Abstract
Quercetin, a flavonoid found in various foodstuffs, has antioxidant properties, increases glutathione (GSH) levels and antioxidant enzyme function. Considerable attention has been focused on increasing the intracellular GSH levels in many diseases, including Alzheimer's disease (AD). Amyloid beta-peptide [Abeta(1-42)], elevated in AD brain, is associated with oxidative stress and neurotoxicity. The present study was aimed to investigate the protective effects of quercetin on Abeta(1-42)-induced oxidative cell toxicity in cultured neurons. Decreased cell survival in neuronal cultures treated with Abeta(1-42) correlated with increased free radical production measured by dichlorofluorescein fluorescence and an increase in protein oxidation (protein carbonyl, 3-nitrotyrosine) and lipid peroxidation (protein bound 4-hydroxy-2-nonenal). Pretreatment of primary hippocampal cultures with quercetin significantly attenuated Abeta(1-42)-induced cytotoxicity, protein oxidation, lipid peroxidation and apoptosis. A dose-response study suggested that quercetin showed protective effects against Abeta(1-42) toxicity by modulating oxidative stress at lower doses, but higher doses not only were non-neuroprotective, but were toxic. These findings provide motivation to test the hypothesis, that quercetin may provide a promising approach for the treatment of AD and other oxidative stress-related neurodegenerative diseases.
Keywords: Quercetin, Oxidative stress, Aβ (1-42), Alzheimer's disease, Neuroprotection
1. Introduction
Reactive oxygen species (ROS) have long been known to damage tissue through protein oxidation, lipid peroxidation, protein cross linking and DNA cleavage processes. With a high content of oxidizable substrates such as polyunsaturated fatty acids, poor catalase activity and low iron-binding capacity, brain is particularly prone to ROS damage [1]. Oxidative stress is associated with neurodegenerative diseases, such as Alzheimer's disease [2] and Parkinson's disease [3]. It has been found that oxidative stress causes damage in neuronal cell nuclei and mitochondria DNA [4, 5], decreases the activities of antioxidant enzymes and increase lipid peroxidation products [6]. Two major ROS produced by living tissue are superoxide anion (O2-) and hydrogen peroxide (H2O2). Although H2O2 is not a free radical, this non-polar molecule can cross biological membranes, as can O2- via an anion channel [7] or as the non-charged HO2• radical [8].
One family of naturally occurring compounds (flavonoids) possess free radical scavenging properties and neuroprotection from oxidative injury by their ability to modulate intracellular signals promoting cellular survival [9]. These flavonoids are found in fruits, vegetables and plant-derived beverages and may have important roles as dietary components via cytoprotective actions in many organs [10, 11]. Flavonoids act as vasodilator [12], anti-carcinogenic, anti-inflammatory, antibacterial, immune-stimulating anti-allergic and antiviral compounds [13]. In the past decade, the antioxidant activity of flavonoids has been given much attention, since many flavonoids such as quercetin, luteolin and catechins may be better antioxidants than the antioxidant nutrients vitamin C, vitamin E and β-carotene [14]. It is observed that quercetin, in addition to many other biological benefits, contributes significantly to the protective effects of neuronal cells from oxidative stress-induced neurotoxicity [15].
Neuronal loss in AD is preceded by the extracellular accumulation of Aβ (1-40, 1-42). Quercetin can increases the resistance of neurons against oxidative stress and exitotixcity by modulation of cell death mechanisms [16]. Aβ (1-40, 1-42 and 25-35) reduce neuronal Cl--ATPase activity and elevated intracellular Cl- concentrations in primary rat hippocampus neurons, probably by lowering PIP levels, and this property may reflect a pro-apoptotic condition in early pathophysiological profiles of AD. The activity of Cl--ATPase is attenuated by an inhibitor of PI-kinase, quercetin [17].
Polyphenols (flavonoids) dose-dependently inhibit the formation of fibrilar Aβ and destabilize fibrils. The modulation of cyclooxygenase-2 and inducible nitric synthase by flavonoids may be important in the prevention of memory deficits, one of the symptoms related to AD [18]. Therefore, flavonoids could in principle be a key class of molecules for the development of therapeutics for AD [19].
We performed the current study to test the hypothesis that there exits a dose-dependent protective effect of the red wine flavonoid quercetin in primary neuronal culture against the oxidant Aβ (1-42).
2. Materials and Methods
2.1. Reagents
All chemicals were purchased from Sigma–Aldrich (St. Louis, MO, USA) unless stated otherwise [20]. Aβ (1–42) was dissolved in sterile water and stirred and pre-incubated for 24 h at 37°C before adding to the culture at a final concentration of 10 μM. Quercetin was dissolved in DMSO diluted in DMEM media used according to the concentration required. The cells were preincubated with quercitin (Fig.1) for 1 h before Aβ (1–42) was added. In some experiments, fresh media were added after 1 h preincubation of neuronal cells with quercitin. Assays for cell viability, protein oxidation, lipid peroxidation, apoptosis and ROS were performed 24 h after Aβ (1–42) treatment as previously described [21]. Before starting experimental analysis, photographs for all cultures were taken using a phase contrast microscope.
Fig. 1.
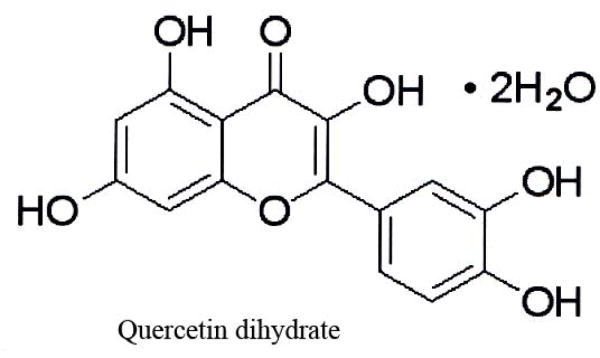
The chemical structure of quercetin dehydrate (2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-4H-1-benzopyran-4-one dihydrate), used in this study.
2.2. Determination of Cell Viability
Cortical neuronal cultures were obtained from 18-day-old Sprague–Dawley rat fetuses as described previously [22] and were plated in 48-well plates. Neuronal mitochondrial function as cell viability was determined by the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] reduction assay. Briefly, 24 h after exposure of cells to Aβ (1–42), MTT stock solution in PBS was added to each well with final concentration of 1.0 mg/ml, and incubated for 1 h. The dark blue formazan crystals formed in intact cells were extracted with 200 ml of DMSO, and absorbance at 595 nm was measured with a microplate reader (Bio-Tek). Results were expressed as the percent of MTT reduction, assuming that the absorbance of control cells was 100%. Cell viability also was qualitatively examined by phase-contrast microscopy as previously described [20,22]. Briefly, features of primary rat neuronal cells treated with Aβ (1-42) with and without quercetin, such as vacuolated soma, fragmented neurites, membrane blebbing and cell shrinkage, were taken as indicative of damaged neurons.
2.3. Measurement of Protein Carbonyls
Protein carbonyls are an index of protein oxidation and were determined as described previously [23]. Briefly, the cell extract (5μg of protein) were derivatized with 10 mM 2,4-dinitrophenylhydrazine in the presence of 5ml of 12% SDS for 20 min at room temperature. The samples were neutralized with 7.5ml of the neutralization solution (2 M Tris in 30% glycerol). Derivatized protein samples were blotted onto nitrocellulose membranes with a slot- blot apparatus (250 ng/slot). The membrane was then washed with wash buffer [10 mM Tris–HCl (pH 7.5), 150 mM NaCl, 0.05% Tween 20], blocked by incubation in the presence of 5% BSA. This step was followed by incubation with rabbit polyclonal anti-DNPH antibody as the primary antibody for 1 h. The membranes were washed with wash buffer and further incubated with alkaline phosphatase (ALP)-conjugated goat anti-rabbit antibody as the secondary antibody for 1 h. Blots were developed using Sigma Fast tablets (BCIP/NBT) and were quantified using Scion Image (PC version of Macintosh compatible NIH Image) software.
2.4. Measurement of 3-Nitrotyrosine (3-NT)
The content of 3-NT was determined by incubating the sample with Laemmli sample buffer (0.125 M Trizma base, pH 6.8, 4% SDS, 20% glycerol) for 20 min. Then 250 ng of protein were blotted onto the nitrocellulose paper using the slot-blot apparatus and immunochemical methods as described above for protein carbonyls. The mouse anti-nitrotyrosine antibody was used as primary antibody and ALP- conjugated anti-mouse secondary antibody was used for detection. Controls in which the primary antibody was reacted with free 3-NT resulted in no detection of protein-bound 3-NT in Aβ-treated cells (data not shown). Densitometric analysis of bands in images of the blots was used to calculate levels of 3-NT.
2.5. Measurement HNE Levels
Levels of HNE were quantified by slot-blot analysis as described previously [24]. Anti-HNE antibody raised in rabbit was used as the primary antibody. Controls in which the primary antibody was reacted with free HNE resulted in faint, non-specific binding of the antibody (data not shown). However, since both Aβ(1-42)- and Aβ(1-42) +quercetin-treated samples used the same antibody, background correction was identical in both samples.
2.6. Estimation of Protein
The protein concentrations were measured using the BCA method as described [20] to determine equal protein concentrations in experiments.
2.7. Statistical Analysis
Analysis of variance (ANOVA) followed by Student's t-test was used. Statistical significance was assumed for p < 0.05.
3. Results
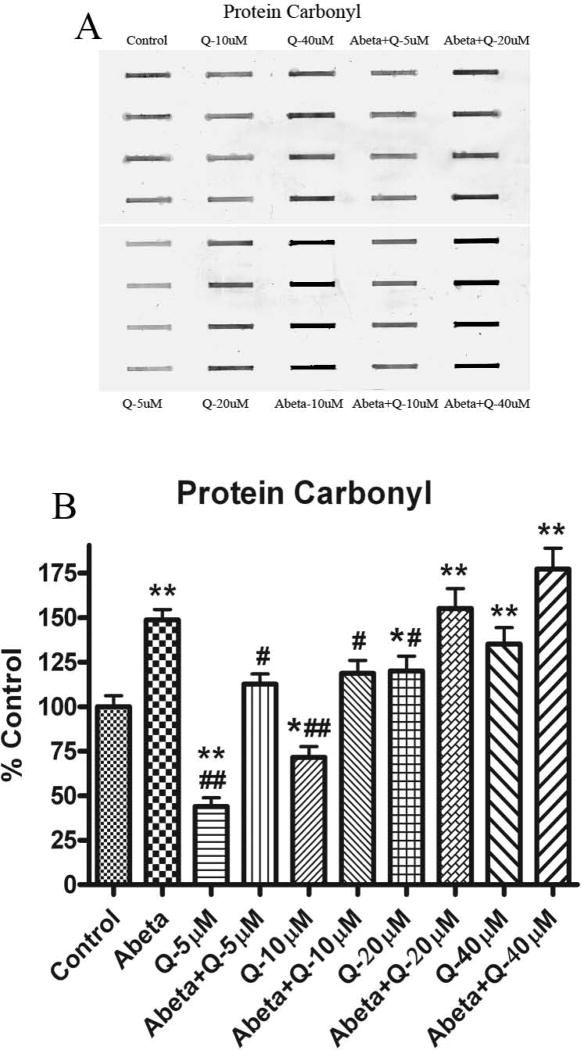
3.1. Protein carbonyls
Fig. 2 shows the levels of protein carbonyls in neuronal cultures treated with Aβ (1-42). As shown previously [20], the levels of protein carbonyls were found to be significantly increased with Aβ (1-42) treatment compared to control. Pretreatment of neurons with quercetin and subsequently treated with Aβ (1-42) showed significantly decreased protein carbonyl levels compared to control, but this effect was found only at lower doses, 5 and 10 μM. Higher concentrations of quercetin (20 and 40 μM) increased protein carbonyl levels significantly.
Fig. 2.
(A) Shows representative blot for protein carbonyl, developed with a primary antibody against the hydrazone formed by reaction of carbonyl groups with DNPH. (B) Shows the increment to protein carbonyl formation in cultured neurons treated with Aβ (1-42) as compared to controls. The dose dependent effect of quercetin shows protection against protein carbonyls formation by Aβ (1-42) treatment. *p<0.01 and **p<0.001 compared to control and #p<0.01 and ##p<0.001 compared to Aβ (1-42) (10 μM) treatment. The data are presented as mean ± SEM expressed as percentage of control (n=6).
3.2. 3-Nitrotyrosine (3-NT)
Fig. 3 shows the levels of 3-NT production in neuronal culture. As shown previously [20] the levels of 3-NT was found to be significantly increased with Aβ (1-42) treatment compared to control. Pretreatment of neurons with quercetin and subsequently treated with Aβ (1-42) showed significantly decreased 3-NT levels compared to control, but this effect was found only at lower doses (5 and 10 μM). Higher concentrations of quercetin (20 and 40 μM) increased 3-NT formation significantly.
Fig. 3.
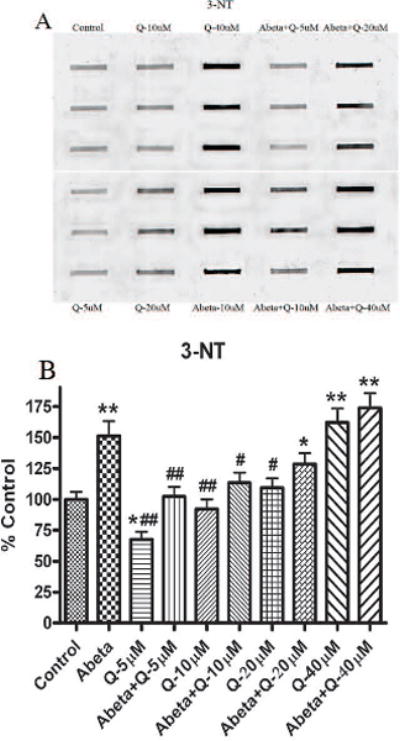
(A) Shows representative blot for 3-NT, developed with a primary antibody against 3-nitrotyrosine in proteins). (B) Shows the increment in 3-NT levels formation in cultured neurons treated with Aβ (1-42) as compared to controls. The dose dependent effect of quercetin shows protection against 3-NT formation by Aβ (1-42) treatment. *p<0.01 and **p<0.001 compared to control and #p<0.01 and ##p<0.001 compared to Aβ (1-42) (10 μM) treatment. The data are presented as mean ± SEM expressed as percentage of control (n=6).
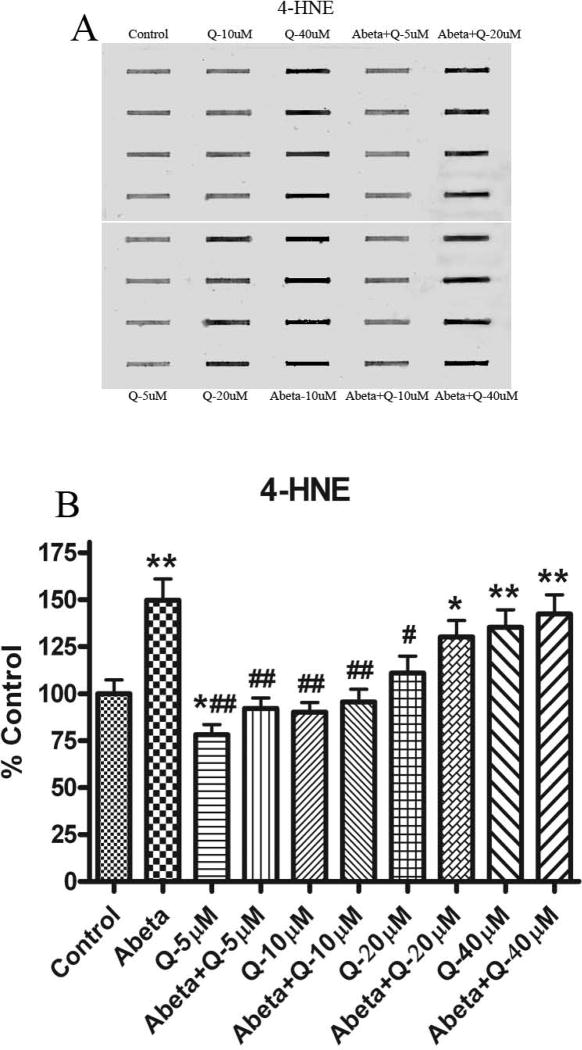
3.3. Protein-Bound 4 –Hydroxynonenal (HNE, an index of lipid peroxidation)
Fig. 4 shows the levels of protein-bound HNE production in neuronal cultures. As shown previously the levels of HNE were found significantly increased with Aβ (1-42) treatment compared to control [20, 24]. Pretreatment of neurons with quercetin subsequently treated with Aβ (1-42) showed significantly decreased HNE levels compared to control, but this effect was found only at lower doses (5 and 10 μM). Higher concentrations of quercetin (20 and 40 μM) increased HNE formation significantly.
Fig. 4.
(A) Shows representative blot for 4-HNE-bound proteins, developed with a primary antibody against the Michael adduct of HNE with proteins. (B) Shows the increment in 4-HNE formation in cultured neurons treated with Aβ (1-42) compared to the control. The dose dependent effect of quercetin shows protection against 4-HNE formation by Aβ (1-42) treatment. *p<0.01 and **p<0.001 compared to control and #p<0.01 and ##p<0.001 compared to Aβ (1-42) (10 μM) treatment. The data are presented as mean ± SEM expressed as percentage of control (n=6).
3.4. Effect of Quercetin on Cell Toxicity Induced by Aβ(1–42)
As shown in Fig. 5, exposure of neuronal cultures to Aβ(1–42) (10μM) for 24 h reduced cell viability. Pretreatment of neurons with quercetin for 1 h significantly attenuated Aβ (1–42)-induced cytotoxicity at the two lower doses (5 and 10 μM), but the high doses (20 and 40 μM) of quercetin did not attenuate significantly the ability of neuronal mitochondria to reduce MTT. Quercetin attenuated Aβ (1–42)- induced cell loss at lower doses, and good effects were observed at 5 μM. When neurons were treated with lower doses (0.1, 0.2, 0.4, 0.5, 0.75 and 1 μM quercetin), there was no significant protection against Aβ (1–42)-induced neurotoxicity (data not shown).
Fig. 5.
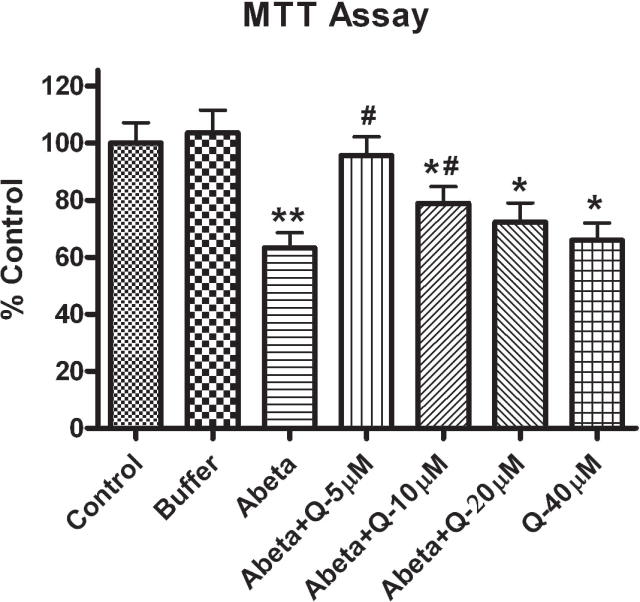
Shows the effect of varying concentrations of quercetin on cell viability that is reduced by Aβ (1–42) in primary cultured rat neurons. Quercetin was added to the culture 1 h prior to 10 μM Aβ (1–42) addition, and the cells were incubated for 24 h. Cell viability was assessed using the MTT reduction assay. The data are presented as mean±SEM expressed as percentage of control values. *p<0.01 and **p<0.001 compared to control and #p<0.01 and ##p<0.001 compared to oxidant treatment.
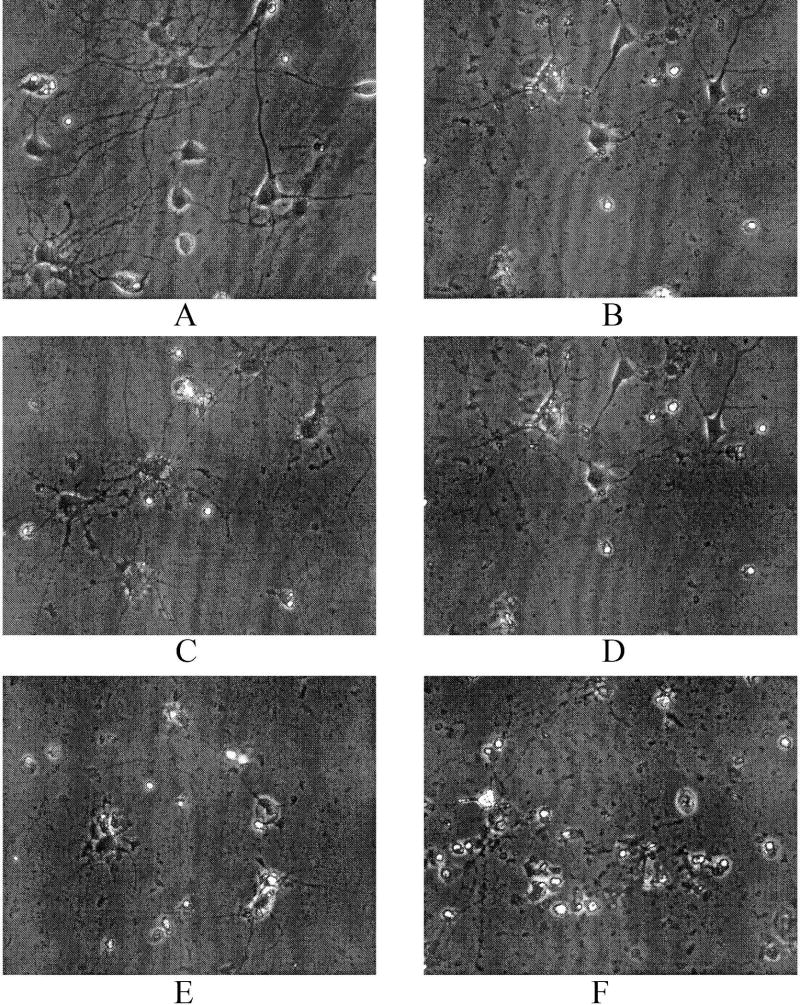
3.5. Quercetin Inhibited Aβ (1–42)-induced Apoptotic Cell Death
Fig. 6 shows phase contrast photomicrographs of primary rat neuronal cells treated with Aβ (1-42) with and without quercetin. Aβ (1-42)-treated neurons demonstrated vacuolated soma and fragmented neurites, membrane blebbings and cell shrinkage. Quercetin mitigated morphological alterations induced by Aβ (1–42) at the 5 μM and 10 μM doses. All of the higher doses of quecitin did not show protective effects against Aβ (1–42), but did induce apoptotic-like morphology. Quercetin alone also led cell death at higher (40 μM) dose.
Fig. 6.
Quercetin protects against Aβ (1–42)-induced neuronal death, indexed by phase contrast microscopy. (A) Control; (B) neurons treated with Aβ (1–42); (C) neurons treated with Aβ (1–42)+5 μM quercetin; (D) neurons treated with Aβ (1–42)+10 μM quercetin; (E) neurons treated with Aβ (1–42)+20 μM quercetin; and (F) neurons treated with 40 μM quercetin only.
4. Discussion
Oxidative stress defines a marked imbalance between reactive oxygen species (ROS) and its removal by anti-oxidant systems. This imbalance may originate from an overproduction of ROS or from a reduction in antioxidant defenses [1] An inverse relationship between lipid peroxidation and antioxidant system is well known [25, 26]. In general, a reduction in antioxidant may impair H2O2 clearance and promote hydroxyl radical formation, thus increasing the free radical load, which triggers oxidative stress [25, 26]. Reduced levels of antioxidants have been observed in oxidative stress-related disorders [27] in specific regions of the central nervous system of AD patients [28]. Studies have shown that an increased endogenous antioxidant levels by dietary means or by pharmacological intake of antioxidant precursors or GSH mimetics or substrates protect GSH from oxidative depletion and protect brain against oxidative stress [29-31].
There is evidence that oxidative stress including free radicals plays a key role in AD and PD [22, 32]. Brain membrane lipids are rich in polyunsaturated fatty acids, which are especially sensitive to free radical-induced lipid peroxidation. H2O2 is a reactive non-radical molecule that can easily permeate through biological cell membranes, while O2•- can only move through an anion channel [7] or diffuse as HO2•. It has been proposed that the phenolic phytochemical quercetin exert positive health effects in chronic disease states, including cancer and neurodegenerative disorders [33]. Antioxidant glycosides, such as quercetin rutoside, quench superoxide production without interfering with the electron transfer activity of the reductase [34]. Many physiological benefits of flavonoids have been attributed to their antioxidant and free radical scavenging properties [14].
Quercetin has been thoroughly investigated for its abilities to express antiproliferative and (Csokay et al., 1997) protective effects in various systems [35, 36]. Lipid peroxidation caused by oxidative stress can lead to changes in membrane integrity and fluidity [37]. Quercetin protects mouse hippocampal cell line HT-22 from glutamate-induced oxidative toxicity and lipid peroxidation, by blocking ROS production [38]. Quercetin is protective against agents in neuroleptic-induced orofacial dyskinesia [39]. In addition, a hydrophobic antioxidant may easily pass into the cytoplasm where ROS are generated and modulate oxidative glutamate toxicity [38]. Quercetin has the specific structure to prevent GSH oxidation, thereby protecting oxidative stress-induced neurotoxicity [38, 40]. It has been reported that quercetin can flux into brain regions [41]. Therefore, it is possible that quercetin with beneficial antioxidant and biological functions is able to penetrate the BBB and protect brain against H2O2-induced cytotoxicity [15].
Increased lipid peroxidation with its consequent decline in GSH and its dependent enzymes [42], as well as diminished SOD and catalase levels are significantly reversed by quercetin treatment in an in vivo system [43]. Chronic quercetin treatment reverses cognitive deficits due to ageing and ethanol-intoxication, effects that are associated with its antioxidant property [43].
Flavonoids such as quercetin have the potential to be therapeutically effective because of their free radical quenching, iron chelating, and anti-inflammatory properties [44]. Aβ-induced oxidative toxicity on neuronal cells is proposed as a principal route in neuronal loss in AD [2]. The flavonoid quercetin strongly inhibited Aβ fibril formation, and protected HT22 murine neuroblastoma cells from Aβ (25-35) oxidative attack [36, 40]. The inhibition of HSP70 by quercetin correlated with a decreased expression of procaspase-3 and enhancement of specific cleavage of poly (ADP-ribose) polymerase into apoptotic fragments [18]. Quercetin inhibited only the oxidative stress but not the heat shock induced expression of Hsp68. This differential regulation was observed after exposing cells to arachidonic acid during stress [45].
During oxidative stress several lipid peroxidation products are formed, including HNE, which is one of the most abundant and toxic lipid-derived aldehydes, that can induce oxidative stress [24]. Lipid peroxidation products such as HNE and acrolein are known to cause damage to biomembranes, proteins and other biomolecules in AD brain [24, 46]. These alkenals react with an immediate substrate, GSH [47], and these lipid peroxidation products are known to be involved in apoptosis, which may derived from GSH depletion [48].
Glutathione protects cultured neurons against oxidative damage resulting from amyloid β-peptide, iron, and HNE [48]. GSH can also protect brain from damage by peroxynitrite, hydroxyl free radicals, or reactive alkenals [49]. HNE can alter α-ketoglutarate dehydrogenase (KGD) [50], decrease cell survival [51] (decrease MTT reduction) that could be reversed by quercetin treatment (Kim et al., 2005). Aβ increases lipid derived free radical production [52], resulting in elevated protein carbonylation, HNE formation and 3-NT production in neuronal culture. Decreased protein oxidation was observed in neuronal cell culture treated with quercetin on incubation with Aβ (1-42). The results shown in this in vitro study demonstrate that quercetin acts as an antioxidant at the lower doses, but at higher doses toxic effects are observed. Consequently, quercetin potentially could be a key molecule for the development of therapeutics for AD, but in this case its effective concentration must be observed.
Acknowledgments
This research was supported in parts by NIH grants [AG-10836; AG-05117].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Halliwell B, Gutteridge JMC. Oxford University Press; Oxford: 1999. Free Radicals in Biology and Medicine. [Google Scholar]
- 2.Butterfield DA, et al. Roles of amyloid beta-peptide-associated oxidative stress and brain protein modifications in the pathogenesis of Alzheimer's disease and mild cognitive impairment. Free Radic Biol Med. 2007;43(5):658–77. doi: 10.1016/j.freeradbiomed.2007.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fahn S, Cohen G. The oxidant stress hypothesis in Parkinson's disease: evidence supporting it. Ann Neurol. 1992;32(6):804–12. doi: 10.1002/ana.410320616. [DOI] [PubMed] [Google Scholar]
- 4.Mecocci P, MacGarvey U, Beal MF. Oxidative damage to mitochondrial DNA is increased in Alzheimer's disease. Ann Neurol. 1994;36(5):747–51. doi: 10.1002/ana.410360510. [DOI] [PubMed] [Google Scholar]
- 5.Greenamyre JT, Betarbet R, Sherer TB. The rotenone model of Parkinson's disease: genes, environment and mitochondria. Parkinsonism Relat Disord. 2003;9 2:S59–64. doi: 10.1016/s1353-8020(03)00023-3. [DOI] [PubMed] [Google Scholar]
- 6.Markesbery WR. Oxidative stress hypothesis in Alzheimer's disease. Free Radic Biol Med. 1997;23(1):134–47. doi: 10.1016/s0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- 7.Multhaup G, et al. Reactive oxygen species and Alzheimer's disease. Biochem Pharmacol. 1997;54(5):533–9. doi: 10.1016/s0006-2952(97)00062-2. [DOI] [PubMed] [Google Scholar]
- 8.Gao Z, Huang K, Xu H. Protective effects of flavonoids in the roots of Scutellaria baicalensis Georgi against hydrogen peroxide-induced oxidative stress in HS-SY5Y cells. Pharmacol Res. 2001;43(2):173–8. doi: 10.1006/phrs.2000.0761. [DOI] [PubMed] [Google Scholar]
- 9.Mercer LD, et al. Dietary polyphenols protect dopamine neurons from oxidative insults and apoptosis: investigations in primary rat mesencephalic cultures. Biochem Pharmacol. 2005;69(2):339–45. doi: 10.1016/j.bcp.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 10.Youdim KA, Joseph JA. A possible emerging role of phytochemicals in improving age-related neurological dysfunctions: a multiplicity of effects. Free Radic Biol Med. 2001;30(6):583–94. doi: 10.1016/s0891-5849(00)00510-4. [DOI] [PubMed] [Google Scholar]
- 11.Paganga G, Miller N, Rice-Evans CA. The polyphenolic content of fruit and vegetables and their antioxidant activities. What does a serving constitute? Free Radic Res. 1999;30(2):153–62. doi: 10.1080/10715769900300161. [DOI] [PubMed] [Google Scholar]
- 12.Duarte J, et al. Vasodilator effects of quercetin in isolated rat vascular smooth muscle. Eur J Pharmacol. 1993;239(13):1–7. doi: 10.1016/0014-2999(93)90968-n. [DOI] [PubMed] [Google Scholar]
- 13.Brown JP. A review of the genetic effects of naturally occurring flavonoids, anthraquinones and related compounds. Mutat Res. 1980;75(3):243–77. doi: 10.1016/0165-1110(80)90029-9. [DOI] [PubMed] [Google Scholar]
- 14.Rice-Evans CA, et al. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic Res. 1995;22(4):375–83. doi: 10.3109/10715769509145649. [DOI] [PubMed] [Google Scholar]
- 15.Heo HJ, Lee CY. Protective effects of quercetin and vitamin C against oxidative stress-induced neurodegeneration. J Agric Food Chem. 2004;52(25):7514–7. doi: 10.1021/jf049243r. [DOI] [PubMed] [Google Scholar]
- 16.Bate C, Salmona M, Williams A. Ginkgolide B inhibits the neurotoxicity of prions or amyloid-beta1-42. J Neuroinflammation. 2004;1(1):4. doi: 10.1186/1742-2094-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yagyu K, et al. Protective effects of estradiol against amyloid beta protein-induced inhibition of neuronal Cl(-)-ATPase activity. Neuropharmacology. 2002;43(8):1297–304. doi: 10.1016/s0028-3908(02)00304-0. [DOI] [PubMed] [Google Scholar]
- 18.Schroeter H, et al. Flavonoids protect neurons from oxidized low-density-lipoprotein-induced apoptosis involving c-Jun N-terminal kinase (JNK), c-Jun and caspase-3. Biochem J. 2001;358(Pt 3):547–57. doi: 10.1042/0264-6021:3580547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patil CS, et al. Protective effect of flavonoids against aging- and lipopolysaccharide-induced cognitive impairment in mice. Pharmacology. 2003;69(2):59–67. doi: 10.1159/000072357. [DOI] [PubMed] [Google Scholar]
- 20.Sultana R, et al. Ferulic acid ethyl ester protects neurons against amyloid beta-peptide(1-42)-induced oxidative stress and neurotoxicity: relationship to antioxidant activity. J Neurochem. 2005;92(4):749–58. doi: 10.1111/j.1471-4159.2004.02899.x. [DOI] [PubMed] [Google Scholar]
- 21.Butterfield DA, Lauderback CM. Lipid peroxidation and protein oxidation in Alzheimer's disease brain: potential causes and consequences involving amyloid beta-peptide-associated free radical oxidative stress. Free Radic Biol Med. 2002;32(11):1050–60. doi: 10.1016/s0891-5849(02)00794-3. [DOI] [PubMed] [Google Scholar]
- 22.Butterfield DA, et al. Amyloid beta-peptide-associated free radical oxidative stress, neurotoxicity, and Alzheimer's disease. Methods Enzymol. 1999;309:746–68. doi: 10.1016/s0076-6879(99)09050-3. [DOI] [PubMed] [Google Scholar]
- 23.Butterfield DA. beta-Amyloid-associated free radical oxidative stress and neurotoxicity: implications for Alzheimer's disease. Chem Res Toxicol. 1997;10(5):495–506. doi: 10.1021/tx960130e. [DOI] [PubMed] [Google Scholar]
- 24.Lauderback CM, et al. The glial glutamate transporter, GLT-1, is oxidatively modified by 4-hydroxy-2-nonenal in the Alzheimer's disease brain: the role of Abeta1-42. J Neurochem. 2001;78(2):413–6. doi: 10.1046/j.1471-4159.2001.00451.x. [DOI] [PubMed] [Google Scholar]
- 25.Ahmad M, et al. Ginkgo biloba affords dose-dependent protection against 6-hydroxydopamine-induced parkinsonism in rats: neurobehavioural, neurochemical and immunohistochemical evidences. J Neurochem. 2005;93(1):94–104. doi: 10.1111/j.1471-4159.2005.03000.x. [DOI] [PubMed] [Google Scholar]
- 26.Ursini F. In: Thoughts on physiological functions from enzymology and molecular structure in oxidative processes and antioxidants. Paoletti R, editor. New York: Raven; 1994. pp. 25–31. [Google Scholar]
- 27.Bains JS, Shaw CA. Neurodegenerative disorders in humans: the role of glutathione in oxidative stress-mediated neuronal death. Brain Res Brain Res Rev. 1997;25(3):335–58. doi: 10.1016/s0165-0173(97)00045-3. [DOI] [PubMed] [Google Scholar]
- 28.Benzi G, Moretti A. Are reactive oxygen species involved in Alzheimer's disease? Neurobiol Aging. 1995;16(4):661–74. doi: 10.1016/0197-4580(95)00066-n. [DOI] [PubMed] [Google Scholar]
- 29.Anderson ME, Luo JL. Glutathione therapy: from prodrugs to genes. Semin Liver Dis. 1998;18(4):415–24. doi: 10.1055/s-2007-1007174. [DOI] [PubMed] [Google Scholar]
- 30.Drake J, et al. Elevation of brain glutathione by gamma-glutamylcysteine ethyl ester protects against peroxynitrite-induced oxidative stress. J Neurosci Res. 2002;68(6):776–84. doi: 10.1002/jnr.10266. [DOI] [PubMed] [Google Scholar]
- 31.Pocernich CB, La Fontaine M, Butterfield DA. In-vivo glutathione elevation protects against hydroxyl free radical-induced protein oxidation in rat brain. Neurochem Int. 2000;36(3):185–91. doi: 10.1016/s0197-0186(99)00126-6. [DOI] [PubMed] [Google Scholar]
- 32.LeWitt PA. New drugs for the treatment of Parkinson's disease. Pharmacotherapy. 2000;20(1 Pt 2):26S–32S. doi: 10.1592/phco.20.2.26s.34631. [DOI] [PubMed] [Google Scholar]
- 33.Edwin Shackelford R. Pharmacological manipulation of ataxia-telangiectasia kinase activity as a treatment for Parkinson's disease. Med Hypotheses. 2005;64(4):736–41. doi: 10.1016/j.mehy.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 34.Marchbanks RM, et al. A mitochondrial DNA sequence variant associated with schizophrenia and oxidative stress. Schizophr Res. 2003;65(1):33–8. doi: 10.1016/s0920-9964(03)00011-2. [DOI] [PubMed] [Google Scholar]
- 35.Eberhardt MV, Lee CY, Liu RH. Antioxidant activity of fresh apples. Nature. 2000;405(6789):903–4. doi: 10.1038/35016151. [DOI] [PubMed] [Google Scholar]
- 36.Kim H, et al. Effects of naturally occurring compounds on fibril formation and oxidative stress of beta-amyloid. J Agric Food Chem. 2005;53(22):8537–41. doi: 10.1021/jf051985c. [DOI] [PubMed] [Google Scholar]
- 37.Prasad MR, et al. Regional membrane phospholipid alterations in Alzheimer's disease. Neurochem Res. 1998;23(1):81–8. doi: 10.1023/a:1022457605436. [DOI] [PubMed] [Google Scholar]
- 38.Ishige K, Schubert D, Sagara Y. Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free Radic Biol Med. 2001;30(4):433–46. doi: 10.1016/s0891-5849(00)00498-6. [DOI] [PubMed] [Google Scholar]
- 39.Naidu PS, Singh A, Kulkarni SK. Quercetin, a bioflavonoid, attenuates haloperidol-induced orofacial dyskinesia. Neuropharmacology. 2003;44(8):1100–6. doi: 10.1016/s0028-3908(03)00101-1. [DOI] [PubMed] [Google Scholar]
- 40.Bastianetto S, Quirion R. Natural extracts as possible protective agents of brain aging. Neurobiol Aging. 2002;23(5):891–97. doi: 10.1016/s0197-4580(02)00024-6. [DOI] [PubMed] [Google Scholar]
- 41.Youdim KA, et al. Flavonoid permeability across an in situ model of the blood-brain barrier. Free Radic Biol Med. 2004;36(5):592–604. doi: 10.1016/j.freeradbiomed.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 42.Sanders RA, Rauscher FM, Watkins JB., 3rd Effects of quercetin on antioxidant defense in streptozotocin-induced diabetic rats. J Biochem Mol Toxicol. 2001;15(3):143–9. doi: 10.1002/jbt.11. [DOI] [PubMed] [Google Scholar]
- 43.Singh A, Naidu PS, Kulkarni SK. Reversal of aging and chronic ethanol-induced cognitive dysfunction by quercetin a bioflavonoid. Free Radic Res. 2003;37(11):1245–52. doi: 10.1080/10715760310001616014. [DOI] [PubMed] [Google Scholar]
- 44.Juurlink BH, Paterson PG. Review of oxidative stress in brain and spinal cord injury: suggestions for pharmacological and nutritional management strategies. J Spinal Cord Med. 1998;21(4):309–34. doi: 10.1080/10790268.1998.11719540. [DOI] [PubMed] [Google Scholar]
- 45.Gosslau A, Rensing L. Induction of Hsp68 by oxidative stress involves the lipoxygenase pathway in C6 rat glioma cells. Brain Res. 2000;864(1):114–23. doi: 10.1016/s0006-8993(00)02195-8. [DOI] [PubMed] [Google Scholar]
- 46.O'Brien PJ. In: Cellular Antioxidant Defense Mechanism. Chow CK, editor. Vol. 1. Boca Raton, FL: CRC; 1988. pp. 73–87. [Google Scholar]
- 47.Lovell MA, Xie C, Markesbery WR. Decreased glutathione transferase activity in brain and ventricular fluid in Alzheimer's disease. Neurology. 1998;51(6):1562–6. doi: 10.1212/wnl.51.6.1562. [DOI] [PubMed] [Google Scholar]
- 48.Mark RJ, et al. A role for 4-hydroxynonenal, an aldehydic product of lipid peroxidation, in disruption of ion homeostasis and neuronal death induced by amyloid beta-peptide. J Neurochem. 1997;68(1):255–64. doi: 10.1046/j.1471-4159.1997.68010255.x. [DOI] [PubMed] [Google Scholar]
- 49.Koppal T, Drake J, Butterfield DA. In vivo modulation of rodent glutathione and its role in peroxynitrite-induced neocortical synaptosomal membrane protein damage. Biochim Biophys Acta. 1999;1453(3):407–11. doi: 10.1016/s0925-4439(99)00014-9. [DOI] [PubMed] [Google Scholar]
- 50.Pocernich CB, Butterfield DA. Acrolein inhibits NADH-linked mitochondrial enzyme activity: implications for Alzheimer's disease. Neurotox Res. 2003;5(7):515–20. doi: 10.1007/BF03033161. [DOI] [PubMed] [Google Scholar]
- 51.Ramachandran V, et al. Ethanol-induced oxidative stress precedes mitochondrially mediated apoptotic death of cultured fetal cortical neurons. J Neurosci Res. 2003;74(4):577–88. doi: 10.1002/jnr.10767. [DOI] [PubMed] [Google Scholar]
- 52.Boyd-Kimball D, et al. Role of phenylalanine 20 in Alzheimer's amyloid beta-peptide (1-42)-induced oxidative stress and neurotoxicity. Chem Res Toxicol. 2004;17(12):1743–9. doi: 10.1021/tx049796w. [DOI] [PubMed] [Google Scholar]