Abstract
Objective
To examine the association of plasma lipid levels to changes in cognitive function in the elderly without dementia.
Methods
We examined changes in performance in tests of memory, visuospatial/cognitive and language abilities in 1147 elderly individuals without dementia or cognitive impairment at baseline followed for seven years using generalized estimating equations.
Results
Performance in all cognitive domains declined significantly over time, while there was no association between levels of any plasma lipid or lipid lowering treatment and memory, cognitive/visuospatial or language performance at any interval. Higher age at baseline was related to lower scores in all three domains at each interval, while higher education and Caucasian ethnicity were associated with higher scores in all domains. Analyses relating plasma lipids to performance in color trails tests using proportional hazards regression showed no association.
In subsequent analyses excluding subjects with incident dementia, memory performance declined over time, while cognitive/visuospatial and language performance did not. Higher plasma HDL and total cholesterol were associated with higher scores in language performance at baseline; this domain declined faster among individuals with higher total cholesterol, but this result was not significant after taking multiple comparisons into account. Plasma triglycerides, LDL, or treatment with lipid lowering agents were not associated to changes in cognitive performance.
Conclusions
Plasma lipid levels or treatment with lipid lowering agents in the elderly were not associated with changes in cognitive function.
Keywords: plasma lipids, memory performance, cognitive performance
INTRODUCTION
Dyslipidemia and dementia are among the most common diseases in western societies. About 1 percent of people aged 65–69 years develop dementia, and the prevalence increases to more than 60 percent for people over the age of 95 (1). More than 50 percent of the US population age 20 years or older suffer from cholesterol 200 mg/dl or higher, and more than 18 percent show cholesterol levels equal to or over 240 mg/dl (2). There is conflicting data showing that dyslipidemia, a modifiable risk factor, is associated with a higher risk of cognitive impairment or dementia. Reduced high-density lipoprotein cholesterol (HDL-C) (3–9) and apolipoprotein A-1 levels (3), as well as increased levels of lipoprotein (a) (5) have been observed in dementia in some but not all studies. There also have been contradictory results in studies relating total cholesterol (10, 11) and low-density lipoprotein cholesterol (LDL-C) (6, 8, 11) to dementia.
Interest in these relationships has been increased by the observation that the use of widely available lipid lowering agents, HMG-COA-reductase-inhibitors (statins), may be associated with a lower risk of dementia (12). In addition, cholesterol alters the degradation of the amyloid precursor protein (APP), which plays a major role in the pathogenesis of Alzheimer’s disease (AD) (13). Moreover, vascular disease, which is associated with dyslipidemia, may be related to the risk of cognitive decline (14, 15) and dementia. We previously reported an association between high levels of total and LDL-C and vascular dementia (16), but no association between LDL-C and AD.
Our objective in this study was to examine the association between plasma lipid levels in the elderly and decline in memory and other cognitive functions.
METHODS
Subjects and Setting
Participants were enrolled in a longitudinal cohort study by a random sampling of Medicare recipients 65 years or older residing in northern Manhattan (Washington Heights, Hamilton Heights, Inwood). The sampling procedures have been described elsewhere (17). Each participant underwent an in-person interview of general health and function at the time of study entry followed by a standard assessment, including medical history, physical and neurological examination as well as a neuropsychological battery (18). Baseline data were collected from 1992 through 1994. Follow-up data were collected during evaluations at sequential intervals of approximately 18 months, performed from 1994 to 1996, 1996 to 1997, and 1997 to 1999. In this elderly population, some participants did not complete follow up at all intervals due to refusal to participate further, relocation or death. About one half of participants were evaluated at the third follow-up visit. This study was approved by the institutional review board of the Columbia-Presbyterian Medical Center.
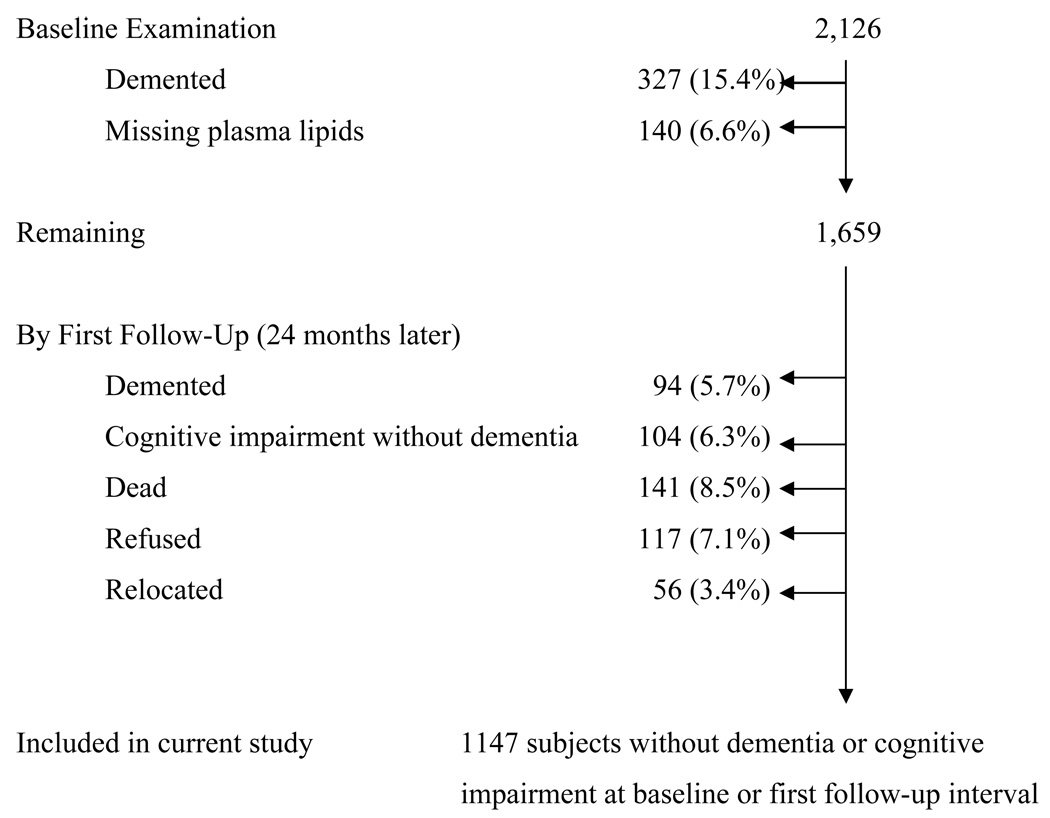
The sample for this study were individuals with lipid levels obtained at the first follow-up interval, without dementia or cognitive impairment at baseline and the first interval, and with complete neuropsychological information in at least 3 follow-up intervals. Of the 2126 individuals who underwent clinical assessment at baseline, 327 individuals were excluded due to dementia at baseline. Plasma lipids were unavailable in 140 cases, and at first follow-up visit 94 subjects were excluded due to prevalent dementia, 104 subjects due to cognitive impairment without dementia (Clinical Dementia Rating Scale Score of 0.5) (19), and 141 subjects were dead, 117 refused to participate further and 56 were relocated (Figure 1).
Figure 1.
Description of Sample Size.
Thus we restricted the sample for these analyses to 1147 individuals without dementia (AD or other forms), and without cognitive impairment without dementia, stroke, Parkinson’s disease or other major neurological disorders at baseline or first follow-up interval. Subjects who developed cognitive impairment or dementia after the first follow-up visit were included in the main analyses.
Clinical assessments
Data included medical, neurological, and neuropsychological evaluations (18, 20). All participants underwent a standardized neuropsychological test battery that examined multiple domains in either English or Spanish (21). Orientation was evaluated using parts of the modified Mini-Mental State Examination (22). Language was assessed using the Boston Naming Test (23), the Controlled Word Association Test (24), category naming, and the Complex Ideational Material and Phrase Repetition subtests from the Boston Diagnostic Aphasia Evaluation (25). Abstract Reasoning was evaluated using WAIS-R Similarities subtest (26), and the non-verbal Identities and Oddities subtest of the Mattis Dementia Rating Scale (27). Visuospatial ability was examined using the Rosen Drawing Test (28), and a matching version of the Benton Visual Retention Test (29). Memory was evaluated using the multiple choice version of the Benton Visual Retention Test (29) and the seven subtests of the Selective Reminding Test (30): total recall, long-term recall, long-term storage, continuous long-term storage, words recalled on last trial, delayed recall, and delayed recognition. This neuropsychological test battery has established norms for the same community (31). Results from the neurological, psychiatric and neuropsychological examinations were reviewed in a consensus conference comprised of physicians, neurologists, neuropsychologists and psychiatrists. Based on this review all participants were assigned to one of three categories: normal cognitive function, cognitive impairment without dementia, or dementia. Dementia was defined by DSM-IV criteria (32) and required cognitive impairment in several domains and functional impairment (Clinical Dementia Rating (CDR)≥1) (19). Cognitive impairment without dementia was diagnosed in participants who had abnormal results in cognitive tests, but had no significant cognitive impairment (CDR=0.5). Color trails were available only in the 1999 follow-up. Thus, analyses with the color trails were conducted separately only in individuals who were in the study beyond 1999 and had information on color trails (n=453). The color trails were not part of the calculated cognitive scores and were dichotomized for prospective analyses.
Plasma Lipids and APOE Genotyping
Fasting plasma total cholesterol and triglyceride levels were determined at the first follow-up interval using standard enzymatic techniques. HDL-C levels were determined after precipitation of apolipoprotein B containing lipoproteins with phosphotungstic acid (33). LDL-C was recalculated using the formula of Friedewald et al. (34). APOE genotypes were determined as described by Hixson and Vernier (35) with slight modification (36). We classified persons as homozygeous or heterozygeous for the APOE ε4 allele or not having any ε4 allele.
Statistical Methods
A factor analysis was performed using data from all visits of the analytic sample with the 15 neuropsychological measures using a principal component analysis with varimax rotation and Kaiser normalization (37). This analysis resulted in three factors: 1) a memory factor, in which the seven subtests of the Selective Reminding Test were the main contributors; 2) a visuospatial/cognitive factor, where visuospatial and tests of reasoning were the main contributors; and 3) a language factor, in which language measures from the Boston Naming Test (23), Controlled Oral Word Association Test (24), and the WAIS-R Similarities (26) were the main contributors. We calculated cognitive scores for each participant at each visit by adding the scores of the measures that contributed most to each factor (tests with correlations of 0.5 or higher). Each factor score was normally distributed. These factors remained stable when we excluded subjects who developed dementia during follow-up, and were reproducible at baseline and at each follow-up interval.
Analysis of prospective change in the memory score was performed by applying generalized estimating equations (GEE) (38) with repeated measures. This statistical method takes into account the multiple observations per subject which are likely to be correlated, and treats them as clusters. The dependent variables were the calculated cognitive scores, and the independent variables were plasma lipid levels of total cholesterol, HDL-C, triglycerides and LDL-C, time (included as a continuous variable), and the interaction of plasma lipids and time. Plasma lipid levels were examined first dichotomized by the median and in subsequent models using the accepted limits of normal as cutoff points (240 mg/dl for total cholesterol, 40 mg/dl for HDL-C, 200 mg/dl for triglycerides and 160 mg/dl for LDL-C)(2). Gender, age, education and ethnic group were included as covariates in subsequent analyses. Because the distribution of HDL-C and triglycerides was skewed, logarithmic transformation of these data was carried out before statistical tests were performed.
The GEE analysis yields coefficient values which represent the associations between a factor score and variables included in the model. There were three main coefficients of interest in each model: one comparing the lipid groups at baseline, one relating the change in cognitive scores with time, and an interaction term for time and lipid group. A significant p value for the coefficient comparing lipids at baseline indicates a difference between two groups at baseline. A significant p value for the coefficient of time indicates a statistically significant change in a cognitive score over the total duration of follow-up. A significant p value for the interaction coefficient indicates a difference in the rate of change in a factor score depending on the plasma lipid level; this is the main variable of interest for the interpretation of the analyses.
We also conducted analyses restricted to the subjects with data on color trails. We dichotomized the color trails time by the 75th percentile, and conducted proportional hazards models relating plasma lipid levels with poor performance in color trails, adjusting for gender and age, baseline cognitive scores, and other variables. The time-to-event variable was age-at-onset of low performance in color trails. Information on covariates was obtained at baseline. Data analysis was performed using SPSS version 12.0.
RESULTS
The mean age was 76.3 years, and 68.4% of the study population were women, 46.3% were Hispanic, 20.8% were White, and 32.3% were Black (Table 1). The mean of years of education was 8.6, and 27.5% were homozygous or heterozygous for the APOE-ε4 allele. The mean level of total cholesterol was 203.1, of HDL-C 47.1, of triglycerides 185.2 and of LDL-C 118.9 mg/dl. The mean body mass index was 27.1, and 15.8% of the subjects reported having diabetes, 50.3% hypertension and 14.7% heart disease. Use of lipid lowering agents was reported by 59 subjects (5.1%). There were 7217 person-years of follow-up, and the mean duration of follow-up was 5.6 ± 2.3 years. Women had higher levels of total cholesterol, HDL, triglycerides and LDL than men (Table 2). Hispanics had lower levels of total cholesterol, HDL and LDL, and higher levels of triglycerides than Whites and Blacks.
Table 1.
Demographic characteristics of the 1147 individuals in the study population
| Men | 363 (31.6) |
| Women | 784 (68.4) |
| Education, mean (SD), year | 8.6 (4.6) |
| Age, mean (SD), year | 76.3 (5.8) |
| Body mass index, mean (SD) | 27.1 (5.1) |
| Ethnic group‡ | |
| White/Non-Hispanic | 239 (20.8) |
| Black/Non-Hispanic | 371 (32.3) |
| Hispanic | 531 (46.3) |
| APOE genotype 4/4 | 22 (1.9) |
| APOE genotype 4/− | 294 (25.6) |
| APOE genotype −/− | 6821 (71.6) |
| Cholesterol (mg/dl), mean (SD) | 203.1 (40.7) |
| HDL (mg/dl), mean (SD) | 47.1 (15.8) |
| Triglycerides (mg/dl), mean (SD) | 185.2 (95.7) |
| LDL (mg/dl), mean (SD) | 118.9 (36.4) |
| No Diabetes | 852 (74.3) |
| Diabetes, not treated | 47(3.6) |
| Diabetes, treated | 140 (12.2) |
| No heart disease | 871 (75.9) |
| Heart disease, not treated | 41 (3.6) |
| Heart disease, treated | 127 (11.1) |
| No hypertension | 458 (39.9) |
| Hypertension, not treated | 173 (15.1) |
| Hypertension, treated | 404 (35.2) |
| Use of lipid lowering agents | |
| no | 763 (66.5) |
| yes | 59 (5.1) |
Values are expressed as number (percentage) unless otherwise indicated. Some percentages are based on an incomplete sample due to small amounts of missing data.
Classified by self-report using the format of the 1990 US census (54).
Table 2.
Comparison of lipid levels by demographics in 1147 subjects
| Cholesterol (mg/dl) | HDL (mg/dl) | Triglycerides (mg/dl) | LDL (mg/dl) | |
|---|---|---|---|---|
| Men | 188.8 (39.2) | 41.9 (12.5) | 182.5 (103.4) | 110.5 (35.8) |
| Women | 209.7 (39.7) * | 49.4 (16.5) * | 186.5 (92.1) | 122.8 (36.0) * |
| Ethnic group † | ||||
| White/Non-Hispanic | 209.3 (40.4)* | 47.4 (16.3) * | 186.5 (94.3) | 124.6 (33.3)* |
| Black/Non-Hispanic | 203.4 (40.7) | 51.1 (16.3) * | 158.7 (78.1) | 120.6 (37.4)* |
| Hispanic | 199.9 (40.8) | 43.8 (46.3) | 203.8 (103.4)* | 115.2 (36.9) |
Values are expressed as number (SD) unless otherwise indicated. Some percentages are based on an incomplete sample due to small amounts of missing data.
Significant at a 0.05 level versus lowest value within lipid group, based on analysis of variance for continuous data and χ2 test for categorical data.
Classified by self-report using the format of the 1990 US census (54).
In the GEE analysis performance in all cognitive domains declined significantly over time, while there was no association between levels of any plasma lipid or lipid lowering treatment and memory, cognitive/visuospatial or language performance at any interval (Table 3, Table 4 and Table 5). These results remained unchanged when not the median but accepted limits of normal were used as cutoff points for plasma lipid levels, or when analyses were stratified by APOEε4 genotype or ethnic group. Higher age at baseline was related to lower scores in all three domains at each interval, while higher education and Caucasian ethnicity were associated with higher scores in all domains.
Table 3.
Relationship of Plasma lipids and Time of Follow-up to Memory Performance in Healthy Elderly Over 7 years
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| Variable | Estimated β (SE) | p-value | Estimated β (SE) | p-value |
| Time | −6.8 (0.6) | 0.000 | −6.8 (0.6) | 0.000 |
| Total cholesterol | 3.5 (3.0) | 0.3 | −0.6 (2.8) | 0.8 |
| Time*total cholesterol | −0.4 (0.8) | 0.6 | −0.4 (0.8) | 0.6 |
| Time | −7.3 (0.6) | 0.000 | −7.3 (0.6) | 0.000 |
| HDL | −3.3 (3.0) | 0.3 | −1.2 (2.8) | 0.7 |
| Time*HDL | 0.5 (0.8) | 0.5 | 0.6 (0.8) | 0.5 |
| Time | −6.9 (0.6) | 0.000 | −6.9 (0.6) | 0.000 |
| Triglycerides | 0.5 (3.0) | 0.9 | 2.3 (2.8) | 0.4 |
| Time*triglycerides | −0.3 (0.8) | 0.7 | −0.1 (0.8) | 0.9 |
| Time | −7.1 (0.6) | 0.000 | −6.9 (0.6) | 0.000 |
| LDL | 1.2 (3.0) | 0.7 | −0.9 (2.8) | 0.7 |
| Time*LDL | 0.2 (0.8) | 0.8 | −0.1 (0.8) | 0.9 |
Model 1 is adjusted for age and gender, Model 2 is adjusted for age, gender, education, ethnic group and APOEε4
Table 4.
Relationship of Plasma lipids and Time of Follow-up to Cognitive Performance in Healthy Elderly Over 7 years
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| Variable | Estimated β (SE) | p-value | Estimated β (SE) | p-value |
| Time | −1.0 (0.2) | 0.000 | −1.0 (0.2) | 0.000 |
| Total cholesterol | 1.4 (1.5) | 0.4 | −1.7 (1.2) | 0.1 |
| Time*total cholesterol | 0.3 (0.3) | 0.4 | 0.2 (0.3) | 0.4 |
| Time | −0.9 (0.2) | 0.000 | −0.9 (0.2) | 0.000 |
| HDL | −1.9 (1.6) | 0.2 | 0.1 (1.3) | 0.9 |
| Time*HDL | −0.1 (0.3) | 0.6 | −0.1 (0.3) | 0.8 |
| Time | −0.9 (0.2) | 0.000 | −0.9 (0.2) | 0.000 |
| Triglycerides | −2.3 (1.5) | 0.1 | −0.1 (1.2) | 0.9 |
| Time*triglycerides | 0.0 (0.3) | 0.9 | 0.2 (0.3) | 0.5 |
| Time | −1.2 (0.2) | 0.000 | −1.1 (0.2) | 0.000 |
| LDL | −0.1 (1.5) | 0.9 | −1.8 (1.2) | 0.1 |
| Time*LDL | 0.5 (0.3) | 0.06 | 0.4 (0.3) | 0.2 |
Model 1 is adjusted for age and gender, Model 2 is adjusted for age, gender, education, ethnic group and APOEε4
Table 5.
Relationship of Plasma lipids and Time of Follow-up to Language Performance in Healthy Elderly Over 7 years
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| Variable | Estimated β (SE) | p-value | Estimated β (SE) | p-value |
| Time | −0.2 (0.1) | 0.000 | −0.2 (0.1) | 0.000 |
| Total cholesterol | 0.2 (0.3) | 0.4 | 0.1 (0.3) | 0.7 |
| Time*total cholesterol | −0.1 (0.1) | 0.7 | −0.1 (0.1) | 0.6 |
| Time | −0.2 (0.1) | 0.000 | −0.2 (0.1) | 0.002 |
| HDL | −0.3 (0.3) | 0.3 | −0.2 (0.3) | 0.5 |
| Time*HDL | 0.0 (0.1) | 0.6 | 0.1 (0.1) | 0.5 |
| Time | −0.2 (0.1) | 0.005 | −0.3 (0.1) | 0.000 |
| Triglycerides | −0.1 (0.3) | 0.9 | 0.2 (0.3) | 0.5 |
| Time*triglycerides | 0.1 (0.1) | 0.4 | 0.1 (0.1) | 0.1 |
| Time | −0.2 (0.1) | 0.006 | −0.2 (0.1) | 0.002 |
| LDL | 0.1 (0.3) | 0.9 | 0.1 (0.3) | 0.9 |
| Time*LDL | 0.1 (0.1) | 0.4 | 0.1 (0.1) | 0.5 |
Model 1 is adjusted for age and gender, Model 2 is adjusted for age, gender, education, ethnic group and APOEε4
Cox proportional hazards analysis relating plasma lipid levels and the incidence of low performance in color trail tasks also showed no association (total cholesterol: HR 1.0, 95% CI 0.9–1.1; HDL-C: HR 0.9, 95% CI 0.9–1.1, triglycerides: 1.1, 95% CI 0.9–1.0; LDL-C: HR 1.0, 95% CI 0.9–1.0).
In subsequent analyses we excluded subjects who developed dementia during follow-up (n=198). While memory performance declined significantly over time, cognitive/visuospatial and language performance did not change. There was no association between plasma levels of triglycerides or LDL and performance on any of the three cognitive factors at any time interval. While increased levels of total cholesterol and HDL-C were associated with higher scores in language performance, there was a statistically significant total cholesterol*time (duration of follow-up) interaction indicating that language performance declined at a faster rate among individuals with higher total cholesterol levels compared to subjects with lower level. This association remained significant after adjusting for age, gender, ethnic group, education and APOE allele. However, this association was not significant considering Bonferroni correction for multiple comparisons (39). There was no similar relationship between total cholesterol*time effect and memory or visuospatial/cognitive factors. However, scores of both factors were normally distributed at each time interval indicating that the lack of a total cholesterol*time interaction was not the result of a ceiling or floor effect. Treatment with lipid lowering agents was not associated with better scores on any of the three cognitive factors at any time interval, and cox proportional hazards analysis relating plasma lipid levels and the incidence of low performance in color trail tasks also showed no association (total cholesterol: HR 0.9, 95% CI 0.9–1.0; HDL-C: HR 1.2, 95% CI 0.7–42.2, triglycerides: 1.0, 95% CI 0.2–4.2; LDL-C: HR 0.9, 95% CI 0.9–1.0).
DISCUSSION
In this study performance in all cognitive domains declined significantly over time in elderly individuals without dementia or cognitive impairment, while there was no association between levels of any plasma lipid or lipid lowering treatment and memory, cognitive/visuospatial or language performance at any interval. Higher age at baseline was related to lower scores in all three domains at each interval, while higher education and Caucasian ethnicity were associated with higher scores in all domains.
The role of dyslipidemia in the development of cognitive impairment remains unclear. Brain cholesterol alters the degradation of APP(2), which contributes to the pathogenesis of AD (13). Several lines of evidence indicate that lowering plasma cholesterol levels prevents AD development by reducing Aβ production and secretion (40). These findings seem to contradict previous studies demonstrating that cholesterol protects PC12 cells from fibrillar Aβ peptide, that cholesterol depletion induces AD-type injuries in cultured hippocampal slices (40), and that brain cholesterol is almost entirely synthesized in situ and not transferred from the plasma into the brain (41). Few studies have examined the association of plasma lipid levels to cognitive function, and they reported inconsistent results (5, 7, 42–44). Results in animal studies (45, 46), and studies relating plasma lipid lowering treatment to cognitive functioning (7, 12, 43, 47, 48) have also been conflicting. Most observational studies were cross-sectional (9, 43, 49–51), and some of the few longitudinal studies included individuals with QD or AD and did not provide methods to limit inclusion of such individuals (52). Our results are consistent with the idea that lipid levels do not affect cognition directly.
There are several potential explanations for our findings of no association of plasma lipids and lipid lowering treatment to cognitive change. One explanation is measurement error. We had only one measure of plasma lipids which may not take into account intrapersonal variation. If the measurement error was random, this would have underestimated the association between lipids and cognitive changes, thus resulting in finding of no association. Another possibility is that our sample was relatively homogeneous in plasma lipid levels, thus not permitting enough variability to detect an association. Another potential explanation is bias related to selection into this study. It is possible that plasma lipid levels are related to cognitive decline in younger individuals but not the older sample in our study. Our sample was older than 65 years with a mean age of 75.7 years. It is possible that individuals with adverse outcomes related to plasma lipid levels did not survive to inclusion in our study, or that the plasma lipid levels at the age of entry in the study did not reflect lipid levels earlier in life. Finally, it is possible that plasma lipid levels are not related to cognitive decline as indicated by our results.
The main limitation of this study is that we used only one measurement of lipid levels, which could have led to measurement error due to intraperson variability and underestimation of the association between lipid levels and cognitive impairment.
This study has important strengths. This is a prospective cohort study designed for the diagnosis of cognitive decline, and with complete clinical and neuropsychological evaluation at each interval. Our study has sensitive measures of cognitive change in several specific domains including memory. In addition, we had the ability to diagnose dementia and cognitive impairment without dementia at baseline, thus allowing us to follow an unbiased sample. Other longitudinal studies used global cognitive assessments or may not have had the ability to detect early stages of cognitive impairment at baseline (43, 52, 53).
An important consideration in the interpretation of the results of this study is its generalizability. This study was conducted in an urban multiethnic elderly community with a high prevalence of risk factors for mortality and dementia. Thus, our results may not be generalizable to cohorts with younger individuals or to cohorts with participants with a lower morbidity burden.
Acknowledgments
Funding
This study was supported by grants AG07232 and AG07702 from the National Institute on Aging (Washington, DC), the Charles S. Robertson Memorial Gift for Research in Alzheimer's disease, the Blanchette Hooker Rockefeller Foundation, and the New York City Council Speaker's Fund for Public Health Research (New York, NY).
REFERENCES
- 1.Fratiglioni L, De Ronchi D, Aguero-Torres H. Worldwide prevalence and incidence of dementia. Drugs Aging. 1999;15(5):365–375. doi: 10.2165/00002512-199915050-00004. [DOI] [PubMed] [Google Scholar]
- 2.American Heart Association. Heart Disease and Stroke Statistics - 2004 Update. Dallas TAHA: [Google Scholar]
- 3.Kuriyama M, Takahashi K, Yamano T, et al. Low levels of serum apolipoprotein A I and A II in senile dementia. Jpn J Psychiatry Neurol. 1994;48(3):589–593. doi: 10.1111/j.1440-1819.1994.tb03019.x. [DOI] [PubMed] [Google Scholar]
- 4.Muckle TJ, Roy JR. High-density lipoprotein cholesterol in differential diagnosis of senile dementia. Lancet. 1985;1(8439):1191–1193. doi: 10.1016/s0140-6736(85)92866-1. [DOI] [PubMed] [Google Scholar]
- 5.Kuriyama M, Hokezu Y, Togo S, et al. Serum lipids, lipoproteins and apolipoproteins in patients with senile dementia. Nippon Ronen Igakkai Zasshi. 1992;29(7–8):559–564. doi: 10.3143/geriatrics.29.559. [DOI] [PubMed] [Google Scholar]
- 6.Kuo YM, Emmerling MR, Bisgaier CL, et al. Elevated low-density lipoprotein in Alzheimer's disease correlates with brain abeta 1–42 levels. Biochem Biophys Res Commun. 1998;252(3):711–715. doi: 10.1006/bbrc.1998.9652. [DOI] [PubMed] [Google Scholar]
- 7.Michikawa M. Cholesterol paradox: is high total or low HDL cholesterol level a risk for Alzheimer's disease? J Neurosci Res. 2003;72(2):141–146. doi: 10.1002/jnr.10585. [DOI] [PubMed] [Google Scholar]
- 8.Wieringa GE, Burlinson S, Rafferty JA, et al. Apolipoprotein E genotypes and serum lipid levels in Alzheimer's disease and multi-infarct dementia. Int J Geriatr Psychiatry. 1997;12(3):359–362. [PubMed] [Google Scholar]
- 9.van Exel E, de Craen AJ, Gussekloo J, et al. Association between high-density lipoprotein and cognitive impairment in the oldest old. Ann Neurol. 2002;51(6):716–721. doi: 10.1002/ana.10220. [DOI] [PubMed] [Google Scholar]
- 10.Lesser G, Kandiah K, Libow LS, et al. Elevated serum total and LDL cholesterol in very old patients with Alzheimer's disease. Dement Geriatr Cogn Disord. 2001;12(2):138–145. doi: 10.1159/000051248. [DOI] [PubMed] [Google Scholar]
- 11.Scacchi R, De Bernardini L, Mantuano E, et al. DNA polymorphisms of apolipoprotein B and angiotensin I-converting enzyme genes and relationships with lipid levels in Italian patients with vascular dementia or Alzheimer's disease. Dement Geriatr Cogn Disord. 1998;9(4):186–190. doi: 10.1159/000017045. [DOI] [PubMed] [Google Scholar]
- 12.Hajjar I, Schumpert J, Hirth V, et al. The impact of the use of statins on the prevalence of dementia and the progression of cognitive impairment. J Gerontol A Biol Sci Med Sci. 2002;57(7):M414–M418. doi: 10.1093/gerona/57.7.m414. [DOI] [PubMed] [Google Scholar]
- 13.Burns M, Duff K. Cholesterol in Alzheimer's disease and tauopathy. Ann N Y Acad Sci. 2002;977:367–375. doi: 10.1111/j.1749-6632.2002.tb04839.x. [DOI] [PubMed] [Google Scholar]
- 14.Breteler MM, Claus JJ, Grobbee DE, et al. Cardiovascular disease and distribution of cognitive function in elderly people: the Rotterdam Study. Bmj. 1994;308(6944):1604–1608. doi: 10.1136/bmj.308.6944.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalmijn S, Feskens EJ, Launer LJ, et al. Cerebrovascular disease, the apolipoprotein e4 allele, and cognitive decline in a community-based study of elderly men. Stroke. 1996;27(12):2230–2235. doi: 10.1161/01.str.27.12.2230. [DOI] [PubMed] [Google Scholar]
- 16.Moroney JT, Tang MX, Berglund L, et al. Low-density lipoprotein cholesterol and the risk of dementia with stroke. Jama. 1999;282(3):254–260. doi: 10.1001/jama.282.3.254. [DOI] [PubMed] [Google Scholar]
- 17.Tang MX, Stern Y, Marder K, et al. The APOE-epsilon4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. Jama. 1998;279(10):751–755. doi: 10.1001/jama.279.10.751. [DOI] [PubMed] [Google Scholar]
- 18.Stern Y, Andrews H, Pittman J, Sano M, Tatemichi T, Lantigua R, et al. Diagnosis of dementia in a heterogeneous population. Development of a neuropsychological paradigm-based diagnosis of dementia and quantified correction for the effects of education. Arch Neurol. 1992;49(5):453–460. doi: 10.1001/archneur.1992.00530290035009. [DOI] [PubMed] [Google Scholar]
- 19.Hughes CP, Berg L, Danziger WL, et al. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 20.Pittman J, Andrews H, Tatemichi T, et al. Diagnosis of dementia in a heterogeneous population. A comparison of paradigm-based diagnosis and physician's diagnosis. Arch Neurol. 1992;49(5):461–467. doi: 10.1001/archneur.1992.00530290043010. [DOI] [PubMed] [Google Scholar]
- 21.Stern Y, Andrews H, Pittman J, et al. Diagnosis of dementia in a heterogeneous population. Development of a neuropsychological paradigm-based diagnosis of dementia and quantified correction for the effects of education. Arch Neurol. 1992;49(5):453–460. doi: 10.1001/archneur.1992.00530290035009. [DOI] [PubMed] [Google Scholar]
- 22.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Philadelphia, PA: Lea & Febiger; 1983. [Google Scholar]
- 24.Benton A, editor. FAS Test. Victoria, B.C.: University of Victoria; 1967. [Google Scholar]
- 25.Goodglass H, Kaplan E. 2.ed. Philadelphia, PA: Lea & Febiger; 1983. The Assessment of Aphasia and Related Disorders. [Google Scholar]
- 26.Wechsler D. New York, NY: The Psychological Corporation; 1981. Wechsler Adult Intelligence Scale-Revised. [Google Scholar]
- 27.Mattis S. Bellak L, Karasu TB. Geriatric Psychiatry. New York, NY: Grune & Stratton; 1976. Mental status examination for organic mental syndrome in the elderly patient; pp. 77–121. [Google Scholar]
- 28.Rosen W. Bronx, NY: Veterans Administration Medical Center; 1981. The Rosen Drawing Test. [Google Scholar]
- 29.Benton A. The Benton Visal Retention Test. New York: The Psychological Corporation; 1955. [Google Scholar]
- 30.Buschke H, Fuld PA. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology. 1974;24(11):1019–1025. doi: 10.1212/wnl.24.11.1019. [DOI] [PubMed] [Google Scholar]
- 31.Stricks L, Pittman J, Jacobs DM, et al. Normative data for a brief neuropsychological battery administered to English-and Spanish-speaking community-dwelling elders. J Int Neuropsychol Soc. 1998;4(4):311–318. [PubMed] [Google Scholar]
- 32.American Psychiatric Association. Fourth Edition. Washington DC: American Psychiatric Association; 1994. Diagnostic and Statistical Manual of Mental Disorders; pp. 143–147. [Google Scholar]
- 33.Lopes-Virella MF, Stone P, Ellis S, et al. Cholesterol determination in high-density lipoproteins separated by three different methods. Clin Chem. 1977;23(5):882–884. [PubMed] [Google Scholar]
- 34.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 35.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31(3):545–548. [PubMed] [Google Scholar]
- 36.Mayeux R, Ottman R, Maestre G, et al. Synergistic effects of traumatic head injury and apolipoprotein-epsilon 4 in patients with Alzheimer's disease. Neurology. 1995;45(3 Pt 1):555–557. doi: 10.1212/wnl.45.3.555. [DOI] [PubMed] [Google Scholar]
- 37.Kleinbaum D, Klipper L, Muller K. 2nd ed. Boston: PWS-Kent; 1988. Applied regression analysis and other multivariable methods; pp. 601–631. [Google Scholar]
- 38.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44(4):1049–1060. [PubMed] [Google Scholar]
- 39.Curran-Everett D. Multiple comparisons: philosophies and illustrations. Am J Physiol Regul Integr Comp Physiol. 2000;279(1):R1–R8. doi: 10.1152/ajpregu.2000.279.1.R1. [DOI] [PubMed] [Google Scholar]
- 40.Sponne I, Fifre A, Koziel V, Oster T, Olivier JL, Pillot T. Membrane cholesterol interferes with neuronal apoptosis induced by soluble oligomers but not fibrils of amyloid-beta peptide. Faseb J. 2004;18(7):836–838. doi: 10.1096/fj.03-0372fje. [DOI] [PubMed] [Google Scholar]
- 41.Dietschy JM, Turley SD. Cholesterol metabolism in the brain. Curr Opin Lipidol. 2001;12(2):105–112. doi: 10.1097/00041433-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Teunissen CE, De Vente J, von Bergmann K, et al. Serum cholesterol, precursors and metabolites and cognitive performance in an aging population. Neurobiol Aging. 2003;24(1):147–155. doi: 10.1016/s0197-4580(02)00061-1. [DOI] [PubMed] [Google Scholar]
- 43.Yaffe K, Barrett-Connor E, Lin F, et al. Serum lipoprotein levels, statin use, and cognitive function in older women. Arch Neurol. 2002;59(3):378–384. doi: 10.1001/archneur.59.3.378. [DOI] [PubMed] [Google Scholar]
- 44.Henderson VW, Guthrie JR, Dennerstein L. Serum lipids and memory in a population based cohort of middle age women. J Neurol Neurosurg Psychiatry. 2003;74(11):1530–1535. doi: 10.1136/jnnp.74.11.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sparks DL, Schreurs BG. Trace amounts of copper in water induce beta-amyloid plaques and learning deficits in a rabbit model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2003;100(19):11065–11069. doi: 10.1073/pnas.1832769100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schreurs BG, Smith-Bell CA, Lochhead J, et al. Cholesterol modifies classical conditioning of the rabbit (Oryctolagus cuniculus) nictitating membrane response. Behav Neurosci. 2003;117(6):1220–1232. doi: 10.1037/0735-7044.117.6.1220. [DOI] [PubMed] [Google Scholar]
- 47.Gibellato MG, Moore JL, Selby K, et al. Effects of lovastatin and pravastatin on cognitive function in military aircrew. Aviat Space Environ Med. 2001;72(9):805–812. [PubMed] [Google Scholar]
- 48.Muldoon MF, Barger SD, Ryan CM, et al. Effects of lovastatin on cognitive function and psychological well-being. Am J Med. 2000;108(7):538–546. doi: 10.1016/s0002-9343(00)00353-3. [DOI] [PubMed] [Google Scholar]
- 49.Orengo CA, Kunik ME, Molinari VA, et al. Association of serum cholesterol and triglyceride levels with agitation and cognitive function in a geropsychiatry unit. J Geriatr Psychiatry Neurol. 1996;9(2):53–56. doi: 10.1177/089198879600900201. [DOI] [PubMed] [Google Scholar]
- 50.Geroldi C, Ferrucci L, Bandinelli S, et al. Mild cognitive deterioration with subcortical features: prevalence, clinical characteristics, and association with cardiovascular risk factors in community-dwelling older persons (The InCHIANTI Study) J Am Geriatr Soc. 2003;51(8):1064–1071. doi: 10.1046/j.1532-5415.2003.51353.x. [DOI] [PubMed] [Google Scholar]
- 51.Atzmon G, Gabriely I, Greiner W, et al. Plasma HDL levels highly correlate with cognitive function in exceptional longevity. J Gerontol A Biol Sci Med Sci. 2002;57(11):M712–M715. doi: 10.1093/gerona/57.11.m712. [DOI] [PubMed] [Google Scholar]
- 52.Kim JM, Stewart R, Shin IS, et al. Low cholesterol, cognitive function and Alzheimer s disease in a community population with cognitive impairment. J Nutr Health Aging. 2002;6(5):320–323. [PubMed] [Google Scholar]
- 53.Paganini-Hill A, Henderson VW. The effects of hormone replacement therapy, lipoprotein cholesterol levels, and other factors on a clock drawing task in older women. J Am Geriatr Soc. 1996;44(7):818–822. doi: 10.1111/j.1532-5415.1996.tb03740.x. [DOI] [PubMed] [Google Scholar]
- 54.Census of Population and Housing. STF 1A database., 1990. Washington DC: Bureau of Census; 1991. Summary Tape File1, Technical Documentation (computer program) [Google Scholar]