Abstract
The shutoff of active intermediates in the phototransduction cascade and the reconstitution of the visual pigment play key roles in the recovery of sensitivity after the exposure to bright light in both rod and cone photoreceptors. Physiological evidence from bleached salamander rods suggests this recovery of sensitivity occurs faster at the outer segment base compared with the tip. Microfluorometric measurements of similarly bleached salamander rods demonstrate that the reduction of all-trans retinal to all-trans retinol also occurs more rapidly at the outer segment base than at the tip. The experiments reported here were designed to test the hypothesis that these two phenomena are linked, e.g., that slowed recovery of sensitivity at the tip of outer segments is rate limited by the reduction of all-trans retinal and results from a shortage of cytosolic nicotinamide adenine dinucleotide phosphate (NADPH), the reducing agent for all-trans retinal reduction. Extracellular measurements of membrane current and sensitivity were made from isolated salamander rods under dark-adapted and bleached conditions while intracellular NADPH concentration was varied by dialysis from a micropipette attached to the inner segment. Sensitivity at the base and tip of the outer segment was assessed before and after bleaching. After exposure to a light that photoactivates 50% of the visual pigment, rods were completely insensitive for nearly 10 minutes, after which the base recovered sensitivity and responsiveness with a time constant of ∼200 seconds, but tip sensitivity recovered more slowly with a time constant of ∼680 seconds. Dialysis of 5 mM NADPH into the rod promoted an earlier recovery and eliminated the previously observed tip/base difference. Dialysis of 1.66 mM NADPH failed to eliminate the tip/base recovery difference, suggesting the steady-state NADPH concentration in rods is ∼1 mM. These results indicate the inner segment is the primary source of reducing equivalents after pigment bleaching, with the reduction of all-trans retinal to all-trans retinol playing a key step in the recovery of sensitivity.
INTRODUCTION
The visual pigment, rhodopsin (Rh) in rods, is one of a large family of G protein–coupled receptors that is embedded in the photoreceptor outer segment and consists of a seven α-helical transmembrane protein (opsin) covalently bound to the light-absorbing chromophore 11-cis retinal. Photon absorption by Rh triggers a rapid cis to trans photoisomerization of the retinal chromophore, leading to the formation of an activated form called Meta II, or Rh*. This initial step in visual transduction activates a G protein–coupled receptor biochemical cascade that results in the destruction of cGMP, the closure of cGMP-gated membrane channels, a hyperpolarization of the rod cell membrane potential, and, finally, the reduction in the release of glutamate onto second order cells in the retina.
Exposure to bright light that photoactivates (bleaches) a significant fraction of the visual pigment in rod and cone photoreceptors leads to a persistent loss of visual sensitivity, a condition referred to as bleaching adaptation. The recovery of sensitivity that occurs in subsequent darkness, termed dark adaptation, is dependent on the efficient and timely quenching of the visual pigment’s catalytic activity and its reconstitution to the dark-adapted ground state. This latter process occurs through an ensemble of biochemical reactions collectively referred to as the visual cycle (for review see Lamb and Pugh, 2004). In rods, the visual cycle begins with the decay of Rh*’s catalytic activity when all-trans retinal dissociates from the chromophore binding pocket of Rh and is reduced to all-trans retinol by retinol dehydrogenase (RDH), which uses nicotinamide adenine dinucleotide phosphate (NADPH) as a cofactor (Futterman et al., 1970). All-trans retinol is then transported to the retinal pigment epithelium where it is reconverted to 11-cis retinal and returned to the photoreceptor, where it forms a Schiff base with the opsin apoprotein, thereby reconstituting the visual pigment.
Several biochemical studies have implicated RDH in limiting the rate of response recovery of rods after pigment bleaching. For instance, Palczewski et al. (1994) suggested that the RDH could influence the phototransduction cascade by regulating the activities of transducin, Rh kinase, and arrestin, as well as the rate of pigment regeneration. Based on other studies, Rattner et al. (2000) also argued that the activity of this enzyme is essential for regulating the recovery of sensitivity after bleaching light. Their conclusions were based on the observation that all-trans retinal can combine in vitro with opsin to form complexes that have significant G protein activity (Jäger et al., 1996), thereby maintaining the activity of the transduction cascade at a high level beyond the time of illumination. The assumption implicit in this argument is that unreduced retinal within the photoreceptor can recombine with opsin to generate these active forms.
Recent physiological and optical studies of intact salamander rod photoreceptors have provided evidence that unreduced retinal may slow the recovery of sensitivity after pigment bleaching by persistently activating phototransduction. Electrophysiological studies of the rate of sensitivity recovery in intact rods exposed to bright bleaching light have demonstrated that visual pigment quenching along the rod outer segment is nonuniform, leading to a wavelike recovery of sensitivity along the outer segment (Jin et al., 1994). Sensitivity recovery occurs fastest at the base and slowest at the tip of the outer segment. Direct measurements of retinol fluorescence along outer segments of uniformly bleached intact rods demonstrate that retinal is reduced at a greater rate at the base than at the tip of the outer segment (Tsina et al., 2004). Finally, microspectrophotometric measurements indicate that the rate of Meta II (Rh*) decay is uniform along the length of the rod outer segment (Ala-Laurila et al., 2006). Collectively, a reasonable interpretation of these observations is that a persistence of retinal, unreduced to retinol, within distal regions of substantially bleached outer segments interacts with opsin to maintain transducin activity at a high level. Because RDH is expressed evenly in all regions within the rod outer segment (Luo et al., 2004), the differential rate of sensitivity recovery along the outer segment may occur secondary to a gradient of NADPH concentration (Ala-Laurila et al., 2006; Sakmar, 2006; Kolesnikov et al., 2007). For example, Kolesnikov et al. (2007) showed in saponin-permeabilized gecko photoreceptors that the introduction of several metabolites, including NADPH, abolishes a base-to-tip gradient in all-trans retinol production.
In the present experiments, we test the role played by NADPH in the differential rate of sensitivity recovery along the intact salamander rod outer segment after visual pigment bleaching. Using a combination of suction electrode recording, to measure the photocurrent, and patch recording, to dialyze internally into the rod various concentrations of NADPH, we show that saturating the rod with NADPH eliminates the concentration gradient along the outer segment and also abolishes the differential rate of recovery of sensitivity at the base and tip of the outer segment. These results allow us to connect causally the recovery of sensitivity in the outer segment with the clearance of all-trans retinal after pigment bleaching, and are explained by a model whereby NADPH, or a precursor, produced predominantly in the inner segment diffuses through the rod photoreceptor cilium and along the length of the outer segment, where it is an obligate participant in the reduction of all-trans retinal.
MATERIALS AND METHODS
Preparation and materials
Experiments were performed on intact rod photoreceptors isolated from the retinae of larval tiger salamanders, Ambystoma tigrinum. Animals were dark-adapted overnight and sacrificed in accordance with protocols approved by the Institutional Animal Care and Use Committee of the University of Southern California and in accordance with the Guide for the Care and Use of Laboratory Animals and the Animal Welfare Act. Animals were killed by decapitation and double pithed. All manipulations were performed either in darkness or under infrared illumination with the aid of infrared image converters. Eyes were enucleated, the lens was removed, and eyecups were stored in darkness on ice in Ringer’s solution bubbled with 100% O2. Ringer’s solution used both for dissociation of the retinae and in which experiments were performed consisted of: 146 mM NaCl, 2.5 mM KCl, 1.6 mM MgCl2, 1.0 mM CaCl2, 10 mM HEPES, 10 mM glucose, and 100 mg/L BSA, pH adjusted to 7.5 with NaOH. Before recordings the retina was finely chopped with a razor blade. The resulting suspension of cells and cellular debris was placed into the recording chamber. Individual cells where then allowed to settle while being superfused with physiological solution at room temperature (17°C).
Electrophysiological recording and light stimulation
The suction pipettes that were used to record the rod outer segment current (Baylor et al., 1979) were made with thin-wall borosilicate glass. These pipettes were pulled to a large tip diameter using a standard micropipette puller (Sutter Instrument Co.), and then their tips melted to yield an open tip whose resistance when filled with physiological solution was measured to be ∼0.5 MΩ. Resistance more than doubled when the cell was drawn into the pipette. Patch electrodes made with borosilicate glass had resistances of 10–15 MΩ. The pipette internal solution (to which NADPH was added) consisted of: 115 mM KAsp, 10 mM KCl, 0.5 mM CaCl2, 5 mM NMG-HEDTA, 10 mM HEPES, 1 mM ATP-Mg, 0.2 mM GTP-Tris, and 0.2 mM MgCl. The pH was adjusted to 7.5, and osmolarity was measured at 270 mOsm. The ground and reference electrodes were coupled to Ringer’s solution with Ag/AgCl bridges.
During dual patch and suction recordings, the rod outer segment was drawn into the suction electrode, whereas whole cell recordings were made from the inner segment. Currents from both recording configurations were collected with Axopatch 200B amplifiers (MDS Analytical Technologies), low-pass filtered at 20 Hz with an 8-pole Bessel filter (Frequency Devices), and digitized and stored for offline analysis using MATLAB (MathWorks). Rod sensitivity was measured from dim flash responses and defined as the amplitude of the flash response (pA) divided by the flash strength (photons/µm2). Offline processing enabled the data to be corrected for drift. Plots of the time course of recovery of sensitivity in darkness after bright light exposure were fitted with single-exponential functions.
Test flashes and bleaching lights were provided by a light stimulator (Cornwall et al., 1990; Ala-Laurila et al., 2007). Light from a 100-W tungsten light source was condensed, passed through an electronic shutter (UNIBLITZ; Vincent Associates), through a 520-nm interference filter (Chroma Technology Corp.), and finally focused as a circular spot of uniform intensity at the level of the preparation. The output of the light stimulator was calibrated at the level of the preparation before each experiment; test flashes were 30 ms in duration. The fractional bleach of visual pigment, F, when rods were exposed to bright light from the photostimulator was calculated according to the relation:
| (1) |
where I is the intensity of the bleaching light, P is the photosensitivity of the visual pigment (6 × 10−9; Jones et al., 1996), and t is the duration of exposure in seconds.
Internal dialysis of rod photoreceptors
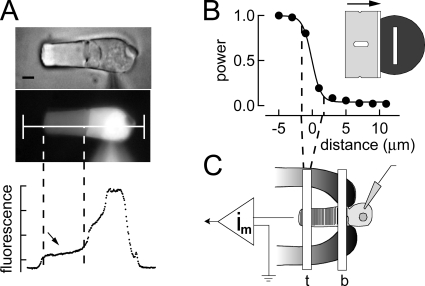
Introduction of NADPH into rod photoreceptors was achieved via a micropipette attached in the whole cell recording mode to the inner segment of the rod. The access resistance during whole cell recordings was ∼15 MΩ. Dialysis recordings were made for ∼2 min before bleaching to ensure that the contents of the patch electrode were in equilibrium with the cytosol. To verify that this period of dialysis was sufficient, we made recordings of outer segment fluorescence after dialysis of rods with Lucifer yellow, a substance of comparable molecular size to NADPH (see Fig. 1). Cellular exchange estimated in this way has been previously measured to be complete within tens of seconds (Holcman and Korenbrot, 2004; Wu et al., 2006). Fluorescence measurements as illustrated in Fig. 1 A were made from rod photoreceptors at 15-s intervals after whole cell break in and reached steady state within 2 min. The roughly uniform loading of dialysate within the outer segment along the long axis of the rod outer segment is illustrated in Fig. 1 A. Based on data such as these, we assume that NADPH is able to diffuse quickly into the rod outer segment.
Figure 1.
Experimental patch dialysis design and characterization of slit width. (A) Bright field and fluorescence images of a salamander rod dialyzed for 2 min with Lucifer yellow by patch pipette. The line drawn down the central axis of the rod indicates the regions from which pixels were selected, and fluorescence was plotted as a function of distance. The region designated between the dashed lines is the outer segment. After 2 min of dialysis, this region’s fluorescence indicates relatively even loading. Bar, ∼5 µm. (B) Measurement of light slit. The power of light that passed through the slit gradually declined as a function of the distance from its edge; this was measured by passing a razor blade from left to right immediately under it and between it and a photo detector. The smooth black curve is a sigmoid curve fitted to the data points. Dashed lines are drawn indicating the drop in power from 90 to 10%, providing an estimate of the fall of light intensity beyond the nominal edge of the slit of ∼3.5 µm. Inset depicts the slit measurement arrangement as seen from above. (C) Schematic representation of the experimental approach. A rod photoreceptor is held in a suction electrode connected to a recording amplifier. Whole cell patch clamp recordings were made with pipettes that either contained control solution, or a solution that included varying concentrations of NADPH. These were made from the inner segment. After bleaching, the slit was positioned at either the base (b) or the tip (t) of the outer segment, and dim flashes were recorded to monitor localized recovery.
Slit characterization
Local measurements of sensitivity along the outer segments of rods were made by measuring flash responses to brief narrow slits of light focused at the base and tip of the outer segments. The spatial resolution and width of this focused stimulus were characterized by measuring the profile of the light intensity detected by a photodiode placed below the microscope stage in the microscope light path. A razor blade mounted to a micromanipulator was advanced in graded increments across the focused image of the slit at the plane of the preparation until the light through the slit was fully occluded. The graph in Fig. 1 B plots the light intensity measured as a function of distance as the slit was moved from left to right. In this plot, the position of the slit is set to zero on the distance axis. The measured slit width was 3.5 µm. The relative intensity changed ∼10-fold over 4 µm. Thus, given an outer segment length of ∼20 µm, we estimate that the light intensity difference due to stimulus spread between the stimulated and nonstimulated ends of the outer segment to be at least 30-fold. Movement of the slit with respect to the outer segment was accomplished by moving the suction pipette holding the cell rather than the slit, as illustrated in Fig. 1 C. Thus, the same slit was used for both tip and base sensitivity measurements; for measurements at the tip, the cell was placed with the stimulus focused at (t), and when measurements were made at the base, the slit was positioned at (b).
RESULTS
Alterations in base and tip sensitivity after pigment bleaching
Exposure of isolated intact rod photoreceptors to bright light that bleaches a large fraction of the visual pigment leads to a persistent loss of outer segment dark current and sensitivity (Cornwall et al., 1990; Jones et al., 1996). This steady-state desensitization has been demonstrated to result from two factors: first, a photochemical destruction of visual pigment, and second, a persistent activation of the transduction cascade by free opsin (Cornwall and Fain, 1994). Its persistence derives from the fact that isolation from the retinal pigment epithelium does not permit regeneration of visual pigment that normally occurs in the intact eye. Thus, in practice, isolation of rods from the RPE allows the experimental separation of recovery of sensitivity attributable to pigment regeneration from recovery that occurs as the activated visual pigment decays.
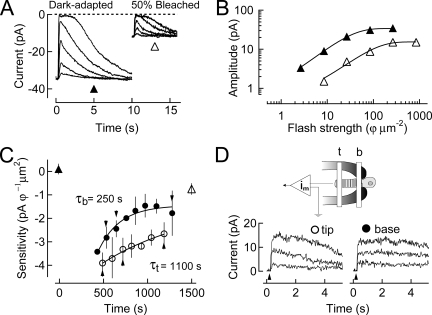
In isolated salamander rods, the recovery of the dark current and sensitivity progress at different rates in different regions of the outer segment, faster at the base and slower at the tip (Jin et al., 1994). An example of this behavior is illustrated in Fig. 2, which shows results from an isolated rod exposed to a light that bleached ∼50% of the visual pigment. Fig. 2 A illustrates families of flash responses elicited when the rod was dark-adapted (left), and then 20 min after exposure to the bleaching light (right). The graph in Fig. 2 B illustrates that in the steady state after bleaching, both response amplitude (dark current) and sensitivity were significantly reduced. In addition, as illustrated in Fig. 2 C, bleaching led to a complete suppression of the dark current for a period of ∼10 min, after which a monotonic recovery occurred, fastest at the base of the outer segment and slowest at the tip. The rates of recovery of sensitivity at the base and the tip of the outer segment were determined by measuring the responses to flashes of light delivered locally to these regions, as illustrated diagrammatically in Fig. 2 D. The intensities of these flashes were chosen to evoke a response that suppressed <25% of the dark current. Accordingly, the time constant for sensitivity recovery was ∼250 s at the base and ∼1,100 s at the tip, a difference of approximately fourfold. In steady state after pigment bleaching, the dark current was reduced by 35% (Fig. 2 A), and the sensitivity remained depressed 40-fold. Such persistent desensitization is characteristic of bleaching adaptation in the absence of pigment regeneration and is significantly greater than the loss predicted by the fraction of pigment bleached (Jones et al., 1996). Experiments similar to this were performed on a total of seven rods. The average ratio of tip/base post-bleach recovery time constants as measured in these cells was ∼3.4 (Table I).
Figure 2.
Recovery of sensitivity after a 50% bleach of the visual pigment. (A) Response families are shown for a dark-adapted cell and at steady state after a 50% bleach. Flash strengths were 2.6, 8.5, 26, 85, and 260 photons/µm2 for the dark-adapted family, and 8.5, 26, 85, 260, and 850 photons/µm2 for the post-bleach family. (B) The amplitude of the response was plotted versus the strength for the dark-adapted and bleached cell shown in A. (C) Changes in log sensitivity after pigment bleaching plotted as a function of time. A 50% bleach was given at time 0 s. Sensitivity before pigment bleaching is denoted by an upward filled triangle. Sensitivity was undetectably low during the period of time where the dark current remained totally suppressed. After this period, there was gradual recovery of sensitivity at both the base (filled circles) and tip (open circles) of the rod outer segment (plotted as mean ± SEM). These recoveries were fit with single-exponential functions that indicate the base recovers to steady state with a time constant of ∼250 s, and the tip with a time constant of ∼1,100 s. Sensitivity once steady state was reached is denoted by an upward open triangle. (D) Suction electrode–only recording configuration for the experiment. Superimposed lower traces show responses to saturating flashes at selected time points during recovery in C.
TABLE I.
Time constants of recovery of sensitivity at the base and tip of the rod outer segment
| n | τb | τt | Tip/Base | |
| s | s | |||
| No whole cell, 50% bleach | 7 | 200 ± 30 | 680 ± 140 | 3.4 ± 0.29 |
| Whole cell (0 mM NADPH), 50% bleach | 5 | 290 ± 20 | 860 ± 80 | 3.1 ± 0.29a |
| Whole cell (1.66 mM NADPH), 50% bleach | 4 | 220 ± 30 | 640 ± 110 | 3.1 ± 0.45 |
| Whole cell (5 mM NADPH), 50% bleach | 5 | 280 ± 50 | 330 ± 60 | 1.2 ± 0.04a |
| No whole cell, 90% bleach | 5 | 210 ± 40 | — | — |
The mean time constant at the base of the outer segment (τb) and the tip of the outer segment (τt), and the tip/base time constant ratio are reported. All values are mean ± SEM for a number of cells, n.
Student’s t test demonstrates a statistically significant difference between whole cell control solution (0 mM NADPH) and whole cell (5 mM NADPH); P < 0.005.
The wavelike recovery of sensitivity observed in Fig. 2 and summarized in Table I is consistent with the results of microfluorometry studies that show that there is a base-to-tip gradient for the reduction of all-trans retinal to all-trans retinol in outer segments of isolated bleached salamander rods (Tsina et al., 2004; Ala-Laurila et al., 2006). These workers suggested that this base-to-tip difference in retinal reduction was secondary to a shortage of NADPH, the cofactor of RDH (see also Kolesnikov et al., 2007).
Excess NADPH eliminates the difference in base-to-tip sensitivity recovery
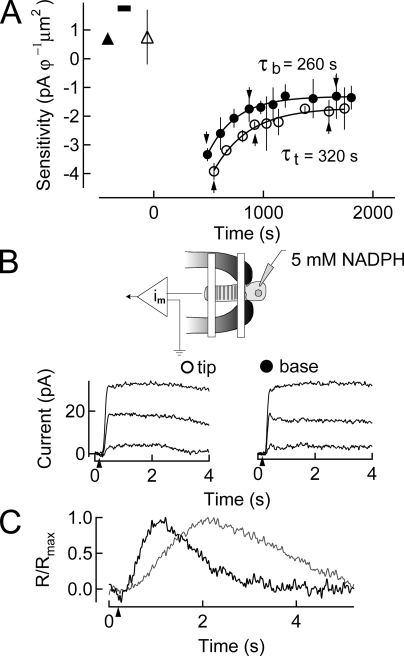
The experiment illustrated in Fig. 3 was designed to test the hypothesis that the slower rate of recovery of sensitivity at the tip compared with the base of isolated bleached rod outer segments arises secondary to a concentration gradient of free NADPH along the length of the rod outer segment. This experiment also tests the hypothesis proposed by Saari et al. (1998) that reduction of all-trans retinal to all-trans retinol by RDH rate limits the recovery of rod sensitivity after large pigment bleaches (see also Discussion). In this experiment, we internally dialyzed rod photoreceptor inner segments with excess NADPH (5 mM) before pigment bleaching, and then followed the time course of sensitivity recovery that occurred in darkness thereafter. The concentration of NADPH loaded into the dialysis pipette was chosen to ensure that all regions of the outer segment had sufficient NADPH to allow reduction of all-trans retinal resulting from a total bleach of the visual pigment.
Figure 3.
Dialysis of 5 mM NADPH into rods before a 50% bleach of the visual pigment. (A) Changes in log sensitivity before and after pigment bleaching were plotted as a function of time. Sensitivity before (upward filled triangle) and after (upward open triangle) dialysis with internal solution containing 5 mM NADPH (black bar) is plotted (mean ± SEM). A 50% bleach was given at time 0 s. Recovery at the base and tip of the outer segment commenced earlier in time than in Fig. 2, with a time constant at the base of the outer segment of ∼260 s, and at the tip of the outer segment of ∼320 s. (B) Suction electrode configuration while dialyzing 5 mM NADPH into the rod inner segment. Lower superimposed traces show responses to saturating flashes at selected time points during recovery in A. (C) Dim flash responses in the dark-adapted cell after dialysis (gray) and at steady state after pigment bleaching (black) indicating the response speeding characteristic of bleaching adaptation.
The time courses of sensitivity changes observed before and after visual pigment bleach and then after dialysis of 5 mM NADPH are illustrated in Fig. 3 A. Sensitivity was determined in dark-adapted conditions before (filled triangle) and after dialysis (open triangle), and then at chosen times during recovery after bleaching (Fig. 3 A). The duration of dialysis is indicated by the horizontal bar at the upper edge of the plot. After the bleach (t = 0 s), the cell was completely unresponsive (dark current = 0 pA) for a period of ∼8 min, after which recovery of responsiveness commenced. In this experiment, the base recovered sensitivity with a time constant of ∼260 s, and the tip with a time constant of ∼320 s. The tip/base ratio was ∼1.2. Also, the superimposed just-saturating flash responses elicited during the response recovery phase (Fig. 3 B) displayed similar time courses, whether elicited at the tip or base of the receptor. The similarity of these response time courses also indicates that sensitivity recovery in these different regions took place at a similar rate. The results from five other experiments on bleached rods dialyzed with 5 mM NADPH yielded an average tip/base sensitivity recovery ratio of ∼1.2, significantly different from the tip/base ratio observed in the absence of dialysis (Table I). Interestingly, dialysis with NADPH also induced a slight slowing of the kinetics of dim flash responses (Fig. 3 C) that was further exacerbated when the internal solution contained 15 mM NADPH (not depicted). However, the post-bleach tip and base flash responses still showed a faster time to peak and an accelerated recovery compared with flash responses elicited before bleach. This is consistent with previous work from others that demonstrates that after Meta II decay, the remaining free opsin persistently activates transducin.
The results presented above are consistent with the notion that dialysis of 5 mM NADPH into the inner segment of the rod provided sufficient reducing power within the outer segment to permit retinal reduction to take place at equal rates along its entire length. These results also highlight the importance of retinal reduction in quenching transducin activation and allowing translocation of all-trans retinol from the outer segment.
Lower NADPH concentrations are insufficient to eliminate the difference in base-to-tip sensitivity recovery
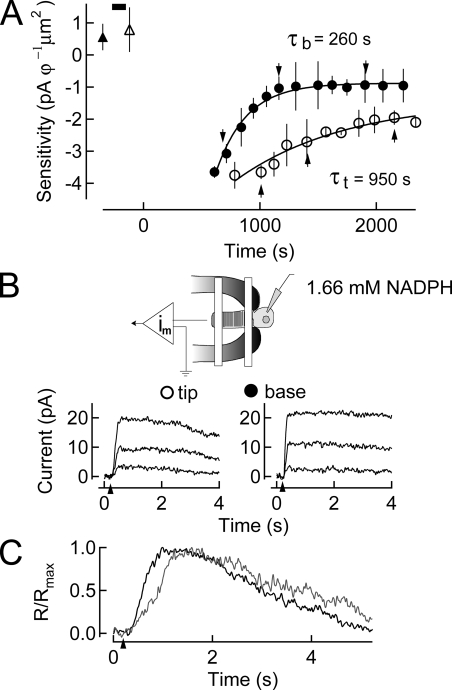
Having shown that 5 mM NADPH can eliminate the tip/base sensitivity recovery difference, we sought to determine if a lower concentration of NADPH could also have a similar effect. The experiment illustrated in Fig. 4 was performed in an effort to estimate the concentration of NADPH at which no effect of dialysis could be observed on the rates of recovery in the tip and base regions of the outer segment. Accordingly, we reduced the NADPH concentration by threefold to 1.66 mM and again dialyzed rod photoreceptors before bleaching.
Figure 4.
Dialysis of 1.66 mM NADPH into rods before a 50% bleach of the visual pigment. (A) Changes in log sensitivity after pigment bleaching plotted as a function of time. Sensitivity before (upward filled triangle) and after (upward open triangle) dialysis with internal solution containing 1.66 mM NADPH (black bar) is plotted (mean ± SEM). A 50% bleach is given at time 0 s. Recovery of sensitivity at the base of the outer segment commenced with a time constant of ∼260 s, and at the tip of the outer segment of ∼950 s. (B) Suction electrode configuration while dialyzing 1.66 mM NADPH into the rod inner segment. On the bottom, responses to saturating flashes at selected time points during recovery in A are shown. (C) Dim flash responses in the dark-adapted cell after dialysis (gray) and at steady state after pigment bleaching (black) indicating the response speeding characteristic of bleaching adaptation.
As was the case in Figs. 2 and 3, sensitivity was little changed by dialysis alone (compare open and filled triangles in Fig. 4 A, top left). About 10 min after the bleach, responsiveness commenced recovery. The time constant of base recovery was measured to be ∼260 s; that at the tip was ∼950 s. For this cell, the tip/base ratio of recovery rate was ∼3.7. In four experiments of this type we observed that the tip/base ratio was ∼3.0 (Table I). This ratio is not significantly different from that observed in the absence of NADPH dialysis. Thus, we conclude that 1.66 mM NADPH does not significantly influence the difference in the time course of recovery of the tip and base regions of the outer segment (Fig. 4 B). Interestingly, the introduction of this extra NADPH, even at a lower concentration, still resulted in slowed flash responses. However, dim flash responses elicited at the base and tip after pigment bleaching share a similar time course and are characteristically accelerated in relation to pre-bleach dim flash responses (Fig. 4 C). The induced slowing of the response due to the presence of excess NADPH presented an opportunity to place an upper limit on the size of the NADPH concentration gradient.
The inability of 1.66 mM NADPH to impact the difference in recovery at the base and tip implies that its outer segment concentration is no longer saturating and that its resting concentration is likely ∼1 mM. It also suggests that the dialysis-induced slowing of the responses observed for dim flash responses in both Figs. 3 and 4 may be independent of NADPH-dependent quenching of transduction activation.
Whole cell dialysis of inner segment does not alter kinetics or recovery
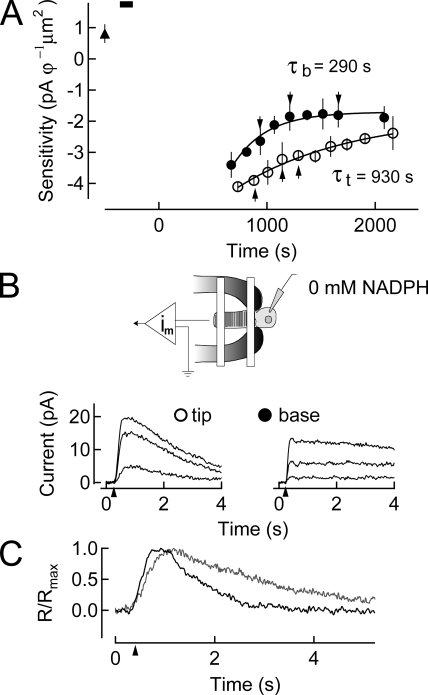
By dialyzing the inner segment, we took advantage of the cell’s compartmentalized structure, which allows for the diffusion of metabolites such as NADPH through the narrow ciliated neck between the inner segment and the outer segment, but prevents the loss of larger proteins required for phototransduction or the retinal reduction. This was demonstrated when we performed similar experiments in which the outer segment was dialyzed directly. These latter experiments resulted in a dramatic slowing of the tip time course of recovery (not depicted). To address the possibility that dialyzing the cell with the patch pipette may have altered the kinetics of recovery, we dialyzed the inner segment of the rods with a control solution that closely matches the internal environment of the cell. Such an experiment is illustrated in Fig. 5. Although we found that recovery of sensitivity at the base of the outer segment was marginally slower after pigment bleaching, we could find no statistically significant difference in the tip/base ratio of recovery rates between cells recorded with control solution (0 mM NADPH) dialysis compared with those recorded without dialysis (Table I). Thus, the kinetics of the dark-adapted response and the post-dialysis response remain for the most part unchanged, and the bleach-adapted tip and base response are sped in relation to them. More importantly, whole cell dialysis with a control internal solution (0 mM NADPH) does not alter the wavelike recovery of sensitivity (not depicted).
Figure 5.
Dialysis of NADPH-free solution into rods before a 50% bleach of the visual pigment. (A) Changes in log sensitivity after pigment bleaching plotted as a function of time. Sensitivity before (upward filled triangle) dialysis with internal solution containing 0 mM NADPH (black bar) is plotted (mean ± SEM). A 50% bleach is given at time 0 s. Recovery of sensitivity at the base of the outer segment commenced with a time constant of ∼290 s, and at the tip of the outer segment of ∼930 s. (B) Suction electrode configuration while dialyzing solution free of NADPH into the rod inner segment. On the bottom, responses to saturating flashes at selected time points during recovery in A are shown. (C) Dim flash responses in the dark-adapted cell after dialysis (gray) and at steady-state after pigment bleaching (black) indicating the response speeding characteristic of bleaching adaptation.
Substantial bleaches result in an even greater tip/base difference in recovery
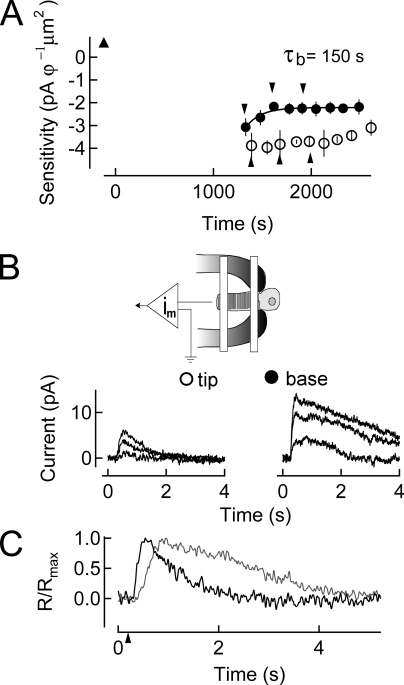
We exposed the photoreceptors to longer durations of bright light that resulted in >90% of the visual pigment bleached. We reasoned that a greater fractional bleach would result in a correspondingly higher outer segment concentration of all-trans retinal and would thus induce additional demands on the limited supplies of NADPH. We anticipated that this increase in metabolic demand would manifest itself by increasing the time during which the cell was refractory to incremental flashes, and would also increase the differential of the tip/base recovery time.
As shown in Fig. 6, after a 90% bleach the cell remained completely unresponsive to incremental flashes of any intensity for a period of ∼20 min. Under this severe bleaching condition, the recovery of responsiveness at the tip of rods was exceedingly slow and sometimes completely absent. As a result of the substantial amount of visual pigment bleached, dim flash responses were difficult to distinguish from the underlying noise. However, the time constant for sensitivity recovery at the base of the outer segment (∼150 s; see Table I) was roughly the same as that which was measured after a 50% bleach.
Figure 6.
Recovery of sensitivity after a 90% bleach of the visual pigment. (A) Changes in log sensitivity after pigment bleaching plotted as a function of time. Sensitivity before pigment bleaching (upward filled triangle) is plotted (mean ± SEM). A 90% bleach is given at time 0 s. Recovery of sensitivity began at much later times than for the 50% bleach shown in Fig. 2 due to the longer period of dark current suppression for the 90% bleach. Recovery of sensitivity at the base of the outer segment commenced with a time constant of ∼150 s but could not be accurately fit at the tip of the outer segment. (B) Suction electrode–only recording configuration for the experiment. On the bottom, responses to saturating flashes at selected time points during recovery in A are shown. (C) Dim flash responses in the dark-adapted cell after dialysis (gray) and at steady state after pigment bleaching (black) indicating the response speeding characteristic of bleaching adaptation.
Thus, after even the brightest bleaching light intensity, retinal reduction at the base of the outer segment is not limited by the availability of NADPH. This suggests that there is sufficient RDH activity to reduce all-trans retinal even at the highest bleaches, and that NADPH concentration becomes limiting progressively from the base to the tip of the outer segment. Finally, this result suggests that under bleaching conditions, the inner segment plays a significant role in supplying NADPH or its precursors to the outer segment to facilitate the retinal reduction. The lack of recovery at the tip suggests that the persistence of all-trans retinal directly desensitizes the cell in this region.
DISCUSSION
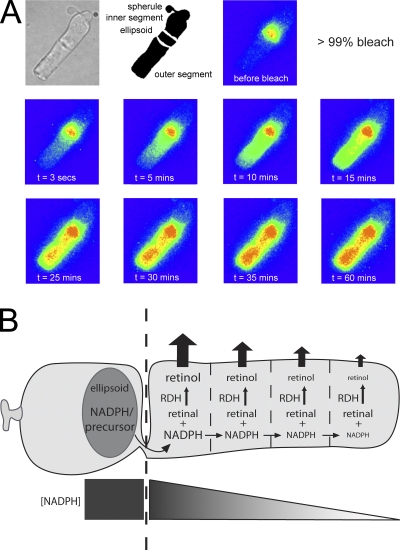
Key to the recovery of sensitivity after pigment bleaching is the quenching of the active intermediates of phototransduction and the reconstitution of visual pigment. The mechanisms that limit this recovery are thus critical for setting the time course of sensitivity recovery. These experiments demonstrate that the photoreceptor’s ability to clear itself of the isomerized chromophore (all-trans retinal) by reducing it to a soluble alcohol (all-trans retinol) is crucial for restoring sensitivity after substantial bleaching of the visual pigment. As long as all-trans retinal lingers, it can recombine with opsin and increase the activity of the phototransduction cascade (Jäger et al., 1996). In salamander rods, the recovery of sensitivity after exposure to bleaching light follows a wavelike pattern, with recovery proceeding from the base of the outer segment to the tip (Jin et al., 1994). Furthermore, measurements of all-trans retinol fluorescence from bleached rods show that its production after a bright bleach follows a similar wavelike pattern, increasing initially at the base of the outer segment and eventually increasing at the tip (Fig. 7 A; see also Tsina et al., 2004). The experiments reported here tie these previous observations together mechanistically by demonstrating that the wavelike pattern in both the recovery of sensitivity and in retinol fluorescence can be explained by an NADPH concentration gradient. We show that dialysis of a saturating concentration of NADPH into the rod cytosol to abolish this gradient eliminates the tip-to-base gradient in the recovery of sensitivity.
Figure 7.
A model describing the role of NADPH in the clearance of all-trans retinal in salamander rods. (A) Fluorescence measurements of all-trans retinol production as described in Tsina et al. (2004) (unpublished data). The top row shows a bright field and diagrammatic image of a salamander rod, as well as the localization of fluorescence before pigment bleaching. After a 99% bleach of the visual pigment, all-trans retinol fluorescence was measured at the noted intervals. At early times after pigment bleaching, retinol fluorescence increased at the base of the outer segment and gradually increases at the tip with time. (B) The wavelike evolution of all-trans retinol fluorescence can be explained if NADPH or a precursor is produced mainly in the ellipsoid region of the inner segment where mitochondria are located. NADPH then diffuses into the outer segment through the rod’s thin ciliary neck, where it is gradually consumed as it progresses to the tip. On the bottom, a gradient of NADPH is generated at steady state.
What physiological step limits the recovery of sensitivity after pigment bleaching?
If the clearance of all-trans retinal is critical for the recovery of sensitivity after pigment bleaching, the rate-limiting step in this process must play a key role in setting its time course. The reduction of all-trans retinal to all-trans retinol may be potentially limited by one of three steps: (1) the availability of all-trans retinal to be reduced, (2) the enzymatic rate of RDH itself, or (3) the NADPH concentration, which is a required cofactor for RDH activity.
Salamander rods provide a powerful model system for identifying the limiting component for the clearance of all-trans retinal because the outer segment is sufficiently large, such that each end is its own microenvironment. In these experiments, we observed that regardless of the magnitude of the bleach, or the NADPH concentration dialyzed into the rod inner segment, the time course of sensitivity recovery is nearly the same at the base of the outer segment. This suggests that the NADPH concentration at the base of the outer segment is never limiting to reduce all-trans retinal (even for a 90% bleach; see Fig. 6). However, at the tip of the rod outer segment, the recovery of sensitivity was significantly speeded by dialysis of saturating NADPH (Fig. 3), and thus is limited by the NADPH concentration. These results suggest the model illustrated in Fig. 7 B, where NADPH, or its precursors, generated primarily in the ellipsoid region of the inner segment diffuses through the ciliary neck connecting the inner to the outer segment. There it is consumed first at the base and finally at the tip of the outer segment to reduce all-trans retinal to all-trans retinol. Limited availability of NADPH along the outer segment would limit the RDH rate, explaining the slower evolution of retinol fluorescence at the tip of the outer segment after bleaching light exposures (Fig. 7 A).
Although NADPH limits the recovery of sensitivity at the tip of the salamander rod outer segment after pigment bleaching, at the base of the outer segment the invariance in the time course of recovery in sensitivity with the magnitude of the bleach or NADPH concentration indicates that NADPH does not appear to limit this recovery. Thus, at the base of the outer segment either the availability of all-trans retinal or the maximal RDH activity plays the limiting role in the recovery of sensitivity. Previous work has suggested that the RDH activity does not become saturated, even for the largest bleaches. For instance, the exogenous application of all-trans retinal to bleached salamander rods produces a faster and larger increase in fluorescence compared with that generated after a 99% bleach (Tsina et al., 2004). Recent evidence from isolated mouse rods additionally shows that pigment regeneration is not limited by all-trans retinal reduction (Chen et al., 2009). Instead, the presence of all-trans retinal appears to play the limiting role in the recovery of sensitivity after pigment bleaching in isolated cells.
The availability of all-trans retinal for reduction to all-trans retinol after the decay of the activated (Meta II) state can be limited by two different processes. First, the dissociation of all-trans retinal from the opsin protein requires the hydrolysis of the Schiff base bond linking the chromophore to the protein. This is followed by its diffusion into the disc membranes. The second limiting step may be the extrusion of all-trans retinal to the cytosolic side of the disc membrane by an ABCR transporter (Sun et al., 1999). In principle, either of these steps may limit the availability of all-trans retinal to RDH. For instance, the lifetime of Meta II Rh after pigment bleaching has been measured in salamander rods and decays evenly across the outer segment with a time constant of ∼500 s (Ala-Laurila et al., 2006), broadly similar to the recovery time course of dark current and sensitivity after pigment bleaching (see Fig. 2). However, if removal of all-trans retinal from the disc membranes by ABCR limits its reduction, alterations in this activity would be expected to influence dark adaptation. Indeed, studies from one group indicate that dark adaptation is delayed in mice with half the expression of ABCR (ABCR+/−; Mata et al., 2001), and even further delayed in the full knockout (Weng et al., 1999). However, more recent evidence suggests that ABCR’s effect may either speed or slow dark adaptation dependent on the magnitude of the bleach (Pawar et al., 2008). Thus, the limiting step controlling the availability of all-trans retinal to the RDH reaction remains controversial.
Outer segment metabolism contributes negligibly to recovery from pigment bleaching
The key metabolic component for the reduction of all-trans retinal is cytosolic NADPH, which serves as a necessary cofactor for RDH. Several lines of evidence point to the ellipsoid as the primary source of this NADPH after bright bleaches, as depicted in Fig. 7 B. First, the recovery of sensitivity at the base of the outer segment after pigment bleaching is invariant with the magnitude of the bleach or the NADPH concentration introduced into the outer segment before bleaching. Thus, the NADPH concentration at the base of the outer segment appears saturating for RDH activity and can be reestablished quickly after pigment bleaching. Second, a gradient both in the recovery of sensitivity after pigment bleaching (Jin et al., 1994) and in outer segment all-trans retinol fluorescence (Tsina et al., 2004) can be explained by a gradient in NADPH concentration that declines along the length of the outer segment. In addition, rod outer segments separated from the inner segment fail to show an all-trans retinol gradient after pigment bleaching (Tsina et al., 2004; Kolesnikov et al., 2007), presumably due to loss of a source of reducing equivalents from the ellipsoid. Limited NADPH production in the outer segment is consistent with the lack of glutathione expression (Hsu and Molday, 1994; Huster et al., 1998). These data together highlight the significant role played by rod inner segment metabolism to the reduction of all-trans retinal after pigment bleaching, and thus the recovery of visual sensitivity.
Several metabolic pathways that generate NADPH have been identified, but their contributions to NADPH production after bleaching light exposure in rods are less well understood (Hsu and Molday, 1994; Chen et al., 2005; Kolesnikov et al., 2007). For instance, NADPH may be produced in the cytosol from: (1) NADH generated in the citric acid cycle as it is transferred out of the mitochondria in the ellipsoid region, (2) the oxidative steps of the pentose phosphate (shunt) pathway, or (3) through an NADPH dehydrogenase (diaphorase) that directly converts NADP+ to NADPH (Berg et al., 2002). Although early indirect measurements of pentose shunt activity in vitro suggested that NADPH production in bovine outer segments was sufficient for supplying retinal reduction (Hsu and Molday, 1991, 1994), recent proteomic studies fail to detect some enzymes that are primarily responsible for the production of NADPH in rod outer segments, such as glucose 6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase (Kwok et al., 2008). The strong contribution of the rod inner segment to the production of reducing equivalents instead suggests the species that diffuses along the length of the outer segment must be consumed along the way to create the observed gradient. Under these conditions, we suggest that NADPH production in the ellipsoid may generate the dominant source of NADPH after pigment bleaching.
Control of NADPH concentration may be critical for the setting functional properties of the rod photoresponse. We observe that the dialysis of high concentrations of NADPH (i.e., in excess of 1.66 mM) slows the light response compared with the dialysis of a control solution (see Figs. 3 and 4). Thus, the resting concentration of NADPH in the outer segment may be set to provide sufficient quantity to reduce rapidly all-trans retinal generated by relatively dim light exposure without significant slowing of the light response. However, it appears that NADPH can be synthesized quickly to accommodate for larger bleaches.
Implications for mammalian rods and disease
The smaller rod outer segment volume of mammalian rods compared with salamander rods would be expected to limit spatial effects of diffusion and thus make their recovery of sensitivity after pigment bleaching, similar to the situation seen in the salamander rod outer segment base. Indeed, no gradient in outer segment fluorescence is observed in isolated mouse rods (Chen et al., 2009), consistent with the idea that the NADPH is uniform throughout the outer segment, similar to the expression of RDH (Luo et al., 2004). Furthermore, Chen et al. (2009) indicate that the reduction of all-trans retinal does not appear to be limiting for the regeneration of the photopigment. Thus, just as for the salamander rod outer segment base, the limiting reaction for the reduction of all-trans retinal in mouse rods may be the availability of all-trans retinal after bleaching. Visual impairment and blindness have been linked to deficits in the ability of rods to terminate the light-evoked response and return to the dark state through regeneration of the visual pigment (Woodruff et al., 2003). Accumulation of all-trans retinal has also been shown to lead to A2E formation, a toxic detergent-like byproduct that is a major component of lipofuscin (Sparrow et al., 2003b). A2E has been shown to damage the retinal pigment epithelium by acting as a photosensitizer (Schütt et al., 2000; Sparrow et al., 2003a; Rózanowska et al., 2004) and an inhibitor of lysosomal enzymes (Finnemann et al., 2002). Thus, the efficient clearance of all-trans retinal is critical for rod function and survival.
Acknowledgments
We thank Dr. Jim Hurley for helpful discussions, Cyrus Arman for help with image analysis, Dr. Haruhisa Okawa for assistance with fluorescence imaging, Drs. Yiannis Koutalos and Johan Pahlberg for comments on the manuscript, and Dr. Efthemia Tsina for help with retinol fluorescence images.
This work was supported by National Institutes of Health grants EY-17606 (to A.P. Sampath) and EY-01157 (to M.C. Cornwall), an award from the Karl Kirschgessner Foundation (to A.P. Sampath), and a National Science Foundation Graduate Research Fellowship (to K.J. Miyagishima).
Edward N. Pugh Jr. served as editor.
Footnotes
Abbreviations used in this paper:
- NADPH
- nicotinamide adenine dinucleotide phosphate
- RDH
- retinol dehydrogenase
- Rh
- rhodopsin
References
- Ala-Laurila P., Kolesnikov A.V., Crouch R.K., Tsina E., Shukolyukov S.A., Govardovskii V.I., Koutalos Y., Wiggert B., Estevez M.E., Cornwall M.C. 2006. Visual cycle: dependence of retinol production and removal on photoproduct decay and cell morphology.J. Gen. Physiol. 128:153–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ala-Laurila P., Donner K., Crouch R.K., Cornwall M.C. 2007. Chromophore switch from 11-cis-dehydroretinal (A2) to 11-cis-retinal (A1) decreases dark noise in salamander red rods.J. Physiol. 585:57–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D.A., Lamb T.D., Yau K.W. 1979. The membrane current of single rod outer segments.J. Physiol. 288:589–611 [PMC free article] [PubMed] [Google Scholar]
- Berg J.M., Tymoczko J.L., Stryer L. 2002. Biochemistry. Vol 6th edition W.H. Freeman and Company, New York: 1120 pp [Google Scholar]
- Chen C., Tsina E., Cornwall M.C., Crouch R.K., Vijayaraghavan S., Koutalos Y. 2005. Reduction of all-trans retinal to all-trans retinol in the outer segments of frog and mouse rod photoreceptors.Biophys. J. 88:2278–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Blakeley L.R., Koutalos Y. 2009. Formation of all-trans retinol after visual pigment bleaching in mouse photoreceptors.Invest. Ophthalmol. Vis. Sci. doi:10.1167/iovs.08-3336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwall M.C., Fain G.L. 1994. Bleached pigment activates transduction in isolated rods of the salamander retina.J. Physiol. 480:261–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwall M.C., Fein A., MacNichol E.F., Jr 1990. Cellular mechanisms that underlie bleaching and background adaptation.J. Gen. Physiol. 96:345–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnemann S.C., Leung L.W., Rodriguez-Boulan E. 2002. The lipofuscin component A2E selectively inhibits phagolysosomal degradation of photoreceptor phospholipid by the retinal pigment epithelium.Proc. Natl. Acad. Sci. USA. 99:3842–3847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futterman S., Hendrickson A., Bishop P.E., Rollins M.H., Vacano E. 1970. Metabolism of glucose and reduction of retinaldehyde in retinal photoreceptors.J. Neurochem. 17:149–156 [DOI] [PubMed] [Google Scholar]
- Holcman D., Korenbrot J.I. 2004. Longitudinal diffusion in retinal rod and cone outer segment cytoplasm: the consequence of cell structure.Biophys. J. 86:2566–2582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S.C., Molday R.S. 1991. Glycolytic enzymes and a GLUT-1 glucose transporter in the outer segments of rod and cone photoreceptor cells.J. Biol. Chem. 266:21745–21752 [PubMed] [Google Scholar]
- Hsu S.C., Molday R.S. 1994. Glucose metabolism in photoreceptor outer segments. Its role in phototransduction and in NADPH-requiring reactions.J. Biol. Chem. 269:17954–17959 [PubMed] [Google Scholar]
- Huster D., Hjelle O.P., Haug F.M., Nagelhus E.A., Reichelt W., Ottersen O.P. 1998. Subcellular compartmentation of glutathione and glutathione precursors. A high resolution immunogold analysis of the outer retina of guinea pig.Anat. Embryol. (Berl.). 198:277–287 [DOI] [PubMed] [Google Scholar]
- Jäger S., Palczewski K., Hofmann K.P. 1996. Opsin/all-trans-retinal complex activates transducin by different mechanisms than photolyzed rhodopsin.Biochemistry. 35:2901–2908 [DOI] [PubMed] [Google Scholar]
- Jin J., Jones G.J., Cornwall M.C. 1994. Movement of retinal along cone and rod photoreceptors.Vis. Neurosci. 11:389–399 [DOI] [PubMed] [Google Scholar]
- Jones G.J., Cornwall M.C., Fain G.L. 1996. Equivalence of background and bleaching desensitization in isolated rod photoreceptors of the larval tiger salamander.J. Gen. Physiol. 108:333–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnikov A.V., Ala-Laurila P., Shukolyukov S.A., Crouch R.K., Wiggert B., Estevez M.E., Govardovskii V.I., Cornwall M.C. 2007. Visual cycle and its metabolic support in gecko photoreceptors.Vision Res. 47:363–374 [DOI] [PubMed] [Google Scholar]
- Kwok M.C., Holopainen J.M., Molday L.L., Foster L.J., Molday R.S. 2008. Proteomics of photoreceptor outer segments identifies a subset of SNARE and Rab proteins implicated in membrane vesicle trafficking and fusion.Mol. Cell. Proteomics. 7:1053–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T.D., Pugh E.N., Jr 2004. Dark adaptation and the retinoid cycle of vision.Prog. Retin. Eye Res. 23:307–380 [DOI] [PubMed] [Google Scholar]
- Luo W., Marsh-Armstrong N., Rattner A., Nathans J. 2004. An outer segment localization signal at the C terminus of the photoreceptor-specific retinol dehydrogenase.J. Neurosci. 24:2623–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata N.L., Tzekov R.T., Liu X., Weng J., Birch D.G., Travis G.H. 2001. Delayed dark-adaptation and lipofuscin accumulation in abcr+/− mice: implications for involvement of ABCR in age-related macular degeneration.Invest. Ophthalmol. Vis. Sci. 42:1685–1690 [PubMed] [Google Scholar]
- Palczewski K., Jäger S., Buczyłko J., Crouch R.K., Bredberg D.L., Hofmann K.P., Asson-Batres M.A., Saari J.C. 1994. Rod outer segment retinol dehydrogenase: substrate specificity and role in phototransduction.Biochemistry. 33:13741–13750 [DOI] [PubMed] [Google Scholar]
- Pawar A.S., Qtaishat N.M., Little D.M., Pepperberg D.R. 2008. Recovery of rod photoresponses in ABCR-deficient mice.Invest. Ophthalmol. Vis. Sci. 49:2743–2755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattner A., Smallwood P.M., Nathans J. 2000. Identification and characterization of all-trans-retinol dehydrogenase from photoreceptor outer segments, the visual cycle enzyme that reduces all-trans-retinal to all-trans-retinol.J. Biol. Chem. 275:11034–11043 [DOI] [PubMed] [Google Scholar]
- Rózanowska M., Pawlak A., Rózanowski B., Skumatz C., Zareba M., Boulton M.E., Burke J.M., Sarna T., Simon J.D. 2004. Age-related changes in the photoreactivity of retinal lipofuscin granules: role of chloroform-insoluble components.Invest. Ophthalmol. Vis. Sci. 45:1052–1060 [DOI] [PubMed] [Google Scholar]
- Saari J.C., Garwin G.G., Van Hooser J.P., Palczewski K. 1998. Reduction of all-trans-retinal limits regeneration of visual pigment in mice.Vision Res. 38:1325–1333 [DOI] [PubMed] [Google Scholar]
- Sakmar T.P. 2006. Timing is everything: direct measurement of retinol production in cones and rods.J. Gen. Physiol. 128:147–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schütt F., Davies S., Kopitz J., Holz F.G., Boulton M.E. 2000. Photodamage to human RPE cells by A2-E, a retinoid component of lipofuscin.Invest. Ophthalmol. Vis. Sci. 41:2303–2308 [PubMed] [Google Scholar]
- Sparrow J.R., Cai B., Fishkin N., Jang Y.P., Krane S., Vollmer H.R., Zhou J., Nakanishi K. 2003a. A2E, a fluorophore of RPE lipofuscin: can it cause RPE degeneration? Adv. Exp. Med. Biol. 533:205–211 [DOI] [PubMed] [Google Scholar]
- Sparrow J.R., Fishkin N., Zhou J., Cai B., Jang Y.P., Krane S., Itagaki Y., Nakanishi K. 2003b. A2E, a byproduct of the visual cycle.Vision Res. 43:2983–2990 [DOI] [PubMed] [Google Scholar]
- Sun H., Molday R.S., Nathans J. 1999. Retinal stimulates ATP hydrolysis by purified and reconstituted ABCR, the photoreceptor-specific ATP-binding cassette transporter responsible for Stargardt disease.J. Biol. Chem. 274:8269–8281 [DOI] [PubMed] [Google Scholar]
- Tsina E., Chen C., Koutalos Y., Ala-Laurila P., Tsacopoulos M., Wiggert B., Crouch R.K., Cornwall M.C. 2004. Physiological and microfluorometric studies of reduction and clearance of retinal in bleached rod photoreceptors.J. Gen. Physiol. 124:429–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng J., Mata N.L., Azarian S.M., Tzekov R.T., Birch D.G., Travis G.H. 1999. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt’s disease from the phenotype in abcr knockout mice.Cell. 98:13–23 [DOI] [PubMed] [Google Scholar]
- Woodruff M.L., Wang Z., Chung H.Y., Redmond T.M., Fain G.L., Lem J. 2003. Spontaneous activity of opsin apoprotein is a cause of Leber congenital amaurosis.Nat. Genet. 35:158–164 [DOI] [PubMed] [Google Scholar]
- Wu Q., Chen C., Koutalos Y. 2006. Longitudinal diffusion of a polar tracer in the outer segments of rod photoreceptors from different species.Photochem. Photobiol. 82:1447–1451 [DOI] [PubMed] [Google Scholar]