Abstract
Corneal allografts transplanted into hosts with allergic conjunctivitis experience an increased incidence and swifter tempo of immune rejection compared to corneal allografts transplanted to nonallergic hosts. Previous findings suggested that increased risk for rejection was not a local effect produced by an inflamed eye, but was due to perturbation of the systemic immune responses to alloantigens on the corneal allograft. We tested the hypothesis that another allergic disease, airway hyperreactivity (AHR), would also increase the risk for corneal allograft rejection. Induction of AHR with either ovalbumin (OVA) or short ragweed (SRW) extract prior to keratoplasty resulted in a steep increase in the speed and incidence of corneal allograft rejection. Delayed-type hypersensitivity (DTH) responses to corneal alloantigens were closely associated with corneal allograft rejection. However, the deleterious effect of AHR on corneal allograft survival was not reflected in a heightened magnitude of allospecific DTH, cytotoxic T lymphocyte and lymphoproliferative responses to the alloantigens on the corneal allograft. Unlike Th2-based immediate hypersensitivity, CD8+ T-cell-based contact hypersensitivity to oxazolone did not increase the risk for corneal allograft rejection. Thus, Th2-based allergic diseases significantly reduce the immune privilege of the corneal allograft and represent important risk factors for consideration in the atopic patient.
Keywords: Allergy, AHR, atopy, corneal allografts, DTH, rejection
Introduction
It was previously believed that corneal allograft rejection was solely mediated by CD4+ Th1 immune responses. Th1 cells produce interferon-γ (IFN-γ ) and IL-2 and mediate delayed-type hypersensitivity (DTH) to the organ donor’s histocompatibility antigens, both of which are closely associated with graft rejection. In the classical T-helper cell paradigm, type 2 T-helper cells (Th2) secrete IL-4, IL-5 and IL-13, as well as other cytokines, which cross-regulate Th1 cytokines, thereby suppressing clonal expansion of Th1 cells (1). Thus, it has been proposed that tilting the alloimmune response toward a Th2 pathway favors corneal allograft survival (2). However, deviation to a Th2 immune response by elimination of the Th1 cell responses does not prevent graft rejection, but instead culminates in an eosinophil-mediated rejection of cardiac and skin allografts in mice (3–5). Previous studies involving the transplantation of corneal allografts to Th2-deviated hosts have produced conflicting results ranging from complete corneal allograft acceptance to complete rejection (2,6). The notion that Th2 immune responses might be deleterious, rather than beneficial, is supported by clinical observations indicating that patients with severe ocular allergies are at a higher risk of corneal transplant rejection (7–9).
Recently, our lab employed a model of allergic conjunctivitis induced by short ragweed (SRW) pollen to determine if a classical form of Th2-based allergy elicited by a well-recognized allergen (i.e. SRW pollen) would affect the fate of orthotopic corneal allografts. The results unequivocally demonstrate that: (a) allergic conjunctivitis promotes systemic Th2 immune responses to the alloantigens expressed on the corneal allograft, (b) corneal allografts transplanted to atopic hosts experience an increased incidence and a swifter tempo of rejection, (c) increased rejection is closely correlated with systemic Th2 cell-mediated responses to donor alloantigens, not local allergic inflammation and (d) corneal allograft rejection in atopic hosts does not require the direct involvement of infiltrating eosinophils (10). A recently completed study by Flynn and coworkers confirmed our findings that allergic conjunctivitis exacerbated corneal allograft rejection in mice (11). The same investigators have also reported that topical treatment with antihistamines alone did not prevent the exacerbation of corneal allograft rejection that was associated with SRW pollen conjunctivitis (12). The latter finding is consistent with our conclusion that the exacerbation of corneal allograft rejection in hosts expressing allergic conjunctivitis is due to a systemic Th2 immune response to donor histocompatibility antigens and not simply a nonspecific local effect produced by an inflamed ‘hot eye’. With this in mind, we tested the hypothesis that an allergic disease manifested in a distant organ, the lungs, would also affect the fate of corneal allografts.
Materials and Methods
Animals
C57BL/6 (H-2b) and BALB/c (H-2d) mice were purchased from Taconic Farms (Germantown, NY). Animals used in grafting experiments were females, 8–12 weeks in age. All animals used in these experiments were housed and cared for in accordance with the Association for Research in Vision and Ophthalmology (ARVO) statement for the use of animals in ophthalmic and vision research.
Surgical technique
Full-thickness penetrating orthotopic corneal grafts were performed as previously described (13), with a few modifications. Mice were anesthetized systemically with an intraperitoneal (i.p.) injection of 115 mg/kg of ketamine HCl (Fort Dodge Laboratories, Fort Dodge, IA) and 5.6 mg/kg of xylazine (Bayer Corporation, Shawnee Mission, KS). Proparacaine HCl ophthalmic solution (USP 0.5%; Alcon Laboratories, Ft. Worth, TX) was used as a topical anesthetic. Donor grafts and recipient graft beds were scored with 2.0-mm trephines and the corneas were excised with Vannas scissors. Donor grafts were sewn into place using running 11–0 nylon sutures (Ethicon, Sommerville, NJ), and sutures were removed on day 7 posttransplantation. Topical antibiotic (Akorn, Decatur, IL) was applied immediately after surgery as well as immediately after removal of sutures. No immunosuppressive drugs were used, either topically or systemically.
Clinical evaluation of grafted corneas
Corneal grafts were examined 2–3 times a week with a slit-lamp biomicroscope (Carl Zeiss, Oberkochen, Germany). Graft opacity was scored using a scale of 0–4. Degree of graft opacity was scored as follows: 0 = clear; 1+ = minimal superficial opacity; 2+ = mild deep stromal opacity with pupil margin and iris visible; 3+ = moderate stromal opacity with pupil margin visible, but iris structure obscured; and 4+ = complete opacity, with pupil and iris totally obscured. Corneal grafts were considered rejected upon two successive scores of 3+.
DTH assay
DTH was measured using a conventional ear swelling assay (14). An eliciting dose of 4 × 106 γ -irradiated (3000 rad) C57BL/6 spleen cells in 20 µL of Hanks’ balanced salt solution (HBSS) was inoculated into the right-ear pinna of BALB/c mice. The left-ear pinna served as a negative control and was injected with 20 µL of HBSS without cells. Results were expressed as specific ear swelling = (24-h measurement–0-h measurement) for experimental ear–(24-h measurement–0-h measurement) for negative control ear.
Cytotoxic T lymphocyte (CTL) assay
A standard 4-h 51Cr-release assay was used as previously described (15). Briefly, single-cell suspensions of spleen cells were prepared from BALB/c mice 1–7 days after they rejected their C57BL/6 corneal allografts. Lymphocytes were suspended in RPMI-1640 medium (BioWhittaker, Cambrex Bioscience, Walkersville, MD) containing 10% heat-inactivated fetal bovine serum(FBS; HyClone Laboratories, Logan, UT), 2-mM L-glutamine (BioWhittaker), 1-mM sodium pyruvate (BioWhittaker), 1% penicillin—streptomycin–fungizone (BioWhittaker), 1% nonessential amino acids (BioWhittaker), 1% HEPES buffer (BioWhittaker) and 5 × 10 − 5 M 2-mercaptoethanol (Sigma Chemical Co., St. Louis, MO). Experimental and control lymphocytes were boosted in vitro (described below), washed in complete RPMI-1640 and dispensed into 96 well microtiter plates (Corning Inc., Corning, NY) along with 2×104 51Cr-labeled C57BL/6 Concanavalin A spleen cell blasts in a total volume of 200 µL/well. Assays were performed in triplicate using effector to target cell ratios ranging from 50:1 to 200:1. Plates were centrifuged at 45 × G for 3 min before incubating at 37°C for 4 h. Plates were centrifuged at 110 × G for 6 min before harvesting 100 µL of the supernatant from each well and counting in a gamma counter (Packard BioScience, Meriden, CT). Cytotoxicity was determined by the amount of 51Cr released by the target cells and the specific lysis was calculated as follows:
Allergic airway hyperreactivity (AHR)
A well-characterized mouse model of allergic pulmonary inflammation was employed to induce AHR in the mouse (16–19). AHR mimics the key features of allergic asthma, including: (a) impaired pulmonary ventilation, (b) the presence of eosinophilic pulmonary inflammation and (c) the presence of Th2 cytokines in bronchoalveolar lavage fluids (16–18,20). To induce AHR with ovalbumin (OVA), BALB/c mice were immunized i.p. with 10 ug of OVA (Sigma-Aldrich, St. Louis, MO) mixed with alum on day 0 and day 14. On days 25–27, mice were challenged intranasally (i.n.) with OVA. On day 28, bronchoalveolar lavage fluid (BALF) was collected and cytospins were prepared for Giemsa and hematoxylin and eosin staining. To induce AHR with SRW extract, BALB/c mice were immunized i.p. with 40 ug of SRW extract (Greer, Lenoir, NC) mixed with alum on day 0 and day 7. On days 14, 15, 21 and 22, mice were challenged i.n. with 125 µg of SRW extract. On day 23, BALF was collected and cytospins were prepared for Giemsa and hematoxylin and eosin staining. AHR was assessed by methacholine-induced airflow obstruction in conscious mice placed in a whole-body plethysmograph and the results were represented as the enhanced pause (Penh) in breathing (17).
Mixed lymphocyte reactions (MLR)
An indirect MLR was used to determine the effect of AHR on the indirect pathway of alloimmune stimulation as described elsewhere (21). The indirect MLR was selected instead of the conventional MLR, as the indirect pathway of alloantigen presentation has been found to be the predominant pathway in the immune rejection of corneal allografts in low-risk eyes (22,23). For the indirect MLR, spleen cells from naϊve C57BL/6 mice were treated with a NH4Cl erythrocyte lysis solution, washed and resuspended in 1-mL complete RPMI without FBS. C57BL/6 cell lysates were prepared by freezing C57BL/6 spleen cells at 80°C for 10 min and thawing at 37°C for 5 min for two cycles. BALB/c splenocytes were isolated, treated with a NH4Cl erythrocyte lysis solution, washed and resuspended in 10-mL complete RPMI containing 10% FBS. The cell suspension was plated onto two 100-mm Primaria plates (5 mL each plate) and incubated at 37°C for 1 h. Nonadherent cells were removed by vigorous washing. Adherent antigen-presenting cells (APCs) were cultured in one 100-mm Primaria plate containing 3-mL complete RPMI containing 10% FBS and pulsed by the addition of the C57BL/6 cell lysate (1 ml) to the APC culture medium for 4 h at 37°C (21). As a control, nonpulsed APCs were cultured in a 100-mm Primaria plate containing 3-mL complete RPMI supplemented with 10% FBS for 4 h at 37°C. Cell proliferation was assessed after 72 h by addition of 3H-thymidine during the last 18 h of culture.
Vascularized corneal graft beds
Vascularized, high-risk graft bed were produced as previously described (23). Briefly, three interrupted sutures (11–0 nylon, 50-µm diameter needle; Sharpoint, Vanguard, Houston, TX) were placed through the central cornea of the right eyes of BALB/c recipient mice 2 weeks prior to their receiving orthotopic C57BL/6 corneal allografts.
Contact hypersensitivity (CHS)
CHS was induced by skin painting with oxazolone (OX) dissolved in olive oil as previously described (24). Briefly, 1.25% solution of OX (Sigma-Aldrich) was applied to the shaved abdominal skin of BALB/c mice on day 0. Seven days later the right ears were challenged with a topical application of 0.5% OX in olive oil. Left ears served as background controls and were challenged topically with the olive oil vehicle. Ear swelling was measured 24 h later using an engineer’s micrometer.
Statistics
Statistical significance of DTH results was determined using Student’s t-test. Median survival times (MST) for corneal allografts were determined and compared using the Mann–Whitney U-test.
Results
AHR increases the risk for corneal allograft rejection
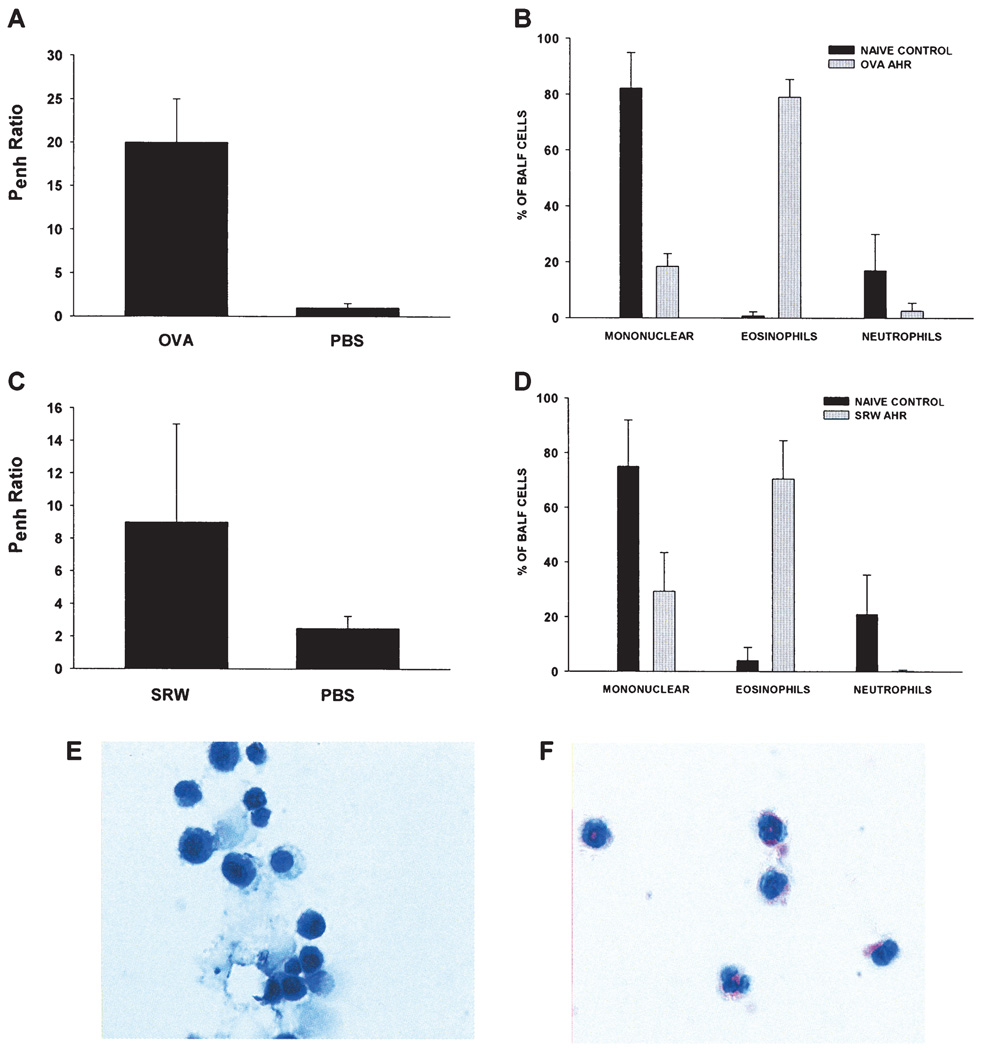
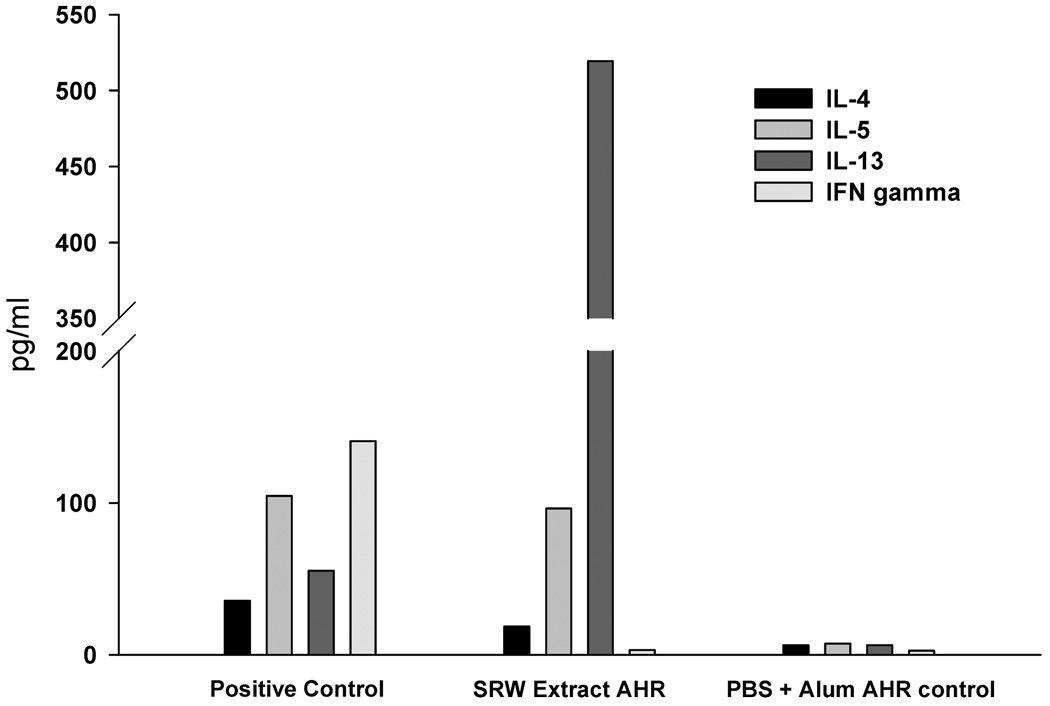
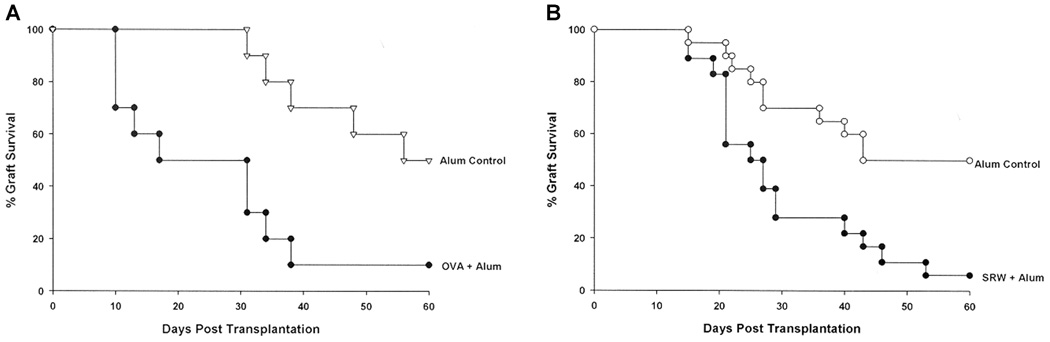
We have previously shown that allergic conjunctivitis is associated with a sharp increase in the tempo and incidence of corneal allograft rejection, but has no adverse effect on the survival of corneal autografts (10). AHR was induced in BALB/c mice to determine if an allergic disease in a distant organ affected the fate of corneal allografts. Accordingly, mice were sensitized intraperitoneally and intranasally with either SRW extract or OVA and AHR was assessed by methacholine-induced airflow obstruction in conscious mice placed in a whole-body plethysmograph. The results demonstrated that AHR was induced by sensitization with either SRW or OVA, as shown by enhanced pause (Penh) in breathing (Figure 1A,C). Cytospin analysis of BALF confirmed that animals with AHR displayed a sharp increase in the numbers of eosinophils in the pulmonary lavages compared to naϊve controls (Figure 1B,D,E,F). BALF from SRW-induced AHR contained a typical Th2 cytokine profile (IL-4, IL-5 and IL-13) (Figure 2). Similar results were found using BALF from mice with OVA-induced AHR (data not shown). AHR was associated with a dramatic increase in the tempo and incidence of corneal allograft rejection. Corneal allografts transplanted to BALB/c mice demonstrating OVA-induced AHR underwent rejection in 90% of the hosts compared to a 50% incidence of rejection in the alum control group (Figure 3A). A similar exacerbation of corneal allograft rejection was found in BALB/c mice that expressed SRW pollen AHR (Figure 3B).
Figure 1. Airway hyperreactivity (AHR) in BALB/c mice using either OVA or SRW extract.
Airflow obstruction was assessed by whole-body plethysmography and recorded as enhanced pause (Penh)(A, C). Bronchoalveolar lavage fluid (BALF) was collected on day 28 (OVA) and day 23 (SRW) and inflammatory cell counts of cytospins were analyzed by compound microscopy (B, D) and stained with Geimsa to distinguish mononuclear cells (E) from eosinophils (F). Results in A–D represent means ± standard deviations. There were at least five mice in each of the BALF groups.
Figure 2. Th2 cytokine expression in bronchoalveolar lavage fluid (BALF) in mice with AHR in BALB/c mice using SRW extract.
BALF was collected on day 23 and was examined for expression of IL-4, IL-5, IL-13 and IFN-γ by ELISA. There were five mice in each group.
Figure 3. Allograft rejection in mice with AHR. BALB/c mice were immunized with either OVA + alum or alum alone (A) or SRW extract + alum or alum alone (B).
AHR was confirmed by plethysmography 28 days later. Animals were challenged 3 days later with a C57BL/6 corneal allograft. Alum control mice did not demonstrate altered Penh and were challenged with C57BL/6 corneal allografts at the same time as the OVA + alum group. There were 10 mice in each group. p = 0.0046 (A) and p = 0.007 (B).
AHR does not produce qualitative or quantitative changes in DTH responses to donor alloantigens
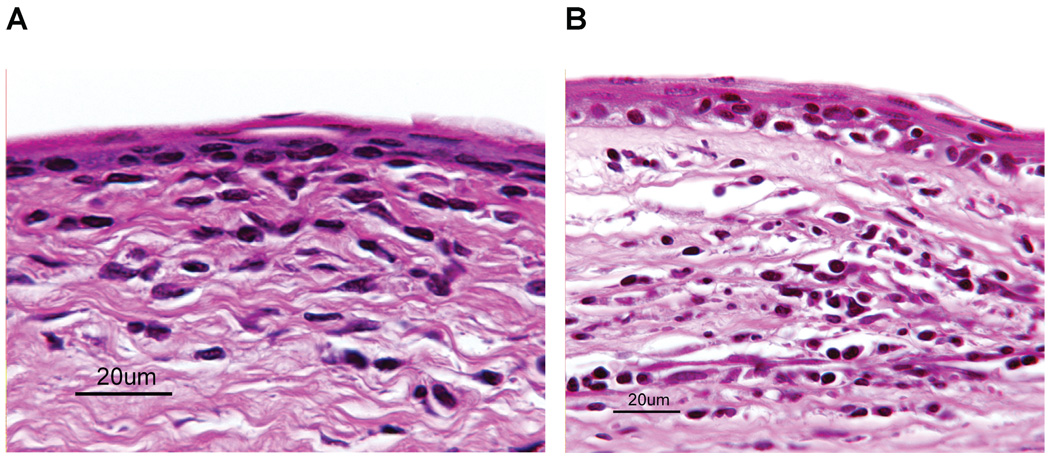
We have previously reported that allergic conjunctivitis results in an increased tempo and incidence of corneal allograft rejection. Corneal allografts that underwent rejection in mice with allergic conjunctivitis were characterized by an inflammatory infiltrate that predominantly comprised eosinophils. By contrast, rejected corneal allografts in AHR mice were not significantly different from those in the alum control groups (Figure 4). In both groups of mice, the inflammatory infiltrate was predominantly mononuclear cells, with occasional neutrophils and virtually no discernible eosinophils.
Figure 4. Histopathological features of rejected corneal allografts in mice with SRW extract-induced AHR.
Mice were sensitized with SRW extract + alum(A) or alum alone (B). C57BL/6 corneal allograftswere transplanted 6 h after AHR was confirmed by whole-body plethysmography. Eyes were enucleated 24 h after corneal allografts were deemed rejected (two consecutive clinical scores of 3+). Inflammatory cell infiltrates were predominantly mononuclear cells. Inflammation in the anterior chamber was comprised predominantly of neutrophils (not shown).
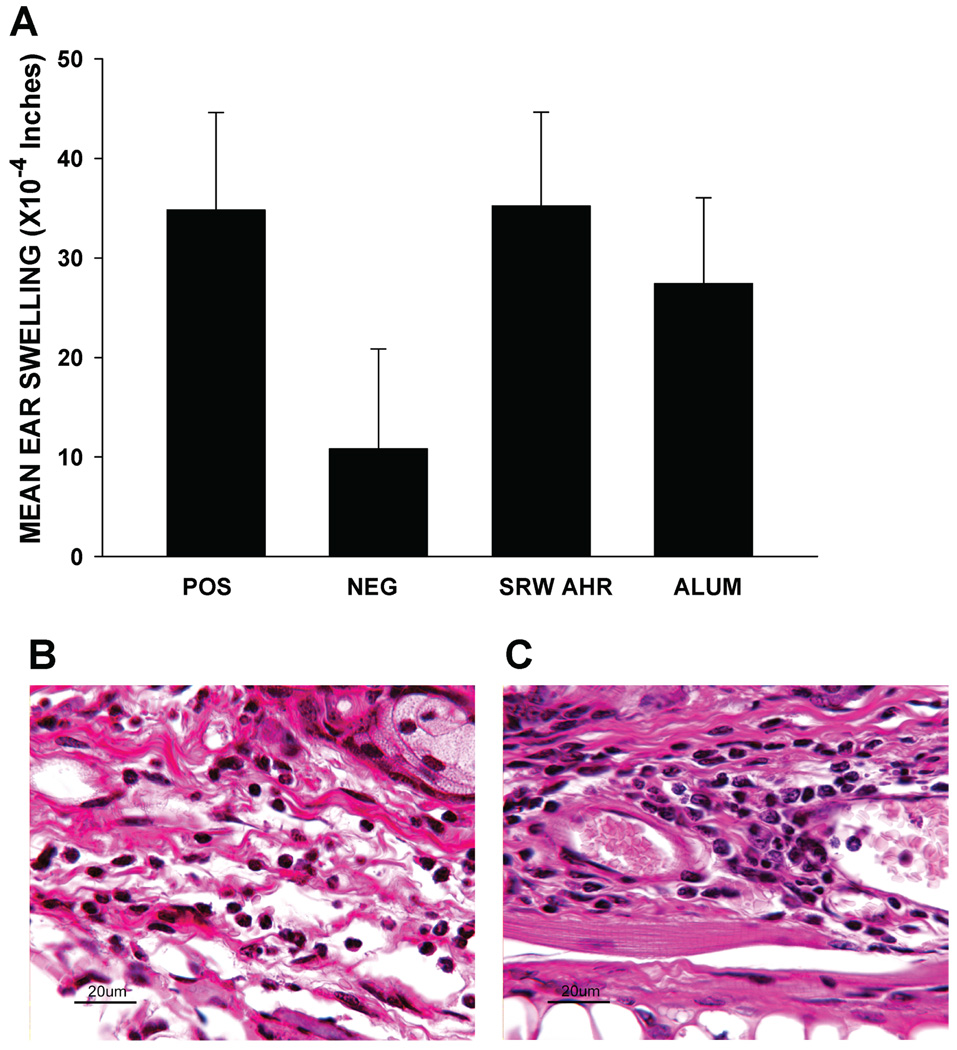
DTH responses to donor alloantigens were assessed in SRW-induced AHR mice and control mice 7 days after corneal allograft rejection. SRW-induced AHR mice and control mice that had rejected corneal allografts mounted significant DTH responses to the donor alloantigens (Figure 5A). Interestingly, the DTH responses in the AHR corneal allograft rejector group were not significantly different from those in the alum control group. The DTH lesions in both the AHR and alum control groups predominantly comprised mononuclear cells with only occasional neutrophils and eosinophils (Figure 5B,C).
Figure 5. DTH responses to C57BL/6 alloantigens in mice with SRW-induced AHR following corneal allograft rejection.
BALB/c mice were sensitized with either SRW extract + alum or alum alone. C57BL/6 corneal allografts were grafted 6 days after AHR was confirmed by plethysmography in the SRW + alum group. AHR was absent in the alum-treated group. Results in ‘A’ represent mean ear swelling ± standard deviation. There were at least five mice in each group. DTH responses in the positive control, SRW AHR, and alum groups were all significantly different from the negative control (p < 0.05), but not different from each other (p > 0.05)(A). Inflammatory infiltrates in the ears of SRW-sensitized mice following measurement of DTH (B) and ears of mice injected with alum only (C). In both groups the inflammatory infiltrates were predominantly mononuclear, with occasional neutrophils and rare eosinophils. There were five mice in each group.
Lymphoproliferative responses to donor alloantigens in mice
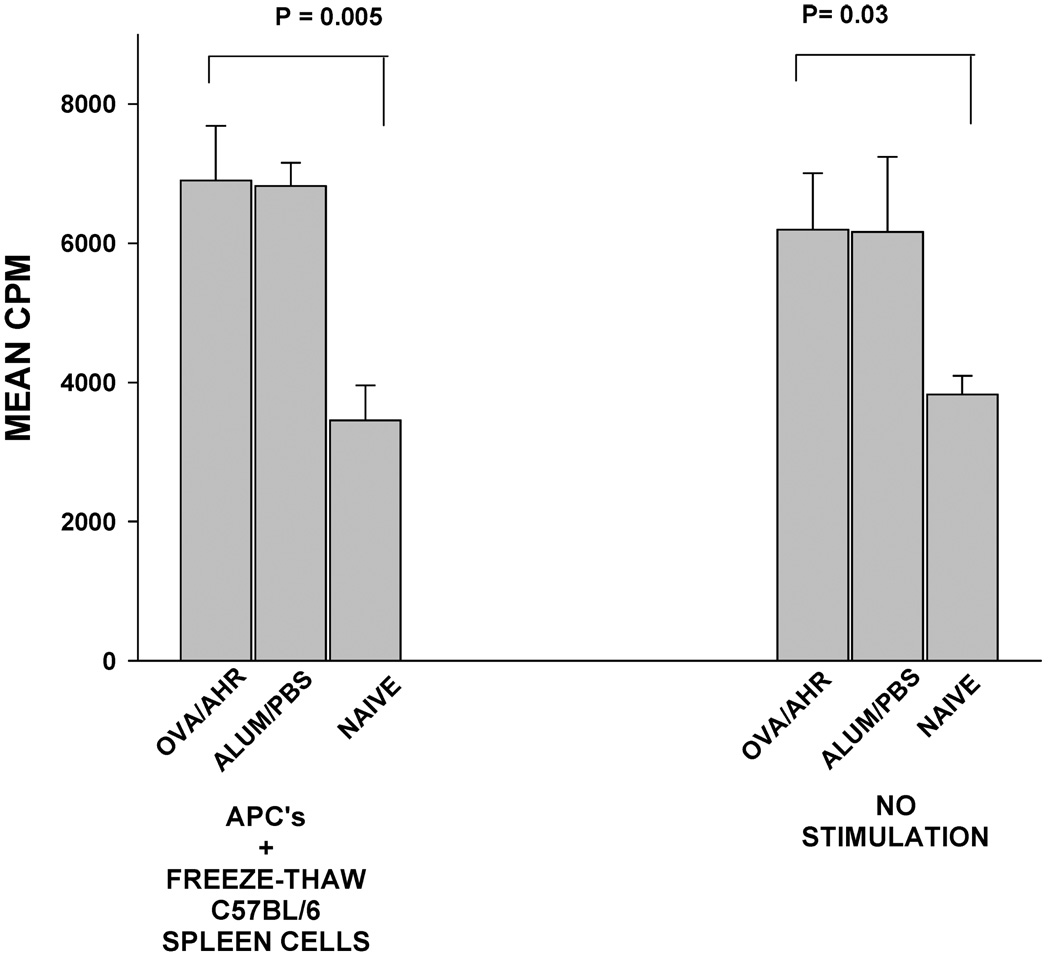
The enhanced rejection of corneal allografts in BALB/c mice with OVA-induced AHR might be the result of an enhanced primary T-cell-based immune response as a result of the AHR protocol. This was examined by determining the lymphoproliferative responses of spleen cells in mice with OVA-induced AHR and mice that were treated with the alum adjuvant in the absence of OVA. Spleen cells from both groups of mice were tested for their responses to C57BL/6 alloantigens via the indirect pathway of allostimulation. In the indirect pathway for MLR, BALB/c APCs were pulsed with freeze/thaw lysates of C57BL/6 spleen cells and cocultured with BALB/c spleen cells from OVA AHR mice, mice treated with the alum adjuvant without OVA or naϊve mice (Figure 6). Lymphoproliferative responses were similar in mice subjected to OVA AHR and mice treated with the alum adjuvant alone. Interestingly, both groups of mice displayed lymphoproliferative responses to C57BL/6 alloantigens that were significantly higher than naϊve negative control mice (p = 0.005 and p = 0.03, respectively). However, the enhanced lymphoproliferative responses of the OVA and alum-treated mice were found even in the absence of in vitro allostimulation.
Figure 6. Lymphoproliferative responses to C57BL/6 alloantigens in BALB/c mice with OVA-induced AHR.
Indirect MLR cultures were prepared in which BALB/c responder lymphocytes were cocultured with BALB/c APC that had been pulsed with freeze/thaw lysates of C57BL/6 spleen cells. Lymphocyte proliferation was determined by 3H-thymidine incorporation 72 h later. CPM = counts per minute. This experiment was performed twice with similar results.
AHR does not promote the generation of allospecific CTL responses
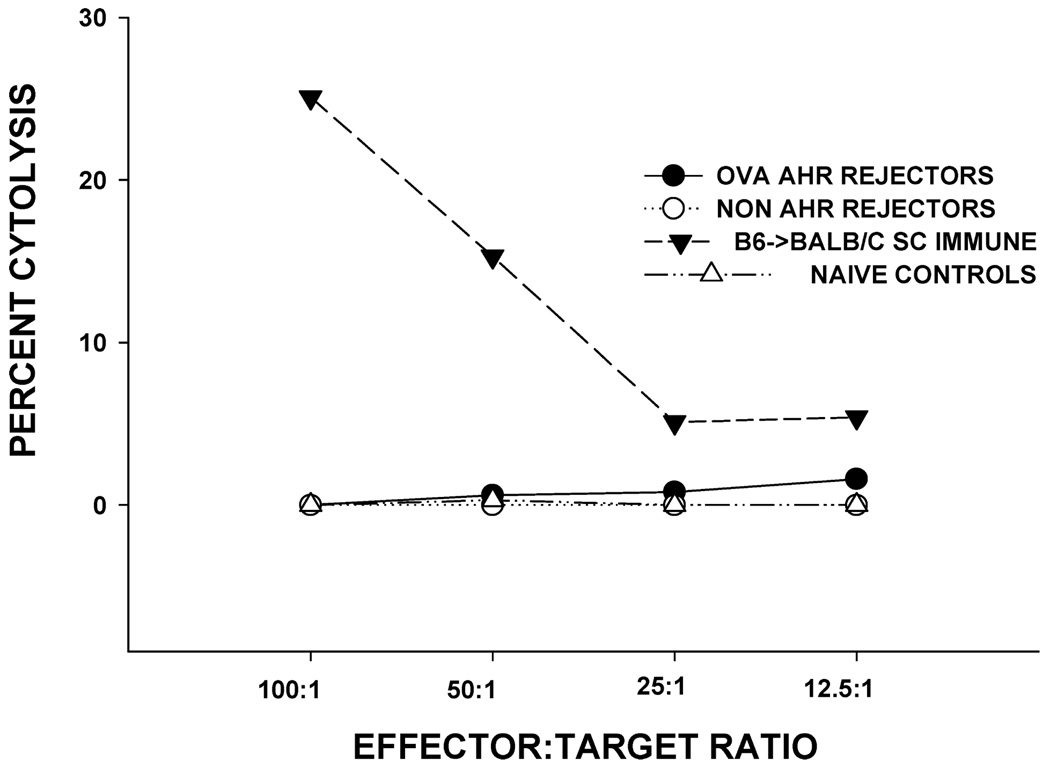
Previous studies have shown that corneal allografts in normal risk hosts do not induce allospecific CTL responses, but hosts with vascularized graft beds develop donor-specific CTL responses and display a dramatically increased incidence and tempo of corneal allograft rejection (14,25). Accordingly, we considered the hypothesis that mice with AHR were high-risk hosts due to the induction of allospecific CTL responses. Spleen cells were isolated from AHR mice within 1–7 days after corneal allograft rejection and were tested for donor-specific CTL responses. The results showed that unlike other high-risk hosts (i.e. mice with prevascularized graft beds), AHR mice did not develop significant donor-specific CTL responses (Figure 7).
Figure 7. CTL responses to C57BL/6 alloantigens in BALB/c mice with OVA-induced AHR and challenged with C57BL/6 corneal allografts.
BALB/c mice were sensitized with OVA + alum and C57BL/6 corneal allografts were transplanted orthotopically 6 days after AHR was confirmed by plethysmography. For comparison, BALB/c mice were not sensitized with OVA, but received C57BL/6 corneal allografts. CTL responses were assessed after corneal graft rejection was complete. Controls consisted of naϊve mice (negative control) and BALB/c mice immunized subcutaneously (SC) with C57BL/6 spleen cells.
AHR-induced exacerbation of corneal allograft rejection dissipates if allergen challenge is terminated
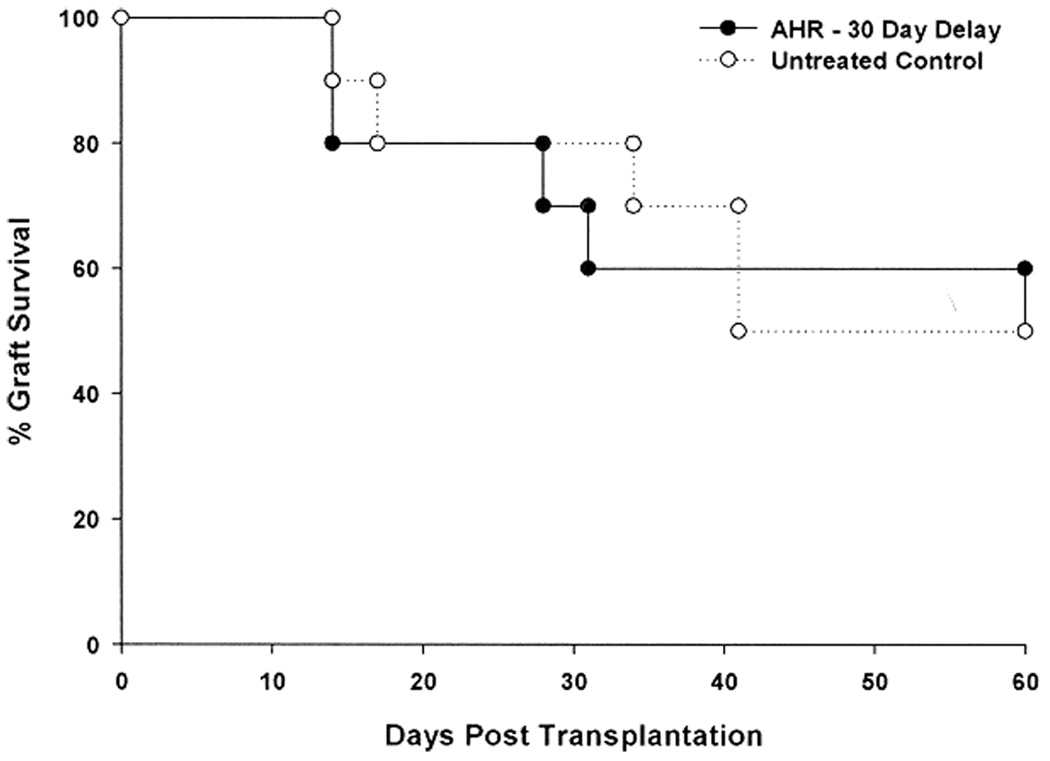
Additional experiments were performed to determine if the AHR-induced exacerbation of corneal allograft rejection was permanent. AHR was induced as before; however, instead of applying an orthotopic corneal allograft 24 h after the final i.n. challenge with SRW extract, corneal transplantation was delayed 30 days. The results show that suspending i.n. challenge with allergen restored the hosts to their original rejection phenotype. The incidence and tempo of corneal allograft rejection in the AHR group and untreated control groups were not significantly different (Figure 8).
Figure 8. Exacerbation of corneal allograft rejection dissipates if allergen challenge is terminated.
AHR was induced with SRW extract + alum. BALB/c mice were challenged intranasally with SRW extract on days 14, 15, 21 and 22. On day 23 AHR was confirmed by whole-body plethysmography. Mice were returned to the vivarium on day 23 and no additional intranasal challenges were performed. Thirty days later, the SRW-sensitized mice and untreated control mice were challenged with C57BL/6 corneal allografts. There were 10 mice in each group. p > 0.05.
CD8+ T-cell-based CHS does not exacerbate corneal allograft survival
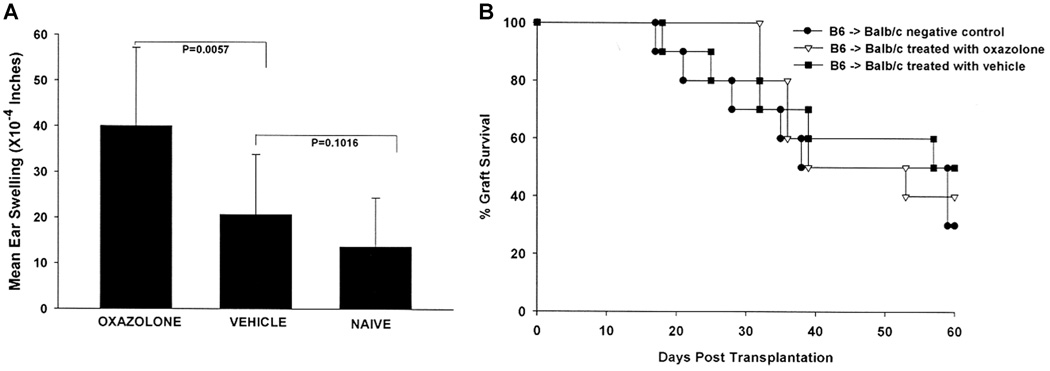
Both allergic conjunctivitis and AHR are Th2-based hypersensitivity reactions. The question arises as to whether other forms of hypersensitivity, such as CHS, might also jeopardize corneal allograft survival. CHS is a well-described form of antigen-specific T-cell-based hypersensitivity that is mediated by CD8+ T cells (26–28). Accordingly, we examined the effect of OX-induced CHS on corneal allograft survival. As expected, skin painting with OX induced DTH to OX, which was confirmed using a conventional ear swelling assay (Figure 9A). C57BL/6 corneal allografts were transplanted orthotopically to OX-sensitized BALB/c mice 1 day after the ear swelling assays were completed. Unlike hosts with ongoing Th2-based hypersensitivity, BALB/c mice that demonstrated CHS did not have an increased incidence or tempo of corneal allograft rejection. Both vehicle-treated and OX-sensitized BALB/c mice rejected 60% of their C57BL/6 corneal allografts with MSTs that were not significantly different (Figure 9B).
Figure 9. Effect of contact hypersensitivity on corneal allograft survival.
Contact hypersensitivity (CHS) was induced in BALB/c mice by abdominal skin painting with oxazolone (OX). DTH responses to OX were determined by skin painting ears with OX 7 days after initial sensitization with OX and measuring specific ear swelling 24 h later (A). C57BL/6 corneal allografts were applied to OX-sensitized BALB/c mice and control mice 24 h after ear swelling was measured. There were 10 mice in each group. p > 0.05 for all groups.
Corneal allograft rejection elicited by corneal inflammation and neovascularization is a local effect
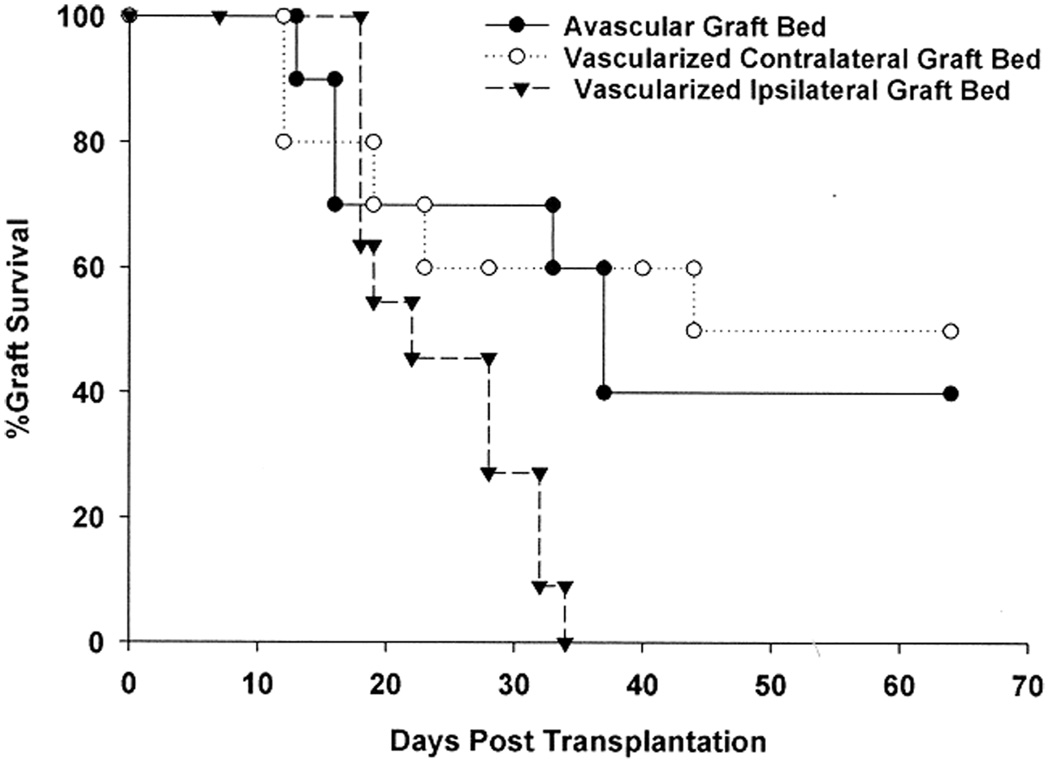
It is widely recognized that corneal neovascularization and inflammation of the ocular surface dramatically increase the risk for corneal allograft rejection in keratoplasty patients and in animal models of penetrating keratoplasty (23,29–32). It is widely assumed that the mechanisms responsible for increased rejection under these conditions are based on local perturbations of the graft bed and are not systemically manifested. Prior to our study on allergic conjunctivitis, it was widely assumed that the increased risk for corneal allograft rejection in patients with ocular allergies was due to the local effects of the inflamed ‘hot eye’. Additional experiments were performed to firmly establish that allergic diseases are different from other risk factors for corneal graft rejection and are due to systemic, not local, perturbations of the host’s response. To determine if the inflammation elicited by corneal neovascularization exerts a local versus a systemic influence on corneal allograft survival, two experimental groups were prepared. Corneal inflammation and neovascularization were induced by placing nylon sutures through the central corneas of the right eyes of BALB/c mice. Two weeks later, C57BL/6 corneal allografts were transplanted either into the vascularized ipsilateral right eyes or the nonvascularized contralateral left eyes. As expected, all of the C57BL/6 corneal allografts placed into prevascularized, inflamed graft beds underwent rejection (Figure 10). By contrast, only 50% of the C57BL/6 corneal allografts underwent rejection when grafted into avascular graft beds in BALB/c hosts that simultaneously expressed inflamed, vascularized corneas in the contralateral eyes. Thus, ocular surface inflammation elicited by a foreign body-like reaction (i.e. sutures) does not produce the same risk for corneal allograft rejection that is associated with Th2-based allergic inflammation.
Figure 10. Effect of corneal inflammation provoked by foreign body reactions on corneal allograft survival.
Nylon sutures were placed into the right eyes of BALB/c mice to induce neovascularization of the cornea. Sutures were removed 2 weeks later and C57BL/6 corneal allografts were transplanted into either the right (vascularized graft bed) or left (nonvascularized graft bed) eyes of the suture-treated mice. A third group of BALB/c mice were not treated with sutures, but received C57BL/6 corneal allografts. There were 10 mice in each group. p > 0.05 for the untreated versus vascularized contralateral graft bed groups.
Discussion
There are widespread anecdotal reports and a limited number of clinical studies suggesting that atopic patients have a significantly elevated risk for corneal allograft rejection (7–9,33). Although there are anecdotal reports that patients with allergic asthma have an increased risk for corneal allograft rejection, there are no published studies on this topic at the present time. We and others tested this hypothesis in a prospective setting and reported that an atopic disease of the ocular surface, allergic conjunctivitis, adversely affects the survival of corneal allografts in mice (10,11). This study sought to determine if other allergic diseases would produce similar adverse effects on corneal allograft survival.
In this study, we used the murine model of AHR to test the hypothesis that an atopic disease in a distant organ would adversely affect the survival of orthotopic corneal allografts. The rationale for testing allergic asthma is 2-fold. Asthma currently affects 14–15 million people in the United States and its incidence appears to be increasing worldwide (34,35). The second reason for studying asthma is that the allergic inflammation is restricted to an organ distant from the eye and thus, any adverse effect on corneal allograft survival would clearly be due to a systemic effect of a Th2-based immune response. The pathophysiology of asthma is associated with a Th2-based inflammatory response that involves the generation of a cytokine pattern that includes IL-4, IL-5 and IL-13, and bronchial inflammation that contains a preponderance of eosinophils. The present murine model of AHR recapitulates these sequelae and has been used extensively to study allergic asthma and the regulation of Th2-based inflammation.
This study demonstrates that allergic AHR elicited at the time of corneal transplantation has a profoundly deleterious effect on corneal allograft survival. The increased tempo and incidence of rejection occur regardless of the allergen used. That is, AHR induced with either OVA or SRW results in a similar acceleration in corneal allograft rejection. In a previous study, we demonstrated that allergic conjunctivitis also produced a steep increase in the incidence of corneal allograft rejection (10). Moreover, allergic conjunctivitis exerted a deleterious effect even if the allergic reaction was elicited in only one eye and the corneal allograft was placed into the contralateral, nonallergic eye. It is possible that SRW pollen applied to the nongrafted eye might have been transferred to the contralateral eye by the grooming behavior of the experimental mice. However, it is unlikely that significant quantities of SRW pollen were deposited on the contralateral grafted eye, as we never observed clinical evidence of allergic conjunctivitis or histopathological evidence of eosinophil-based inflammation in either the rejected corneal grafts or the conjunctivae of the grafted eyes inmice sensitized with SRW in the contralateral eye. These findings demonstrating that AHR exacerbates corneal allograft rejection provide further evidence that the untoward effect of Th2-based inflammation on corneal allograft survival is a systemic effect and not due to local inflammatory changes in the corneal graft bed. Although there are many possible mechanisms to account for the increased speed and incidence of immune rejection that is associated with AHR, a heightened alloimmune DTH response is not one of them, as the DTH responses to C57BL/6 alloantigens were of the same magnitude in AHR-sensitized and alum-treated control mice that had rejected their corneal allografts. Similarly, OVA AHR did not promote the development of allospecific MLR or CTL responses that were significantly greater than control mice treated with the alum adjuvant alone.
The histopathological features of rejected corneal allografts in eyes with allergic conjunctivitis were suggestive of a Th2-based inflammation, as eosinophils were the predominant inflammatory cell population (10). However, corneal allografts that were transplanted into the ‘quiet’ contralateral eyes in mice with allergic conjunctivitis were invariably rejected, yet the inflammatory infiltrates in these rejected corneal allografts were predominantly mononuclear and not eosinophilic. A similar histopathological picture emerged in the rejected corneal allografts in mice with AHR; that is, the inflammatory infiltrate was largely mononuclear, with only occasional eosinophils. Although these results tend to rule out a role for eosinophils in the rejection of corneal allografts in mice with either allergic conjunctivitis or AHR, it is still possible that under certain circumstances Th2-associated eosinophils might contribute to corneal allograft rejection.
These results also demonstrate that the exacerbation of corneal allograft rejection that is associated with allergic conjunctivitis and AHR is not simply a nonspecific consequence of ongoing immune-mediated (CHS) or nonimmune-mediated inflammation (suture-induced inflammation of the corneal graft bed). Hosts with CHS did not experience a swifter tempo or a higher incidence of corneal allograft rejection than naϊve control mice. It might be argued that the proinflammatory cytokine production and the systemic effects produced by CHS were not of the same magnitude as those elicited by AHR and as a result, CHS did not affect corneal allograft rejection. However, it is noteworthy that CHS is a CD8+ T-cell-dependent immune-mediated inflammatory response (26–28). We and others have previously shown that in normal risk hosts, corneal allograft rejection does not require the participation of CD8+ T cells and it occurs in the absence of detectable allospecific CTL activity (14,25). Thus, it is not surprising that provoking CD8+ T-cell-mediated inflammation does not exacerbate corneal allograft rejection.
The risk for corneal allograft rejection is sharply increased in hosts treated with the insertion of silk sutures into the central cornea (23,29,32). However, this effect is local and not systemic. Pretreatment of the future corneal allograft recipient’s cornea with intracorneal silk sutures produces multiple local effects, which increase the induction and expression of alloimmunity. These include: (a) the activation and centripetal migration of ocular surface antigen-presenting Langerhans cells (LC) (32), (b) the induction of new lymphatic vessels that penetrate the graft bed and corneal allograft (36,37) and (c) growth and penetration of new blood vessels within the graft, which facilitates the trafficking and extravasation of activated immune cells into the body of the corneal allograft (36,37). As stated earlier, the effect of intracorneal silk sutures is local and does not affect the fate of corneal allografts placed into contralateral ‘quiet’ eyes.
It is important to note that the deleterious effects of AHR on corneal allograft survival are not permanent and dissipate in 30 days. That is, the speed and incidence of corneal allograft rejection returned to baseline levels when corneal transplantation was delayed until 30 days after the last intranasal challenge with SRW extract. This finding supports the concept that aggressive management of allergic diseases and stabilizing the atopic patient prior to corneal transplantation will reduce the risk of corneal allograft rejection.
Acknowledgments
This work was supported by National Institutes of Health grants EY07641 and EY016664 and an unrestricted grant from Research to Prevent Blindness, Inc., New York, NY.
References
- 1.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 2.Yamada J, Yoshida M, Taylor AW, Streilein JW. Mice with Th2-biased immune systems accept orthotopic corneal allografts placed in "high risk" eyes. J Immunol. 1999;162:5247–5255. [PubMed] [Google Scholar]
- 3.Le Moine A, Flamand V, Demoor FX, et al. Critical roles for IL-4, IL-5, and eosinophils in chronic skin allograft rejection. J Clin Invest. 1999;103:1659–1667. doi: 10.1172/JCI5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piccotti JR, Chan SY, Goodman RE, Magram J, Eichwald EJ, Bishop DK. IL-12 antagonism induces T helper 2 responses, yet exacerbates cardiac allograft rejection. Evidence against a dominant protective role for T helper 2 cytokines in alloimmunity. J Immunol. 1996;157:1951–1957. [PubMed] [Google Scholar]
- 5.VanBuskirk AM, Wakely ME, Orosz CG. Transfusion of polarized TH2-like cell populations into SCID mouse cardiac allograft recipients results in acute allograft rejection. Transplantation. 1996;62:229–238. doi: 10.1097/00007890-199607270-00014. [DOI] [PubMed] [Google Scholar]
- 6.Hargrave SL, Hay C, Mellon J, Mayhew E, Niederkorn JY. Fate of MHC-matched corneal allografts in Th1-deficient hosts. Invest Ophthalmol Vis Sci. 2004;45:1188–1193. doi: 10.1167/iovs.03-0515. [DOI] [PubMed] [Google Scholar]
- 7.Ghoraishi M, Akova YA, Tugal-Tutkun I, Foster CS. Penetrating keratoplasty in atopic keratoconjunctivitis. Cornea. 1995;14:610–613. [PubMed] [Google Scholar]
- 8.Hargrave S, Chu Y, Mendelblatt D, Mayhew E, Niederkorn J. Preliminary findings in corneal allograft rejection in patients with keratoconus. Am J Ophthalmol. 2003;135:452–460. doi: 10.1016/s0002-9394(02)02055-x. [DOI] [PubMed] [Google Scholar]
- 9.Lyons CJ, Dart JK, Aclimandos WA, Lightman S, Buckley RJ. Sclerokeratitis after keratoplasty in atopy. Ophthalmology. 1990;97:729–733. doi: 10.1016/s0161-6420(90)32523-x. [DOI] [PubMed] [Google Scholar]
- 10.Beauregard C, Stevens C, Mayhew E, Niederkorn JY. Cutting edge: Atopy promotes Th2 responses to alloantigens and increases the incidence and tempo of corneal allograft rejection. J Immunol. 2005;174:6577–6581. doi: 10.4049/jimmunol.174.11.6577. [DOI] [PubMed] [Google Scholar]
- 11.Flynn TH, Ohbayashi M, Ikeda Y, Ono SJ, Larkin DF. Effect of allergic conjunctival inflammation on the allogeneic response to donor cornea. Invest Ophthalmol Vis Sci. 2007;48:4044–4049. doi: 10.1167/iovs.06-0973. [DOI] [PubMed] [Google Scholar]
- 12.Larkin DF, Flynn TH, Ohbayashi M, Ikeda Y, Ono SJ. Early treatment of perioperative allergic conjunctivitis prolongs corneal allograft survival (Abstract) (program #1302) Invest Ophthalmol Vis Sci. 2006;46 Suppl [Google Scholar]
- 13.He Y, Mellon J, Apte R, Niederkorn JY. Effect of LFA-1 and ICAM-1 antibody treatment on murine corneal allograft survival. Invest Ophthalmol Vis Sci. 1994;35:3218–3225. [PubMed] [Google Scholar]
- 14.Hegde S, Niederkorn JY. The role of cytotoxic T lymphocytes in corneal allograft rejection. Invest Ophthalmol Vis Sci. 2000;41:3341–3347. [PubMed] [Google Scholar]
- 15.He YG, Mellon J, Niederkorn JY. The effect of oral immunization on corneal allograft survival. Transplantation. 1996;61:920–926. doi: 10.1097/00007890-199603270-00014. [DOI] [PubMed] [Google Scholar]
- 16.Akbari O, Stock P, Meyer E, et al. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nature Med. 2003;9:582–588. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- 17.Lisbonne M, Diem S, de Castro Keller A, et al. Cutting edge: Invariant V alpha 14 NKT cells are required for allergen-induced airway inflammation and hyperreactivity in an experimental asthma model. J Immunol. 2003;171:1637–1641. doi: 10.4049/jimmunol.171.4.1637. [DOI] [PubMed] [Google Scholar]
- 18.Santeliz JV, Van Nest G, Traquina P, Larsen E, Wills-Karp M. Amb a 1-linked CpG oligodeoxynucleotides reverse established airway hyperresponsiveness in a murine model of asthma. J Allergy Clin Immunol. 2002;109:455–462. doi: 10.1067/mai.2002.122156. [DOI] [PubMed] [Google Scholar]
- 19.Wang B, Huang X, Wolters PJ, et al. Cutting edge: Deficiency of macrophage migration inhibitory factor impairs murine airway allergic responses. J Immunol. 2006;177:5779–5784. doi: 10.4049/jimmunol.177.9.5779. [DOI] [PubMed] [Google Scholar]
- 20.Zhang-Hoover J, Finn P, Stein-Streilein J. Modulation of ovalbumin-induced airway inflammation and hyperreactivity by tolerogenic APC. J Immunol. 2005;175:7117–7124. doi: 10.4049/jimmunol.175.11.7117. [DOI] [PubMed] [Google Scholar]
- 21.Goddard RV, Prentice AG, Copplestone JA, Kaminski ER. Generation in vitro of B-cell chronic lymphocytic leukaemia-proliferative and specific HLA class-II-restricted cytotoxic T-cell responses using autologous dendritic cells pulsed with tumour cell lysate. Clin Exp Immunol. 2001;126:16–28. doi: 10.1046/j.1365-2249.2001.01617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boisgerault F, Liu Y, Anosova N, Ehrlich E, Dana MR, Benichou G. Role of CD4 +and CD8+ T cells in allorecognition: Lessons from corneal transplantation. J Immunol. 2001;167:1891–1899. doi: 10.4049/jimmunol.167.4.1891. [DOI] [PubMed] [Google Scholar]
- 23.Sano Y, Ksander BR, Streilein JW. Murine orthotopic corneal transplantation in high-risk eyes. Rejection is dictated primarily by weak rather than strong alloantigens. Invest Ophthalmol Vis Sci. 1997;38:1130–1138. [PubMed] [Google Scholar]
- 24.Matsue H, Edelbaum D, Shalhevet D, et al. Generation and function of reactive oxygen species in dendritic cells during antigen presentation. J Immunol. 2003;171:3010–3018. doi: 10.4049/jimmunol.171.6.3010. [DOI] [PubMed] [Google Scholar]
- 25.Ksander BR, Sano Y, Streilein JW. Role of donor-specific cytotoxic T cells in rejection of corneal allografts in normal and high-risk eyes. Transpl Immunol. 1996;4:49–52. doi: 10.1016/s0966-3274(96)80034-7. [DOI] [PubMed] [Google Scholar]
- 26.Gocinski BL, Tigelaar RE. Roles of CD4+ and CD8+ T cells in murine contact sensitivity revealed by in vivo monoclonal antibody depletion. J Immunol. 1990;144:4121–4128. [PubMed] [Google Scholar]
- 27.Xu H, Banerjee A, Dilulio NA, Fairchild RL. Development of effector CD8+ T cells in contact hypersensitivity occurs independently of CD4+ T cells. J Immunol. 1997;158:4721–4728. [PubMed] [Google Scholar]
- 28.Xu H, DiIulio NA, Fairchild RL. T cell populations primed by hapten sensitization in contact sensitivity are distinguished by polarized patterns of cytokine production: Interferon gamma-producing (Tc1) effector CD8+ T cells and interleukin (Il) 4/Il-10-producing (Th2) negative regulatory CD4+ T cells. J Exp Med. 1996;183:1001–1012. doi: 10.1084/jem.183.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen L, Hamrah P, Cursiefen C, et al. Vascular endothelial growth factor receptor-3 mediates induction of corneal alloimmunity. Nature Med. 2004;10:813–815. doi: 10.1038/nm1078. [DOI] [PubMed] [Google Scholar]
- 30.Group CCTSR. The collaborative corneal transplantation studies (CCTS). Effectiveness of histocompatibility matching in high-risk corneal transplantation. Arch Ophthalmol. 1992;110:1392–1403. [PubMed] [Google Scholar]
- 31.Volker-Dieben HJ, Kok-van Alphen CC, Lansbergen Q, Persijn GG. Different influences on corneal graft survival in 539 transplants. Acta Ophthalmol. 1982;60:190–202. doi: 10.1111/j.1755-3768.1982.tb08373.x. [DOI] [PubMed] [Google Scholar]
- 32.Yamada J, Dana MR, Zhu SN, Alard P, Streilein JW. Interleukin 1 receptor antagonist suppresses allosensitization in corneal transplantation. Arch Ophthalmol. 1998;116:1351–1357. doi: 10.1001/archopht.116.10.1351. [DOI] [PubMed] [Google Scholar]
- 33.Kuchle M, Cursiefen C, Nguyen NX, et al. Risk factors for corneal allograft rejection: Intermediate results of a prospective normal-risk keratoplasty study. [Graefe’s Arch Clin Exp Ophthalmol] Albrecht von Graefes Arch Klin Exp Ophthalmol. 2002;240:580–584. doi: 10.1007/s00417-002-0496-5. [DOI] [PubMed] [Google Scholar]
- 34.Howarth PH. Is allergy increasing?–early life influences. Clin Exp Allergy. 1998;28 Suppl 6:2–7. doi: 10.1046/j.1365-2222.1998.0280s6002.x. [DOI] [PubMed] [Google Scholar]
- 35.von Mutius E. The rising trends in asthma and allergic disease. Clin Exp Allergy. 1998;28 Suppl 5:45–49. doi: 10.1046/j.1365-2222.1998.028s5045.x. discussion 50–41. [DOI] [PubMed] [Google Scholar]
- 36.Cursiefen C, Cao J, Chen L, et al. Inhibition of hemangiogenesis and lymphangiogenesis after normal-risk corneal transplantation by neutralizing VEGF promotes graft survival. Invest Ophthalmol Vis Sci. 2004;45:2666–2673. doi: 10.1167/iovs.03-1380. [DOI] [PubMed] [Google Scholar]
- 37.Cursiefen C, Chen L, Dana MR, Streilein JW. Corneal lymphangiogenesis: Evidence, mechanisms, and implications for corneal transplant immunology. Cornea. 2003;22:273–281. doi: 10.1097/00003226-200304000-00021. [DOI] [PubMed] [Google Scholar]