Abstract
OBJECTIVE
Advances in neonatal care have resulted in children born pre-maturely with respiratory distress syndrome (RDS) successfully reaching school age. It is unknown how many will be ready for school and what factors affect school readiness in these children at high risk. Our objective was to assess readiness of children born prematurely with RDS in the last decade for entry into public school, and determine risk factors associated with lack of school readiness in this population.
METHODS
This was a single-center prospective cohort study. Follow-up data were collected for 135 of 167 (81%) surviving premature infants with RDS requiring surfactant-replacement therapy and mechanical ventilation. The children were seen between July 2005 and September 2006 (average age: 5.7 ± 1.0 years) and underwent standardized neurodevelopmental and health assessments and socioeconomic status classification. A 4-level school-readiness score was constructed by using each child’s standardized scores on assessments of basic concepts (Bracken School-Readiness Assessment), perceptual skills (Visual-Motor Integration Test), receptive vocabulary (Peabody Picture Vocabulary Test, Third Edition), daily living functional skills (Pediatric Functional Independence Measure), and presence of sensory impairments or autism. Proportional odds models were used to identify risk factors predicting lower school-readiness levels.
RESULTS
Of the children examined, the mean birth weight was 1016 ± 391 g, and the mean gestational age was 27.5 ± 2.6 weeks. Ninety-one (67%) children were school-ready. Using multivariate analysis, male gender, chronic lung disease, and severe intraventricular hemorrhage or periventricular leukomalacia were associated with lower school-readiness levels. However, the most powerful factor determining school-readiness level was low socioeconomic status.
CONCLUSION
Interventions targeting neonatal morbidities may be much less effective at improving overall performance at school age compared with the effect of the impoverished social environment.
Keywords: chronic lung disease, developmental outcome, intraventricular hemorrhage, kindergarten readiness, neurodevelopmental outcome, periventricular leukomalacia, prematurity, respiratory distress, school-age follow-up, social disadvantage, socioeconomic status, very low birth weight
WHAT’S KNOWN ON THIS SUBJECT
Premature infants are at increased risk for abnormal neurodevelopmental outcomes at 2 years’ postconceptional age. It is not known what factors increase the risk that these infants will be unready to enter school at an appropriate age.
WHAT THIS STUDY ADDS
Using a multidimensional assessment in a school-aged cohort of premature infants, we identify risk factors for lack of school readiness. Low socioeconomic status increases the risk of lower school-readiness levels far more than bronchopulmonary dysplasia or severe IVH/PVL.
In comparison with term children, infants having birth weights of <2000 g have increased risks of adverse neurodevelopmental outcomes, including cerebral palsy (CP), impaired cognition, blindness, and deafness.1–6 Assessments of neurodevelopmental outcomes have been routinely performed at corrected ages of 1 to 2 years. The developmentally limited repertoire of behaviors in these infants necessarily restricts assessments of higher-level cognition, communication, and motor coordination employed during later development.7 Thus, results of neurodevelopmental assessments performed at 2 years of age may not reliably predict performance in later childhood.8–10 Impairments of motor function and cognition, detected at 2 years of age, may be transient, such that some children are able to catch-up by early school age.11–13 In contrast, children in which CP is not found at age 2 may subsequently exhibit CP.11,12 Finally, subtle cognitive problems may not be detectable until school age, when the complexity of cognitive and adaptive demands increase markedly in academic and social spheres.14,15
The goal of the present study was to assess school readiness at 5 to 6 years of age in a cohort of infants having birth weights of <2000 g with moderate respiratory distress syndrome (RDS),16 and to identify the risk factors associated with lack of school readiness. We defined school readiness as preparedness to learn to read, write, follow directions, interact socially, and function independently in activities of daily living. Disorders in these domains have been associated with increased risk for grade repetition, need for special education, and academic underachievement.17,18
PATIENTS AND METHODS
Study Population
The study population consisted of school-aged survivors from a cohort of 207 patients with RDS requiring surfactant-replacement therapy and mechanical ventilation. Data for this study were obtained between July 2005 and September 2006. The protocol was approved by the institutional review board of the University of Chicago. Informed consent was obtained from all parents.
Neonatal Course
Each child’s birth weight, gestational age, Apgar scores, and neonatal clinical course were obtained from a research database populated during the infant’s initial hospitalization, as was the presence of chronic lung disease (CLD) (oxygen requirement at 36 weeks’ postconceptional age), severe intraventricular hemorrhage (IVH) (grade III and IV), periventricular leukomalacia (PVL), necrotizing enterocolitis, sepsis, and multiple gestation.
Follow-up Assessments
Children and families were assessed in a single visit. Caretakers completed questionnaires regarding race, their child’s need for special education services, and ongoing morbidities. We classified ongoing morbidities as none/mild (eg, allergies, eczema), chronic (eg, failure to thrive, asthma on controller medication), or multiple chronic morbidities/technology dependence (eg, ventriculo-peritoneal shunt, gastrostomy tube).
Information on socioeconomic status and the child’s functioning and behavior was collected using standardized instruments. Socioeconomic status was determined by using the Hollings head Index of Social Position (ISP),19 a 2-factor index based on the head of household’s highest educational attainment and current occupation. Independent functioning was determined by using the Pediatric Functional Independence Measure (WeeFIM),20 an 18-item instrument measuring performance in essential daily functional skills across the domains of self-care, mobility, and cognition. Behavior was assessed using the National Initiative for Children’s Healthcare Quality Vanderbilt Parent Assessment Scale,21 a screen for common pediatric behavioral disorders including inattentiveness and hyperactivity related to attention-deficit/hyperactivity disorder, oppositional-defiant disorder, conduct disorder, anxiety, and depression. Children were classified as having any of the above disorders according to the scoring instructions of the Vanderbilt Scale.
Children were evaluated by a pediatrician and/or a developmental and behavioral specialist unaware of the children’s perinatal history at the University of Chicago Hospitals, La Rabida Children’s Hospital (Chicago, IL), or by home visit. Children underwent a detailed physical and neurologic examination. Children with abnormalities of tone and posture were classified as having a CP syndrome, and the Gross Motor Function Classification System (GMFCS)22 level was assigned (level 1: mild; levels 2–3: moderate; levels 4–5: severe-profound).
All children underwent a battery of developmental assessments: the Bracken School-Readiness Assessment23 evaluated understanding of foundational concepts in the categories of colors, letters, numbers, sizing, comparisons, and shapes. The Peabody Picture Vocabulary Test, Third Edition (PPVT-III)24 assessed receptive vocabulary. The Beery Test of visual-motor integration (VMI)25 assessed the ability to copy geometric forms. Raw scores were converted to standard scores (mean: 100; SD: 15). To identify autism, children with developmental delay in communication, concepts, and/or behavior were administered the Childhood Autism Rating Scale.26
Use of a hearing aid constituted the criterion for hearing loss. Children with hearing aids who were unable to communicate were presumed deaf. Visual acuity was assessed with Lea symbols.27 Children with corrected visual acuity between 20/60 and 20/200 were considered visually impaired, and children with acuity worse than 20/200 were considered blind.
School-Readiness Assessment
School-readiness levels were derived from scores on the WeeFIM, Bracken School-Readiness Assessment, PPVT-III, and Beery Test of VMI. Using these scores and the presence of sensory or motor impairment, children were assigned to 1 of 4 levels of school readiness (Table 1).
TABLE 1.
Classification of School Readiness (Highest to Lowest)
| School-ready | |
| Level 4 | No more than 1 developmental assessment score between 70 and 85 |
| Level 3 | No more than 2 developmental assessment scores between 70 and 85 |
| Not school-ready | |
| Level 2 (1 of the following) | 3 developmental assessment scores between 70 and 85 |
| 1 test developmental assessment score 370 | |
| Visual impairment | |
| Hearing impairment | |
| CP: GMFCS level 1–3 (mild-moderate) | |
| Level 1 (1 of the following) | Bracken score 350 |
| Blindness | |
| Deafness | |
| CP: GMFCS level 4–5 (severe-profound) | |
| Autism | |
Developmental assessments: WeeFIM, Bracken School-Readiness Assessment, PPVT-III, and Beery Test of VMI.
We categorized children scoring >85 on all assessments, or scoring 70 to 85 on only 1 assessment, as level 4 (ie, school-ready). Children scoring 70 to 85 on no more than 2 assessments were level 3 (ie, also school-ready, but less ready than children in level 4). These children did not have either impairments in multiple domains or mental retardation that would render them unready for school. We categorized children scoring 70 to 85 on ≥3 assessments as level 2; these children had impairments in multiple domains, and therefore, were not school-ready. Children scoring <70 on any single assessment were also categorized as level 2; these children had mental retardation or severe communicative, perceptual, or adaptive developmental disorders. We categorized children with deafness, blindness, or an inability to speak as level 1 (ie, also not school-ready). Children with severe autism (score of >36, and moderately severe abnormal ratings on 3 of the 5 subscales of the Childhood Autism Rating Scale) were also categorized as level 1.
Statistical Analysis
We assessed the roles played by demographics, socioeconomic status, and neonatal morbidities in predicting the level of school readiness using proportional odds models.28 We examined each factor in a univariate analysis. Factors with a P value of < .15 in the univariate analysis were included in subsequent multivariate models. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated from the proportional odds model as the estimate of the strength of association. A P value of ≤ .05 was considered statistically significant.
RESULTS
Patient Population
Data were obtained for 135 of the 167 (81%)surviving children at a mean age of 5.7 years (SD: ±1 year). Thirty-two children were lost to follow-up. For the 5 children who could not be seen, we administered the parent questionnaire, the WeeFIM, and the Vanderbilt Scale by telephone. With this information, school-readiness levels were easily assigned. One child was reported to be blind (level 1). From recent medical charts, a second child had documented global developmental delay (level 1). Two children were reported to have CP, and, using directed questioning, were assigned GMFCS levels of 1 and 3. These children were classified as school-readiness level 2. The fifth child was in kindergarten without special services and, based on reports of parent and teacher, was classified as level 4.
Maternal demographic factors and infant birth data are presented in Table 2. The mean birth weight was 1016 g (SD: ±391 g), and the mean gestational age was 27.5 weeks (SD: ±2.6 weeks). There were no significant differences between the birth weights (1136 ± 380 g; P = .12) and gestational ages (27.9 ± 2.7 weeks; P = .44) of the infants who were lost to follow-up compared with those in the study. The majority of children (58%) came from families classified by the Hollingshead Index of Social Position as lowest socioeconomic status (levels 4 and 5). Only 12% of children came from families with highest social position (levels 1 and 2).
TABLE 2.
Demographics and Clinical Characteristics
| Mean birth weight, g (±1 SD) | 1016 ± 391 |
| Gestational age, wk | 27.5 ± 2.6 |
| Male, n (%) | 67 (50) |
| Race (identified by maternal report), n (%) | |
| Black | 95 (70) |
| White | 23 (17) |
| Hispanic | 16 (12) |
| Other | 1 (1) |
| Hollingshead ISP, n (%) | |
| Levels 1–2 | 16 (12) |
| Level 3 | 41 (30) |
| Level 4 | 37 (27) |
| Level 5 | 41 (31) |
| 1-min Apgar score, median (interquartile range) | 5 (4–6) |
| 5-min Apgar score, median (interquartile range) | 7 (6–8) |
| Ventilator type, n (%) | |
| Conventional | 69 (51) |
| High-frequency oscillatory ventilation | 66 (49) |
| Corticosteroids, n (%) | |
| Antenatal | 78 (59) |
| Postnatal (>7 d) | 15 (11) |
| CLD, n (%) | 63 (47) |
| Severe IVH/PVL, n (%) | 23 (17) |
School-Readiness Outcomes
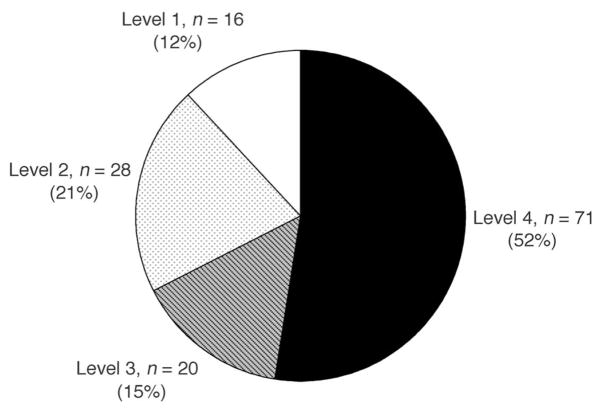
Children who were ready for school (level 3 and 4) constituted 67% of the cohort (Fig 1). Of the children who were scored as not ready for school, 37% were classified as having the lowest school-readiness level (level 1).
FIGURE 1.
Distribution of school-readiness levels.
We next asked whether epidemiologic factors contributed to lack of school readiness. Gender,15,29–35 maternal nonwhite race,31,33,34 and birth weight4,10,31,34,36,37 adversely affect neurodevelopmental outcome. In our study, boys had more than 2 times the odds of having a lower school-readiness level compared with girls (Table 3). Infants born to black mothers were at a similar disadvantage compared with infants born to nonblack mothers (Table 3). The differences in mean birth weights of children grouped by school-readiness levels were not statistically significant (P = .11).
TABLE 3.
Intrinsic Factors and School Readiness
| Risk Factor | School-Readiness Levels |
OR (95% CI) | P | |||
|---|---|---|---|---|---|---|
| 4 (N = 71) | 3 (N = 20) | 2 (N = 28) | 1 (N = 16) | |||
| Birth weight, mean ± SD, g | 1046 ± 426 | 1135 ± 298 | 937 ± 387 | 871 ± 282 | — | |
| Per 100-g increment | — | — | — | — | 0.92 (0.86–1.02) | .11 |
| Gender, n (%) | ||||||
| Male | 28 (42) | 14 (21) | 13 (19) | 12 (18) | 2.19 (1.15–4.21) | .02 |
| Female | 43 (63) | 6 (9) | 15 (22) | 4 (6) | — | |
| Maternal race, n (%) | ||||||
| Black | 44 (46) | 15 (16) | 24 (25) | 12 (13) | 2.29 (1.08–4.87) | .03 |
| Nonblack | 27 (68) | 5 (13) | 4 (10) | 4 (10) | — | |
We then asked to what extent an infant’s neonatal course and ongoing morbidities at follow-up determined lack of school readiness. Prolonged postnatal steroid treatment has been associated with abnormal neurodevelopment.35,38–40 In our study, dexamethasone was the only systemic cortico-steroid used. No significant difference in school-readiness levels was observed between children exposed to prolonged postnatal dexamethasone (>7 days) and those not receiving prolonged dexamethasone (Table 4).
TABLE 4.
Neonatal Morbidities, Chronic Disease Burden, and School Readiness
| Risk Factor | School-Readiness Levels |
OR (95% CI) | P | |||
|---|---|---|---|---|---|---|
| 4 (N = 71) | 3 (N = 20) | 2 (N = 28) | 1 (N = 16) | |||
| Severe IVH/PVL, n (%) | ||||||
| Yes | 7 (30) | 6 (26) | 4 (17) | 6 (26) | 2.61 (1.15–5.94) | .02 |
| No | 64 (57) | 14 (13) | 24 (21) | 10 (9) | — | |
| CLD, n (%) | ||||||
| Yes | 26 (41) | 10 (16) | 15 (24) | 12 (19) | 2.53 (1.32–4.85) | .005 |
| No | 45 (63) | 10 (14) | 13 (18) | 4 (6) | — | |
| Postnatal steroids (>7 d), n (%) | ||||||
| Yes | 6 (40) | 1 (7) | 4 (27) | 4 (27) | 2.34 (0.84–6.51) | .10 |
| No | 63 (53) | 19 (16) | 24 (20) | 12 (10) | — | |
| Ongoing morbidities, n (%) | ||||||
| None/mild | 55 (63) | 14 (16) | 14 (16) | 4 (5) | 1.0 | <.001 |
| Chronic | 14 (39) | 5 (14) | 11 (31) | 6 (17) | 3.04 (1.44–6.40) | |
| Multiple chronic/technological dependence | 0 (0) | 1 (9) | 3 (27) | 5 (45) | 11.7 (3.32–41.0) | |
CLD4,8,35,41,42 and the presence of severe IVH/PVL4,8,29,34,35,37,42–44 adversely affect neurodevelopment. In this study, infants with CLD or severe IVH/PVL were >2½ times more likely to have lower school-readiness levels (Table 4). Because chronic illness may slow physical and intellectual development, we assessed the role played by ongoing morbidities. As the number of ongoing morbidities increased, the likelihood of lower school-readiness levels increased. The odds of having lower school-readiness levels increased approximately threefold in children classified as having 1 chronic morbidity, while increasing 11-fold in children with multiple chronic morbidities or technology dependence (Table 4).
Lower socioeconomic status has been linked to decreased cognition at 2 years of age and during kindergarten.4,5,8,10,30,31,33,43,45 To assess the role of socioeconomic class and school-readiness levels, we classified families by Hollings head ISP scores and compared school-readiness levels of upper class families (levels 1 and 2) with each of the other ISP scores. Overall, worse ISP scores were significantly associated with increased odds of having lower school-readiness levels (P = .003). Most strikingly, children from ISP class 5, the lowest socioeconomic class, had a more than four fold increased likelihood of having lower school-readiness levels compared with children from ISP class 1–2 (Table 5).
TABLE 5.
Socioeconomic Status and School Readiness
| Risk Factor | School-Readiness Levels |
OR (95% CI) | P | |||
|---|---|---|---|---|---|---|
| 4 (N = 71) | 3 (N = 20) | 2 (N = 28) | 1 (N = 16) | |||
| Hollingshead ISP | ||||||
| Level 1–2 | 12 (75) | 1 (6) | 2 (13) | 1 (6) | 1.0 (reference) | .003 |
| Level 3 | 27 (66) | 5 (12) | 4 (10) | 5 (12) | 1.56 (0.43–5.68) | |
| Level 4 | 14 (38) | 11 (30) | 10 (27) | 2 (5) | 3.30 (0.94–11.6) | |
| Level 5 | 18 (44) | 3 (7) | 12 (29) | 8 (20) | 4.42 (1.24–15.8) | |
Receiving early intervention (EI) services might be expected to improve functional outcomes. Furthermore, access to EI may depend on socioeconomic class. Accordingly, we asked whether receipt of EI depended on socioeconomic status. All infants were referred for EI, and 86% received it. Children who received EI and were more socially advantaged (ISP class 1–3) were more likely to be school-ready than children who received EI and were less socially advantaged (OR: 2.74 [95% CI: 1.30–5.74] P = .008). Only 19 children did not receive EI; whether a child received EI was not dependent on social position (P = .94).
To understand which of the risk factors identified by univariate analysis were independent, we performed serial multivariate analyses, whereby a multivariate model was sequentially pruned of variables found not to be significant. The presence of chronic morbidities was not employed in the multivariate model, because it was measured at the same time as school readiness. In the first model, we considered birth weight, race, gender, ISP level, CLD, and IVH/PVL. Subsequent iterations removed birth weight and race for lack of significance. The final model, therefore, included as significant factors gender, CLD, IVH/PVL, and Hollingshead ISP (Table 6). In this model, socioeconomic class played the most significant role, with children in ISP level 5 having a greater than sevenfold increased likelihood of having lower school-readiness levels compared with children in ISP level 1–2.
TABLE 6.
Risk Factors for Lack of School Readiness: Multivariate Analyses
| Risk Factor | All Variables |
Without Birth Weight |
Without Race and Birth Weight |
|||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Birth weight, per 100 g | 0.95 (0.86–1.06) | .36 | — | — | ||
| Maternal race | ||||||
| Black vs nonblack | 2.11 (0.88–5.02) | .093 | 2.18 (0.91–5.19) | .079 | — | |
| Male gender | 2.30 (1.10–4.78) | .026 | 2.09 (1.03–4.24) | .04 | 2.09 (1.04–4.20) | .04 |
| Hollingshead ISP | ||||||
| Level 1–2 | 1.00 (reference) | .008 | 1.00 (reference) | .007 | 1.00 (reference) | .001 |
| Level 3 | 1.90 (0.47–7.70) | 1.93 (0.47–7.94) | 2.32 (0.58–9.36) | |||
| Level 4 | 3.37 (0.85–13.4) | 3.31 (0.82–13.3) | 4.50 (1.16–17.4) | |||
| Level 5 | 5.10 (1.24–21.0) | 5.25 (1.25–21.9) | 7.63 (1.92–30.4) | |||
| CLD | 2.23 (1.02–4.89) | .045 | 2.62 (1.29–5.32) | .007 | 2.39 (1.19–4.79) | .01 |
| Severe IVH/PVL | 2.61 (1.03–6.61) | .044 | 2.71 (1.07–6.86) | .035 | 2.54 (1.01–6.36) | .05 |
DISCUSSION
In this follow-up study of school-aged children who were premature infants with RDS and treated with surfactant-replacement therapy, we used historical, physical, and neurodevelopmental assessments to measure readiness for entry into public school and the risk factors determining lack of school readiness. Male gender, CLD, and severe IVH/PVL, factors well-known to predispose to abnormal neurodevelopment, constituted risks for lower school-readiness levels. However, decreased socioeconomic class constituted the strongest barrier to achieving school readiness.
Assessing intercenter differences in neurodevelopmental outcome of extremely low birth weight premature infants at 18 to 22 months of age, Vohr et al46 found that the use of postnatal steroids and increased duration of mechanical ventilation increased rates of neurodevelopmental impairment. We also found that longer duration of ventilatory support was associated with decreased school-readiness levels. However, after adjusting for CLD in a multivariate analysis, duration of ventilatory support was not an independent risk factor. In addition, having a prolonged postnatal dexamethasone course was not associated with lack of school readiness. However, the small number of children receiving prolonged postnatal steroids (15 of 135 infants) is likely too small to detect significant differences.
A child’s readiness for school requires age-appropriate physical, behavioral, communicative, visual, motor, and conceptual skills. Accordingly, in designing our assessment of school readiness, we used a multidimensional battery of well-validated cognitive, language visual-motor, functional, and behavioral assessments, supplemented with diagnoses of CP and sensory impairment, to assign school-readiness levels. Children scoring between 1 and 2 SDs below the mean on ≤2 measures (level 3) have been considered to have minor impairments and may need special education services.17 Accordingly, we considered these children ready for school but at an increased disadvantage compared with their peers. Children unable to perform better than 2 SDs below the mean in any of the measures employed (level 2), as well as those having significant sensory or motor impairment (level 1), clearly cannot participate in the regular classroom.
Neurodevelopmental follow-up studies of preterm infants at school age have used full-scale IQ scores or disability diagnoses (CP, blindness, deafness) as primary outcome variables.15,47,48 However, preterm infants are at increased risk for learning disabilities,45 which by current definition occur in the setting of normal IQ scores.49,50 Children having learning disabilities require special education services,51 and these needs will not be identified by standard IQ assessments alone. A school-readiness evaluation, such as ours, that assesses performance in these domains more completely describes a child’s overall learning ability. Our assessment distinguishes important, subtle, functional delays leading to poor school performance. Future long-term neurodevelopmental follow-up studies may benefit from adding multidimensional school-readiness evaluations to standard assessments of IQ and disability.
The ability to perform MRI in premature children has allowed for a better understanding of brain development and the potential contribution to long-term adverse outcome in this vulnerable population. By using MRI, Peterson52 measured regional brain volumes in 8-year-old children who were preterm and term infants who had their IQ measured. In the brain regions of preterm infants where the greatest abnormalities in regional brain volumes were observed, the degree of abnormality correlated with IQ,52 suggesting a contribution of morphologic abnormality to functional outcome. It is conceivable that school-readiness levels correlate similarly with regional brain volume abnormalities.
By using functional MRI, Ment et al53 demonstrated that, at 8 years of age, preterm boys who received indomethacin in the NICU may demonstrate improved phonologic processing compared with saline-treated boys, and comparable with boys born at term. In our study, all infants with birth weights <1250 g received prophylactic indomethacin. To the extent that this subtle improvement in linguistic processing may improve performance on the school-readiness tests we employed, school-readiness levels of those males in our study who received prophylactic indomethacin may have been improved. However, the profound effects we observed of low socioeconomic status on school readiness are likely to dwarf any possibly beneficial effect of indomethacin.
A school-readiness measure has, to our knowledge, been employed in only 1 previous study, in which risk factors associated with need for special education services in kindergarten were assessed in premature infants (23–28 weeks’ gestation).17 This retrospective cohort study defined kindergarten-readiness as having no major impairments or no more than 1 minor impairment. In this population, only 35% of children were considered kindergarten-ready, consistent with the study being performed before the routine use of surfactant and antenatal steroids. Interestingly, neither IVH nor CLD was found to be a significant risk factor for lack of kindergarten readiness. Nonetheless, socioeconomic class was found to be as powerful a predictor of lack of kindergarten-readiness as in the present population.
We found that the strongest risk for decreased school-readiness levels was low socioeconomic status. Although where a child received early intervention was independent of socioeconomic status, it is unclear whether the detrimental effect of socioeconomic status is related to the quality of early intervention received or the social milieu of impoverished families. Impoverished families are at higher risk for parents with decreased education,31,54–59 having a single parent household,58,60 having decreased access to resources,60–62 poor nutrition,59,63–65 and increased psychological distress.66,67 These factors likely contribute to impoverished environments for child development, including a low stimulation environment,68,69 impoverished oral language exposure,57,60,68 and decreased exposure to cognitively stimulating materials,62,67,70 factors potentially affecting neurodevelopmental outcome. To the extent that families in our study in the lowest socioeconomic stratum are similarly at risk, our data demonstrate that this social environment conspires to retard cognitive and functional development to a far greater extent than physical morbidities acquired as a result of prematurity or its complications.
We found that 33% of school-aged children born prematurely with RDS were, in fact, not ready for school and 15%, although ready, were at risk for needing special education. How do these statistics compare to the general population? To our knowledge, no information is systematically compiled by public school districts on school readiness of children entering kindergarten. Therefore, a direct comparison of our cohort with the general population is not possible. Similarly, without a term control group matched for socioeconomic status, we are unable to assess whether decreased socioeconomic status plays as significant a role in determining school readiness in term infants.
CONCLUSIONS
In the current era of neonatology, an adverse socioeconomic environment increases the risk that a child born prematurely will not be prepared to enter school on time. This risk is disproportionately high among children who live in poverty. Our findings demonstrate the risk of neonatal morbidities in determining school readiness. However, decreased socioeconomic status plays a far greater role in determining school readiness than these biomedical risks. Consequently, interventions targeting neonatal morbidities are likely to be less effective at improving school readiness in the setting of an impoverished socioeconomic environment.
Acknowledgments
Dr Patrianakos-Hoobler was supported in part by the American Academy of Pediatrics Resident Research Grant. Dr Schreiber was supported by an investigator-initiated grant from INO Therapeutics/IKARIA.
This work benefited from the assistance of Larry Gray, MD, Emily Msall, Jennifer Park, MA, Scott Schreiber, and Danielle Zageris.
ABBREVIATIONS
- CI
confidence interval
- CLD
chronic lung disease
- CP
cerebral palsy
- EI
early intervention
- GMFCS
Gross Motor Function Classification System
- IVH
intraventricular hemorrhage
- ISP
Index of Social Position
- OR
odds ratio
- PPVT-III
Peabody Picture Vocabulary Test Third Edition
- PVL
periventricular leukomalacia
- RDS
respiratory distress syndrome
- VMI
visual-motor integration
- WeeFIM
Pediatric Functional Independence Measure
Footnotes
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
References
- 1.National Center for Health Statistics. [Accessed October 16, 2007];Final natality data, 2004. Available at: www.cdc.gov/nchs.
- 2.Doyle LW, Anderson PJ Victorian Infant Collaborative Study Group. Improved neurosensory outcome at 8 years of age of extremely low birthweight children born in Victoria over three distinct eras. Arch Dis Child Fetal Neonatal Ed. 2005;90(6):F484–F488. doi: 10.1136/adc.2004.063362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hack M, Taylor HG, Drotar D, et al. Chronic conditions, functional limitations, and special health care needs of school-aged children born with extremely low-birth-weight in the 1990s. JAMA. 2005;294(3):318–325. doi: 10.1001/jama.294.3.318. [DOI] [PubMed] [Google Scholar]
- 4.Hack M, Taylor HG, Klein N, Eiben R, Schatschneider C, Mercuri-Minich N. School-age outcomes in children with birthweights under 750g. N Engl J Med. 1994;331(12):753–759. doi: 10.1056/NEJM199409223311201. [DOI] [PubMed] [Google Scholar]
- 5.Kilbride HW, Thorstad K, Daily D. Preschool outcome of less than 801-gram preterm infants compared with full-term siblings. Pediatrics. 2004;113(4):742–747. doi: 10.1542/peds.113.4.742. [DOI] [PubMed] [Google Scholar]
- 6.Taylor HG, Klein N, Drotar D, Schluchter M, Hack M. Consequences and risks of <1000-g birthweight for neuropsychological skills, achievement, and adaptive functioning. J Dev Behav Pediatr. 2006;27(6):459–469. doi: 10.1097/00004703-200612000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Hack M, Taylor G, Drotar D, et al. Poor predictive validity of the Bayley Scales of Infant Development for cognitive function of extremely low birthweight children at school age. Pediatrics. 2005;116(2):333–341. doi: 10.1542/peds.2005-0173. [DOI] [PubMed] [Google Scholar]
- 8.Msall ME. Neurodevelopmental surveillance in the first 2 years after extremely preterm birth: evidence, challenges, and guidelines. Early Hum Dev. 2006;82(3):157–166. doi: 10.1016/j.earlhumdev.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 9.Victorian Infant Collaborative Study Group. Eight year outcome in infants with birthweights of 500–999 grams: continuing regional study of 1979 and 1980 births. J Pediatr. 1991;118(5):761–767. doi: 10.1016/s0022-3476(05)80044-7. [DOI] [PubMed] [Google Scholar]
- 10.Koller H, Lawson K, Rose S, Wallace I, McCarton C. Patterns of cognitive development in very low birthweight children during the first six years of life. Pediatrics. 1997;99(3):383–389. doi: 10.1542/peds.99.3.383. [DOI] [PubMed] [Google Scholar]
- 11.Nelson K, Ellenberg J. Children who “outgrew” cerebral palsy. Pediatrics. 1982;69(5):529–536. [PubMed] [Google Scholar]
- 12.Ross G, Lipper E, Auld P. Consistency and change in the development of premature infants weighting les than 1501 grams at birth. Pediatrics. 1985;76(6):885–891. [PubMed] [Google Scholar]
- 13.Ford G, Kitchen W, Doyle L, Rickards A, Kelly E. Changing diagnosis of cerebral palsy in very low birthweight children. Am J Perinatol. 1990;7(2):178–181. doi: 10.1055/s-2007-999475. [DOI] [PubMed] [Google Scholar]
- 14.Aylward GP. Neurodevelopmental outcomes of infants born prematurely. J Dev Behav Pediatr. 2005;26(6):427–440. doi: 10.1097/00004703-200512000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Marlow N, Wolke D, Bracewell M, Samara M. Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med. 2005;352(1):9–19. doi: 10.1056/NEJMoa041367. [DOI] [PubMed] [Google Scholar]
- 16.Schreiber MD, Gin-Mestan K, Marks JD, Huo D, Lee G, Srisuparp P. Inhaled nitric oxide in premature infants with the respiratory distress syndrome. N Engl J Med. 2003;349(22):2099–2107. doi: 10.1056/NEJMoa031154. [DOI] [PubMed] [Google Scholar]
- 17.Msall ME, Buck GM, Rogers BT, Catanzaro NL. Kindergarten readiness after extreme prematurity. Am J Dis Child. 1992;146(11):1371–1375. doi: 10.1001/archpedi.1992.02160230129033. [DOI] [PubMed] [Google Scholar]
- 18.Vohr BR, Msall ME. Neuropsychological and functional outcomes of very low birthweight infants. Semin Perinatol. 1997;21(3):202–220. doi: 10.1016/s0146-0005(97)80064-x. [DOI] [PubMed] [Google Scholar]
- 19.Hollingshead AB. Two Factor Index of Social Position. New Haven, CT: ••••; 1957. [Google Scholar]
- 20.System for Medical Rehabilitation and the Center for Functional Assessment Research. Uniform Data System for Medical Rehabilitation. Buffalo, NY: University of Buffalo; 1998. [Google Scholar]
- 21.Caring for Children With ADHD: A Resource Toolkit for Clinicians. Cambridge, MA: American Academy of Pediatrics and National Initiative for Children’s Healthcare Quality; [Accessed July 8, 2005]. Available at: www.nichq.org/NICHQ/Topics/ChronicConditions/ADHD/Tools/ADHD.htm. [Google Scholar]
- 22.Palisano R, Rosenbaum P, Walter S, Wood E, Galuppi B. Gross Motor Function Classification System for cerebral palsy. Dev Med Child Neurol. 1997;39(4):214–223. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 23.Bracken B. Bracken School Assessment (Administrative Manual) ••••: Harcourt Assessment, Inc; 2002. [Google Scholar]
- 24.Dunn L, Dunn L. Peabody Picture Vocabulary Test. 3. ••••: American Guidance Service; 1997. [Google Scholar]
- 25.Beery K, Beery N. BEERYVMI (The Beery-Buktenica Developmental Test of Visual-Motor Integration. 5. ••••: NCS Pearson, Inc; 2004. [Google Scholar]
- 26.Schopler E, Reichler R, Rochen-Renner B. Childhood Autism Rating Scale (CARS) Los Angeles, CA: Western Psychological Services; 1988. [Google Scholar]
- 27.Hyvärinen L, Näsänen R, Laurinen P. New visual acuity test for pre-school children. Acta Ophthalmol (Copenh) 1980;58(4):507–511. doi: 10.1111/j.1755-3768.1980.tb08291.x. [DOI] [PubMed] [Google Scholar]
- 28.McCullagh P. Regression models for ordinal data. J R Stat Soc Ser B Stat Soc. 1980;42(•••):109–142. [Google Scholar]
- 29.Mikkola K, Ritari N, Tommiska V, et al. Neurodevelopmental outcome at 5 years of age of a national cohort of extremely low birthweight infants who were born in 1996–1997. Pediatrics. 2005;116(6):1391–1400. doi: 10.1542/peds.2005-0171. [DOI] [PubMed] [Google Scholar]
- 30.Hille E, Ouden A, Bauer L, van den Oudenrijn C, Brand R, Verloove-Vanhorick S. School performance at nine years of age in very premature and very low birthweight infants: perinatal risk factors and predictors at five years of age. J Pediatr. 1994;125(3):426–434. doi: 10.1016/s0022-3476(05)83290-1. [DOI] [PubMed] [Google Scholar]
- 31.Resnick MB, Gueorguieva RV, Carter RL, et al. The impact of low birthweight, perinatal conditions, and sociodemographic factors on educational outcome in kindergarten. Pediatrics. 1999;104(6) doi: 10.1542/peds.104.6.e74. Available at: www.pediatrics.org/cgi/content/full/104/6/e74. [DOI] [PubMed]
- 32.Wood NS, Marlow N, Costeloe K, Gibson AT, Wilkinson AR. Neurological and developmental disability after extremely preterm birth. N Engl J Med. 2000;343(6):378–384. doi: 10.1056/NEJM200008103430601. [DOI] [PubMed] [Google Scholar]
- 33.Msall ME, Buck GM, Rogers BT, Duffy LC, Mallen SR, Catanzaro NL. Predictors of mortality, morbidity, and disability in a cohort of infants ≤28 weeks’ gestation. Clin Pediatr (Phila) 1993;32(9):521–527. doi: 10.1177/000992289303200903. [DOI] [PubMed] [Google Scholar]
- 34.Msall ME, Buck GM, Rogers BT, et al. Multivariate risks among extremely premature infants. J Perinatol. 1994;14(1):41–46. [PubMed] [Google Scholar]
- 35.Vohr BR, Wright LL, Dusick AM, et al. Neurodevelopmental and functional outcomes of extremely low birthweight infants in the National Institutes of Health and Human Development Neonatal Research Network, 1993–1994. Pediatrics. 2000;105(6):1216–1226. doi: 10.1542/peds.105.6.1216. [DOI] [PubMed] [Google Scholar]
- 36.Horwood LJ, Mogridge N, Darlow BA. Cognitive, educational, and behavioural outcomes at 7 to 8 years in a national very low birthweight cohort. Arch Dis Child Fetal Neonatal Ed. 1998;79(1):F12–F20. doi: 10.1136/fn.79.1.f12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Halsey CL, Collin MF, Anderson CL. Extremely low-birth-weight children and their peers: a comparison of school-age outcomes. Arch Pediatr Adolesc Med. 1996;150(8):790–794. doi: 10.1001/archpedi.1996.02170330016003. [DOI] [PubMed] [Google Scholar]
- 38.Finer NN, Craft A, Vaucher YE, Clark RH, Sola A. Postnatal steroids: short-term gain, long-term pain? J Pediatr. 2000;137(1):9–13. doi: 10.1067/mpd.2000.107799. [DOI] [PubMed] [Google Scholar]
- 39.Merz U, Peschgens T, Kusenbach G, Hornchen H. Early versus late dexamethasone treatment in preterm infants at risk for chronic lung disease: a randomized pilot study. Eur J Pediatr. 1999;158(4):318–322. doi: 10.1007/s004310051081. [DOI] [PubMed] [Google Scholar]
- 40.Eichenwald EC, Stark AR. Are postnatal steroids ever justified to treat severe bronchopulmonary dysplasia? Arch Dis Child Fetal Neonatal Ed. 2007;92(5):F334–F337. doi: 10.1136/adc.2006.106583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Böhm B, Katz-Salamon M. Cognitive development at 5.5 years of children with chronic lung disease of prematurity. Arch Dis Child Fetal Neonatal Ed. 2003;88(2):F101–F105. doi: 10.1136/fn.88.2.F101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mestan KL, Marks J, Hecox K, Huo D, Schreiber MD. Neurodevelopmental outcomes of premature infants treated with inhaled nitric oxide. N Engl J Med. 2005;353(1):23–32. doi: 10.1056/NEJMoa043514. [DOI] [PubMed] [Google Scholar]
- 43.Gross SJ, Metterlman BB, Dye TD. Impact of family structure and stability on academic outcome in preterm children at 10 years of age. J Pediatr. 2001;138(2):169–175. doi: 10.1067/mpd.2001.111945. [DOI] [PubMed] [Google Scholar]
- 44.Vollmer B, Roth S, Baudin J, Stewart AL, Neville BG, Wyatt JS. Predictors of long-term outcome in very preterm infants: gestational age versus neonatal cranial ultrasound. Pediatrics. 2003;112(5):1108–1114. doi: 10.1542/peds.112.5.1108. [DOI] [PubMed] [Google Scholar]
- 45.Saigal S, Szatmari P, Rosenbaum P, Campbell D, King S. Cognitive abilities and school performance of extremely low birthweight children and matched term control children at age 8 years: a regional study. J Pediatr. 1991;118(5):751–760. doi: 10.1016/s0022-3476(05)80043-5. [DOI] [PubMed] [Google Scholar]
- 46.Vohr BR, Wright LL, Dusick AM, et al. for the Neonatal Research Network. Center differences and outcomes of extremely low birthweight infants. Pediatrics. 2004;113(4):781–789. doi: 10.1542/peds.113.4.781. [DOI] [PubMed] [Google Scholar]
- 47.Doyle LW Victorian Infant Collaborative Study. Outcome at 5 years of age of children 23 to 27 weeks’ gestation: refining the prognosis. Pediatrics. 2001;108(1):134–141. doi: 10.1542/peds.108.1.134. [DOI] [PubMed] [Google Scholar]
- 48.Hoekstra RE, Ferrara TB, Couser RJ, Payne NR, Connett JE. Survival and long-term neurodevelopmental outcome of extremely premature infants bornat 23–26 weeks’ gestational age at atertiary center. Pediatrics. 2004;113(1 pt 1) doi: 10.1542/peds.113.1.e1. Available at: www.pediatrics.org/cgi/content/full/113/1/e1. [DOI] [PubMed]
- 49.US Department of Education, President’s Commission on Excellence in Special Education. [Accessed September 15, 2008];A new era: revitalizing special education for children and their families. 2002 Available at: www.ed.gov/inits/commissionsboards/whspecialeducation.
- 50.Rispens J, van Y, peren TA, van Duijn GA. The irrelevance of IQ to the definition of learning disabilities: some empirical evidence. J Learn Disabil. 1991;24(7):434–438. doi: 10.1177/002221949102400709. [DOI] [PubMed] [Google Scholar]
- 51.Saigal S, Hoult LA, Streiner DL, Stoskopf BL, Rosenbaum PL. School difficulties at adolescence in a regional cohort of children who were extremely low birthweight. Pediatrics. 2000;105(2):325–331. doi: 10.1542/peds.105.2.325. [DOI] [PubMed] [Google Scholar]
- 52.Peterson BS. Brain imaging studies of the anatomical and functional consequences of preterm birth for human brain development. Ann N Y Acad Sci. 2003;1008:219–237. doi: 10.1196/annals.1301.023. [DOI] [PubMed] [Google Scholar]
- 53.Ment LR, Peterson BS, Meltzer JA, et al. A functional magnetic resonance imaging study of the long-term influences of early indomethacin exposure on language processing in the brains of prematurely born children. Pediatrics. 2006;118(3):961–970. doi: 10.1542/peds.2005-2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alexander KL, Entwisle DR, Dauber SL. First-grade behavior: its short-and long-term consequences for school performance. Child Dev. 1993;64(3):801–814. [PubMed] [Google Scholar]
- 55.Duncan GJ, Brooks-Gunn J, Klebanov P. Economic deprivation and early childhood development. Child Dev. 1994;65(2 Spec No):296–318. [PubMed] [Google Scholar]
- 56.Escalona S. Babies at double hazard: early development of infants at biologic and social risk. Pediatrics. 1982;70(5):670–676. [PubMed] [Google Scholar]
- 57.Ment LR, Vohr B, Walter A, et al. Change in cognitive function over time in very low-birth-weight infants. JAMA. 2003;289(6):705–711. doi: 10.1001/jama.289.6.705. [DOI] [PubMed] [Google Scholar]
- 58.Duncan GJ, Brooks-Gunn J. What Money Can’t Buy: The Effect of Parental Income on Children’s Outcomes. Cambridge, MA: Harvard University Press; 1997. [Google Scholar]
- 59.Korenman S, Miller JE, Sjaastad JE. Long-term poverty and child development in the United States: results from the NLSY. Child Youth Serv Rev. 1995;17(•••):127–155. [Google Scholar]
- 60.Ramey CT, Ramey SL. Prevention of intellectual disabilities: early interventions to improve cognitive development. Prev Med. 1998;27(2):224–232. doi: 10.1006/pmed.1998.0279. [DOI] [PubMed] [Google Scholar]
- 61.Sameroff AJ, Seifer R, Barocas R, Zax M, Greenspan S. Intelligence quotient scores of 4-year-old children: social-environmental risk factors. Pediatrics. 1987;79(3):343–350. [PubMed] [Google Scholar]
- 62.Brooks-Gunn J, Duncan GJ. The effects of poverty on children. Future Child. 1997;7(2):55–71. [PubMed] [Google Scholar]
- 63.Pollitt E, Golub M, Gorman K, et al. A reconceptualization of the effects of under-nutrition on children’s biological, psychosocial, and behavioral development. Soc Policy Rep. 1996;10(•••):1–24. [Google Scholar]
- 64.Oski F. Iron deficiency in infancy and childhood. N Engl J Med. 1993;329(3):190–193. doi: 10.1056/NEJM199307153290308. [DOI] [PubMed] [Google Scholar]
- 65.Raisler J, Alexander C, O’Campo P. Breast-feeding and infant illness: adoseresponse relationship? Am J Public Health. 1999;89(1):25–30. doi: 10.2105/ajph.89.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McLoyd VC. Socioeconomic disadvantage and child development. Am Psychol. 1998;53(2):185–204. doi: 10.1037//0003-066x.53.2.185. [DOI] [PubMed] [Google Scholar]
- 67.Shonkoff JP, Phillips DA. From Neurons to Neighborhoods: The Science of Early Childhood Development. Washington, DC: National Academy Press; 2000. [PubMed] [Google Scholar]
- 68.Yeung J, Linver M, Brooks-Gunn J. How money matters for young children’s development: parental investment and family processes. Child Dev. 2002;73(6):1861–1879. doi: 10.1111/1467-8624.t01-1-00511. [DOI] [PubMed] [Google Scholar]
- 69.Weisglas-Kuperus N, Baerts W, Smrkovsky M, Sauer PJ. Effects of biological and social factors on the cognitive development of very low birthweight children. Pediatrics. 1993;92(5):658–665. [PubMed] [Google Scholar]
- 70.Bradley RH, Corwyn RF, Burchinal M, McAdoo HP, Coll C. The home environments of children in the United States. Part II: relations with behavioral development through age thirteen. Child Dev. 2001;72(6):1868–1886. doi: 10.1111/1467-8624.t01-1-00383. [DOI] [PubMed] [Google Scholar]