Abstract
Background
The Survivor’s Health and Reaction (SHARE) study examined health-related quality of life (HRQL) in breast cancer patients who had participated in CALGB 8541 from 1985–1991.
Methods
A total of 245 survivors (78% of eligible patients) who were 9.4–16.5 years post-diagnosis (mean 12.5 years) completed HRQL surveys relating to 5 domains. Analyses examined HRQL domains by the three chemotherapy doses administered in the original treatment trial: low-dose=Cyclophosphamide/Doxorubicin/Flouracil (CAF) at 300/30/300×2 mg/m2 over 4 cycles, standard-dose=CAF at 400/40/400×2 mg/m2over 4 cycles, and high-dose=CAF at 600/60/600 mg/m2over 4 cycles.
Results
In univariate analyses, a statistically significant difference was found in SF-36 physical role functioning by treatment group, with the standard treatment arm showing lower mean scores (mean=65.05) compared to the low-dose (mean=74.66) or high-dose (mean=84.94) arms (p=<0.0001). Multivariate analysis, however, revealed that treatment arm was no longer statistically significant, while the following factors were associated with decreased physical role functioning: age ≥60 (OR=3.55, p=0.006), increased comorbidity interference total score (OR=1.64, p=0.005), lower vitality (OR=1.05, p=0.0002) and increased menopausal symptoms (OR=1.04, p=0.02).
Conclusions
At 9.4–16.5 years post-diagnosis, differences in physical role functioning among breast cancer survivors who received three chemotherapy doses were explained by clinical and demographic variables, such as age, fatigue, menopausal symptoms and comorbidities. Prospective studies are needed to further assess the role of these factors in explaining HRQL and physical role functioning among long-term survivors.
Keywords: breast cancer, survivorship, quality of life, chemotherapy
Introduction
Advances in early detection and treatment have led to increased numbers of breast cancer survivors, totaling approximately 2.4 million in 2007.1, 2 This increase in survivors raises concerns about the long-term effects of cancer treatment on health-related quality of life (HRQL).
Several studies have reported good overall HRQL among long-term survivors, but have identified issues such as sexual concerns, psychosocial problems and physical symptoms, including pain and lymphedema.3–7 Adverse effects of systemic adjuvant therapy (chemotherapy) on global HRQL, physical functioning, bodily pain and sexual functioning have been shown to worsen 5–10 years after diagnosis among breast cancer patients.3,6
Ahles et al. revealed gaps in knowledge regarding the effects of cancer treatment in long-term breast cancer survivors, as few studies exist.8 Ganz et al. found few differences in the effects of adjuvant treatment on HRQL and emotional functioning in breast cancer survivors 3 years post-treatment, but discovered significant differences in global HRQL, general health, physical and social functioning after 6 years of follow-up.9 Bottomley et al. reported declines in HRQL 3 months after treatment, but found that these effects had largely diminished 3 years post-treatment.10 Ahles et al. also emphasized the importance of assessing the impact of chemotherapy on HRQL to make cancer survivors aware of potentially negative outcomes of cancer treatment, and for the development of interventions to cope with negative side effects of treatment.8 Thus, past studies have shown inconsistent results regarding the effects of adjuvant chemotherapy on long-term HRQL. This issue has important implications for breast cancer survivors, and, therefore, needs further study.
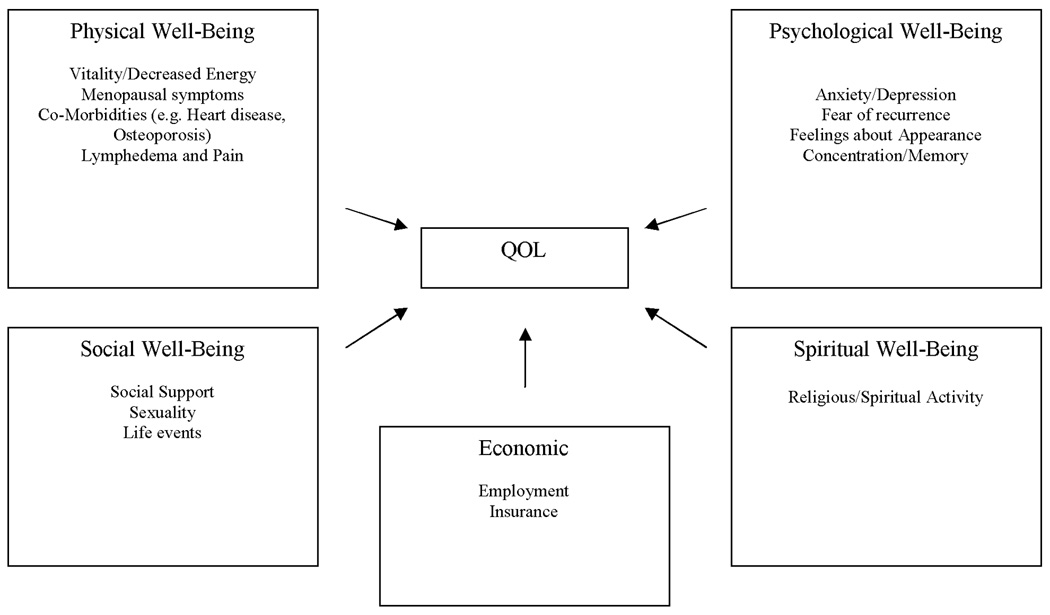
The theoretical framework for this study was derived from the Quality of Life model adapted for cancer survivors by Dow, Ferrell and colleagues (Figure 1).5, 11 The model identifies four major areas of HRQL in cancer patients: physical, psychological, social and spiritual well-being. It has been tested in several studies with specific issues identified within each domain.12, 13 In the present study, the social well-being area was subdivided into social and economic well-being. Thus, 5 domains plus medical and demographic variables were assessed.
Figure 1. Quality of Life Model Adapted for Breast Cancer Survivors*.
The primary goal of this paper was to assess whether adjuvant chemotherapy dose of a commonly used breast cancer treatment regimen (Cyclophosphamide/Doxorubicin/Flouracil (CAF)) was associated with differences in 5 HRQL domains (physical, psychological, social, spiritual, and economic) among long-term breast cancer survivors (9–16 years post-diagnosis) who had participated in Cancer and Leukemia Group B (CALGB) treatment trial 8541. Due to conflicting evidence from past studies and because these women had survived 9–16 years, we hypothesized that there would be differences among the treatment arms, which could result in higher or lower HRQL. The secondary goal was to identify factors that currently exhibited significant differences by treatment arm, such as co-morbidities, treatment variables and demographic variables, including age, education and socioeconomic status (SES). Identification of these factors may be useful for interventions to improve HRQL among long-term survivors.
Methods
1. Setting
The current study (CALGB 79804) examined HRQL in breast cancer patients who had participated in CALGB 8541 from 1985–1991. The goal of CALGB 8541 was to determine whether a dose-dependent relationship existed between disease-free survival, dose, and dose-intensity for stage II breast cancer patients randomly assigned to receive one of the following (CAF) adjuvant therapy regimens for surgically resected breast cancer: low-dose (CAF 300/30/300×2 mg/m2 over 4 cycles), standard-dose (CAF 400/40/400×2 mg/m2 over 6 cycles); and high-dose (CAF 600/60/600 mg/m2 over 4 cycles).14,15
The results of the trial showed that after 3.4 median follow-up years, women treated with high- or standard-dose/intensity had significantly longer disease-free survival (p<0.001) and overall survival (p=0.004) than low-dose/intensity patients in log rank comparisons (3 degrees-of-freedom).14, 15 The difference in survival between the two groups treated with standard- or high-dose/intensity was not significant.
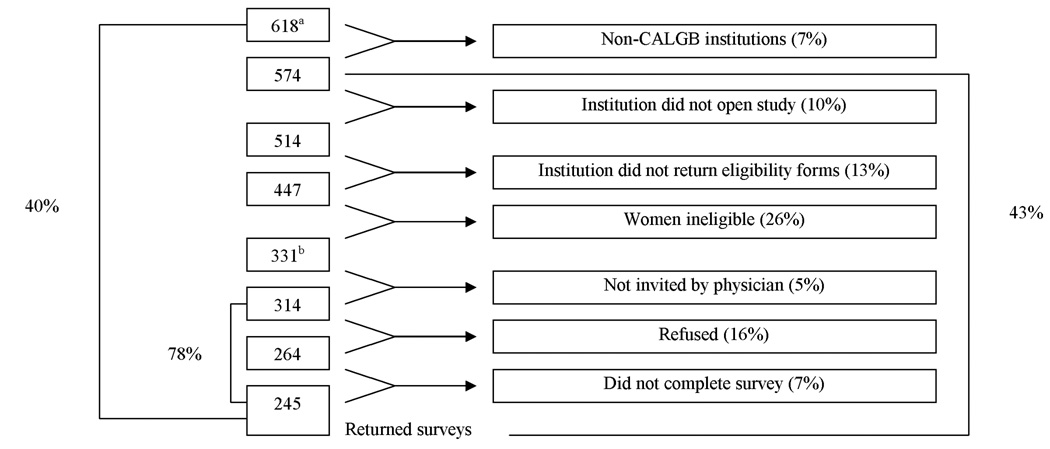
Of 1,572 women randomized to CALGB 8541, approximately 618 were alive and cancer-free when the present study began in 1999. Since accrual for CALGB 8541 occurred from 1985–1991, participants in this study were 9.4 to 16.5 years post-diagnosis (mean 12.5 years). The study was approved by the Institutional Review Boards of each participating institution.
2. Procedures
Details of the study methods have been provided elsewhere.16 In brief, clinical research associates (CRAs) at CALGB treating institutions were notified of patient eligibility by the CALGB Statistical Center. CRAs confirmed patient disease status (alive and disease-free) and informed the treating physician of the study. CRAs then either contacted the patient about the study or an introductory letter was sent from the principal investigator (EP) noting the physician’s permission to contact the patient. A consent form and questionnaire were sent, which were completed, signed and returned by women who wished to participate. Non-respondents were contacted by phone to complete the survey (N=8), if necessary. Upon questionnaire completion, patients were registered with the CALGB Statistical Center.
In total, 618 women from 42 CALGB institutions were identified by the CALGB Statistical Center for potential participation in this study. Eligibility criteria consisted of: participation in CALGB 8541, breast cancer-free for 12 months, free of other cancers in the past 5 years (except basal/squamous skin or in-situ cervical cancers), physician approval to participate, and could complete English-language questionnaire. Figure 2 shows exclusions that were made. Reasons for ineligibility included death or disease recurrence and lack of physician approval. Thus, 314 women were eligible to participate and 245 (78%) returned the surveys. Participants did not differ significantly from non-participants (CALGB 8541 survivors who were not eligible to participate in the follow-up study) by age, treatment arm, number of nodes or age/year of entry in CALGB 8541 (data not shown). More white survivors, however, versus non-white survivors chose to participate (93% versus 81%, p<0.0001).
Figure 2. Accrual/Eligibility to SHARE.
aThere were 618 potentially eligible patients
bThere were 331 Eligible patients
3. Measures
The following questionnaires were used to assess HRQL domains by treatment arm in this study, with higher scores representing increased levels of the outcome being assessed.
Quality of Life
a.) SF-36
Overall HRQL was assessed using eight dimensions: physical functioning, role limitations, bodily pain, general health perceptions, vitality (fatigue), social functioning, emotional well-being, and perceived changes in health status.17, 18 Subscales were scored from 0–100, with higher scores indicating better HRQL. Lower vitality scores indicated greater fatigue.
Psychological Well-being
b.) CES-D (20 items)
Depression was measured with a total score that was dichotomized using a score ≥16 to reflect the possible presence of depression.19
c.) Breast Cancer Anxiety and Screening Behavior Scale(BCAS)
This modified 21-item scale assessed the emotional and cognitive aspects of breast cancer,20 showing high validity with breast cancer worries and generalized anxiety scales.20–23 All subscales were examined, including intrusive and avoidant thoughts, and total cognitive distress.
Social Well-being
d.) MOS Social Support Survey
This validated 20-item survey measured 4 areas of perceived social support: emotional/informational, tangible, affectionate, and positive social interaction.24, 25 All subscale scores and total score were examined.
e.) Life Events Scale
Stress was assessed with this 11-item survey, which has been used in past studies.26 Both the number and frequency of events were examined.
Spiritual Well-being
f.) System of Beliefs Inventory
Religious/spiritual beliefs were measured with this 15-item scale.27 Two subscales, spiritual beliefs practices and community social support, and total score, were examined.
Economic Well-being
g.) Employment and Insurance Difficulties Attributed to Cancer
The overall impact of breast cancer diagnosis on employment and insurance was assessed with 2 questions from this survey,28 which were developed for prior CALGB survivor studies.7,29
Physical Well-being
h.) Your Health-Short Form
This modified version of the validated OARS Co-Morbidity list30 assessed the following illnesses/comorbidities (no/yes): heart disease, osteoporosis, high blood pressure, diabetes, circulation problems in arms/legs, stroke, depression, chronic liver/kidney disease, stomach/intestinal disorders, osteoporosis, arthritis, glaucoma, and emphysema. An “Interference Score” (with daily activities) was assessed using a 3-point scale (‘not at all’=1, ‘somewhat’=2, ‘a great deal’=3).30
i.) Pain and Lymphedema Questionnaire
This 12-item module documented the occurrence and duration of treatment-related swelling and pain in the arms/hands.31
j.) Menopause and Reproductive Health Questionnaire
This 47-item survey asked participants if they had experienced particular physical symptoms (yes/no) and if the severity was mild, moderate or severe. A total score assessed both frequency and severity.
Medical Information/Demographics
k.) The medical file in the CALGB 8541 database provided demographics and the following information: date of study entry, treatment arm, menopausal status (at diagnosis), number of positive nodes at diagnosis, tumor size, histological grade, estrogen receptor (ER) status, and performance status (Karnofsky Performance Scale32). Current demographics, such as age, education, income and insurance status were obtained from a supplemental demographic survey for the current study.
5. Analysis
Statistical analyses were performed by statisticians at the CALGB Statistical Center (JEH and KD). Descriptive statistics were used to characterize HRQL domains and clinical characteristics of survivors by treatment arm. Power analyses for this study revealed 90% power to detect a clinically significant difference in physical role functioning, where one group differed from the other groups by more than 0.5 standard deviations, assuming alpha = 0.05 (2-sided) and sample sizes of 74, 93, and 78 for the low, standard and intensive-dose groups, respectively.33
Due to skewed distributions and a ceiling effect for many of the survey scores, the non-parametric Kruskal-Wallis test was used to compare survey scores by treatment arm. All other categorical data were analyzed using Fisher’s exact test. The Jonckhere-Terpstra34 test was used to test for a dose-response relationship between treatment dose and survey score. Statistical tests were calculated using exact methods from Monte-Carlo simulations and were two-sided, using alpha=0.05. The null hypothesis in these comparisons was that there were no differences in survey scores between treatment arms. The alternative hypothesis was that at least one arm had a significantly higher or lower score compared to the other arms.
The relationship between treatment arm and physical role functioning was analyzed using logistic regression. Physical role functioning, which was the outcome for this analysis since it was the only HRQL variable that showed a statistically significant association with treatment arm (p=0.001), was dichotomized (100 versus <100) due to the discrete nature of the distribution. Higher SES was defined as having private health insurance, household income ≥$20,000, and being a high school graduate; otherwise, women were classified as having lower SES.35 Other variables in the logistic regression analysis were: age at interview (≥60 years versus <60), number of co-morbidities (0 versus ≥1), type of surgery (lumpectomy versus mastectomy), ER status (negative versus positive), hormone therapy (no versus yes), radiation therapy (no versus yes), high blood pressure interfering with daily life (no/not applicable versus yes), diabetes interfering with daily life (no/not applicable versus yes), vitality score, menopausal symptom score, comorbidity interference total score, and time since diagnosis, which was stratified by its median (12.3 years). Variables significant at the 25% level based on Wald’s chi-square test were included in the multivariate model using stepwise selection methods. The selection criterion was based on the Score chi-square statistic, using alpha=0.05. The Wald chi-square statistic was used to determine if a factor should remain in the model (alpha=0.05).
Results
Table 1 shows the distribution of demographic and clinical characteristics of participants by treatment arm. Seventy-four patients (30%) had received low-dose chemotherapy, 93 patients (38%) had received the standard dose, and 78 patients (32%) had received the highest dose. Age and race did not differ significantly between groups. Differences were evident regarding education, whereby patients who received standard therapy were somewhat less-educated (p=0.04). No significant differences were found in the distribution of co-morbidities interfering with daily life by treatment group (results not shown).
Table 1.
Demographic and Clinical Characteristics of Study Participants by Treatment Trial Arm*
| Variable | Low Dose | Standard Dose | Intensive Dose | All Patients | |
|---|---|---|---|---|---|
| n = 74 | n = 93 | n = 78 | n = 245 | ||
| Total # of Patients | n(%) | n(%) | n(%) | n(%) | P-Value |
| Age (years) | 0.31 | ||||
| 30 – 39 | 1(1) | 0(0) | 2(3) | 3(1) | |
| 40 – 49 | 4(5) | 10(11) | 6(8) | 20(8) | |
| 50 – 59 | 28(38) | 26(28) | 24(31) | 78(32) | |
| 60 – 69 | 30(41) | 32(34) | 30(38) | 92(38) | |
| 70 + | 11(15) | 25(27) | 16(21) | 52(21) | |
| Mean(SD) | 61.3(8.8) | 63.4(10.5) | 61.1(9.8) | 62.0(9.8) | |
| Race | 0.34 | ||||
| White | 70(95) | 84(90) | 75(96) | 229(94) | |
| Other | 4(5) | 9(10) | 3(4) | 16(6) | |
| Education | 0.04 | ||||
| 0 – 12 years | 29(41) | 54(61) | 39(54) | 122(55) | |
| 13 + years | 42(59) | 34(39) | 33(46) | 109(47) | |
| Income | 0.12 | ||||
| Under $10,000 | 4(6) | 3(4) | 5(8) | 12(6) | |
| $10,000 – $19,999 | 9(14) | 13(16) | 8(13) | 30(14) | |
| $20,000 – $29,999 | 4(6) | 19(24) | 7(11) | 30(14) | |
| $30,000 – $44,999 | 10(16) | 18(23) | 10(16) | 38(18) | |
| $45,000 – $59,999 | 9(14) | 6(8) | 11(17) | 26(13) | |
| $60,000 – $79,000 | 8(13) | 9(11) | 10(16) | 27(13) | |
| $80,000 + | 20(31) | 12(15) | 13(20) | 45(22) | |
| Socioeconomic Status | 0.51 | ||||
| Lower | 32(47) | 47(54) | 31(46) | 110(49) | |
| Higher | 36(53) | 40(46) | 37(54) | 113(51) | |
| Type of Treatment | 0.12 | ||||
| Mastectomy | 62(84) | 75(81) | 55(71) | 192(78) | |
| Breast Conservation | 12(16) | 18(19) | 23(29) | 53(22) | |
| Received Radiation Therapy | 0.13 | ||||
| Yes | 13(18) | 19(20) | 24(31) | 56(23) | |
| No |
60(82) | 74(80) | 53(69) | 187(77) | |
| Received Tamoxifen | 0.89 | ||||
| Yes | 32(44) | 42(45) | 32(42) | 106(44) | |
| No |
41(56) | 51(55) | 45(58) | 137(56) | |
| Received Any Hormone | 0.89 | ||||
| Yes | 34(46) | 44(47) | 34(44) | 112(46) | |
| No | 40(54) | 49(53) | 44(56) | 133(54) | |
| Estrogen Receptor Status | 0.55 | ||||
| Negative | 26(35) | 25(27) | 25(32) | 76(31) | |
| Positive | 46(62) | 65(70) | 48(62) | 159(65) | |
| Borderline | 2(3) | 3(3) | 1(1) | 6(3) | |
| Time Since Diagnosis (years) | |||||
| Mean(SD) | 12.4(1.9) | 12.5(1.7) | 12.5(1.9) | 12.5(1.8) | 0.89 |
Note: Frequencies within education, income, socioeconomic status, Tamoxifen use, estrogen receptor status, and prior radiation therapy columns do not sum to column total due to missing data
Table 2 shows the distribution of SF-36 subscales by treatment arm. The mean scores were highest for the intensive treatment arm and lowest for the standard arm for all but the mental health subscale, with the only statistically significant difference seen in physical role functioning (p<0.0001). Trends toward significance were seen for general health perceptions (p=0.06), emotional role functioning (p=0.07), and vitality (p=0.09), with lower scores found in the standard treatment arm. There were no significant dose-response relationships by treatment arm (results not shown).
Table 2.
SF-36 Subscales and Treatment Arm for Patients Enrolled in CALGB 79804
| Treatment Arm | |||||||
|---|---|---|---|---|---|---|---|
| Low (n=74) | Standard (n=93) | Intensive (n=78) | P-value* | ||||
| MOS SF-36 Subscale: | Mean(SD) | Median | Mean(SD) | Median | Mean(SD) | Median | |
| Physical Function | 75.14(30.64) | 85.00 | 73.01(30.74) | 85.00 | 82.31(22.91) | 90.00 | 0.17 |
| Role Functioning-Physical | 74.66(39.42) | 100.00 | 65.05(41.56) | 75.00 | 84.94(30.24) | 100.00 | <0.0001 |
| Role Functioning-Emotional | 86.94(31.12) | 100.00 | 77.78(36.23) | 100.00 | 87.89(26.99) | 100.00 | 0.07 |
| Social Function | 87.50(23.77) | 100.00 | 82.07(27.06) | 100.00 | 87.18(21.13) | 100.00 | 0.36 |
| Bodily Pain | 73.69(24.14) | 84.00 | 71.37(24.34) | 74.00 | 77.27(21.22) | 84.00 | 0.32 |
| Mental Health | 77.70(16.56) | 80.00 | 76.58(17.35) | 84.00 | 76.08(15.86) | 80.00 | 0.74 |
| Vitality | 61.40(22.65) | 65.00 | 56.81(22.06) | 55.00 | 64.62(17.33) | 70.00 | 0.09 |
| General Health Perceptions | 74.50(21.49) | 75.00 | 68.92(20.58) | 72.00 | 75.35(17.99) | 77.00 | 0.06 |
Kruskal-Wallis test.
Table 3 displays the HRQL domain items examined by treatment arm. Overall, approximately 80% of women indicated having one or more comorbidities and 20% of women had possible depression (CES-D≥16). They also showed high levels of MOS social support (mean scores>78). In examining the social support systems of belief subscale, a trend towards significantly higher social support was seen in the standard treatment arm (p=0.07). There were no significant dose-response relationships by treatment arm (results not shown).
Table 3.
Quality of Life Domains by Treatment Arm for Patients Enrolled in CALGB 79804
| Treatment Arm | ||||
|---|---|---|---|---|
| Low (n=74) | Standard (n=93) | Intensive (n=78) | ||
| Domain/Items | Mean(SD)a | Mean(SD)a | Mean(SD)a | P-Value |
| Physical Well-Being | ||||
| Menopausal Symptoms Score | 22.53(18.16) | 24.53(17.99) | 21.21(18.45) | 0.31b |
| Vitality (Fatigue) | 61.40(22.65) | 56.81(22.06) | 64.62(17.33) | 0.09b |
| Lymphedema & Pain | ||||
| Swelling Since Surgery | 0.55c | |||
| Yes | 26(35%) | 28(30%) | 21(27%) | |
| No | 48(64%) | 65(70%) | 57(73%) | |
| Arm/Hand Pain | 0.76c | |||
| Yes | 18(24%) | 18(19%) | 18(23%) | |
| No | 55(74%) | 73(78%) | 60(77%) | |
| Co-morbidities: | ||||
| None | 15(20%) | 23(25%) | 18(23%) | 0.81c |
| >1 | 59(80%) | 70(75%) | 60(77%) | |
| Interference Total Score | 1.35(2.27) | 1.80(2.34) | 1.10(1.79) | 0.13b |
| Psychological Well-Being | ||||
| CES-D Total Score | 9.52(9.96) | 9.78(8.01) | 9.30(7.79) | 0.64b |
| Score≥16 | 15(20%) | 21(23%) | 15(19%) | 0.88c |
| Score<16 | 59(80%) | 72(77%) | 63(81%) | |
| Breast Cancer Anxiety & Screening Subscales: | ||||
| Total Cognitive Distress | 10.88(6.44) | 11.31(6.21) | 10.57(6.33) | 0.86b |
| Intrusive Thoughts | 4.16(4.26) | 4.15(3.70) | 4.00(4.18) | 0.81b |
| Avoidant Thoughts | 2.90(3.08) | 3.39(3.13) | 2.69(2.68) | 0.34b |
| Appearance Assessment | 26.09(8.12) | 26.16(7.35) | 24.97(7.19) | 0.54b |
| Difficulty Concentrating | ||||
| Yes | 10(14%) | 17(18%) | 17(22%) | 0.45c |
| No | 61(82%) | 75(81%) | 60(77%) | |
| Social Well-Being | ||||
| MOS Social Support Score MOS Subscales: | 83.04(17.96) | 77.53(23.27) | 79.16(20.43) | 0.34b |
| Positive Interaction | 85.70(17.96) | 79.71(24.96) | 82.59(22.32) | 0.44b |
| Affection | 85.59(22.89) | 81.54(26.20) | 83.33(20.81) | 0.29b |
| Emotional Support | 83.24(17.79) | 78.14(23.51) | 78.41(22.61) | 0.41b |
| Tangible Support | 80.07(23.03) | 73.12(27.04) | 76.12(23.25) | 0.20b |
| Life Events Score | 5.79(5.79) | 7.26(6.38) | 5.85(4.91) | 0.14b |
| Spiritual Well-Being | ||||
| Systems of Belief Score | 2.31(0.78) | 2.39(0.78) | 2.29(0.73) | 0.12b |
| Religious-Spiritual | 2.49(0.79) | 2.54(0.72) | 2.45(0.72) | 0.30b |
| Social Support | 1.96(0.94) | 2.12(1.00) | 1.95(0.86) | 0.07b |
| Economic Well-Being | ||||
| Perceived Negative Socio-Economic Impact | 0.01(0.12) | 0.08(0.27) | 0.06(0.25) | 0.20b |
| Perceived Positive Socio-Economic Impact | 0.03(0.16) | 0.02(0.03) | 0.03(0.16) | 1.00b |
Frequencies and percentages are displayed for categorical variables
Kruskal-Wallis test
Fisher’s exact test
The association of demographic and medical characteristics with decreased physical role functioning were analyzed using logistic regression. The univariate results (Table 4) showed a highly significant relationship between treatment arm and decreased physical role functioning (OR=3.17, p=0.0009). Other factors associated with reduced physical role functioning were: lower SES (OR=2.76, p=0.0004); age≥60 (OR=1.85, p=0.03); ≥1 co-morbidities (OR=4.76, p=0.0001); lower vitality (OR=1.06, p<0.0001); increasing menopausal symptom score (OR=1.07, p<0.0001), higher comorbidity interference total score (OR=2.81, p<0.0001), and positive ER status (OR=2.17, P=0.009).
Table 4.
Univariate Logistic Regression Analysis: Association of Demographic and Medical Characteristics With Decreased Physical Role Functioning
| Variable | Odds Ratio | 95% CI | P-Value |
|---|---|---|---|
| Treatment Arm: | |||
| Low vs. High | 1.62 | 0.80,3.28 | 0.75 |
| Standard vs. High | 3.17 | 1.64,6.12 | 0.0009 |
| Age at Time of Interview: ≥60 years old vs. <60 |
1.85 | 1.08,3.23 | 0.03 |
| Number of Co-Morbidities: * None vs. ≥1 |
4.76 | 2.13,10.0 | 0.0001 |
| Type of Surgery: Lumpectomy vs. Mastectomy |
0.97 | 0.52,1.79 | 0.91 |
| Estrogen Receptor Status: Negative vs. Positive |
2.17 | 1.22,4.00 | 0.009 |
| Hormone Therapy: No vs. Yes |
0.64 | 0.38,1.09 | 0.10 |
| Radiation Therapy: No vs. Yes |
0.91 | 0.49,1.68 | 0.77 |
| High Blood Pressure Interference: No or N/A vs. Yes |
0.11 | 0.04,0.33 | 0.0001 |
| Diabetes Interference: No or N/A vs. Yes |
0.12 | 0.03,0.43 | 0.001 |
| Vitality Score (Fatigue) | 1.06 | 1.05,1.09 | <0.0001 |
| Menopausal Symptom Total Score | 1.07 | 1.05,1.09 | <0.0001 |
| SES: Lower vs Higher | 2.76 | 1.57,4.85 | 0.0004 |
| Time Since Diagnosis <12.3 vs. ≥12.3 |
1.14 | 0.68,1.92 | 0.62 |
| Comorbidity Interference Total Score | 2.81 | 2.10,3.76 | <0.0001 |
Comorbidities included (interfered with daily life): other cancers/leukemia, arthritis/rheumatism/other connective tissue disorder, glaucoma, emphysema/chronic bronchitis, high blood pressure, heart disease, circulation problems in legs/arms, diabetes, stomach/intestinal disorders, osteoporosis, chronic liver/kidney disease, stroke, and depression
Variables significant at the 25% level in the univariate analysis were included in the stepwise selection process to derive the multivariate model (Table 5). Vitality, menopause symptoms, age and comorbidity interference total score all had significant associations with physical role functioning, but treatment arm and SES were no longer significant. Older patients (OR=3.55, p=0.006), patients with higher comorbidity total interference scores (OR=1.64, p=0.005), patients with higher menopausal symptom scores (OR=1.04, p=0.02), and women with reduced vitality (fatigue) (OR=1.05, p=0.0002) were more likely to report decreased physical role functioning.
Table 5.
Multivariate Logistic Regression Analysis: Association of Demographic and Medical Characteristics With Decreased Physical Role Functioning
| Variable | Odds Ratio | 95% CI | P-Value |
|---|---|---|---|
| Age at time of interview: ≥60 years old vs. <60 |
3.55 | 1.45,8.62 | 0.006 |
| Vitality Score (Fatigue) | 1.05 | 1.02,1.08 | 0.0002 |
| Menopausal Symptom Total Score | 1.04 | 1.01,1.07 | 0.02 |
| Comorbidity Interference Total Score | 1.64 | 1.17,2.31 | 0.005 |
In further exploring these associations, two specific co-morbidities reported as interfering with daily life, high blood pressure and diabetes, were highly related to decreased physical role functioning in univariate analyses (p<0.001). Patients with a high school education or less tended to have more health problems compared to those having at least some college education (not shown). Significant differences by education level were seen in circulation trouble (p=0.01), depression level (p=0.01), and diabetes (p=0.06) interfering with daily life.
Discussion
The primary purpose of this study was to compare differences by treatment group in HRQL domains (physical, psychological, social, spiritual and economic) among long-term breast cancer survivors. When HRQL measures were examined by treatment arm, physical role functioning emerged as the only statistically significant outcome. Trends toward significance were seen in variables such as general health perceptions, vitality, emotional role functioning and social functioning. These results are similar to Ganz et al., who found that 6-year survivors treated with adjuvant therapy reported lower levels of physical role functioning, as well as general health, bodily pain, physical functioning, and social functioning.6,36 Ahles et al. also found significantly lower scores in social and physical domains among 10-year survivors who received chemotherapy versus local therapy.37 Others have reported poorer physical role functioning among patients 5 years post-chemotherapy compared to patients without cancer38 and to patients who received adjuvant chemotherapy.3
Unlike previous studies, where a dose-response relationship was found, the present study showed the lowest SF-36 subscale means in the standard group, followed by the low-dose and high-dose groups. Survivors in the standard arm also showed significantly higher levels of systems of belief social support in the spiritual domain. These findings may be due to survivors in the standard group having less education or perhaps being less healthy than the other groups. Future studies should examine similar dose-intensities and their effect on HRQL.
In further examining the relationship of physical role functioning by treatment arm, logistic regression analyses revealed that treatment arm was no longer significantly associated with HRQL after adjusting for factors such as age, fatigue, menopausal symptoms and comorbidities interfering with daily life. A recent prospective study found lower levels of physical role functioning among women one year after receiving high-dose chemotherapy, which remained stable over 4 years but had small effect sizes that were clinically irrelevant.39 In that study, when age and menopausal status were assessed as covariates, postmenopausal women exhibited lower physical role functioning scores. Other studies have also shown lower levels of HRQL in physical/role functioning domains in high-dose chemotherapy patients that returned to baseline 1 year post-treatment.40–42 The current results, however, provide information on who may be at risk for reduced physical role functioning following the receipt of any dose of this adjuvant therapy regimen.
Age was significantly related to physical role functioning in this study, and has typically been associated with lower HRQL in past studies. Older survivors have reported decreased physical role functioning,43, 44 more physical problems, depressed mood and days affected by fatigue.45,46
Comorbidities interfering with daily life, which were significantly related to physical role functioning in this study, have consistently been shown to affect HRQL. Depression, diabetes and circulation trouble were also evident in women with lower education levels. Past studies have shown that poorer HRQL among lower SES groups may be related to increased co-morbidities and reduced access to care. Patients with lower education level have reported more physical symptoms, such as tiredness, decreased sexual interest and painful muscles.39
Fatigue was significantly related to physical role functioning in this study. Fatigue is often reported as a long-term side effect of breast cancer treatment that persists years after active treatment.6,7,47,48 Co-morbidities, such as high blood pressure, which were prevalent in this study, have been shown to be related to fatigue in previous studies.48 The presence of joint and muscle pain have also been associated with fatigue.49,50,51 Patients who most frequently reported symptoms, such as pain and fatigue 5 years post-treatment, scored significantly lower on HRQL at baseline compared to other patients.39 Future studies should assess these domains at baseline, and possible interactions with other factors, as differences may be predictive of future outcomes.
Regarding menopausal symptoms, which showed a statistically significantly association with physical role functioning, Schultz et al. concluded that despite “complex interactions” between HRQL indicators and physiologic effects of treatment, menopausal symptoms may not be different for breast cancer survivors and should not be confused with quality of life/psychosocial issues.52 Others have demonstrated that HRQL differences could not be explained by menopausal symptoms alone and that more research is needed in this area.38
There were several strengths of this study. First, it focused on the HRQL domain of physical role functioning, and factors influencing this domain, which have not been explored in previous studies. Second, it examined survivors who were 9–16 years post-diagnosis, which few studies have included. Third, the women were diagnosed at relatively the same disease stage received one of 3 known chemotherapy regimens within the same clinical trial, thus reducing variability due to treatment and stage of diagnosis. Many previous studies have used heterogeneous populations in examining stage and treatment.
Limitations of this study include reliance on self-reported co-morbidities, such as lymphedema and osteoporosis. Information on temporal changes in HRQL was not assessed since HRQL was examined at only one time point. Also, a survival bias may have occurred, whereby patients with better HRQL were more likely to be long-term survivors, and thus, eligible to participate in the follow-up study. However, analyses showed few differences between CALGB 8541 survivors who did and did not participate in this study. These limitations emphasize the need for prospective long-term studies of HRQL in breast cancer survivors from treatment through survivorship.
While chemotherapy provides a great survival benefit for cancer patients, it provides potential long-term side effects that may greatly impact HRQL. The current study demonstrated that while adjuvant chemotherapy dose was initially related to lower HRQL in physical role functioning, this effect was actually explained by demographic and clinical factors, which can be used in targeting HRQL interventions for long-term survivors. The clinical significance of these factors and their role as potential areas for interventions in improving HRQL needs to be further explored in prospective studies of HRQL in long-term breast cancer survivors.
Acknowledgement
*The authors acknowledge the following individuals for their contribution to the SHARE study: Eric Winer MD, Charles Shapiro MD, Gini Fleming PhD, Marcy List PhD, and Karleen Habin RN. This study was funded by the National Institutes of Health Grants: AG16602, CA79883, and CA57707
Footnotes
IRB statement: All patients gave informed consent before participating in this study.
REFERENCES
- 1.American Cancer Society: Breast Cancer Facts and Figures. 2007–2008 [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57(2):43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 3.Casso D, Buist DS, Taplin S. Quality of life of 5–10 year breast cancer survivors diagnosed between age 40 and 49. Health Qual Life Outcomes. 2004;2:25. doi: 10.1186/1477-7525-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dorval M, Maunsell E, Deschenes L, et al. Long-term quality of life after breast cancer: comparison of 8-year survivors with population controls. J Clin Oncol. 1998;16:487–494. doi: 10.1200/JCO.1998.16.2.487. [DOI] [PubMed] [Google Scholar]
- 5.Dow KH, Ferrell BR, Leigh S, et al. An evaluation of the quality of life among long-term survivors of breast cancer. Breast Cancer Res Treat. 1996;39(3):261–273. doi: 10.1007/BF01806154. [DOI] [PubMed] [Google Scholar]
- 6.Ganz PA, Desmond KA, Leedham B, et al. Quality of life in long-term disease-free survivors of breast cancer: a follow-up study. J Natl Cancer Inst. 2002;94:39–49. doi: 10.1093/jnci/94.1.39. [DOI] [PubMed] [Google Scholar]
- 7.Kornblith AB, Herndon JE, Weiss RB, et al. Long-term adjustment of survivors of early-stage breast carcinoma 20 years after adjuvant chemotherapy. Cancer. 2003;98(4):679–689. doi: 10.1002/cncr.11531. [DOI] [PubMed] [Google Scholar]
- 8.Ahles TA, Saykin AJ, Furstenberg CT, et al. Quality of life of long-term survivors of breast cancer and lymphoma treated with standard-dose chemotherapy or local therapy. J Clin Oncol. 2005;23:4399–4405. doi: 10.1200/JCO.2005.03.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ganz PA, Rowland JH, Merowitz BE, et al. Impact of different adjuvant therapy strategies on quality of life in breast cancer survivors. Recent Results Cancer Res. 1998;16:501–514. doi: 10.1007/978-3-642-45769-2_38. [DOI] [PubMed] [Google Scholar]
- 10.Bottomley A, Therasse P, Piccart M, et al. Health-related quality of life in survivors of locally advanced breast cancer: an international randomised controlled phase III trial. Lancet Oncol. 2005;6:287–294. doi: 10.1016/S1470-2045(05)70100-5. [DOI] [PubMed] [Google Scholar]
- 11.Ferrell BR, Dow KH, Grant M. Measurement of the quality of life in cancer survivors. Qual Life Res. 1995;4:523–531. doi: 10.1007/BF00634747. [DOI] [PubMed] [Google Scholar]
- 12.Dow KH, Ferrell BR, Haberman MR, Eaton L. The meaning of quality of life in cancer survivorship. Oncol Nurs Forum. 1999;26:519–528. [PubMed] [Google Scholar]
- 13.Ersek M, Ferrell BR, Dow KH, Melancon CH. Quality of life in women with ovarian cancer. West J Nurs Res. 1997;19:334–350. doi: 10.1177/019394599701900305. [DOI] [PubMed] [Google Scholar]
- 14.Wood WC, Budman DR, Korzun AH, et al. Dose and dose intensity of adjuvant chemotherapy for stage II, node-positive breast carcinoma. N Engl J Med. 1994;330(18):1253–1259. doi: 10.1056/NEJM199405053301801. [DOI] [PubMed] [Google Scholar]
- 15.Budman D, Berry DA, Cirrincione CT, et al. Dose and dose intensity as determinants of outcome in the adjuvant treatment of breast cancer. J Natl Cancer Inst. 1998;90(16):1205–1211. doi: 10.1093/jnci/90.16.1205. [DOI] [PubMed] [Google Scholar]
- 16.Paskett ED, Herndon JE, II, Day JM, et al. Applying a Conceptual Model for Examining Health-Related Quality of Life in Long-Term Breast Cancer Survivors: CALGB Study 79804. Psycho-oncology. 2007 doi: 10.1002/pon.1329. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey Manual and Interpretation Guide. Boston, MA: New England Medical Center, The Health Institute; 1993. [Google Scholar]
- 18.Stewart AL, Ware J. Measure of Functioning and Well-Being: The Medical Outcomes Study Approach. Durham, NC: Duke University Press; 1992. [Google Scholar]
- 19.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Applied Psychol Measurement. 1977;1:385–401. [Google Scholar]
- 20.Kash KM, Jacobsen PB, Holland JC, et al. Measuring breast cancer anxiety; Proceedings of the 42nd annual meeting of the Academy of Psychosomatic Medicine; 1995. Nov, p. 13. [Google Scholar]
- 21.Lerman C, Trock B, Rimer B. Psychological Side Effects of Breast Cancer Screening. Health Psychology. 1991;10(4):259–267. doi: 10.1037//0278-6133.10.4.259. [DOI] [PubMed] [Google Scholar]
- 22.Spielberger CD, Gorsuch RL, Lushene RD. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- 23.Taylor JA. A personality scale of manifest anxiety. J Abnorm Psychol. 1953;48:285–290. doi: 10.1037/h0056264. [DOI] [PubMed] [Google Scholar]
- 24.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32(6):705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 25.Anderson D, Bilodeau B, Deshaies G, et al. French-Canadian validation of the MOS Social Support Survey. Can J Cardiol. 2005;21:867–873. [PubMed] [Google Scholar]
- 26.Wilcox S, Aragaki A, Mouton CP, et al. The effects of widowhood on physical and mental health, health behaviors, and health outcomes: The Women's Health Initiative. Health Psychol. 2003;22:513–522. doi: 10.1037/0278-6133.22.5.513. [DOI] [PubMed] [Google Scholar]
- 27.Holland JC, Kash KM, Passik S, et al. A brief spiritual beliefs inventory for use in quality of life research in life-threatening illnesses. Psycho-Oncology. 1998;7:460–469. doi: 10.1002/(SICI)1099-1611(199811/12)7:6<460::AID-PON328>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 28.Holland JC, Herndon J, Kornblith AB, et al. A sociodemographic data collection model for cooperative clinical trials. Proc ASCO. 1992;445:157. [Google Scholar]
- 29.Greenberg DB, Kornblith AB, Herndon JE, et al. Quality of life of adult leukemia survivors treated on clinical trials of the Cancer and Leukemia Group B from 1971–1988: Predictors for later psychological distress. Cancer. 1997;80:1936–1944. doi: 10.1002/(sici)1097-0142(19971115)80:10<1936::aid-cncr10>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 30.George LK, Fillenbaum G. OARS methodology: A decade of experience in geriatric assessment. J Am Geriatrics Soc. 1985;33:607–615. doi: 10.1111/j.1532-5415.1985.tb06317.x. [DOI] [PubMed] [Google Scholar]
- 31.Paskett E. Lymphedema: knowledge, treatment and impact among breast cancer survivors. Breast Journal. 1999;6(6):373–378. doi: 10.1046/j.1524-4741.2000.99072.x. [DOI] [PubMed] [Google Scholar]
- 32.Crooks V, Waller S, Smith T, Hahn TJ. The use of the Karnofsky Performance Scale in determining outcomes and risk in geriatric outpatients. J Gerontol. 1991;46:M139–M144. doi: 10.1093/geronj/46.4.m139. [DOI] [PubMed] [Google Scholar]
- 33.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. New York: Academic Press; 1988. [Google Scholar]
- 34.Jonckheere AR. A distribution-free k-sample test against ordered alternatives. Biometrika. 1954;41:133–145. [Google Scholar]
- 35.Paskett E, Tatum C, Rushing J, et al. Randomized trial of an intervention to improve mammography utilization among a triracial rural population of women. J Natl Cancer Inst. 2006;98:1226–1237. doi: 10.1093/jnci/djj333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ganz PA, Rowland JH, Desmond K, et al. Life after breast cancer: understanding women's health-related quality of life and sexual functioning. J Clin Oncol. 1998;16:501–514. doi: 10.1200/JCO.1998.16.2.501. [DOI] [PubMed] [Google Scholar]
- 37.Ahles TA, Saykin AJ, Furstenberg CT, et al. Quality of life of long-term survivors of breast cancer and lymphoma treated with standard-dose chemotherapy or local therapy. J Clin Oncol. 2005;23:4399–4405. doi: 10.1200/JCO.2005.03.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Broeckel JA, Jacobsen PB, Balducci L, et al. Quality of life after adjuvant chemotherapy for breast cancer. Breast Cancer Res Treat. 2000;62:141–150. doi: 10.1023/a:1006401914682. [DOI] [PubMed] [Google Scholar]
- 39.Buijs C, Rodenhuis S, Seynaeve CM, et al. Prospective study of long-term impact of adjuvant high-dose and conventional-dose chemotherapy on health-related quality of life. J Clin Oncol. 2007;25(34):5403–5409. doi: 10.1200/JCO.2007.11.2813. [DOI] [PubMed] [Google Scholar]
- 40.Bottomley A, Therasse P, Piccart M, et al. Health-related quality of life in survivors of locally advanced breast cancer: an international randomised controlled phase III trial. Lancet Oncol. 2005;6:287–294. doi: 10.1016/S1470-2045(05)70100-5. [DOI] [PubMed] [Google Scholar]
- 41.Connor-Spady BL, Cumming C, Nabholtz JM, et al. A longitudinal prospective study of health-related quality of life in breast cancer patients following high-dose chemotherapy with autologous blood stem cell transplantation. Bone Marrow Transplant. 2005;36:251–259. doi: 10.1038/sj.bmt.1705032. [DOI] [PubMed] [Google Scholar]
- 42.Peppercorn J, Herndon J, Kornblith AB, et al. Quality of life among patients with Stage II and III breast carcinoma randomized to receive high-dose chemotherapy with autologous bone marrow support or intermediate-dose chemotherapy: results from Cancer and Leukemia Group B 9066. Cancer. 2005;104:1580–1589. doi: 10.1002/cncr.21363. [DOI] [PubMed] [Google Scholar]
- 43.Fehlauer F, Tribius S, Mehnert A, Rades D. Health-related quality of life in long term breast cancer survivors treated with breast conserving therapy: impact of age at therapy. Breast Cancer Res Treat. 2005;92:217–222. doi: 10.1007/s10549-005-2420-2. [DOI] [PubMed] [Google Scholar]
- 44.Peuckmann V, Ekholm O, Rasmussen NK, et al. Health-related quality of life in long-term breast cancer survivors: nationwide survey in Denmark. Breast Cancer Res Treat. 2007;104:39–46. doi: 10.1007/s10549-006-9386-6. [DOI] [PubMed] [Google Scholar]
- 45.Cimprich B, Ronis DL, Martinez-Ramos G. Age at diagnosis and quality of life in breast cancer survivors. Cancer Practice. 2002;10:85–93. doi: 10.1046/j.1523-5394.2002.102006.x. [DOI] [PubMed] [Google Scholar]
- 46.Robb C, Haley WE, Balducci L, et al. Impact of breast cancer survivorship on quality of life in older women. Crit Rev Oncol Hematol. 2007;62:84–91. doi: 10.1016/j.critrevonc.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 47.Meeske K, Smith AW, Alfano CM, et al. Fatigue in breast cancer survivors two to five years post diagnosis: a HEAL Study report. Qual Life Res. 2007;16:947–960. doi: 10.1007/s11136-007-9215-3. [DOI] [PubMed] [Google Scholar]
- 48.Bower JE, Ganz PA, Desmond KA, et al. Fatigue in long-term breast carcinoma survivors: a longitudinal investigation. Cancer. 2006;106:751–758. doi: 10.1002/cncr.21671. [DOI] [PubMed] [Google Scholar]
- 49.Ganz PA. Quality of life across the continuum of breast cancer care. Breast Journal. 2000;6(5):324–330. doi: 10.1046/j.1524-4741.2000.20042.x. [DOI] [PubMed] [Google Scholar]
- 50.Nieboer P, Buijs C, Rodenhuis S, et al. Fatigue and relating factors in high-risk breast cancer patients treated with adjuvant standard or high-dose chemotherapy: a longitudinal study. J Clin Oncol. 2005;23:8296–8304. doi: 10.1200/JCO.2005.10.167. [DOI] [PubMed] [Google Scholar]
- 51.Burckhardt CS, Jones KD. Effects of chronic widespread pain on the health status and quality of life of women after breast cancer surgery. Health Qual Life Outcomes. 2005;3:30. doi: 10.1186/1477-7525-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schultz PN, Klein MJ, Beck ML, et al. Breast Cancer: relationship between menopausal symptoms, physiologic health effects of cancer treatment and physical constraints on quality of life in long-term survivors. J Clin Nursing. 2005;14:204–211. doi: 10.1111/j.1365-2702.2004.01030.x. [DOI] [PubMed] [Google Scholar]