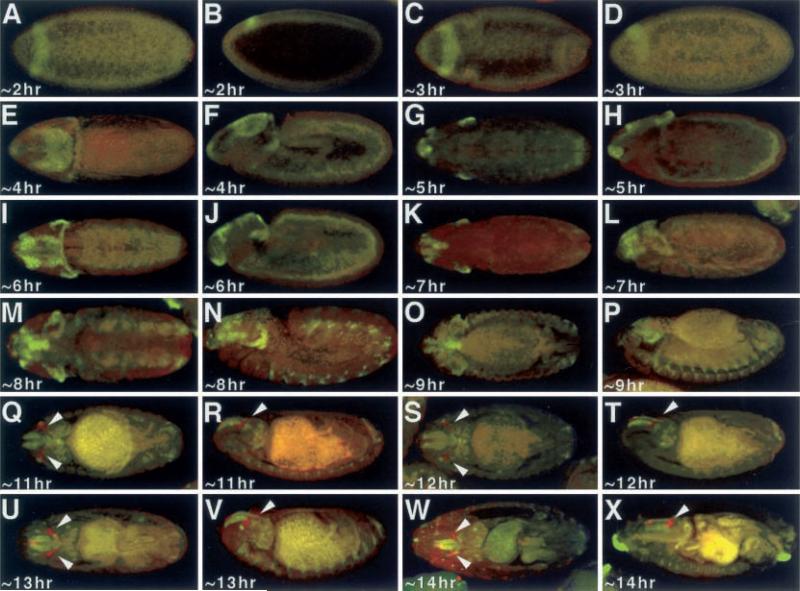
Ey directs the transcription of eya by binding to regulatory regions within the eya promoter (Halder et al. 1998; Bui et al. 2000; Zimmerman et al. 2000). Ectopic expression of eya was not however observed to induce eye transcription (Bonini et al. 1997). It is therefore possible to see cells that are Eya positive and Ey negative, but any cell that is Ey positive should also be Eya positive. To determine if this is indeed the case we compared the expression of ey-lacZ with that of eya, with the expectation that all cells (especially the eye imaginal disc primordia) that express ey-lacZ will also express eya. Surprisingly, eya expression begins even earlier than that of dac and is obviously also independent of ey regulation. Eya protein is first seen at approximately 2 h AED at which the embryo is still in the synctial blastoderm time point and can be seen as a band of cells that runs along the dorsal surface of the embryo (Fig. 3A–D). By approximately 4 h AED this band is transformed into a crown that extends more laterally (Fig. 3E–J). As is the case with dac, eya begins to be expressed in subsets of cells within the embryonic brain by approximately 7 h AED, but these cells are distinct from those that express dac (Fig. 3K, L). Unlike dac, eya is not expressed within the ventral nerve cord, but rather is found in a small clustering of cells within the segmental grooves of the embryo (Fig. 3M–P). From the onset of ey-lacZ expression at approximately 11 h AED through the end of embryogenesis, eya is not expressed within the eye imaginal disc (Fig. 3Q–X). These results are consistent with those reported in Bonini et al. (1998). Eya protein is first detected in the eye imaginal disc during the first larval instar (Bui et al. 2000).
Fig. 3A–X.
Expression of Eya and ey-lacZ. A, C, E, G, I, K, M, O, Q, S, U, W Dorsal view. B, D, F, H, J, L, N, P, R, T, V, X Lateral view. Arrowheads denote the eye imaginal discs. Age of embryos listed at bottom left of each panel. Red ey-lacZ, green Eya. Note that Eya is not co-expressed with ey-lacZ at any time point in development. Note: each panel has a low level of background autofluoresence, while older embryos have high levels of background staining in the presumptive gut region
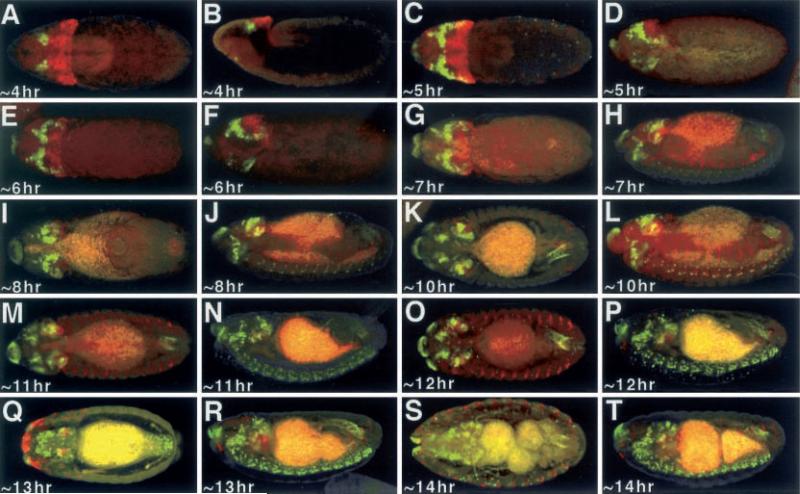
Recently it has been shown that the patterning genes hedgehog (hh) and decapentaplegic (dpp) are required for the specification in the eye (Chen et al. 1999; Curtiss and Mlodzik 2000). In an interesting model it has been proposed that Hh signals to Eya which then in turn induces (directly or indirectly) the transcription of both so and dac (Curtiss and Mlodzik 2000). This would then suggest that during embryogenesis all three proteins should have overlapping expression patterns during the allocation of the eye disc. We first compared the expression of a so-lacZ transgene with that of dac. Interestingly, while the onset of expression of both genes are first detected at approximately 4 h AED, their expression patterns abut each other and are not overlapping. While dac is expressed in two clusters of dorsal medially located cells, so-lacZ expression is seen in a broad swathe of cells that extends from one lateral surface to another. Its expression appears to be delimited by the more-anterior domain of dac expression and the cephalic furrow (Fig. 4A, B). By approximately 5 h AED there is a cluster of cells along the lateral margins just anterior to the cephalic furrow in which both so-lacZ and dac are co-expressed (Fig. 4C–F). However, the vast majority of so-lacZ and dac expression is non-overlapping. Not unlike eya, so-lacZ is expressed in a subset of cells within the developing brain but is not expressed in the ventral nerve cord (Fig. 4G–T). There is considerable overlap between the dac and so-lacZ expression patterns within the developing brain lobes. In the segmental grooves so-lacZ expression can be seen much like that of eya (Fig. 4K–T). At approximately 11−14 h AED there is no expression of so-lacZ within the developing eye imaginal disc (Fig. 4M–T).
Fig. 4A–T.
Expression of Dac and so-lacZ. A, C, E, G, I, K, M, O, Q, S Dorsal view. B, D, F, H, J, L, N, P, R, T Lateral view. Arrowheads denote the eye imaginal discs. Age of embryos listed at bottom left of each panel. Red so-lacZ, green Dac. Note that Dac and so-lacZ is not found within the embryonic eye imaginal disc. Note: each panel has a low level of background autofluoresence, while older embryos have high levels of background staining in the presumptive gut region
Eya can form a complex with So in vitro (Pignoni et al. 1997). Within the eye imaginal disc of second and third instar larvae, both genes are expressed in overlapping, although not completely exact, patterns (Cheyette et al 1994; Mardon et al. 1994; Serikaku and O'Tousa 1994; Kumar and Moses 2001). It has also been shown that so and eya act synergistically in promoting ectopic eye development (Bui et al. 2000). We compared the expression of so-lacZ with that of eya. Eya protein is first detected in the cellular blastoderm at approximately 2 h AED (Fig. 5A–D), while so-lacZ expression is not seen until approximately 4 h AED (Fig. 5E. F). The dorsal expression of both genes overlaps considerably (Fig. 5E–L). There is also considerable overlap in their expression patterns within the developing brain lobes (Fig. 5M–R). By the end of embryogenesis so-lacZ expression is severely reduced (Fig. 5S, T). Interestingly, the patterns of expression of so-lacZ and eya within the segmental grooves are not overlapping (Fig. 5N–Q). However, the degree of overlap between the patterns within the embryo and the late second and third eye imaginal discs furthers supports the in vitro biochemical evidence that these genes do interact.
Fig. 5A–T.
Expression of Eya and so-lacZ. A, C, E, G, I, K, M, O, Q, S Dorsal view. B, D, F, H, J, L, N, P, R, T Lateral view. Arrowheads denote the eye imaginal discs. Age of embryos listed at bottom left of each panel. Red so-lacZ, green Eya. Note that Eya and so-lacZ is not found within the embryonic eye imaginal disc. Note: each panel has a low level of background autofluoresence, while older embryos have high levels of background staining in the presumptive gut region
Discussion
The dramatic differences in morphology and optical properties between invertebrate and vertebrate eyes have been used to justify the argument that the eye has a polyphyletic origin. In fact it has been argued that eye evolution has occurred independently at least 40 if not 65 times throughout history (Salvini-Plawen and Mayr 1997). It has been estimated that eyes can evolve in a relatively short period of time, furthering the polyphyletic argument (Nilsson and Pelger 1994). In addition to the morphological and optical differences, the differences in lens components and phototransduction machinery have been used to forcefully argue the polyphyletic origin of the eye (Land and Fernald 1992; Fernald 1997, 2000).
An alternate view of eye evolution came with the dramatic finding that the fruit fly Drosophila contained a homolog of the Pax6 gene (eyeless) and it was sufficient on its own to direct eye development (Quiring et al. 1994; Halder et al. 1995a). The demonstration that vertebrate Pax6 genes could direct ectopic eye development in flies identified a functional conservation that was unexpected given the tremendous differences in eye morphology (Halder et al. 1995a). Over the last few years, Pax6 genes have been identified throughout the animal kingdom and homologs from several different phyla have been shown to function in flies. These findings have led to the proposal that the eye does in fact have a monophyletic origin (Gehring 1996; Gehring and Ikeo 1999).
A number of additional genes have been identified in Drosophila that are essential for the specification of the eye and are capable of inducing ectopic retinal development (Treisman 1999; Heberlein and Treisman 2000). They have a similar ability to induce ectopic eyes and have been also considered master control genes for eye specification. The simplest explanation for the complex genetic, molecular, and biochemical interactions among these genes is that they work in concert within the same cells to direct the fate of the eye. We have previously reported that all seven of these eye specification genes are co-expressed during the second larval instar, a point in time that is surprisingly later than expected considering that the fate of the eye was thought to be determined during embryogenesis (Kumar and Moses 2001). In fact only three eye specification genes (toy, ey, and eyg) are expressed in the embryonic eye imaginal disc (Quiring et al. 1994; Jones et al 1998; Czerny et al. 1999). If these are the only eye specification genes to be expressed in the eye primordium then it is unlikely that eye specification could occur during embryogenesis in light of the results of Kumar and Moses (2001).
In this report we provide a detailed description of the ey, so, eya, and dac expression patterns during all time points of embryogenesis. We found that (1) so, eya, and dac are expressed in several regions that are not fated to become eyes, (2) so, eya, and dac expression begins prior to ey expression, (3) ey does not induce the transcription of so, eya, or dac in the eye imaginal disc during embryogenesis, and (4) the patterns of so, eya, and dac are dynamic and in most instances non-overlapping.
These results have several implications for our current thinking on how the Drosophila eye is specified. It has been shown that the ectopic expression of each of the eye specification genes (with the exception of so) is sufficient to induce the formation of ectopic eyes. What prevents the induction of retinal tissue throughout the embryo? Based on these and our previous findings we would like to argue that expression of all eye specification genes are required for eye determination. Within the embryo we do not find a region in which all these factors are present. It is not until the second larval instar that all genes are expressed within the same tissue (Kumar and Moses 2001). Within the embryo, positive or repressive mechanisms must be in place to prevent the eye specification genes from being co-expressed. For example, ey is capable of directly inducing the transcription of both so and eya within the mature eye imaginal disc, but within the eye anlagen these genes are not expressed, although Ey protein is present. The nature of this regulatory mechanism is unknown, but its existence is supported by the finding that the Notch receptor and thus Notch signaling is highly upregulated within the eye imaginal disc at the time at which all eye specification genes are co-expressed. At earlier time points Notch is seen even throughout the eye and antennal primordium (Kumar and Moses 2001).
It is still unclear as to why only three eye specification genes (toy, ey, and eyg) are expressed within the embryonic eye primordium. Is the expression of these three genes within the eye primodium a priming step for the eventual specification of the eye or is it simply a step that distinguishes one disc from another? Since Ey protein has been shown to directly bind to the so and eya promoters, there must be an inhibitory signal within the eye disc that prevents the transcription of these genes from being induced. This repression is first released for eya transcription as it is localized to the first instar eye disc. The inhibition upon the remaining genes is released during the second larval instar. Unraveling this mystery will certainly require extensive molecular and biochemical analysis on embryonic and early larval eye discs.
Another lingering question focuses on the fates of the cells that are derived from the initial expression of so, eya, and dac. All three of these genes are expressed very early; for instance eya is expressed in a cluster of cells at the cellular blastoderm time point. Do these cells contribute to the formation of the visual field? Are these three proteins committing cells to adopt an eye imaginal disc fate, an event that will occur much later in embryogenesis? Such questions can only be addressed by precise single cell fate mapping experiments. Only by labeling a single cell and tracing its progeny will we be able to know if the earliest cells that express so, eya, and dac will later become cells of the eye imaginal disc. How the expression patterns described here correlate with the genetic, molecular, and biochemical interactions of the eye specification genes is an interesting problem that will undoubtedly require the identification of additional instructive and inhibitory signals.
Finally, are the earliest expression patterns of these eye specification genes homologous between vertebrates and invertebrates? This is certainly a much more-difficult question to answer. A decade ago this question would be easily answered with a resounding “no”. Now as more molecular and physiological similarities between the visual systems of vertebrates and invertebrates are being discovered the answer to this developmental question may not be as easily or as negatively answered. It would be truly remarkable if a common developmental history underlies the use of identical molecules to create the different types of eyes seen throughout the animal kingdom. The key to such questions may lie in the precise fate mapping of individual cells that express each of the genes responsible for eye specification.
Acknowledgements
We would like to thank Graeme Mardon, Grace Panganiban, and the Developmental Studies Hybridoma Bank for fly stocks and antibodies. This work was supported by NIH grants (EY-12537 and IBN 9807892) to Kevin Moses and a NIH postdoctoral fellowship (5F32 EY06763) to Justin P. Kumar.
References
- Abdelhak S, Kalatzis V, Heilig R, Compain S, Samson D, Vincent C, LeviAcobas F, Cruaud C, Le Merrer M, Mathieu M, Konig R, Vigneron J, Weissenbach J, Petit C, Weil D. Clustering of mutations responsible for branchio-oto-renal (BOR) syndrome in the eyes absent homologous region (eyaHR) of EYAL. Hum Mol Genet. 1997a;6:2247–2255. doi: 10.1093/hmg/6.13.2247. [DOI] [PubMed] [Google Scholar]
- Abdelhak S, Kalatzis V, Heilig R, Compain S, Samson D, Vincent C, Weil D, Cruaud C, Sahly I, Leibovici M, Bitner-Glindzicz M, Francis M, Lacombe D, Vigneron J, Charachon R, Boven K, Bedbeder P, Van Regemorter N, Weissenbach J, Petit C. A human homologue of the Drosophila eyes absent gene underlies branchio-oto-renal (BOR) syndrome and identifies a novel gene family. Nat Genet. 1997b;15:157–164. doi: 10.1038/ng0297-157. [DOI] [PubMed] [Google Scholar]
- Azuma N, Hirakiyama A, Inoue T, Asaka A, Yamada M. Mutations of a human homologue of the Drosophila eyes absent gene (EYAI) detected in patients with congenital cataracts and ocular anterior segment anomalies. Hum Mol Genet. 2000;9:363–366. doi: 10.1093/hmg/9.3.363. [DOI] [PubMed] [Google Scholar]
- Bonini NM, Leiserson WM, Benzer S. The eyes absent gene: genetic control of cell survival and differentiation in the developing Drosophila eye. Cell. 1993;72:379–395. doi: 10.1016/0092-8674(93)90115-7. [DOI] [PubMed] [Google Scholar]
- Bonini NM, Bui QL, Gray-Board GL, Warrick IM. The Drosophila eyes absent gene directs ectopic eye formation in a pathway conserved between flies and vertebrates. Development. 1997;124:4819–4826. doi: 10.1242/dev.124.23.4819. [DOI] [PubMed] [Google Scholar]
- Bonini NM, Lieserson WM, Benzer S. Multiple roles of the eyes absent gene in Drosophila. Dev Biol. 1998;196:42–57. doi: 10.1006/dbio.1997.8845. [DOI] [PubMed] [Google Scholar]
- Bui QT, Zimmerman JE, Liu H, Gray-Board GL, Bonini NM. Functional analysis of an eye enhancer of the Drosophila eyes absent gene: differential regulation by eye specifcation genes. Dev Biol. 2000;221:355–364. doi: 10.1006/dbio.2000.9688. [DOI] [PubMed] [Google Scholar]
- Callaerts P, Halder G, Gehring WJ. PAX–6 in development and evolution. Annu Rev Neurosci. 1997;20:483–532. doi: 10.1146/annurev.neuro.20.1.483. [DOI] [PubMed] [Google Scholar]
- Caubit X, Thangarajah R, Theil T, Wirth J, Nothwang HG, Ruther U, Krauss S. Mouse Dac, a novel nuclear factor with homology to Drosophila dachshund shows a dynamic expression in the neural crest, the eye, the neocortex, and the limb bud. Dev Dyn. 1999;214:66–80. doi: 10.1002/(SICI)1097-0177(199901)214:1<66::AID-DVDY7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Chen R, Amoui M, Zhang Z, Mardon G. Dachshund and Eyes Absent proteins form a complex and function synergistically to induce ectopic eye development in Drosophila. Cell. 1997;91:893–903. doi: 10.1016/s0092-8674(00)80481-x. [DOI] [PubMed] [Google Scholar]
- Chen R, Halder G, Zhang Z, Mardon G. Signaling by the TGF-b homolog decapentaplegic functions reiteratively within the network of genes controlling retinal cell fate determination in Drosophila. Development. 1999;126:935–943. doi: 10.1242/dev.126.5.935. [DOI] [PubMed] [Google Scholar]
- Cheyette BN, Green PJ, Martin K, Garren H, Hartenstein V, Zipursky SL. The Drosophila sine oculis locus encodes a homeodomain-containing protein required for the development of the entire visual system. Neuron. 1994;12:977. doi: 10.1016/0896-6273(94)90308-5. [DOI] [PubMed] [Google Scholar]
- Curtiss J, Mlodzik M. Morphogenetic furrow initiation and progression during eye development in Drosophila: the roles of decapentaplegic, hedgehog and eyes absent. Development. 2000;127:1325–1336. doi: 10.1242/dev.127.6.1325. [DOI] [PubMed] [Google Scholar]
- Czerny T, Halder G, Kloter U, Souabni A, Gehring WJ, Busslinger M. twin of eyeless, a second Pax-6 gene of Drosophila, acts upstream of eyeless in the control of eye development. Mol Cell. 1999;3:297–307. doi: 10.1016/s1097-2765(00)80457-8. [DOI] [PubMed] [Google Scholar]
- Davis RJ, Shen W, Heanue TA, Mardon G. Mouse Dach, a homologue of Drosophila dachshun rly is expressed in the developing retina, brain and limbs. Dev Genes Evol. 1999;209:526–536. doi: 10.1007/s004270050285. [DOI] [PubMed] [Google Scholar]
- Davis RJ, Shen W, Sandler YI, Heanue TA, Mardon G. Characterization of mouse Dacht, a homologue of Drosophila dachshund. Mech Dev. 2001;102:169–179. doi: 10.1016/s0925-4773(01)00307-0. [DOI] [PubMed] [Google Scholar]
- Dong PD, Chu J, Panganiban G. Coexpression of the homeobox genes distal-less and homothorax determines Drosophila antennal identity. J Dev. 2000;127:209–216. doi: 10.1242/dev.127.2.209. [DOI] [PubMed] [Google Scholar]
- Fasano L, Roder L, Core N, Alexandre E, Vola C, Jacq B, Kerridge S. The gene teashirt is required for the development of Drosophila embryonic trunk segments and encodes a protein with widely spaced zinc finger motifs. Cell. 1991;64:63–79. doi: 10.1016/0092-8674(91)90209-h. [DOI] [PubMed] [Google Scholar]
- Fernald RD. The evolution of eyes. Brain Behav Evol. 1997;50:253–259. doi: 10.1159/000113339. [DOI] [PubMed] [Google Scholar]
- Fernald RD. Evolution of eyes. Curr Opin Neurobiol. 2000;10:444–50. doi: 10.1016/s0959-4388(00)00114-8. [DOI] [PubMed] [Google Scholar]
- Gehring WJ. The master control gene for morphogenesis and evolution of the eye. Genes Cells. 1996;1:11–15. doi: 10.1046/j.1365-2443.1996.11011.x. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Ikeo K. 1'ax 6: mastering eye morphogenesis and eye evolution. Trends Genet. 1999;15:371–377. doi: 10.1016/s0168-9525(99)01776-x. [DOI] [PubMed] [Google Scholar]
- Grindley JC, Davidson DR, Hill RE. The role of Pax-6 in eye and nasal development. Development. 1995;121:1433–1442. doi: 10.1242/dev.121.5.1433. [DOI] [PubMed] [Google Scholar]
- Halder G, Callaerts P, Gehring WJ. Induction of ectopic eyes by target expression of the eyeless gene in Drosophila. Science. 1995a;267:1788–1792. doi: 10.1126/science.7892602. [DOI] [PubMed] [Google Scholar]
- Halder G, Callaerts P, Gehring WJ. New perspectives on eye evolution. Curr Opin Genet Dev. 1995b;5:602–9. doi: 10.1016/0959-437x(95)80029-8. [DOI] [PubMed] [Google Scholar]
- Halder G, Callaerts P, Flister S, Walldorf U, Kloter U, Gehring WJ. Eyeless initiates the expression of both sine oculis and eyes absent during Drosophila compound eye development. Development. 1998;125:2181–2191. doi: 10.1242/dev.125.12.2181. [DOI] [PubMed] [Google Scholar]
- Hammond KL, Hanson IM, Brown AG, Lettice LA, Hill RE. Mammalian and Drosophila dachshund genes are related to the Ski protooncogene and are expressed in eye and limb. Mech Dev. 1998;74:121–131. doi: 10.1016/s0925-4773(98)00071-9. [DOI] [PubMed] [Google Scholar]
- Heberlein U, Treisman JE. Early retinal development in Drosophila. In: Fini ME, editor. Vertebrate eye development. Springer; Berlin Heidelberg New York: 2000. p. 288. [Google Scholar]
- Hill RE, Favor J, Hogan BL, Ton CC, Saunders GF, Hanson IM, Prosser J, Jordan T, Hastie ND, Heyningen V van. Mouse small eye results from mutations in a paired-like homeobox-containing gene. Nature. 1991;354:522–525. doi: 10.1038/354522a0. [DOI] [PubMed] [Google Scholar]
- Hogan BL, Horsburgh G, Cohen J, Hetherington CM, Fisher G, Lyon M. Small eyes (Sey): a homozygous lethal mutation an chromosome 2 which affects the differentiation of both lens and nasal. placodes in the mouse. J Embryol Exp Morphol. 1986;97:95–110. [PubMed] [Google Scholar]
- Jones NA, Kuo YM, Sun YH, Beckendorf SK. The Drosophila Pax gene eye gone is required for embryonic salivary duct development. Development. 1998;125:4163–4174. doi: 10.1242/dev.125.21.4163. [DOI] [PubMed] [Google Scholar]
- Jun S, Wallen RV, Goriely A, Kalionis B, Desplan C. Lune%ye gone, a Pax-like protein, uses a partial paired dornain and a homeodomain for DNA recognition. Proc Natl Acad Sci U S A. 1998;95:13720–13725. doi: 10.1073/pnas.95.23.13720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klesert TR, Cho DH, Clark JI, Maylie J, Adelman J, Snider L, Yuen EC, Soriano P, Tapscott SJ. Mice deficient in Six5 develop cataracts: implications for myotonic dystrophy. Nat Genet. 2000;25:105–109. doi: 10.1038/75490. [DOI] [PubMed] [Google Scholar]
- Kumar JP, Moses K. EGF receptor and Notch signaling act upstream of EyelesslPax6 to control eye specification. Cell. 2001;104:687–697. doi: 10.1016/s0092-8674(01)00265-3. [DOI] [PubMed] [Google Scholar]
- Land MF, Fernald RD. The evolution of eyes. Annu Rev Neurosci. 1992;15:1–29. doi: 10.1146/annurev.ne.15.030192.000245. [DOI] [PubMed] [Google Scholar]
- Leiserson WM, Benzer S, Bonini NM. Dual functions of the Drosophila eyes absent gene in the eye and embryo. Mech Dev. 1998;73:193–202. doi: 10.1016/s0925-4773(98)00052-5. [DOI] [PubMed] [Google Scholar]
- Leppert GS, Yang JM, Sundin OH. Sequence and location of SIX3, a homeobox gene expressed in the human eye. Ophthalmic Genet. 1999;20:7–21. doi: 10.1076/opge.20.1.7.2298. [DOI] [PubMed] [Google Scholar]
- Mardon G, Solomon NM, Rubin GM. dachshund encodes a nuclear protein required for normal eye and leg development in Drosophila. Development. 1994;120:3473–3486. doi: 10.1242/dev.120.12.3473. [DOI] [PubMed] [Google Scholar]
- Niimi T, Seimiya M, Kloter U, Flister S, Gehring WJ. Direct regulatory interaction of the eyeless protein with an eye-specific enhancer in the sine oculis gene during eye induction in Drosophila. Development. 1999;126:2253–2260. doi: 10.1242/dev.126.10.2253. [DOI] [PubMed] [Google Scholar]
- Nilsson DE, Pelger S. A pessimistic estimate of the time required for an eye to evolve. Proc R Soc Lond B Biol Sci. 1994;256:53. doi: 10.1098/rspb.1994.0048. [DOI] [PubMed] [Google Scholar]
- Nishina S, Kohsaka S, Yamaguchi Y, Handa H, Kawakami A, Fujisawa H, Azuma N. PAXÖ expression in the developing human eye. Br J Ophthalmol. 1999;83:723–727. doi: 10.1136/bjo.83.6.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver G, Mailhos A, Wehr R, Copeland NG, Jenkins NA, Gruss P. Six3, a murine homologue of the sine oculis gene, demarcates the most anterior border of the developing neural plate and is expressed during eye development. Development. 1995;21:4045–4055. doi: 10.1242/dev.121.12.4045. [DOI] [PubMed] [Google Scholar]
- Pan D, Rubin GM. Targeted expression of teashirt induces ectopic eyes in Drosophila. Proc Natl Acad Sci USA. 1998;95:15508–15512. doi: 10.1073/pnas.95.26.15508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquier L, Dubourg C, Blayau M, Lazaro L, Le Marec B, David V, Odent S. A new mutation in the six-domain of SIX3 gene causes holoprosencephaky. Eur J Hum Genet. 2000;8:797–800. doi: 10.1038/sj.ejhg.5200540. [DOI] [PubMed] [Google Scholar]
- Pignoni F, Hu B, Kenton HZ, Xiao J, Garrity PA, Zipurksy SL. The eyespecif cation proteins So and Eya form a complex and regulate multiple steps in Drosophila eye development. Cell. 1997;91:881–891. doi: 10.1016/s0092-8674(00)80480-8. [DOI] [PubMed] [Google Scholar]
- Postlethwait JH, Schneiderman HA. A clonal analysis of development in Drosophila melanogaster: morphogenesis, determination and growth in the wild type antenna. Dev Biol. 1971;24:477–519. doi: 10.1016/0012-1606(71)90061-3. [DOI] [PubMed] [Google Scholar]
- Quiring R, Walldorf U, Kloter U, Gehring WJ. Homology of the eyeless gene of Drosophila to the Small eye gene in mice and Aniridia in humans. Science. 1994;265:785–789. doi: 10.1126/science.7914031. [DOI] [PubMed] [Google Scholar]
- Salvini-Plawen LV, Mayr E. On the evolution of photoreceptors and eyes. Evol Biol. 1997;10:207–263. [Google Scholar]
- Sarkar PS, Appukuttan B, Han J, Ito Y, Ai C, Tsai W, Chai Y, Stout JT, Reddy S. Heterozygous loss of Six5 in mice is sufficient to cause ocular cataracts. Nat Genet. 2000;25:110–114. doi: 10.1038/75500. [DOI] [PubMed] [Google Scholar]
- Seimiya M, Gehring WJ. The Drosophila homeobox gene optix is capable of inducing ectopic eyes by an eyeless-independent mechanism. Development. 2000;127:1879–1886. doi: 10.1242/dev.127.9.1879. [DOI] [PubMed] [Google Scholar]
- Serikaku MA, O'Tousa JE. sine oculis is a homeobox gene required for Drosophila visual system development. Genetics. 1994;138:1137–1150. doi: 10.1093/genetics/138.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Mardon G. Ectopic eye development in Drosophila induced by directed dachshund expression. Development. 1997;124:45–52. doi: 10.1242/dev.124.1.45. [DOI] [PubMed] [Google Scholar]
- Terzic J, Saraga-Babic M. Expression pattern of PAX3 and PAX6 genes during human embryogenesis. Int J Dev Biol. 1999;43:501. [PubMed] [Google Scholar]
- Ton CC, Hirvonen H, Miwa H, Weil MM, Monaghan P, Jordan T, Heyningen V van, Hastie ND, Meijers-Heijboer H, Drechsler M, et al. Positional cloning and characterization of a paired box- and homeobox-containing gene from the aniridia region. Cell. 1991;67:1059–1074. doi: 10.1016/0092-8674(91)90284-6. [DOI] [PubMed] [Google Scholar]
- Treisman JE. A conserved blueprint for the eye? Bioessays. 1999;21:843–850. doi: 10.1002/(SICI)1521-1878(199910)21:10<843::AID-BIES6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Wallis DE, Roessler E, Hehr U, Nanni L, Wiltshire T, Richieri-Costa A, Gillessen-Kaesbach G, Zackai EH, Rommens J, Muenke M. Mutations in the homeodomain of the human SIX3 gene cause holoprosencephalv. Nat Genet. 1999;22:196. doi: 10.1038/9718. [DOI] [PubMed] [Google Scholar]
- Wawersik S, Purcell P, Maas RL. Pax6 and the genetic control of early eye development. Results Probl Cell Differ. 2000;31:15–36. doi: 10.1007/978-3-540-46826-4_2. [DOI] [PubMed] [Google Scholar]
- Winchester CL, Ferrier RK, Sermoni A, Clark BJ, Johnson KJ. Characterization of the expression of DMPK and SIX5 in the human eye and implications for pathogenesis in myo-tonic dystrophy. Hum Mol Genet. 1999;8:481–492. doi: 10.1093/hmg/8.3.481. [DOI] [PubMed] [Google Scholar]
- Xu PX, Woo I, Her H, Beier DR, Maas RL. Mouse Eya homologues of the Drosophila eyes absent gene require Pax6 for expression in lens and nasal placode. Development. 1997;124:219–231. doi: 10.1242/dev.124.1.219. [DOI] [PubMed] [Google Scholar]
- Younossi-Hartenstein A, Tepass U, Hartenstein V. Embryonic origin of the imaginal discs of the head of Drosophila melanogaster. Roux's Arch Dev Biol. 1993;203:60–73. doi: 10.1007/BF00539891. [DOI] [PubMed] [Google Scholar]
- Zimmerman JE, Bui QT, Steingrimsson E, Nagle DL, Fu W, Genin A, Spinner NB, Copeland NG, Jenkins NA, Bucan M, Bonini NM. Cloning and characterization of two vertebrate homologs of the Drosophila eyes absent gene. Genome Res. 1997;7:128–141. doi: 10.1101/gr.7.2.128. [DOI] [PubMed] [Google Scholar]
- Zimmerman JE, Bui QT, Liu H, Bonini NM. Molecular genetic analysis of Drosophila eyes absent mutants reveals an eye enhancer element. Genetics. 2000;154:237–246. doi: 10.1093/genetics/154.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]