Abstract
Insufficient duration of sleep is a highly prevalent behavioral pattern in society that has been shown to cause an increase in spontaneous pain and sensitivity to noxious stimuli. Prostaglandins (PG), in particular PGE2, are key mediators of inflammation and pain, and we investigated whether PGE2 is a potential mediator in sleep-loss induced changes in nociceptive processing. Twenty-four participants (7 females, age 35. 17.1yrs) stayed for 7 days in the Clinical Research Center. After two baseline days, participants were randomly assigned to either three days of 88 hours of total sleep deprivation (TSD, N=15) or 8 hours of sleep per night (N=9), followed by a night of recovery sleep. Participants rated the intensity of various pain-related symptoms every two hours across waking periods on computerized visual analog scales. PGE2 was measured in 24h-urine collections during baseline and third sleep deprivation day. Spontaneous pain, including headache, muscle pain, stomach pain, generalized body pain, and physical discomfort significantly increased by 5 to 14 units on a 100-unit scale during TSD, compared to the sleep condition. Urinary PGE2 metabolite significantly increased by about 30% in TSD over sleep condition. TSD-induced increase in spontaneous pain, in particular headache and muscle pain, was significantly correlated with increase in PGE2 metabolite. Activation of the PGE2 system appears to be a potential mediator of increased spontaneous pain in response to insufficient sleep.
Keywords: Sleep deprivation, prostaglandin, pain, headache, muscle pain, inflammation
INTRODUCTION
Experimental evidence has accumulated over the past 30 years supporting a reciprocal relationship between sleep and pain: While the experience of pain can disrupt sleep [19], experimental sleep loss itself has been shown to cause changes in the nociceptive system neuroplasticity in animal and humans, such as hyperalgesia and the development of spontaneous pain, including muscle pain, headache, and back pain [13, 30].
Despite the well established link between sleep and pain, there is surprisingly very little knowledge about the underlying mechanisms that interface sleep and pain. However, this knowledge is essential for the development of interventions that would mitigate the sleep-pain interaction, and improve physical well-being in those undergoing periods of insufficient sleep, such as patients suffering from chronic pain, health care workers performing or serving on-call duty, military personnel, parents attending to infants and toddlers, time zone travellers, shift workers, among others.
Activation of inflammatory components is a central feature in various types of painful conditions, a finding frequently reported under conditions of experimental sleep loss. Partial and acute sleep deprivation in healthy volunteers have been shown to increase proinflammatory cytokines such as interleukin (IL)-6, tumor necrosis factor (TNF)-alpha, its soluble receptor p55, and C-reactive protein (CRP) [8, 18, 29, 35, 36]. Little is known about the effects of sleep loss on the prostaglandin system, though it plays a significant role in sleep-wake regulation in animals [9], and is well-studied for its involvement in the origin of inflammatory pain [7].
Prostaglandins (PGs) are synthesized from membrane-released arachidonic acid when cells are activated by mechanical trauma, or by contact with cytokines, growth factors, and other inflammatory processes [6]. Cyclooxygenases (COX) catalyse the conversion of arachidonic acid to unstable PGH2, which then is converted to the functionally important prostanoids PGD2, E2, F2, I2, and thromoboxane by specific synthases. PGs can be synthesised by virtually every cell of the body, including macrophages and endothelial cells.
PGE2 appears to be a principle mediator of inflammation and promotes the development of inflammatory signs, including pain. At a peripheral level, PGE2 is able to sensitize the nociceptive system through binding to EP receptors located on peripheral terminals of primary sensory neurons, resulting in an increased sensitivity to noxious stimuli (i.e. hyperalgesia). On a central level, numerous studies have shown that PGE2 is able to change neuronal excitability and synaptic dis-inhibition in the spinal cord [34] that manifest in hyperalgesia and has been suggested to play a role in the development of spontaneous pain in the absence of any sensory input [37].
Given that the PG system is critically involved in nociceptive processing and sleep-wake regulation in animals, we hypothesised that up-regulation of PGE2, a potent mediator of pain hypersensitivity, is associated with the level of spontaneous pain experienced by healthy participants in response to prolonged total sleep deprivation.
METHODS AND MATERIAL
Participants
The study was approved by the Institutional Review Board for the Protection of Human Subjects at the Beth Israel Deaconess Medical Center. The current study is part of a larger study in which participants received either placebo or esmolol. Here, only participants who were randomized to placebo were included (N=32). Drop-out rate of this study arm was 25% of participants entering the in-hospital part (2 participants did not tolerate sleep deprivation, 3 participants did not tolerate the study environment/equipment, 1 participant dropped out due to iv line problems, 2 participants dropped out due to other reasons). The remaining 24 participants were randomized to the condition sleep (N=9) or sleep deprivation (N=15). Participants had to meet the following screening criteria after signing the informed consent form: Age between 21–55 years, body mass index (BMI) between 18 and 35 kg/m2, no current or past history of psychiatric, neurological, immune, cardiovascular, chronic pain or current active pain, or sleep disorder, no history of drug dependence/abuse, non-smoking for at least 6 months prior to study start, normal physical examination by a licensed physician, normal blood chemistry, negative blood and urine toxicology, no shift work/time zone changes within the last 3 months, habitual sleep duration between 6.5 and 9 hours as verified by 10–14 days of sleep log records, habitual bedtimes within one hour of study bedtimes (11pm-7am), and no signs of sleep disorders as evaluated by polysomnography during the first adaptation night. The week prior to study start, participants were instructed to follow study bedtimes (11pm-7am), reduce caffeine consumption to ½ cup/day, and to not take any anti-inflammatory medication.
Study Protocol
Participants stayed for 6 nights and 7 days in a private room in the Clinical Research Center (CRC). Following an adaptation and baseline day with a sleep opportunity of 8 hours (11pm-7am), participants were randomized to either 88 hours of continuous wakefulness (TSD condition), or had a sleep opportunity of 8h/night for three consecutive nights (sleep condition). The study design had an imbalanced randomization plan with two-thirds of the subjects randomized to TSD condition and one-third of the subjects randomized to the sleep condition. The last day was a recovery day with a sleep opportunity of 12 hours (11pm-11am). Starting on the baseline day until the end of the study, participants had an intravenous catheter in the superficial forearm for hourly blood draws, continuous polysomnographic and actigraphic recordings, continuous rectal temperature recordings, and continuous BP recordings during the nighttime periods.
Urine was collected for 24h-intervals across study days and an aliquot was kept frozen at −80C until assayed. Every two hours during the waking periods of the study, participants were asked to rate the intensity of their mood and pain symptoms on computerized visual analog scales (VAS). Participants had hourly, 3–10min long walks during the scheduled waking periods in order to provide and control for exercise during their stay in the CRC. Caloric- and electrolyte balanced meals were served at 800h, 1300h, 1800h, and a light snack at 2150h. Equal amounts of water were provided hourly throughout waking periods, totaling a daily fluid intake of 40 ml/kg body weight/day. Water amount was adjusted by 5ml/kg body weight/day if participants felt thirsty.
Participants were accompanied by trained research assistants during all waking periods, in order to ensure that they stayed on the study schedule and maintained wakefulness. During the TSD vigil, participants remained in bed in a semisupine position between 11pm and 7am, with lights dimmed to <40 lux. Usual activities included talking with research assistants and nurses, playing board games, and watching movies. Participants were able to maintain their social relationships by having visitors and access to email and phone.
Assessment of Mood and Spontaneous Pain
Participants rated the intensity of their current mood and spontaneous pain on 100mm long Visual Analog Scales (VAS) presented every two hours on a tablet computer throughout the waking period. Participants used a pen to slide the cross hatch on the scale either to the left or right, and the rating was stored on the computer in measurement units between 0 and 100. Pain-related items included generalized body pain, physical discomfort, headache, back pain, stomach pain, muscle pain, and joint pain. Intensity ratings of single pain items were compiled to a global pain score, labeled spontaneous pain, and ratings were averaged across each study days (baseline day, 1st, 2nd, and 3rd day of TSD, recovery day).
PG E2 measurement
Prostaglandin E metabolite (PGE-M) assay was measured to provide a reliable estimate of PGE2 production. PGE-M was assayed in 24h-urine collection with an Enzyme Immunoassay (EIA, Cayman Chemicals, Ann Arbor, MI). A 20-fold dilution was found to be optimal. PGE-M was measured in triplicates, and intra- and inter-assay coefficients of variation were 5.7 3.6 and 12.5 6.6, respectively. PGE-M was expressed as amount per 24h-urine output. PGE-M was measured on baseline day and again on the 3rd day of TSD.
Quantification of excreted urinary metabolites is thought to be the most accurate index of endogenous PG production, because PGs are rapidly metabolized and appear to act on a local rather then systemic level [2]. In addition, urinary quantification of major metabolites is able to reflect changes in PG turnover, for example induced by COX inhibition [22]. With respect to pharmacokinetics of PGs, PG metabolites increase in urine collected within 3 hours after stimulation of PG synthesis with endotoxin administration in humans [17]. This suggests that measurement of excreted urine metabolites in 24h-collections lags actual PG production by a few hours.
Statistics
General Linear Model (GLM) for repeated measures was used to analyze timecourse differences of spontaneous pain ratings and PGE-M levels, with days as within-subject factor and group (sleep vs. sleep deprivation) as between-subject factor. Greenhouse Geisser correction was used if the test of sphericity was significant. Univariate tests were used to test significant differences at single time points. To account for differences between groups at baseline, baseline was used as a covariate in univariate testing. Pearson correlation coefficient was used to examine the strength of association between change in PGE-M and change in pain ratings. Pain ratings and PGE-M values were both positively skewed and therefore normalized using a natural logarithm transformation. Mean SD is given for data in text; mean SEM is given for data in graphs. Alpha level of rejection was set to P<0.05.
RESULTS
Twenty-four participants were randomly assigned to Sleep (N= 9) or TSD (N=15). Sleep and TSD groups did not differ with respect to female/male ratio (3/6 vs. 4/11, ns) and BMI (23.2 3.4 vs. 25.5 4.2 m2/kg, ns). The sleep group was younger than the TSD group (30.4 5.6 vs. 37.9 6.5 years, p<0.05). However, there was no correlation between the increase in PGE-M excretion or pain scores with age.
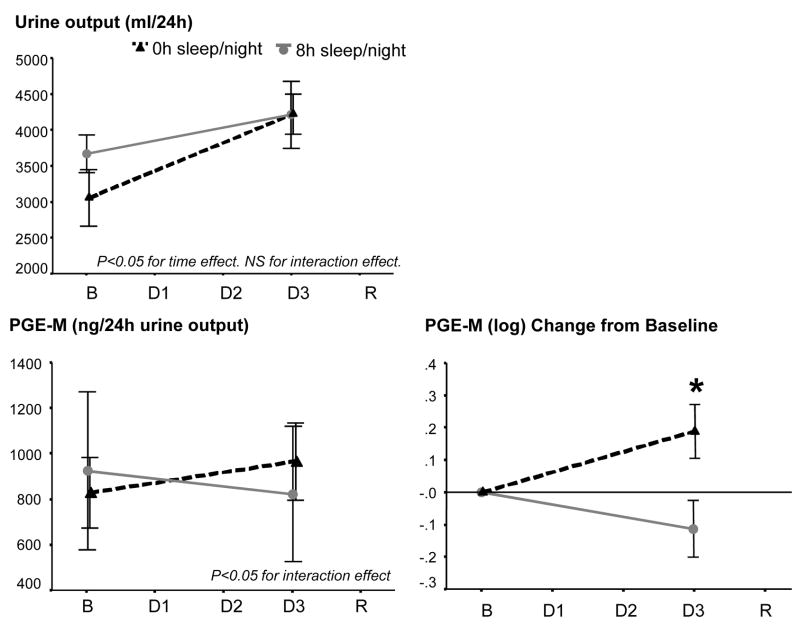
Urine output from baseline to the third experimental day in the CRC increased significantly increased in both TSD and sleep group (F[1,22]=20.84, p<0.01 for time effect), but did not differ between groups (F[1,22]=2.81, NS, for interaction effect; see figure 1 top panel).
Figure 1.
Top left: Urine output in 24h collections at baseline and 3rd day under conditions of TSD (N=15) or control sleep (N=9).
Lower left: Urinary PGE2 metabolite levels/24h urine output at baseline and 3rd day of TSD (N=15) or control sleep (N=9). Lower right: Change of log-transformed urinary PGE2 metabolite levels/24h urine output from baseline to 3rd day of TSD or control sleep. For statistical analysis, data were log-transformed due to skewed distribution. Asterisk indicate p<0.05 between groups.
B=Baseline, Day 1 to 3=Days of TSD or control sleep, R=Recovery.
Urinary PGE-M levels showed large inter-individual differences ranging from 215 to 3553 pg/24h urine output at baseline. The lower left panel of figure 1 depicts the raw data at baseline and third day of SD. The lower right panel of figure 1 shows the change from baseline of log-transformed data. GLM analysis of PGE-M levels showed a significant time by group interaction effect (F[1,22]=5.42, p<0.05). This was due to a significant increase in the TSD group from baseline to third day of TSD (F[1,14]=4.97, p<0.05), while the sleep group showed a slight non-significant decrease (F[1,8]=1.60, NS). Results of GLM analysis of PGE-M levels did not change after controlling for age and for sex (F[1,21]>5.00, p<0.05).
Univariate testing with baseline as covariate showed sig. higher values in the SD group compared to the sleep group on the third day of SD (F[1,21]=5.96, p<0.05). PGE-M increased in eleven out of 15 participants increased in the TSD condition, compared to one out of nine participants in the sleep condition.
Spontaneous pain
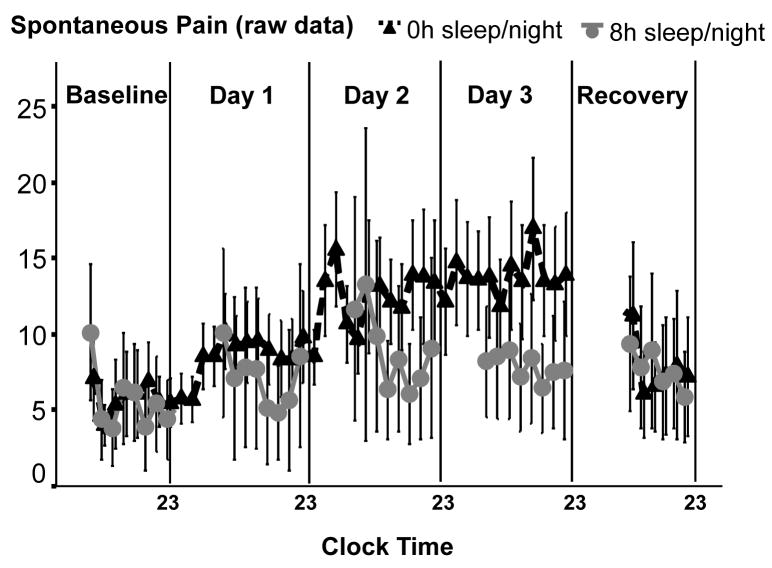
One participant in the sleep group had missing data due to computer failure, reducing sample size in the sleep group to N=8. Figure 2 shows the raw data of intensity ratings of spontaneous pain (averaged across all pain items, i.e. headache, muscle pain etc.). Figure 3 (top left panel) shows log-transformed spontaneous pain ratings presented as change from baseline and averaged across study days. GLM analysis of spontaneous pain ratings showed a significant time by group interaction effect (F[3,60]= 5.00, p<0.05). This was due do an increase of spontaneous pain in the TSD group (F[4,56]=11.46, p<0.001 for time effect), while pain ratings in the sleep group stayed stable across days (F[4,28]=0.15, NS for time effect). GLM analysis of spontaneous pain ratings controlled for age and for sex did not change results (F[3,57]>4.7, p<0.05).
Figure 2.
Intensity ratings of spontaneous measured every 2 hours throughout the protocol. For statistical analysis, ratings were averaged across days and log-transformed (see figure 3)
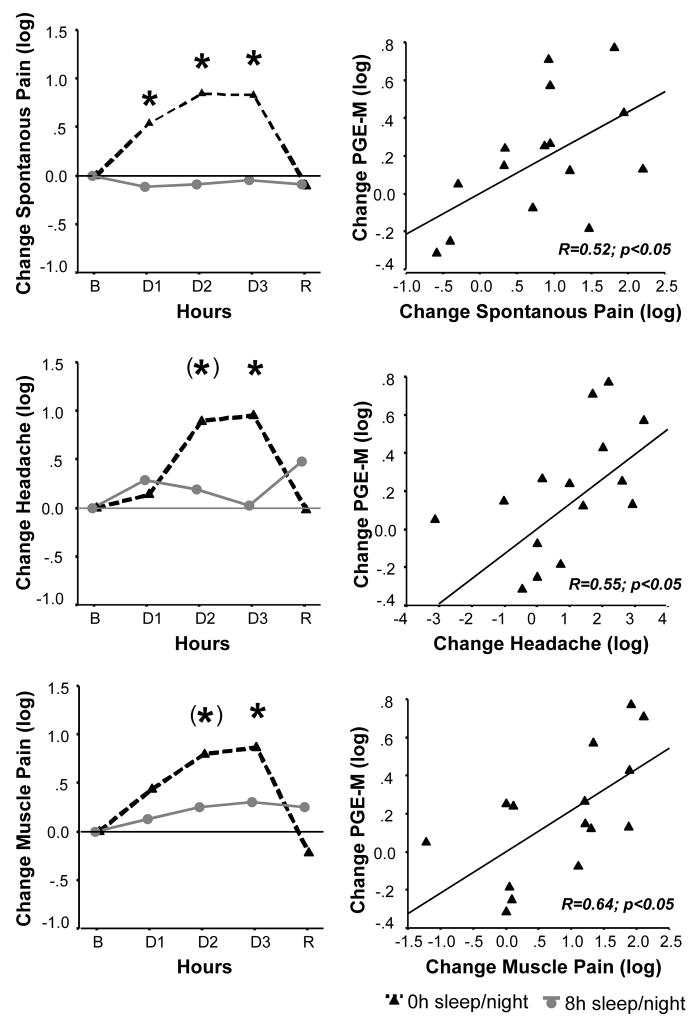
Figure 3.
Left panel: Intensity ratings of spontaneous pain (averaged across days), headache, and muscle pain significantly increased throughout three nights of TSD (N=15) compared to 8h control sleep (N=8, p<0.05 for interaction effect).
B=Baseline, Day 1 to 3 = days of TSD or control sleep, R=Recovery. Right panel: Correlation between change in urinary PGE2 metabolite and pain symptoms from baseline to the 3rd day of TSD.
Data are log-transformed due to skewed distribution and presented as differences from baseline. Asterisks indicate p<0.05 between groups. Asterisks in parenthesis indicate trend towards significance (p<0.10) between groups.
With respect to group differences at single time points (baseline taken as covariate in univariate testing), ratings were significantly higher in the TSD group compared to the sleep group starting on the first TSD night, and went back to baseline after a night of recovery sleep.
With respect to single pain items, participants in the TSD group experienced increased generalized body pain, physical discomfort, headache, stomach pain, and muscle pain compared to participants with a regular sleep schedule (all F[3,60]>4.0, p<0.05 for interaction or group effect, see middle and lower left panel in figure 2 for the pain items headache and muscle pain). No significant interaction or group effects were observed for joint pain and back pain.
Change of spontaneous pain from baseline to third TSD day was 9 12 units on a 100-unit scale. Higher changes could be seen in ratings assessing unspecific pain items (i.e. generalized body pain and physical discomfort (14 17 units and 14 19 units, resp.)) than localized pain (i.e. headache, stomach pain, and muscle pain (6 12, 5 12, and 9 19 units, resp.)).
Under baseline conditions, PGE-M levels were not significantly correlated with intensity ratings of spontaneous pain (R=−.28, NS).
Change in PGE-M metabolite levels from baseline to 3rd TSD day was positively correlated with spontaneous pain (R=.52, p<0.05) and localized pain items of headache (R=.55, p<0.05), muscle pain (R=.64, p<0.05), back pain (R=.65, p<0.01), and joint pain (R=.58, p<0.05), indicating that sleep deprivation-induced increase in PGE-M is associated with an increase in spontaneous pain complaints (see right panel in figure 3).
DISCUSSION
The current findings show that total sleep deprivation of 88 hours carried out under controlled in-lab conditions increased spontaneous pain (i.e. pain in the absence of any noxious sensory input) and this effect was associated with an increased production of urinary PGE2 metabolite. While a reciprocal connection between sleep loss and pain has been supported by experimental and epidemiological findings [5, 13, 19], the current findings suggest that PGE2 may constitute a mechanistic mediator underlying the pain-enhancing effect of sleep loss.
Activation of PGD2, E2, and F2, has been shown in rats after moderate sleep deprivation [26]. Since the effect of sleep loss in that study appeared not to be restricted to a specific PG, the authors suggested that sleep loss may target the production of cyclooxygenase (COX), resulting in increased production of PGH2 and subsequently the synthesis of functional prostaglandins. We found an average increase of PGE2 metabolite by almost 30% during the third night of total sleep deprivation compared to non sleep-deprived participants [22, 32]. PGs have rarely been studied in the context of human sleep loss. We recently reported a non-significant increase of urinary PGD2 and E2 metabolite of about 20% after 10 days of sleep restriction to four hours/night [8]. Present study results corroborate activation of the PG system in response to partial or total sleep deprivation in humans.
We observed a wide interindividual variability of PG E2 metabolite levels at baseline as well as in response to sleep deprivation in this sample of healthy participants. A wide heterogeneity between subjects in the expression of the COX-2 gene and other inflammatory genes that encode enzymes related to PG production has been reported [12, 15]. Thus, genetic variability very likely contributes to these large interindividual basal and response differences in the PG system.
We here found that the sleep loss-induced increase in PGE-M was associated with the extent to which participants experienced spontaneous pain. The association was evident for headache and muscle pain. The mechanism through which PGE2 contributes to pain likely involves peripheral as well as central actions. In the periphery, PGE2 sensitizes the nociceptive system by increasing excitability of primary nociceptive afferent nerve fibers, leading to a lower response threshold to noxious stimuli. This mechanism is able to contribute to hyperalgesia, i.e. lowered pain thresholds to various stimuli under conditions of total or partial sleep deprivation, which has been demonstrated in several studies [4, 11, 16, 20, 21, 24, 25, 27]. In the central nervous system, PGE2 causes its pro-nociceptive effect through activation of various signalling pathways. Spinal PGs are able to facilitate excitatory glutamatergic transmission [23]), and to reduce synaptic inhibition The loss of inhibitory pain control mediated by PGE2 probably accounts for most symptoms of chronic pain, including the occurrence of spontaneous pain [37]. Here we measured changes in PGE-M production of systemic origin and therefore cannot delineate the contribution of peripheral and central PG production to sleep-loss induced spontaneous pain reports.
In the present study, spontaneous pain complaints increased by 5–14 units on 100-unit visual analog scales during three nights of sleep deprivation. It is important to keep in mind that current findings have been obtained in healthy participants without any history of chronic pain-related complaints, and under short-term sleep loss conditions. A 5–14 unit increase under these conditions may further develop into a more pronounced state under long-term sleep loss conditions and/or in vulnerable populations.
To date, quantification of excreted urinary PGE-M is thought to be a reliable method to estimate systemic PG production in humans [22]. It is likely that urinary PGE-M is COX-2 derived. This is based on observations that non-selective COX-1/2 inhibitors (ibuprofen) and selective COX-2 inhibitors (refecoxib) lead to an almost equal decrease in urinary PGE-M excretion in healthy volunteers [22]. The cellular origin of PGE-M in response to sleep loss is uncertain. COX-2 is normally absent in most cells, but can be induced by inflammatory stimuli in immune cells or by shear forces in endothelial cells [31]. It is well established that sleep loss activates various inflammatory components, including monocytes [1, 3], and nuclear factor (NF)-kB [10], a gene transcription factor controlling the expression of various inflammatory signals. NF-kB as well as proinflammatory cytokines are able to activate COX-2 and consequently PG production [14]. COX-2 has been also found activated in endothelial cells in response to shear stress [33]. Blood pressure has been reported to increase while undergoing sleep deprivation [28], and increase in shear stress may serve as a potential mechanism of sleep loss-induced activation of PGE-M production.
In summary, we here show that increase in PGE2 metabolite levels in response to sleep loss is associated with the degree to which participants report spontaneous pain complaints. This correlational finding gives us first insight that PG activation serves as a potential mechanism underlying sleep loss-induced pain. Interventional studies that involve blocking PG and other inflammatory markers are necessary to test causality.
Acknowledgments
This work was supported by NIH grant HL075501 (JMM) and RR01032 (GCRC).
Footnotes
Conflict of interest: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Born J, Lange T, Hansen K, Mölle M, Fehm HL. Effects of sleep and circadian rhythm on human circulating immune cells. J Immunol. 1997;158:4454–4464. [PubMed] [Google Scholar]
- 2.Catella F, Nowak J, FitzGerald GA. Measurement of renal and nonrenal eicosanoid synthesis. Am J Med. 1986;81:23–29. doi: 10.1016/0002-9343(86)90905-8. [DOI] [PubMed] [Google Scholar]
- 3.Dinges DF, Douglas SD, Zaugg L, Campbell DE, McMann JM, Whitehouse WG, Orne EC, Kapoor SC, Icaza E, Orne MT. Leukocytosis and natural killer cell function parallel neurobehavioral fatigue induced by 64 hours of sleep deprivation. J Clin Invest. 1994;93:1930–1939. doi: 10.1172/JCI117184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drewes AM, Nielsen KD, Rasmussen C, Arima T, Svensson P, Rossel P, Arendt-Nielsen L. The effects of controlled delta sleep deprivation on experimental pain in healthy subjects. Journal of Musculoskeletal Pain. 2000;8:49–67. [Google Scholar]
- 5.Edwards RR, Almeida DM, Klick B, Haythornthwaite JA, Smith MT. Duration of sleep contributes to next-day pain report in the general population. Pain. 2008;137:202–207. doi: 10.1016/j.pain.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Funk CD. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 7.Griffiths RJ. Prostaglandins and inflammation. In: Gallin J, Synderman R, editors. Inflammation: Basic principles and clinical correlates. Philadelphia, PA: Lippincott Williams and Wilkins; 1999. pp. 349–360. [Google Scholar]
- 8.Haack M, Sanchez E, Mullington JM. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep. 2007;30:1145–1152. doi: 10.1093/sleep/30.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang ZL, Urade Y, Hayaishi O. Prostaglandins and adenosine in the regulation of sleep and wakefulness. Current Opinion in Pharmacology. 2007;7:33–38. doi: 10.1016/j.coph.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Irwin MR, Wang M, Ribeiro D, Cho HJ, Olmstead R, Breen EC, Martinez-Maza O, Cole S. Sleep loss activates cellular inflammatory signaling. Biol Psychiatry. 2008;64:538–540. doi: 10.1016/j.biopsych.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kundermann B, Spernal J, Huber MT, Krieg JC, Lautenbacher S. Sleep deprivation affects thermal pain thresholds but not somatosensory thresholds in healthy volunteers. Psychosom Med. 2004;66:932–937. doi: 10.1097/01.psy.0000145912.24553.c0. [DOI] [PubMed] [Google Scholar]
- 12.LaCroix-Fralish ML, Mogil JS. Progress in Genetic Studies of Pain and Analgesia. Annual Review of Pharmacology and Toxicology. 2009;49:97–121. doi: 10.1146/annurev-pharmtox-061008-103222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lautenbacher S, Kundermann B, Krieg JC. Sleep deprivation and pain perception. Sleep Med Rev. 2006;10:357–369. doi: 10.1016/j.smrv.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Lee KM, Kang BS, Lee HL, Son SJ, Hwang SH, Kim DS, Park JS, Cho HJ. Spinal NF-kB activation induces COX-2 upregulation and contributes to inflammatory pain hypersensitivity. Eur J Neurosci. 2004;19:3375–3381. doi: 10.1111/j.0953-816X.2004.03441.x. [DOI] [PubMed] [Google Scholar]
- 15.Lee YS, Kim H, Wu TX, Wang XM, Dionne RA. Genetically mediated interindividual variation in analgesic responses to cyclooxygenase inhibitory drugs. Clin Pharmacol Ther. 2006;79:407–418. doi: 10.1016/j.clpt.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Lentz MJ, Landis CA, Rothermel J, Shaver JL. Effects of selective slow wave sleep disruption on musculoskeletal pain and fatigue in middle aged women. J Rheumatol. 1999;26:1586–1592. [PubMed] [Google Scholar]
- 17.McAdam BF, Mardini IA, Habib A, Burke A, Lawson JA, Kapoor S, FitzGerald GA. Effect of regulated expression of human cyclooxygenase isoforms on eicosanoid and isoeicosanoid production in inflammation. J Clin Invest. 2000;105:1473–1482. doi: 10.1172/JCI9523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, Dinges DF, Mullington JM. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43:678–683. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 19.Menefee LA, Cohen MJM, Anderson WR, Doghramji K, Frank ED, Lee H. Sleep disturbance and nonmalignant chronic pain: A comprehensive review of the literature. Pain Medicine. 2000;1:156–172. doi: 10.1046/j.1526-4637.2000.00022.x. [DOI] [PubMed] [Google Scholar]
- 20.Moldofsky H, Scarisbrick P. Induction of neurasthenic musculoskeletal pain syndrome by selective sleep stage deprivation. Psychosom Med. 1976;38:35–44. doi: 10.1097/00006842-197601000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Moldofsky H, Scarisbrick P, England R, Smythe H. Musculosketal symptoms and non-REM sleep disturbance in patients with “fibrositis syndrome” and healthy subjects. Psychosom Med. 1975;37:341–351. doi: 10.1097/00006842-197507000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Murphey LJ, Williams MK, Sanchez SC, Byrne LM, Csiki I, Oates JA, Johnson DH, Morrow JD. Quantification of the major urinary metabolite of PGE(2) by a liquid chromatographic/mass spectrometric assay: determination of cyclo oxygenase-specific PGE(2) synthesis in healthy humans and those with lung cancer. Analytical Biochemistry. 2004;334:266–275. doi: 10.1016/j.ab.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 23.Nishihara I, Minami T, Watanabe Y, Ito S, Hayaishi O. Prostaglandin E(2) stimulates glutamate release from synaptosomes of rat spinal cord. Neurosci Lett. 1995;196:57–60. doi: 10.1016/0304-3940(95)11839-o. [DOI] [PubMed] [Google Scholar]
- 24.Older SA, Battafarano DF, Danning CL, Ward JA, Grady EP, Derman S, Russell IJ. The effects of delta wave sleep interruption on pain thresholds and fibromyalgia-like symptoms in healthy subjects; correlations with insulin-like growth factor I. J Rheumatol. 1998;25:1180–1186. [PubMed] [Google Scholar]
- 25.Onen SH, Alloui A, Gross A, Eschallier A, Dubray C. The effects of total sleep deprivation, selective sleep interruption and sleep recovery on pain tolerance thresholds in healthy subjects. J Sleep Res. 2001;10:35–42. doi: 10.1046/j.1365-2869.2001.00240.x. [DOI] [PubMed] [Google Scholar]
- 26.Ram A, Pandey HP, Matsumura H, Kasahara-Orita K, Nakajima T, Takahata R, Satoh S, Terao A, Hayaishi O. CSF levels of prostaglandins, especially the level of prostaglandin D2, are correlated with increasing propensity towards sleep in rats. Brain Res. 1997;751:81–89. doi: 10.1016/s0006-8993(96)01401-1. [DOI] [PubMed] [Google Scholar]
- 27.Roehrs T, Hyde M, Blaisdell B, Greenwald M, Roth T. Sleep loss and REM sleep loss are hyperalgesic. Sleep. 2006;29:145–151. doi: 10.1093/sleep/29.2.145. [DOI] [PubMed] [Google Scholar]
- 28.Sanchez A, Haack M, Toth M, Serrador J, Mullington J. Effects of sleep deprivation on blood pressure and vascular cellular adhesion molecules. Sleep. 2008;31:A137. [Google Scholar]
- 29.Shearer WT, Reuben JM, Mullington JM, Price NJ, Lee BN, Smith EO, Szuba MP, Van Dongen HP, Dinges DF. Soluble TNF- receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J Allergy Clin Immunol. 2001;107:165–170. doi: 10.1067/mai.2001.112270. [DOI] [PubMed] [Google Scholar]
- 30.Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev. 2004;8:119–132. doi: 10.1016/S1087-0792(03)00044-3. [DOI] [PubMed] [Google Scholar]
- 31.Smith WL. Prostaglandin biosynthesis and its compartmentation in vascular smooth-muscle and endothelial cells. Annual Review of Physiology. 1986;48:251–262. doi: 10.1146/annurev.ph.48.030186.001343. [DOI] [PubMed] [Google Scholar]
- 32.Stichtenoth DO, Marhauer V, Tsikas D, Gutzki FM, Frolich JC. Effects of specific COX-2-inhibition on renin release and renal and systemic prostanoid synthesis in healthy volunteers. Kidney International. 2005;68:2197–2207. doi: 10.1111/j.1523-1755.2005.00676.x. [DOI] [PubMed] [Google Scholar]
- 33.Topper JN, Cai J, Falb D, Gimbrone MA., Jr Identification of vascular endothelial genes differentially responsive to fluid mechanical stimuli: cyclooxygenase-2, manganese superoxide dismutase, and endothelial cell nitric oxide synthase are selectively up-regulated by steady laminar shear stress. Proc Natl Acad Sci U S A. 1996;93:10417–10422. doi: 10.1073/pnas.93.19.10417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vanegas H, Schaible HG. Prostaglandins and cyclooxygenases in the spinal cord. Prog Neurobiol. 2001;64:327–363. doi: 10.1016/s0301-0082(00)00063-0. [DOI] [PubMed] [Google Scholar]
- 35.Vgontzas AN, Papanicolaou DA, Bixler EO, Lotsikas A, Zachman K, Kales A, Prolo P, Wong ML, Licinio J, Gold PW, Hermida RC, Mastorakos G, Chrousos GP. Circadian interleukin-6 secretion and quantity and depth of sleep. J Clin Endocrinol Metab. 1999;84:2603–2607. doi: 10.1210/jcem.84.8.5894. [DOI] [PubMed] [Google Scholar]
- 36.Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Follett H, Kales A, Chrousos GP. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89:2119–2126. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- 37.Zeilhofer HU, Zeilhofer UB. Spinal dis-inhibition in inflammatory pain. Neurosci Lett. 2008;437:170–174. doi: 10.1016/j.neulet.2008.03.056. [DOI] [PubMed] [Google Scholar]