Abstract
Background
An estimated 34,000 cases of squamous cell carcinomas of the head and neck (HNSCC) will be diagnosed in 2007 with 7,500 estimated deaths. Radiation is commonly used to treat these patients. Preclinical studies have suggested that sirolimus may be an effective radiosensitizer in HNSCC.
Methods
The present case report describes a patient, status post liver transplant, who was switched to sirolimus for immunosupression. The patient subsequently underwent radiation therapy for a T2N0M0 SCC of the larynx.
Results
The patient had an unusually early response to radiation, with a clinical complete response after seven fractions of radiation. However, the patients also had toxicity earlier than expected and required a break from radiation after eleven fractions.
Conclusions
To the authors’ knowledge, this is the first observation to suggest that sirolimus is an effective radiosensitizer in patients with HNSCC. We hope that our results will create interest in future clinical studies.
Introduction
An estimated 34,000 cases of squamous cell carcinomas of the head and neck (HNSCC) will be diagnosed in 2007 with 7,500 estimated deaths (1). Locally advanced disease can be devastating, with a 5-year survival rate of less than 50% with little improvement over the last three decades (2). Generally, in early stage disease, a large portion of patients can be cured, however, even in these cases, radiation doses of 6,000 to 7,000 cGy are needed for disease control and clinical complete responses are usually not seem until at least 4,000 cGy. The present case is interesting because it describes the rare occurrence where a patient on sirolimus monotherapy for immunosupression, with a diagnosis of a T2N0M0 squamous cell carcinoma (SCC) of the epiglottis, was treated with radiation. The laryngeal location of the tumor allowed frequent evaluation with NPL. Unexpectedly, the patient’s tumor had a profound response to a relatively low dose of radiation. To the authors’ knowledge, this is the first clinical report suggesting that sirolimus may be an effective radiosensitizer in squamous cell carcinoma of the head and neck.
Case Report
A 59-year-old gentleman with a past medical history of non-alcoholic steatohepatitis (NASH) treated with liver transplant approximately three years ago presented with several months of hoarseness and voice changes. He had a history of significant tobacco use. He was evaluated by his primary care physician who treated him for thrush over a three month period with various antifungal agents, which failed to relieve his symptoms. He then underwent flexible laryngoscopy which revealed an exophytic white lesion on the laryngeal surface of the epiglottis just superior to the false vocal cords. The lesion predominantly involved the right side of the epiglottis measuring approximately 3 cm in the greatest dimension. There was no lateral involvement of the piriform sinuses bilaterally or anterior involvement into the vallecula. No other masses were seen, vocal cord motion was normal, and there were no palpable neck nodes. Biopsy of this lesion demonstrated a moderately to poorly differentiated invasive squamous cell carcinoma (SCC). PET scan demonstrated increased uptake in the supra and infrahyoid epiglottis with and SUV of 6.3, consistent with malignancy. No distant disease or pathological cervical or mediastinal lymph nodes were noted on PET or MRI scans. The patient was staged as T2N0M0.
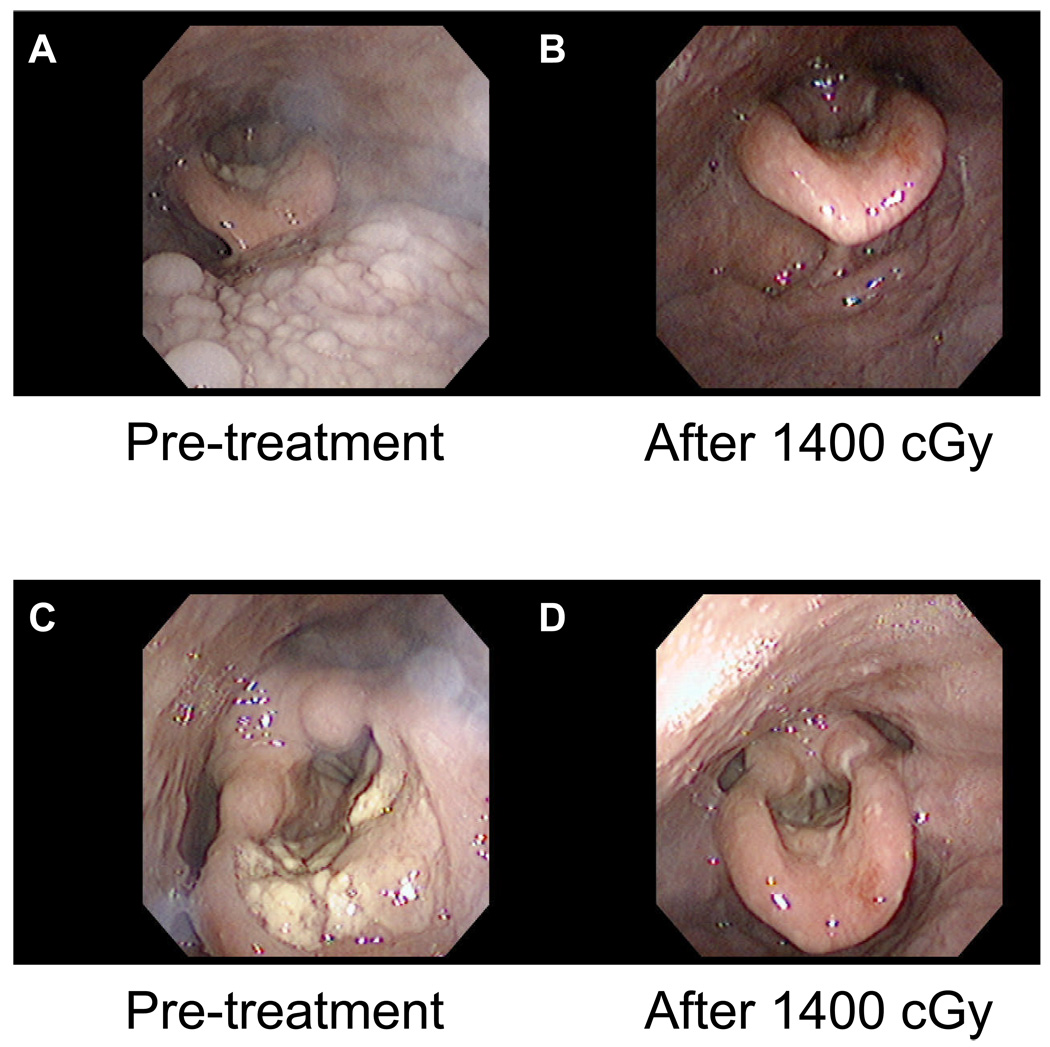
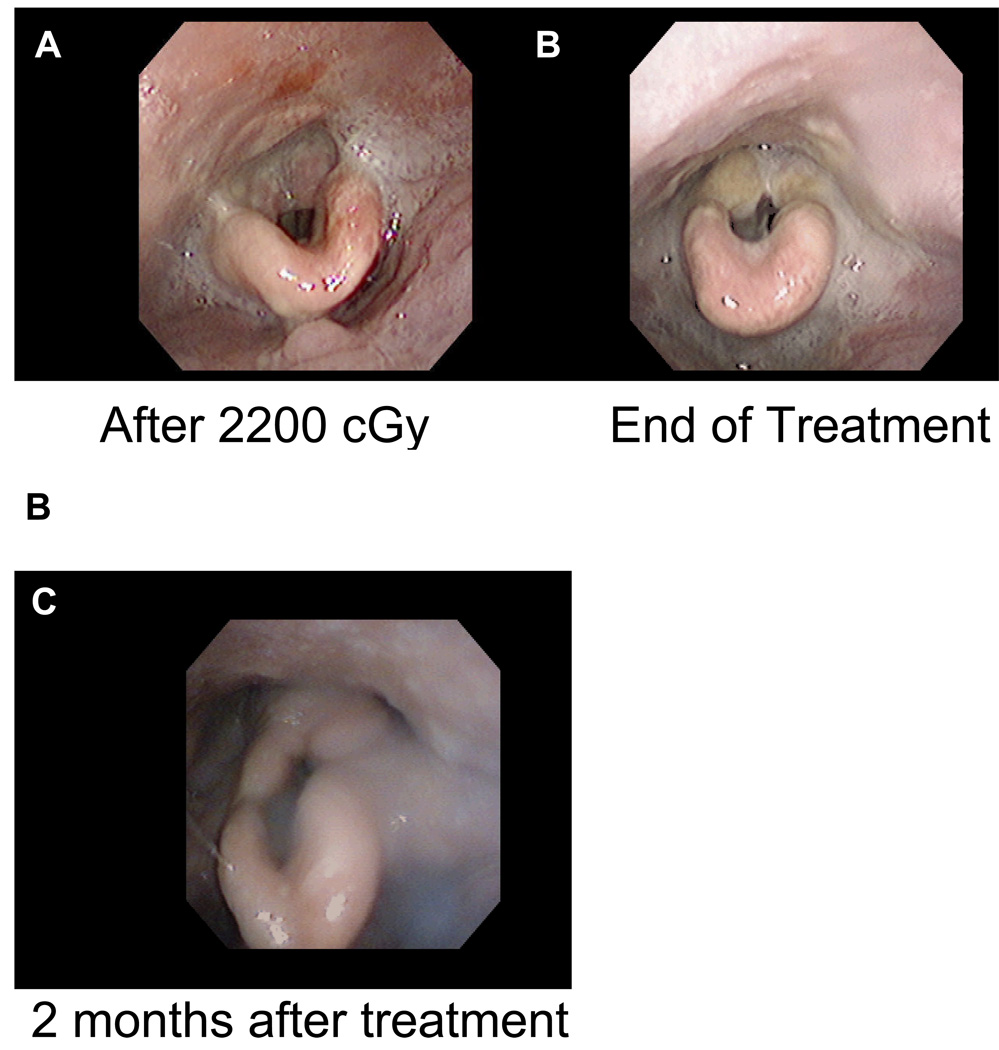
Due to the patient’s numerous other comorbidities he was deemed a poor candidate for surgery. During consultation with his transplant physician, the patient was given the option of switching to sirolimus (2 mg daily) from tacrolimus (Prograf) (90 mg, twice a day) as sirolimus is used as an immunosuppressant and has theoretical oncologic benefit due to its antiproliferative activity. The patient elected to be switch to sirolimus. Prior to treatment, the patient underwent nasopharyngeal laryngoscopy (NPL) to assess pretreatment tumor size (figure I. A and C). Two and a half weeks prior to radiation treatment the patient’s sirolimus level was 8.6 ug/L (therapeutic range 4.0–12.0 ug/L). The sirolimus level was checked again at the start of treatment and was 14.4 ug/L, slightly supratherapeutic. The sirolimus dose was subsequently reduced to 1 mg daily. Definitive radiation therapy was planned for the patient using 200 cGy fractions to a dose of 4,400 cGy followed by a conedown to a dose of 2,600 cGy, giving a total dose of 7,000 cGy. Initial fields were opposed laterals treating the primary tumor, encompassing the patient’s neck bilaterally from the base of skull to the to the level IV cervical lymph nodes. After seven 200 cGy fractions of radiation to a dose of 1,400 cGy the patient had a repeat NPL and a dramatic response was seen (figure I. B and D), with complete resolution of the tumor. The patient developed worsening odynophagia as treatment continued and after approximately two weeks of treatment to a dose of 2,200 cGy the patient required a three day break from radiation. NPL was repeated at that time which demonstrated mucositis (figure IIa). There was a concern that his sirolimus levels might still be supratherapeutic. The sirolimus level was repeated three weeks into treatment and was 11.6 ug/L. One week later it was 9.9 ug/L. Two weeks after completing treatment the patient’s serum sirolimus was 5.5 ug/L. The patient had an additional two day treatment break secondary to an admission for nephrolithiasis during the fourth week of treatment and a one day break due to pneumonia during the fifth week of treatment. The patient was able to complete 35 fractions of 200 cGy to a final dose of 7,000 cGy, without further problems (figure IIb), over 57 days (35 treatment days, 16 weekend days, 5 days of treatment break). The patient was doing well at two month follow-up and appeared disease free (figure IIc). The patient was last seen for one year follow up and had no signs of recurrence on NPL or PET/CT.
Figure I.
Shown are the patient’s Nasopharyngolaryngoscopy (NPL) exams from prior to radiation (A, C) and after seven fractions of radiation (B, D) to a dose of 1400 cGy. Note that there has been a dramatic improvement in the tumor in only seven treatments.
Figure II.
Shown are Nasopharyngolaryngoscopy (NPL) exams at 2200 cGy with mucositis (A), and at the end of treatment (B).
Discussion
Sirolimus (Rapamycin) was first isolated from the bacterium, Streptomyces hygroscopicus found in a soil sample from the island Rapa Nui. It is a member of the macrolide family of antibiotics and was first used as an antifungal agent. It was subsequently used in transplant patients to prevent rejection after its immunosupressive and antiproliferative effects were discovered. Studies have demonstrated that sirolimus has antitumor effects at clinically relevant doses in HNSCC (3) and that sirolimus is an effective in vivo radiosensitizer in several different cancer cell lines (4, 5). Several studies have found that sirolimus can suppress progression of malignancies associated with immunosupression, such as Kaposi sarcoma and other cutaneous malignancies (6–8), post-transplant lymphoproliferative disease, hepatoblastoma (9), as well as several others (7).
A modified version of sirolimus, temsirolimus (CCI-779), demonstrated no immunosuppressive effects and limited, reversible side effects in a recent phase I trial (10). There have been several phase I/II clinical trials suggesting the safety and efficacy of mammalian target of rapamycin (mTOR) inhibition in several different types of cancer, such as renal cancer (11, 12), breast cancer (13), glioblastoma multiforme (GBM) (14), and various other advance staged tumors (15). However, other Phase II trials using CCI-779 in cancers such as malignant melanoma, neuroendocrine tumors, and recurrent GBM showed disappointing results (16–18). The combined use of radiation and sirolimus was recently studied in a phase I trial (19). Patients with stage III non-small cell lung cancer were treated with a combination of sirolimus, cisplatin, and radiation. Significant odynophagia due to mucositis was seen in 3/7 patients, however, this resolved within four weeks of the end of treatment in all patients. Patients were initially treated with 2 mg/day of sirolimus and had no dose limiting toxicity. Serum sirolimus levels were drawn at three weeks and were 3.4, 3.9, and 8.6 ug/L. Subsequent patients were treated with 5 mg/day of sirolimus and had serum concentrations of 4.7, 14.2 and 7.5 ug/L. One patient had dose limiting mucositis in the 5 mg/day group. Three weeks into treatment the patient in the present study had a serum sirolimus level of 11.6 ug/L, despite having had his dose of sirolimus decreased to 1 mg/day, perhaps due to his liver transplant. The patient in the present study developed mucositis and odynophagia earlier than expected, likely reflecting radiosensitization of the normal tissues by sirolimus. However, later, with lower serum sirolimus levels, the patient had no further dose limiting toxicity. This may suggest that the dose of sirolimus is an important factor in normal tissue radiosensitization.
The molecular mechanism for sirolimus’s antitumoral effects may be due to its role in inhibiting components of the PI3K/Akt pathway. Studies have demonstrated that Akt (also known as Protein kinase B) is persistently active in HNSCC cell lines (20, 21). mTOR is a downstream target of Akt and may be responsible for Akt’s regulation of cell growth. mTOR exerts its effect by phosphorylating p70-S6 kinase and 4E binding protein 1 (4E-BP1) (22). Phosphorylation of 4E-BP1 inhibits it from repressing Eukaryotic initiation factor 4E (eIF4E), which can then upregulate several genes related to cell growth. eIF4E has been associated with malignant progression in HNSCC and its expression at tumor margins can predict for recurrence (23, 24). Staining for S6 kinase phosphorylation in pathological specimens from patients with dysplastic oral mucosa as well as well differentiated, moderately differentiated, and poorly differentiated HNSCC all demonstrated significantly increased phosphorylated S6 compared with normal mucosa (3).
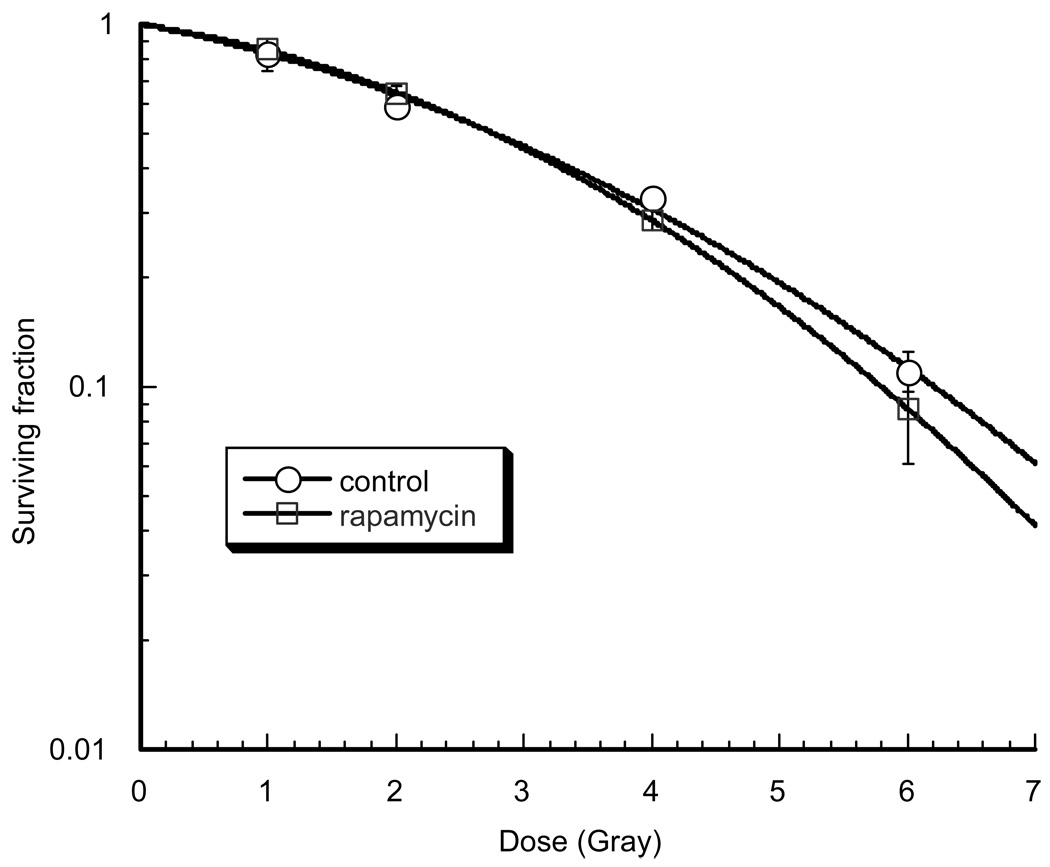
There have been conflicting results regarding the radiosensitizing effects of mTOR inhibition. Clonogenic studies conducted with a HNSCC cell line treated with sirolimus found no radiosensitizing effects (figure III), which is in agreement with previous studies in other cancer cell lines (4, 25). On the other hand, some studies have shown radiosensitization in response to treatment with mTOR inhibitors in other cancer cell lines in vitro (5). Interestingly, several in vivo studies have demonstrated radiosensitization of tumors treated with sirolimus, some of which were not radiosensitized after in vitro treatment with sirolimus (4, 5, 25–27). There are several explanations for the discordance between the in vivo and in vitro effects of sirolimus. Studies have demonstrated that malignant cells treated with sirolimus that were not radiosensitive in clonogenic studies, did demonstrate radiosensitization when grown as spheroids (25). The authors concluded that oxygenation may play a role and that sirolimus may directly radiosensitize malignant cells. Further studies demonstrated that the vascular endothelium can be sensitized by mTOR inhibition and cause regression of tumor vasculature in vivo, resulting in reduced tumor growth (4). This discordance has been seen with several other agents, such as cetuximab in HNSCC where the in vivo radiosensitizing effects are much greater than the in vitro effects, possibly due to antiangiogenic effects (28). Other studies with nelfinavir and bevacizumab have shown increased radiosensitization in vivo compared with in vitro and suggest that antiangionic effects leading to vascular normalization and improved oxygenation may be responsible for this difference (29–31). Based on the in vitro results from this study, and the results of the studies mentioned above, we hypothesize that sirolimus may be exerting a radiosensitizing effect on the vasculature of HNSCC tumors. Caution is necessary as normal tissue toxicity may also be increased however; these pre-clinical findings suggest that mTOR inhibition might be an effective radiosensitizer in HNSCC. This case report is the first to suggest that sirolimus is an effective radiosensitizer in HNSCC. Clearly further clinical studies are required to further evaluate this clinical observation. We hope this case study will spark interest in further evaluation of sirolimus as a radiosensitizer in HNSCC.
Figure III.
Shown are clonogenic survival curves performed in the human head and neck squamous cell cancer cell line, SQ20B. SQ20B cells in exponential growth phase were treated with sirolimus (10 nM) or control solvent followed by one, two, four or six gray. No significant difference in response to radiation was seen between the two groups. This dose of sirolimus has been shown to inhibit downstream signaling from mTOR using western blotting. After 24 hours, cells were trypsinized to make a single cell suspension and seeded into 60-mm dishes in sirolimus-free media. They were allowed to attach and then irradiated with a Mark I cesium irradiator (J.L. Shepherd, San Fernando, CA) at a dose rate of 1.6 Gy/min. Colonies were stained and counted 14 days after irradiation. A colony by definition had >50 cells. The surviving fraction was calculated by dividing the number of colonies formed by the number of cells plated times plating efficiency. Each point on the survival curve represents the mean surviving fraction from three replicates.
References
- 1.Society AC. Cancer Facts & Figures 2007. 2007 [Google Scholar]
- 2.Jemal A, Clegg LX, Ward E, et al. Annual report to the nation on the status of cancer, 1975–2001, with a special feature regarding survival. Cancer. 2004;101(1):3–27. doi: 10.1002/cncr.20288. [DOI] [PubMed] [Google Scholar]
- 3.Amornphimoltham P, Patel V, Sodhi A, et al. Mammalian target of rapamycin, a molecular target in squamous cell carcinomas of the head and neck. Cancer Res. 2005;65(21):9953–9961. doi: 10.1158/0008-5472.CAN-05-0921. [DOI] [PubMed] [Google Scholar]
- 4.Shinohara ET, Cao C, Niermann K, et al. Enhanced radiation damage of tumor vasculature by mTOR inhibitors. Oncogene. 2005;24(35):5414–5422. doi: 10.1038/sj.onc.1208715. [DOI] [PubMed] [Google Scholar]
- 5.Albert JM, Kim KW, Cao C, Lu B. Targeting the Akt/mammalian target of rapamycin pathway for radiosensitization of breast cancer. Mol Cancer Ther. 2006;5(5):1183–1189. doi: 10.1158/1535-7163.MCT-05-0400. [DOI] [PubMed] [Google Scholar]
- 6.Lebbe C, Euvrard S, Barrou B, et al. Sirolimus conversion for patients with posttransplant Kaposi's sarcoma. Am J Transplant. 2006;6(9):2164–2168. doi: 10.1111/j.1600-6143.2006.01412.x. [DOI] [PubMed] [Google Scholar]
- 7.Campistol JM, Eris J, Oberbauer R, et al. Sirolimus therapy after early cyclosporine withdrawal reduces the risk for cancer in adult renal transplantation. J Am Soc Nephrol. 2006;17(2):581–589. doi: 10.1681/ASN.2005090993. [DOI] [PubMed] [Google Scholar]
- 8.Stallone G, Schena A, Infante B, et al. Sirolimus for Kaposi's sarcoma in renal-transplant recipients. N Engl J Med. 2005;352(13):1317–1323. doi: 10.1056/NEJMoa042831. [DOI] [PubMed] [Google Scholar]
- 9.Jimenez-Rivera C, Avitzur Y, Fecteau AH, Jones N, Grant D, Ng VL. Sirolimus for pediatric liver transplant recipients with post-transplant lymphoproliferative disease and hepatoblastoma. Pediatr Transplant. 2004;8(3):243–248. doi: 10.1111/j.1399-3046.2004.00156.x. [DOI] [PubMed] [Google Scholar]
- 10.Raymond E, Alexandre J, Faivre S, et al. Safety and pharmacokinetics of escalated doses of weekly intravenous infusion of CCI-779, a novel mTOR inhibitor, in patients with cancer. J Clin Oncol. 2004;22(12):2336–2347. doi: 10.1200/JCO.2004.08.116. [DOI] [PubMed] [Google Scholar]
- 11.Boni JP, Leister C, Bender G, et al. Population pharmacokinetics of CCI-779: correlations to safety and pharmacogenomic responses in patients with advanced renal cancer. Clin Pharmacol Ther. 2005;77(1):76–89. doi: 10.1016/j.clpt.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 12.Atkins MB, Hidalgo M, Stadler WM, et al. Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J Clin Oncol. 2004;22(5):909–918. doi: 10.1200/JCO.2004.08.185. [DOI] [PubMed] [Google Scholar]
- 13.Chan S, Scheulen ME, Johnston S, et al. Phase II study of temsirolimus (CCI-779), a novel inhibitor of mTOR, in heavily pretreated patients with locally advanced or metastatic breast cancer. J Clin Oncol. 2005;23(23):5314–5322. doi: 10.1200/JCO.2005.66.130. [DOI] [PubMed] [Google Scholar]
- 14.Galanis E, Buckner JC, Maurer MJ, et al. Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: a North Central Cancer Treatment Group Study. J Clin Oncol. 2005;23(23):5294–5304. doi: 10.1200/JCO.2005.23.622. [DOI] [PubMed] [Google Scholar]
- 15.Hidalgo M, Buckner JC, Erlichman C, et al. A phase I and pharmacokinetic study of temsirolimus (CCI-779) administered intravenously daily for 5 days every 2 weeks to patients with advanced cancer. Clin Cancer Res. 2006;12(19):5755–5763. doi: 10.1158/1078-0432.CCR-06-0118. [DOI] [PubMed] [Google Scholar]
- 16.Duran I, Kortmansky J, Singh D, et al. A phase II clinical and pharmacodynamic study of temsirolimus in advanced neuroendocrine carcinomas. Br J Cancer. 2006;95(9):1148–1154. doi: 10.1038/sj.bjc.6603419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Margolin K, Longmate J, Baratta T, et al. CCI-779 in metastatic melanoma: a phase II trial of the California Cancer Consortium. Cancer. 2005;104(5):1045–1048. doi: 10.1002/cncr.21265. [DOI] [PubMed] [Google Scholar]
- 18.Chang SM, Wen P, Cloughesy T, et al. Phase II study of CCI-779 in patients with recurrent glioblastoma multiforme. Invest New Drugs. 2005;23(4):357–361. doi: 10.1007/s10637-005-1444-0. [DOI] [PubMed] [Google Scholar]
- 19.Sarkaria JN, Schwingler P, Schild SE, et al. Phase I trial of sirolimus combined with radiation and cisplatin in non-small cell lung cancer. J Thorac Oncol. 2007;2(8):751–757. doi: 10.1097/JTO.0b013e3180cc2587. [DOI] [PubMed] [Google Scholar]
- 20.Amornphimoltham P, Sriuranpong V, Patel V, et al. Persistent activation of the Akt pathway in head and neck squamous cell carcinoma: a potential target for UCN-01. Clin Cancer Res. 2004;10(12 Pt 1):4029–4037. doi: 10.1158/1078-0432.CCR-03-0249. [DOI] [PubMed] [Google Scholar]
- 21.Gupta AK, McKenna WG, Weber CN, et al. Local recurrence in head and neck cancer: relationship to radiation resistance and signal transduction. Clin Cancer Res. 2002;8(3):885–892. [PubMed] [Google Scholar]
- 22.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18(16):1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 23.Sorrells DL, Meschonat C, Black D, Li BD. Pattern of amplification and overexpression of the eukaryotic initiation factor 4E gene in solid tumor. J Surg Res. 1999;85(1):37–42. doi: 10.1006/jsre.1999.5653. [DOI] [PubMed] [Google Scholar]
- 24.Nathan CA, Amirghahri N, Rice C, Abreo FW, Shi R, Stucker FJ. Molecular analysis of surgical margins in head and neck squamous cell carcinoma patients. Laryngoscope. 2002;112(12):2129–2140. doi: 10.1097/00005537-200212000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Eshleman JS, Carlson BL, Mladek AC, Kastner BD, Shide KL, Sarkaria JN. Inhibition of the mammalian target of rapamycin sensitizes U87 xenografts to fractionated radiation therapy. Cancer Res. 2002;62(24):7291–7297. [PubMed] [Google Scholar]
- 26.Cao C, Subhawong T, Albert JM, et al. Inhibition of mammalian target of rapamycin or apoptotic pathway induces autophagy and radiosensitizes PTEN null prostate cancer cells. Cancer Res. 2006;66(20):10040–10047. doi: 10.1158/0008-5472.CAN-06-0802. [DOI] [PubMed] [Google Scholar]
- 27.Weppler SA, Krause M, Zyromska A, Lambin P, Baumann M, Wouters BG. Response of U87 glioma xenografts treated with concurrent rapamycin and fractionated radiotherapy: Possible role for thrombosis. Radiother Oncol. 2007;82(1):96–104. doi: 10.1016/j.radonc.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Harari PM, Huang SM. Head and neck cancer as a clinical model for molecular targeting of therapy: combining EGFR blockade with radiation. Int J Radiat Oncol Biol Phys. 2001;49(2):427–433. doi: 10.1016/s0360-3016(00)01488-7. [DOI] [PubMed] [Google Scholar]
- 29.Pore N, Gupta AK, Cerniglia GJ, et al. Nelfinavir down-regulates hypoxia-inducible factor 1alpha and VEGF expression and increases tumor oxygenation: implications for radiotherapy. Cancer Res. 2006;66(18):9252–9259. doi: 10.1158/0008-5472.CAN-06-1239. [DOI] [PubMed] [Google Scholar]
- 30.Duda DG, Jain RK, Willett CG. Antiangiogenics: the potential role of integrating this novel treatment modality with chemoradiation for solid cancers. J Clin Oncol. 2007;25(26):4033–4042. doi: 10.1200/JCO.2007.11.3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gorski DH, Beckett MA, Jaskowiak NT, et al. Blockage of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Res. 1999;59(14):3374–3378. [PubMed] [Google Scholar]