Abstract
Background
Human immunodeficiency virus (HIV) therapy includes a backbone of nucleoside reverse-transcriptase inhibitors (NRTIs). Toxicities associated with NRTIs are not fully defined in children.
Methods
We studied 2233 children ≤13 years of age who were perinatally infected with HIV and were receiving ≥2 NRTIs, to determine the relative toxicities of the 5 most common NRTI pairs: zidovudine (ZDV)/lamivudine (3TC), ZDV/didanosine (ddI), stavudine (d4T)/3TC, d4T/ddI, and ddI/3TC. Incidence rates for clinical and laboratory toxicities were estimated, and NRTI pairs were compared with regard to the time to the first toxicity.
Results
The most common clinical toxicities noted were hepatitis, peripheral neuropathy, lipodystrophy/lipoatrophy, and pancreatitis, whereas the most common laboratory toxicities were an elevated anion gap, an increased total amylase level, neutropenia, and thrombocytopenia. Overall, regimens containing ZDV were associated with a significantly lower rate of clinical toxicities than were those containing d4T (adjusted hazard ratio [HR], 0.49; P = .02); regimens containing ddI were associated with a significantly lower rate of laboratory toxicities than were those containing 3TC (adjusted HR, 0.78; P = .04). ZDV/3TC was associated with a lower rate of clinical toxicities than were d4T/ddI and ddI/3TC and with a higher rate of laboratory toxicities than was ZDV/ddI. ZDV/ddI was associated with a lower rate of clinical toxicities than was d4T/3TC.
Conclusions
In children, regimens containing ZDV have less toxicity than do those containing d4T, thereby supporting their use in first-line regimens. D4T/3TC, d4T/ddI, and ddI/3TC have similar toxicity rates and are appropriate for second-line therapy.
Highly active antiretroviral therapy (HAART), which typically includes a “backbone” of 2 nucleoside reverse-transcriptase inhibitors (NRTIs) plus either a protease inhibitor (PI) or a nonnucleoside reverse transcriptase inhibitor (NNRTI), is the most commonly used regimen for children with pediatric HIV infection [1]. According to the 2008 US Public Health Service (PHS) guidelines for antiretroviral therapy (ART) for HIV-infected children, the NRTI pairs that are “preferred” for the initial treatment of preadolescent children are abacavir (ABC) or zidovudine (ZDV) plus lamivudine (3TC) or emtricitabine (FTC); or didanosine (ddI) plus FTC [2]. ZDV/ABC and ZDV/ddI are classified as “alternative” pairs, and d4T/3TC or d4T/FTC may be used in special circumstances. Although HAART improves the outcomes of HIV-infected children [3], it is associated with such toxicities as bone marrow suppression, lactic acidosis, lipoatrophy, hyperlipidemia, peripheral neuropathy, hepatitis, pancreatitis, and hypersensitivity [2, 4].
In both the pediatric and adult PHS guidelines, d4T/ddI is “not recommended” [5]. In initial small pediatric [6, 7] and adult [8, 9] studies, d4T/ddI demonstrated good antiviral activity and was well tolerated. Subsequent larger studies of adults revealed that this combination, when compared with ZDV/3TC, was associated with a significantly increased rate of lactic acidosis, peripheral neuropathy, pancreatitis, lipodystrophy, and hepatitis [10–15]. Serious, sometimes fatal lactic acidosis with hepatic steatosis, with or without pancreatitis, has been reported in pregnant women receiving d4T/ddI [16–18]. These adverse events are believed to result from mitochondrial toxicity [17, 19].
Because few pediatric studies compare the tolerance of different NRTI combinations [20], we used information from the largest United States–based prospective study of HIV-infected children to study the relative toxicities of the 5 NRTI pairs most commonly used in children: ZDV/3TC, ZDV/ddI, d4T/3TC, d4T/ddI, and ddI/3TC. Our hypothesis is that, in children, ddI/d4T has a rate of toxicity similar to that of other NRTI pairs, making it appropriate for second-line therapy.
METHODS
We used data from the Pediatric AIDS Clinical Trials Group (PACTG) 219C cohort study, which enrolled HIV-infected and HIV-exposed but uninfected children in the United States from September 2000 through April 2006 and was closed to follow-up in May 2007 [3]. Children enrolled in the PACTG 219C study were eligible for the present evaluation if they were perinatally infected with HIV and had received an ART regimen containing ≥2 NRTIs at ≤13 years of age. Although older children were also enrolled in PACTG 219C, we used an age limit of 13 years to conform to pediatric treatment guidelines published elsewhere [2]. The institutions participating in the study obtained approval from their institutional review board (IRB); each child’s parent or guardian provided written, informed consent; and each child provided assent according to the regulations of the local IRB.
At the time of enrollment in the PACTG 219C study, the clinical records of the children were abstracted to obtain demographic information and the medical history, including information on all previous ART received, with dates of treatment initiation and discontinuation. In addition, the percentage of CD4-positive T lymphocytes (i.e., the “CD4+ percentage”) and the HIV load were determined. ART use, HIV immunologic and virologic parameters, results of routine laboratory tests, and clinical diagnoses were recorded at follow-up visits that occurred every 3 months. Approximately one-half (49%) of the perinatally infected children in PACTG 219C had previously participated in PACTG 219. For children who started receiving a particular NRTI pair before enrollment in PACTG 219C, measurements of the CD4+ percentage were obtained from PACTG 219, when available.
The toxicities evaluated included both clinical diagnoses and laboratory test abnormalities. The targeted clinical diagnoses included lipodystrophy or lipoatrophy, metabolic acidosis, lactic acidosis, peripheral neuropathy, pancreatitis, and hepatitis. The targeted laboratory test abnormalities included metabolic acidosis, as reflected by an increase in the anion gap (Na+-[Cl− + CO2] > 16 mEq/L); a decrease in the albumin level (<2 g/dL); an elevation (grade 3 or higher) in levels of aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, bilirubin, creatinine, creatinine phosphokinase (CPK), total amylase, pancreatic serum amylase, or lipase; or a decrease (grade 3 or higher) in hemoglobin, the white blood cell count, the absolute neutrophil count (ANC), or the platelet count. Grading of these laboratory test abnormalities was based on a table provided by the National Institutes of Health Division of AIDS for grading the severity of adverse events in adults and children [21].
In evaluating targeted toxicities, we included those that occurred while the subject was receiving a particular dual NRTI regimen for the first time and for 1 month after stopping that regimen. Toxicities were summarized separately for clinical diagnoses and laboratory test abnormalities, and data include the number and percentage of subjects experiencing targeted toxicities, the incidence rates and corresponding confidence intervals, and the Kaplan-Meier estimates for the time until the first targeted toxicity was noted. In evaluating clinical toxicities, we relied on the fact that a complete history of major diagnoses and ART had been collected at entry into the study, and, thus, we included all age-eligible subjects who met the criteria described above (i.e., the full cohort). However, in our evaluation of laboratory test abnormalities, we restricted our evaluation to the subset of subjects who had ≥1 targeted laboratory evaluation recorded while receiving the dual NRTI regimen of interest (or in the month after stopping therapy), because they were monitored for laboratory test abnormalities (i.e., the laboratory cohort).
Although evaluation of the incidence rates of targeted toxicities allows a descriptive comparison of the 5 NRTI pairs, these incidence rates cannot be compared directly, because some subjects may have received >1 of the 5 dual NRTI regimens. Instead, pairwise comparisons of toxicities using Cox proportional hazards models were conducted to determine the time to the first targeted clinical diagnosis and laboratory test abnormality. If a subject had received both NRTI pairs being compared, only their experience with the first pair that they received was considered. In addition, a small number of subjects who simultaneously received all NRTIs compared were excluded from the pairwise comparison (e.g., a subject receiving ZDV/3TC/ddI was excluded from the comparison of ZDV/3TC and ZDV/ddI).
For each pairwise comparison, we calculated Kaplan-Meier estimates of the probability of remaining event free for each regimen, as well as a log-rank test comparing the 2 regimens. Subjects without any targeted toxicities were censored 1 month after stopping the regimen or at a maximum of 5 years after initiation of the regimen. Multivariate Cox proportional hazards models were used to adjust the pairwise comparisons for potential confounders, including age and calendar year (before 1997, 1997–2000, or after 2000) at the time of initiation of the regimen, the number of ART regimens previously received (≤2, 3–9, or ≥10), and concurrent use of PIs, NNRTIs, and/or other NRTIs. Final adjusted models included covariates for which P < .10. In the evaluation of targeted clinical diagnoses, there were insufficient data on the CD4+ percentage before treatment with NRTIs was initiated. However, for evaluation of laboratory toxicities, for the subset of subjects for whom the CD4+ percentage was available, a sensitivity analysis was conducted by further adjusting the multivariate Cox model for the most recent CD4+ percentage level (<15%, 15%–25%, or >25%) before initiation of the regimen.
All analyses were conducted using SAS software (version 9; SAS Institute) and included data submitted for the study by April 2006. Comparisons of dual NRTI backbones were based on a prespecified plan of analysis. Two-sided P values of <.05 were considered to be statistically significant.
RESULTS
Of 2571 children and adolescents perinatally infected with HIV and enrolled in the PACTG 219C study, 2233 (87%) received a regimen that included ≥2 NRTIs before 13 years of age. These 2233 subjects form the basis for the evaluation of targeted clinical diagnoses (full cohort). The number of children who received each of the 5 NRTI pairs is summarized in table 1, on the basis of the first course of treatment with each combination. In accordance with pediatric treatment guidelines [2], ZDV/3TC was the most commonly used combination, followed by d4T/3TC, ddI/d4T, and ddI/3TC. ZDV/ddI tended to be used in earlier years than were the other combinations; therefore, the percentage of children who also received a PI or NNRTI is much lower than that associated with other combinations. In addition, ZDV/ddI tended to be used in younger children. In contrast, d4T/ddI tended to be used in later years, which may have reflected its use as a second-line regimen. The median duration of a regimen (i.e., before switching to another regimen) was longest for d4T/3TC and ZDV/ddI and was shortest for ddI/3TC.
Table 1.
Summary of the use of 5 dual nucleoside reverse-transcriptase inhibitor (NRTI) regimens, based on the first treatment course for each regimen.
| Cohort, regimen | Ever followed a regimen, no. (%) of subjects |
Also received a PI, % of subjects |
Also received an NNRTI, % of subjects |
Treatment initiation date, median (IQR) |
Duration of regimen, median (IQR), months |
Age at start of regimen, median (IQR), years |
|---|---|---|---|---|---|---|
| Fulla (n = 2233) | ||||||
| ZDV/3TC | 1336 (60) | 32 | 9 | Mar 1997 (Aug 1996–Jan 1998) | 12.4 (5.5–26.5) | 4.9 (1.8–7.5) |
| ZDV/ddI | 1022 (46) | 9 | 8 | Aug 1995 (Nov 1994–May 1996) | 17.0 (8.3–31.4) | 3.7 (1.5–6.4) |
| d4T/3TC | 1154 (52) | 67 | 19 | Mar 1998 (Jun 1997–Sep 1999) | 19.9 (6.4–49.3) | 6.0 (3.2–8.7) |
| d4T/ddI | 772 (35) | 59 | 23 | Nov 1998 (Aug 1997–Jun 2000) | 15.1 (5.9–35.1) | 6.4 (3.6–9.1) |
| ddI/3TC | 258 (12) | 40 | 14 | Jul 1997 (Oct 1996–Oct 2000) | 9.0 (3.0–20.6) | 6.6 (3.7–9.9) |
| Laboratoryb (n = 1553) |
||||||
| ZDV/3TC | 584 (38) | 42 | 13 | Apr 1997 (Sep 1996–Nov 1998) | 23.0 (11.8–41.7) | 5.2 (1.7–7.9) |
| ZDV/ddI | 386 (25) | 15 | 12 | Sep 1995 (Nov 1994–Jun 1996) | 25.0 (14.9–51.7) | 4.3 (1.9–7.0) |
| d4T/3TC | 688 (44) | 73 | 23 | Jul 1998 (Aug 1997–May 2000) | 40.5 (18.9–65.1) | 6.4 (3.4–9.0) |
| d4T/ddI | 439 (28) | 63 | 24 | Jun 1999 (Feb 1998–Jan 2001) | 27.6 (13.8–55.4) | 6.6 (3.8–9.2) |
| ddI/3TC | 118 (8) | 53 | 16 | Mar 2000 (Dec 1996–Aug 2002) | 17.1 (8.3–32.4) | 8.0 (6.0–10.6) |
NOTE. d4T, stavudine; ddI, didanosine; IQR, interquartile range; NNRTI, nonnucleoside reverse-transcriptase inhibitor; PI, protease inhibitor; 3TC, lamivudine; ZDV, zidovudine.
All age-eligible subjects who met the study criteria described in the Methods section.
The subset of subjects monitored for laboratory test abnormalities, defined as having ≥1 targeted laboratory evaluation while receiving the dual NRTI regimen of interest (or in the month after stopping therapy).
Of the 2233 patients who started receiving a dual NRTI–based regimen, 1553 (70%) could be included in the laboratory cohort. Use of the 5 NRTI pairs in this cohort is summarized in table 1, on the basis of the first course of treatment with each combination. The duration of treatment tended to be longer for the laboratory cohort than for the full cohort, in part because of the requirement that there be ≥1 laboratory test performed while the treatment regimen was received; that is, first courses of treatment that were too short to include routine laboratory monitoring were excluded. The laboratory cohort also tended to include older children who started receiving therapy later than children in the full cohort.
The occurrence of targeted clinical diagnoses for each of the 5 dual NRTI regimens is summarized in table 2. Hepatitis was the most common diagnosis associated with ZDV/3TC, ZDV/ddI, and d4T/3TC; pancreatitis was most often associated with d4T/ddI; and lipodystrophy/lipoatrophy was most commonly associated with ddI/3TC.
Table 2.
Occurrence of specific targeted clinical and laboratory test abnormalities by dual nucleoside reverse-transcriptase inhibitor (NRTI) combination.
| Targeted finding | ZDV/3TC | ZDV/ddI | d4T/3TC | d4T/ddI | ddI/3TC |
|---|---|---|---|---|---|
| Clinical diagnosis | (n = 1336) | (n = 1022) | (n = 1154) | (n = 772) | (n = 258) |
| Peripheral neuropathy | 8 (0.6) | 5 (0.5) | 13 (1.1) | 5 (0.6) | 2 (0.8) |
| Hepatitis | 22 (1.6) | 16 (1.6) | 18 (1.6) | 5 (0.6) | 2 (0.8) |
| Pancreatitis | 0 | 2 (0.2) | 14 (1.2) | 13 (1.7) | 2 (0.8) |
| Acidosis | 0 | 2 (0.2) | 5 (0.4) | 3 (0.4) | 2 (0.8) |
| Lipodystrophy/lipoatrophy | 1 (0.1) | 0 | 13 (1.1) | 6 (0.8) | 4 (1.6) |
| Total diagnoses | 31 (2.3) | 25 (2.4) | 63 (5.5) | 32 (4.1) | 12 (4.7) |
| Subjects with ≥1 diagnosis | 27 (2.0) | 19 (1.9) | 47 (4.1) | 25 (3.2) | 10 (3.9) |
| Laboratory test abnormalitya | n = 584 | n = 386 | n = 688 | n = 439 | n = 118 |
| Metabolic acidosisb | 79 (13.5) | 32 (8.3) | 145 (21.1) | 76 (17.3) | 15 (12.7) |
| Increased level | |||||
| AST | 2 (0.3) | 1 (0.3) | 1 (0.1) | 3 (0.7) | 0 |
| ALT | 1 (0.2) | 0 | 1 (0.1) | 0 | 0 |
| Alkaline phosphatase | 2 (0.3) | 1 (0.3) | 5 (0.7) | 2 (0.5) | 0 |
| Bilirubin | 1 (0.2) | 1 (0.3) | 4 (0.6) | 3 (0.7) | 2 (1.7) |
| Creatinine | 0 | 1 (0.3) | 5 (0.7) | 1 (0.2) | 0 |
| CPK | 5 (0.9) | 1 (0.3) | 14 (2.0) | 2 (0.5) | 2 (1.7) |
| Total amylase | 12 (2.1) | 15 (3.9) | 17 (2.5) | 18 (4.1) | 3 (2.5) |
| Pancreatic serum amylase | 0 | 0 | 0 | 1 (0.2) | 0 |
| Lipase | 3 (0.5) | 3 (0.7) | 7 (1.0) | 4 (0.9) | 0 |
| Decreased value | |||||
| Albumin level | 2 (0.3) | 1 (0.3) | 5 (0.7) | 1 (0.2) | 0 |
| Hemoglobin | 2 (0.3) | 1 (0.3) | 4 (0.6) | 0 | 1 (0.8) |
| WBC | 6 (1.0) | 0 | 4 (0.6) | 5 (1.1) | 0 |
| ANC | 13 (2.2) | 2 (0.5) | 12 (1.7) | 4 (0.9) | 2 (1.7) |
| Platelet count | 5 (0.9) | 1 (0.3) | 19 (2.8) | 7 (1.6) | 3 (2.5) |
| Total toxicities | 133 (22.8) | 60 (15.5) | 243 (35.3) | 127 (28.9) | 28 (23.7) |
| Subjects with ≥1 toxicity | 119 (20.5) | 57 (14.8) | 202 (29.4) | 111 (25.3) | 24 (20.3) |
NOTE. Data are no. (%) of subjects. ALT, alanine transaminase; ANC, absolute neutrophil count; AST, aspartate transaminase; CPK, creatinine phosphokinase; d4T, stavudine; ddI, didanosine; 3TC, lamivudine; WBC, white blood cell; ZDV, zidovudine.
More than 97% of subjects had ≥1 laboratory measurement of AST, ALT, creatinine, albumin, alkaline phosphatase, and bilirubin levels; hemoglobin; WBC; ANC; and platelet count. A total of 90%–95% of subjects had laboratory measurements of CPK and amylase levels available; 73%–92% had measurements of the lipase level available; 67%–86% had measurements of acidosis available; and <3% had measurements of the pancreatic serum amylase level available.
As defined by an increase in the anion gap: Na+−(Cl− + CO2 −) >16 mEq/L
The occurrence of abnormalities within the laboratory cohort is also summarized in table 2. For each of the 5 NRTI pairs, the most common abnormality was an elevated anion gap, which occurred in 8%–21% of children. It occurred most often in subjects receiving d4T/3TC and d4T/ddI and least often in those receiving ZDV/ddI. An elevated total serum amylase level occurred in 2%–4% of subjects and was most common among those receiving d4T/ddI and ZDV/ddI. A smaller number of subjects had an elevated lipase level, which was seen most commonly in association with d4T/3TC and d4T/ddI, and an elevated CPK level, which was seen most commonly in association with d4T/3TC and ddI/3TC. A decreased ANC was seen most commonly with ZDV/3TC, d4T/3TC, and ddI/3TC, and a decreased platelet count with d4T/3TC, ddI/3TC, and d4T/ddI. All other laboratory test abnormalities occurred in <2% of the subjects. Although the pancreatic serum amylase level is a more specific indicator of pancreatitis than is the total amylase level, the total amylase level was routinely monitored in the PACTG 219C study (90%–95% of subjects had ≥1 measurement obtained), whereas <3% of subjects had pancreatic serum amylase levels measured. All other laboratory measurements were evaluated in >97% of subjects, with the exception of the CPK level (90%–95% of subjects), the lipase level (73%–92% of subjects), and the anion gap (67–86% of subjects).
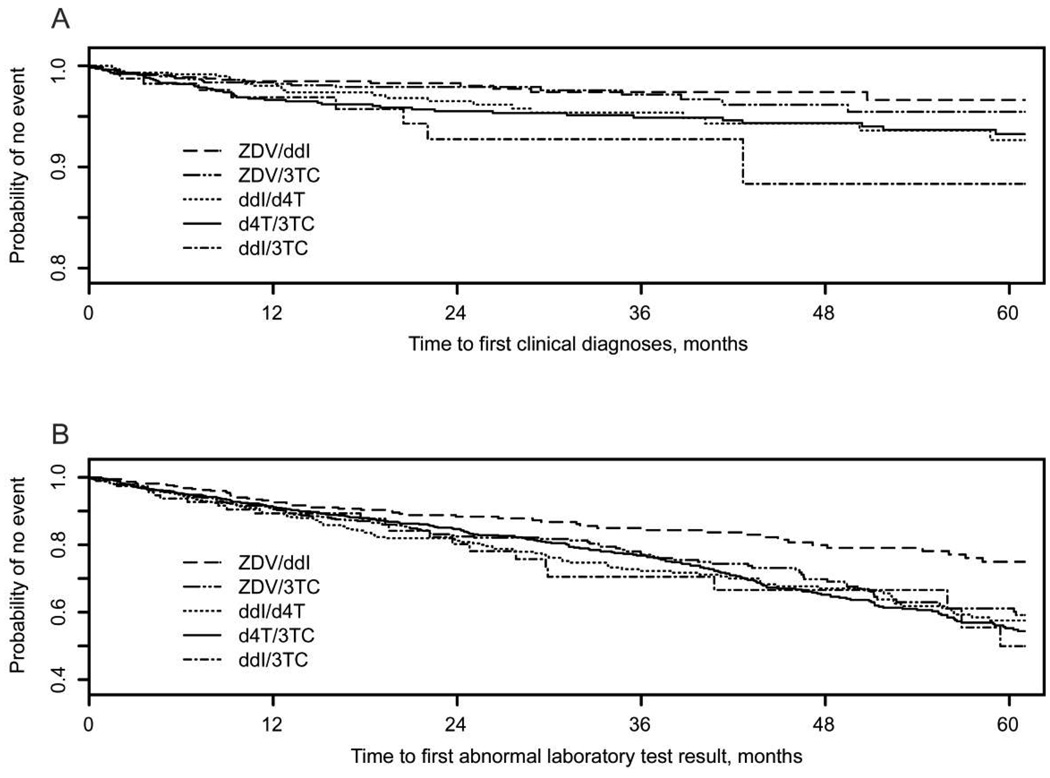
Table 3 summarizes both the percentage of subjects with targeted clinical diagnoses and laboratory test abnormalities and the incidence rates adjusted for exposure time for each NRTI pair. The percentage of subjects with a targeted clinical diagnosis ranged from 1.9% (for those receiving ZDV/ddI) to 4.1% (for those receiving d4T/3TC), whereas the percentage of subjects with any targeted laboratory test abnormality ranged from 15% (for those receiving ZDV/ddI) to 29% (for those receiving d4T/3TC). Subjects who received d4T/3TC had the highest proportion of both clinical and laboratory toxicities. This combination was also associated with the longest median duration of exposure per patient (table 1) and the largest total number of person-years of exposure (table 3). Patients receiving ddI/3TC had the highest incidence of clinical diagnosis (3.0 cases/100 person-years), followed by those receiving d4T/3TC and d4T/ddI. The incidence rate of laboratory test abnormalities was also highest for ddI/3TC (10 cases/100 person-years) and lowest for ZDV/ddI. The incidence rates in table 3 suggest that ZDV/ddI has the “safest” toxicity profile, with the lowest incidence rates for both targeted clinical diagnoses and targeted laboratory test abnormalities. The time to the first targeted toxicity, summarized via Kaplan-Meier estimates in figure 1, again demonstrated that ZDV/ddI was associated with the lowest probability of both clinical and laboratory toxicities.
Table 3.
Incidence of targeted clinical diagnoses and laboratory toxicities for each dual nucleoside reverse-transcriptase inhibitor (NRTI) regimen.
| Cohort, regimen | Time exposed to regimen, person-years |
Subjects with targeted finding,a no. (%) |
IR (95% CI) |
|---|---|---|---|
| Fullb | |||
| ZDV/3TC (n = 1336) | 2189.5 | 27 (2.0) | 1.23 (0.81–1.79) |
| ZDV/ddI (n = 1022) | 1982.5 | 19 (1.9) | 0.96 (0.58–1.50) |
| d4T/3TC (n = 1154) | 2653.7 | 47 (4.1) | 1.77 (1.30–2.36) |
| d4T/ddI (n = 772) | 1516.5 | 25 (3.2) | 1.65 (1.07–2.43) |
| ddI/3TC (n = 258) | 336.4 | 10 (3.9) | 2.97 (1.43–5.47) |
| Laboratoryc | |||
| ZDV/3TC (n = 584) | 1396.5 | 119 (20.4) | 8.52 (7.06–10.20) |
| ZDV/ddI (n = 386) | 1029.2 | 57 (14.8) | 5.54 (4.19–7.18) |
| d4T/3TC (n = 688) | 2219.1 | 202 (29.4) | 9.10 (7.89–10.45) |
| d4T/ddI (n = 439) | 1216.3 | 111 (25.3) | 9.13 (7.51–10.99) |
| ddI/3TC (n = 118) | 234.2 | 24 (20.3) | 10.2 (6.57–15.25) |
NOTE. CI, exact 95% confidence interval based on Poisson distribution; d4T, stavudine; ddI, didanosine; IR, incidence rate per 100 person-years of exposure; 3TC, lamivudine; ZDV, zidovu-dine.
Clinical diagnosis or laboratory test abnormality.
All age-eligible subjects who met the study criteria described in the Methods section.
The subset of subjects monitored for laboratory test abnormalities, defined as having ≥1 targeted laboratory evaluation while receiving the dual NRTI regimen of interest (or in the month after stopping therapy).
Figure 1.
Kaplan-Meier estimates of the probability of remaining event free for each of 5 dual nucleoside reverse-transcriptase inhibitor (NRTI) regimens, where the event is a targeted clinical diagnosis (A) or laboratory toxicity (B). d4T, stavudine; ddI, didanosine; 3TC, lamivudine; ZDV, zidovudine.
As previously noted, the incidence rates presented in table 3 and figure 1 cannot be compared directly, because some subjects received >1 of the 5 dual NRTI combinations. Thus, pairwise comparisons were conducted for the time to the first targeted clinical and laboratory toxicity. The unadjusted (univariate) hazard ratios (HRs) and the adjusted HRs from multivariate Cox models adjusting for covariates for which P < .10 are summarized in table 4, as are their corresponding confidence intervals and P values. After adjustment for potential confounders, ZDV/3TC was associated with a significantly lower risk of a targeted clinical diagnosis than was ddI/3TC (HR, 0.34; P = .05), but it was also associated with a significantly higher risk of laboratory toxicities than was ZDV/ddI (HR, 1.44; P = .05). No significant differences in clinical or laboratory toxicity rates were detected when d4T/ddI was compared with d4T/3TC or ddI/3TC. However, d4T/ddI was associated with a significantly higher rate of clinical toxicities than was ZDV/3TC (HR, 2.56; P = .01). Although the unadjusted analysis indicated that a significantly higher risk of clinical and laboratory toxicities was associated with d4T/ddI than with ZDV/ddI, these associations did not persist in the final model. Finally, d4T/3TC was associated with a significantly higher risk of targeted clinical diagnoses and a marginally higher risk of targeted laboratory toxicities than was ZDV/ddI (HR, 2.63 [P = .001] and 1.39 [P = .08], respectively).
Table 4.
Cox proportional hazards models for pairwise comparisons of dual nucleoside reverse-transcriptase inhibitor (NRTI) regimens based on time to first targeted toxicity.
| Subjects in each group,a no. | Unadjusted test | Adjusted test (final model)b | ||||
|---|---|---|---|---|---|---|
| Cox model, pairs compared | N1 | N2 | HR | P | HR | P |
| Time to first clinical diagnosis | ||||||
| d4T/ddI vs. ZDV/3TC | 333 | 1265 | 1.96 (1.02–3.78) | .04 | 2.56 (1.28–5.10) | .01 |
| d4T/ddI vs. d4T/3TC | 537 | 987 | 0.85 (0.47–1.53) | .59 | 0.82 (0.45–1.46) | .49 |
| d4T/ddI vs. ZDV/ddI | 474 | 995 | 2.11 (1.12–3.95) | .02 | 1.45 (0.66–3.17) | .36 |
| d4T/ddI vs. ddI/3TC | 678 | 166 | 0.69 (0.26–1.82) | .45 | 0.92 (0.33–2.54) | .87 |
| ZDV/3TC vs. d4T/3TC | 1251 | 688 | 0.65 (0.37–1.15) | .14 | 0.65 (0.37–1.16) | .15 |
| ZDV/3TC vs. ZDV/ddI | 867 | 921 | 1.10 (0.55–2.17) | .79 | 0.95 (0.44–2.07) | .90 |
| ZDV/3TC vs. ddI/3TC | 1249 | 111 | 0.48 (0.17–1.38) | .17 | 0.34 (0.12–1.00) | .05 |
| d4T/3TC vs. ZDV/ddI | 647 | 977 | 2.63 (1.48–4.68) | .001 | 2.63 (1.48–4.68) | .001 |
| d4T/3TC vs. ddI/3TC | 1051 | 172 | 0.82 (0.35–1.93) | .64 | 0.82 (0.35–1.93) | .64 |
| ZDV/ddI vs. ddI/3TC | 967 | 95 | 0.35 (0.12–1.04) | .06 | 1.05 (0.22–5.09) | .95 |
| Time to first laboratory test abnormality | ||||||
| d4T/ddI vs. ZDV/3TC | 319 | 569 | 1.00 (0.75–1.33) | .99 | 0.90 (0.65–1.24) | .51 |
| d4T/ddI vs. d4T/3TC | 367 | 634 | 0.95 (0.74–1.22) | .67 | 0.89 (0.69–1.15) | .36 |
| d4T/ddI vs. ZDV/ddI | 386 | 380 | 1.78 (1.29–2.46) | <.001 | 1.14 (0.76–1.71) | .52 |
| d4T/ddI vs. ddI/3TC | 413 | 84 | 1.05 (0.59–1.87) | .88 | 1.10 (0.60–2.01) | .76 |
| ZDV/3TC vs. d4T/3TC | 561 | 603 | 0.90 (0.71–1.14) | .40 | 0.94 (0.72–1.21) | .62 |
| ZDV/3TC vs. ZDV/ddI | 484 | 363 | 1.55 (1.11–2.16) | .01 | 1.44 (1.01–2.06) | .05 |
| ZDV/3TC vs. ddI/3TC | 566 | 81 | 0.96 (0.57–1.61) | .87 | 1.28 (0.71–2.31) | .41 |
| d4T/3TC vs. ZDV/ddI | 565 | 378 | 1.71 (1.27–2.31) | <.001 | 1.39 (0.96–2.00) | .08 |
| d4T/3TC vs. ddI/3TC | 660 | 82 | 1.04 (0.59–1.82) | .91 | 1.16 (0.64–2.08) | .63 |
| ZDV/ddI vs. ddI/3TC | 374 | 76 | 0.52 (0.30–0.89) | .02 | 0.80 (0.41–1.55) | .51 |
NOTE. d4T, stavudine; ddI, didanosine; 3TC, lamivudine; ZDV, zidovudine.
“N1” denotes the no. of subjects receiving the first of the NRTI pairs listed in column 1, and “N2” denotes the no. of subjects receiving the second of the NRTI pairs listed in column 1.
Adjusted for covariates for which P < .10 in the full model.
In a sensitivity analysis conducted for subjects in the laboratory cohort for whom measurements of the CD4+ percentage were available (70%–82% of the cohort, depending on the NRTI pair), adjustment for the CD4+ percentage yielded consistent or slightly stronger effects. There was strong evidence for an increased risk of laboratory toxicity associated with regimens containing ZDV/3TC, d4T/3TC, and ddI/3TC, compared with regimens containing ZDV/ddI (HR, 1.65 [P = .02], 1.65 [P = .04], and 2.63 [P < .001], respectively).
In general, subjects who started receiving an NRTI pair at a younger age tended to have a lower risk of targeted clinical and laboratory toxicities than did subjects who started receiving an NRTI pair at an older age. However, in some of the pairwise comparisons of laboratory toxicities, the risk decreased with increasing age at initiation of therapy up to 7–8 years of age, and it then began to increase slightly with further increasing age. Other covariates found to be associated with an increased risk of targeted clinical diagnoses included a larger number of prior regimens and the inclusion of other agents (PIs, NNRTIs, or an additional NRTI) with the dual NRTI backbone. Covariates associated with an increased risk of targeted laboratory toxicities included initiation of the regimen in later calendar years and a larger number of prior regimens received.
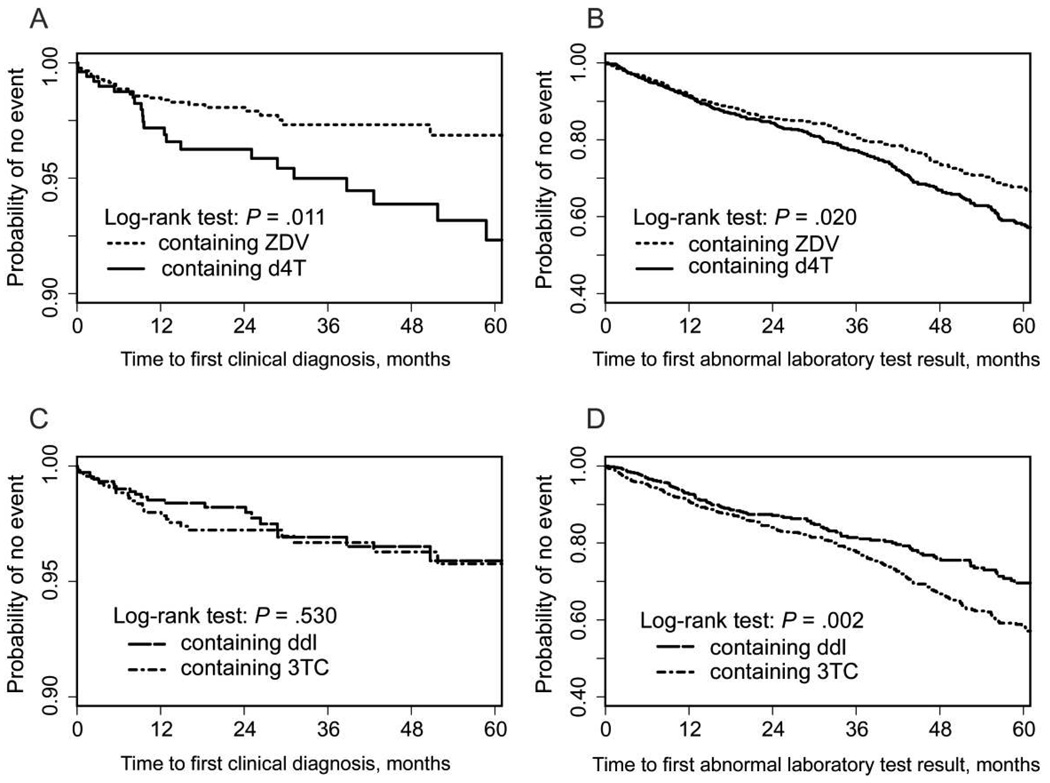
The results in table 4 suggest that, of all the regimens considered, dual NRTI backbones containing ZDV were associated with a lower rate of targeted toxicities than were those containing d4T. As a supporting analysis, we assessed the first course of regimens containing ZDV and the first course of regimens containing d4T. The Kaplan-Meier curves for the time to the first targeted clinical and laboratory test abnormality are shown in figure 2A and 2B. As expected, the regimens containing ZDV were associated with a significantly lower risk of both clinical diagnoses and laboratory toxicities; the difference in the risk of clinical diagnoses persisted after adjustment for all potential confounders (HR, 0.49; P = .02). In a similar comparison of regimens containing ddI and those containing 3TC, there was no difference in the time to the first clinical diagnosis (figure 2C). However, there was a significantly lower risk of laboratory toxicity associated with regimens containing ddI (figure 2D), which persisted after adjustment for potential confounders (HR, 0.78; P = .04).
Figure 2.
Pairwise comparison of dual nucleoside reverse-transcriptase inhibitor (NRTI) regimens containing both zidovudine (ZDV) and stavudine (d4T) (A and B) and pairwise comparison of dual NRTI regimens containing both didanosine (ddI) and lamivudine (3TC) (C and D), with Kaplan-Meier estimates of the probability of remaining event free and associated P values (by log-rank test). The event is a targeted clinical diagnosis (A and C) or a targeted abnormal laboratory test result (B and D).
DISCUSSION
Of the 2233 children who were perinatally infected with HIV and were ≤13 years of age when they started receiving ART, those who received regimens containing ZDV had lower rates of both clinical and laboratory toxicities than did those receiving regimens containing d4T, thereby supporting the inclusion of ZDV in recommended first-line regimens. Regimens containing d4T/3TC, d4T/ddI, and ddI/3TC had similar rates of toxicities and are appropriate for second-line therapy in children.
ZDV/ddI, when compared with ZDV/3TC, was associated with a similar risk of clinical diagnoses but a significantly lower risk of laboratory test abnormalities. Overall, of the 5 NRTI pairs, ZDV/ddI was associated with the lowest rate of toxicities. However, it should be noted that its use was more common in earlier calendar years, and, thus, it was less often used as a part of HAART than were other combinations. In addition, it was used in younger children. However, even after adjustment for calendar year and subject age at initiation of therapy, pairwise comparisons still generally favored the use of ZDV/ddI. Thus, either ZDV/ddI or ZDV/3TC is appropriate for inclusion in first-line therapy, with the choice being informed by their differing toxicity profiles and formulations and the need to administer ddI without food. These pairs had similar toxicity profiles, except that neutropenia was more commonly associated with the use of ZDV/3TC, whereas pancreatitis and acidosis were slightly more commonly associated with the use of ZDV/ddI. Previous pediatric trials comparing ddI and ZDV demonstrated a significantly higher rate of anemia or neutropenia in association with ZDV and a higher rate of elevated liver enzyme levels in association with ddI [22, 23].
D4T/ddI, compared with the first-line backbone regimen ZDV/3TC, was associated with a significantly higher rate of clinical diagnoses but not of laboratory test abnormalities. The clinical diagnoses that were more commonly associated with d4T/ddI included pancreatitis and lipoatrophy/lipodystrophy, whereas hepatitis was more commonly associated with ZDV/3TC. No significant differences in clinical or laboratory toxicity rates were observed when d4T/ddI was compared with d4T/3TC, ZDV/ddI, or ddI/3TC.
D4T/3TC, compared with ZDV/ddI, was associated with a significantly higher rate of clinical diagnoses, as well as a higher rate of laboratory test abnormalities, which approached significance. As noted above, d4T/ddI was associated with a significantly higher rate of clinical diagnoses than was ZDV/3TC. Both of these pairwise comparisons involve a comparison between d4T and ZDV, with the addition of a second NRTI (either 3TC or ddI). These results, together with the supporting analysis comparing all NRTI pairs containing ZDV with those containing d4T, illustrate a significantly lower toxicity rate associated with regimens containing ZDV than with those containing d4T. Of pairs containing d4T, d4T/ddI and d4T/3TC had similar rates of toxicities, suggesting that each is appropriate for second-line therapy.
The combination of ddI/3TC was associated with a significantly higher risk of clinical diagnoses than was that of ZDV/3TC, with a higher risk of lipodystrophy/lipoatrophy but a lower risk of hepatitis; the rate of laboratory test abnormalities was similar. DDI/3TC was associated with rates of clinical abnormalities and laboratory test abnormalities similar to those associated with the remaining NRTI pairs. This finding suggests that ddI/3TC should be reserved for second-line therapy, although it might be considered as initial therapy for a child with preexisting liver disease.
Many of the adverse events associated with NRTI therapy are believed to result from mitochondrial toxicity, because these agents inhibit mitochondrial DNA (mtDNA) polymerase γ, resulting in inhibition of mtDNA synthesis [24, 25]. Toxicities related to mitochondrial toxicity include peripheral neuropathy, lactic acidosis, hepatitis, hepatic steatosis, myopathy, cardiomyopathy, and, possibly, lipoatrophy/lipodystrophy. The NRTIs most active in inhibiting DNA polymerase γ are zalcitabine, d4T, and ddI [26, 27]. Studies in adults have demonstrated lower concentrations of PBMC mtDNA in subjects receiving d4T/ddI than in those receiving other NRTI combinations [28, 29]. Mitochondrial haplogroup T has been associated with a significantly increased risk of developing peripheral neuropathy among white adult subjects, particularly among those who receive d4T/ddI [30]. Because most HIV-infected children in the United States are African American or Hispanic, it is possible that mtDNA polymorphisms that predispose to d4T/ddI-induced mitochondrial toxicity are uncommon in these populations. Thus, both younger age and race may account for the modest rates of toxicity that we observed among children receiving d4T/ddI.
Because this was a retrospective study, we were able to examine only those laboratory evaluations dictated by the protocol. For instance, although pancreatic serum amylase is a much more specific marker of pancreatitis than is total amylase, its collection was not specified in the protocol, so results were available for very few subjects. The most common abnormality detected in the laboratory was an elevated anion gap, which occurred in 8%–13% of subjects. This abnormality was included as a marker for metabolic acidosis, which might suggest lactic acidosis. We recognize that the anion gap is neither a specific nor a sensitive indicator of metabolic acidosis. It would have been preferable to measure lactic acid directly, and future prospective studies may benefit from the availability of rapid point-of-care devices to measure blood concentrations of lactic acid.
A limitation of the present analysis is the use of observational data rather than a randomized design, raising the possibility of selection bias. Subjects who experience failure of a particular regimen are likely to be at an increased risk for treatment-related toxicities with subsequent regimens. We attempted to control for such potential confounding by adjusting for the number of previous ART regimens that each subject received and for whether subjects were concurrently receiving a PI, an NNRTI, or an additional NRTI, in which case the toxicities are unlikely to be solely attributed to the NRTIs. Although our ability to control for HIV disease status was limited by the lack of viral load information (a large proportion of regimens were initiated before the availability of viral load quantification), we conducted a sensitivity analysis controlling for the CD4+ percentage at the time of initiation, which generally gave similar if not stronger results.
The conclusions of our analysis must be viewed as somewhat exploratory in nature, given the fact that 10 comparisons were conducted for each of the 2 endpoints, and no adjustment was made for multiple comparisons. However, before conducting our analysis, we developed a detailed analysis plan for the stated protocol objectives, which clearly specified the comparisons of interest. In addition, all comparisons are presented rather than selecting post hoc comparisons on the basis of the observed results. Given the multiple comparisons conducted, we emphasize consistency across analyses and endpoints in drawing conclusions.
Finally, the small number of children in the PACTG 219C study who were receiving FTC, ABC, and tenofovir prevented their inclusion in the analysis. Currently, FTC and ABC are among the preferred first-line agent for children, and all 3 agents are preferred for adults. There is no pediatric formulation of tenofovir, and it is not approved for use in children <18 years of age. [5] Additional studies are needed to fully define the safety profile of these agents in children.
In summary, regimens containing ZDV are preferred for initial therapy in children, because of their low rates of toxicity. However, regimens containing d4T and ddI/3TC have similar rates of toxicity, which are only modestly higher than those of regimens containing ZDV, and are appropriate for second-line therapy.
Acknowledgments
We thank the children and families for their participation in the Pediatric AIDS Clinical Trials Group study, as well as the individuals and institutions involved in the conduct of the study.
Financial support: National Institute of Allergy and Infectious Diseases (NIAID; grant U01AI068632); the Eunice Kennedy Shriver National Institute of Child Health and Human Development (contract NO1 HD33345); Statistical and Data Analysis Center, Harvard School of Public Health (under the NIAID cooperative agreements U01 AI41110 and U01 AI068616); National Center for Research Resources National Institutes of Health General Clinical Research Center (grant RR05096).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases, Eunice Kennedy Shriver National Institute of Child Health and Human Development, or the National Institutes of Health.
Footnotes
Potential conflicts of interest: none reported.
Presented in part: XVIth International AIDS Conference, Toronto, Canada, 13–18 August 2006, (abstract WEPE0141).
References
- 1.Brogly S, Williams P, Seage G, Oleske J, Van Dyke R, MacIntosh K for the PACTG 219C. Antiretroviral treatment in pediatric HIV in the United States: from clinical trials to clinical practice. JAMA. 2005;293:2213–2220. doi: 10.1001/jama.293.18.2213. [DOI] [PubMed] [Google Scholar]
- 2.Working Group on Antiretroviral Therapy and Medical Management of HIV-Infected Children. Guidelines for the use of antiretroviral agents in pediatric HIV infection. [Accessed 22 April 2008];2008 February 28; Available at: http://aidsinfo.nih.gov/ContentFiles/PediatricGuidelines.pdf.
- 3.Gortmaker S, Hughes M, Cervia J, et al. Effect of combination therapy including protease inhibitors on mortality among children and adolescents infected with HIV-1. N Engl J Med. 2001;345:1522–1528. doi: 10.1056/NEJMoa011157. [DOI] [PubMed] [Google Scholar]
- 4.Ene L, Goetghebuer T, Hainaut M, et al. Prevalence of lipodystrophy in HIV-infected children: a cross-sectional study. Eur J Pediatr. 2007;166:13–21. doi: 10.1007/s00431-006-0193-1. [DOI] [PubMed] [Google Scholar]
- 5.Department of Health and Human Services Panel on Antiretroviral Guidelines for Adult and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. [Accessed 22 April 2008];2008 January 29; Available at: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
- 6.Kline MW, Van Dyke RB, Lindsey JC, et al. The Pediatric AIDS Clinical Trials Group 327 Team. Combination therapy with stavudine (d4T) plus didanosine (ddl) in children with human immunodeficiency virus infection. Pediatrics. 1999;103:e62. doi: 10.1542/peds.103.5.e62. [DOI] [PubMed] [Google Scholar]
- 7.de Mendoza C, Ramos JT, Ciria L, et al. Efficacy and safety of stavudine plus didanosine in asymptomatic HIV-infected children with plasma HIV RNA below 50,000 copies per milliliter. HIV Clin Trials. 2002;3:9–16. doi: 10.1310/FAJF-7A8G-QAR4-Q0X5. [DOI] [PubMed] [Google Scholar]
- 8.Mobley JE, Pollard RB, Schrader S, Adler MH, Kelleher T, McLaren C AI454–143 Team. Virological and immunological responses to once-daily dosing of didanosine in combination with stavudine. AIDS. 1999;13:F87–F93. doi: 10.1097/00002030-199907300-00003. [DOI] [PubMed] [Google Scholar]
- 9.Pollard RB, Peterson D, Hardy D, et al. Safety and antiretroviral effects of combined didanosine and stavudine therapy in HIV-infected individuals with CD4 counts of 200 to 500 cells/mm3. J Acquir Immune Defic Syndr. 1999;22:39–48. doi: 10.1097/00042560-199909010-00005. [DOI] [PubMed] [Google Scholar]
- 10.Boubaker K, Flepp M, Sudre P, et al. Hyperlactatemia and antiretroviral therapy: the Swiss HIV Cohort Study. Clin Infect Dis. 2001;33:1931–1937. doi: 10.1086/324353. [DOI] [PubMed] [Google Scholar]
- 11.Coghlan ME, Sommadossi JP, Jhala NC, Many WJ, Saag MS, Johnson VA. Symptomatic lactic acidosis in hospitalized antiretroviral-treated patients with human immunodeficiency virus infection: a report of 12 cases. Clin Infect Dis. 2001;33:1914–1921. doi: 10.1086/323783. [DOI] [PubMed] [Google Scholar]
- 12.French M, Amin J, Roth N, et al. Randomized, open-label, comparative trial to evaluate the efficacy and safety of three antiretroviral drug combinations including two nucleoside analogues and nevirapine for previously untreated HIV-1 infection: the OzCombo 2 study. HIV Clin Trials. 2002;3:177–185. doi: 10.1310/9n21-1hg1-7n1q-jkw1. [DOI] [PubMed] [Google Scholar]
- 13.Amin J, Moore A, Carr A, et al. Combined analysis of two-year follow-up from two open-label randomized trials comparing efficacy of three nucleoside reverse transcriptase inhibitor backbones for previously untreated HIV-1 infection: OzCombo 1 and 2. HIV Clin Trials. 2003;4:252–261. doi: 10.1310/K2U9-QC2V-1Y3V-5DYF. [DOI] [PubMed] [Google Scholar]
- 14.Robbins GK, De Gruttola V, Shafer RW, et al. Comparison of sequential three-drug regimens as initial therapy for HIV-1 infection. NEngl J Med. 2003;349:2293–2303. doi: 10.1056/NEJMoa030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shafer RW, Smeaton LM, Robbins GK, et al. Comparison of four-drug regimens and pairs of sequential three-drug regimens as initial therapy for HIV-1 infection. N Engl J Med. 2003;349:2304–2315. doi: 10.1056/NEJMoa030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perinatal HIV Guidelines Working Group. Public Health Service Task Force recommendations for use of antiretroviral drugs in pregnant HIV-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States. [Accessed 4 January 2008];2008 July 8; Available at http://aidsinfo.nih.gov/contentfiles/PerinatalGL.pdf.
- 17.Bristol-Myers Squibb. Healthcare provider important drug warning letter. 2001 January 5; [Google Scholar]
- 18.Sarner L, Fakoya A. Acute onset lactic acidosis and pancreatitis in the third trimester of pregnancy in HIV-1 positive women taking antiretroviral medication. Sex Transm Infect. 2002;78:58–59. doi: 10.1136/sti.78.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blanco F, García-Benayas T, José de la Cruz J, González-Lahoz J, Soriano V. First-line therapy and mitochondrial damage: different nucleosides, different findings. HIV Clin Trials. 2003;4:11–19. doi: 10.1310/HF1J-3P6K-1K9H-AGPY. [DOI] [PubMed] [Google Scholar]
- 20.Pediatric European Network for Treatment of AIDS. A randomized double-blind trial of the addition of lamivudine or matching placebo to current nucleoside analogue reverse transcriptase inhibitor therapy in HIV-infected children: the PENTA-4 trial. AIDS. 1998;12:F151–F160. [PubMed] [Google Scholar]
- 21.Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health. Table for grading the severity of adult and pediatric adverse events, version 1.0. [Accessed 20 May 2008];2004 December; Available at: http://rcc.tech-res-intl.com/
- 22.Englund JA, Baker CJ, Raskino C, et al. Zidovudine, didanosine, or both as initial treatment for symptomatic HIV-infected children. N Engl J Med. 1997;336:1704–1712. doi: 10.1056/NEJM199706123362403. [DOI] [PubMed] [Google Scholar]
- 23.McKinney RE, Jr, Johnson GM, Stanley K, et al. A randomized study of combined zidovudine-lamivudine versus didanosine monotherapy in children with symptomatic therapy-naive HIV-1 infection. J Pediatr. 1998;133:500–508. doi: 10.1016/s0022-3476(98)70057-5. [DOI] [PubMed] [Google Scholar]
- 24.Johnson AA, Ray AS, Hanes J, et al. Toxicity of antiviral nucleoside analogs and the human mitochondrial DNA polymerase. J Biol Chem. 2001;276:40847–40857. doi: 10.1074/jbc.M106743200. [DOI] [PubMed] [Google Scholar]
- 25.Brinkman K, Smeitink JA, Romijn JA, Reiss P. Mitochondrial toxicity induced by nucleoside-analogue reverse-transcriptase inhibitors is a key factor in the pathogenesis of antiretroviral-therapy-related lipodystrophy. Lancet. 1999;354:1112–1115. doi: 10.1016/S0140-6736(99)06102-4. [DOI] [PubMed] [Google Scholar]
- 26.Martin JL, Brown CE, Matthews-Davis N, Reardon JE. Effects of antiviral nucleoside analogs on human DNA polymerases and mitochondrial DNA synthesis. Antimicrobial Agents Chemother. 1994;38:2743–2749. doi: 10.1128/aac.38.12.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Birkus G, Hitchcock MJM, Cihlar T. Assessment of mitochondrial toxicity in human cells treated with tenofovir: comparison with other nucleoside reverse transcriptase inhibitors. Antimicrob Agents Chemother. 2002;46:716–723. doi: 10.1128/AAC.46.3.716-723.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Côté HC, Yip B, Asselin JJ, et al. Mitochondrial:nuclear DNA ratios in peripheral blood cells from human immunodeficiency virus (HIV)-infected patients who received selected HIV antiretroviral drug regimens. J Infect Dis. 2003;187:1972–1976. doi: 10.1086/375353. [DOI] [PubMed] [Google Scholar]
- 29.Chiappini F, Teicher E, Saffroy R, et al. Prospective evaluation of blood concentration of mitochondrial DNA as a marker of toxicity in 157 consecutively recruited untreated or HAART-treated HIV-positive patients. Lab Invest. 2004;84:908–914. doi: 10.1038/labinvest.3700113. [DOI] [PubMed] [Google Scholar]
- 30.Hulgan T, Haas DW, Haines JL, et al. Mitochondrial haplogroups and peripheral neuropathy during antiretroviral therapy: an adult AIDS Clinical Trials Group study. AIDS. 2005;19:1341–1349. doi: 10.1097/01.aids.0000180786.02930.a1. [DOI] [PubMed] [Google Scholar]