Abstract
DNAzymes are catalytic DNA which bind to target RNA by complementary sequence arms on a Watson-Crick basis and cleave RNA at specific sites. Potential therapeutic applications require DNAzymes that can efficiently cleave their target. Here we investigate factors affecting DNAzyme cleavage efficacy against the muscle acetylcholine receptor (AChR) α-subunit. The 10-23 DNAzymes cleave at Y-R nucleotide motifs, where R is A or G, and Y is U or C. Targeting a series of sites within different regions of the full-coding length cRNA under simulated physiological conditions found that the most efficient motifs for cleavage were in the hierarchy: GU ≥ AU > GC ⋙ AC. This order is consistent with the kinetic analysis of short synthetic RNA substrates that have the same binding arms but different cleavage sites. DNAzymes with longer symmetric binding arms were more efficient than those with shorter arms, while asymmetric DNAzymes with a longer arm I were also more efficient, suggesting a dominant role for arm I in determining cleavage activity. Modification of one DNAzyme by inverted thymidine (iT) or locked nucleic acids (LNA) showed the LNA-modified DNAzyme gave efficient silencing of AChR expression in HEK 293 cells. Our data demonstrate the usefulness of screening in vitro for an efficient DNAzyme prior to cellular applications.
KEYWORDS: DNAzymes, RNA cleavage, AChR α-subunit, gene therapy, LNAzymes, gene silencing
INTRODUCTION
DNAzymes are catalytic DNA analogues of the naturally occurring hammerhead ribozymes that cleave RNA substrates in a sequence-specific manner (Santoro and Joyce, 1997). DNAzymes, like hammerhead ribozymes, have a structure comprised of three domains. A central conserved motif contains the catalytic domain and is activated by metal ions such as Mg2+ cations. The catalytic core is flanked by two recognition-binding arms (arms I and II) that bind to target RNA on a Watson-Crick basis. Santoro and Joyce identified two catalytic DNAzyme motifs, 8-17 and 10-23 (Santoro and Joyce, 1997). Each enzyme motif recognises nucleotide residues within the target RNA resulting in phosphodiester bond cleavage and the generation of two RNA fragments.
DNAzymes have been used to suppress pathogenic genes in a growing number of disease models (reviewed in Khachigian, 2002; Achenbach et al, 2004) and in other in vivo applications (Zhang et al, 2004; Liu et al, 2005). Understanding the design of efficient DNAzymes is of growing interest in the gene therapy field. The cleavage efficiency depends on the properties of the target, such as its structure and therefore cleavage site accessibility, and on properties of the DNAzyme, principally its catalytic activity and how this is optimised. For example, the RY rule for 10-23 DNAzymes gives four potential cleavage sites: GU, GC, AU and AC (Santoro and Joyce, 1997), which may be cleaved with different efficiencies. Screening for an efficient DNAzyme in vitro may give a good indication of its potential cellular efficacy. Reported in vitro optimisation of DNAzyme catalysis has mostly used non-physiological high Mg2+ concentrations and/or on short substrate target molecules (Cairns et al, 2000; Toyoda et al, 2000; Basu et al, 2000; Sriram and Banerjea, 2000; Sioud and Leirdal, 2000; Liu et al, 2001; Kurreck et al, 2002; Wang et al, 2002; Cairns et al, 2003; Schubert et al, 2003; Schubert et al, 2004). However, screening for efficient DNAzymes using full-length in vitro transcribed RNA (cRNA) under simulated physiological conditions is likely, in many cases, to be of greater direct relevance for therapeutic application.
The muscle acetylcholine receptor (AChR) is expressed at the neuromuscular junction, and plays the principal role in nerve to muscle signal transmission. A number of mutations have been characterised in the AChR α-subunit gene which affect receptor function and give rise to congenital myasthenic syndromes (Croxen et al, 1997; Engel and Sine, 2005). Some of these mutations were targeted in an initial report investigating the potential therapeutic applications of DNAzymes and considerable variation in cleavage efficiencies was encountered (Abdelgany et al, 2005). In this study, we investigate the factors affecting DNAzyme cleavage activity using full-coding length cRNA transcripts under simulated physiological conditions and highlight parameters that should be taken into account prior to the design of DNAzymes for cellular applications.
MATERIALS AND METHODS
Design and synthesis of DNAzymes and their synthetic RNA substrates
DNAzymes bearing the 10-23 catalytic motif were designed against the full-coding length of AChR α-subunit cRNA and were obtained from Invitrogen (http://www.invitrogen.com). Chemically modified DNAzymes were synthesised by incorporating a modified nucleotide either side of the binding arms. Two types of modifications were used: 3′-3′-inverted thymidine (iT) obtained from Invitrogen and locked nucleic acids (LNA) obtained from Proligo (http://www.proligo.com). RNA oligonucleotides were synthesised on a DNA/RNA synthesiser (model 394; PE Applied Biosystems, Foster City, CA). The crude deprotected oligonucleotides were purified on an open column and then purified on a 20% (w/v) polyacrylamide gel that contained 7 M urea, and RNA was eluted with milli-Q water (Millipore, Billerica, MA) and desalted by passage through a NAP10 column (Amersham Pharmacia Biotech AB, Uppsala, Sweden). DNAzymes for short substrates were obtained from Hokkaido System Science Co Ltd (Sapporo, Hokkaido, Japan) and Espec Oligo Service Corp (Ibaraki, Japan). DNAs were purified on a 20% (w/v) polyacrylamide gel and preparations were desalted on a NAP10 column. To facilitate DNAzyme nomenclature, DNAzymes will be referred to, for example, as 808GU(13+9), where 808 is the nucleotide position within the cRNA target transcript, GU the cleavage site sequence, and (13+9) represents the lengths of arm II and arm I respectively, i.e., arm II is 13 nucleotides in length and arm I is 9 nucleotides in length (see list of DNAzymes and sites within the AChR α-subunit sequence targeted in Table 1).
Table 1.
DNAzyme nomenclature and target sequences used in this study, with cleavage sites in bold.
| Region | DNAzyme | Target sequence 5′ - 3′ | |||
|---|---|---|---|---|---|
| Arm II | cleavage site | Arm I | |||
| A | 463GU(10+10) | GACGGCUCU | GU | CGUGGCCAU | |
| 463GU(13+9) | CUACGACGGCUCU | GU | CGUGGCCA | ||
| 463GU(9+13) | GACGGCUCU | GU | CGUGGCCAUCAA | ||
| 472AU(10+10) | UGUCAUGGCC | AU | CAACCCGGA | ||
| 472AU(13+9) | CUCUGUCAUGGCC | AU | CAACCCGG | ||
| 472AU(9+13) | GUCAUGGCC | AU | CAACCCGGAAAG | ||
| 476AC(10+10) | AUGGCCAUCA | AC | CCGGAAAGC | ||
| B | 685AC(13+9) | CUCCUUCUUCUUA | AC | UGGCCUGG | |
| 694GU(13+9) | CUUAACUGGCCUG | GU | AUUCUACC | ||
| 696AU(13+9) | UAACUGGCCUGGU | AU | UCUACCUG | ||
| 765GU(13+9) | UGUCUUUGACUGU | GU | UCCUUCUG | ||
| 765GU(9+13) | UUUGACUGU | GU | UCCUUCUGGUCA | ||
| C | 799AC(13+9) | GCUGAUCCCCUCC | AC | GUCCAUUG | |
| 801GU(13+9) | UGAUCCCCUCCAC | GU | CCAUUGCU | ||
| 801GU(9+13) | CCCCUCCAC | GU | CCAUUGCUGUGC | ||
| 805AU(13+9) | CCCCTCCACGTCC | AU | UGCUGUGC | ||
| 808GC(13+9) | CUCCACGUCCAUU | GC | UGUGCCCU | ||
| 811GU(10+10) | GUCCAUUGCU | GU | GCCCUUGAU | ||
| 811GU(13+9) | CACGUCCAUUGCU | GU | GCCCUUGA | ||
| 811GU(9+13) | UCCAUUGCU | GU | GCCCUUGAUUGG | ||
| 811GU(13+13) | CACGUCCAUUGCU | GU | GCCCUUGAUUGG | ||
| Synthetic RNA substrates | 694GU(7+7) | UGGCCUG | GU | AUUCUA | |
| 694AU(7+7) | UGGCCUG | AU | AUUCUA | ||
| 694GC(7+7) | UGGCCUG | GC | AUUCUA | ||
| 694AC(7+7) | UGGCCUG | AC | AUUCUA | ||
| Chemically modified DNAzymes | 463GU(10+10)LNA | CGACGGCUCU | GU | CGUGGCCA | |
| 463GU(10+10)iT | TCGACGGCUCU | GU | CGUGGCCAT | ||
RNA substrate preparation, in vitro transcription and in vitro cleavage reactions
cDNA encoding the human AChR α-subunit was subcloned into pcDNA3.1hygro (Invitrogen). The full coding sequence of α-subunit was synthesised from its cDNA using the Megascript™ T7 in vitro transcription kit (Ambion Biosciences) to generate 32P- labelled RNA substrate (cRNA).
To determine DNAzyme catalytic activity, target cRNA and DNAzyme were incubated under simulated physiological conditions of 2 mM MgCl2, 150 mM KCl, 50 mM Tris-HCl, pH 7.5, at 37° C (Santoro and Joyce, 1997) for 4 hr. Certain reactions were also performed at 10, 25 and 50 mM Mg2+. The reactions were carried out under single turn over conditions using a ratio for DNAzyme:substrate of 10:1. Reactions were terminated by adding 90 μl stop buffer (95% (v/v) formamide, 0.025% (w/v) xylene cyanol, 0.025% (w/v) bromophenol blue, 18 mM EDTA and 0.025% (w/v) SDS) and were run on a 6% (w/v) denaturing polyacrylamide gel.
Analysis of DNAzyme cleavage efficiency
Gels were dried and exposed overnight to a phosphor storage screen and scanned in a PhosphorImager (Fuji). The cleavage efficiency of labelled transcripts was quantified by measuring the band intensity of the cleaved products (P1 and P2) and the remaining substrate (S). The percentage cleavage (%C) was calculated using the equation %C = [(P1+P2)/ (P1+P2+S)] × 100 where P1 and P2 are the 5′ and 3′products, S is the substrate. This equation is modified from Werner and Uhlenbeck (1995). The data presented are the means of at least 3 repeated experiments unless otherwise stated.
Cell culture and transfection
cDNAs encoding the AChR α, β, δ and ε subunits were co-transfected with respective DNAzymes into HEK 293 cells grown in 6-well tissue culture plates. A total of 3 μg of AChR subunit cDNA and 700 pmol of DNAzymes were used per well. Surface AChR expression was determined by incubating the cells, 48 hr post-transfection, in 10 nM 125I-α-bungarotoxin (BuTx) for 1 hr, followed by washing in PBS. Cells were removed from the plate and 125I-α-BuTx binding measured using a gamma counter.
RESULTS
DNAzyme structure: cleavage sites
The cleavage efficiencies for DNAzymes targeting different cleavage sites within the AChR α-subunit cRNA were investigated in three regions (Figure 1, regions A, B and C). In order to minimise the effect of the RNA secondary structure, cleavage sites close to each other within each region were targeted. In region A these were 463GU, 472AU and 476AC. Symmetrical (10+10) DNAzymes against these sites were incubated with AChR α-subunit cRNA under simulated physiological conditions for 4 hr. The results showed that the substrate was cleaved at the GU, AU and AC sites with efficiencies of 62%, 73% and 9%, respectively. The AC site was significantly poor compared to GU and AU sites (P < 0.001 for each) (Figure 1, region A). Region B was targeted at four cleavage sites: 685AC, 689GC, 694GU and 696AU, with asymmetrical (13+9) DNAzymes. Sites GU and AU were efficiently cleaved with over 90% cleavage (Figure 1, region B). By contrast, the AC and GC sites showed significantly lower levels of cleavage than the GU and AU sites (P < 0.001). In the third region, region C, cleavage sites 799AC, 801GU, 805AU and 808GC were targeted. The AC target site was cleaved least (P < 0.001), whereas GU, GC and AU sites were efficiently cleaved (Figure 1, region C). In this region the GC site showed 54% cleavage efficiency in contrast to the result from region B. Cleavage at the AC sites was slightly improved if the reaction conditions used higher Mg2+ concentrations (10 and 50 mM) but was still far below levels of cleavage at the GU and AU sites (data not shown).
Figure 1.
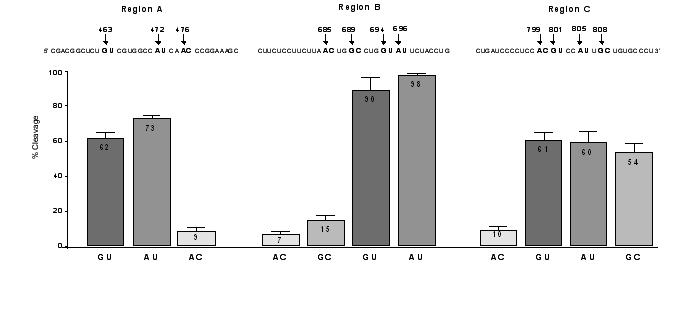
Efficiency of 10-23 DNAzyme at different cleavage sites. A series of different cleavage sites within regions A, B and C of the AChR α-subunit target sequence was studied to investigate 10-23 DNAzyme cleavage efficiency. Each panel shows the substrate sequence targeted by each DNAzyme. Cleavage was carried out under simulated physiological conditions of 2 mM Mg2+ for four hours. In region A three cleavage sites were targeted (AU, GU and AC). In regions B and C four cleavage sites (AC, GC, GU and AU) were targeted.
We next performed a time course of enzymatic cleavage under simulated physiological conditions. DNAzymes targeting region A, 463GU(10+10), 472AU(10+10) and 476AC(10+10) were assessed. For the AU-targeted DNAzyme, 50% of the substrate was cleaved within 30 min, whereas for the GU-targeted DNAzyme this was achieved after approximately 1 hr, and the AC-targeted DNAzyme showed minimal cleavage never reaching 50% (Figure 2A). After 8 hr, maximum cleavage of the substrate of 90% and 77% for the AU and GU sites respectively had been achieved, whereas the AC site was still cleaved by less than 14%. In region B, the DNAzyme 694GU(13+9) demonstrated the highest cleavage efficiency, over 50% of substrate was cleaved after 15 min, increasing to 74% cleavage after 30 min and almost reaching completion by 4 hr. By contrast, 685AC(13+9) showed insignificant cleavage after eight hours (Figure 2 B). In region C, DNAzymes 801GU(13+9) and 808GC(13+9) showed similar catalytic behaviour, achieving about 50% cleavage after approximately three hours, while DNAzyme 799AC(13+9) yielded less than 13% cleavage after eight hours (Figure 2 C).
Figure 2.
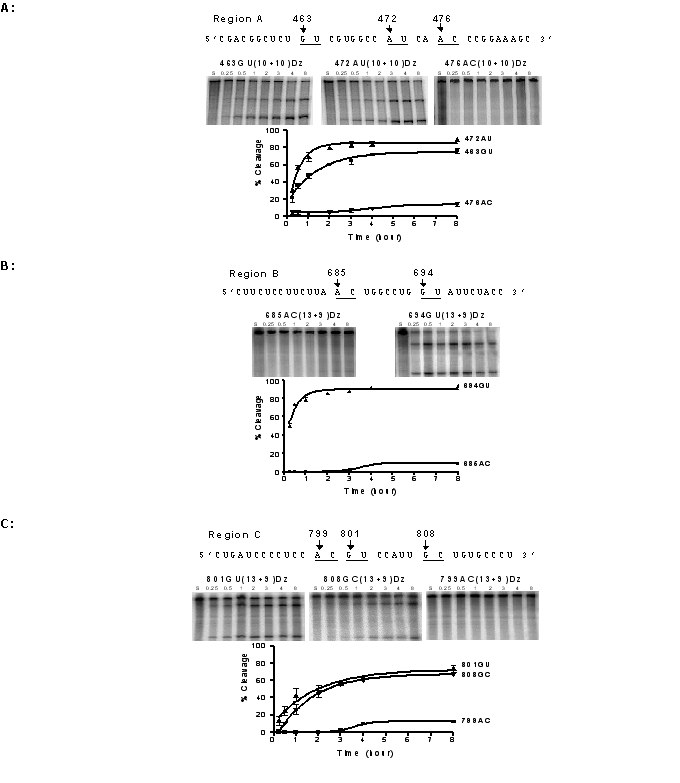
Time course of cleavage reactions. Reactions under simulated physiological conditions were followed with respect to incubation time for DNAzymes in each of the target regions. Samples were analysed at 0.25, 0.5, 1, 2, 3, 4, and 8 hr. Lane S represents uncleaved full length substrate. (A) DNAzymes targeting cleavage sites (GU, AU and AC) in region A, example autoradiographs of in vitro cleavage reactions, and plots % of the cleavage. (B) DNAzymes targeting cleavage sites (AC and GU) in region B, example autoradiographs of in vitro cleavage reactions and plots of % cleavage. (C) DNAzymes targeting cleavage sites (AC, GU, and GC) in region C, autoradiographs of in vitro cleavage reactions and plots of % cleavage.
Kinetic analysis of cleavage efficiency for the alternative RY nucleotide pairs
The above results suggest a hierarchy for cleavage efficiency as follows: AU = GU > GC ⋙ AC. To confirm this hierarchy, we investigated their cleavage efficiency using kinetic analysis. 694GU was found to be the most efficiently cleaved site and so was used for this analysis. Four short RNA substrates were synthesised differing only by the cleavage site residues. Thus, the 15-nucleotide sequence UGGCCUG GU AUUCUA was used as substrate and the GU cleavage site replaced by AU, GC or AC to give four respective separate RNA oligonucleotides. Each of the four synthetic substrates was incubated with the relevant DNAzyme and cleavage reactions were performed under DNAzyme-saturated (single-turnover) conditions at 37°C. In order to exclude any effects from low Mg2+ levels, cleavage reactions were performed in high Mg2+ concentration (25 mM MgCl2). The cleavage rates were analysed for each of the DNAzymes by calculating the kobs (min−1) ×10−3 (Table 2). The results show that the GU site cleaved twice as fast as the AU, and the AU was 75 times faster than the GC. By contrast, the AC site was the slowest. From the results of this experiment the hierarchy for cleavage efficiency may be arranged in the descending order GU>AU> GC>AC.
Table 2.
Cleavage rate constants of substrates were measured in 50 mM Tris-HCl (pH 7.0) and 25 mM MgCl2 under enzyme-saturated (single-turnover) conditions at 37°C.
| DNAzyme | Cleavage site | kobs (min−1) ×10−3 |
|---|---|---|
| 694GU(7+7) | GU | 63.30 |
| 694AU(7+7) | AU | 30.80 |
| 694GC(7+7) | GC | 00.41 |
| 694AC(7+7) | AC | 00.14 |
DNAzyme structure: length and symmetry of the binding arms
DNAzyme catalytic activity is influenced by the stability of binding to the RNA substrate (Ota et al, 1998; Lustig et al, 1995) and thus will be affected by the length of the binding arms. We first studied symmetrical DNAzymes targeted to the 801GU site. A symmetrical 801GU(10+10) DNAzyme yielded 41% substrate cleavage after 4 hr, and lengthening the binding arms in the DNAzyme 801GU(13+13) increased the cleavage efficiency to 77% (Figure 3A). Two symmetrical DNAzymes 805AU(10+10) and 805AU(13+13) yielded 55% and 76% cleavage respectively, but when the length of the arms was increased to 805AU(21+21) cleavage efficiency was reduced to 40% (data not shown).
Figure 3.
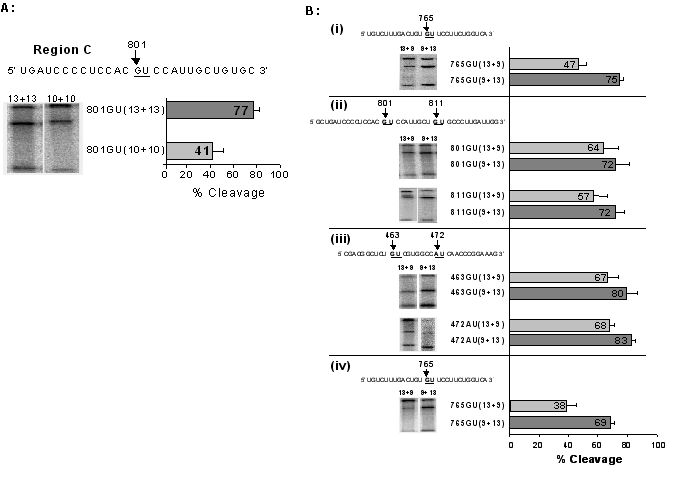
Binding arm length and asymmetric DNAzymes. (A) The 801GU cleavage site in region C was targeted by two DNAzymes with differing binding arm lengths (13+13 and 10+10) under simulated physiological conditions. Example autoradiographs of in vitro cleavage reactions for the 13+13 and 10+10 DNAzymes are shown. The cleavage efficiency after four hours, determined from four independent experiments in each case, is shown following quantification of 32P signals by phosphoimage analysis. (B) Pairs of asymmetrical DNAzymes with differing binding arm lengths were targeted at the five different cleavage sites using simulated physiological conditions. In each case example autoradiographs of in vitro cleavage reactions and the cleavage efficiency after four hours, determined from four independent experiments, are shown. (i) Cleavage of a short 459 nucleotide AChR α-subunit cRNA substrate at the 764GU site, by 764GU(13+9) and 764GU(9+13) DNAzymes. (ii) Cleavage of full-length α-subunit cRNA substrate at two cleavage sites 801GU and 811GU. (iii) Cleavage of full-length α-subunit cRNA substrate at two cleavage sites 463GU and 472AU. (iv) Cleavage of a full-length α-subunit cRNA substrate at the 764GU site at 10 mM Mg2+.
To investigate further the effect of each binding arm, two DNAzymes with asymmetrical binding arms, 765GU(13+9) and 765GU(9+13), were designed to target a short 459 nucleotide AChR α-subunit cRNA substrate. The 765GU(13+9) DNAzyme cleaved 47% of the target whereas the 765GU(9+13) DNAzyme cleaved with a higher efficiency of 75% (Figure 3B(i)). We then examined the effect of alternative lengths of arm I and arm II on catalytic activity by designing pairs of asymmetric DNAzymes, (13+9) and (9+13) and targeting the full length AChR α-subunit cRNA at sites in regions A and C. GU or AU cleavage sites were targeted in the three different regions and in each case the DNAzyme with longer arm I (9+13) gave greater cleavage of the substrate (Figure 3B). Pairwise comparison of (9+13) versus (13+9) DNAzymes for the five target sites showing greater cleavage efficiency gave a statistically significant p value of 0.00002 by Student's t-test. This greater cleavage efficiency for asymmetric DNAzymes with longer arm I was also maintained when the Mg2+ levels were increased to 10 mM (Figure 3B(iv)).
Cellular cleavage of α-subunit AChR
Chemical modifications may be used to stabilise DNAzymes against attack by cellular nucleases. The cellular efficacy of modified DNAzymes targeted in region A was assessed. Modified 463GU(10+10) DNAzymes with either 3′-3′-inverted thymidine (iT) or locked nucleic acids (LNA) containing a 2′-O, 4′-C methylene bridge were first assessed in vitro. Modifying the DNAzyme with the (iT) dramatically reduced its catalytic activity: 13% cleavage versus 67% for the standard DNAzyme (Figure 4 A). By contrast, the LNA-DNAzyme gave enhanced cleavage of the cRNA substrate, 87% versus 67%. The modified DNAzymes were next targeted at AChR α-subunit mRNA transcript in cells. We first established efficient transfection into HEK 293 cells using a FITC-labelled DNAzyme (Figure 4B). Subsequently, cDNA encoding the AChR α, β, δ and ε subunits were co-transfected with standard iT- or LNA-DNAzymes into HEK293 cells and the expression of AChR was determined by measuring the binding of 125I-labelled α-bungarotoxin (125I-α-BuTx) to the surface of the cells. Measurements were normalised to surface expression in cells co-transfected with a non-specific DNAzyme. Whereas both the standard DNAzyme and the iT-DNAzyme show only a modest effect on AChR expression, the LNA-DNAzyme efficiently down-regulated surface expression of the AChR to only 13% of the positive control value. Co-transfection of an LNA-modified DNAzyme targeted at EGFP had no inhibitory effect on surface AChR expression (Figure 4C).
Figure 4.
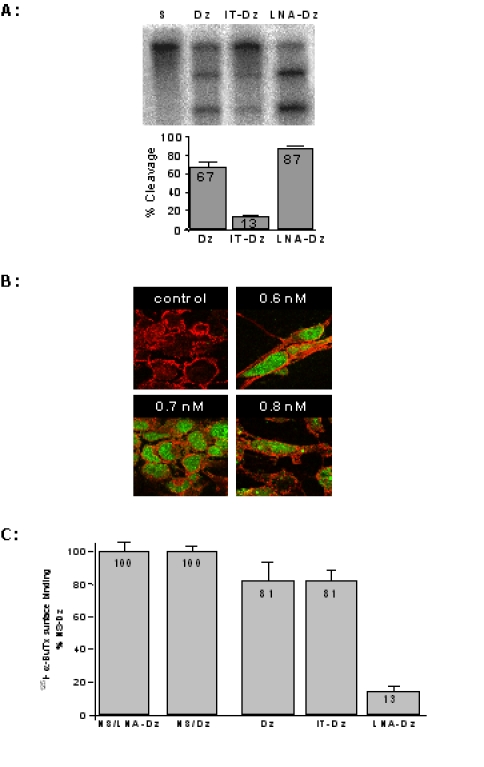
Cleavage and cellular silencing of AChR α-subunit cRNA by modified DNAzymes. (A) In vitro cleavage by modified DNAzymes of full coding region AChR α subunit cRNA using a standard DNAzyme, inverted T (iT) and locked nucleic acid (LNA) modified DNAzymes. Cleavage was performed under simulated physiological conditions for 4 hr. Reaction products were size fractionated on a 6% (w/v) sequencing gel and cleavage was determined by phosphorimage analysis. % cleavage was determined from three independent experiments. (B) Transfection of DNAzymes into HEK 293 cells. HEK 293 cells were transfected with varying amounts of FITC-labelled 463GU(10+10) DNAzyme (green) and visualised using confocal microscopy 48 hr post-transfection. Cells were visualised by immunoflorescence of a membrane-protein CD44 (red). (C) Silencing of AChR expression by an LNA-modified DNAzyme. cDNA encoding the human muscle AChR α, β, δ and ε subunits were co-transfected with a non-specific DNAzyme (NS-Dz), standard DNAzyme (Dz), iT-DNAzyme (iT-Dz) or LNA-DNAzyme (LNA-Dz) into HEK 293 cells. Total 125I- α-BuTx binding to the surface of the transfected HEK 293 cells was measured 48 hr post-transfection. Results are normalised to 125I- α-BuTx binding to the α β δ ε + NS-Dz transfection and represent the mean ± SD of five experiments.
DISCUSSION
Understanding the requirements for optimal DNAzyme activity under cellular conditions will establish the knowledge base necessary for efficient in vivo applications of these molecules. We were therefore interested to optimise the cleavage process for a cRNA transcript under simulated physiological conditions, and in a cellular model. As a model we have studied the AChR α-subunit cRNA transcript as a target substrate, in which pathogenic mutations have been previously described (recently reviewed in Engel and Sine, 2005). Here we demonstrate efficient cleavage of the target RNA transcript in regions where mutations have been identified. 10-23 DNAzymes show preferential cleavage, at the four possible cleavage sites that can be arranged in the following hierarchical order: GU ≥ AU > GC ⋙ AC. This order is closely aligned to the AU = GU ≥ GC ≫ AC hierarchy reported by Cairns et al (2003), where the reduced activity against AC was ascribed to the stronger Watson-Crick interaction of rC-dG, which leads to reduced catalysis. Modification of DNAzymes which weakened the Watson-Crick interaction at this residue dramatically enhanced activity against the AC cleavage site. As expected, longer symmetrical binding arms confer greater cleavage, but our investigations also revealed that arm I plays a dominant role in determining activity. Based upon our in vitro studies, we were able to create an LNA-modified DNAzyme that was highly effective in silencing AChR expression in HEK 293 cells.
Cleavage sites
Comparison of the four 10-23 DNAzyme sites, AC, AU, GC and GU, in three different regions revealed differences in their cleavage efficiencies as follows: GU ≥ AU > GC ⋙ AC. Kinetic analysis of cleavage efficiency used short synthetic substrates in order to minimize the influence of RNA secondary structure. Nevertheless we found the results using cRNAs harbouring the full AChR α-subunit coding region under simulated physiological conditions correlate well with our results from the kinetic analysis and with the previous reported analysis of the cleavage sites in short RNA substrates (Cairns et al, 2003). The difference in cleavage efficiency obtained between the regions, A, B and C of the AChR α-subunit mRNA are likely due to differing secondary structure and sequence context of the binding arms. Within the three regions we observed little difference between GU and AU cleavage sites, but did encounter an unexpectedly efficient GC site in region C. Our data support previous findings (Cairns et al, 2003) of AC sites being the least efficient for DNAzyme cleavage and may well provide an alternative explanation for a previous report in which lack of cleavage by two DNAzymes targeting AC sites in the TAT RNA HIV-1 gene was attributed to RNA secondary structure (Unwalla and Banerjea, 2001).
Asymmetrical DNAzymes
The cleavage process depends on DNAzyme-substrate binding substrate (Ota et al, 1998; Lustig et al, 1995), which, in turn, is affected by the length of the DNAzyme recognition arms. Not surprisingly, under single turnover conditions, long-armed DNAzymes were found to be more efficient than short ones in agreement with previous findings (Shimayama et al, 1995; Oketani et al, 1999). However, the respective arm length of arms I or II seems also to play a role in determining the DNAzyme activity as observed in comparing asymmetric DNAzymes. Those asymmetric molecules with a shorter arm I showed lower catalytic efficiency than those with a shorter arm II. In our results this effect was consistent at five different cleavage sites with pairs of asymmetric DNAzymes under simulated physiological conditions (Figure 3B). Interestingly, a dominant role for arm I in determining catalytic activity has been reported for the hammerhead ribozyme equivalent (helix I) (Hertel et al, 1996). Crystallographic studies have revealed that hammerhead ribozyme helices I (equivalent to DNAzyme arm I) and II (the catalytic core) are required to be in close proximity for function (Tuschl et al, 1994; Lustig et al, 1995), and recently it was also shown that some residues in helix I (e.g., positions 1.5 and 1.6) specifically bind the catalytic core (Dunham et al, 2003). Given that the catalytic mechanism for DNAzymes is thought to be similar to that of hammerhead ribozymes (He et al, 2002), our data, together with these reports, suggest a dominant role for arm I over arm II in determining catalytic efficiency. This is consistent with the observation that the activity of asymmetrical DNAzymes is more sensitive to mismatches in arm I than arm II (Abdelgany et al, 2005).
Silencing of AChR expression in HEK293 cells
Preventing the rapid cellular degradation of DNAzymes is crucial for most therapeutic applications. We show that an LNA-modified DNAzyme has a significant cellular efficacy resulting in efficient silencing of AChR in cells with a reduction in surface 125I-α-BuTx binding to 13% of the control. To examine the effects of chemical modifications on cleavage efficiency, we selected an efficient DNAzyme, 463GU(10+10) and modified it either by in-corporating inverted thymidines (iT) or the locked nucleic acid (LNA) method. Chemical modifications are likely to influence DNAzyme catalytic activity; the LNA modification has previously been shown, for example, to enhance catalytic activity (Vester et al, 2002). The modified DNAzymes were first tested in vitro, and while the iT-DNAzyme activity dropped dramatically, the LNA-DNAzyme activity increased. The stronger binding affinity of LNA/RNA duplex over DNA/RNA (Crinelli et al, 2002) might account for this. The reduced activity of the iT-DNAzyme was unexpected since previous reports have found this modification has minimal effects on kinetic behaviour (Schubert et al, 2003). When tested in cells, the gene silencing of the modified DNAzymes correlated well with their in vitro activity, contrasting with the unmodified DNAzyme, which showed little silencing in the cellular environment. This may well be due to resistance to nuclease degradation conferred by the LNA modification. Although not strictly comparable, the gene silencing effect achieved in HEK293 cells with the LNA-modified DNAzyme was similar to the gene silencing for the AChR expression in HEK 293 cells achieved using siRNA directed against the AChR α-subunit mRNA (Abdelgany et al, 2003). Our results highlight the usefulness of defining optimal DNAzymes in vitro prior to cellular applications.
CONCLUSIONS
10-23 DNAzyme sites were cleaved in the hierarchy GU ≥ AU > GC ⋙ AC in agreement with results of Cairns et al (2003).
Asymmetric DNAzymes with a longer arm I were more efficient than those with a longer arm II, suggesting a role for arm I in enhancing the DNAzyme activity.
In vitro testing of DNAzymes under simulated physiological conditions may help identify efficient DNAzymes for cellular applications.
ACKNOWLEDGMENTS
This work was supported by the Wellcome Trust, the MRC (UK), and the Muscular Dystrophy Campaign / Myasthenia Gravis Association and the Oxford University Challenging Seed Fund (UCSF-94).
STATEMENT OF COMPETING INTERESTS
The authors declared no competing interests.
REFERENCES
- Abdelgany A, Ealing J, Wood M, Beeson D. Selective DNAzyme-mediated cleavage of AChR mutant transcripts by targeting the mutation site or through mismatches in the binding arm. J RNAi Gene Silenc. 2005;1:32–37. [PMC free article] [PubMed] [Google Scholar]
- Abdelgany A, Wood M, Beeson D. Allele-specific silencing of a pathogenic mutant acetylcholine receptor subunit by RNA interference. Hum Mol Genet. 2003;12:2637–2644. doi: 10.1093/hmg/ddg280. [DOI] [PubMed] [Google Scholar]
- Achenbach JC, Chiuman W, Cruz RP, Li Y. DNAzymes: from creation in vitro to application in vivo. Curr Pharm Biotechnol. 2004;5:321–336. doi: 10.2174/1389201043376751. [DOI] [PubMed] [Google Scholar]
- Basu S, Sriram B, Goila R, Banerjea AC. Targeted cleavage of HIV-1 coreceptor-CXCR-4 by RNA-cleaving DNA-enzyme: inhibition of coreceptor function. Antiviral Res. 2000;46:125–134. doi: 10.1016/s0166-3542(00)00075-9. [DOI] [PubMed] [Google Scholar]
- Cairns MJ, Hopkins TM, Witherington C, Sun LQ. The influence of arm length asymmetry and base substitution on the activity of the 10-23 DNA enzyme. Antisense Nucleic Acid Drug Dev. 2000;10:323–332. doi: 10.1089/oli.1.2000.10.323. [DOI] [PubMed] [Google Scholar]
- Cairns MJ, King A, Sun LQ. Optimisation of the 10-23 DNAzyme-substrate pairing interactions enhanced RNA cleavage activity at purine-cytosine target sites. Nucleic Acids Res. 2003;31:2883–2889. doi: 10.1093/nar/gkg378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crinelli R, Bianchi M, Gentilini L, Magnani M. Design and characterization of decoy oligonucleotides containing locked nucleic acids. Nucleic Acids Res. 2002;30:2435–2443. doi: 10.1093/nar/30.11.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxen R, Newland C, Beeson D, et al. Mutations in different functional domains of the human muscle acetylcholine receptor alpha subunit in patients with the slow-channel congenital myasthenic syndrome. Hum Mol Genet. 1997;6:767–774. doi: 10.1093/hmg/6.5.767. [DOI] [PubMed] [Google Scholar]
- Dunham CM, Murray JB, Scott WG. A helical twist-induced conformational switch activates cleavage in the hammerhead ribozyme. J Mol Biol. 2003;332:327–336. doi: 10.1016/s0022-2836(03)00843-x. [DOI] [PubMed] [Google Scholar]
- Engel AG, Sine SM. Current understanding of congenital myasthenic syndromes. Curr Opin Pharmacol. 2005;5:308–321. doi: 10.1016/j.coph.2004.12.007. [DOI] [PubMed] [Google Scholar]
- He QC, Zhou JM, Zhou DM, Nakamatsu Y, Baba T, Taira K. Comparison of metal-ion-dependent cleavages of RNA by a DNA enzyme and a hammerhead ribozyme. Biomacromolecules. 2002;3:69–83. doi: 10.1021/bm010095c. [DOI] [PubMed] [Google Scholar]
- Hertel KJ, Herschlag D, Uhlenbeck OC. Specificity of hammerhead ribozyme cleavage. EMBO J. 1996;15:3751–3757. [PMC free article] [PubMed] [Google Scholar]
- Khachigian LM. DNAzymes: cutting a path to a new class of therapeutics. Curr Opin Mol Ther. 2002;4:119–121. [PubMed] [Google Scholar]
- Kurreck J, Bieber B, Jahnel R, Erdmann VA. Comparative study of DNA enzymes and ribozymes against the same full-length messenger RNA of the vanilloid receptor subtype I. J Biol Chem. 2002;277:7099–7107. doi: 10.1074/jbc.M107206200. [DOI] [PubMed] [Google Scholar]
- Liu C, Cheng R, Sun LQ, Tien P. Suppression of plate-let-type 12-lipoxygenase activity in human erythroleukemia cells by an RNA-cleaving DNAzyme. Biochem Biophys Res Commun. 2001;284:1077–1082. doi: 10.1006/bbrc.2001.5077. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang Y, Wan C, et al. The role of mPer1 in morphine dependence in mice. Neuroscience. 2005;130:383–388. doi: 10.1016/j.neuroscience.2004.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig B, Lin NH, Smith SM, Jernigan RL, Jeang KT. A small modified hammerhead ribozyme and its conformational characteristics determined by mutagenesis and lattice calculation. Nucleic Acids Res. 1995;23:3531–3538. doi: 10.1093/nar/23.17.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oketani M, Asahina Y, Wu CH, Wu GY. Inhibition of hepatitis C virus-directed gene expression by a DNA ribonuclease. J Hepatol. 1999;31:628–634. doi: 10.1016/s0168-8278(99)80341-9. [DOI] [PubMed] [Google Scholar]
- Ota N, Warashina M, Hirano K, Hatanaka K, Taira K. Effects of helical structures formed by the binding arms of DNAzymes and their substrates on catalytic activity. Nucleic Acids Res. 1998;26:3385–3391. doi: 10.1093/nar/26.14.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro SW, Joyce GF. A general purpose RNA-cleaving DNA enzyme. Proc Natl Acad Sci USA. 1997;94:4262–4266. doi: 10.1073/pnas.94.9.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert S, Furste JP, Werk D, Grunert HP, Zeichhardt H, Erdmann VA, Kurreck J. Gaining target access for deoxyribozymes. J Mol Biol. 2004;339:355–363. doi: 10.1016/j.jmb.2004.03.064. [DOI] [PubMed] [Google Scholar]
- Schubert S, Gul DC, Grunert HP, Zeichhardt H, Erdmann VA, Kurreck J. RNA cleaving ‘10-23’ DNAzymes with enhanced stability and activity. Nucleic Acids Res. 2003;31:5982–5992. doi: 10.1093/nar/gkg791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimayama T, Nishikawa S, Taira K. Extraordinary enhancement of the cleavage activity of a DNA-armed hammerhead ribozyme at elevated concentrations of Mg2+ ions. FEBS Lett. 1995;368:304–306. doi: 10.1016/0014-5793(95)00682-y. [DOI] [PubMed] [Google Scholar]
- Sioud M, Leirdal M. Design of nuclease resistant protein kinase calpha DNA enzymes with potential therapeutic application. J Mol Biol. 2000;296:937–947. doi: 10.1006/jmbi.2000.3491. [DOI] [PubMed] [Google Scholar]
- Sriram B, Banerjea AC. In vitro-selected RNA cleaving DNA enzymes from a combinatorial library are potent inhibitors of HIV-1 gene expression. Biochem J. 2000;352(Pt 3):667–673. [PMC free article] [PubMed] [Google Scholar]
- Toyoda T, Imamura Y, Takaku H, et al. Inhibition of influenza virus replication in cultured cells by RNA-cleaving DNA enzyme. FEBS Lett. 2000;481:113–116. doi: 10.1016/s0014-5793(00)01974-8. [DOI] [PubMed] [Google Scholar]
- Tuschl T, Gohlke C, Jovin TM, Westhof E, Eckstein F. A three-dimensional model for the hammerhead ribozyme based on fluorescence measurements. Science. 1994;266:785–789. doi: 10.1126/science.7973630. [DOI] [PubMed] [Google Scholar]
- Unwalla H, Banerjea AC. Novel mono- and di-DNA-enzymes targeted to cleave TAT or TAT-REV RNA inhibit HIV-1 gene expression. Antiviral Res. 2001;51:127–139. doi: 10.1016/s0166-3542(01)00144-9. [DOI] [PubMed] [Google Scholar]
- Vester B, Lundberg LB, Sorensen MD, Babu BR, Douthwaite S, Wengel J. LNAzymes: incorporation of LNA-type monomers into DNAzymes markedly increases RNA cleavage. J Am Chem Soc. 2002;124:13682–13683. doi: 10.1021/ja0276220. [DOI] [PubMed] [Google Scholar]
- Wang DY, Lai BH, Feldman AR, Sen D. A general approach for the use of oligonucleotide effectors to regulate the catalysis of RNA-cleaving ribozymes and DNAzymes. Nucleic Acids Res. 2002;30:1735–1742. doi: 10.1093/nar/30.8.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner M, Uhlenbeck OC. The effect of base mis-matches in the substrate recognition helices of hammerhead ribozymes on binding and catalysis. Nucleic Acids Res. 1995;23:2092–2096. doi: 10.1093/nar/23.12.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Dass CR, Sumithran E, Di Girolamo N, Sun LQ, Khachigian LM. Effect of deoxyribozymes targeting c-Jun on solid tumor growth and angiogenesis in rodents. J Natl Cancer Inst. 2004;96:683–696. doi: 10.1093/jnci/djh120. [DOI] [PubMed] [Google Scholar]


