Abstract
Cell cycle re-entry of quiescent T lymphocytes regulated by cdk2 is required for antigen-specific clonal expansion and generation of productive T cell responses. Recently, we determined that induction of antigen-specific T cell tolerance results in impaired cdk2 activity leading to enhanced Smad3 transactivation, upregulation of p15 and blockade of cell cycle progression. Here we report that pharmacologic inhibition of cdk2 with (R)-roscovitine blocked expansion of alloreactive T cells in vitro and in vivo and protected from lethal acute GvHD. In addition to inhibiting alloreactive T cell responses, (R)-roscovitine prevented TNFα-mediated activation of NFκB pathway, which is involved in the inflammatory process leading to the development of GvHD. The combined anti-proliferative and anti-inflammatory properties of (R)-roscovitine make it an attractive treatment modality toward control of GvHD.
Keywords: alloreactive T cells, cell cycle, cdk inhibitors, NFκB, TNFα, GvHD
Introduction
Cellular immune responses require expansion of antigen-specific T cell clones from the pool of resting T lymphocytes that perform immune surveillance. Highly controlled regulation of this proliferative potential is critical for defense against pathogens and foreign antigens with simultaneous avoidance of autoimmunity.1,2 Cell cycle re-entry of quiescent T lymphocytes is required for generation of productive antigen-specific T cell responses. Naïve T cells are unable to efficiently produce effector cytokines, but these cells gain effector functions after several rounds of cell division following initial activation.3
Cyclin-dependent kinases (cdk), particularly cdk2, have an essential role in cell cycle re-entry. Cdk2 promotes phosphorylation of Rb and related pocket proteins thereby reversing their ability to sequester E2F transcription factors.4 Besides Rb, cdk2 phosphorylates Smad2 and Smad3.5 Smad3 inhibits cell cycle progression from G1to S phase, and impaired phosphorylation on the cdk-mediated sites renders it more effective in executing this function. In contrast, cdk-mediated phosphorylation of Smad3 reduces Smad3 transcriptional activity and antiproliferative function. Recently, we determined that induction of antigen-specific T cell tolerance results in impaired cdk2 activity, leading to reduced levels of Smad3 phosphorylation on cdk-specific sites and increased Smad3 transactivation and antiproliferative function due to upregulation of p15.6
Since cdk2 is involved in multiple steps of the cell activation process,4 we hypothesized that pharmacologic inhibition of cdk2 during alloantigen-mediated T cell stimulation might provide an effective strategy to control T cell clonal expansion and induce tolerance. Such approach might have a significant clinical application for the control of graft versus host disease (GvHD), which is induced by donor T cells stimulated by recipient’s alloantigens and remains a frequent and severe complication of allogeneic hematopoietic stem cell transplantation (HSCT). Experimental and clinical data suggest that host antigen-presenting cells, presenting alloantigen to donor T cells, initiate a cascade of events resulting in development of cytotoxic effectors and release of inflammatory cytokines and chemokines. Activated donor leukocytes subsequently traffic to specific host tissues where along with soluble factors cause organ damage and dysfunction.7 In the context of hematologic malignancies a delicate balance exists between the harmful consequences of GvHD and the beneficial effects incurred when donor T cells attack recipient malignant cells, a process referred to as the graft versus leukemia effect (GvL). A major limitation to the preventive and therapeutic approaches for control of GvHD is the compromise of the GvL effect of allo-HSCT.
To evaluate whether pharmacologic inhibition of cdk2 might control GvHD, we employed (R)-roscovitine (CYC202), a potent inhibitor of cdk2-cyclin E with a 50% inhibitory concentration (IC50) of 0.1 μM as well as complexes of cdk7-cyclin H, cdk9-cyclin T1 and cdk5-p35-p25 with IC50 of 0.4 μM, 0.8 μM and 0.16 μM respectively.8 Roscovitine was recently shown to induce long-lasting arrest of murine polycystic kidney disease9,10 to limit glomerulonephritis and extend the lifespan of mice with systemic lupus11 and to prolong survival of kidney allografts in a rat model of fully MHC-mismatched kidney transplantation model.12 Here, we determined that roscovitine suppressed expression and activation of alloreactive T cells in vitro and in vivo and protected from acute lethal GvHD. Mechanistic studies revealed that roscovitine exerted its activity on alloreactive T cells in multiple levels. Roscovitine blocked expansion of alloreactive T cells by inhibiting cdk2 activity, promoted apoptosis by suppressing RNA pol II-mediated expression of Mcl-1, and prevented TNFα-mediated activation of the NFκB pathway that is involved in the development of GvHD. These results suggest that the combined anti-proliferative and anti-inflammatory activity of roscovitine might be exploited for therapeutic purposes to control GvHD.
Results
(R)-roscovitine inhibits T-cell expansion and activation
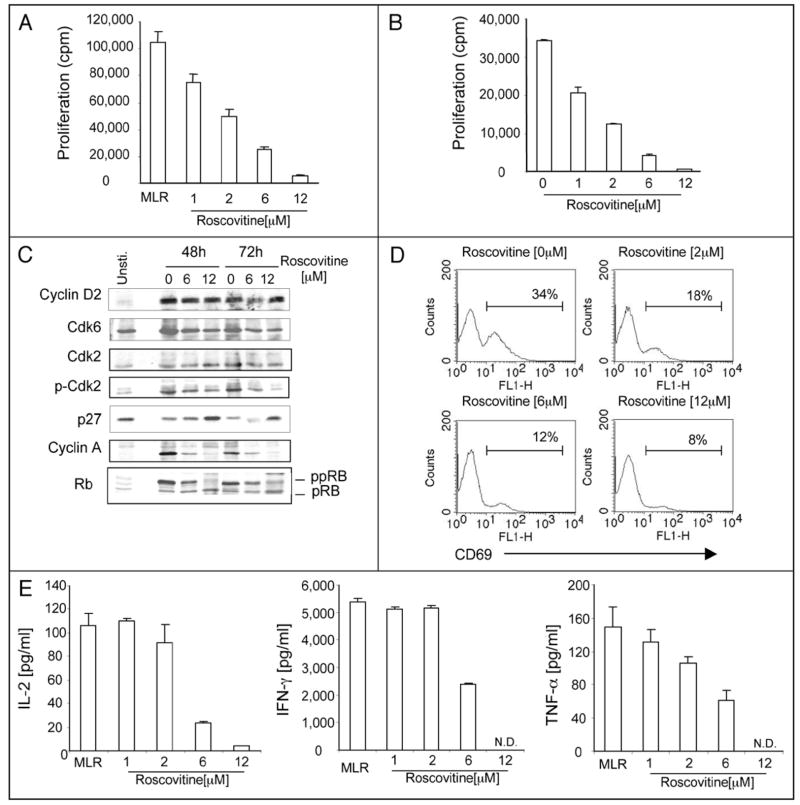
To determine whether roscovitine might affect alloreactive T cell responses in vitro we used mixed lymphocyte reaction (MLR) cultures, in which C57/B6 T cells were stimulated with MHC disparate Balb/c splenocytes. (R)-roscovitine used as low as 2 μM resulted in reduction of cell proliferation by 50% (Fig. 1A). A comparable inhibitory effect was observed when T-cell proliferation was induced by anti-CD3 and anti-CD28 antibodies (Fig. 1B). Biochemical analysis revealed that roscovitine allowed synthesis of cyclin D2 indicating that antigen-specific T cells had entered the G1 phase of the cell cycle (Fig. 1C). Under these conditions, roscovitine prevented phosphorylation of cdk2, down-regulation of p27, phosphorylation of Rb and synthesis of cyclin A (Fig. 1C), suggesting an effective G1/S cell cycle block. Besides cell cycle progression, roscovitine also significantly inhibited T cell activation as determined by diminished expression of surface activation marker CD69 (Fig. 1D).
Figure 1.
Roscovitine inhibits expansion and effector function of T cells in response to antigen stimulation. Purified T cells were cultured with irradiated allogeneic splenocytes (A and C–E) or with anti-CD3 and antiCD-28 antibodies (B) in the absence or the presence of titrated dose of roscovitine or vehicle control. Proliferative capacity was assessed by incorporation of [3H] thymidine at day 3 of the culture (A and B). Results are expressed as mean ± standard deviation (n = 3) and are representative of four independent experiments. (C) Cell lysates were prepared at the indicated time intervals and after SDS-PAGE expression of cell cycle regulators was analyzed by immunoblot with indicated antibodies. Results are representative of three independent experiments. (D) Cells were harvested at day 3 of the culture and surface expression of CD69 was analyzed on gated T lymphocytes by flow cytometry. Similar pattern of results was obtained in three separate experiments. (E) Culture supernatants from experiments described in (A) were collected on day 2 (IL-2, IFNγ) or day 3 (TNFα) of culture and concentration of cytokines was measured by ELISA. 0 μM of roscovitine stands for vehicle (DMSO) alone.
To examine the effect of roscovitine on the effector function of alloreactive T cells, we assessed cytokine production. As shown in Figure 1E, unlike proliferation that was inhibited by low concentrations of roscovitine (Fig. 1A and B), 1 to 2 μM of roscovitine did not alter levels of IL-2 and IFNγ production, whereas 2 μM of roscovitine had a moderate inhibitory effect on production of TNFα. However, alloreactive T cells cultured with ≥6 μM roscovitine produced significantly reduced cytokines in comparison to T cells cultured with vehicle alone (Fig. 1E). These results indicate that suppression of T cell proliferation by low concentrations of roscovitine was mediated via a direct effect of roscovitine on cell cycle progression and not via inhibition of autoregulatory cytokine production. However, at higher concentrations, roscovitine, also inhibited cytokine production.
Proliferating T cells are sensitive to roscovitine-mediated apoptosis
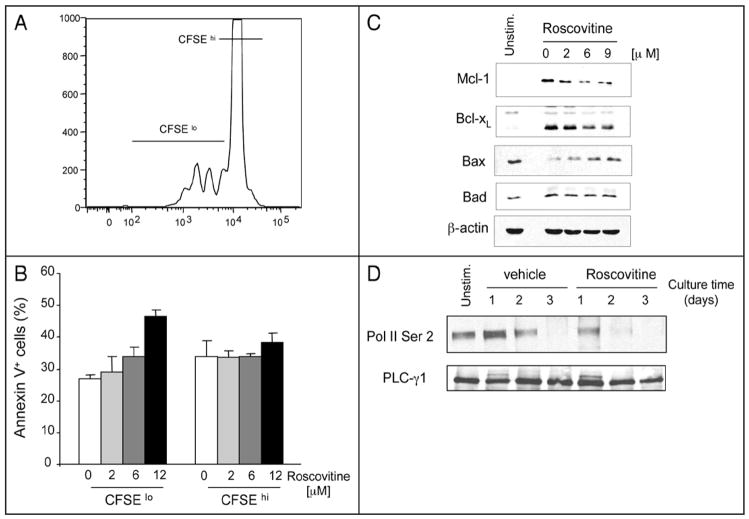
It has been reported that roscovitine induces not only cell cycle arrest but also apoptosis in cancer cells.14–21 Thus, we sought to investigate whether roscovitine induced apoptosis in T cells and, if so, to determine whether roscovitine might equally affect viability of proliferating or non-dividing T cells. Assessment of cell division by CFSE dye dilution indicated that although roscovitine significantly suppressed T cell proliferation compared to control cultures (data not shown), a small proportion of cells were capable of undergoing cell division (Fig. 2A). Assessment of apoptosis by surface binding of annexin V demonstrated that roscovitine induced a dose-dependent increase of annexin V+ apoptotic cells in the CFSElo dividing T cell population but did not affect survival of CFSEhigh non-dividing T cells (Fig. 2A and B). These results suggest that proliferating T cells were the selective target of roscovitine-mediated apoptosis.
Figure 2.
Roscovitine increases apoptosis of proliferating cells by altering expression of Mcl-1 and Bax. (A and B) CFSE-labeled T cells were stimulated with anti-CD3 and anti-CD28 antibodies for 48 hrs and viability of proliferating cells (CFSElo) and non-proliferating cells (CFSEhi) was determined by expression of Annexin V. Results shown in (B) represent mean values of two independent experiments (p = 0.02). (C and D) Purified T cells were stimulated with anti-CD3 and anti-CD28 antibodies in the absence or the presence of roscovitine. Cells were cultured for 48 hours with the indicated concentrations of roscovitine (C) or with 12 μM roscovitine for the indicated time points (D), cell lysates were prepared and protein expression was analyzed by SDS-PAGE and immunoblot with the indicated antibodies. Immunoblots for b-actin and PLC-g1 were used as loading control for (C and D), respectively.
Next, we evaluated the potential molecular mechanisms by which proliferating cells might be sensitive to roscovitine-mediated cytotoxicity. During antigenic stimulation, the fate of T lymphocytes toward survival or cell death is determined by the balanced expression of pro-apoptotic and anti-apoptotic regulators of the bcl-2 superfamily. Among them, Bcl-2, Bcl-xL and Mcl-1 promote survival whereas Bax, Bad and Bcl-xs promote apoptosis.22 Studies in other cell types have shown that roscovitine promotes apoptosis of cancer cells by downregulating expression levels of Mcl-1.16,17 Analysis of cell lysates at various times after T cell culture showed that roscovitine had a potent inhibitory effect on Mcl-1 induction during T cell activation (Fig. 2C). In contrast to its effects on Mcl-1, roscovitine had a minor inhibitory effect on activation-dependent upregulation of Bcl-xL (Fig. 2C). Expression of Bad also remained unaltered but roscovitine induced a dose-dependent increase of Bax expression in activated T cells (Fig. 2C).
MCL-1 is an important transcriptional target of RNA pol II and its expression is downregulated by roscovitine in other cell types.16–20 In addition to cdk2, roscovitine has significant inhibitory activity against cdk7 and cdk9, which regulate transcription by phosphorylating the carboxy-terminal domain of RNA polymerase II (RNA pol II).9,16,18,19 As shown in Figure 2D, unstimulated T cells expressed detectable levels of RNA pol II phosphorylation on serine 2. Stimulation resulted in increased phosphorylation of RNA pol II that peaked on day 1 of culture and gradually declined thereafter until day 3 (Fig. 2D, lanes 2–4). In contrast, roscovitine abrogated increase of RNA pol II phosphorylation (Fig. 2D, lanes 5–7).
(R)-roscovitine affects TNFα-mediated NFκB activation
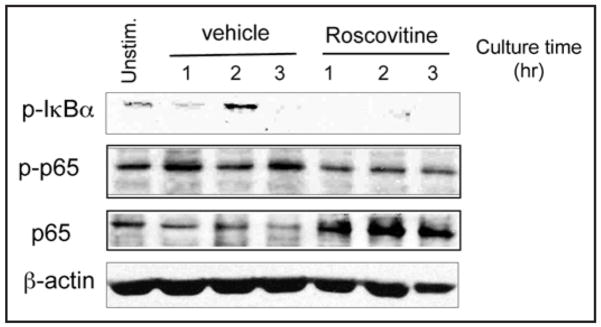
In cancer cells, roscovitine downregulates NFκB activation in response to TNFα and suppresses NFκB-mediated gene transcription.23 During allogeneic HSCT, recognition of host alloantigens activates alloreactive donor T cells and stimulates cytokine production among which TNFα has a central role in the development of GvHD.24–27 For these reasons, we investigated whether roscovitine might have a similar effect on TNFα-mediated activation of NFκB in T lymphocytes as in cancer cells. Phosphorylation of IkBa by IkB kinase (IKK) is required for IkBa degradation and subsequent nuclear accumulation of NFκB.28 IKK also phosphorylates NFκB p65 and this event is mandatory for p65 nuclear translocation and NFκB transcription function.29 Addition of roscovitine in T cell cultures incubated with TNFα resulted in defective phosphorylation of IkBa and p65 (Fig. 3, first and second panels). Moreover, roscovitine prevented nuclear translocation and resulted in cytoplasmic accumulation of p65 (Fig. 3, third panel). Because phosphorylation on serine 536 of p65, which is required for TNFα-induced NFκB transcription, is considered a bona fide site of IkB kinase (IKK) site,28 these results indicate that roscovitine is an inhibitor of canonical IKK signaling in T cells.
Figure 3.
Roscovitine regulates TNFα-mediated NFκB activation. Purified T cells were treated with TNFα (100 ng/ml) in the absence or the presence of 12 μM roscovitine for indicated time points. Cytoplasmic cell lysates were prepared and effects of roscovitine on TNFα induced phosphorylation of IkBa and phosphorylation of Ser536 of p65 were analyzed by immunoblotting with specific antibodies.
(R)-roscovitine protects from lethal acute GvHD in vivo
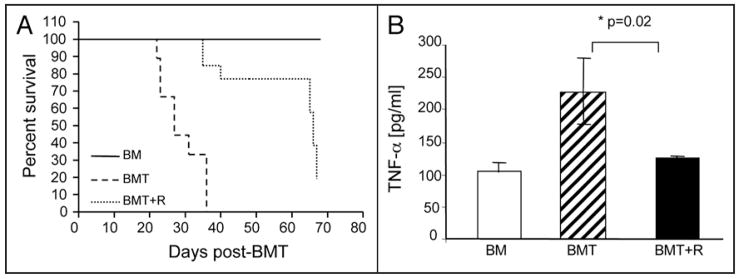
Our data showed that roscovitine reversed both TCR-mediated clonal expansion of alloreactive T cells and TNFα-mediated inflammatory responses. Because TCR-mediated clonal expansion of alloreactive T cells and TNFα-mediated inflammatory processes are directly involved in the pathophysiology of GvHD we hypothesized that roscovitine might represent a novel therapeutic approach toward control of GvHD. To investigate this hypothesis, we employed an in vivo mouse model of allogeneic bone marrow transplantation.13 Recipient B6D2F1 mice were lethally irradiated and were subsequently infused with bone marrow cells and splenocytes—as source of allogeneic T cells—(BMT) from parental B6 donors. Roscovitine or vehicle-control was administered at the time of allogeneic BMT and on a daily basis thereafter for three weeks. Administration of roscovitine resulted in protection against acute GvHD, as determined by significantly prolonged survival (Fig. 4A). Systemic levels of TNFα, a biochemical marker that correlates with the severity of acute GvHD,27 was significantly increased in B6D2F1 recipients infused with bone marrow and splenocytes from B6 donors (BMT group), compared to control recipients that were infused with bone marrow only without donor splenocytes (BM group) (Fig. 4B). Notably, in BMT recipients treated with roscovitine (BMT + R group), the serum TNFα level was comparable to that in the control (BM) group, suggesting that roscovitine inhibited the in vivo inflammatory process associated with GvHD (Fig. 4B).
Figure 4.
Roscovitine protects from lethal acute GvHD in vivo. Lethally irradiated (1,000 cGy) B6D2F1 mice (H-2b/d) underwent transplantation with either bone marrow alone (BM) (n = 5) or with bone marrow and splenocytes from parental B6 (H-2b) mice, as described in Materials and Methods. Mice that received bone marrow and splenocytes (n = 11 to 15 per group) were subsequently treated with vehicle (BMT) or with roscovitine (BMT + R) on the day of transplantation and daily thereafter for a total of three weeks. Survival (A) was monitored after transplantation and significantly delayed mortality of lethal acute GvHD was observed in roscovitine treated mice (BMT + R) compared with control-treated mice (BMT) (p = 0.001). (B) Serum was obtained on day 7 after transplantation and concentration of TNFα was determined by ELISA. Results are expressed as mean value from 3–7 mice in each group ± standard deviation.
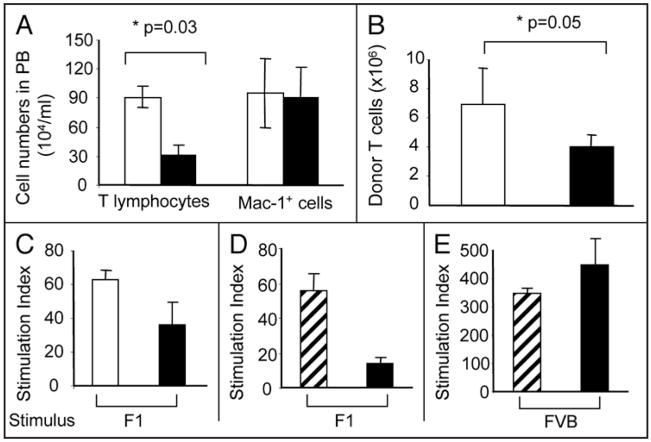
To obtain insight to the action of roscovitine on T cells in vivo, we analyzed its effect on proliferation of alloreactive T lymphocytes in recipient animals. Seven days after infusion of bone marrow cells and allogeneic splenocytes, B6D2F1 recipients treated with vehicle alone had a high number of in vivo expanded B6 donor-derived alloreactive T cells in the peripheral blood (Fig. 5A). In contrast, roscovitine treated recipients displayed significantly reduced in vivo expansion of alloreactive donor T cells (Fig. 5A). Notably, roscovitine exerted its inhibitory effect predominantly on T lymphocytes as determined by the presence of comparable numbers of macrophages (Mac-1+) in mice treated with roscovitine or vehicle control (Fig. 5A). Similarly to the reduced expansion of alloreactive donor T cells in the peripheral blood, significant suppression of in vivo expanded alloreactive donor T cells was detected in the spleen of B6D2F1 recipients treated with roscovitine (Fig. 5B). Rechallenge of B6 donor-derived T cells isolated from these recipients with host splenocytes (B6D2F1) indicated that alloreactive responses of donor T cells against host were reduced in roscovitine treated recipients compared to responses of vehicle treated recipients (Fig. 5C). Strikingly, a number of roscovitine-treated mice achieved prolonged survival (≥3 months) after discontinuation of roscovitine treatment. These mice developed full donor chimerism (data not shown) indicating that thymic function was not affected by treatment with roscovitine. Rechallenge of T cells from these long-term survivors with splenocytes from either host (B6D2F1) mice or from third-party (FVB) mice revealed that host-specific T cell responses were abrogated (Fig. 5D) but responses against third-party stimulators were preserved (Fig. 5E), indicating that host-specific tolerance had been achieved.
Figure 5.
Roscovitine inhibits in vivo expansion of allogeneic donor T cells after BMT transplantation. Lethally irradiated B6D2F1 mice (H-2b/d) underwent transplantation with bone marrow cells and splenocytes from parental B6 donors as described in Materials and Methods. Recipients were given either roscovitine (filled bars) or vehicle (open bars) as described in Figure 4. (A) Seven days after BMT transplantation, total peripheral blood nucleated cells were counted; donor T cell (CD3+) and myeloid cell (Mac-1+) populations were determined by flow cytometry as described in Materials and Methods. Donor T cell expansion was significantly reduced (p = 0.03) in roscovitine treated recipients (n = 8) compared to non-treated recipients (n = 9). (B) Three weeks after BMT transplantation, total splenocytes were counted and donor T cells were determined by flow cytometry. Roscovitine significantly inhibited (p = 0.05) in vivo expansion of allogeneic T cells (n = 5 per group). (C–E) Three weeks (C) or 3 months (D and E) after BMT transplantation, T cells from roscovitine treated (filled bars), non-treated (open bars) recipients, or wild type B6 mice (hatched bars) were stimulated with irradiated allogeneic splenocytes from B6D2F1 or FVB mice and proliferative response was determined by [3H] incorporation. Stimulation index is expressed as the ratio of proliferation in response to host antigens (B6D2F1) or third party antigens (FVB) over proliferation in response to donor antigens (B6) and results are expressed as mean ± standard deviation (n = 3 for results shown in C, and n = 2 for results shown in D and E).
Treatment with (R)-roscovitine preserves anti-tumor activity in vivo
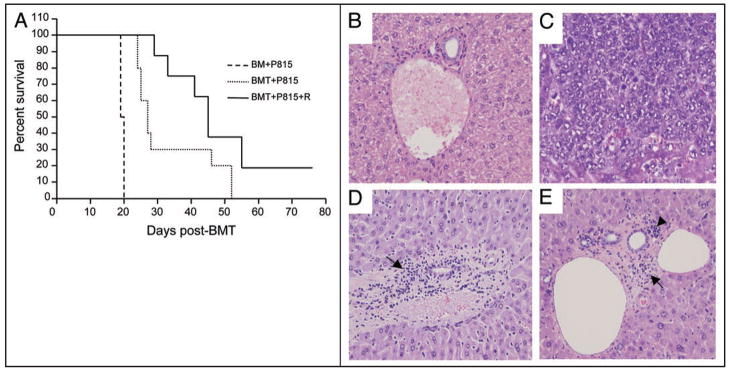
Alloreactive donor T cells transferred with the allogeneic HSC graft are directly associated with GvHD but also mediate GvL, an effect that is desired and highly beneficial in patients with malignant hematologic diseases.7,30 Therefore, we used a previously established model13 to examine whether in addition to suppressing GvHD, roscovitine might also compromise GvL during allogeneic HSCT. B6D2F1 recipient mice were lethally irradiated and subsequently received bone marrow transplants without concomitant infusion of allogeneic (B6) T cells or with concomitant infusion of allogeneic (B6) T cells followed by treatment with either roscovitine or vehicle control. In all treatment groups, host-type (H-2d) P815 tumor cells were added to the bone marrow inoculum at the time of infusion. All animals receiving bone marrow only (BM + P815) died of disseminated P815 tumor cell infiltration by day 19 to 20 (Fig. 6A and C). In contrast, recipients of allogeneic T cells (BMT + P815) effectively rejected their tumor as they had neither evidence of macroscopic tumor growth in the autopsy nor findings of microscopic tumor infiltration in the liver (Fig. 6D), indicating that a GvL effect was mediated by the allogeneic T lymphocytes. Survival of these animals was prolonged (p = 0.009) but, eventually, died of GvHD by day 50 post-transplant (Fig. 6A) consistent with previous observations in this experimental model.13 Administration of roscovitine significantly reduced severity of liver GvHD as determined by reduced inflammatory changes and lymphocyte infiltration in the portal areas (Fig. 6E, compare with D). Roscovitine (BMT + P815 + R) further prolonged survival (Fig. 6A) compared to recipients of P815 tumor and bone marrow only (BM + P815) (p = 0.002) with concomitant preservation of GvL activity as determined by the absence of macroscopic or microscopic evidence of tumor growth (Fig. 6E).
Figure 6.
Roscovitine preserves anti-tumor activity induced after administration of allogeneic T cells in vivo. (A) Lethally irradiated F1 mice were infused with bone marrow alone or with T cells from parental B6 mice as described in Materials and Methods. P815 (H-2d) tumor cells were added to the BM inoculum on day 0 of transplantation. Subsequently, animals receiving allogeneic T cells were treated with either vehicle (BMT + P815) or with roscovitine (BMT + P815 + R) as described in Figure 4 (n = 8 to 10 per group). Anti-tumor activity induced after administration of allogeneic T cells is maintained during treatment with roscovitine (p = 0.009, survival of P815 recipients transplanted with bone marrow and allogeneic T cells vs. P815 recipients transplanted with bone marrow only; p = 0.002, survival of P815 recipients transplanted with bone marrow and allogeneic T cells treated with roscovitine vs. P815 recipients transplanted with bone marrow only). (B–E) Histopathology of the liver was assessed for GvHD severity and tumor infiltration (n = 5 to 9 per group). (B) Normal liver as control, (C) P815 recipients transplanted with bone marrow only, (D) P815 recipients transplanted with bone marrow and allogeneic T cells, (E) P815 recipients transplanted with bone marrow and allogeneic T cells, treated with roscovitine. Roscovitine treated animals displayed reduced inflammatory changes and lymphocyte infiltration in the portal areas (arrows), without evidence of tumor growth. Arrowhead indicates hematopoietic progenitors engrafted in the liver. Original magnification ×200.
Discussion
In the present study we examined the effects of the cdk inhibitor R-roscovitine on the responses of alloreactive T cells in vitro and in vivo using a mouse model that is directly related to disease pathophysiology. Our data showed that roscovitine inhibited clonal expansion of alloreactive T cells in vitro and in vivo. Furthermore, both in vitro and in vivo, roscovitine reduced levels of TNFα, a major cytokine mediating tissue damage in GvHD.26,27 Besides activated donor T cells, sources of TNFα during acute GvHD are inflammatory process secondary to tissue damage induced by conditioning therapy.7 Roscovitine markedly enhances resolution of established neutrophil-dependent inflammation by inducing apoptosis of inflammatory cells.31 Thus, in vivo, roscovitine may inhibit alloreactive T cell expansion and TNFα production, similarly to its effects on T cells activated in vitro, but may also promote resolution of tissue inflammation by its effects on other cell types, thereby controlling GvHD via multiple mechanisms.
An additional novel observation of our studies was that roscovitine inhibited TNFα mediated activation of the canonical NFκB pathway in T cells. NFκB controls the expression of a number of genes important for mediating immune and inflammatory responses.32 The role of NFκB pathway in the induction of GvHD is well established. PS-1145, a specific IKK inhibitor that inhibits phosphorylation and degradation of IKBa induced by TNFα, protects from lethal GvHD.33 Roscovitine inhibits IKK activity and suppresses TNFα-mediated phosphorylation and p65 in cancer cells.23 Our present results showed that roscovitine inhibited TNFα mediated activation of IKK in T lymphocytes as determined by inhibition of IKBa phosphorylation, inhibition of p65 phosphorylation at serine 536 and impaired nuclear translocation of p65. In our system, we did not observe inhibition of TCR-mediated NFκB activation when roscovitine was used in the same concentration range that inhibited TNFα-mediated signals (Li L and Boussiotis VA, unpublished observations). This observation indicates that activation of NFκB through TNFα and through TCR display distinct sensitivity to roscovitine and suggest that activation of NFκB by antigen (i.e., pathogens or tumor antigens) might not be affected at doses that would inhibit TNFα mediated activation of NFκB.
Our studies indicated that roscovitine did not compromise thymic function, as determined by development of full donor chimerism in long-term surviving allogeneic bone marrow recipients. These results are consistent with previous observations that hematopoiesis and thymic maturation were not affected by the loss of ckd2 activity.34 Notably, detailed studies on the effect of roscovitine on hematopoietic progenitors showed that roscovitine had significant inhibitory effect on CFU-GM, CFU-GEMM and BF-U growth only when bone marrow cells were treated in vitro with high concentrations of roscovitine for prolonged periods of time. In contrast, in vivo administration of roscovitine had a mild and transient effect on BFU-E and no significant impact on CFU-E and CFU-GEMM growth.35
At a mechanistic level, we determined that the inhibitory effects of roscovitine on T cell expansion were at least two fold. Roscovitine inhibited cdk2 activation resulting in blockade of cell cycle progression. In addition, roscovitine inhibited phosphorylation of RNA pol II. This mechanism may have additional suppressive effects on expansion of activated T cells because it regulates expression of survival genes, as previously described in kidney epithelial cells and cancer.9,16 Consistent with this hypothesis, we observed that although roscovitine significantly suppressed T cell proliferation, a small proportion of cells were capable of undergoing cell division and roscovitine selectively induced apoptosis in this dividing population. Previous studies have shown that in extensively dividing cell populations, roscovitine stimulates apoptosis. Conversely, in non-dividing differentiated cells, such as neurons and thymocytes, roscovitine has protective effect.36 Induction of apoptosis by roscovitine has been associated with its effects on genes of the BCL-2 family.16,17,37 In myeloma cell lines, roscovitine inhibited cdks that phosphorylate the C-terminal domain of the large subunit of RNA polymerase II, thus, inhibiting its transcriptional activity and resulting in rapid downregulation of MCL-1 mRNA, and inhibition of Mcl-1 protein synthesis.16 Roscovitine also promotes neutrophil apoptosis by reducing concentrations of Mcl-1.31 Our present studies showed that Mcl-1 was upregulated upon T cell activation and this event was abrogated in the presence of roscovitine. These observations suggest that during acute GvHD roscovitine might preferentially lead to elimination of activated, replicating alloreactive T lymphocytes, without targeting non-dividing T cells thus, without loss of T cell subsets with specificity for other antigens such as pathogens and tumor antigens. The combined anti-proliferative and anti-inflammatory properties of (R)-roscovitine make it an attractive treatment modality toward control of GvHD.
Materials and Methods
Cell line, antibodies and reagents
P815 (H-2d) from ATCC (Manassas, VA) is a mastocytoma cell line of DBA/2 mouse origin. Fluorochrome-labeled anti-murine antibodies against CD69, CD3, H-2d and CD11b were obtained from eBioscience (San Diego, CA). Antibodies against Cyclin D2, Cyclin A, Cdk6, Cdk2, p27, Rb, b-actin and PLC-g1 were obtained from Santa Cruz (Santa Cruz, CA). Antibodies against p-Cdk2, Bax, p-IkBa and p-p65 were purchased from cell signaling Technology, Inc., (Danvers, MA). Antibodies against Bcl-xL, Mcl-1, p65 and p-RNA polymerase II were obtained from Abcam (Cambridge, MA). Anti-mouse CD3 antibody was from Bioexpress (West Lebanon, NH), anti-CD28 antibody from BD Pharmingen (San Jose, CA) and recombinant TNFα from R&D systems (Minneapolis, MN).
Mice
Female C57BL/6 (B6, H-2b) mice were purchased from Charles River (Wilmington, MA), and female C57/B6xDBA/2 F1 (B6D2F1) (H-2b/d) mice were obtained from Jackson Laboratory (Bar Harbor, Maine). The mice used in this study were 10–15 weeks old and their care was in compliance with NIH guidelines. Animal protocol was approved by Subcommittee on Research Animal Care at Beth Israel Deaconess Medical Center.
Cell preparation
Splenocytes were collected from B6 and B6D2F1 mice and CD90+ T cells were isolated using a Pan T cell Isolation Kit (Miltenyi Biotec, Auburn, CA) according to the instructions of the manufacturer. For CFSE labeling, T cells (5 × 106 cells/ml) were incubated with 25 μM CFSE (Molecular Probes) according to the manufacturer’s instructions.
Cell culture
To examine T cell immune responses, purified T cells from B6 mice were cultured with T-cell depleted, irradiated (3,000 rad) allogeneic splenocytes from Balb/c mice. Alternatively, purified T cells (1 × 106 cell/ml) were stimulated with soluble anti-CD3 and anti-CD28 antibodies at a final concentration of 1 μg/ml. For rechallenge experiments B6-donor derived T cells were stimulated with T-cell depleted, irradiated splenocytes from either B6D2F1 or FVB mice. Proliferation of responder cells was measured by [3H] thymidine incorporation; for measurement of IL-2, IFNγ and TNFα production, supernatants were collected at day 1 to day 4 of the culture and were analyzed by enzyme-linked immunosorbent assay (ELISA) using reagents purchased from eBioscience (San Diego, CA). For biochemical analyses, T cells (10 × 106 cells) were incubated with soluble anti-CD3 and anti-CD28 antibodies (10 ug/ml each) for 10 min at 37°C. To examine TNFα mediated NFκB activation, purified T cells (10 × 106 cells/ml) were cultured with 100 ng/ml of recombinant TNFα for 1–3 hrs. Roscovitine was prepared in DMSO and stock concentration was 10 mM. Where indicated, titrated amounts of roscovitine were added to the culture and final concentration of DMSO used was below 0.12% (vol/vol). Cell culture medium contains RPMI 1640, 10% fetal bovine serum, 10 mM Hepes, 100 IU/ml penicillin-streptomycin and 5 × 10−5 M 2-mercaproethanol.
Flow cytometric analysis
For flow cytometry, cells were stained with FITC-conjugated antibody against MHC class I (H-2d) combined with PE-conjugated antibodies either against CD3 or Mac-1(CD11b), followed by analysis on FACSCaliber (Becton-Dickinson, San Jose, CA). Donor cells from B6 (H-2b) mice were defined as H-2d negative populations. For assessment of apoptosis, the AnnexinV/PI Apoptosis Detection Kit (BD Pharmingen) was used according to manufacturer’s instructions. Apoptotic cells were assessed within T cell populations expressing either high levels of CFSE or low levels of CFSE, using BD LSR II System.
Western blotting
T cells were cultured with various stimulators for the indicated time points and cell lysates were prepared with ice-cold lyses buffer (containing 0.1% Nonidet P40). Equal amounts of protein lysates were resolved by SDS-PAGE, transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA) and immunoblots were probed with the indicated antibodies.
Induction of GvHD
B6D2F1 mice were subjected to lethal dose of total body irradiation (TBI, 1,000 cGy) from a 137Cesium source. Irradiation was followed by infusion of 1.5 × 107 parental B6 bone marrow cells, with or without 3 × 107 B6 splenocytes (yielding 7 × 106 T cells) as a source of allogeneic T cells according to a previously described protocol. Acute GvHD was evaluated by daily monitor of survival, as described previously.13
Leukemia induction and assessment of tumor growth
B6D2F1 mice received 1,300 cGy total body irradiation split into 2 doses, 3 hrs apart. Allografts consisted of 5 × 106 parental B6 bone marrow cells with or without 2 × 106 T cells were injected intravenously into B6D2F1 mice along with 5,000 P815 cells. Survival was monitored daily. In GvL experiments, we used a lower dose of donor T cells than in GvHD experiments in order to decrease the severity of GvHD so that GvHD-mediated mortality does not temporally coincide with tumor-mediated mortality, thereby allowing assessment of GvL. P815 tumor growth was assessed by the occurrence of either macroscopic tumor nodules in liver or spleen on autopsy or hind leg paralysis, as described previously.13 Regardless of the presence or absence of macroscopic tumor growth at autopsy, all P815 tumor recipients underwent histopathological examinations of liver and spleen. Organs were preserved in 10% formalin and were subsequently embedded in paraffin, sectioned and stained with hematoxilin/eosin. Sections were evaluated at the Rodent Pathology Core Facility of the Dana-Farber/Harvard Cancer Center.
Administration of roscovitine
Roscovitine (Sigma, MO) was solubilized in DMSO (Sigma, St. Louis, MO). Roscovitine was injected daily intraperitoneally into B6D2F1 receipts (10 mg/kg) starting on the day of BMT for total 3 weeks post-transplant.
Statistics
Survival data were plotted by Kaplan-Meier survival curve and analyzed by the log-rank test. In vitro assays were analyzed by the unpaired Student t test as indicated. A p value of <0.05 was considered significant.
Acknowledgments
This work was supported by NIH grants: AI43552, CA104596, CA123855, HL087870.
References
- 1.Abbas AK, Lohr J, Knoechel B. Balancing autoaggressive and protective T cell responses. J Autoimmun. 2007;28:59–61. doi: 10.1016/j.jaut.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appleman LJ, Boussiotis VA. T cell anergy and costimulation. Immunol Rev. 2003;192:161–80. doi: 10.1034/j.1600-065x.2003.00009.x. [DOI] [PubMed] [Google Scholar]
- 3.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–92. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 4.Sherr CJ, Roberts JM. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 2004;18:2699–711. doi: 10.1101/gad.1256504. [DOI] [PubMed] [Google Scholar]
- 5.Matsuura I, Denissova NG, Wang G, He D, Long J, Liu F. Cyclin-dependent kinases regulate the antiproliferative function of Smads. Nature. 2004;430:226–31. doi: 10.1038/nature02650. [DOI] [PubMed] [Google Scholar]
- 6.Li L, Iwamoto Y, Berezovskaya A, Boussiotis VA. A pathway regulated by cell cycle inhibitor p27Kip1 and checkpoint inhibitor Smad3 is involved in the induction of T cell tolerance. Nat Immunol. 2006;7:1157–65. doi: 10.1038/ni1398. [DOI] [PubMed] [Google Scholar]
- 7.Ferrara JL, Reddy P. Pathophysiology of graft-versus-host disease. Semin Hematol. 2006;43:3–10. doi: 10.1053/j.seminhematol.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Meijer L, Borgne A, Mulner O, Chong JP, Blow JJ, Inagaki N, et al. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur J Biochem. 1997;243:527–36. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- 9.Bukanov NO, Smith LA, Klinger KW, Ledbetter SR, Ibraghimov-Beskrovnaya O. Long-lasting arrest of murine polycystic kidney disease with CDK inhibitor roscovitine. Nature. 2006;444:949–52. doi: 10.1038/nature05348. [DOI] [PubMed] [Google Scholar]
- 10.Park JY, Schutzer WE, Lindsley JN, Bagby SP, Oyama TT, Anderson S, et al. p21 is decreased in polycystic kidney disease and leads to increased epithelial cell cycle progression: roscovitine augments p21 levels. BMC Nephrol. 2007;8:12. doi: 10.1186/1471-2369-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zoja C, Casiraghi F, Conti S, Corna D, Rottoli D, Cavinato RA, et al. Cyclin-dependent kinase inhibition limits glomerulonephritis and extends lifespan of mice with systemic lupus. Arthritis Rheum. 2007;56:1629–37. doi: 10.1002/art.22593. [DOI] [PubMed] [Google Scholar]
- 12.Pezzotta A, Mister M, Monteferrante G, Cassis L, Azzollini N, Aiello S, et al. Effect of seliciclib (CYC202, R-roscovitine) on lymphocyte alloreactivity and acute kidney allograft rejection in rat. Transplantation. 2008;85:1476–82. doi: 10.1097/TP.0b013e31816f240c. [DOI] [PubMed] [Google Scholar]
- 13.Tsukada N, Kobata T, Aizawa Y, Yagita H, Okumura K. Graft-versus-leukemia effect and graft-versus-host disease can be differentiated by cytotoxic mechanisms in a murine model of allogeneic bone marrow transplantation. Blood. 1999;93:2738–47. [PubMed] [Google Scholar]
- 14.Alessi F, Quarta S, Savio M, Riva F, Rossi L, Stivala LA, et al. The cyclin-dependent phase by kinase inhibitors olomoucine and roscovitine arrest human fibroblasts in G1 specific inhibition of CDK2 kinase activity. Exp Cell Res. 1998;245:8–18. doi: 10.1006/excr.1998.4216. [DOI] [PubMed] [Google Scholar]
- 15.Hahntow IN, Schneller F, Oelsner M, Weick K, Ringshausen I, Fend F, et al. Cyclin-dependent kinase inhibitor Roscovitine induces apoptosis in chronic lymphocytic leukemia cells. Leukemia. 2004;18:747–55. doi: 10.1038/sj.leu.2403295. [DOI] [PubMed] [Google Scholar]
- 16.MacCallum DE, Melville J, Frame S, Watt K, Anderson S, Gianella-Borradori A, et al. Seliciclib (CYC202, R-Roscovitine) induces cell death in multiple myeloma cells by inhibition of RNA polymerase II-dependent transcription and downregulation of Mcl-1. Cancer Res. 2005;65:5399–407. doi: 10.1158/0008-5472.CAN-05-0233. [DOI] [PubMed] [Google Scholar]
- 17.Raje N, Kumar S, Hideshima T, Roccaro A, Ishitsuka K, Yasui H, et al. Seliciclib (CYC202 or R-roscovitine), a small-molecule cyclin-dependent kinase inhibitor, mediates activity via downregulation of Mcl-1 in multiple myeloma. Blood. 2005;106:1042–7. doi: 10.1182/blood-2005-01-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lacrima K, Valentini A, Lambertini C, Taborelli M, Rinaldi A, Zucca E, et al. In vitro activity of cyclin-dependent kinase inhibitor CYC202 (Seliciclib, R-roscovitine) in mantle cell lymphomas. Ann Oncol. 2005;16:1169–76. doi: 10.1093/annonc/mdi217. [DOI] [PubMed] [Google Scholar]
- 19.Lacrima K, Rinaldi A, Vignati S, Martin V, Tibiletti MG, Gaidano G, et al. Cyclin-dependent kinase inhibitor seliciclib shows in vitro activity in diffuse large B-cell lymphomas. Leuk Lymphoma. 2007;48:158–67. doi: 10.1080/10428190601026562. [DOI] [PubMed] [Google Scholar]
- 20.Alvi AJ, Austen B, Weston VJ, Fegan C, MacCallum D, Gianella-Borradori A, et al. A novel CDK inhibitor, CYC202 (R-roscovitine), overcomes the defect in p53-dependent apoptosis in B-CLL by downregulation of genes involved in transcription regulation and survival. Blood. 2005;105:4484–91. doi: 10.1182/blood-2004-07-2713. [DOI] [PubMed] [Google Scholar]
- 21.Lu W, Chen L, Peng Y, Chen J. Activation of p53 by roscovitine-mediated suppression of MDM2 expression. Oncogene. 2001;20:3206–16. doi: 10.1038/sj.onc.1204412. [DOI] [PubMed] [Google Scholar]
- 22.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 23.Dey A, Wong ET, Cheok CF, Tergaonkar V, Lane DP. R-Roscovitine simultaneously targets both the p53 and NFkappaB pathways and causes potentiation of apoptosis: implications in cancer therapy. Cell Death Differ. 2008;15:263–73. doi: 10.1038/sj.cdd.4402257. [DOI] [PubMed] [Google Scholar]
- 24.Speiser DE, Bachmann MF, Frick TW, McKall-Faienza K, Griffiths E, Pfeffer K, et al. TNF receptor p55 controls early acute graft-versus-host disease. J Immunol. 1997;158:5185–90. [PubMed] [Google Scholar]
- 25.Cooke KR, Hill GR, Gerbitz A, Kobzik L, Martin TR, Crawford JM, et al. Tumor necrosis factor-alpha neutralization reduces lung injury after experimental allogeneic bone marrow transplantation. Transplantation. 2000;70:272–9. doi: 10.1097/00007890-200007270-00006. [DOI] [PubMed] [Google Scholar]
- 26.Schmaltz C, Alpdogan O, Muriglan SJ, Kappel BJ, Rotolo JA, Ricchetti ET, et al. Donor T cell-derived TNF is required for graft-versus-host disease and graft-versus-tumor activity after bone marrow transplantation. Blood. 2003;101:2440–5. doi: 10.1182/blood-2002-07-2109. [DOI] [PubMed] [Google Scholar]
- 27.Choi SW, Kitko CL, Braun T, Paczesny S, Yanik G, Mineishi S, et al. Change in plasma tumor necrosis factor receptor 1 levels in the first week after myeloablative allogeneic transplantation correlates with severity and incidence of GVHD and survival. Blood. 2008;112:1539–42. doi: 10.1182/blood-2008-02-138867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pahl HL. Activators and target genes of Rel/NFkappaB transcription factors. Oncogene. 1999;18:6853–66. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 29.Zhong H, Voll RE, Ghosh S. Phosphorylation of NFkappaB p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol Cell. 1998;1:661–71. doi: 10.1016/s1097-2765(00)80066-0. [DOI] [PubMed] [Google Scholar]
- 30.Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–62. [PubMed] [Google Scholar]
- 31.Rossi AG, Sawatzky DA, Walker A, Ward C, Sheldrake TA, Riley NA, et al. Cyclin-dependent kinase inhibitors enhance the resolution of inflammation by promoting inflammatory cell apoptosis. Nat Med. 2006;12:1056–64. doi: 10.1038/nm1468. [DOI] [PubMed] [Google Scholar]
- 32.Hacker H, Karin M. Regulation and function of IKK and IKK-related kinases. Sci STKE. 2006;2006:13. doi: 10.1126/stke.3572006re13. [DOI] [PubMed] [Google Scholar]
- 33.Vodanovic-Jankovic S, Hari P, Jacobs P, Komorowski R, Drobyski WR. NFkappaB as a target for the prevention of graft-versus-host disease: comparative efficacy of bortezomib and PS-1145. Blood. 2006;107:827–34. doi: 10.1182/blood-2005-05-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berthet C, Rodriguez-Galan MC, Hodge DL, Gooya J, Pascal V, Young HA, et al. Hematopoiesis and thymic apoptosis are not affected by the loss of Cdk2. Mol Cell Biol. 2007;27:5079–89. doi: 10.1128/MCB.00029-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song H, Vita M, Sallam H, Tehranchi R, Nilsson C, Siden A, et al. Effect of the Cdk-inhibitor roscovitine on mouse hematopoietic progenitors in vivo and in vitro. Cancer Chemother Pharmacol. 2007;60:841–9. doi: 10.1007/s00280-007-0431-x. [DOI] [PubMed] [Google Scholar]
- 36.Sallam H, Jimenez P, Song H, Vita M, Cedazo-Minguez A, Hassan M. Age-dependent pharmacokinetics and effect of roscovitine on Cdk5 and Erk1/2 in the rat brain. Pharmacol Res. 2008;58:32–7. doi: 10.1016/j.phrs.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 37.Chen S, Dai Y, Harada H, Dent P, Grant S. Mcl-1 downregulation potentiates ABT-737 lethality by cooperatively inducing Bak activation and Bax translocation. Cancer Res. 2007;67:782–91. doi: 10.1158/0008-5472.CAN-06-3964. [DOI] [PubMed] [Google Scholar]