Abstract
Hepatic ischemia/reperfusion (I/R) injury is a complication of liver surgery, transplantation and shock and is known to be age-dependent. Our laboratory has recently shown that peroxisome proliferator-activated receptor-gamma (PPARγ) is downregulated during hepatic ischemia and that this exacerbates injury. Here we examined whether activation of PPARγ during ischemia was age-dependent. Male mice of different ages (young: 4–5 weeks; adult: 10–12 weeks; old: 10–12 months) were subjected to up to 90 minutes of hepatic ischemia. PPARγ activation occurred throughout ischemia in young mice, whereas activation in adult and old mice was lost after 30 minutes. No significant differences were noted in PPARγ ligand expression amongst the age groups. However, in young mice we observed a predominance of PPARγ1 in the nucleus, whereas in old mice this isoform remained largely in the cytoplasm. Finally, the degree of PPARγ activation was associated with autophagy in the liver, a mechanism of self-preservation.
Conclusion
PPARγ activation is prolonged in young mice as compared to older mice. This appears to be mediated by a selective retention of PPARγ1 in the nucleus and is associated with increased autophagy. The data suggest that PPARγ activation is an important component of the age-dependent response to hepatic I/R injury.
Keywords: liver injury, transcription, autophagy, hepatocytes
Introduction
Hepatic ischemia/reperfusion (I/R) injury is a complication of a number of diverse clinical settings such as trauma, vascular surgery, liver resection and transplantation(1). Hepatic I/R injury has been modeled in rodents and consists of initial phase mediated by reactive oxygen species released by Kupffer cells (2). The later phase of injury is dependent upon the recruitment of activated neutrophils that cause direct damage to hepatocytes by release of oxidants and proteases (2). We have shown that there is a distinct difference in the injury response to hepatic I/R in mice of different ages (3). These studies demonstrated that mice 10–12 weeks of age had significantly less liver injury than mice 9–12 months of age. These ages correspond approximately to 10–15 year old and 40–50 year old humans, respectively (3). Interestingly, trauma and critical care physicians have long noted a significant divergence in the responses of pediatric and adult populations to severe trauma (4). There is also evidence that age is an important factor in liver transplantation as well as liver resection with or without ischemic-preconditioning (5, 6). The underlying mechanism(s) by which age alters the injury response to liver injury is unknown.
Peroxisome proliferators-activated receptors (PPARs) belong to the hormone nuclear receptor superfamily and consist of three isoforms, PPARα, PPARβ/δ and PPARγ. Of these, we and others have recently shown that PPARγ is an important regulator of post-ischemic liver injury (7, 8). We found that activation of PPARγ was suppressed during the ischemic period and that treatment with a synthetic PPARγ ligand restored activation and reduced injury (7). In the present study, we examined whether PPARγ activation in the liver during ischemia was age-dependent. Our data provide direct evidence that PPARγ activation is preserved in animals corresponding in age to a pediatric human population, and may represent a significant age-related regulatory mechanism of I/R injury in the liver.
Materials and Methods
Hepatic ischemia/reperfusion injury model
Male C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) were used. The project was approved by the University of Cincinnati Animal Care and Use Committee and was in compliance with the National Institutes of Health guidelines. Three ages of mice were utilized: “young” mice were 4–5 weeks old, “adult” mice were 10–12 weeks of age, and “old” mice were 10–12 months old. Mice underwent either sham surgery or partial hepatic ischemia as described elsewhere (9). Briefly, mice were anaesthetized with pentobarbital (60 mg/kg, i.p). A midline laparotomy was made and an atraumatic clip was placed at the portal confluence to interrupt the blood supply to the left and median lobes of the liver which consists about 70% of the total liver. Animals underwent either 30, 60, or 90 minutes of ischemia after which time ischemic lobes of the liver were harvested. Sham control mice underwent the same operation without vascular clamping. Core body temperature was maintained between 34–36 °C from time of anaesthesia to sacrifice. Some adult mice were pretreated orally with methycellulose (vehicle) or 10 mg/kg rosiglitazone dissolved in methylcellulose 30 minutes prior to the induction of ischemia.
Electrophoretic Mobility Shift Assay (EMSA)
Nuclear extracts of liver tissue were prepared by the method of Deryckere and Gannon (10). Double-stranded consensus oligonucleotides to PPAR (Santa Cruz Biotechnology, Santa Cruz, CA) was end labeld with γ [32P] adenosine triphosphate (3000 Ci/mmol at 10 mCi/mL; GE heath). Binding reactions (total of 15 μL) containing 20 μg of protein extract and 35fmol of oligonucleotide were incubated for 30 minutes at room temperature. Binding reaction products were separated in a 4% polyacrylamide gel.
Western Blot Analyses
Liver nuclear or cytosolic extracts containing 50μg of protein were separated in a denaturing polyacrylamide gel. Proteins were transferred to a 0.45μm pore polyvinyulidene difluoride membrane and blocked for 1 hour at room temperature with 5% nonfat dry milk in tris-buffered saline with 0.1% Tween 20. Membranes were then incubated with antibodies for PPARγ (Santa Cruz Biotechnology, sc 7273) and LC3B (Cell Signaling #2775) according to the manufacture protocol. Secondary antibodies conjugated to horseradish peroxidase (Santa Cruz Biotechnology) was detected by chemiluminescence (Denville Scientific Inc., South Plainfield, NJ). Protein loading was confirmed by staining for β-actin.
Tissue Analysis
Levels of 15-deoxy-delta-12,14-prostaglandin J2 (15d-PGJ2) in the liver were measured by ELISA using reagents obtained from Assay Designs Inc. (Ann Arbor, MI). Methods for extracting samples for tissue ELISA are described elsewhere (7). The measurement of liver fatty acid composition was performed by gas chromatography at the Mouse Metabolic Phenotyping Center at the University of Cincinnati.
Statistical analysis
All data are expressed as mean ± SEM. Data were analyzed with a one-way analysis of variance with subsequent Student-Newman-Keuls test. Differences were considered significant when P<0.05.
Results
PPAR γ activation decreases with ischemia but is prolonged in young mice
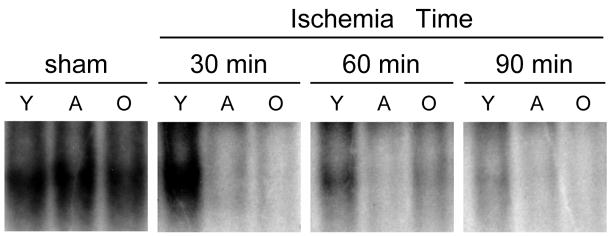
In order to assess the activation of PPARγ during hepatic ischemia, liver nuclear extracts were assayed by EMSA. In sham-operated controls, there was constitutive activation of PPARγ which was similar in all age groups (Figure 1). After 30 minutes of ischemia, activation of PPARγ did not change from sham levels in young mice, but almost completely disappeared in adult and old mice. After 60 minutes of ischemia, PPARγ activation was reduced, but still present in young mice, but lacking in adult and old mice. After 90 minutes of ischemia, there was limited activation of PPARγ in young mice and none in adult or old mice (Figure 1). The specificity for PPARγ binding was verified by unlabelled olionucleotide competition and PPARγ antibody supershift assays as previously described (7).
Figure 1.
Hepatic activation of PPARγ during ischemia in different age groups of mice. Liver nuclear extracts were analyzed by EMSA. Y, young mice (4–5 weeks of age); A, adult mice (10–12 weeks of age); O, old mice (10–12 months of age). Results are representative of 4 sets of experiments.
Activation of PPARγ is not due to age-dependent differences in ligand expression
15d-PGJ2 is thought to be a primary endogenous ligand of PPARγ (11). We have previously shown that in adult mice, 15d-PGJ2 levels progressively decrease with ischemia reaching a nadir at 90 minutes of ischemia (7). Here we show similar results, and that there are significant reductions in hepatic levels of 15d-PGJ2 in old mice (Figure 2). Interestingly, the level of 15d-PGJ2 in young mice was lower than in the older groups and did not significantly change during the ischemic period. We also investigated the hepatic levels of other known PPARγ ligands (12), including arachidonic acid, linoleic acid, and petroselenic acid as well as possible ligands, cervonic acid, palmitic acid and stearic acid. We observed no differences in the hepatic levels of these mediators (Figure 2).
Figure 2.
Hepatic expression of endogenous ligands (left) and potential ligands (right) for PPARγ. 15d-PGJ2 was measured in liver extracts by ELISA. Fatty acids were determined by gas chromatography at the Mouse Metabolic Phenotyping Center at the University of Cincinnati. Data are means ± SEM with n=4 per group. *P<0.05 compared with sham-operated mice.
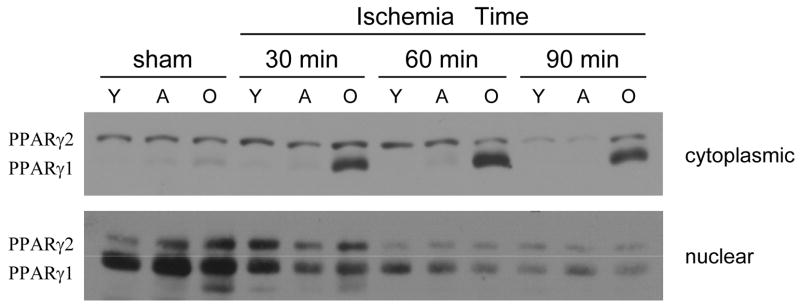
Divergent compartmentalization of PPARγ1 in young and old mice
To further evaluate the nature of PPARγ in liver cells, we examined the composition of the PPARγ in cytoplasmic and nuclear extracts from livers of young, adult and old mice during ischemia. PPARγ has two isoforms, PPARγ1 and PPARγ2, generated by alternative promoter usage and splicing(13). PPARγ2 is 30 amino acids longer than PPARγ1 in the N2-terminus and therefore has a lower mobility by gel electrophoresis. In the cytoplasm, expression of PPARγ2 was similar amongst age groups at all times examined (Figure 3). Interestingly, PPARγ1 was abundant in the cytoplasm of old mice during hepatic ischemia (Figure 3). In nuclear extracts from sham-operated mice, PPARγ1 was the predominant isoform present and had similar expression amongst the age groups (Figure 3). After 30 minutes of ischemia, the expression of PPARγ1 was maintained in the young but was significantly reduced in the older mice. After 60 minutes of ischemia, there was further loss of PPARγ1 expression but still with greater level in the young mice. By 90 minutes of ischemia, there was limited expression of PPARγ in all age groups.
Figure 3.
Expression of PPARγ isoforms in liver cytoplasmic and nuclear extracts during hepatic ischemia. PPARγ1 and PPARγ2 expression were determined by Western blot. Y, young mice (4–5 weeks of age); A, adult mice (10–12 weeks of age); O, old mice (10–12 months of age). Results are representative of 3 sets of experiments.
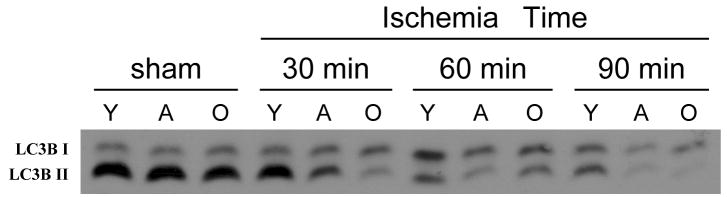
Extent of autophagy is associated with the degree of PPARγ activation
Autophagy is a mechanism of self-protection during stress in which energy is produced by the recycling of old proteins and organelles via the lysosomal degradation (14). PPARγ activation has been previously linked with increased autophagy (15). Therefore, we examined the extent of autophagy in the liver at various times of ischemia. Autophagy was determined by immunblotting for LC3B, a specific marker of autophagy (16). Sham-operated controls of all age groups showed high levels of constitutive autophagy activity as indicated by strong staining of LC3B-II (Figure 4). With progressive ischemia, autophagy decreased in all groups. However, young mice had no discernable change in autophagy after 30 minutes of ischemia and a less marked reduction in autophagy after 60 or 90 minutes of ischemia, compared to adult and old mice (Figure 4).
Figure 4.
Autophagy in the liver during hepatic ischemia. Liver lysates were analyzed for autophagy activity by Western blot for LC3B. Conversion of LC3B-I to LC3B-II is an indicator of autophagy. Y, young mice (4–5 weeks of age); A, adult mice (10–12 weeks of age); O, old mice (10–12 months of age). Results are representative of 4 sets of experiments.
Activation of PPAR γ with rosiglitazone increases autophagy during ischemia
To access if PPARγ activation is directly responsible for the observed differences in autophagy, we pretreated adult mice with 10 mg/kg rosiglitazone 30 minutes prior to ischemia. We have shown previously that rosiglitazone pretreatment increases PPARγ activation in the liver and is associated with decreased I/R injury (7). Treatment with rosiglitazone increased the autophagy at all ischemic time points when compared to the vehicle treated groups (Figure 6).
Discussion
The present study provides strong evidence that PPARγ activation is preserved in the livers of young mice and that this may represent an important age-dependent protective mechanism against hepatic I/R injury. Our recent studies have demonstrated that pretreatment of adult mice with rosiglitazone activated PPARγ in hepatocytes which significantly reduced liver I/R injury (7). In contrast, PPAR γ+/− mice, which have diminished PPAR γactivity, had more severe liver injury than the wild type mice. Interestingly, the rosiglitazone treatment resulted primarily in restoring PPARγ activation during the early ischemic period only. Thus, the activation of PPARγ induced by rosiglitazone treatment in adult mice is nearly identical to that observed in young mice in the current study. This suggests that prolonged activation of PPARγduring hepatic ischemia is an age-dependent protective mechanism that is lost in adult and old mice.
To determine if the differences in PPARγ activation were due to differences in endogenous ligands, we measured the expression of several ligands during ischemia. In agreement with our previous study, level of PPARγ ligand 15d-PGJ2 decreased sharply during ischemia in the adult/old mice. Surprisingly, the level of 15d-PGJ2 was lowest in the young mice and did not change during ischemia. Furthermore, there was no significant difference in the levels of other PPARγ ligands between age groups. These data suggest that the differences in PPARγ activation between young mice and the older groups are not related to ligand expression.
PPARγ has two isoforms, PPARγ1 and PPARγ2, generated by alternative promoter usage and splicing (13). PPARγ1 is the predominant isoform found in all tissues that express PPARγ, except in the adipocytes where PPARγ2 is the major isoform. In the present studies, we found a differential compartmentalization of PPARγ isoforms in the liver. In the cytoplasm, expression of PPARγ2 is similar in all age groups and does change significantly with ischemia. Interestingly, PPARγ1 was observed in the cytoplasm only in the old mice during ischemia. However, in the nucleus there was much stronger expression of PPARγ1 compared to PPARγ2. In all age groups, nuclear expression of PPARγ1 was highest at baseline and gradually diminished during ischemia. In young mice, loss of PPARγ1 expression was significantly delayed. PPARγ1 expression in the nucleus is strikingly similar to PPARγ-DNA-binding kinetics, suggesting that PPARγ1 may be the active isoform in the hepatocytes. Another notable finding of our study is that in the old mice, the loss of nuclear PPARγ1 during ischemia is accompanied by its appearance in the cytoplasm. These observations raise the possibility that the rapid loss of PPARγ activity in the old mice may be due to selective trafficking of PPARγ1 to the cytoplasm during ischemia in this age group.
Although others have reported that PPARγ regulates hepatic I/R injury by modulating inflammation (8), our collective studies suggest that other, non-inflammatory mechanisms are important. Our previous study (7) showed that activation of PPARγ reduced injury without affecting the inflammatory response. Furthermore, that study showed that the majority of PPARγ-DNA-binding activity in the liver was found in hepatocytes. However, the mechanism by which PPARγ activation could be directly hepatoprotective was not discerned. Our current study suggests that increased PPARγ activation is associated with increased autophagy during ischemia. Importantly, we demonstrate that rosiglitazone treatment, which increases PPARγ activation in hepatoctyes, also increases autophagy. This suggests a direct link between PPARγ activation and induction of autophagy. Autophagy is an evolutionarily conserved cellular process for recycling of old proteins and organelles via the lysosomal degradation (14). It has a constitutive function in resting cells where it is involved in the routine turnover of proteins and organelles. Autophagy can also be activated in stressful stimuli such as nutrient deprivation, I/R and oxidative stress (16, 17). Induction of autophagy in certain situations has been shown to be protective. For example, autophagy induction after nutrient deprivation is essential for cell survival (14). Recent discoveries suggest that autophagy during ischemia may have protective function by providing cells with the energy derived from lysosomal degradation of cellular materials (18). Thus, it is plausible that prolonged activation of PPARγ in young mice confers hepatoprotection by more effective recycling of cellular components for energy.
A previous study from our lab showed that another PPAR, PPARα, was protective against hepatic I/R injury (19). However, that study did not assess the activation kinetics of PPARα, or whether there was any difference in PPARα activation relevant to age. Whether PPARα has similar regulatory effects as PPARγ in this response warrants further study.
In summary, we show that during hepatic ischemia, PPARγ activation is significantly prolonged in young mice compared to older mice. This increased activity was associated with higher expression of PPARγ1 in the nucleus. PPARγ activation was also associated with autophagy in the liver. Stimulation of PPARγ in adult mice with rosiglitazone treatment resulted in increased autophagy, demonstrating a direct link between PPARγ activation and induction of autophagy. These data indicate an age-dependent difference in PPARγ activity and suggest that previously documented hepatoprotective effects of PPARγ may be related to induction of autophagy.
Figure 5.
Effect of rosiglitazone on hepatic autophagy during ischemia in adult mice. Mice were treated orally with methylcellulose (veh) or 10 mg/kg rosiglitazone (rosi) dissolved in methylcellulose 30 minutes prior to the induction of ischemia. Liver lysates were analyzed for autophagy activity by Western blot for LC3B. Results are representative of 2 sets of experiments.
Acknowledgments
This work was supported by National Institutes of Health grants AG025881 and DK56029 to A.B.L. Special thanks to Dr. Ronald Jandacek and Therese Rider at the Mouse Metabolic Phenotyping Center at the University of Cincinnati (supported by DK59630) for performing the fatty acid analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lentsch AB, Kato A, Yoshidome H, McMasters KM, Edwards MJ. Inflammatory mechanisms and therapeutic strategies for warm hepatic ischemia/reperfusion injury. Hepatology. 2000;32:169–173. doi: 10.1053/jhep.2000.9323. [DOI] [PubMed] [Google Scholar]
- 2.Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 2003;284:G15–26. doi: 10.1152/ajpgi.00342.2002. [DOI] [PubMed] [Google Scholar]
- 3.Okaya T, Blanchard J, Schuster R, Kuboki S, Husted T, Caldwell CC, Zingarelli B, Wong H, Solomkin JS, Lentsch AB. Age-dependent responses to hepatic ischemia/reperfusion injury. Shock. 2005;24:421–427. doi: 10.1097/01.shk.0000181282.14050.11. [DOI] [PubMed] [Google Scholar]
- 4.Calkins CM, Bensard DD, Moore EE, McIntyre RC, Silliman CC, Biffl W, Harken AH, Partrick DA, Offner PJ. The injured child is resistant to multiple organ failure: a different inflammatory response? J Trauma. 2002;53:1058–1063. doi: 10.1097/00005373-200212000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Clavien PA, Selzner M, Rudiger HA, Graf R, Kadry Z, Rousson V, Jochum W. A prospective randomized study in 100 consecutive patients undergoing major liver resection with versus without ischemic preconditioning. Ann Surg. 2003;238:843–850. doi: 10.1097/01.sla.0000098620.27623.7d. discussion 851–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Markmann JF, Markmann JW, Markmann DA, Bacquerizo A, Singer J, Holt CD, Gornbein J, Yersiz H, Morrissey M, Lerner SM, McDiarmid SV, Busuttil RW. Preoperative factors associated with outcome and their impact on resource use in 1148 consecutive primary liver transplants. Transplantation. 2001;72:1113–1122. doi: 10.1097/00007890-200109270-00023. [DOI] [PubMed] [Google Scholar]
- 7.Kuboki S, Shin T, Huber N, Eismann T, Galloway E, Schuster R, Blanchard J, Zingarelli B, Lentsch AB. Peroxisome proliferator-activated receptor-gamma protects against hepatic ischemia/reperfusion injury in mice. Hepatology. 2007;47:215–224. doi: 10.1002/hep.21963. [DOI] [PubMed] [Google Scholar]
- 8.Akahori T, Sho M, Hamada K, Suzaki Y, Kuzumoto Y, Nomi T, Nakamura S, Enomoto K, Kanehiro H, Nakajima Y. Importance of peroxisome proliferator-activated receptor-gamma in hepatic ischemia/reperfusion injury in mice. J Hepatol. 2007;47:784–792. doi: 10.1016/j.jhep.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 9.Lentsch AB, Yoshidome H, Cheadle WG, Miller FN, Edwards MJ. Chemokine involvement in hepatic ischemia/reperfusion injury in mice: roles for macrophage inflammatory protein-2 and Kupffer cells. Hepatology. 1998;27:507–512. doi: 10.1002/hep.510270226. [DOI] [PubMed] [Google Scholar]
- 10.Deryckere F, Gannon F. A one-hour minipreparation technique for extraction of DNA-binding proteins from animal tissues. Biotechniques. 1994;16:405. [PubMed] [Google Scholar]
- 11.Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 12.Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS, Devchand P, Wahli W, Willson TM, Lenhard JM, Lehmann JM. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc Natl Acad Sci U S A. 1997;94:4318–4323. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu Y, Qi C, Korenberg JR, Chen XN, Noya D, Rao MS, Reddy JK. Structural organization of mouse peroxisome proliferator-activated receptor gamma (mPPAR gamma) gene: alternative promoter use and different splicing yield two mPPAR gamma isoforms. Proc Natl Acad Sci U S A. 1995;92:7921–7925. doi: 10.1073/pnas.92.17.7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yorimitsu T, Klionsky DJ. Autophagy: molecular machinery for self-eating. Cell Death Differ. 2005;12 (Suppl 2):1542–1552. doi: 10.1038/sj.cdd.4401765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodway HA, Hunt AN, Kohler JA, Postle AD, Lillycrop KA. Lysophosphatidic acid attenuates the cytotoxic effects and degree of peroxisome proliferator-activated receptor gamma activation induced by 15-deoxyDelta12,14-prostaglandin J2 in neuroblastoma cells. Biochem J. 2004;382:83–91. doi: 10.1042/BJ20040107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takagi H, Matsui Y, Sadoshima J. The role of autophagy in mediating cell survival and death during ischemia and reperfusion in the heart. Antioxid Redox Signal. 2007;9:1373–1381. doi: 10.1089/ars.2007.1689. [DOI] [PubMed] [Google Scholar]
- 17.Levine B, Yuan J. Autophagy in cell death: an innocent convict? J Clin Invest. 2005;115:2679–2688. doi: 10.1172/JCI26390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100:914–922. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 19.Okaya T, Lentsch AB. Peroxisome proliferator-activated receptor-α regulates postischemic liver injury. Am J Physiol Gastrointest Liver Physiol. 2004;286:G606–G612. doi: 10.1152/ajpgi.00191.2003. [DOI] [PubMed] [Google Scholar]