Abstract
Pro–brain-derived neurotrophic factor (proBDNF) and mature BDNF utilize distinct receptors to mediate divergent neuronal actions. Using new tools to quantitate endogenous BDNF isoforms, we found that mouse neurons secrete both proBDNF and mature BDNF. The highest levels of proBDNF and p75 were observed perinatally and declined, but were still detectable, in adulthood. Thus, BDNF actions are developmentally regulated by secretion of proBDNF or mature BDNF and by local expression of p75 and TrkB.
BDNF critically regulates neuronal function by promoting survival, enhancing synaptic plasticity and altering spine morphology via TrkB1,2. However, BDNF is initially synthesized as a precursor, proBDNF, which is trafficked to the regulated secretory pathway by chaperone proteins, including sortilin3. Although proBDNF may be cleaved intracellulary to release mature BDNF in an activity-dependent manner4,5, it is not clear how efficient this processing is and how much proBDNF is secreted by neurons. Because recombinant proBDNF does not activate TrkB, but rather activates p75 to promote cell death and attenuate synaptic transmission6,7, this is an important question.
To reliably measure the very low levels of endogenous proBDNF, we generated a monoclonal antibody (mAb287) that was specific for the prodomain. This antibody detected a 32-kDa immunoreactive species in hippocampal lysates from Bdnf+/+, but not Bdnf −/−, littermates (Supplementary Fig. 1 and Supplementary Methods online), that corresponded in molecular mass to recombinant proBDNF; however, we did not observe a cleaved prodomain (of 14–16 kDa; Supplementary Fig. 1). BDNF prodomain immunoreactivity was present in cultured neurons from Bdnf+/+, but not Bdnf−/−, mice hippocampal granule neurons of the dentate gyrus and most prominently in their mossy fiber projections from tissue sections of Bdnf+/+, but not Bdnf−/−, mice (cultured neurons from Bdnf+/+, but not Bdnf−/−, mice (Supplementary Fig. 1). We also observed prodomain immunoreactivity in pyramidal cell bodies of CA2 and CA3 and the stratum lucidum, consistent with prior immunolocalization of BDNF in rats8. These results indicated that intact proBDNF was regionally expressed at substantial levels in the adult brain, particularly in hippocampal mossy fibers.
To facilitate quantitative detection of proBDNF and mature BDNF, we generated a knock-in mouse in which the endogenous Bdnf coding exon was replaced with the murine Bdnf sequence with a C-terminal hemagglutinin (HA) epitope tag (Bdnf-HA; Supplementary Fig. 2 online). Both homozygous (Bdnf-HA/Bdnf-HA) and heterozygous (Bdnf-HA/+) mice were viable, fertile and indistinguishable from wild-type littermates. Regional expression of BDNF by ELISA documented comparable levels of BDNF in cortex and hippocampus in Bdnf-HA/+ mice and wild-type littermates (Supplementary Fig. 2). In addition, the levels of proBDNF in the hippocampus of wild-type and Bdnf-HA/Bdnf-HA mice were comparable by western blot (Supplementary Fig. 1).
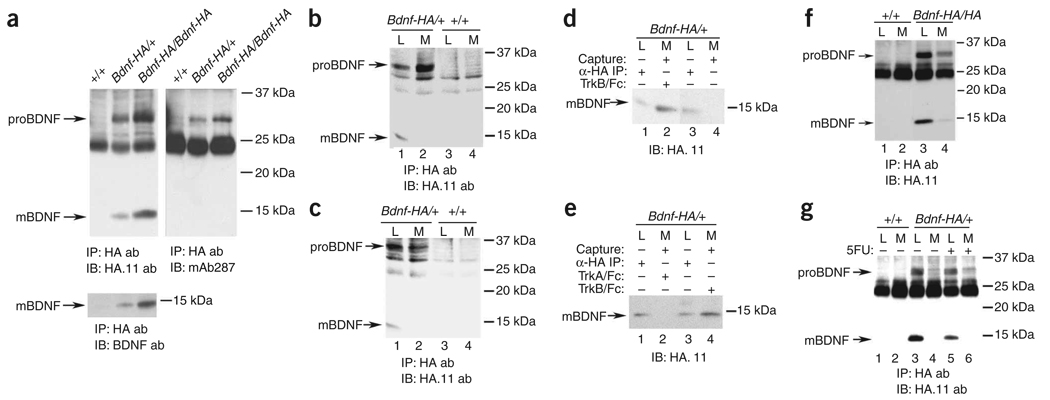
To quantitate proBDNF and mature BDNF, we immunoprecipitated hippocampal lysates from wild-type, Bdnf-HA/+ and Bdnf-HA/Bdnf-HA with antibodies to HA and probed for HA immunoreactivity. Mature BDNF-HA (~14.2 kDa = ~13.5-kDa mature BDNF + ~0.7-kDa HA) and proBDNF-HA (~32.7 kDa = ~32-kDa proBDNF + ~0.7-kDa HA) were readily detectable from Bdnf-HA/+ and Bdnf-HA/Bdnf-HA, but not wild-type hippocampal lysates (Fig. 1a); the B32.7-kDa proBDNF band was confirmed by probing with antisera to proBDNF (Fig. 1a) and the ~14.2-kDa mature BDNF was also detected with antibody to mature BDNF (Fig. 1a). Our analysis confirmed that BDNF-HA was quantitatively precipitated, with BDNF-HA isoforms in Bdnf-HA/+ being detected at approximately 50% of the level of BDNF-HA isoforms in Bdnf-HA/Bdnf-HA mice (Fig. 1a).
Figure 1.
Detection of proBDNF and mature BDNF in mouse hippocampus and secretion of proBDNF and mature BDNF by cultured hippocampal neurons. (a) Hippocampal lysates of 4-week-old mice of the indicated genotype were immunoprecipitated with antibody to HA and immunoblotted as indicated. mAb287 is the monoclonal antibody to the BDNF prodomain that we characterized in Supplementary Figure 1. (b,c) Neurons were cultured from P0 mice of the indicated genotype for 7 d in the presence (b) or absence (c) of alpha 2 anti-plasmin. Media (M) and cell lysates (L) were immmunoprecipitated (IP) with antibody to HA. proBDNF and mature BDNF were detected with antibody to HA.11. (d,e) Neuronal cultures were established as in b. We added TrkB-Fc (d) or TrkB-Fc or TrkA-Fc (e) to the media 3 d before harvest. Neurons cultured in the absence of TrkB-Fc were analyzed as controls. Lysates were immunoprecipitated; Fc proteins were captured by Protein A-Sepharose (capture). (f) Embryonic day 16.5 (E16.5) neurons were cultured as in b and stimulated with 56 mM KCl for 90 min. The media and cell lysates were processed as indicated. (g) E16.5 hippocampal neurons were cultured as described previously9, with or without fluorouracil for 2 d. At 7 d in vitro, cells were stimulated with 56 mM KCl for 90 min, and immunoprecipitation/western blot analysis was carried out as indicated. All of our animal studies were approved by the Institutional Animal Care and Use Committee of Weill Cornell Medical College.
To determine whether proBDNF was indeed secreted, we cultured hippocampal neurons from Bdnf-HA/+ or wild-type mice in conditions to reduce glia contamination, using alpha 2 anti-plasmin to prevent cleavage of secreted proBDNF. Following neuronal maturation in vitro, BDNF isoforms were collected from media and cell lysates using immunoprecipitation/western blot analysis with antibodies to HA. Both proBDNF and mature BDNF were readily detectable in hippocampal neuron lysates (Fig. 1b). Unexpectedly, only proBDNF was detectable in the media of neurons at 7 or 14 d in vitro (Fig. 1b and Supplementary Fig. 3 online, respectively). ProBDNF was also detected at reduced levels in the media of cultures lacking a plasmin inhibitor (Fig. 1c compared with Fig. 1b), suggesting that secreted proBDNF is processed extracellularly. The absence of mature BDNF in the media was surprising to us, and we considered whether secreted mature BDNF binds and is internalized by neuronal TrkB. Therefore, we added TrkB-Fc receptor bodies to the media of established cultures to capture secreted mature BDNF. On precipitation of TrkB-Fc, mature BDNF was detected in the media (Fig. 1d,e), but was not observed in media lacking TrkB-Fc (Fig. 1d) or with TrkA-Fc (Fig. 1e). In addition, we documented the release of proBDNF and mature BDNF by depolarizing neuronal cultures with 56 mM KCl for 90 min (Fig. 1f). These studies indicate that both mature and proBDNF are secreted from depolarized hippocampal neurons.
These results are substantially different from those of a recent report9, where mature BDNF was the predominant form detected in cell lysates and secreted proBDNF was not observed. In that study, differences in experimental design may have impaired detection of proBDNF, including the use of mixed cultures of neurons and glia, omission of plasmin inhibitors, and prolonged treatment with 50 µM bicuculline (24 h), which may have led to excitotoxicity. We directly compared our culture conditions with those used in the recent report9, using Bdnf-HA/+ or wild-type hippocampal neurons and brief KCl treatment (90 min) to induce secretion of BDNF isoforms. In mixed neuronal/glial cultures that lack a plasmin inhibitor, proBDNF was detectable in cell lysates and in the media (Fig. 1g), but the levels of secreted proBDNF were reduced compared with neuronal cultures depleted of glia that lack a plasmin inhibitor (Fig. 1g). These results strongly suggest that glia may enhance proBDNF processing or uptake, observations that are consistent with the high levels of proteases, including tissue plasminogen activator, synthesized by glia10. Moreover, the generation and use of an epitope-tagged BDNF mouse substantially enhanced our detection of BDNF isoforms as compared with BDNF mature domain antibodies (Supplementary Fig. 1).
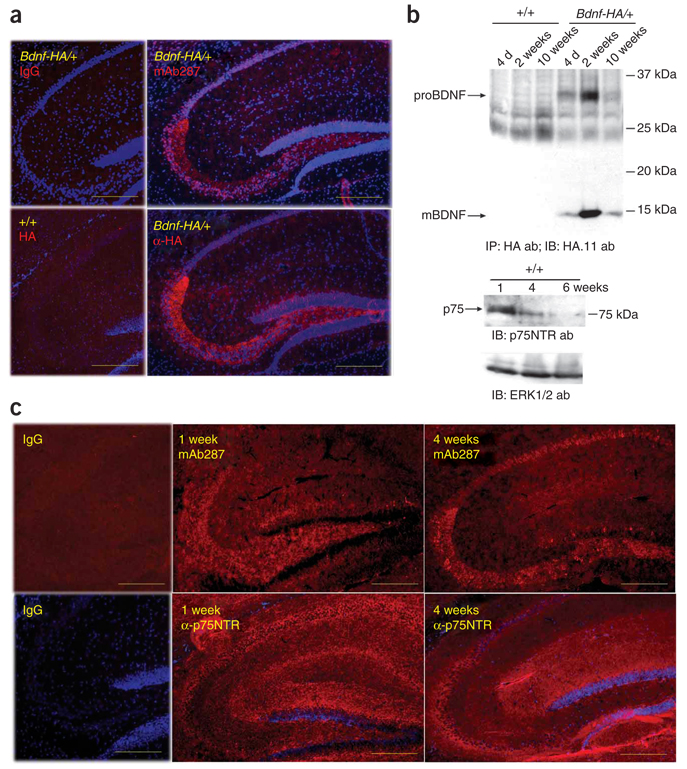
Because proBDNF and mature BDNF show distinct actions on cell survival and synaptic plasticity, we next asked whether proBDNF was more highly expressed when axonal projections are being established and synapses are forming. The cellular localization of prodomain immunoreactivity and HA immunoreactivity were comparable and prominent in dentate granule neurons, particularly in mossy fibers (Fig. 2a) from Bdnf-HA/+ or wild-type brains, and therefore we evaluated hippocampal proBDNF expression at postnatal days (P) 3–21, when mossy fiber projection and synaptogenesis occurs. Indeed, proBDNF expression was highest during the second postnatal week, with levels dropping, but still being detectable, in mature hippocampi (Fig. 2b). In contrast, mature BDNF was expressed throughout postnatal development and was the prominent isoform in the adult. In parallel, hippocampal expression of p75 was highest in the first postnatal week, with substantial reductions at 4 and 6 weeks (Fig. 2b). Immunolocalization in the postnatal hippocampus revealed that p75 and proBDNF were widely expressed at postnatal week 1, but became more restricted at later ages to predominantly the stratum lucidum(Fig. 2c). These results suggested that the spatial and temporal expression of proBDNF and p75 were coordinated, with high levels being observed during of the perinatal window when axonal outgrowth and synapse maturation was robust, and more localized, but maintained, expression occurring during adolescence/adulthood.
Figure 2.
Developmental regulation of proBDNF and p75 expression. (a) Immunofluorescence detection of BDNF in mouse hippocampi (P28) using mAb287, antibodies to HA and Cy3-conjugated streptavidin, shown in serial sections of Bdnf-HA/+ or wild-type brain. Non-immune mouse IgG was used as a control. Scale bars represent 250 µm. (b) Hippocampal lysates from mice of the indicated genotype and ages were subjected to immunoprecipitation/western blot analysis for BDNF isoforms (top), probed to detect p75 (middle) or probed for ERK as a loading control (bottom). (e) Immunofluorescence detection of proBDNF or p75 using 1-week-old and 4-week-old mouse brains. Non-immune IgG was used as a control. Scale bars represent 250 µm.
Our results suggest that proBDNF is not a transient biosynthetic intermediate, but is effectively expressed and transported in mossy fibers during postnatal development. Live imaging of BDNF-GFP in neurons shows that 60% of the BDNF is rapidly transported anterogradely11, consistent with prior studies suggesting that BDNF is sorted to dense-core vesicles12 and is released on depolarization. These results support a model in which BDNF isoforms, probably both proBDNF and mature BDNF, can be targeted and released to modulate synaptic plasticity. The relatively high levels of proBDNF in the early postnatal brain, followed by more efficient conversion of proBDNF to mature BDNF in adolescence and adulthood, indicate that the efficiency of proBDNF conversion is developmentally regulated. However, the mechanisms that prevent efficient proBDNF conversion in the perinatal period are undefined, as pro-convertase 2, which is known to be able to intracellularly cleave proBDNF, is broadly expressed in the CNS by mid-gestation13. However, the coordinate regulation of p75 and proBDNF in the postnatal hippocampus underscores the tightly regulated temporal and regional patterns of proBDNF:p75 receptor pairing. Collectively, these studies suggest that proBDNF actions may be most robust during postnatal development when axonal extension, dendritic spine pruning and synaptic maturation are prevalent, whereas proBDNF effects are more regionally restricted, but are maintained in adulthood.
Supplementary Material
Note: Supplementary information is available on the Nature Neuroscience website.
ACKNOWLEDGMENTS
We thank K. Teng and members of the Hempstead laboratory for their input. These studies were supported by grants from the National Institute of Neurological Disorders and Stroke (NS30687 to B.L.H. and NS52819 to F.S.L.), National 973 Basic Research Program of China (No. 2006CB503803 and 2009CB941403 to Z.-Y.C.) and the Intramural Program of the National Institute of Child Health and Human Development and the National Institute of Mental Health (to B.L.).
Footnotes
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/
References
- 1.Huang EJ, Reichardt LF. Annu. Rev. Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rex CS, et al. J. Neurosci. 2007;27:3017–3029. doi: 10.1523/JNEUROSCI.4037-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen ZY, et al. J. Neurosci. 2005;25:6156–6166. doi: 10.1523/JNEUROSCI.1017-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mowla SJ, et al. J. Biol. Chem. 2001;276:12660–12666. doi: 10.1074/jbc.M008104200. [DOI] [PubMed] [Google Scholar]
- 5.Kolarow R, Brigadski T, Lessmann V. J. Neurosci. 2007;27:10350–10364. doi: 10.1523/JNEUROSCI.0692-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teng HK, et al. J. Neurosci. 2005;25:5455–5463. doi: 10.1523/JNEUROSCI.5123-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woo NH, et al. Nat. Neurosci. 2005;8:1069–1077. doi: 10.1038/nn1510. [DOI] [PubMed] [Google Scholar]
- 8.Yan Q, et al. Neuroscience. 1997;78:431–448. doi: 10.1016/s0306-4522(96)00613-6. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto T, et al. Nat. Neurosci. 2008;11:131–133. doi: 10.1038/nn2038. [DOI] [PubMed] [Google Scholar]
- 10.Tsirka SE, Guslandris A, Amaral DG, Strickland S. Nature. 1995;377:340–344. doi: 10.1038/377340a0. [DOI] [PubMed] [Google Scholar]
- 11.Adachi N, Kohara K, Tsumoto T. BMC Neurosci. 2005;6:42–52. doi: 10.1186/1471-2202-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu YJ, et al. J. Neurosci. Res. 2004b;75:825–834. doi: 10.1002/jnr.20048. [DOI] [PubMed] [Google Scholar]
- 13.Zheng M, Streck RD, Scott RE, Seidah NG, Pintar JE. J. Neurosci. 1994;14:4656–4673. doi: 10.1523/JNEUROSCI.14-08-04656.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Note: Supplementary information is available on the Nature Neuroscience website.