Abstract
Acid-sensing ion channels (ASICs) are ligand-gated cation channels activated by a drop in extracellular pH. They are enriched in the mammalian brain with a high synaptic density. Accumulating evidence suggests that ASIC1 contributes to synaptic activity related to learning/memory and fear conditioning, and also plays critical roles in neurodegenerative diseases. In this study, we explored the effect of the psychostimulant, cocaine, on protein expression of ASICs in the mouse forebrain in vivo. We found that chronic systemic injection of cocaine (20 mg/kg, once daily for 5 consecutive days; 14 days of withdrawal) increased ASIC1, but not ASIC2, protein levels in the striatum, including the dorsal (caudate putamen) and the ventral (nucleus accumbens) striatum. No significant changes in ASIC1 or 2 protein levels in the median prefrontal cortex and the hippocampus were observed following the chronic cocaine administration. These data demonstrate that chronic cocaine exposure can upregulate ASIC expression in the striatum in a subunit-selective manner.
Keywords: ASICs, Caudate, Nucleus accumbens, Cortex, Hippocampus, Addiction, Dopamine, Drug abuse
Acid-sensing ion channels (ASICs) are proton-gated, voltage-insensitive cation ion channels [16, 20, 21]. They belong to the superfamily of degenerin/epithelial sodium channels [15]. To date, seven subtypes of this family have been cloned [16, 24, 26]. Compared to the topology of other ion channels and receptors, ASICs only have two transmembrane domains with a large cysteine-rich extracellular loop, and short intracellular amino and carboxy termini [20]. ASICs form into homomeric and hetromeric functional channels with trimer complex [11].
ASICs are highly expressed in peripheral sensory and brain neurons [1, 16, 21, 24]. Among the seven subtypes of ASICs, three of them (ASIC1a, ASIC2a and ASIC2b) are expressed in brain neurons [3, 24, 26]. Of particular interest is ASIC1a, which is permeable to Na+ as well as Ca2+ [5, 20, 28, 29]. The calcium permeability is a unique property of homomeric ASIC1a channels as it differs from other ASIC subunits [26, 29]. In addition, ASIC1a channels are localized at synaptic sites and contribute to synaptic plasticity related to learning/memory and fear conditioning [22 - 24]. Recent studies also indicate that ASIC1a is involved in acidosis-mediated neuronal injury during brain ischemia [9, 19, 26, 28] and seizure [30], and plays critical roles in neurodegenerative diseases such as multiple sclerosis, Parkinson's disease and Huntington's disease [2, 8, 25].
In contrast to homomeric ASIC1a channels, which have a pH value for half maximal activation (pH50) between 6.2 to 6.8 [5, 20], homomeric ASIC2a channels have a low sensitivity to proton, with a pH50 of 4.4 [16, 21]. However, ASIC2a can assemble with ASIC1a to form heteromeric channels [3]. Different from homomeric ASIC2a, ASIC2b subunits do not appear to respond to low pH drops when expressed in homomeric form, but may associate with other ASIC subunits to constitute heteromultimeric channels [10, 17]. The expression level of ASIC2 is subject to modulation by some neurological disorders. For example, transient global ischemia upregulates ASIC2a expression in surviving neurons [13] while status epilepticus downregulates ASIC2b expression in hippocampal neurons [4]. Functionally, activation of ASIC2 contributes to the maintenance of retinal integrity in brain [7].
ASIC1 and ASIC2 are densely expressed in the striatum [1, 4, 22]. Our recent studies indicate that functional homomeric ASIC1a is the dominant subtype in the medium spiny neurons of striatum [12]. Homomeric ASIC1a channel has a unique property in the calcium permeability [5, 20, 29], and has been considered to be involved in synaptic plasticity [22, 23]. Thus, it is likely that ASIC1a channels may also contribute to the synaptic modification during drug addiction. Since the striatum is a key structure in reward circuits involved in biological actions of addictive drugs, it is intriguing to explore whether striatal ASIC expression is subject to the modulation by psychoactive drugs. In this study, we thus investigated the possible effect of psychostimulatant administration on basal ASIC gene expression in the striatum in vivo. Chronic injection of cocaine (20 mg/kg, daily i.p. for 5 consecutive days) was given to adult mice. Alterations in basal levels of both ASIC1 and ASIC2 protein abundance in the dorsal (caudate putamen, CPu) and ventral (nucleus accumbens, NAc) striatum were examined after chronic cocaine injection. We also investigated whether ASIC1 and ASIC2 expression in other forebrain regions such as the median prefrontal cortex (mPFC) and the hippocampus is subject to the modulation by chronic cocaine administration.
Adult male C57BL/6J mice weighing 21 – 26 g (Jackson Lab, New York, NY) were individually housed in clear plastic cages. An at least 5-day accommodation period was allowed prior to the commencement of the experiment. Animals were maintained on a 12/12-h light–dark cycle; lights were turned on at 7:00 a.m. The housing environment was maintained at 23°C and humidity at 50 ± 10% with food and water available ad libitum. All procedures performed were approved by the Institutional Animal Care and Use Committee and were in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Cocaine hydrochloride (Sigma–Aldrich, Saint Louis, MO) was injected at 20 mg/kg daily for 5 consecutive days intraperitoneally (i.p.). Doses of cocaine were calculated on salt. Saline was injected i.p. as control. Mice were anesthetized with Equithesin (9 ml/kg, i.p.) and decapitated at 14 days after the final cocaine injection. Brains were removed, and different brain regions, including the CPu, the NAc, the mPFC, and the hippocampus, were separately removed into a 1.5-ml microtube containing ice-cold sample buffer (20 mM Tris–HCl, pH 7.4, 1 mM dithiothreitol, 10 mM NaF, 2 mM Na3VO4, 1 mM EDTA, 1 mM EGTA, 5 μM microcystin-LR, and 0.5 mM phenylmethylsulfonyl fluoride). The sample was homogenized by sonication. The homogenate was centrifuged at 700 × g for 10 min at 4 °C. The supernatant was used for Western blot analysis of ASIC proteins. Protein concentrations were determined. The equal amount of protein was separated on SDS NuPAGE Novex 4 – 12% gels (Invitrogen, Carsbad, CA). Proteins were transferred to polyvinylidene fluoride membrane (Millipore, Bedford, MA) and blocked in blocking buffer (5% nonfat dry milk in phosphate-buffered saline and 0.1% Tween 20) for 1 h. The blots were washed and incubated in the blocking buffer containing a primary rabbit antibody against ASIC1 (Sigma–Aldrich, Saint Louis, MO), ASIC2 (Alpha Diagnostic International, San Antonio, TX) or actin (Santa Cruz Biotechnology, Santa Cruz, CA) usually at 1: 1000 overnight at 4 °C. This was followed by 1 h incubation in a goat horseradish peroxidase-linked secondary antibody against rabbit (Jackson Immunoresearch Laboratory, West Grove, PA) at 1:5000. Immunoblots were developed with the enhanced chemiluminescence reagents (ECL; Amersham Pharmacia Biotech, Piscataway, NJ), and captured into Kodak Image Station 2000R. Kaleidoscope-prestained standards (Bio-Rad, Hercules, CA) and MagicMark XP Western protein standards (Invitrogen) were used for protein size determination. The density of immunoblots was measured using the Kodak 1D Image Analysis software [18].
The results are presented as mean ± S.E.M., and were evaluated using a one- or two-way analysis of variance, as appropriate, followed by a Bonferroni (Dunn) comparison of groups using least squares-adjusted means. Probability levels of < 0.05 were considered statistically significant.
ASIC1 and ASIC2 are widely expressed in brain regions including the cerebral cortex, hippocampus and striatum [1, 4, 22]. A rabbit antibody against ASIC1 or ASIC2 was used to assay changes in ASIC1 or ASIC2 protein abundance in these regions in response to chronic cocaine stimulation. The selectivity of the antibodies was first verified in a series of control experiments using striatal protein extracts. Omission of the anti-ASIC1 or ASIC2 antibody in Western blot analysis produced no visible immunoreactive bands (data not shown). Addition of the ASIC1 antibody produced a strong immunoreactive band at a molecular weight predicted for ASIC1 (∼78 kDa) (data not shown). Using the ASIC2 antibody labeled an immunoreactive band at ∼ 60 KDa (data not shown). This 60 KDa protein was also detected in retina from ASIC2 +/+, but not from ASIC2 -/-, mice [7]. Thus, it is considered as ASIC2 immunoreactive band.
We then used ASIC1 or ASIC2 antibody to probe changes in ASIC1 and ASIC2 protein levels in different brain regions following chronic cocaine administration in vivo. After 14 days of withdrawal from chronic cocaine injection (20 mg/kg daily, i.p., for 5 consecutive days), a marked increase in ASIC1 proteins was displayed in the CPu (Fig. 1A). The ASIC1 protein level in cocaine-treated mice was increased to 139.8 ± 8.9% (p < 0.05) of that in saline-treated mice. However, there was no statistically significant difference in the ASIC2 protein level between cocaine- and saline-treated mice (Fig. 1B; saline-treated mice: 100 ± 2.5%; cocaine-treated mice: 102.5 ± 6.2%; p > 0.05). These results demonstrate a sensitive nature of ASIC1 expression in the CPu to chronic cocaine exposure. In contrast to ASIC1, ASIC2 expression in the same region is resistant to cocaine stimulation.
Fig. 1.
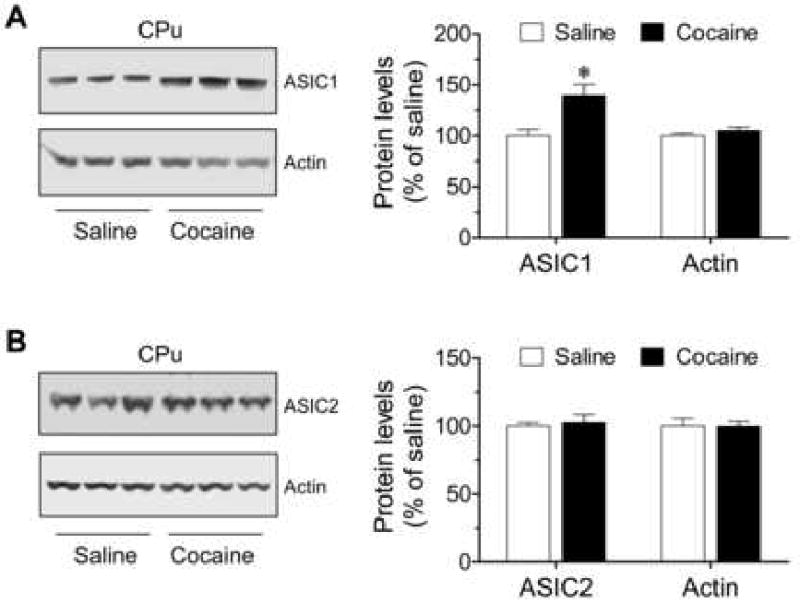
Effects of chronic cocaine injections on ASIC protein expression in the CPu in vivo. (A) Chronic cocaine injections increase ASIC1 expression in the CPu. (B) Chronic cocaine injections had no effect on ASIC2 expression in the CPu. Representative immunoblots of ASIC and actin are shown left to the quantification of immunoblot results. Cocaine (20 mg/kg daily for 5 consecutive days) or saline was injected i.p. Mice were sacrificed 14 days after the final drug injection. The quantified data are expressed as mean ± S.E.M. (n = 5 – 9 per group). *p < 0.05 versus saline. CPu represents caudate putamen.
We also detected the changes in ASIC1 and ASIC2 protein levels in the NAc at 14 days after the final cocaine injection. Similar to the CPu, the ASIC1 protein level in cocaine-treated mice was increased to 132.5 ± 8.9% of that in saline-treated mice (Fig. 2A; p < 0.05). The ASIC2 protein level in cocaine-treated mice was slightly decreased to 87.1 ± 19.4% of that in saline-treated mice, although it revealed an insignificant difference between cocaine- and saline-treated mice (Fig. 2B; p > 0.05). These data suggest that chronic cocaine exposure can induce a significant increase in ASIC1, but not ASIC2, proteins in the NAc, as seen in the CPu.
Fig. 2.
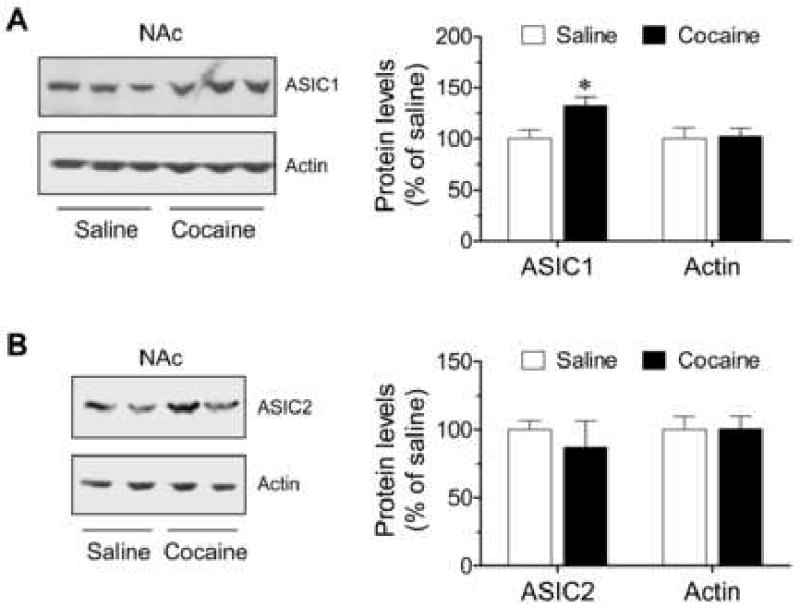
Effects of chronic cocaine injections on ASIC protein expression in the NAc in vivo. (A) Chronic cocaine injections increase ASIC1 expression in the NAc. (B) Chronic cocaine injections had no effect on ASIC2 expression in the NAc. Representative immunoblots of ASIC and actin are shown left to the quantification of immunoblot results. Cocaine (20 mg/kg daily for 5 consecutive days) or saline was injected i.p. Mice were sacrificed 14 days after the final drug injection. The quantified data are expressed as mean ± S.E.M. (n = 5 – 9 per group). *p < 0.05 versus saline. NAc represents nucleus accumbens.
Other forebrain structures, including the mPFC and hippocampus, densely express ASIC1 and ASIC2 [1, 4, 22, 23]. We then investigated whether ASIC1 and ASIC2 expression in these regions is subject to the modulation by chronic cocaine administration. The ASIC1 protein level in the mPFC and hippocampus showed no change in mice treated with chronic cocaine injections compared to mice treated with saline injections (Fig. 3A and 3B; p > 0.05). The ASIC2 level both in the mPFC and hippocampus was slightly increased in cocaine-treated mice. However, there were no significant differences between saline- and cocaine-treated mice (Fig. 3B; p > 0.05). These data demonstrate that ASIC1 and ASIC2 in these two structures are insensitive to chronic cocaine exposure.
Fig. 3.
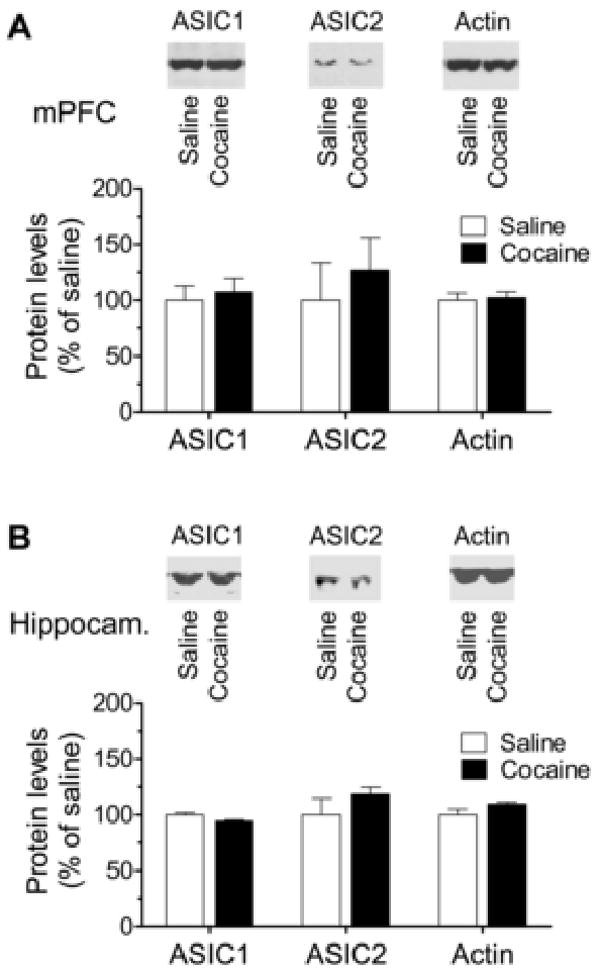
Effects of chronic cocaine injections on ASIC1 and ASIC2 protein expression in the mouse mPFC (A) and hippocampus (B) in vivo. Representative immunoblots of ASIC and actin are shown top to the quantification of immunoblot results. Cocaine (20 mg/kg daily for 5 consecutive days) or saline was injected i.p. Mice were sacrificed 14 days after the final drug injection. The quantified data are expressed as mean ± S.E.M. (n = 5 – 9 per group). p > 0.05 versus saline. mPFC represents median prefrontal cortex; Hippocam. represents hippocampus.
Typical behavioral responses to a daily injection of cocaine were observed. After each injection, mice showed an increased level of locomotor activity, which usually lasted for 1 to 2 h. A higher level of locomotor responses to the last injection of cocaine was observed as compared to the responses to the first cocaine injection, indicating the development of behavioral sensitization. At 14 days of withdrawal, no abnormal motor activity was observed in all animals receiving either saline or cocaine injection.
This study investigated the effect of chronic cocaine administration on ASIC expression in the mouse forebrain in vivo. The results indicate that ASIC1, but not ASIC2, is a sensitive target of cocaine as its protein expression can be readily regulated by the drug. Chronic cocaine exposure induced a profound increase in ASIC1, but not ASIC2, protein abundance in the striatum (CPu and NAc), indicating the subunit-dependent modulation by cocaine. Furthermore, ASIC1 and ASIC2 protein levels in the other forebrain regions such as the mPFC and hippocampus remained relatively stable after chronic cocaine injections, suggesting a region-specific modulation by chronic cocaine administration. The mechanism underlying the upregulation of ASIC1 protein expression in response to chronic cocaine exposure is unclear and requires further experimental investigation. A recent study by Ziemann et al., showed different expression levels of ASICs in pyramidal neurons and interneurons [30]. Thus, the upregulation of ASIC1 in pyramidal neurons or in interneurons will have totally different physiological consequences. Therefore, further studies are required to explore which types of neurons (pyramidal neurons vs. interneurons) are regulated by chronic cocaine administration in the striatum.
ASIC1 channels are enriched at synaptic sites in the mammalian brain [22, 23]. This enrichment situates the channel well to closely modulate synaptic activity and plasticity. Accumulative evidence has already shown that ASIC1 is actively involved in synaptic plasticity related to learning and memory and pathogenesis of several long-lasting mental disorders [6, 24, 27]. Drug addiction is a persistent mental illness that is characterized by compulsive drug craving, seeking, and ingestion in spite of severe adverse consequences. Repeated cocaine administration is known to cause behavioral sensitization in experimental animals, which corresponds to the intensification of drug craving in human addicts. The molecular mechanism for behavioral sensitization has been linked to plastic changes in excitatory synapses on spiny neurons in the striatum. For instance, several types of synaptic plasticity, such as long-term potentiation or depression, in the NAc are subject to the modification in response to chronic stimulant exposure [14]. Such modification is believed to be important steps in the remodeling of excitatory synapses, leading to enduring behavioral plasticity. Given the fact that ASIC1 is abundant at synaptic sites and its expression level is sensitive to drug exposure as observed in this study, it is possible that this channel might contribute to the reshaping of excitatory synapses. In concert with glutamate receptors, ASIC1 channels control synaptic and behavioral adaptations to cocaine. Our study represents an initial effort towards defining the role of ASIC channels. Future studies will need to elucidate the precise role of ASIC1 and other subtypes in processing synaptic and behavioral adaptations to drugs.
Acknowledgments
This project was sponsored in part by American Heart Association Scientist Development Grant 0735092N (X.-P.C), NIH grants DA 10355 (J.Q.W) and MH 61469 (J.Q.W).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alvarez de la Rosa D, Krueger SR, Kolar A, Shao D, Fitzsimonds RM, Canessa CM. Distribution, subcellular localization and ontogeny of ASIC1 in the mammalian central nervous system. J Physiol (Lond) 2003;546:77–87. doi: 10.1113/jphysiol.2002.030692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arias RL, Sung ML, Vasylyev D, Zhang MY, Albinson K, Kubek K, Kagan N, Beyer C, Lin Q, Dwyer JM, Zaleska MM, Bowlby MR, Dunlop J, Monaghan M. Amiloride is neuroprotective in an MPTP model of Parkinson's disease. Neurobiol Dis. 2008;31:334–341. doi: 10.1016/j.nbd.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Askwith CC, Wemmie JA, Price MP, Rokhlina T, Welsh MJ. Acid-sensing ion channel 2 (ASIC2) modulates ASIC1 H+-activated currents in hippocampal neurons. J Biol Chem. 2004;279:18296–18305. doi: 10.1074/jbc.M312145200. [DOI] [PubMed] [Google Scholar]
- 4.Biagini G, Babinski K, Avoli M, Marcinkiewcz M, Seguela P. Regional and subunit-specific downregulation of acid-sensing ion channels in the pilocarpine model of epilepsy. Neurobiol Dis. 2001;8:45–58. doi: 10.1006/nbdi.2000.0331. [DOI] [PubMed] [Google Scholar]
- 5.Chu XP, Miesch J, Johnson M, Root L, Zhu XM, Chen D, Simon RP, Xiong ZG. Proton-gated channels in PC12 cells. J Neurophysiol. 2002;87:2555–2561. doi: 10.1152/jn.00741.2001. [DOI] [PubMed] [Google Scholar]
- 6.Dwyer JM, Sukoff Rizzo SJ, Neal SJ, Lin Q, Jow F, Arias RL, Rosenzweig-Lipson S, Dunlop, Beyer CE. Acid-sensing ion channel (ASIC) inhibitors exhibit anxiolytic-like activity in preclinical pharmacological models. Psychopharmacology. 2009;203:41–52. doi: 10.1007/s00213-008-1373-7. [DOI] [PubMed] [Google Scholar]
- 7.Ettaiche M, Guy N, Hofman P, Lazdunski M, Waldmann R. Acid-sensing ion channel 2 is important for retinal function and protects against light-induced retinal degeneration. J Neurosci. 2004;24:1005–1012. doi: 10.1523/JNEUROSCI.4698-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friese MA, Craner MJ, Etzensperger R, Vergo S, Wemmie JA, Welsh MJ, Vincent A, Fugger L. Acid-sensing ion channel-1 contributes to axonal degeneration in autoimmune inflammation of the central nervous system. Nat Med. 2007;13:1483–1489. doi: 10.1038/nm1668. [DOI] [PubMed] [Google Scholar]
- 9.Gao J, Duan B, Wang DG, Deng XH, Zhang GY, Xu L, Xu TL. Coupling between NMDA receptor and acid-sensing ion channel contributes to ischemic neuronal death. Neuron. 2005;48:635–646. doi: 10.1016/j.neuron.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Hesselager M, Timmermann DB, Ahring PK. pH Dependency and desensitization kinetics of heterologously expressed combinations of acid-sensing ion channel subunits. J Biol Chem. 2004;279:11006–11015. doi: 10.1074/jbc.M313507200. [DOI] [PubMed] [Google Scholar]
- 11.Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 A° resolution and low pH. Nature. 2007;449:316–323. doi: 10.1038/nature06163. [DOI] [PubMed] [Google Scholar]
- 12.Jiang Q, Li MH, Papasian CJ, Branigan D, Xiong ZG, Wang JQ, Chu XP. Characterization of acid-sensing ion channels in medium spiny neurons of mouse striatum. Neuroscience. 2009 doi: 10.1016/j.neuroscience.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 13.Johnson MB, Jin K, Minami M, Chen D, Simon RP. Global ischemia induces expression of acid-sensing ion channel 2a in rat brain. J Cereb Blood Flow Metab. 2001;21:734–740. doi: 10.1097/00004647-200106000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Kalivas PW, Hu XT. Exciting inhibition in psychostimulant addiction. Trends Neurosci. 2006;29:610–616. doi: 10.1016/j.tins.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Review. 2002;82:735–767. doi: 10.1152/physrev.00007.2002. [DOI] [PubMed] [Google Scholar]
- 16.Krishtal O. The ASICs: signaling moleculars? modulators? Trends Neurosci. 2003;26:477–483. doi: 10.1016/S0166-2236(03)00210-8. [DOI] [PubMed] [Google Scholar]
- 17.Lingueglia E, de Weille JR, Bassilana F, Heurteaux C, Sakai H, Waldmann R, Lazdunski M. A modulatory subunit of acid sensing ion channels in brain and dorsal root ganglion cells. J Biol Chem. 1997;272:29778–29783. doi: 10.1074/jbc.272.47.29778. [DOI] [PubMed] [Google Scholar]
- 18.Liu XY, Chu XP, Mao LM, Wang M, Lan HX, Li MH, Zhang GC, Parelkar NK, Fibuch EE, Haines M, Neve KA, Liu F, Xiong ZG, Wang JQ. Modulation of D2R-NR2B interactions in response to cocaine. Neuron. 2006;52:897–909. doi: 10.1016/j.neuron.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 19.Pignataro G, Simon RP, Xiong ZG. Prolonged activation of ASIC1a and the time window for neuroprotection in cerebral ischemia. Brain. 2007;130:151–158. doi: 10.1093/brain/awl325. [DOI] [PubMed] [Google Scholar]
- 20.Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature. 1997;386:173–177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- 21.Waldmann R, Lazdunski M. H+-gated cation channels: neuronal acid sensors in the ENaC/DEG family of ion channels. Curr Opin Neurobiol. 1998;8:418–424. doi: 10.1016/s0959-4388(98)80070-6. [DOI] [PubMed] [Google Scholar]
- 22.Wemmie JA, Askwith CC, Lamani E, Cassell MD, Freeman JH, Jr, Welsh MJ. Acid-sensing ion channel 1 is localized in brain regions with high synaptic density and contributes to fear conditioning. J Neurosci. 2003;23:5496–5502. doi: 10.1523/JNEUROSCI.23-13-05496.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wemmie JA, Chen J, Askwith CC, Hruska-Hageman AM, Price MP, Nolan BC, Yoder PG, Lamani E, Hoshi T, Freeman JH, Jr, Welsh MJ. The acid-activated ion channel ASIC contributes to synaptic plasticity, learning, and memory. Neuron. 2002;34:463–477. doi: 10.1016/s0896-6273(02)00661-x. [DOI] [PubMed] [Google Scholar]
- 24.Wemmie JA, Price MP, Welsh MJ. Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci. 2006;29:578–586. doi: 10.1016/j.tins.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 25.Wong HK, Bauer PO, Kurosawa M, Goswami A, Washizu C, Machida Y, Tosaki A, Yamada M, Knöpfel T, Nakamura T, Nukina N. Blocking acid-sensing ion channel 1 alleviates Huntington's disease pathology via an ubiquitin-proteasome system-dependent mechanism. Hum Mol Genet. 2008;17:3223–3235. doi: 10.1093/hmg/ddn218. [DOI] [PubMed] [Google Scholar]
- 26.Xiong ZG, Chu XP, Simon RP. Ca2+-permeable acid-sensing ion channels and ischemic brain injury. J Membr Biol. 2006;209:59–68. doi: 10.1007/s00232-005-0840-x. [DOI] [PubMed] [Google Scholar]
- 27.Xiong ZG, Pignataro G, Li M, Chang SY, Simon RP. Acid-sensing ion channels (ASICs) as pharmacological targets for neurodegenerative diseases. Curr Opin Pharmacol. 2008;8:25–32. doi: 10.1016/j.coph.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wemmie JA, Price M, Welsh MJ, Simon RP. Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell. 2004;118:687–698. doi: 10.1016/j.cell.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 29.Yermolaieva O, Leonard AS, Schnizler MK, Abboud FM, Welsh MJ. Extracellular acidosis increases neuronal cell calcium by activating acid-sensing ion channel 1a. Proc Natl Acad Sci U S A. 2004;101:6752–6757. doi: 10.1073/pnas.0308636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ziemann AE, Schnizler MK, Albert GW, Severson MA, Howard MA, Welsh MJ, Wemmie JA. Seizure termination by acidosis depends on ASIC1a. Nat Neurosci. 2008;11:816–822. doi: 10.1038/nn.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]


