Abstract
Recent studies suggest that hypertension associated with low renin status and hyperaldosteronism is associated with increased risk for end-organ damage and cardiovascular events compared with other forms of hypertension. Additionally, experimental studies have demonstrated impaired nitric oxide-mediated bioactivity in these states. To investigate the relation between renin/aldosterone status and resistance vessel function, we examined plasma renin activity, serum aldosterone level, and forearm blood flow responses to the endothelium-dependent vasodilator methacholine and the endothelium-independent vasodilators sodium nitroprusside and verapamil using venous occlusion plethysmography in 130 volunteers (43 hypertensive, 87 normotensive). Low renin status was associated with impaired responses to methacholine and nitroprusside in patients with hypertension. Peak methacholine response was 8.7±5.6 mL/min per dL in the lowest renin quartile (0.1 to 0.3 ng/mL per hour) versus 14.3±7.3 mL/min per dL in the highest 3 renin quartiles combined (0.4 to 4.6 ng/mL per hour; P<0.001). Peak nitroprusside response was 5.6±2.3 mL/min per dL in the lowest renin quartile versus 13.3±4.1 mL/min per dL in the highest 3 renin quartiles combined (P<0.001). Blood pressure and other clinical characteristics were similar in all 4 quartiles. Vasodilator responses to verapamil did not relate to renin activity. Methacholine and nitroprusside responses did not relate to renin status in normotensive controls (P=0.34). Importantly, hypertensive patients with a high aldosterone/renin ratio also had impaired responses to methacholine. This study demonstrates that low-renin hypertension is associated with marked impairment of nitric oxide-mediated vasodilation of resistance vessels in the forearm vasculature of humans. This impairment could contribute to adverse outcomes in patients with low-renin hypertension and relative aldosterone excess.
Keywords: aldosterone, blood flow, endothelium, mineralocorticoids, renin
Approximately one-third of patients with hypertension have “low-renin hypertension,”1 and such patients are more likely to be salt-sensitive and may respond better to diuretic therapy.2–4 The spectrum of low-renin hypertension includes patients with normokalemic primary hyperaldosteronism (mostly idiopathic hyperaldosteronism), which occurs in up to 15% of hypertensive subjects5–8 and may represent an end-stage in the evolution of neurohormonal changes in “essential” hypertension. 7,8 Despite early evidence that patients with low renin levels might have a relatively favorable prognosis,1,9 more recent epidemiological studies suggest that patients with low-renin and/or salt-sensitive hypertension have increased risk of end-organ damage, cardiovascular events, and mortality compared with other hypertensives.3,10–12
These observations might seem difficult to reconcile with our current understanding of the vascular consequences of neurohormonal activation. For example, experimental and clinical studies strongly suggest that activation of the reninangiotensin-aldosterone system leads to endothelial dysfunction, 13,14 which contributes importantly to the pathogenesis of cardiovascular events in hypertension.15,16 In this regard, treatment with angiotensin II, aldosterone, or deoxycorticosterone and salt in rats reduces the bioavailability of endothelium-derived NO, increases superoxide production, and produces other pathological changes in the vasculature.17–19 In humans, patients with elevated aldosterone levels20,21 or renovascular hypertension20 have impaired endothelium-dependent vasodilation in forearm microvessels, and intra-arterial aldosterone infusion impairs endothelium-dependent vasodilation.22 Finally, treatment of hypertensive patients with angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and aldosterone receptor blockers generally improves endothelial function in hypertensive patients, providing further support for a link between endothelial dysfunction and increased activity of the renin-angiotensin system in hypertension.21,23
On the basis of this experimental and clinical work, we hypothesized that increased activity of the renin-aldosterone system would be associated with endothelial dysfunction. The present study investigated this hypothesis in forearm microvessels of normotensive and hypertensive patients. Our finding that lower, rather than higher, renin activity is associated with endothelial dysfunction in hypertensive patients was unexpected, but may be explained by the observation that patients with relative aldosterone excess in the setting of low renin activity have impaired function. These findings are consistent with available epidemiological evidence and may support the adverse effects of relative hyper-aldosteronism among patients with essential hypertension.
Methods
Study Participants
We prospectively recruited patients with a clinical history of hypertension (on treatment and/or systolic blood pressure >140 mm Hg and/or diastolic blood pressure >90 mm Hg) and normotensive volunteers by advertisement. Race was defined by self-report. Volunteers were excluded if they had a history of coronary artery disease, congestive heart failure, peripheral vascular disease, diabetes mellitus (on hypoglycemic medications or with a fasting glucose >140 mg/dL), serum low-density lipoprotein >190 mg/dL, or end-stage renal disease.24 All participants provided written informed consent as approved by the institutional review board of Boston Medical Center.
Study Protocol
Participants discontinued all vasoactive medications for 48 hours before study. In addition, we asked participants to fast overnight and refrain from alcohol, caffeine, and cigarette smoking (if applicable) for at least 12 hours before study. The studies were performed in a quiet, dimmed, temperature-controlled vascular laboratory (24°C). Using sterile conditions and local anesthesia, a 20-gauge polyethylene catheter (Arrow International) was inserted in the nondominant brachial artery for measurement of blood pressure and infusion of drugs. After catheter insertion, 5% dextrose in water (Baxter Healthcare Co) was infused at 0.4 mL/min for at least 30 minutes while stable baseline flow and blood pressure conditions were established. Forearm blood flow was measured by venous occlusion plethysmography with calibrated mercury-in-silastic strain gauges and automatic venous-cuff occlusion at 40 mm Hg (Hokanson, Inc), as previously described.25,26
Serial 5-minute infusions of methacholine (0.3, 1.0, 3.0, and 10 µg/min; Metapharm, Inc), sodium nitroprusside (0.3, 1.0, 3.0, and 10 µg /min; Baxter Anesthesia and Critical Care), or verapamil (10, 30, 100, and 300 µg/min; Abbott Hospital Products) were delivered into the brachial artery. Forearm blood flow was measured during the last 2 minutes of each infusion. Dextrose control was infused for 30 minutes between agonists to reestablish control conditions. The order of drugs was randomized, but not all participants had all agonists.
Biochemical Analyses
Arterial blood samples were collected from the brachial artery catheter into Vacutainer tubes after the participants lay in a supine position for at least 15 minutes. Serum total cholesterol, high-density lipoprotein cholesterol, triglycerides, glucose, and creatinine were measured using an automated analyzer (Hitachi model 917; Hitachi Instruments). LDL cholesterol was calculated using the Friedewald formula.27 Serum aldosterone levels and plasma renin activity (blood anticoagulated with ethylene diamine tetra-acetic acid) were measured using immunochemiluminescence and radioimmunoassay on a commercial basis by Quest Diagnostics, Inc.
Statistical Analysis
We completed separate analyses of patients with hypertension and normotensive healthy volunteers. For each analysis, we categorized participants according to quartile of renin activity and compared clinical characteristics using the χ2 test or 1-way ANOVA for categorical and continuous variables, respectively. We examined forearm blood flow responses to methacholine and sodium nitroprusside by quartile of renin activity, quartile of aldosterone to renin ratio, or hypertension status (present or absent) using 2-way repeated measures ANOVA. We completed a similar analysis using the renin cutpoint of 1 ng/mL per hour, which is commonly used clinically to define renin status.21,28 Post hoc comparisons were made using least significant difference testing. The relation between verapamil responses and renin status was examined using the renin activity cutpoint of 1 ng/mL per hour rather than quartile, because of smaller sample size. Among the measured clinical characteristics, only serum creatinine varied significantly by quartile of renin status in hypertensive patients and, for this reason, we also completed an analysis of the relation between forearm blood responses and quartile of renin status with serum creatinine level included as a covariate in the model. Statistical analyses were performed using SPSS for Windows version 12.0 (SPSS Inc). Data are mean±SD, except in the Figures (mean±SE), and P<0.05 was considered significant.
Results
Baseline Clinical Characteristics
We enrolled 43 patients with hypertension and 87 normotensive controls. The clinical characteristics of these 2 groups of participants according to quartile of plasma renin activity are presented in Table 1 and Table 2, respectively. There was no significant difference in the duration of hypertension in the 4 quartiles. No patient had clinical evidence of target organ damage. Among hypertensive patients (Table 1), we observed a wide range of plasma renin activities (0.1 to 4.6 ng/mL per hour); however, there were no significant relations between quartile of renin activity and age, gender, body mass index, blood pressure, or lipids, except that HDL tended to decrease with increasing renin activity. Patients in the lowest quartile of renin activity had lower serum creatinine levels than the other 3 quartiles. Interestingly, serum aldosterone level did not differ according to quartile of renin activity but, as expected,4,21 there was a graded reduction in the aldosterone/renin ratio with increasing renin activity.
TABLE 1.
Hypertensives by Quartile of Renin Activity
| Quartiles of Plasma Renin Activity (range: ng/mL per hour) | |||||
|---|---|---|---|---|---|
| Parameter | 1 (0.1–0.3) | 2 (0.4–0.8) | 3 (0.9–1.8) | 4 (1.9–4.6) | P |
| No. | 10 | 11 | 11 | 11 | |
| Age (y) | 54±14 | 52±9 | 51±13 | 48±12 | 0.63 |
| BMI (kg/m2) | 29±5 | 30±7 | 30±5 | 29±5 | 0.92 |
| Female, n (%) | 4 (40) | 5 (45) | 4 (36) | 3 (27) | 0.84 |
| Black, n (%) | 5 (50) | 6 (55) | 3 (27) | 3 (27) | 0.41 |
| Systolic BP (mm Hg) | 152±24 | 158±15 | 145±8 | 143±21 | 0.23 |
| Diastolic BP (mm Hg) | 91±16 | 98±15 | 95±11 | 91±11 | 0.57 |
| Total cholesterol (mg/dL) | 191±48 | 216±41 | 189±26 | 197±36 | 0.36 |
| LDL (mg/dL) | 115±39 | 138±44 | 110±31 | 125±28 | 0.30 |
| HDL (mg/dL) | 52±12 | 49±15 | 47±11 | 38±7 | 0.03 |
| Triglycerides (mg/dL) | 117±61 | 145±114 | 162±106 | 174±85 | 0.55 |
| Glucose (mg/dL) | 101±29 | 107±24 | 99±11 | 107±13 | 0.72 |
| Creatinine (mg/dL) | 0.66±0.17 | 0.85±0.18 | 0.96±0.20 | 0.82±0.21 | 0.008 |
| Renin (ng/mL per hour) | 0.20±0.05 | 0.49±0.12 | 1.25±0.31 | 2.63±0.78 | <0.001 |
| Aldosterone (ng/dL) | 9.9±8.5 | 7.8±3.4 | 8.8±4.5 | 10.9±6.3 | 0.64 |
| Aldosterone/renin | 51.7±42.4 | 16.6±7.4 | 7.1±3.3 | 4.3±2.6 | <0.001 |
Data are mean±SD. P value refers to overall ANOVA.
BMI indicates body mass index; BP, blood pressure.
TABLE 2.
Normotensives by Quartile of Renin Activity
| Quartiles of Plasma Renin Activity (range: ng/mL per hour) | |||||
|---|---|---|---|---|---|
| Parameter | 1 (0.2–0.6) | 2 (0.6–1.0) | 3 (1.1–1.8) | 4 (2.0–12.5) | P |
| No. | 22 | 22 | 22 | 21 | |
| Age (y) | 39±13 | 39±14 | 34±13 | 39±13 | 0.59 |
| BMI (kg/m2) | 27±5 | 27±5 | 25±4 | 26±5 | 0.53 |
| Female, n (%) | 9 (41) | 8 (36) | 10 (45) | 7 (33) | 0.86 |
| Black, n (%) | 15 (68) | 11 (50) | 8 (36) | 9 (43) | 0.18 |
| Systolic BP (mm Hg) | 129±15 | 122±12 | 118±11 | 119±11 | 0.02 |
| Diastolic BP (mm Hg) | 79±10 | 76±10 | 74±9 | 70±10 | 0.04 |
| Total cholesterol (mg/dL) | 165±34 | 170±31 | 178±35 | 184±32 | 0.24 |
| LDL (mg/dL) | 96±28 | 105±27 | 104±30 | 116±30 | 0.16 |
| HDL (mg/dL) | 50±17 | 47±12 | 54±17 | 50±14 | 0.52 |
| Triglycerides (mg/dL) | 96±61 | 91±51 | 106±81 | 92±47 | 0.85 |
| Glucose (mg/dL) | 101±17 | 93±15 | 101±28 | 98±16 | 0.47 |
| Creatinine (mg/dL) | 0.83±0.20 | 0.77±0.22 | 0.80±0.21 | 0.75±0.14 | 0.55 |
| Renin (ng/mL per hour) | 0.39±0.13 | 0.78±0.15 | 1.35±0.25 | 3.75±2.52 | <0.001 |
| Aldosterone (ng/dL) | 7.8±6.4 | 7.4±2.6 | 6.6±2.3 | 12.3±6.5 | 0.001 |
| Aldosterone/renin | 23.9±27.6 | 9.7±3.6 | 5.0±2.0 | 4.1±2.9 | <0.001 |
Data are mean±SD. P value refers to overall ANOVA.
Among normotensive participants (Table 2), we also observed a wide range of plasma renin activities (0.2 to 12.5 ng/mL per hour). Clinical characteristics were similar among the quartiles of renin activity. However, systolic and diastolic blood pressures were inversely related to renin activity. In this group of normotensive participants, serum aldosterone levels were highest in the highest quartile of plasma renin activity, but there was not a graded increase across quartiles. As expected, we observed a graded decrease in aldosterone to renin ratio with increasing quartile of renin activity.
Comparison of Vascular Function in Hypertensive and Normotensive Participants
Methacholine, sodium nitroprusside, and verapamil produced dose-dependent increases in forearm blood flow in both groups. As has been previously reported,20,25,29 the response to peak methacholine was lower in hypertensive patients compared with normotensives (13.0±6.3 versus 16.0±7.2 mL/min per dL tissue, respectively; P=0.01). In contrast, the response to peak dose of sodium nitroprusside was similar in hypertensive and normotensive subjects (11.1±5.1 and 13.2±5.6 mL/min per dL, respectively; P=0.10). The response to peak dose of verapamil was similar in the 2 groups (13.2±8.7 and 14.4±6.7 mL/min per dL tissue, respectively; P=0.62).
Because the hypertensive group was older than the controls, we selected an age-comparable group of hypertensive (n=41, aged 50±11years) and normotensive (n=42, aged 48±9 years) participants. Despite being comparable in age, hypertensive patients had higher body mass index and serum triglycerides, and tended to have higher total cholesterol and low-density lipoprotein levels. They also had lower high-density lipoprotein levels; findings consistent with the hypertension-associated metabolic syndrome.30 As for the group as a whole, patients with hypertension had impaired endothelium-dependent responses to methacholine compared with age-matched normotensive controls (13.4±6.2 versus 15.9±6.5 mL/min per dL for the peak response; P=0.013). However, their responses to the endothelium-independent vasodilator sodium nitroprusside were preserved (11.4±5.0 versus 12.9±5.7 mL/min per dL for the peak response; P=0.54).
Renin Status and Vascular Function
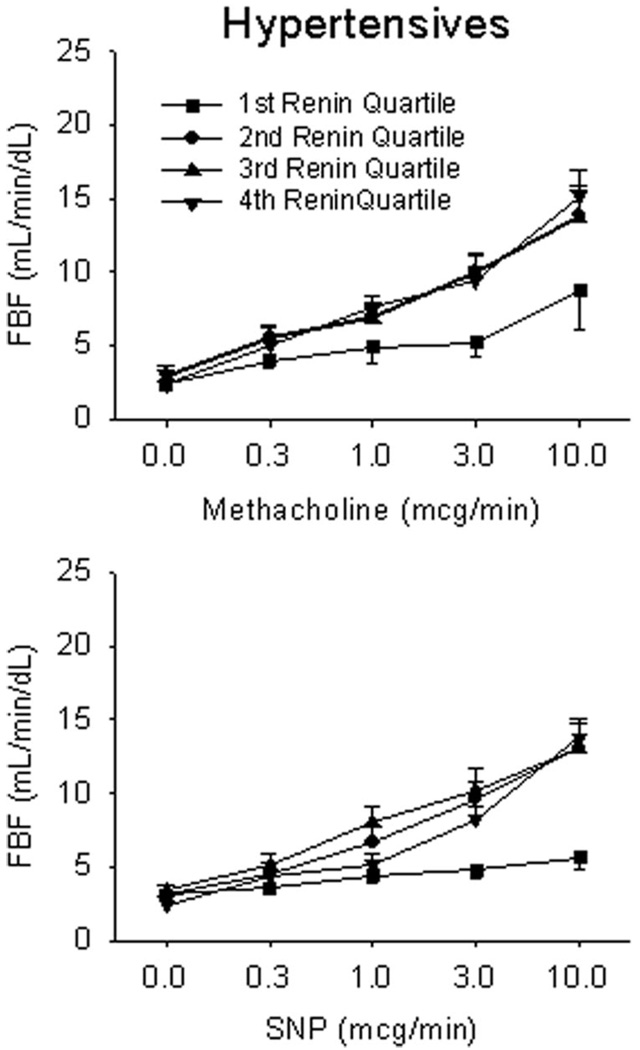
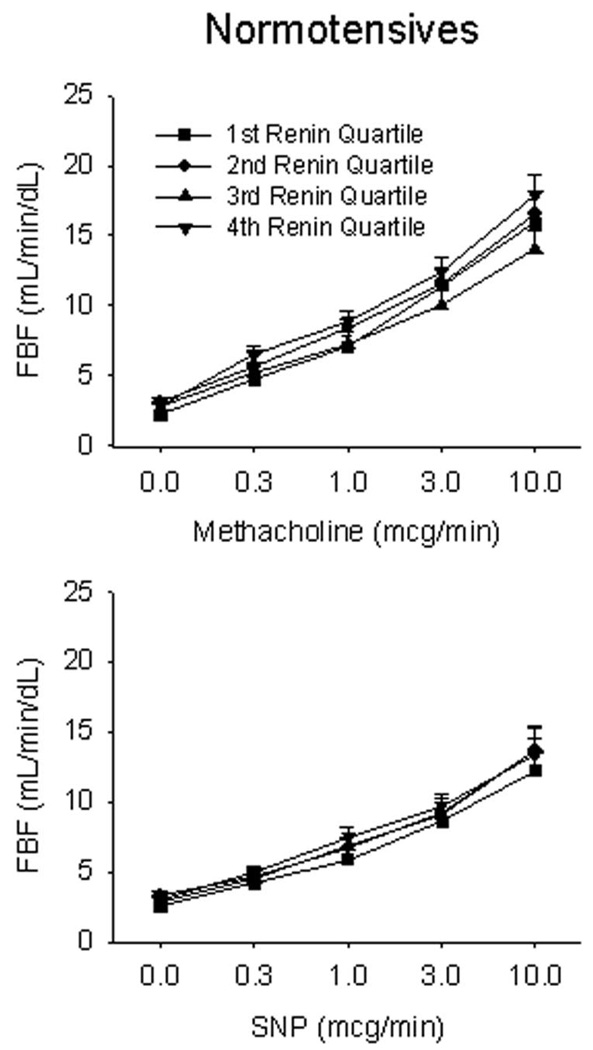
Figure 1 displays the forearm blood flow responses to methacholine and nitroprusside in patients with hypertension. As shown, those in the lowest quartile of renin activity had markedly impaired endothelium-dependent responses to peak methacholine compared with patients in the other three quartiles (8.7±7.3 versus 13.8±5.1, 13.7±6.5, and 15.1±5.3 mL/min per dL, respectively; P<0.001). These low-renin hypertensive patients also had markedly impaired responses to the peak dose of the nitric oxide donor sodium nitroprusside (5.6±2.1 versus 13.1±4.0, 13.1±5.4, and 13.8±2.9 mL/min per dL, respectively; P<0.001). By contrast, normotensive, healthy controls had similar responses in all 4 quartiles of renin activity to methacholine (P=0.34) and sodium nitroprusside (P=0.34) (Figure 2). When patients with hypertension were dichotomized by plasma renin activity above and below 1 ng/mL per hour (a clinical cutoff point for low-renin hypertension),21,28 patients with lower renin activity (n=25) also had impaired vasodilator responses to methacholine (11.0±6.4 versus 15.7±5.2 mL/min per dL for the peak response; P=0.002) and sodium nitroprusside (9.1±4.7 versus 14.1±4.3 mL/min per dL for the peak response; P=0.015).
Figure 1.
Vascular function by quartile of plasma renin activity in hypertensives. Endothelium-dependent and endothelium-independent function was assessed in response to methacholine and sodium nitroprusside (SNP), respectively. Hypertensive patients in the lowest quartile of plasma renin activity had markedly impaired responses to both nitric oxide-mediated vasodilators, P<0.001.
Figure 2.
Vascular function by quartile of plasma renin activity in normotensive controls. Endothelium-dependent and endothelium-independent function was assessed in response to methacholine and SNP, respectively. Normotensive participants had preserved responses to both nitric oxide-mediated vasodilators, irrespective of plasma renin activity.
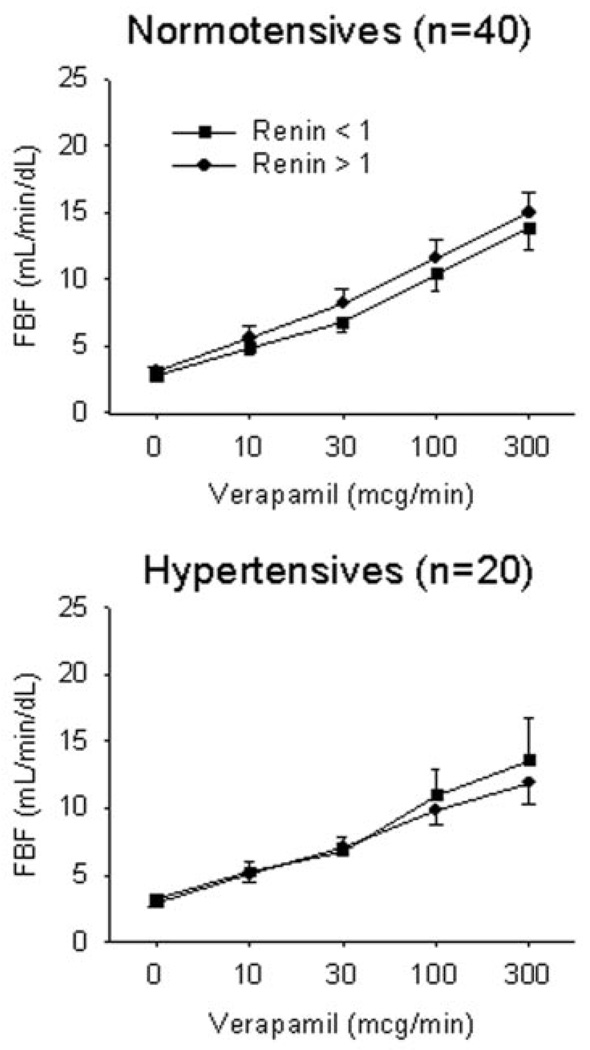
To investigate the relation between renin status and non-nitric oxide-dependent vasodilation, we examined the forearm blood flow response to verapamil in 20 patients with hypertension and 40 normotensive controls. Because of the smaller sample size, we again dichotomized the groups using a cutpoint of 1 ng/mL per hour. Among hypertensives, the peak responses to verapamil were 13.6±10.3 and 11.9±4.9 mL/min per dL for the low (n=10) and high (n=10) plasma renin activity groups, respectively (P=0.93) (Figure 3). Among normotensives, the peak responses to verapamil were 13.9±6.9 and 15.0±6.8 mL/min per dL for the low (n=19) and high (n=21) plasma renin activity groups, respectively (P=0.93) (Figure 3).
Figure 3.
Vasodilator responses to verapamil by plasma renin activity dichotomized above and below 1 ng/mL per hour. Endothelium-independent responses were assessed to the non-nitric oxide-mediated vasodilator verapamil in 20 patients with hypertension and 40 normotensive controls. Verapamil responses were preserved in both cohorts irrespective of plasma renin activity.
Renal dysfunction is associated with endothelial dysfunction. 31 Therefore, we investigated whether variation in renal function confounded our results. As shown in Table 1, we observed that serum creatinine was significantly lower in the lowest quartile of renin activity compared with the other 3 quartiles. Quartile of renin activity remained a significant predictor of the dilator response to peak methacholine dose (P=0.04) and peak sodium nitroprusside dose (P<0.001) after adjusting for serum creatinine level. Because serum creatine was actually lower in the lowest quartile of renin activity, it is unlikely that variation in renal function accounts for the impaired endothelium-dependent or endothelium-independent dilation in the lowest quartile of renin activity.
Aldosterone to Renin Ratio and Vascular Function
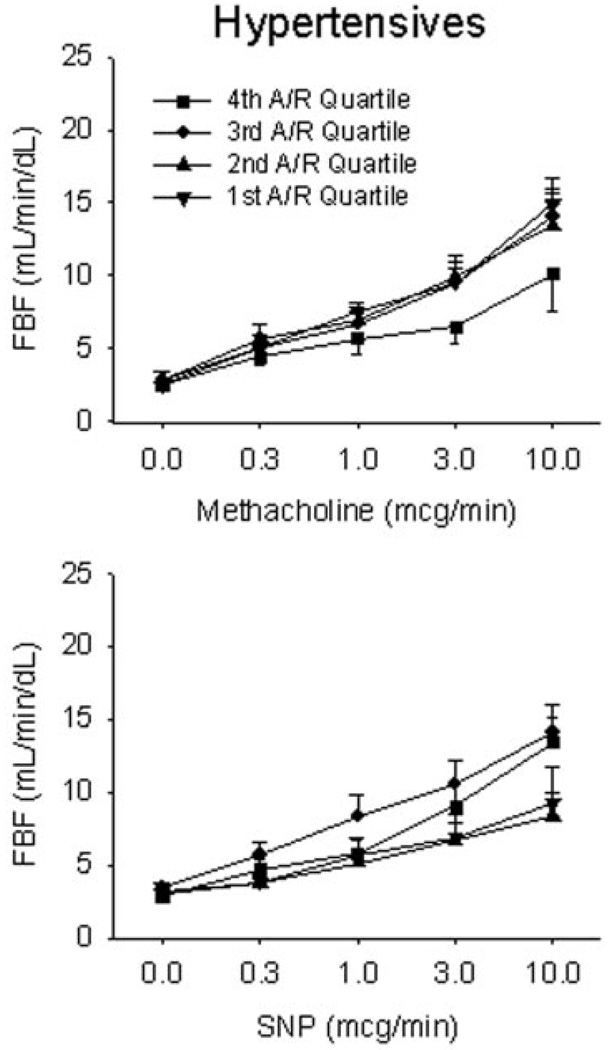
We observed no relation between quartile of serum aldosterone level and vasodilator response to methacholine, sodium nitroprusside, or verapamil (data not shown). However, a high aldosterone to renin ratio reflects “inappropriate” elevation of aldosterone level for a given level of renin activity. This variable has been used as a screen for adrenal adenomas and hyperplasia and may be moderately elevated in low-renin hypertension (30 to 100 ng/dL per ng/mL per hour).4 We therefore investigated the relation between quartile of aldosterone to renin ratio and vascular function in hypertensive and normotensive participants. In the hypertensive group, patients with the highest quartile of aldosterone to renin ratio (19.7 to 155 ng/dL per ng/mL per hour) had lower vasodilator responses to peak methacholine (9.5±7.3 mL/min per dL) compared with the first, second, and third quartiles (15.0±5.9, 14.1±5.9, and 12.8±6.3 mL/min per dL, respectively, P<0.001) (Figure 4). However, there was no significant relation between quartile of aldosterone to renin ratio and the vasodilator response to sodium nitroprusside (Figure 4).
Figure 4.
Vascular function by quartile of aldosterone/renin ratio in hypertensives. Endothelium-dependent and endothelium-independent function was assessed in response to methacholine and SNP, respectively. Hypertensive patients in the highest aldosterone-renin ratio quartile (4th aldosterone-renin quartile) had impaired endothelial function but preserved responses to nitroprusside, P<0.001.
Discussion
In this study, hypertensive patients in the lowest quartile of plasma renin activity had markedly impaired forearm blood flow responses to methacholine and sodium nitroprusside compared with patients in the other three quartiles. This relation between plasma renin activity and vascular function was not observed in normotensive participants. Furthermore, there was no relation between renin status and the vasodilator responses to verapamil in hypertensive or normotensive participants. Because methacholine is a stimulus for production of endothelium-derived nitric oxide and sodium nitroprusside is a nitric oxide donor, these findings are consistent with the possibility that patients with low-renin hypertension have a selective defect in nitric oxide-dependent vasodilation. The preserved vasodilator responses to the calcium channel blocker verapamil argue against a generalized impairment of vasodilator function in hypertensive patients with low renin status. Finally, we observed that hypertensive patients in the highest quartile of aldosterone to renin ratio had the most impaired vasodilator function, raising the possibility that relative aldosterone excess in low-renin hypertension might lead to a reduction in the biological activity of nitric oxide.
Based on animal and human studies,17,20 we had hypothesized that hypertensive patients with the highest rather than the lowest plasma renin activity would display the most severe degree of vasodilator dysfunction. However, the present findings are not entirely inconsistent with previous work. For example, patients with salt-sensitive hypertension tend to have low renin activity, and Bragulat et al observed impaired vasodilator responses to acetylcholine and preserved vasodilator responses to sodium nitroprusside in patients with salt-sensitive hypertension.32 In addition, Omland et al did not detect endothelial dysfunction in volunteers in whom plasma renin activity was increased from 1.0±0.5 to 5.0±2.5 ng/mL per hour by 5 days of a low-salt diet.33 In the present study, renin activity in the highest quartile was 1.9 to 4.6 ng/mL per hour, and it remains possible that patients with more marked elevations of plasma renin activity might display impaired vasodilator function. Although the relation between high renin status and vascular dysfunction in humans remain speculative, the present study points to a marked impairment of nitric oxide-dependent dilation with patients with the lowest renin activity.
Both experimental and clinical studies support that possibility that relative aldosterone excess might account for our findings. In rats, mineralocorticoid treatment (aldosterone or deoxycorticosterone acetate) is associated with low renin activity and endothelial dysfunction.18,19 Consistent with those observations, patients with hyperaldosteronism also display endothelial dysfunction. For example Nishizaka et al observed impaired flow-mediated dilation of the brachial artery in patients with hyperaldosteronism and a mean aldosterone to renin ratio (44.7±29.5) that is comparable to the ratio in the patients in our highest quartile (55.1±39.4).21 Taddei et al also observed impaired endothelial function in forearm microvessels of patients with primary hyperaldosteronism.20 Additionally, treatment with an aldosterone antagonist has been shown to reverse endothelial dysfunction in patients with hypertension and congestive heart failure.21,34 Finally, acute intra-arterial infusion of aldosterone into the forearm of healthy volunteers impaired the dilator response to acetylcholine, but not to sodium nitroprusside, further supporting a link between elevated aldosterone levels and endothelial dysfunction.22
The present study did not examine the mechanisms that might account for vascular dysfunction in patients with low-renin hypertension or a relative increase in aldosterone activity. However, extensive experimental studies have documented increased vascular production of superoxide anion in models of low-renin hypertension.19,35–37 Furthermore, administration of superoxide dismutase corrects vascular dysfunction under these conditions.19 The stimulus for increased production of superoxide in aldosterone-induced hypertension is not clear. However, aldosterone-induced hypertension is associated with activation of NADPH oxidase and endothelial dysfunction in experimental models, and these effects are reversed by spironolactone.18 In addition, recent studies suggest that aldosterone may increase endothelin-1 production, which may also stimulate superoxide production by NADPH oxidase in the arterial wall.35,38 Aldosterone and endothelin-1 may stimulate local arterial inflammation39 that may be associated with production of reactive oxygen species and/or consumption of nitric oxide. Arterial remodeling and fibrosis in this setting may perpetuate vascular dysfunction and hypertension.8 Thus, a state of relative aldosterone excess in low-renin hypertension has the potential to impair nitric oxide-dependent vasodilation by increasing oxidative stress and by promoting local inflammation and remodeling.
Our study has a number of limitations. For example, assessment of plasma renin activity and aldosterone levels were performed after a relatively short period in a supine position and without adjustment for salt intake. However, all participants were consuming typical American diets and may be assumed to be salt replete. Unmeasured variation because of posture or salt intake would have tended to obscure differences between groups and are unlikely to explain the marked impairment in vascular function observed in patients with the lowest renin activity. We were not able to formally exclude primary hyperaldosteronism in our subjects. The study is further limited because verapamil-induced vasodilation was only examined in a subset of patients. However, there was no apparent trend for impaired vasodilator responses in patients with lower renin activity. Strengths of our study include a relatively large sample size for an invasive study of endothelial function, the use of multiple doses of specific agonists for endothelium- and nitric oxide-dependent vasodilation, and the marked impairment in vasodilator function in the patients with low renin. Thus, we have demonstrated impaired nitric oxide-mediated vasodilation in patients with low-renin hypertension and in those with inappropriately raised aldosterone levels. Additional studies will be required to define the mechanisms accounting for these observations.
Perspectives
The present study may have a number of important clinical implications. Low-renin hypertension is associated with worse end organ damage and a worse prognosis compared with other hypertensive patients.3,10–12 Inappropriately elevated aldosterone levels in this setting is a common finding and has piqued interest in normokalemic primary hyperaldosteronism, 28 which has recently been hypothesized to represent an “end stage” of neurohormonal activation in hypertension. 7 This contention is consistent with the observations that salt-sensitivity, low-renin status, increased adrenal sensitivity to angiotensin II, increased aldosterone/renin ratios, and endothelial dysfunction are all seen with advancing age.7,40 Endothelial dysfunction and the loss of the biological activity of endothelium-derived nitric oxide may contribute to the pathogenesis and clinical expression of cardiovascular disease and to adverse arterial and left ventricular remodeling. 41,42 Furthermore, endothelial dysfunction and its failure to improve with antihypertensive treatment identifies hypertensive patients at high risk for cardiovascular events.15,16 Thus, our finding that nitric oxide-dependent dilation is impaired in hypertensive patients with low renin activity and high aldosterone to renin ratio may provide insight into why such patients have increased risk. Targeting treatments more effectively may improve blood pressure control, endothelial function, and prognosis. For example, spironolactone or eplerenone appear to be particularly efficacious in hypertension associated with increased aldosterone/renin ratios43,44 and could have particularly favorable effects on the vascular wall.18 Thus, our findings are consistent with the growing recognition that low-renin hypertension and aldosterone excess represent an end stage of neurohormonal activation in essential hypertension and may provide insight into why such patients have a worse prognosis. In addition, the findings may provide further rationale for targeting antihypertensive therapy to neurohormonal status.
Supplementary Material
Acknowledgments
S.J.D. is supported by a career development award (182830) and a project grant (225107) from the National Health and Medical Research Council of Australia. J.A.V. is supported by a Specialized Center of Research Grant (HL55993), the Boston Medical Center General Clinical Research Center (M01RR00533), and National Institutes of Health grant (HL60886).
Footnotes
Reprints: Information about reprints can be found online at http://www.lww.com/reprints
References
- 1.Brunner HR, Laragh JH, Baer L, Newton MA, Goodwin FT, Krakoff LR, Bard RH, Buhler FR. Essential hypertension: renin and aldosterone, heart attack and stroke. N Engl J Med. 1972;286:441–449. doi: 10.1056/NEJM197203022860901. [DOI] [PubMed] [Google Scholar]
- 2.Weinberger MH, Miller JZ, Luft FC, Grim CE, Fineberg NS. Definitions and characteristics of sodium sensitivity and blood pressure resistance. Hypertension. 1986;8:II127–II134. doi: 10.1161/01.hyp.8.6_pt_2.ii127. [DOI] [PubMed] [Google Scholar]
- 3.Campese VM. Salt sensitivity in hypertension. Renal and cardiovascular implications. Hypertension. 1994;23:531–550. doi: 10.1161/01.hyp.23.4.531. [DOI] [PubMed] [Google Scholar]
- 4.Warnock DG. Low-renin and nonmodulating essential hypertension. Hypertension. 1999;34:395–397. doi: 10.1161/01.hyp.34.3.395. [DOI] [PubMed] [Google Scholar]
- 5.Conn JW, Cohen EL, Rovner DR, Nesbit RM. Normokalemic primary aldosteronism. A detectable cause of curable “essential” hypertension. J Am Med Assoc. 1965;193:200–206. doi: 10.1001/jama.1965.03090030022005. [DOI] [PubMed] [Google Scholar]
- 6.Gordon RD, Stowasser M, Tunny TJ, Klemm SA, Rutherford JC. High incidence of primary aldosteronism in 199 patients referred with hypertension. Clin Exp Pharmacol Physiol. 1994;21:315–318. doi: 10.1111/j.1440-1681.1994.tb02519.x. [DOI] [PubMed] [Google Scholar]
- 7.Lim PO, Struthers AD, MacDonald TM. The neurohormonal natural history of essential hypertension: towards primary or tertiary aldosteronism? J Hypertens. 2002;20:11–15. doi: 10.1097/00004872-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Fritsch Neves M, Schiffrin EL. Aldosterone: a risk factor for vascular disease. Curr Hypertens Rep. 2003;5:59–65. doi: 10.1007/s11906-003-0012-2. [DOI] [PubMed] [Google Scholar]
- 9.Alderman MH, Madhavan S, Ooi WL. Association of the renin-sodium profile with the risk of myocardial infarction in patients with hypertension. N Engl J Med. 1991;324:1098–1104. doi: 10.1056/NEJM199104183241605. [DOI] [PubMed] [Google Scholar]
- 10.Morimoto A, Uzu T, Fujii T, Nishimura M, Kuroda S, Nakamura S, Inenaga T, Kimura G. Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet. 1997;350:1734–1737. doi: 10.1016/S0140-6736(97)05189-1. [DOI] [PubMed] [Google Scholar]
- 11.Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension. 2001;37:429–432. doi: 10.1161/01.hyp.37.2.429. [DOI] [PubMed] [Google Scholar]
- 12.Meade TW, Imeson JD, Gordon D, Peart WS. The epidemiology of plasma renin. Clin Sci (Lond) 1983;64:273–280. doi: 10.1042/cs0640273. [DOI] [PubMed] [Google Scholar]
- 13.Münzel T, Keaney JF., Jr Are ACE-inhibitors a “magic bullet” against oxidative stress? Circulation. 2001;104:1571–1574. doi: 10.1161/hc3801.095585. [DOI] [PubMed] [Google Scholar]
- 14.Schiffrin EL. Vascular and cardiac benefits of angiotensin receptor blockers. Am J Med. 2002;113:409–418. doi: 10.1016/s0002-9343(02)01241-x. [DOI] [PubMed] [Google Scholar]
- 15.Perticone F, Ceravolo R, Pujia A, Ventura G, Iacopino S, Scozzafava A, Ferraro A, Chello M, Mastroroberto P, Verdecchia P, Schillaci G. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation. 2001;104:191–196. doi: 10.1161/01.cir.104.2.191. [DOI] [PubMed] [Google Scholar]
- 16.Modena MG, Bonetti L, Coppi F, Bursi F, Rossi R. Prognostic role of reversible endothelial dysfunction in hypertensive postmenopausal women. J Am Coll Cardiol. 2002;40:505–510. doi: 10.1016/s0735-1097(02)01976-9. [DOI] [PubMed] [Google Scholar]
- 17.Rajagopalan S, Kurz S, Münzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. J Clin Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Virdis A, Neves MF, Amiri F, Viel E, Touyz RM, Schiffrin EL. Spironolactone improves angiotensin-induced vascular changes and oxidative stress. Hypertension. 2002;40:504–510. doi: 10.1161/01.hyp.0000034738.79310.06. [DOI] [PubMed] [Google Scholar]
- 19.Somers MJ, Mavromatis K, Galis ZS, Harrison DG. Vascular superoxide production and vasomotor function in hypertension induced by deoxycorticosterone acetate-salt. Circulation. 2000;101:1722–1728. doi: 10.1161/01.cir.101.14.1722. [DOI] [PubMed] [Google Scholar]
- 20.Taddei S, Virdis A, Mattei P, Salvetti A. Vasodilation to acetylcholine in primary and secondary forms of human hypertension. Hypertension. 1993;21:929–933. doi: 10.1161/01.hyp.21.6.929. [DOI] [PubMed] [Google Scholar]
- 21.Nishizaka MK, Zaman MA, Green SA, Renfroe KY, Calhoun DA. Impaired endothelium-dependent flow-mediated vasodilation in hypertensive subjects with hyperaldosteronism. Circulation. 2004;109:2857–2861. doi: 10.1161/01.CIR.0000129307.26791.8E. [DOI] [PubMed] [Google Scholar]
- 22.Farquharson CA, Struthers AD. Aldosterone induces acute endothelial dysfunction in vivo in humans: evidence for an aldosterone-induced vasculopathy. Clin Sci (Lond) 2002;103:425–431. doi: 10.1042/cs1030425. [DOI] [PubMed] [Google Scholar]
- 23.Taddei S, Virdis A, Ghiadoni L, Sudano I, Salvetti A. Effects of antihypertensive drugs on endothelial dysfunction: clinical implications. Drugs. 2002;62:265–284. doi: 10.2165/00003495-200262020-00003. [DOI] [PubMed] [Google Scholar]
- 24.Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel Spaceiiiqq) J Am Med Assoc. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 25.Sherman DL, Keaney JF, Jr, Biegelsen ES, Duffy SJ, Coffman JD, Vita JA. Pharmacological concentrations of ascorbic acid are required for the beneficial effects on endothelial vasomotor function in hypertension. Hypertension. 2000;35:936–941. doi: 10.1161/01.hyp.35.4.936. [DOI] [PubMed] [Google Scholar]
- 26.Duffy SJ, Biegelsen ES, Holbrook M, Russell JD, Gokce N, Keaney JF, Jr, Vita JA. Iron chelation improves endothelial function in patients with coronary artery disease. Circulation. 2001;103:2799–2804. doi: 10.1161/01.cir.103.23.2799. [DOI] [PubMed] [Google Scholar]
- 27.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 28.Stowasser M. New perspectives on the role of aldosterone excess in cardiovascular disease. Clin Exp Pharmacol Physiol. 2001;28:783–791. doi: 10.1046/j.1440-1681.2001.03523.x. [DOI] [PubMed] [Google Scholar]
- 29.Panza JA, Quyyumi AA, Brush JE, Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med. 1990;323:22–27. doi: 10.1056/NEJM199007053230105. [DOI] [PubMed] [Google Scholar]
- 30.Reaven GM, Oithell H, Landsberg L. Hypertension and associated metabolic abnormalities-The role of insulin resistance and the sympatho-adrenal system. N Engl J Med. 1996;334:374–381. doi: 10.1056/NEJM199602083340607. [DOI] [PubMed] [Google Scholar]
- 31.Perticone F, Maio R, Tripepi G, Zoccali C. Endothelial dysfunction and mild renal insufficiency in essential hypertension. Circulation. 2004;110:821–825. doi: 10.1161/01.CIR.0000138745.21879.27. [DOI] [PubMed] [Google Scholar]
- 32.Bragulat E, de la SA, Antonio MT, Coca A. Endothelial dysfunction in salt-sensitive essential hypertension. Hypertension. 2001;37:444–448. doi: 10.1161/01.hyp.37.2.444. [DOI] [PubMed] [Google Scholar]
- 33.Omland T, Johnson W, Gordon MB, Creager MA. Endothelial function during stimulation of renin-angiotensin system by low-sodium diet in humans. Am J Physiol Heart Circ Physiol. 2001;280:H2248–H2254. doi: 10.1152/ajpheart.2001.280.5.H2248. [DOI] [PubMed] [Google Scholar]
- 34.Farquharson CA, Struthers AD. Spironolactone increases nitric oxide bioactivity, improves endothelial vasodilator dysfunction, and suppresses vascular angiotensin I/angiotensin II conversion in patients with chronic heart failure. Circulation. 2000;101:594–597. doi: 10.1161/01.cir.101.6.594. [DOI] [PubMed] [Google Scholar]
- 35.Li L, Fink GD, Watts SW, Northcott CA, Galligan JJ, Pagano PJ, Chen AF. Endothelin-1 increases vascular superoxide via endothelin(A)-NADPH oxidase pathway in low-renin hypertension. Circulation. 2003;107:1053–1058. doi: 10.1161/01.cir.0000051459.74466.46. [DOI] [PubMed] [Google Scholar]
- 36.Zhou MS, Adam AG, Jaimes EA, Raij L. In salt-sensitive hypertension, increased superoxide production is linked to functional upregulation of angiotensin II. Hypertension. 2003;42:945–951. doi: 10.1161/01.HYP.0000094220.06020.C8. [DOI] [PubMed] [Google Scholar]
- 37.Beswick RA, Dorrance AM, Leite R, Webb RC. NADH/NADPH oxidase and enhanced superoxide production in the mineralocorticoid hypertensive rat. Hypertension. 2001;38:1107–1111. doi: 10.1161/hy1101.093423. [DOI] [PubMed] [Google Scholar]
- 38.Ergul A. Hypertension in black patients: an emerging role of the endothelin system in salt-sensitive hypertension. Hypertension. 2000;36:62–67. doi: 10.1161/01.hyp.36.1.62. [DOI] [PubMed] [Google Scholar]
- 39.Schiffrin EL, Touyz RM. From bedside to bench to bedside: role of renin-angiotensin-aldosterone system in remodeling of resistance arteries in hypertension. Am J Physiol Heart Circ Physiol. 2004;287:H435–H446. doi: 10.1152/ajpheart.00262.2004. [DOI] [PubMed] [Google Scholar]
- 40.Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation. 1995;91:1981–1987. doi: 10.1161/01.cir.91.7.1981. [DOI] [PubMed] [Google Scholar]
- 41.Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 42.Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER, Bedja D, Gabrielson KL, Wang Y, Kass DA. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med. 2005 doi: 10.1038/nm1175. [DOI] [PubMed] [Google Scholar]
- 43.Lim PO, Jung RT, MacDonald TM. Raised aldosterone to renin ratio predicts antihypertensive efficacy of spironolactone: a prospective cohort follow-up study. Br J Clin Pharmacol. 1999;48:756–760. doi: 10.1046/j.1365-2125.1999.00070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krum H, Nolly H, Workman D, He W, Roniker B, Krause S, Fakouhi K. Efficacy of eplerenone added to renin-angiotensin blockade in hypertensive patients. Hypertension. 2002;40:117–123. doi: 10.1161/01.hyp.0000025146.19104.fe. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.