Abstract
Approximately 14 of the more than 1,000 species of microsporidia infect humans, only 2 of which, Enterocytozoon bieneusi and Encephalitozoon intestinalis, cause intestinal microsporidiosis. Clinical isolates of three microsporidia species, E. intestinalis, Encephalitozoon hellem, and the insect parasite, Anncaliia (Brachiola, Nosema) algerae were used in a spore germination assay, and enterocyte attachment and infection assays to model the potential roles of gastric and duodenal environments, and host temperature in determining why only one of these microsporidia species causes intestinal microsporidiosis. Enterocyte infection with A. algerae spores was 10% that of the Encephalitozoon species, a difference not attributable to differences in spore attachment to host cells. Prior spore treatment with pepsin in HCl, pancreatic enzymes or ox bile did not inhibit germination or enterocyte infection by the three microsporidia species. While the Encephalitozoon species differentiated to mature spores within 3 days, the time taken for many enterocytes to turn over, A. algerae took 3 - 5 days to produce mature spores, near the upper limit for enterocyte turnover in vivo. Thus host temperature may contribute to A. algerae not causing human intestinal microsporidiosis, but none of the factors tested account for the inability of E. hellem to cause such an infection.
INTRODUCTION
Approximately 14 species of microsporidia infect humans (Didier, 2005). Microsporidia spores from several of these species are ubiquitous in the environment and have been found in potential drinking water and food sources (Cotte et al, 1999; Dowd et al, 1998; Graczyk et al, 2004; Kwakye-Nuako et al, 2007; Graczyk et al, 2008). However, only two species, Enterocytozoon bieneusi and Encephalitozoon intestinalis, appear to infect the gastrointestinal tract causing significant diarrhea and wasting, particularly in AIDS patients (Kotler and Orenstein, 1998). Franzen and colleagues (2005) found that spores from several species could infect human intestinal epithelial cells in culture, although only one of the tested species causes intestinal disease, and they concluded that the route of administration was a major factor in determining host cell organ specificity.
There are many examples of spores of various microsporidia species readily infecting human and other mammalian cell lines (e.g. Visvesvara et al, 1999; Mathis et al 2005). The present study used three microsporidia species isolated from human infections. The species used were the second most common cause of intestinal disease, E. intestinalis (Kotler and Orenstein, 1998), Encephalitozoon hellem, a cause of ocular and disseminated infections, not generally considered a cause of intestinal disease (Didier, 2005), and Anncaliia (Brachiola, Nosema) algerae, an insect parasite,(Undeen, 1975), that has caused several human infections (Moura et al, 1999; Coyle et al, 2004; Visvesvara et al, 2005). In preliminary studies we found that all three microsporidia species readily infect human Caco2 cells in culture, yet clinically only E. intestinalis appears to cause intestinal microsporidiosis.
Infection by obligate intracellular microsporidia parasites appears to occur in one of two ways. The spore may deploy a tube, impaling a nearby target cell, and then inject its content of infectious sporoplasm, into the host cell. Deployment of the tube is known as germination. Alternatively a spore may be phagocytized by a host cell and intracellular germination follows to cause infection (Franzen, 2004). While phagocytosis is probably favored in phagocytic and perhaps poorly differentiated cells (e.g. Couzinet at al, 2000), impalement by a deployed polar tube seems more likely as the method of infection in differentiated intestinal epithelial cells (Leitch et al, 2005). Spore adherence to target cell glycosaminoglycans also correlates with infection (Hayman et al, 2005; Southern et al, 2007). For these reasons we used a spore germination assay, and a host cell attachment assay and an infection assay using differentiated human intestinal epithelial cells to model the role of host factors in intestinal microsporidiosis.
MATERIAL AND METHODS
Microsporidia culture and spore isolation
Isolates of E. intestinalis and E. hellem were obtained from American Type Culture Collection (ATCC 50651 and 50451 respectively), Manassas, VA, while an isolate of A. algerae (CDC:V422) was obtained from the Centers for Disease Control and Prevention, Atlanta, GA. The microsporidia cultures were maintained in green monkey, Vero, E6 cells in a 37°C CO2 (5%) incubator as described elsewhere (Visvesvara et al, 1999), except in the case of the A. algerae cultures which were maintained at 33°C. The Anncalia cultures were maintained at the lower temperature to provide optimal spore yield and because we have found that this human isolate of A. algerae cultured at 30°C readily germinates in vitro and infected mammalian cells maintained at 37°C (Kucherova et al, 2004). Spores were purified as described previously (Leitch et al, 2005), maintained in water at 4°C and used within 2 weeks of isolation.
Germination assay
In all germination assays purified spores samples were fixed for the measurement of baseline (0 time) germination, while other samples from the same stock of spores were incubated in a test solution for 15 min at 37°C, washed by centrifugation where indicated, incubated in a germination buffer at 37°C for an additional 30 min, and then fixed with 5% neutral formalin. Fixed spore samples were then placed on chambered coverslip slides, the spores allowed to settle overnight, and the percentage of germinated spores determined by phase contrast microscopy as described previously (Leitch et al, 1993). The germination solution used for all three microsporidia species was 140 mM NaCl, buffered to pH 7.5 with 20 mM Hepes to which was added H2O2 to a final concentration of 5%. In one A. algerae experiment a second germination solution was also used consisting of 140 mM NaCl, buffered to pH 9.5 with 20 mM glycine (Frixione et al, 1997)
The test solutions used were PBS (control), 100 mM HCl, HCl containing 1 mg/ml hog pepsin (EC3 4.23.1), PBS containing 1 mg/ml pancreatin (EC 232.468.9), or PBS containing 1 or 10 mg/ml ox bile, all obtained from Sigma Chemical Co. (St. Louis, MO) Following 15 minutes exposure at 37°C, the control and treated spore samples were washed twice at room temperature in PBS by centrifugation. The same washing conditions were used with all samples to control for any effect of washing and/or centrifugation on germination. The samples were then incubated in the germination solution for 30 min at 37°C prior to fixation.
Infection assay
The Caco2 clone, C2Bbel (ATCC CRL-2102), was used in all infection assays. Cells were cultured on collagen coated chamber slides or 1 cm2 transwells and maintained at 37°C in DMEM medium supplemented with 10μg/ml human transferrin and 10% fetal bovine serum. At 7 days post-confluence, by which time cells were significantly differentiated, monolayers were infected with 4 × 105 spores per well of the 8 well-chamber slides or 106 spores per transwell. The slides were used for the estimation of infection using a calcofluor staining method (Didier et al, 1995), while the transwells were used for transmission electron microscopy. Spores were used without pretreatment or following pretreatments that mimicked gastric and duodenal environments as above before being used in the infection assays. Twenty-four hours post infection (PI) the monolayers were washed twice with Opti-MEM containing 1mg/ml chondroitin sulfate to remove spores that had attached to the monolayer but had not yet germinated and infected the host cells (Hayman et al, 2005; Leitch et al, 2005). This first wash was allowed to remain in the wells for 30 minutes and a second wash for 10 minutes. The infected wells were then refilled with medium and 48 hours later (3 days PI) the medium removed and the cells prepared for calcofluor staining.
Calcofluor staining
Cells fixed with 3% paraformaldehyde for 10 minutes were washed 3 X with PBS, exposed to 0.1% triton X100 in PBS for 2 minutes, and washed again 3 X with PBS. Calcofluor (Fluorescent Brightner 28, Sigma Chemical Co, St. Louis, MO) was then used to identify parasite spores (Didier et al, 1995). Calcofluor (50 μl, 1mg/ml in water) was added to each well for 2 minutes, and the wells were again washed 2 X with PBS before adding 0.1% Evans blue in PBS. After 2 additional washes with PBS, 50 μl propidium iodide (1mg/ml water) was added to each well. The propidium iodide staining was used to localize host cell and intracellular parasite nuclei. The wells were then washed 2 X with PBS, allowed to air dry, and coverslips were mounted with Fluoromount-G (Southern Biotech, Birmingham, AL). An Olympus BX41TF microscope with Microfire Digital camera was used to capture images using Picture Frame software, and the number of infected cells counted in randomly chosen 2,300μm2 fields. Because of chamber wall edge effects, the areas adjacent to the site of the chamber slide scaffolding were avoided.
Electron microscopy
Transwells were fixed by emersion of both apical and basolateral surfaces in 4°C, 2.5% glutaraldehyde, buffered to pH 7.4 with 0.1M cacodylate buffer. Preparations were processed as described previously (Visvesvara et al, 1991). All sections were cut at right angles to the transwell surface.
Spore attachment
Spore attachment was assessed in differentiated C2Bbe1 cells by exposing 7 day post-confluent cultures to 4 × 105 spores per chamber of a chamber slide for a period of 4 hours (Leitch et al, 2005). The monlayers were then fixed with 3% paraformaldehyde. The cells were not subsequently permeabilized and spores attached to the apical cell surface were visualized using calcofluor staining.
Statistial evaluation
In germination assays treatment effects were calculated using the percent germination in a sample after correcting for any pretreatment germination (Leitch et al, 1993), comparing pairs of treated and control samples, both from the same stock of purified spores. A minimum of 4 replicate experiments were performed for each treatment. Differences between control and treatment means were evaluated using paired Student t tests.
In the case of infection assays an average of 15 fields were evaluated for the number of infected cells and the significance of differences between means determined by one-way ANOVA of repeated measures using aggregate data sets. Post hoc analyses were performed using Tukey’s tests, and p values of <0.05 were considered significant.
RESULTS
Spore attachment and infection
Figure 1 illustrates the relationship between spore attachment and infection of C2Bbe1 cells with the three microsporidia species under study. Infection was significantly less (∼10%) in the case of A. algerae 3 days PI when compared with either of the Encephalitozoon species. However, this effect did not appear to be due to a difference in spore adherence as there was no significant difference between the three species in their spore attachment to the target cells measured at 4 h PI.
Figure 1.
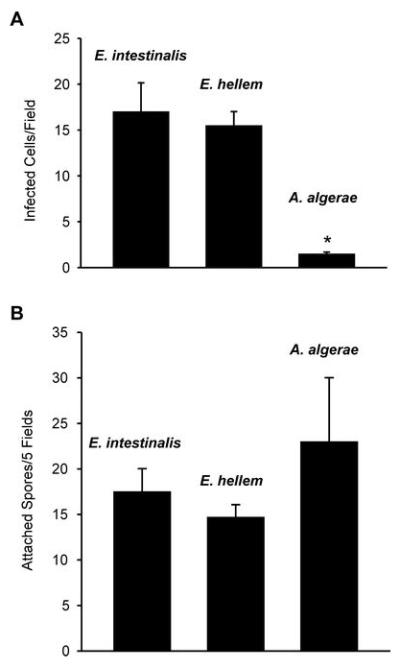
A. Number of infected C2Bbe1 cells per field 3 days PI with spores of E. intestinalis, E. hellem or A. algerae. B. Attachment of E. intestinalis, E.hellem and B. algerae spores to differentiated C2Bbe1 cells 4 hours after inoculation. Means ± SEM. (N=4). * Significantly different from the other two parasite species, p < 0.05.
Gastric and duodenal environment effects
Exposure to HCl, with or without pepsin, a mixture of pancreatic enzymes, or ox bile did not inhibit H2O2-stimulated spore germination in any of the three parasite species used here (Table 1). Similarly, prior exposure to pepsin in 100 mM HCl, pancreatin or ox bile did not reduce infection of differentiated C2Bbe1 cells (Figure 2).
Table 1.
Effects of prior exposure to HCl in the presence or absence of pepsin, pancreatic enzymes in PBS, or bile in PBS on microsporidia spore germination.
| E. intestinalis | E. hellem | B. algerae | |
|---|---|---|---|
| HCl, 100m M | -1.3 ± 2.9a | 3.1 ± 3.3 | 1.1 ± 2.9 |
| HCl/pepsin, 1 mg/ml | -2.0 ± 6.8 | 0.3 ± 0.7 | 4.1 ± 5.8 |
| Pancreatin, 1 mg/ml | 6.7 ± 0.8b | 10.6 ± 5.4 | 16.2 ± 2.8b |
| Ox bile, 1 mg/ml | 6.0 ± 5.5 | 0.8 ± 2.1 | -1.3 ± 1.6 |
| Ox bile, 10 mg/ml | 10.2 ± 10.7 | 7.0 ± 4.9 | 2.2 ± 4.7 |
Results are expressed as percentage difference from the appropriate control replicate experiments.
Means ± SEM or 4 replicate experiments.
Significantly different from control, p<0.05.
Figure 2.
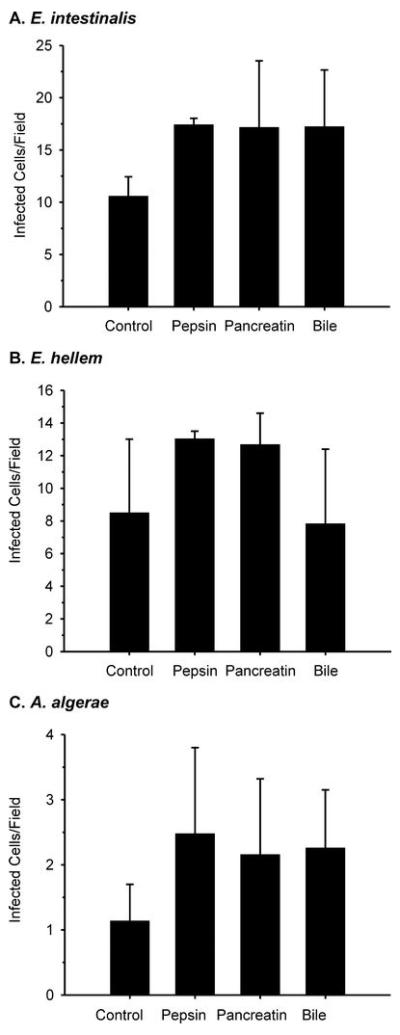
Number of infected differentiated C2Bbe1 cells per field 3 days after inoculation with E. intestinalis, E. hellem or A. algerae spores that had been exposed to PBS (control), 1 mg/ml pepsin in 100 mM HCl, 1 mg/ml pancreatin in PBS, or 10 mg/ml ox bile in PBS . Means ± SEM. (N=3)
Effect of temperature on microsporidia infection of enterocytes
Transmission electron micrographs of infected, differentiated C2Bbe1 cells grown on transwells are shown in figure 3. Encephalitozoon-infected cells were fixed 3 days PI and A. algerae-infected cells were fixed at 3 and 5 days PI. When the cultures were maintained at 37°C mature spores were frequently evident within parasitophorous vacuoles in both E. intestinalis and E. hellem infected cells (Figure3 A and B). However we were unable to detect mature A. algerae spores in 3 day 37°C cultures, while by 5 days cells were observed containing multiple empty (germinated) spores (Figure 3 C). Such infected host cells were more vacuolated than were Encephalitozoon-infected cells. When A. algerae-infected cells were maintained at 33°C for 3 days sporogonial stages were evident and there were examples of intracellular spore germination indicating that differentiation occurred more rapidly with this species at the lower temperature (Figure 3D and insert).
Figure 3.
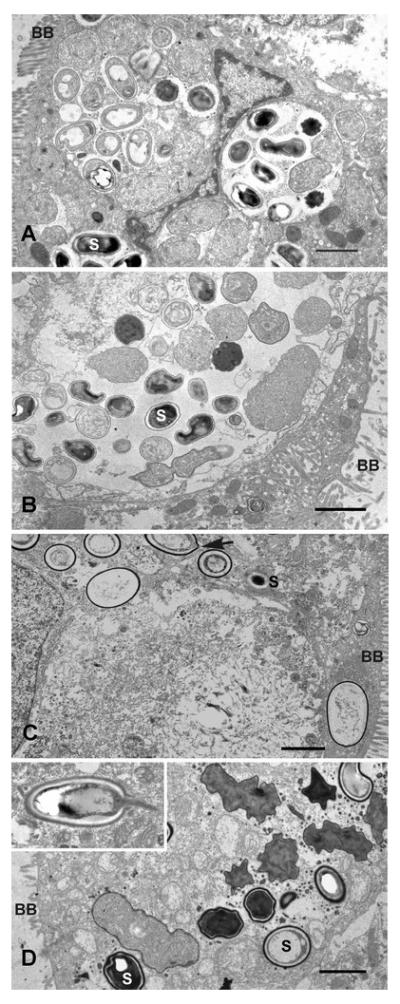
A, B. Transmission electron microscopic appearance of C2Bbe1 cells cultured on transwells at 37°C, 3 days PI. A. Several parasitophorous vacuoles containing E. intestinalis developing stages and matures spores (S). Brush border (BB). B. Large parasitophorous vacuole containing E. hellem developing stages and mature spores. C. Empty A. algerae spores in cells 5 days PI, cultured at 37°C. Arrow indicates a site near polar tube extrusion. D. A. algerae developing stages and mature spores in 33°C culture, 3 days PI. Germinating intracellular spore (inset). Bars, 2μm.
A similar infection experiment was conducted using C2Bbe1 cultures on chamber slides employing a calcofluor stain that identifies the chitinous spore coat of sporogonial stages (Didier et al, 1995). With the calcofluor staining method it was possible to distinguish and count the larger individual intracellular A. algerae spores as these are separate within the host cell cytoplasm and not aggregated within parasitophorous vacuoles, as is the case with the Encephalitozoon microsporidia.. Figure 4 illustrates both the number of A. algerae-infected cells per field and the number of spores per field when C2Bbe1 cells were maintained at 33°C and at 37°C for 3 and 5 days PI. There was a significant increase in both parameters as the incubation continued from 3 to 5 days, and at each period both the number of infected cells and the number of spores were significantly greater in the 33°C cultures than in the 37°C cultures. The spore count included all parasite stages that stained positive with calcofluor and is thus probably an overestimation of mature, infectious spores. On the other hand the low incidence of A. algerae mature spores in 37°C cultures makes it likely that they might be missed with the sampling constraints of transmission electron microscopy. Nevertheless the calcofluor and transmission electron microscopic data indicate that at mammalian body temperature there is a lower likelihood of A. algerae completing its life cycle to infective mature spores within the life span of the target enterocytes than is the case with either of the Encephalitozoon species.
Figure 4.
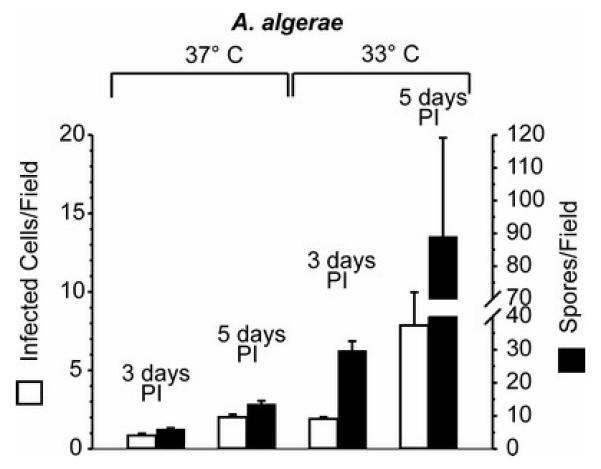
Number of infected cells (open bars) and number of spores (closed bars) detected in fields of C2Bbe1 cell cultures 3 and 5 days after inoculation with A. algerae spores. Cultures were maintained at 33°C or 37°C. Means ± SEM. (N=4).
Effect of temperature on A. algerae spore germination
Anncaliia algerae infection could also have been reduced in the 37°C cultures by a delay or reduction of spore germination. Figure 5 shows that this possibility is unlikely as in vitro spore germination was stable over the temperature range employed in the infection assays, using two different germination media.
Figure 5.
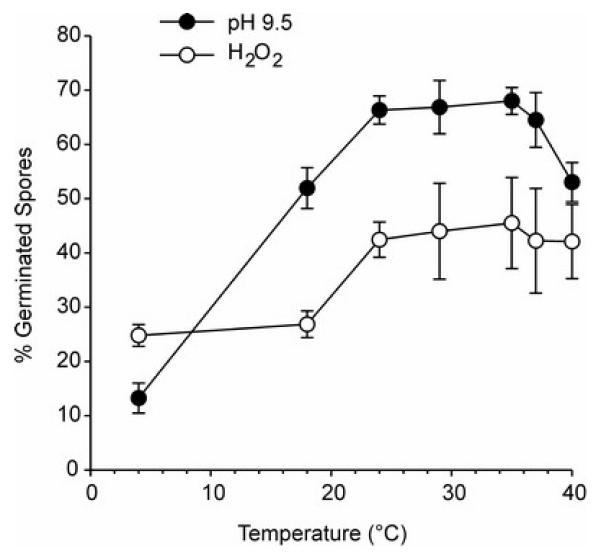
Effect of incubation temperature on the germination of A. algerae spores. Spores were stimulated to germinate using a H2O2 germinating solution or a pH 9.5 germinating solution. Means ± SEM. (N = 4).
DISCUSSION
Microsporidia spores have been recovered from surface water and drinking water sources (Dowd et al, 1998; Cotte et al, 1999). Fecal-oral transmission and person-person transmission have been implicated in microsporidiosis and the gastrointestinal tract and lungs would seem the most probable sites for infection (Mathis et al, 2005). The present study was designed to determine what host gastrointestinal factors contribute to determining why the three microsporidia species under study can all infect enterocytes in culture, but have such different organ specificity in vivo.
In humans, intestinal microsporidiosis is generally limited to two species, Enterocytozoon bieneusi and E. intestinalis (Kotler and Orenstein, 1998; Didier, 2005). Encephalitozoon hellem causes enteritis in birds (Mathis et al, 2005), but is not generally associated with human intestinal microsporidiosis, with the possible exception of two diarrhea cases in related individuals who were HIV negative and were co-infected with Enterocytozoon bieneusi (Muller at al, 2001).
Brachiola (Nosema) algerae has recently been reassigned to the genus Anncaliia (Franzen et al, 2006). This species is probably ubiquitous in the environment (Avery and Undeen, 1987) and has been detected in three clinical cases to date (Coyle et al, 2004:Visvesvara at al, 2005), none involving the gastrointestinal tract It was previously thought that this insect parasite could not infect mammals because of their higher body temperature. In addition to the clinical cases cited above there have been multiple reports of infection of mammalian cell lines and mammalian animal models by this species (Undeen, 1975; Undeen and Alger, 1976; Trammer et al, 1997; Moura et al, 1999; Lowman et al, 2000; Koudela et al, 2001). However spores from clinical isolates were significantly more infectious in mammalian cells cultured at 37°C than were spores from insect isolates (Kucerova et al, 2004).
In this study A. algerae spores infected significantly fewer cultured differentiated intestinal epithelial cells than spores of either of the Encephalitozoon species tested (Figure 1A). Microsporidia spores have been shown to express a protein, EnP1, on the spore coat, particularly in the area of the anchoring disc (Southern et al, 2007), that mediates spore attachment to target cell glycosaminoglycans (Hayman et al, 2005), and there appears to be a direct correlation between such spore attachment and infection. Thus a lower incidence of spore attachment is a potential explanation for the lower infection rate seen with A. algerae spores. However, as seen in Figure 1B there were no differences in spore attachment to C2Bbe1 cells among the three species tested.
When we modeled the effects of the gastric and duodenal environments on microsporidia spore germination and enterocyte infection we found no major antimicrosporidial effects with any of the three parasite species (Table 1, Figure 2). In fact there were modest but significant increases in E. intestinalis and A. algerae spore germination following pancreatin pretreatment, and a trend to increased enterocyte infection will all three species following spore exposure to pepsin, bile or pancreatin.
For a microsporidia parasite to successfully maintain an infection of the intestinal epithelium it must be able to complete its life cycle to the point of producing infectious mature spores before the infected epithelial cell turns over, a period of approximately 3-6 days in the intestine depending on species, location, age, nutritional status and luminal flora (e.g. Lipkin, 1987; Jones and Gore, 1997). Alternatively the host could continuously consume spores. In an in vitro murine model E. intestinalis has been shown to be able to complete its life cycle well within this time frame (Wasson and Barry, 2003). Using transmission electron microscopy of transwell-cultured C2Bbe1 cells maintained at 37°C we were frequently able to detect all parasite stages, including mature spores, in both E. intestinalis- and E. hellem-infected cells 3 days after spore inoculation (Figure 3). While we were unable to find mature A. algerae spores, by 5 days PI there was evidence that intracellular spores had germinated. This contrasts with 33°C A. algerae-infected cells in which empty and germinating intracellular spores were seen by 3 days PI.
When a calcofluor stain was used to monitor infection both the number of infected cells and the number of spores increased from 3 to 5 days PI and were always greater in 33°C cultures than 37°C cultures. The calcofluor stain did not permit distinguishing between mature spores and other sporogonial stages that were establishing a chitinous spore coat (Didier et al, 1995), but these experiments were not subject to the sampling limitations of the transmission electron microscopy. The fact that the number of A. algerae-infected cells increased with time can be explained by the fact that the calcofluor assay only detected sporgony stages, thus underestimating the number of infected cells but overestimating the number of mature spores. Taken together with the electron microscopy data the cacofluor experiments suggest that A. algerae differentiation to mature spores is temperature dependent and, while the life cycle may be completed by 3 – 5 days PI in 37°C cultures, host temperature would be expected to limit the ability of this species to cause intestinal microsporidiosis. This is consistent with other reports (Lowman et al, 2000; Koudela et al, 2001; Kucherova et al, 2004) suggesting that mammalian body temperature slows differentiation in this parasite species. There is no reason to believe that A. algerae spore germination was less in the 37°C cultures than in the 33°C cultures as in vitro germination was stable over a wide temperature range (Figure 5).
This study suggests that gastric, pancreatic and hepatic secretions do not play a major role in determining if an ingested microsporidia spore will infect the intestinal epithelium. The lower infection rate of A. algerae spores could not be attributed to a lower attachment of spores to host cells in culture However, the lower probability of this parasite completing its life cycle before target enterocytes turn over may contribute to the fact that this species has not been implicated in intestinal microsporidiosis. Encephalitozoon intestinalis and E. hellem were both able to complete their life cycles in enterocytes within 3 days, suggesting that other factors are responsible for the fact that only the former Encephalitozoon species is important in infecting the human gastrointestinal tract.
ACKNOWLEDGEMENTS
This work was supported by U.S. PHS grant R21 DK64573-A3.
REFERENCES
- Avery SW, Undeen AH. The isolation of microsporidia and other pathogens from concentrated ditch water. J Am Mosq Control Assoc. 1987;3:54–58. [PubMed] [Google Scholar]
- Cotte L, Rabodonirina M, Chapuis F, Bailly F, Bissuel F, Raynal C, Gelas P, Persat F, Piens M-A, Trepo C. Waterborne outbreak of intestinal microsporidiosis in persons with and without human immunodeficiency virus infection. J Infect Dis. 1999;180:2003–2008. doi: 10.1086/315112. [DOI] [PubMed] [Google Scholar]
- Couzinet S, Cejas E, Schittny J, Deplazes P, Weber R, Zimmerli S. Phagocytic uptake of Encephalitozoon cuniculi by nonprofessional phagocytes. Infect Immun. 2000;68:6939–6945. doi: 10.1128/iai.68.12.6939-6945.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle CM, Weiss LM, Rhodes LV, Cali A, Takvorian PM, Brown DF, Visvesvara GS, Xiao L, Naktin J, Young E, Gareca M, Colasante G, Wittner M. Fatal myositis due to the microsporidia Brachiola algerae, a mosquito pathogen. N Engl J Med. 2004;351:42–47. doi: 10.1056/NEJMoa032655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didier ES. Microsporidiosis: and emerging and opportunistic infection in humans and animals. Acta Trop. 2005;94:61–76. doi: 10.1016/j.actatropica.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Didier ES, Orenstein JM, Aldras A, Bertucci D, Rogers LB, Janney FA. Comparison of three staining methods for detecting microsporidia in fluids. J Clin Microbiol. 1995;33:3138–3145. doi: 10.1128/jcm.33.12.3138-3145.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd SE, Gerba CP, Pepper IL. Confirmation of human-pathogenic microsporidia Enterocytozoon bieneusi, Encephalitozoon intestinalis, and Vittaforma corneae in water. Appl Environ Microbiol. 1998;64:3332–3335. doi: 10.1128/aem.64.9.3332-3335.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzen C. Microsporidia: how can they invade other cells? Trends Parasitol. 2004;20:275–279. doi: 10.1016/j.pt.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Franzen C, Hosl M, Salzberger B, Hartmann P. Uptake of Encephalitozoon spp. and Vittaforma corneae (microsporidia) by different cells. J Parasitol. 2005;91:745–749. doi: 10.1645/GE-468R.1. [DOI] [PubMed] [Google Scholar]
- Franzen C, Nassanova ES, Scholmerich J, Issi IV. Transfer of the members of the genus Brachiola (Microsporidia) to the genus Anncaliia based on ultrastructural and molecular data. J Eukaryot Microbiol. 2006;53:26–35. doi: 10.1111/j.1550-7408.2005.00066.x. [DOI] [PubMed] [Google Scholar]
- Frixione E, Ruiz L, Cerbon J, Undeen AH. Germination of Nosema algerae (Microspora) spores: conditional inhibition by D2O, ethanol and Hg2+ suggests dependence of water influx upon membrane hydration and specific transmembrane pathways. J Eukaryot Microbiol. 1997;44:109–116. doi: 10.1111/j.1550-7408.1997.tb05946.x. [DOI] [PubMed] [Google Scholar]
- Graczyk TK, Conn DB, Lucy F, Minchin D, Tamang L, Moura LN, DaSilva AJ. Human waterborne parasites in zebra mussels (Dreissena polymorpha) from the Shannon River drainage area, Ireland. Parasitol Res. 2004;93:385–391. doi: 10.1007/s00436-004-1142-4. [DOI] [PubMed] [Google Scholar]
- Graczyk TK, Majewska AC, Schwab KJ. The role of birds in dissemination of human waterborne enteropathogens. Trends Parasitol. 2008;24:55–59. doi: 10.1016/j.pt.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Hayman JR, Southern TR, Nash TE. Role of sulfated glycans in adherence of the microsporidian Encephalitozoon intestinalis to host cells in vitro. Infect Immun. 2005;73:841–848. doi: 10.1128/IAI.73.2.841-848.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BA, Gore GJ. Physiology and pathophysiology of apoptosis in epithelial cells of the liver, pancreas and intestine. Am J Physiol. 1997;273:G1174–G1188. doi: 10.1152/ajpgi.1997.273.6.G1174. [DOI] [PubMed] [Google Scholar]
- Kotler DP, Orenstein JM. Clinical syndromes associated with microsporidiosis. Adv Parasitol. 1998;40:321–341. doi: 10.1016/s0065-308x(08)60126-8. [DOI] [PubMed] [Google Scholar]
- Koudela B, Visvesvara GS, Moura H, Vavra J. The human isolate of Brachiola algerae (Phylum Microsporidia): development in SCID mice and description of its fine structure features. Parasitol. 2001;123:153–162. doi: 10.1017/s0031182001008228. [DOI] [PubMed] [Google Scholar]
- Kucerova Z, Moura H, Visvesvara GS, Leitch GJ. Differences between Brachiola (Nosema) algerae isolates of human and insect origin when tested using an in vitro spore germination assay and a cultured cell infection assay. J Eukaryot Microbiol. 2004;51:339–343. doi: 10.1111/j.1550-7408.2004.tb00577.x. [DOI] [PubMed] [Google Scholar]
- Kwakye-Nuako G, Borkettey P, Mansah-Attipoe I, Asmah R, Ayeh-Kumi P. Sachet drinking water in Accra: the potential threats of transmission of enteric pathogenic protozoan organisms. Ghana Med J. 2007;41:62–67. doi: 10.4314/gmj.v41i2.55303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitch GJ, He Q, Wallace S, Visvesvara GS. Inhibition of the spore polar filament extrusion of the microsporidium, Encephalitozoon hellem, isolated from an AIDS patient. J Eukaryot Microbiol. 1993;40:711–717. doi: 10.1111/j.1550-7408.1993.tb04463.x. [DOI] [PubMed] [Google Scholar]
- Leitch GJ, Ward TL, Shaw AP, Newman G. Apical spore phagocytosis is not a significant route of infection of differentiated enterocytes by Encephalitozoon intestinalis. Infect Immun. 2005;73:7697–7704. doi: 10.1128/IAI.73.11.7697-7704.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkin M. Proliferation and differentiation of normal and diseased gastrointestinal cells. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. Raven Press; New York, NY: 1987. pp. 255–284. [Google Scholar]
- Lowman PM, Takvorian PM, Cali A. The effects of elevated temperature and various time-temperature combinations on the development of Brachiola (Nosema) algerae N. Comb. in mammalian cell culture. J Eukaryot Microbiol. 2000;47:221–234. doi: 10.1111/j.1550-7408.2000.tb00041.x. [DOI] [PubMed] [Google Scholar]
- Mathis A, Weber R, Deplazes P. Zoonotic potential of the microsporidia. Clin Microbiol Rev. 2005;18:423–445. doi: 10.1128/CMR.18.3.423-445.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura H, da Silva AJ, Moura IN, Schwartz DA, Leitch GJ, Wallace S, Pieniazek NJ, Wirtz RA, Visvesvara GS. Characterization of Nosema algerae isolates after continuous cultivation in mammalian cells at 37°C. J Eukaryot Microbiol. 1999;46:14S–16S. [PubMed] [Google Scholar]
- Muller A, Bialek R, Kamper A, Fatkenheuer G, Salzberger B, Franzen C. Detection of microsporidia in travelers with diarrhea. J Clin Microbiol. 2001;39:1630–1632. doi: 10.1128/JCM.39.4.1630-1632.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern TR, Jolly CE, Lester ME, Hayman JR. EnP1, a microsporidian spore wall protein that enables spores to adhere to and infect host cells in vitro. Eukaryotic Cell. 2007;6:1354–1362. doi: 10.1128/EC.00113-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trammer T, Dumbrowski F, Doehring M, Maier WA, Seitz HM. Opportunistic properties of Nosema algerae (Microspora), a mosquito parasite, in immunocompromised mice. J Eukaryot Microbiol. 1997;44:258–262. doi: 10.1111/j.1550-7408.1997.tb05709.x. [DOI] [PubMed] [Google Scholar]
- Undeen AH. Growth of Nosema algerae in pig kidney cell culture. J Protozool. 1975;22:107–110. doi: 10.1111/j.1550-7408.1975.tb00951.x. [DOI] [PubMed] [Google Scholar]
- Undeen AH, Alger N. Nosema algerae: infection of the white mouse by a mosquito parasite. Exp Parasitol. 1976;40:86–88. doi: 10.1016/0014-4894(76)90068-0. [DOI] [PubMed] [Google Scholar]
- Visvesvara GS, Leitch GJ, Moura H, Wallace S, Weber R, Bryan RT. Culture, electron microscopy, and immunoblot studies on a microsporidian isolated from the urine of a patient with AIDS. J Protozool. 1991;38:105S–111S. [PubMed] [Google Scholar]
- Visvesvara GS, Moura H, Leitch GJ, Schwartz DA. Culture and propagation of microsporidia. In: Whittner M, Weiss LM, editors. The Microsporidia and Microsporidiosis. ASM Press; Washington, D.C.: 1999. pp. 363–392. [Google Scholar]
- Visvesvara GS, Moura H, Leitch GJ, Schwartz DA, Xiao LX. Public health importance of Brachiola algerae (Microsporidia) — an emerging pathogen of humans. Folia Parasitol (Praha) 2005;52:83–94. doi: 10.14411/fp.2005.011. [DOI] [PubMed] [Google Scholar]
- Wasson K, Barry PA. Molecular characterization of Encephalitozoon intestinalis (Microsporidia) replication kinetics in a murine intestinal cell line. J Eukaryot Microbiol. 2003;50:169–174. doi: 10.1111/j.1550-7408.2003.tb00112.x. [DOI] [PubMed] [Google Scholar]


