Abstract
Study Objective
To compare drug adherence rates among patients with gout, hypercholesterolemia, hypertension, hypothyroidism, osteoporosis, seizure disorders, and type 2 diabetes mellitus by using a standardized approach.
Design
Longitudinal study.
Data Source
Health care claims data from 2001–2004.
Patients
A total of 706,032 adults aged 18 years or older with at least one of the seven medical conditions and with incident use of drug therapy for that condition.
Measurements and Main Results
Drug adherence was measured as the sum of the days’ supply of drug therapy over the first year observed. Covariates were age, sex, geographic residence, type of health plan, and a comorbidity score calculated by using the Hierarchical Condition Categories risk adjuster. Bivariate statistics and stratification analyses were used to assess unadjusted means and frequency distributions. Sample sizes ranged from 4984 subjects for seizure disorders to 457,395 for hypertension. During the first year of drug therapy, 72.3% of individuals with hypertension achieved adherence rates of 80% or better compared with 68.4%, 65.4%, 60.8%, 54.6%, 51.2%, or 36.8% for those with hypothyroidism, type 2 diabetes, seizure disorders, hypercholesterolemia, osteoporosis, or gout, respectively. Age younger than 60 years was associated with lower adherence across all diseases except seizure disorders. Comorbidity burden and adherence varied by disease. As comorbidity increased, adherence among subjects with osteoporosis decreased, whereas adherence among those with hypertension, hypercholesterolemia, or gout increased. Add-on drug therapies and previous experience with taking drugs for the condition increased adherence among subjects with hypertension, type 2 diabetes, hypothyroidism, or seizure disorders but not the other conditions.
Conclusion
This uniform comparison of drug adherence revealed modest variation across six of seven diseases, with the outlier condition being gout.
Keywords: drug adherence, comparative study, type 2 diabetes mellitus, hypertension, osteoporosis, hypercholesterolemia, gout, hypothyroidism, seizure disorders
Effective drug therapies are available for a wide range of chronic medical conditions, yet all are challenged by nonadherence. For example, only 45% of patients with osteoporosis continue to take their drugs after the first year,1 and 54% of patients who newly use statins to manage hypercholesterolemia have periods of nonadherence lasting longer than 90 days.2 Likewise, 21% of patients with diabetes mellitus have gaps exceeding 20% of the year in terms of prescription fills for oral hypoglycemics, antihypertensives, and statins.3 However, whether nonadherence is more problematic for some medical conditions than others is unclear. Learning how nonadherence rates compare across diseases may broaden our understanding of their common issues.
Our assessment of the published empiric evidence revealed few comparisons of drug nonadherence rates across medical conditions. Nearly all adherence studies have focused on a single disease, and comparisons across studies are difficult given the wide variety of methods used to calculate drug nonadherence rates.4 The objective of our study was to apply a uniform method for comparing adherence rates across a range of chronic medical conditions that are commonly treated with long-term drug therapy.
Methods
Study Population and Data Sources
The study sample included approximately 1.3 million individuals aged 18 years or older who had a diagnosis of gout, hypercholesterolemia, hypertension, hypothyroidism, osteoporosis, seizure disorders, or type 2 diabetes during the study period of 2001–2004. These conditions were selected because they are chronic, because they commonly occur in adults, and because regular and persistent drug therapy is recommended as treatment. In addition, the subjects must have started new drug therapy for their condition between January 1, 2002, and December 31, 2003. Table 1 lists the diagnostic codes and drug therapies for these disorders.
Table 1.
Diagnostic Codes and Therapeutic Drug Classes for the Diseases Studied
| Disease | ICD-9-CM Code | Therapeutic Drug Classes or Drugsa |
|---|---|---|
| Type 2 diabetes mellitus | 250.X0, 250.X2 | α-Glucosidase inhibitors, sulfonylureas, thiazolidinediones, meglitinides, biguanides |
| Hypertension | 401.X | β-Adrenergic blockers, ACE inhibitors, angiotensin II receptor blockers, calcium channel blockers, diuretics |
| Hypercholesterolemia | 272.X | Statins |
| Osteoporosis | 733.0X | Bisphosphonates |
| Seizure disorders | 345.X | Hydantoins, carbamazepine, lamotrigine, barbiturates, primidone, topiramate, valproic acid derivatives, sulfonamides |
| Hypothyroidism | 244.X | Thyroid preparations |
| Gout | 274.X | Allopurinol, uricosurics |
ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification; ACE = angiotensin-converting enzyme.
Includes all generic and brand-name drugs, as well as combination products. When determining adherence rates, we also included the days’ supplies dispensed for relatively uncommon agents. These agents were centrally acting antiadrenergics, peripherally acting antiadrenergics, vasodilators, and eplerenone for hypertension; bile acid sequestrants, fibrates, ezetimibe, and niacin for hypercholesterolemia; and succinimides, felbamate, gabapentin, levetiracetam, oxcarbazepine, and tiagabine for seizure disorders.
Our analysis focused on recipients of new drug therapy to compare patient groups at the same time relative to the start of therapy. New drug therapy was defined as a dispensing of a study drug for the patient’s condition after 1 year of continuous membership in the health plan during which no study drug had been dispensed for that condition. Individuals were excluded if values were missing, if the quantity dispensed for the newly started study drug was zero or less (11,972 patients), or if they received less than 1 year of follow-up observation after the study drug was first dispensed (588,278 patients).
The study data came from the 2001–2004 MarketScan Research databases (Medstat, Ann Arbor, MI). The databases contained secondary data sets of employer-sponsored medical care claims, prescription drug claims, and health care encounters data from approximately 45 large employers and public organizations in the United States. For each year, the data set contained medical care information for 3–6 million individuals. Scientific studies based on this data source have been reported in more than 40 peer-reviewed articles.5
The encounter files contained information about age, sex, geographic area of residence, and eligibility. The prescription claims file included national drug codes, dates of purchase, quantities dispensed, days’ supplies, and expenditure information for each dispensing. The medical claims file contained payment information, diagnoses, procedure codes, and types of providers.
For this analysis, we linked the annual files to create a longitudinal panel of continuous observations for each subject.
Measures
We used the medication possession ratio (MPR) to measure adherence.6 The MPR was the days’ supply of the drug dispensed during the follow-up year divided by the number of days in the year. A recent review of adherence measures showed that the MPR was a reliable measure of adherence.4
Our calculation included dispensings of the initial study drug and dispensings of all other study drugs for the condition. For instance, if an individual with hypertension received a prescription for lisinopril, we added the days’ supplies for lisinopril as well as those for angiotensin-converting enzyme inhibitors, β-adrenergic blockers, angiotensin II receptor blockers, calcium channel blockers, diuretics, and combination products containing these drugs.
Overlaps in the dispensing days for different generic drugs were eliminated. Our assumption was that leftover supplies from previous refills were discarded to begin the new drug (e.g., a change in therapy). Overlaps in the dispensing days of the same generic drugs were summed. The assumption here was that the patient continued taking the drug from previous refills as part of the same regimen (e.g., an early refill).
The value of the days’ supply was truncated if the supply extended beyond the period of observation. In addition, MPRs higher than 100% were truncated to 100%. Overadherence was difficult to interpret. We could not differentiate inappropriate behaviors, such as overuse and early refills, from appropriate behaviors, such as changes in drug regimens, combination therapies, or multiple dispensings to achieve a specific dosage. Percentages of patients with MPRs higher than 100% ranged from 12% (patients with gout) to 27% (patients with seizure disorders).
We evaluated the covariates of age, sex, drug history, and comorbidity level. Drug history was defined as the use of other drugs for the condition in the year before the start of new therapy and the number of add-on drugs used to treat the condition in the year after the start of the new therapy. The comorbidity level was generated by using the Diagnostic Cost Group Hierarchical Condition Category system (DxCG, Boston, MA).7, 8 For each individual, the risk adjuster from this system created a single score based on data from the diagnosis fields of the claims records. Using a distribution of all scores, we established cutoff points at the highest and lowest 25% quantiles to create high, medium, and low comorbidity categories.
Each individual was assigned an index date based on the first dispensing of the newly started drug therapy. Data from the year before the index date were used to calculate the subject’s age, previous drug therapy, and comorbidity risk score. Data after the index date were used to measure adherence.
Statistical Analysis
Bivariate statistics were used to calculate 95% confidence intervals (CIs) and to assess the unadjusted means and frequency distributions of the study variables. Stratified analyses were conducted with age, sex, comorbidity and drug history.
Results
We identified 706,032 individuals with at least one of the seven chronic medical conditions and who newly began drug therapy (Table 2). Sample sizes ranged from 4984–457,395. Individuals with osteoporosis were, on average, the oldest group (64.8 yrs), whereas those with gout were youngest (50.1 yrs). Distributions of women ranged from 22.5–92.8%. No apparent cluster pattern by disease was detected for either the type of health plan or the subject’s geographic area of residence. Mean comorbidity scores varied from a low burden of 0.55 for hypothyroidism to a high burden of 1.14 for seizure disorders. Rates of previous drug therapy for the condition before the start of new therapy ranged from 1.6% for gout to 63.3% for hypertension. Additional drug exposures for the condition after the commencement of new drug therapy ranged from 2.9% for gout to 70.9% for hypertension.
Table 2.
Characteristics of the Study Populations by Diagnosis
| Diagnosis |
|||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Hypertension (n=457,395) | Hypercholesterolemia (n=207,676) | Type 2 Diabetes (n=105,225) | Osteoporosis (n=42,007) | Hypothyroidism (n=37,606) | Gout (n=9715) | Seizure Disorders (n=4984) |
| Mean ± SE | |||||||
| Age (yrs) | 59.9 ± 0.02 | 57.7 ± 0.02 | 58.5 ± 0.04 | 64.8 ± 0.6 | 54.0 ± 0.07 | 58.7 ± 0.14 | 50.1 ± 0.24 |
| Comorbidity scorea | 0.67 ± 0.00 | 0.56 ± 0.00 | 0.81 ± 0.00 | 0.69 ± 0.00 | 0.55 ± 0.00 | 0.73 ± 0.00 | 1.14 ± 0.01 |
|
| |||||||
| No. (%) of Patients | |||||||
| Sex | |||||||
| Male | 204,913 (44.8) | 107,576 (51.8) | 54,717 (52.0) | 3025 (7.2) | 7672 (20.4) | 7529 (77.5) | 2088 (41.9) |
| Female | 252,482 (55.2) | 100,100 (48.2) | 50,508 (48.0) | 38,982 (92.8) | 29,934 (79.6) | 2186 (22.5) | 2896 (58.1) |
| Type of health planb | |||||||
| Comprehensive | 161,003 (35.2) | 66,422 (32.0) | 35,987 (34.2) | 17,999 (42.9) | 11,461 (30.5) | 3274 (33.7) | 1585 (31.8) |
| Exclusive provider organization | 3659 (0.8) | 2411 (1.2) | 631 (0.6) | 272 (0.7) | 477 (1.3) | 49 (0.5) | 52 (1.0) |
| Health maintenance organization | 35,219 (7.7) | 16,395 (7.9) | 10,207 (9.7) | 1253 (3.0) | 2524 (6.7) | 787 (8.1) | 509 (10.2) |
| Point of service | 59,004 (12.9) | 26,393 (12.7) | 14,205 (13.5) | 3726 (8.9) | 5115 (13.6) | 1263 (13.0) | 661 (13.3) |
| Preferred provider organization | 172,438 (37.7) | 81,395 (39.2) | 37,986 (36.1) | 16,824 (40.1) | 15,217 (40.5) | 3847 (39.6) | 1810 (36.3) |
| Point of service with cap | 26,072 (5.7) | 14,660 (7.1) | 6209 (5.9) | 1933 (4.6) | 2812 (7.5) | 495 (5.1) | 367 (7.4) |
| Geographic area of residenceb | |||||||
| Northeast | 48,484 (10.6) | 22,137 (10.7) | 10,373 (9.9) | 5387 (12.8) | 3723 (9.9) | 963 (9.9) | 578 (11.6) |
| North Central | 140,878 (30.8) | 68,512 (33.0) | 32,836 (31.2) | 14,774 (35.2) | 11,771 (31.3) | 3049 (31.4) | 1490 (29.9) |
| South | 189,819 (41.5) | 79,692 (38.4) | 44,019 (41.8) | 14,438 (34.4) | 14,478 (38.5) | 3875 (39.9) | 1934 (38.8) |
| West | 78,214 (17.1) | 37,335 (18.0) | 17,997 (17.1) | 7408 (17.6) | 7634 (20.3) | 1828 (18.8) | 982 (19.7) |
| Previous drug therapyc | 289,531 (63.3) | 61,472 (29.6) | 50,403 (47.9) | 2940 (7.0) | 3610 (9.6) | 155 (1.6) | 2148 (43.1) |
| Drugs added to the initial therapyb | |||||||
| 0 | 133,102 (29.1) | 156,795 (75.5) | 46,720 (44.4) | 39,453 (93.9) | 34,485 (91.7) | 9433 (97.1) | 2088 (41.9) |
| 1 | 146,824 (32.1) | 40,705 (19.6) | 37,565 (35.7) | 2554 (6.1) | 2933 (7.8) | 282 (2.9) | 1714 (34.4) |
| ≥ 2 | 177,469 (38.8) | 10,176 (4.9) | 20,940 (19.9) | 0 (0.0) | 188 (0.5) | 0 (0.0) | 1182 (23.7) |
SE = standard error.
Comorbidity scores equal the sum of medical care risk based on diagnoses; a higher score indicates higher risk.
Percentages may not equal 100% due to rounding.
History of drug therapy for the condition in the year before the newly started treatment.
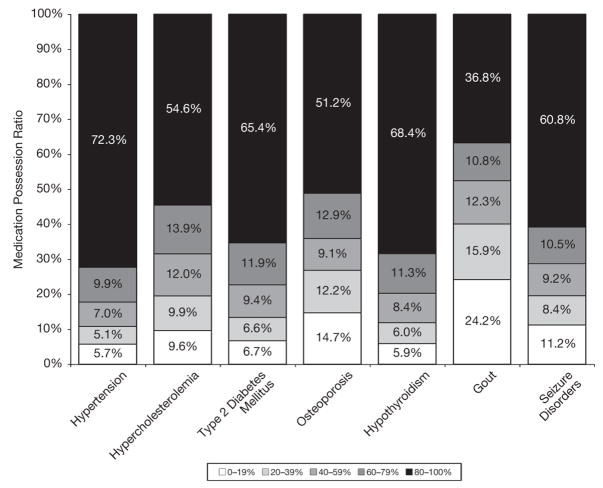
Figure 1 shows modest variation in adherence across six of the seven medical conditions, with the exception being gout. The highest levels of adherence were found for hypertension (72.3% of subjects with MPRs ≥ 80%) followed by hypothyroidism (68.4% with MPRs ≥ 80%). The lowest levels of adherence were detected for gout (24.2% of subjects with MPRs ≤ 19%) and osteoporosis (14.7% with MPRs ≤ 19%).
Figure 1.
Comparison of drug adherence rates across seven medical conditions.
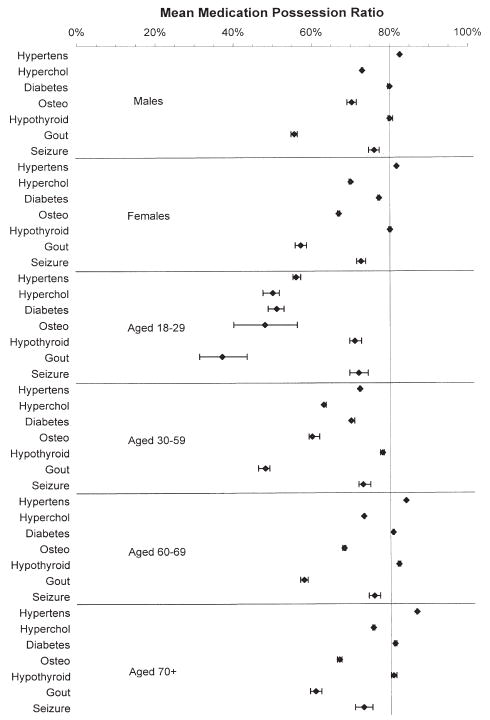
Figures 2 and 3 show the mean adherence levels from the stratified analyses. An MPR exceeding 80% indicated the highest adherence level. In the comparison of adherence by sex, the mean MPR varied little across the seven conditions (Figure 2). In contrast, adherence ratios considerably improved with increasing age for six of the seven conditions, particularly hypertension, type 2 diabetes, and hypothyroidism. For instance, among individuals with hypertension, those aged 18–29 years had a mean MPR of 56.2% (95% CI 55.2–57.2%) compared with 86.7% (95% CI 86.5–86.8%) for those aged 70 years or older. For only seizure disorders was adherence not associated with age. Among individuals with seizure disorders, the mean MPR was 72.0% (95% CI 69.7–74.4%) for those aged 18–29 years compared with 73.0% (95% CI 70.8–75.3%) for those aged 70 years or older.
Figure 2.
Mean (95% confidence interval) medication possession ratios stratified by sex and age (years). Hypertens = hypertension; Hyperchol = hypercholesterolemia; Osteo = osteoporosis.
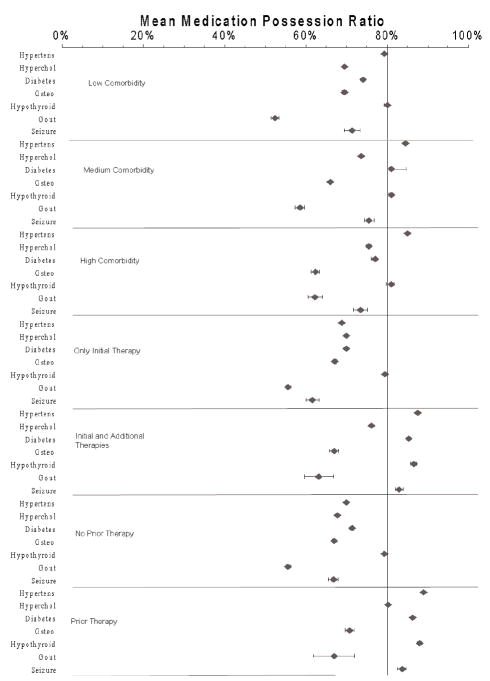
Figure 3.
Mean (95% confidence interval) medication possession ratios stratified by comorbidity and drug history. Hypertens = hypertension; Hyperchol = hypercholesterolemia; Osteo = osteoporosis.
In the analysis stratified according to comorbidity levels, the influence of comorbidity burden was generally small and varied by disease (Figure 3). For individuals with hypertension, gout, or hypercholesterolemia, MPRs increased with increasing comorbidities. The improvement was greatest for individuals with gout. In this group, those with low comorbidity had a mean MPR of 52.3% (95% CI 51.4–53.3%), whereas those with high comorbidity had a mean MPR of 62.2% (95% CI 60.4–64.1%). In contrast, among individuals with osteoporosis, adherence decreased with high comorbidity (mean MPR 62.3%) versus low comorbidity (mean MPR 69.5%). A U-shaped response was observed for individuals with seizure disorders or type 2 diabetes, whereas MPR rates for subjects with hypothyroidism remained at a steady level of 79.5–80.6% across the three comorbidity levels.
The last two stratified analyses revealed adherence based on history of drug use. The first comparison showed that patients with hypertension, type 2 diabetes, hypothyroidism, or seizure disorders improved their adherence if additional therapies were prescribed after they began drug therapy. However, that relationship did not hold true in patients with hypercholesterolemia, osteoporosis, or gout. The next comparison of adherence according to previous experience in taking drugs for that condition showed nearly the same pattern of association as before. Adherence improved in patients with hypertension, type 2 diabetes, hypothyroidism, or seizure disorders if they had a history of trying other drugs for their condition before starting the new therapy. However, a history of drug use did not influence adherence among subjects with osteoporosis or gout.
Discussion
In this study of more than 700,000 privately insured adults aged 18 or older, we found modest variation in the adherence of newly started drug therapies. Most patients with six of seven chronic conditions were able to achieve MPRs of 80% or higher. Patients with hypertension achieved the highest mean MPR (82.1%), followed by patients with hypothyroidism (80%). The two lowest mean MPRs of 56.0% and 67.1% were observed for patients with gout and osteoporosis, respectively.
Stratified analyses showed that adherence increased across all diseases except seizure disorders as subjects aged. By the age of 60 years, most patients with hypertension, type 2 diabetes, or hypothyroidism achieved adherence levels of 80% or better. However, older patients with hypercholesterolemia, osteoporosis, gout, or seizure disorders still struggled with suboptimal adherence, albeit less so than younger patients. Sex exerted little influence on adherence among subjects with any of the diseases. As comorbidity burden increased, adherence increased a small amount across all diseases, with the exception of osteoporosis. The effect of drug history on adherence was strong and varied by disease. Add-on therapy and previous experience with taking drugs for the condition increased adherence in association with hypertension, type 2 diabetes, hypothyroidism, or seizure disorders but not the other conditions.
To our knowledge, this study is among the first to uniformly assess adherence across such a large array of medical conditions. Previous comparisons of adherence across studies have been impossible, given that different measures of adherence, approaches for calculating measures, and sample criteria yielded different results.6 Our analysis overcame these barriers because we applied a standardized approach to measure adherence and to identify the sample by using the same criteria (e.g., all adults aged ≥ 18 yrs). In addition, the stratification studies provide sensitivity analyses that enabled us to determine if certain patient characteristics that dominated in particular diseases influenced our assessment of adherence.
This study had several limitations. The selection of chronic medical conditions was a somewhat arbitrary process that was limited to conditions most often treated with prescription drugs taken on a regular and daily basis. For instance, arthritis was not selected because of the common use of over-the-counter drugs or drugs taken on an as-needed basis.
Also, the samples overlapped because we did not limit the individuals to a primary diagnosis. The largest rate of overlap was 17%, which occurred between the sample with hypertension and that with hypercholesterolemia.
In addition, we used the MPR and pharmacy claims records to measure adherence. Thus, we assessed rates of drug acquisition rather than drug exposure. However, research has demonstrated predictive validity for measuring the cumulative exposure of drugs with acquisition data.9 Indeed, our measure of adherence depended on the accuracy of the days’ supply information. However, we found no evidence of a measurement bias related to the drug class or specific disease state being treated. In addition, we had no information about whether the drug therapy was prescribed for the primary or secondary prevention for certain conditions.
Finally, the type of health plan and the copayment amount certainly influence adherence. However, these factors were not part of this evaluation.
Conclusion
Despite the limitations, we conclude that adherence rates for newly started drug therapies varied modestly for the common medical conditions we examined, with the exception of gout. This evidence supports our understanding of drug nonadherence as a universal problem more than a disease- and drug-specific problem. The clinical implication of these findings is that treatment practices in which clinicians address nonadherence as a bigger problem for some conditions than for others should be discouraged. Furthermore, patient management strategies that have been successful in increasing drug adherence among patients with one condition may be valuable for patients with others.
Acknowledgments
Funded by an unrestricted research grant from Novartis Pharmaceuticals Corporation, East Hanover, New Jersey.
References
- 1.Solomon DH, Avorn J, Katz JN, et al. Compliance with osteoporosis medications. Arch Intern Med. 2005;165:2414–19. doi: 10.1001/archinte.165.20.2414. [DOI] [PubMed] [Google Scholar]
- 2.Brookhart MA, Patrick AR, Dormuth C, et al. Adherence to lipid-lowering therapy and the use of preventive health services: an investigation of the healthy user effect. Am J Epidemiol. 2007;166:348–54. doi: 10.1093/aje/kwm070. [DOI] [PubMed] [Google Scholar]
- 3.Ho PM, Rumsfeld JS, Masoudi FA, et al. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med. 2006;166:1836–41. doi: 10.1001/archinte.166.17.1836. [DOI] [PubMed] [Google Scholar]
- 4.Hess LM, Raebel MA, Conner DA, Malone DC. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother. 2006;40:1280–8. doi: 10.1345/aph.1H018. [DOI] [PubMed] [Google Scholar]
- 5.Briesacher B, Kamal-Bahl S, Hochberg M, Orwig D, Kahler KH. Three-tiered-copayment drug coverage and use of nonsteroidal anti-inflammatory drugs. Arch Intern Med. 2004;164:1679–84. doi: 10.1001/archinte.164.15.1679. [DOI] [PubMed] [Google Scholar]
- 6.Andrade SE, Kahler KH, Frech F, Chan KA. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006;15:565–74. doi: 10.1002/pds.1230. discussion 575–7. [DOI] [PubMed] [Google Scholar]
- 7.Ash AS, Ellis RP, Pope GC, et al. Using diagnoses to describe populations and predict costs. Health Care Financ Rev. 2000;21:7–28. [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao Y, Ellis RP, Ash AS, et al. Measuring population health risks using inpatient diagnoses and outpatient pharmacy data. Health Serv Res. 2001;36:180–93. [PMC free article] [PubMed] [Google Scholar]
- 9.Choo PW, Rand CS, Inui TS, et al. Validation of patient reports, automated pharmacy records, and pill counts with electronic monitoring of adherence to antihypertensive therapy. Med Care. 1999;37:846–57. doi: 10.1097/00005650-199909000-00002. [DOI] [PubMed] [Google Scholar]